
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 31 January 2022
Sec. Veterinary Infectious Diseases
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.824349
This article is part of the Research TopicInsights in Veterinary Infectious Diseases: 2022View all 9 articles
 Lei Qin1,2†
Lei Qin1,2† Fandan Meng1†
Fandan Meng1† Haijuan He3
Haijuan He3 Yong-Bo Yang1
Yong-Bo Yang1 Gang Wang1
Gang Wang1 Yan-Dong Tang1
Yan-Dong Tang1 Mingxia Sun1
Mingxia Sun1 Wenlong Zhang2
Wenlong Zhang2 Xuehui Cai1*
Xuehui Cai1* Shujie Wang1*
Shujie Wang1*Trueperella pyogenes causes disease in cattle, sheep, goats and swine, and is involved occasionally in human disease worldwide. Most reports implicating T. pyogenes have been associated with clinical cases, whereas no report has focused on pathogenicity of T. pyogenes in mouse models or precision-cut lung slice (PCLS) cultures from swine. Here, we isolated and identified a virulent, β-hemolytic, multidrug-resistant T. pyogenes strain named 20121, which harbors the virulence marker genes fimA, fimE, nanH, nanP and plo. It was found to be highly resistant to erythromycin, azithromycin and medemycin. Strain 20121 was pathogenic in mouse infection models, displaying pulmonary congestion and inflammatory cell infiltration, partial degeneration in epithelial cells of the tracheal and bronchiolar mucosa, a small amount of inflammatory cell infiltration in the submucosa, and bacteria (>104 CFU/g) in the lung. Importantly, we used T. pyogenes 20121 to infect porcine precision-cut lung slices (PCLS) cultures for the first time, where it caused severe bronchoconstriction. Furthermore, dexamethasone showed its ability to relieve bronchoconstriction in PCLS caused by T. pyogenes 20121, highlighting dexamethasone may assist antibiotic treatment for clinical T. pyogenes infection. This is the first report of T. pyogenes used to infect and cause bronchoconstriction in porcine PCLS. Our results suggest that porcine PCLS cultures as a valuable 3D organ model for the study of T. pyogenes infection and treatment in vitro.
Trueperella pyogenes is a Gram-positive, non-motile, non-spore-forming, short, rod- to coccobacillus-shaped bacterium that occurs singly, in pairs or in clusters. T. pyogenes was previously called Corynebacterium pyogenes, Actinomyces pyogenes and Arcanobacterium pyogenes, in chronological order (1, 2). In 2011, according to Yassin et al., Arcanobacterium pyogenes was renamed as T. pyogenes based on phylogenetic and chemotaxonomic observations (2).
T. pyogenes expresses several established and putative virulence factors. To date, known virulence factors mainly include exotoxin pyolysin (PLO), and others promote adhesion factors such as fimbriae (Fim), neuraminidase (NanH, NanP) and collagen-binding protein (CbpA) (3, 4). The cytolysin PLO is considered to be a major virulence factor, associated with cell damage induced by T. pyogenes infection (5). Adhesion factors may be associated with mucosal adherence and colonization of host tissues, thereby contributing to the pathogenicity of T. pyogenes (3).
The use of precision-cut lung slices (PCLS) in three-dimensional (3D) organ models is becoming an area of intensive research due to its close similarity to the host environment (6, 7). There are many advantages in the use of PCLS, including isolated tracheal rings and bronchial rings, and the contraction of airway (7). PCLS have been commonly used in the study of viral infections (8), but there have been few studies on bacterial pathogens (9). Porcine PCLS have been reported for the study of Streptococcus suis (10, 11), but never for T. pyogenes.
Here, we used a porcine PCLS model to study a T. pyogenes isolate, showing that infection of lung tissues by strain 20121 (isolated and characterized from lung tissues of clinically diseased pigs) resulted in severe bronchoconstriction. This study demonstrates that porcine PCLS is a suitable 3D organ model for the study of pathogenicity of T. pyogenes in vitro.
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People's Republic of China. Mouse infection experiments (approval number 210119-02) were carried out in the animal biosafety level 2 facilities under the supervision of the Committee on the Ethics of Animal Experiments of the Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences (CAAS) and the Animal Ethics Committee of Heilongjiang Province, China.
The novel T. pyogenes strain 20121 was isolated from the lungs of two sick nursery pigs with pneumonia and severe abdominal effusion from a pig farm in Heilongjiang Province of China in 2020. The lung samples were cultured on Columbia-based blood agar media (ThermoFisher Scientific, Beijing, China) for 36 h at 37 °C. The isolated colonies were cultured for 36–48 h in tryptic soy agar (TSA, Difco, Loveton Circle Sparks, MD, USA) plates supplemented with 5% fetal bovine serum (FBS, CLARK, USA) at 37 °C and inoculated in tryptic soy broth (TSB, Difco, Loveton Circle Sparks, MD, USA) supplemented with 5% FBS for extraction of genomic DNA.
The isolate 20121 in TSB was observed after rapid Gram stain (AOBOX, Beijing, China) following standard procedure using an optical microscope (Primo Star, ZEISS). Furthermore, the isolate was prepared with standard electron microscopy procedures and the morphological structure was observed using a Hitachi H-7650 transmission electron microscope.
Genomic DNA of T. pyogenes 20121 was extracted by a Bacterial DNA Extraction Kit (Tiangen, Beiing, China) following the manufacturer's instructions. T. pyogenes was positively identified by detecting the 16S rRNA (12) products through PCR. The virulence factors of T. pyogenes 20121 were detected by PCR (13), including hemolysin (pyolysin, PLO), collagen-binding protein (CbpA), neuraminidase (NanH and NanP), and fimbriae (FimA, FimC, FimE and FimG). The primer sequences and reaction conditions are listed in Table 1. PCR products were subsequently analyzed by loading to 1% agarose gels with 2000 bp marker (Tiangen, Beiing, China) and purified with a Gel Extraction Kit (OMEGA, New York, USA). Then, the PCR products were cloned into a pMD18-T vector (Takara, Dalian, China) and positive clones were sequenced by Comate Bioscience Company Ltd.
