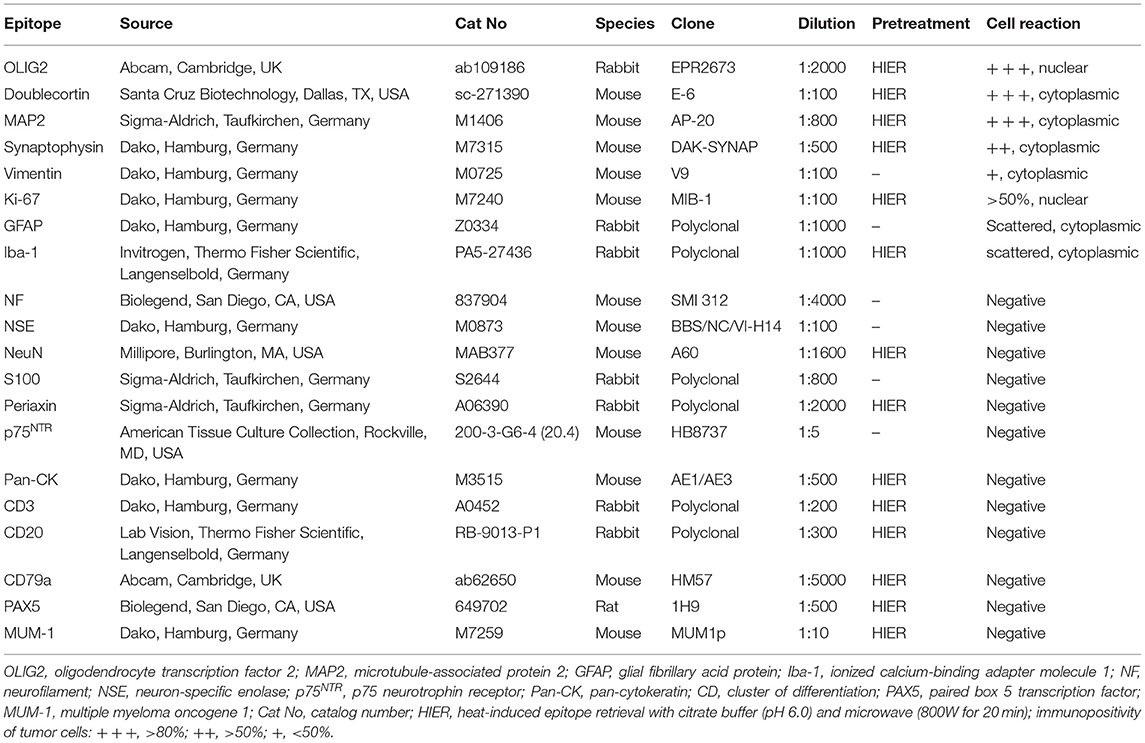
- 1Department of Pathology, University of Veterinary Medicine, Hannover, Germany
- 2Tierärztliche Praxis für Kleintiere Dr. med. vet. Jürgen Weber, Oer-Erkenschwick, Germany
A 2-year-old cat was presented with progressive ataxia. Despite treatment the animal died. Pathomorphological examination revealed a widespread leptomeningeal mass at all levels of the central nervous system accentuated on the cervical spinal cord and the medulla oblongata without presence of a primary intraaxial tumor. The neoplasm was mainly composed of round, uninucleate cells with hyperchromatic nuclei, which were immunopositive for OLIG2, doublecortin, MAP2, synaptophysin, and vimentin, indicating components of both oligodendroglial and neuronal differentiation. Ki-67 immunohistochemistry indicated a high proliferation activity of the neoplasm. Few GFAP positive and Iba-1 positive cells were interpreted as reactive astrocytes and macrophages or microglia, respectively. The tumor was immunonegative for CD3, CD20, PAX5, MUM1, pan-cytokeratin, S100, NSE, p75NTR, NeuN and periaxin. These findings led to the diagnosis of primary diffuse leptomeningeal oligodendrogliomatosis. This is the first reported case of this entity in a young cat, which should be considered as a differential diagnosis for diffuse subarachnoidal round cell infiltrates.
Introduction
Gliomas are the most common primary intracranial neoplasms in humans and, after meningiomas, the second most frequent primary CNS neoplasm in dogs (1, 2). They are uncommon to rare in cats (3–7).
Based on their morphological appearance and immunohistochemical profile, gliomas have been classically grouped into astrocytomas, oligodendrogliomas, and ependymomas with various subtypes. During recent years, genetic alterations have gained importance in classification of human gliomas, which have not been determined yet in animals (8).
Commonly, glial neoplasms develop as intraaxial tumors within the telencephalic white and gray matter including basal nuclei, the diencephalon (thalamus, hypothalamus) and the mesencephalon with only rare occurrence in the pons, medulla oblongata and the spinal cord. Especially anaplastic variants and glioblastomas (WHO grade III and IV) may secondarily infiltrate into adjacent structures, including the leptomeninges. Rarely, this is followed by further diffuse spread via the cerebrospinal fluid (CSF), resulting in secondary diffuse leptomeningeal gliomatosis (9–14).