The susceptibility of T. pyogenes 20121 to different antibiotics was determined by the drug sensitive paper disc diffusion method, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (2016). The following antimicrobials were used: cefazolin, nitrofurantoin, erythromycin, chloramphenicol, kanamycin, ampicillin, ceftazidime, clarithromycin, meropenem, azithromycin, trimethoprim, medemycin, spiramycin, fosfomycin, ceftriaxone, cefoxitin, streptomycin, tetracycline, ciprofloxacin and vancomycin (Tianhe, Hangzhou, China). The quality control strain Streptococcus pneumoniae (ATCC49619) was stored in our lab (12).
The similarities between the nucleotide sequences recovered from isolate 20121 and the reference T. pyogenes sequence published in GenBank were aligned using BLAST online search tool (http://blast.ncbi.nlm.nih.gov). Phylogenetic trees of genes 16S rRNA and plo sequences were constructed with MEGA V 7.0 using the neighbor-joining method and a bootstrap validation with 1,000 replications. Branches corresponding to partitions reproduced in <50% bootstrap replicates were collapsed and shown above the branches. Data from the T. pyogenes 16S rRNA and plo genes used for phylogenetic trees are listed in Tables 2, 3.
To investigate the virulence of T. pyogenes 20121, a mouse survival experiment was carried out. Briefly, 35 six-week-old (18–20 g) female C57BL/6 mice (Changsheng Biotechnology, Liaoning, China) were randomly divided into 5 groups including a non-infection control. The mice were challenged intraperitoneally (i.p.) with 0.2 ml bacterial suspension containing strain 20121 (2 × 106, 2 × 107, 2 × 108 and 2 × 109 CFU) or sterile TSB. Each group contained 6 mice except for the group receiving 2 × 106 CFU, which had 11 mice. Clinical symptoms and mortality were recorded for 14 days, during which any mice exhibiting extreme lethargy were considered moribund and were humanely euthanized. Samples of blood and organs (lung, trachea, heart, liver, ileum, duodenum, spleen and thymus) of infected mice were collected and observed for gross pathological changes. Organ/body weight (g/g) × 100% was calculated and organ samples were fixed immediately in 3.7% formaldehyde (Amresco, Fountain Parkway, USA) for histopathological examination. 5 μl of anticoagulated blood and lung samples were serially diluted in PBS, and plated on blood agar medium for 36 h, and the bacteria in blood and lung samples were quantified by colony counting. 60 μl of anticoagulated blood was tested by a ProCyte Dx automatic blood cell analyzer (IDEXX, USA), determining white blood cell count (WBC), reticulocyte (RET), platelet (PLT), neutrophilic granulocyte percentage (NEUT%), lymphatic number percentage (LYMP%), monocyte percentage (MONO%), eosinophil percentage (EO%) and hematocrit percentage (HCT%).
Fresh lung tissue was obtained after euthanasia from three 3-month-old SPF pigs from Harbin Veterinary Research Institute. The cranial, middle, and intermediate lobes were filled with low-melting agarose (Promega, Madison WI, USA) along the bronchus gently as described previously (14). The filling tissue were covered by ice until the agarose became solidified, then the tissue was stamped out as cylindrical portions with 8-mm tissue coring tool and precision slices were further prepared by a Krumdieck tissue slicer (model MD6000-01; TSE Systems). The PCLS were carefully picked up into 24-well plat (one slice/well) maintained with 1ml fresh medium for additional cultivation for 24 h. Then the airway epithelial cells with 100% ciliary activity were divided randomly into three groups. PCLS were washed 3 times with PBS, then inoculated with T. pyogenes 20121 (8 × 104 CFU or 8 × 105 CFU per slice) in a humidified atmosphere containing 5% CO2 at 37 °C, mock-infected slices were used as control. Infected slices were washed three times with PBS to remove non-attached bacteria at 4 h post-inoculation (hpi), and 1 ml fresh medium (1640, Gibco, Beijing, China) was added for further cultivation.
T. pyogenes 20121-induced bronchoconstriction was detected by imaging and measurement of bronchial cavity area. Initial bronchial cavity area was calculated for each PCLS by imaging at 0 hpi by inverted light microscope (EVOS FL Auto, ThermoFisher Scientific). To quantify the relative bronchoconstriction of the lung tissue, bronchial cavity positions in each infected and mock-infected PCLS were imaged at 4 and 24 hpi. Bronchial cavity areas were measured and calculated using ImageJ/Fiji and the results were presented as bronchial contraction percentage (BCP) using the formula, BCP = [reduced bronchial cavity area / initial bronchial cavity area] × 100%. Treatments were carried out in triplicate and experiments were repeated at least three times.
In order to evaluate the drug intervention effect on bronchoconstriction induced by T. pyogenes 20121, several bronchodilators were selected and applied in this study. Dexamethasone (785 μg/ml in final concentration), atropine (300 μg/ml in final concentration), aminophylline (420.43 μg/ml in final concentration), α-Terpineol (168 μg/ml in final concentration) or salbutamol (250 μg/ml in final concentration) were added at the time of inoculation with strain 20121 (8 × 105 CFU), and then non-attached bacteria were removed at 4 hpi followed by adding fresh medium containing the appropriate drugs.
Numerical data were expressed as the mean ± standard deviation (SD). Statistical analysis of the results was performed with one- or two-way ANOVA using GraphPad Prism version 9.00 (GraphPad, San Diego, CA, USA). Statistical significance was evaluated based on Bonferroni post-tests, and p < 0.05 was considered statistically significant.
Strain 20121 was isolated from two lungs of two sick pigs from Heilongjiang Province of China in December 2020. The isolate shows a narrow zone of β-hemolysis with 0.1-mm hemolytic rings and formed small, white, wet, smooth, and glossy colonies on Columbia blood agar media after culturing for 48 h. It is a Gram-positive coccobacilli or short coryneform occuring in single or pairs (Figure 1A). Electron micrographs of negative staining showed that the isolate were corynebacteria (Figure 1B). Electron micrographs of ultrathin sections showed the strain was most likely encapsulated with 10–25 nm cell wall thickness (Figure 1C). 16S rRNA sequencing indicated that the isolate was T. pyogenes, which harbored virulence factor genes plo, fimA, fimE, nanH and nanP.
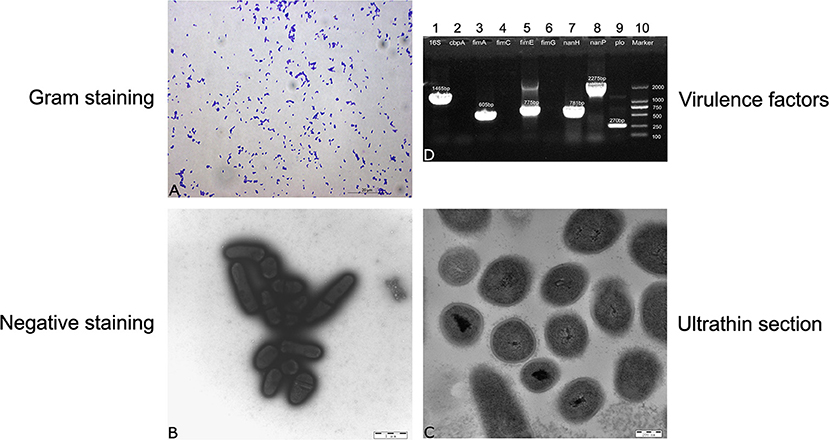
Figure 1. Morphological evaluation and screening of genes encoding virulence factors. (A) Gram staining for strain 20121, (B) negative staining for strain 20121 on transmission electron microscope (C) ultrathin section for strain 20121 on transmission electron microscope, (D) PCR analysis of virulence factors genes, 1: 16S-rRNA, 2: cbpA, 3: fimA, 4: fimC, 5: fimE, 6: fimG, 7: nan-H, 8: nan-P, 9: plo, 10: Marker.
Genes 16S rRNA, plo, fimA, fimE, nanH, nanP of strain 20121 were sequenced and compared to T. pyogenes strains in NCBI GenBank. 16S rRNA sequence of strain 20121 shared >94% nucleotide identity with sequences in GenBank (Figure 1D). In addition, the virulence factors plo (accession MZ189360), fimA (accession MZ189359), fimE (accession MZ579543) and nanP (accession MZ579545) were highly similar, sharing >96, >98, >96, and >97% nucleotide identity, respectively, with sequences in GenBank. The nanH sequence (accession MZ579544) from strain 20121 was less similar, with only 81–89% nucleotide identity with homologous sequences from T. pyogenes strains in GenBank.
To analyze the evolutionary relationship between strain 20121 and other T. pyogenes strains, we constructed phylogenetic trees based on the 16S rRNA Figure 2A and plo Figure 2B. As shown in Figure 2, strain 20121 is located within lineage 1 of the trees based on the 16S rRNA and plo genes, in the same lineage as most Chinese and other Asian isolates, and some European ones. The sequences within lineage 1 shared >99 and >98% nucleotide identity, respectively.
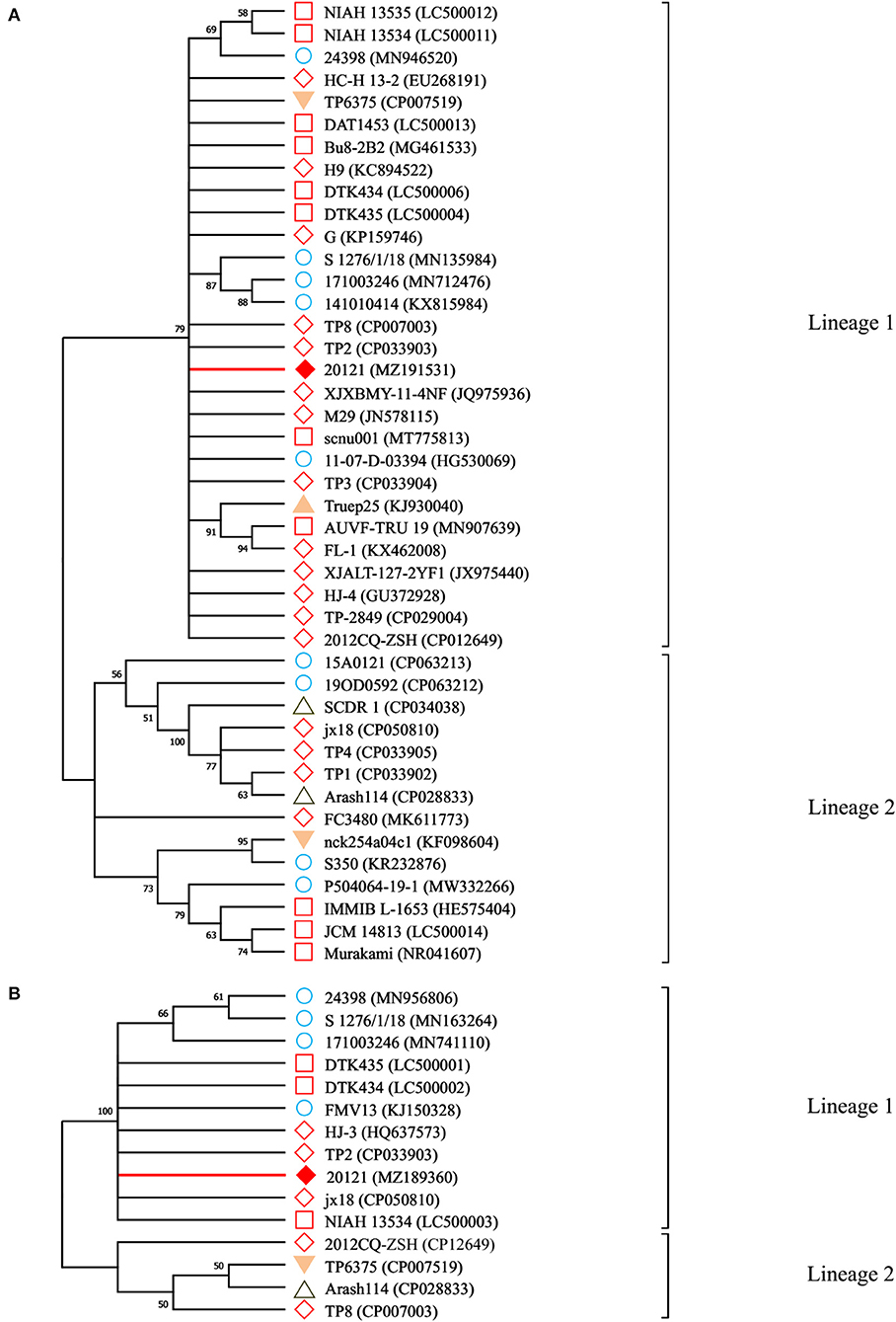
Figure 2. Neighbor-joining phylogenetic trees for 16S RNA and plo genes. A: the phylogenetic trees for 16S RNA gene; B: the phylogenetic trees for plo genes. Branches corresponding to partitions reproduced in <50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown above the branches. denotes the genes sequenced in this study; indicates the reference sequences isolated from China; while indicates sequences isolated from the rest of Asia; are sequences isolated from European countries; △ indicates isolated reports from the Middle Eastern countries, was from a South American country and denotes isolates from North American countries.
In order to determine the drug resistance profile and thus the best way to potentially treat sick animals, we tested strain 20121 for susceptibility to cefazolin, nitrofurantoin, erythromycin, chloramphenicol, kanamycin, ampicillin, ceftazidime, clarithromycin, meropenem, azithromycin, trimethoprim, medemycin, spiramycin, fosfomycin, ceftriaxone, cefoxitin, streptomycin, tetracycline, ciprofloxacin and vancomycin. The test strain was highly resistant to erythromycin, azithromycin and medemycin, with growth exclusion diameters of 6 mm, 10 mm, and 8 mm, respectively, while it was sensitive to the other antibiotics.
To evaluate the virulence of strain 20121 in vivo, we injected six-week-old C57BL/6 mice and tracked their survival and clinical signs. The mice infected with 20121 exhibited depression, trembling, exuberant periocular secretion and fecal adhesions at the anus after injecting 20121, and the clinical signs were bacteria dose-dependent. In the higher dose groups, all of the mice receiving 2 × 109 CFU and most (5/6) of the mice who received 2 × 108 CFU died. The survival percentage of the 2 × 107 CFU and 2 × 106 CFU groups were 50% and 100%, respectively (Figure 3A).
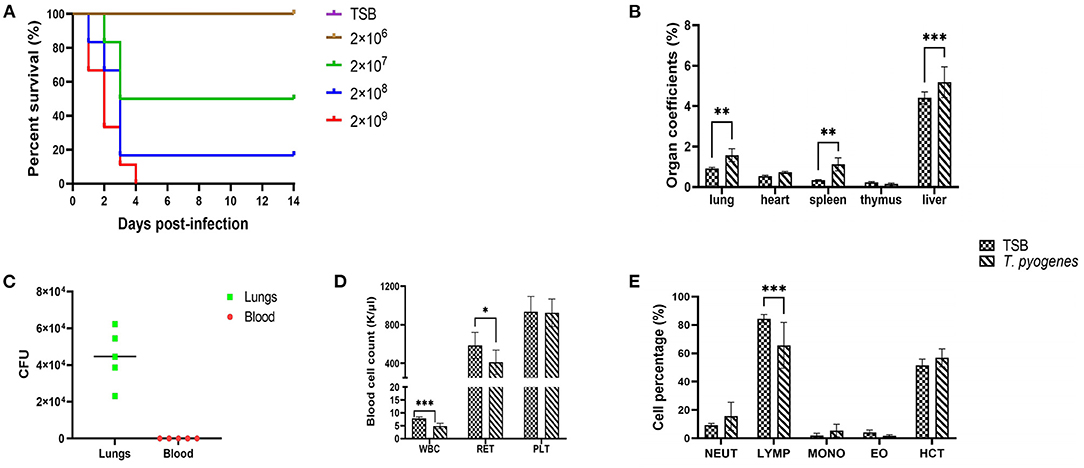
Figure 3. Virulence of T. pyogenes strain 20121 in a mouse model. Mice were challenged intraperitoneally with the strain 20121, as described in the Experimental Procedure. (A) Survival percentage of mice inoculated with strain 20121 or tryptic soy broth (TSB; control). (B) Organ coefficients after 20121 infection at 2 dpi. (C) Bacterial loads in lung (CFU/g of tissue) and blood (CFU/ml). (D) White blood cell (WBC), reticulocyte (RET) and platelet (PLT) count after T. pyogenes infection. (E) Percentage of neutrophilic granulocyte (NEUT), lymphatic number (LYMP), monocyte (MONO), eosinophil (EO), hematocrit (HCT) after T. pyogenes infection. Results are expressed as means ± S.D., and significance was determined using two-way ANOVA and the Sidak multiple-comparison test. *p < 0.05; **p < 0.01; ***p < 0.001.
On 2 dpi, five mice in the 2 × 106 CFU group and three control mice were sacrificed, and the main gross lesions in various organs were visualized. Organ-body weight ratios for lung, heart, spleen, thymus and liver were calculated. As shown in Figure 3B, all coefficients of the lungs, spleen and liver were significantly increased (p < 0.01). In addition, bacteria were detected in the lungs of infected mice, revealing higher levels (>104 CFU/g tissue) on 2 dpi (Figure 3C). No bacteremia was observed at 2 dpi as determined by colony count after plating 5 μl blood on blood agar media (Figure 3C).
We used an automatic blood cell analyzer to detect WBC, RET, PLT, NEUT%, LYMP%, MONO%, EO% and HCT% at 2 dpi. T. pyogenes 20121 infection altered the expression of hematological parameters which can influence immunity. As shown in Figure 3D, the blood levels of WBC decreased significantly (p < 0.001) and RET decreased slightly (p < 0.05) at 2 dpi, and no significant differences in the levels of blood of PLT. Meanwhile, LYMP% in blood of infected mice had a significant decrease (p < 0.001), with 18.8% less than control mice at 2 dpi, but no significant differences in NEUT, MONO, EO, and HCT% in blood (Figure 3E).
Histopathological lesions were determined in mice that were euthanized on 2 dpi (Figure 4A). In 20121-infected mice, the main histopathological lesions observed in the lungs were pulmonary congestion and inflammatory cell infiltration, moderately broadened alveolar diaphragm, partial degeneration in the epithelial cells of the tracheal and bronchial mucosa, and a small amount of inflammatory cell infiltration in the submucosa. In infected livers, extensive degeneration of hepatocytes, partial necrosis, and small focal clusters of Kupffer cells could be seen locally. Extensive necrosis and nuclear pyknosis of thymocytes and infiltration of inflammatory cells into the lamina propria of the chorionic mucosa were seen, along with massive necrosis of lymphocytes in the submucosal lymphatic tissue of the duodenum. A small amount of mucosal epithelial cells degenerated, necrosed and fell off, and Paneth cell degeneration was observed in the ileum (Figure 4B). The main histopathological lesions observed in the spleen were white pulp atrophy and lymphopenia. However, there was no significant changes in the infected heart tissue.
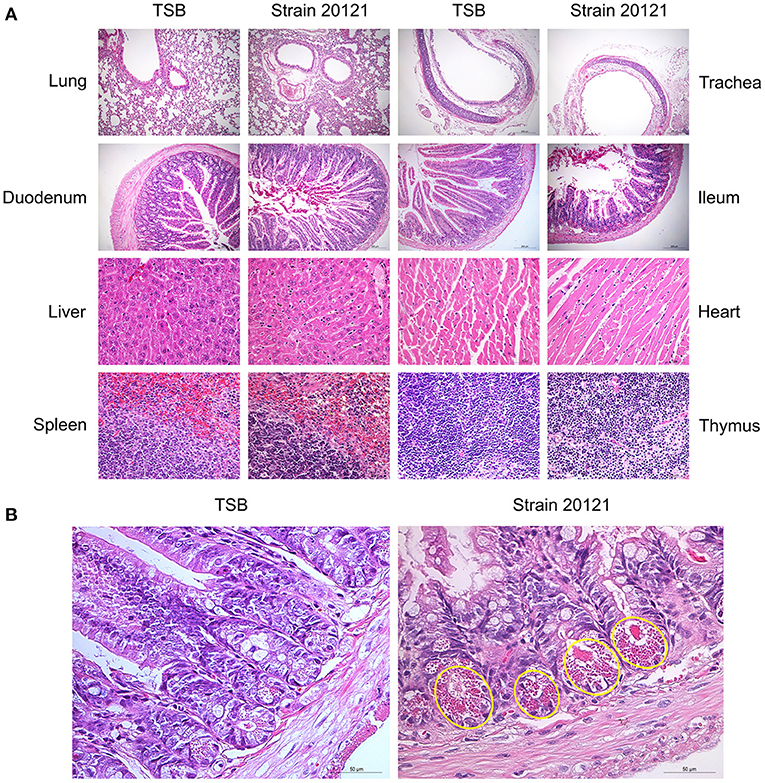
Figure 4. Organ histopathology in mice infected with T. pyogenes. (A) Mice were infected with 2 × 106 CFU of T. pyogenes strain 20121 or inoculated with tryptic soy broth (TSB) as control. Lung, trachea, duodenum, ileum (scale bars = 200 μm), liver, heart, spleen and thymus (scale bars = 50 μm) were collected on 2 dpi for observation of histopathological lesions. (B) Ileum at 2 dpi, showing degeneration of mucosal epithelial cells, necrosis and sloughing, and Paneth cell degeneration (yellow oval); scale bars = 50 μm.
It has been reported that bacterial infection in the respiratory tract may contribute to development of bronchospasm and the progression of chronic obstructive pulmonary disease (COPD) (15). To further evaluate the damage to pig lungs induced by T. pyogenes 20121, we prepared the porcine PCLS and the preparation process was showed in Figure 5A. Then different doses were used to inoculate PCLS for 4 or 24 h. Interestingly, we found that T. pyogenes infection was able to cause obvious bronchoconstriction on PCLS (Figure 5B). A slightly constriction can be observed in bronchus of infected PCLS at 2 hpi. The bronchial cavities of infected PCLS showed obvious reduction in area relative to control PCLS at 4 hpi; the BCP of the 8 × 104 CFU group and 8 × 105 CFU group were nearly 77.11 and 82.44% (Figure 5C), respectively. At 24 hpi, the bronchial cavity area remained nearly 14% (BCP: 86.31%) in the 8 × 104 CFU group, and the area of bronchial cavity almost disappeared completely in 8 × 105 CFU group (BCP: 99.53%) comparing with controls (Figure 5). The observed airway closure was persistent and could not be relieved by the replacement of fresh medium. The high dose infectious group induced a faster bronchoconstriction response, which indicates a dose-dependent capability of strain 20121 to trigger bronchoconstriction.
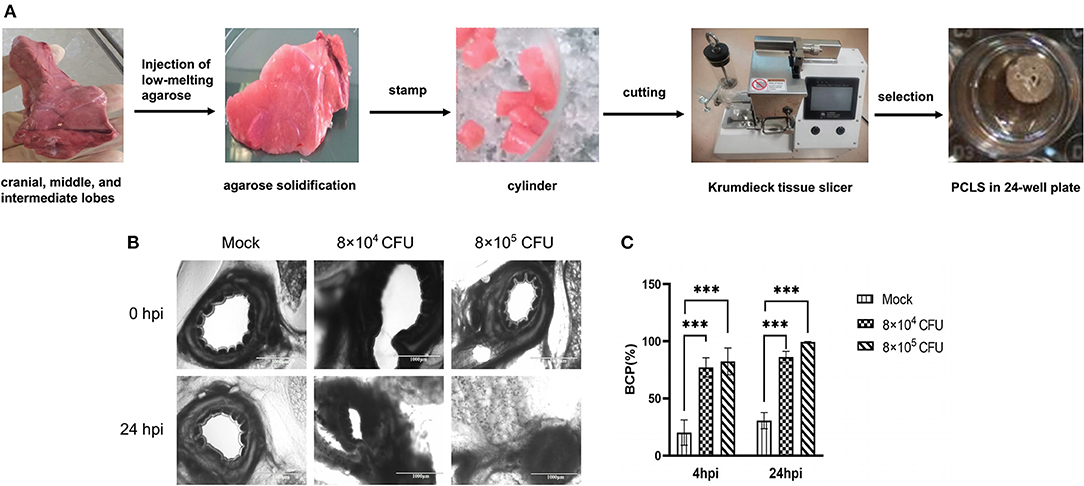
Figure 5. T. pyogenes 20121 induced bronchoconstriction in porcine PCLS cultures. (A) The PCLS preparation process; (B) Light microscope imaging of bronchial cavities in infected (8 × 104 or 8 × 105 CFU) and mock PCLS cultures at 4 and 24 hpi; (C) Bronchial cavity area was measured and calculated as bronchial contraction percentage (BCP). Results are expressed as means ± S.D., and significance was determined using two-way ANOVA and the Tukey multiple-comparison test. ***p < 0.001.
Bronchodilating drugs can dilate the bronchi and bronchioles to improve lung ventilation and relieve wheezing. Five common bronchodilators (dexamethasone, atropine, aminophylline, α-Terpineol and salbutamol) were selected and applied to the PCLS infection model in order to improve bronchospasm and relieve bronchoconstriction caused by T. pyogenes infection. Among the five, our results showed that only dexamethasone relieved the bronchoconstriction caused by T. pyogenes 20121 at 4 hpi and 24 hpi, keeping 14.70 and 19.17% of bronchiole luminal area open, respectively, compared to the no-drug group (Figure 6). Thus, our results indicated that dexamethasone is a candidate for relief of bronchospasm in the treatment of T. pyogenes infection resulting in wheezing.
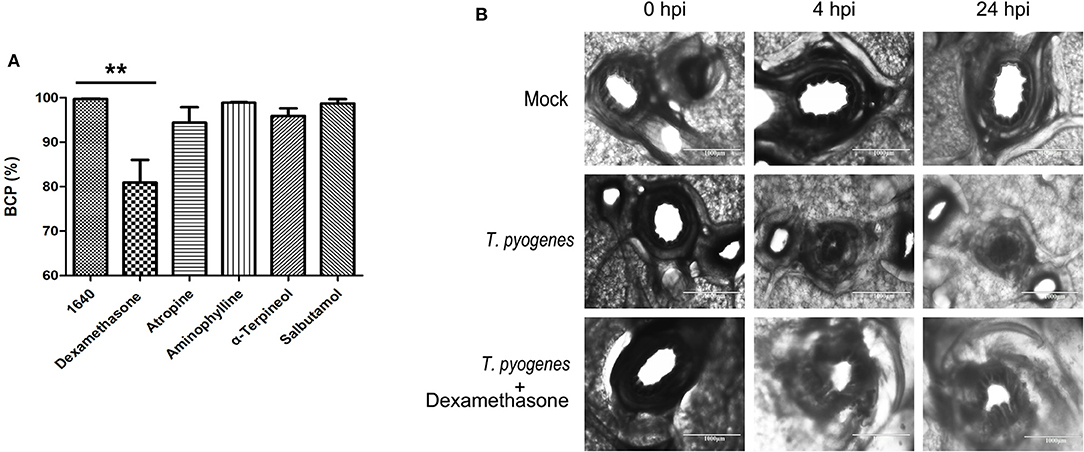
Figure 6. Therapeutic effect of bronchodilators on bronchoconstriction in infected PCLS cultures. (A) The BCP after five common bronchodilators were used to treat bronchoconstriction resulting from T. pyogenes infection. Results are expressed as means ± S.D., and significance was determined using one-way ANOVA and the Tukey multiple-comparison test. **p < 0.01. (B) Therapeutic effect of dexamethasone on bronchoconstriction in PCLS cultures infected with T. pyogenes.
Although T. pyogenes has been especially linked to bovine mastitis, it is a well-known causative agent of diverse clinical presentations among domestic ruminants, pigs, companion animals and gray slender lorises (16). In domestic animals, there have been more bacterial species isolated from cattle and sheep than pigs over the last 10 years (17, 18). However, T. pyogenes infections in pigs have become an increasingly serious clinical problem on large-scale farms (19, 20). T. pyogenes had a high isolation rate in bacterial swine pneumonia in Jilin Province (21). Pneumonia in pigs is caused mainly by viruses and bacteria. In this study, we firstly detected common viruses on the lungs of sick pigs, and the results were all negative. Furthermore, we isolated bacteria and most of clones on the blood plate are T. pyogenes. In order to know the best way to potentially treat the sick animals, we conducted a drug sensitivity experiment and made recommendations to the affected pig farm that resulted in effective control of the disease.
Strain 20121 showed a β-hemolytic phenotype, implying that it could produce a hemolysin that dissolves red blood cells completely. This finding was further confirmed by detecting the virulence factor PLO in the strain. The genotype of virulence factors in strain 20121 was plo/fimA/fimE/nanH/nanP, genes which have also been identified in bovine mastitis, pneumonia and abscesses, as well as encephalitis in goats (22). This is the first report of the genotype plo/fimA/fimE/nanH/nanP identified in swine pneumonia. The sequences of plo, fimA, fimE and nanP were highly similar to sequences in GenBank, indicating that these genes are highly conserved in T. pyogenes species. However, the nanH sequence of strain 20121 was relatively less similar to other nanH sequences from GenBank, suggesting that it may be less conserved among T. pyogenes isolates.
This is the first report of T. pyogenes infection in a mouse model causing massive necrosis of lymphocytes in the submucosal lymphatic tissue of the duodenum. Intestinal Paneth cells are the “gatekeepers” of intestinal innate immunity, thus impacts to Paneth cells can cause intestinal inflammation and inflammatory bowel disease. Therefore, our results suggest that T. pyogenes infection of mice induces abnormal mucosal immune response, thereby downregulating the immune response. Also, the levels of WBC and LYMP% in blood decreased significantly at 2 dpi, indicating that T. pyogenes infection reduced the immune function in mice. What's more, there was extensive necrosis in thymocytes, suggesting that the central immune organ thymus was destroyed after T. pyogenes infection in mice. Altogether, we provided evidence to show that T. pyogenes decreases immune function in the early stages of infection, though much remains undetermined and warrants further investigation.
For the first time in swine PCLS cultures, we showed that infection of T. pyogenes induced severe bronchoconstriction and led to a narrowing of the luminal area of bronchioles, suggesting PCLS are a suitable 3D model for the study T. pyogenes infection. Narrowing of bronchioles would affect lung ventilation and cause severe airflow limitations, which may aggravate damage to the lungs and cause the host to die suddenly from asphyxiation. We speculate that an inflammatory response may responsible for contraction of airway smooth muscle induced by T. pyogenes infection. However, the mechanisms by which T. pyogenes causes bronchoconstriction remain unclear and warrants further study in the future.
PCLS cultures have been reported to preserve lung functions for around 10 days (23), during which the bronchioles can maintain reversible constriction after methacholine treatment (24). Thus, as a good model for analyzing the effect of drugs on lung tissue, PCLS cultures have been applied for toxicological and functional studies (25, 26). In the current study, we found that dexamethasone dilated airways and kept the bronchioles open, showing a protective effect for bronchoconstriction caused by T. pyogenes infection in porcine PCLS. Dexamethasone is a long-acting glucocorticoid that is widely used due to its anti-inflammatory and immunosuppressive properties (27). High-dose glucocorticoid intervention is used for the treatment of mild or severe asthma with sudden onset in human to improve alveolar ventilation while treating the underlying illness (28). With respect to treatment of T. pyogenes infection, we suggest that a combination therapy of dexamethasone and antibiotics should be taken into account in swine clinical practice. Moreover, PCLS can be used in the hunt for more effective therapeutic drugs for T. pyogenes infection.
In conclusion, we isolated and identified a multidrug-resistant T. pyogenes strain from the lungs of sick pigs. It was virulent in a mouse infection model and also led to heavy bronchoconstriction in porcine PCLS cultures. What's more, bronchodilators showed their ability to antagonize bronchoconstriction in the same cultures. In summary, our results highlight porcine PCLS cultures as a valuable 3D organ model for the study of T. pyogenes infection and treatment in vitro.
A virulent, multidrug-resistant T. pyogenes strain induced severe bronchoconstriction in porcine PCLS, highlighting a valuable 3D organ model for the study of T. pyogenes infection in vitro.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, MZ189360; https://www.ncbi.nlm.nih.gov/genbank/, MZ189359; https://www.ncbi.nlm.nih.gov/genbank/, MZ579543; https://www.ncbi.nlm.nih.gov/genbank/, MZ579544; https://www.ncbi.nlm.nih.gov/genbank/, MZ579545.
The animal study was reviewed and approved by the Committee on the Ethics of Animal Experiments of the Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences (CAAS) and the Animal Ethics Committee of Heilongjiang Province, China.
SW, FM, and XC conceived the study and designed the experimental procedures. LQ, FM, and SW performed the experiments. LQ, FM, HH, MS, WZ, and SW analyzed the data. HH, Y-BY, Y-DT, GW, MS, WZ, and XC contributed reagents and materials. LQ, FM, and SW wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (32002249), the Natural Science Foundation of Heilongjiang Province (QC2018030), and the Local Social Science Project (2018QD0031) of Heilongjiang Province and Heilongjiang pig Modern Agricultural Technology Collaborative Innovation System.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Collins MD, Jones D, Kroppenstedt RM, Schleifer KH. Chemical studies as a guide to the classification of Corynebacterium pyogenes and “Corynebacterium haemolyticum”. J Gen Microbiol. (1982) 128:335–41. doi: 10.1099/00221287-128-2-335
2. Yassin AF, Hupfer H, Siering C, Schumann P. Comparative chemotaxonomic and phylogenetic studies on the genus Arcanobacterium 1 emend. Lehnen et al. 2006: proposal for Trueperella gen nov and emended description of the genus Arcanobacterium. Int J Syst Evol Microbiol. (2011) 61:1265–74. doi: 10.1099/ijs.0.020032-0
3. Jost BH, Billington SJ. Arcanobacterium pyogenes: molecular pathogenesis of an animal opportunist. Antonie Van Leeuwenhoek. (2005) 88:87–102. doi: 10.1007/s10482-005-2316-5
4. Yang L, Liang H, Wang B, Ma B, Wang J, Zhang W. Evaluation of the potency of two pyolysin-derived recombinant proteins as vaccine candidates of Trueperella Pyogenes in a mouse model: pyolysin oligomerization and structural change affect the efficacy of pyolysin-based vaccines. Vaccines (Basel). (2020) 8. doi: 10.3390/vaccines8010079
5. Rudnick ST, Jost BH, Billington SJ. Transcriptional regulation of pyolysin production in the animal pathogen, Arcanobacterium pyogenes. Vet Microbiol. (2008) 132:96–104. doi: 10.1016/j.vetmic.2008.04.025
6. Henjakovic M, Martin C, Hoymann HG, Sewald K, Ressmeyer AR, Dassow C, et al. Ex vivo lung function measurements in precision-cut lung slices (PCLS) from chemical allergen-sensitized mice represent a suitable alternative to in vivo studies. Toxicol Sci. (2008) 106:444–53. doi: 10.1093/toxsci/kfn178
7. Weldearegay YB, Müller S, Hänske J, Schulze A, Kostka A, Rüger N, et al. Host-Pathogen Interactions of Mycoplasma mycoides in Caprine and Bovine Precision-Cut Lung Slices (PCLS) Models. Pathogens. (2019) 8. doi: 10.3390/pathogens8020082
8. Dobrescu I, Levast B, Lai K, Delgado-Ortega M, Walker S, Banman S, et al. In vitro and ex vivo analyses of co-infections with swine influenza and porcine reproductive and respiratory syndrome viruses. Vet Microbiol. (2014) 169:18–32. doi: 10.1016/j.vetmic.2013.11.037
9. Ebsen M, Mogilevski G, Anhenn O, Maiworm V, Theegarten D, Schwarze J, et al. Infection of murine precision cut lung slices (PCLS) with respiratory syncytial virus (RSV) and chlamydophila pneumoniae using the Krumdieck technique. Pathol Res Pract. (2002) 198:747–53. doi: 10.1078/0344-0338-00331
10. Meng F, Wu NH, Nerlich A, Herrler G, Valentin-Weigand P, Seitz M. Dynamic virus-bacterium interactions in a porcine precision-cut lung slice coinfection model: swine influenza virus paves the way for Streptococcus suis Infection in a two-step process. Infect Immun. (2015) 83:2806–15. doi: 10.1128/IAI.00171-15
11. Dresen M, Schenk J, Berhanu Weldearegay Y, Vötsch D, Baumgärtner W, Valentin-Weigand P, et al. Streptococcus suis Expression of Cyclooxygenase-2 in Porcine Lung Tissue. Microorganisms. (2021) 9. doi: 10.3390/microorganisms9020366
12. Wang S, Zhang D, Jiang C, He H, Cui C, Duan W, et al. Strain characterization of Streptococcus suis serotypes 28 and 31, which harbor the resistance genes optrA and ant(6)-Ia. Pathogens. (2021) 10. doi: 10.3390/pathogens10020213
13. Zastempowska E, Lassa H. Genotypic characterization and evaluation of an antibiotic resistance of Trueperella pyogenes (Arcanobacterium pyogenes) isolated from milk of dairy cows with clinical mastitis. Vet Microbiol. (2012) 161:153–8.
14. Barton KT, Conklin DR, Ranabhat RS, Harper M, Holmes-Cobb LM, Martinez Soto MH, et al. Methacholine induced airway contraction in porcine precision cut lung slices from indoor and outdoor reared pigs. Am J Transl Res. (2020) 12:2805–13.
15. Cazzola M, Matera MG, Rossi F. Bronchial hyperresponsiveness and bacterial respiratory infections. Clin Ther. (1991) 13:157–71.
16. Ribeiro MG, Risseti RM, Bolaños CA, Caffaro KA, De Morais AC, Lara GH, et al. Trueperella pyogenes multispecies infections in domestic animals: a retrospective study of 144 cases (2002 to 2012). Vet Q. (2015) 35:82–7. doi: 10.1080/01652176.2015.1022667
17. Nagib S, Rau J, Sammra O, Lämmler C, Schlez K, Zschöck M, et al. Identification of Trueperella pyogenes isolated from bovine mastitis by Fourier transform infrared spectroscopy. PLoS ONE. (2014) 9:e104654. doi: 10.1371/journal.pone.0104654
18. Jaureguiberry M, Madoz LV, Giuliodori MJ, Wagener K, Prunner I, Grunert T, et al. Identification of Escherichia coli and Trueperella pyogenes isolated from the uterus of dairy cows using routine bacteriological testing and Fourier transform infrared spectroscopy. Acta Vet Scand. (2016) 58:81. doi: 10.1186/s13028-016-0262-z
19. Galán-Relaño Á, Gómez-Gascón L, Luque I, Barrero-Domínguez B, Casamayor A, Cardoso-Toset F, et al. Antimicrobial susceptibility and genetic characterization of Trueperella pyogenes isolates from pigs reared under intensive and extensive farming practices. Vet Microbiol. (2019) 232:89–95. doi: 10.1016/j.vetmic.2019.04.011
20. Jarosz ŁS, Gradzki Z, Kalinowski M. Trueperella pyogenes infections in swine: clinical course and pathology. Pol J Vet Sci. (2014) 17:395–404. doi: 10.2478/pjvs-2014-0055
21. Dong WL, Liu L, Odah KA, Atiah LA, Gao YH, Kong LC, et al. Antimicrobial resistance and presence of virulence factor genes in Trueperella pyogenes isolated from pig lungs with pneumonia. Trop Anim Health Prod. (2019) 51:2099–103. doi: 10.1007/s11250-019-01916-z
22. Risseti RM, Zastempowska E, Twaruzek M, Lassa H, Pantoja JCF, De Vargas APC, et al. Virulence markers associated with Trueperella pyogenes infections in livestock and companion animals. Lett Appl Microbiol. (2017) 65:125–32. doi: 10.1111/lam.12757
23. Meng F, Punyadarsaniya D, Uhlenbruck S, Hennig-Pauka I, Schwegmann-Wessels C, Ren X, et al. Replication characteristics of swine influenza viruses in precision-cut lung slices reflect the virulence properties of the viruses. Vet Res. (2013) 44:110. doi: 10.1186/1297-9716-44-110
24. Punyadarsaniya D, Liang CH, Winter C, Petersen H, Rautenschlein S, Hennig-Pauka I, et al. Infection of differentiated porcine airway epithelial cells by influenza virus: differential susceptibility to infection by porcine and avian viruses. PLoS ONE. (2011) 6:e28429. doi: 10.1371/journal.pone.0028429
25. Ressmeyer AR, Larsson AK, Vollmer E, Dahlèn SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J. (2006) 28:603–11. doi: 10.1183/09031936.06.00004206
26. Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, et al. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am J Respir Crit Care Med. (2012) 186:225–32. doi: 10.1164/rccm.201202-0368OC
27. Becker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesth Prog. (2013) 60:25–31. doi: 10.2344/0003-3006-60.1.25
Keywords: Trueperella pyogenes, porcine precision-cut lung slices, virulent, bronchoconstriction, infection
Citation: Qin L, Meng F, He H, Yang Y-B, Wang G, Tang Y-D, Sun M, Zhang W, Cai X and Wang S (2022) A Virulent Trueperella pyogenes Isolate, Which Causes Severe Bronchoconstriction in Porcine Precision-Cut Lung Slices. Front. Vet. Sci. 8:824349. doi: 10.3389/fvets.2021.824349
Received: 29 November 2021; Accepted: 23 December 2021;
Published: 31 January 2022.
Edited by:
Mujeeb Ur Rehman, Livestock and Dairy Development Department, PakistanReviewed by:
Ting Huang, Chengdu University, ChinaCopyright © 2022 Qin, Meng, He, Yang, Wang, Tang, Sun, Zhang, Cai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuehui Cai, Y2FpeHVlaHVpQGNhYXMuY24=; Shujie Wang, d2FuZ3NodWppZUBjYWFzLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.