In contrast, primary diffuse leptomeningeal gliomatosis (PDLG) is an even rarer manifestation which is characterized by a diffuse infiltration of the subarachnoid space by neoplastic glial cells without evidence of a primary intraaxial tumor (15).
PDLG was firstly described in humans in 1923 and in dogs in 2013 (16–18). Recently, a case of primary leptomeningeal gliomatosis was described in an older cat (19).
Due to its rarity and unspecific clinical and radiological findings, an intravital diagnosis, predominantly based on MRI findings and exclusion of other diseases, is challenging and requires histopathological confirmation (15, 20, 21). In human medicine, adequate meningeal biopsy is a useful tool allowing a definitive diagnosis (22). However, due to the aggressive nature of the tumor, short clinical course and poor prognosis, in most cases final diagnosis of PDLG is made at necropsy (20).
The present report describes the clinical, pathomorphological, and immunohistochemical findings in a young cat with primary diffuse leptomeningeal oligodendrogliomatosis.
Case Description
Clinical History
A 2-year-old, female-neutered domestic shorthair cat of 3.43 kg body weight was presented with acute back pain and mild ataxia after a jump from a cupboard. Clinical examination revealed no additional specific findings. A presumptive diagnosis of suspect traumatic injury of vertebral bone and/or spinal cord was made and the cat was treated with robenacoxib (Onsior™ 1.75 mg/kg, s.i.d.). After 2 days without clinical improvement, blood count and blood chemistry were performed and revealed a mild hypophosphatemia (3.1 mg/dl, reference range: 3.4–8.5 mg/dl) and monocytosis (6.6%, reference range: 1–3%). A radiological examination of the thorax, abdomen and the proximal hind limbs showed a mildly increased interstitial pattern in the craniodorsal lung, but no abnormalities in the skeleton or the spinal cord (Supplementary Figure 1A). The cat was further treated with meloxicam (Metacam™ 0.05 mg/kg s.i.d.), amoxicillin (Duphamox LA™ 15 mg/kg once every other day) and vitamin B (B-Vitamin-Tabletten™, one pill s.i.d.). Four days after initial presentation, the cat showed mild apathy and severe ataxia with markedly reduced proprioceptive reactions in all four limbs and reduced segmental reflexes in the thoracic limbs. The suspected neuroanatomical localization was the brain or the spinal cord cranial to T2. No cells were found in the cerebrospinal fluid (CSF). An infection with feline coronavirus, bornavirus, Toxoplasma gondii and Bartonella henselae within the CSF was excluded via PCR. Computed tomography (CT) of the entire body was performed but showed no abnormalities of the central nervous system (Supplementary Figures 1B,C). Therefore, the cause of neurological deficits remained undefined. The cat was hospitalized and treated with Ringer infusion (Deltamedica™ 5 ml/kg body weight during the entire time of hospitalization). Further progressive clinical decline with irresponsiveness to treatment resulted in lateral recumbency and death, which was 6 days after initial presentation. The cat was subsequently submitted to the Department of Pathology for pathological examination (necropsy, histopathological examination, immunohistochemistry).
Pathomorphological Findings
At gross examination, a diffuse enlargement of the leptomeninges was noted, mostly pronounced within cervical segments with rostral extension to the medulla oblongata. After formalin fixation, cross sections revealed a well demarcated, gray-beige, soft, subdural thickening within the whole circumference of the spinal cord, involving primarily the cervical part, and the medulla oblongata with partial compression of the neuroparenchyma (Figure 1A). Macroscopically, no intraaxial tumor was found in coronal sections of the brain and spinal cord.
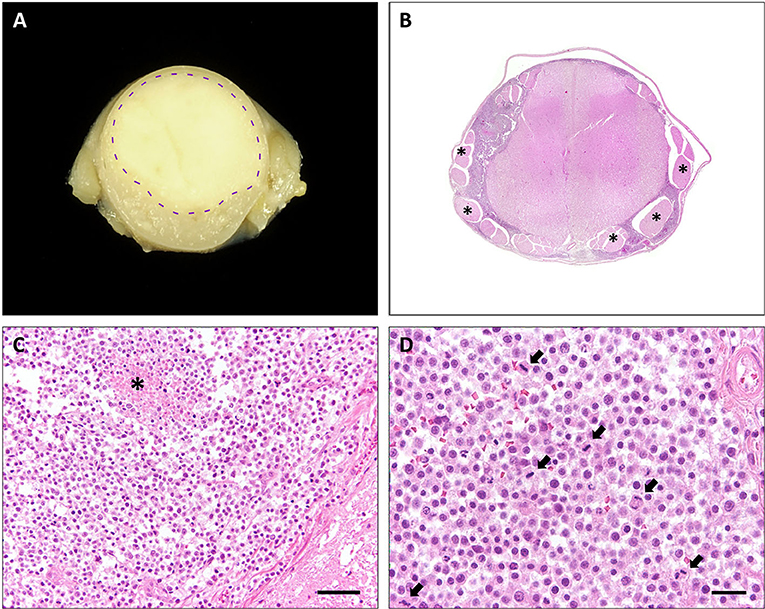
Figure 1. Cervical spinal cord, cat. (A) Transversal section of formalin-fixed spinal cord with leptomeningeal pale, gray-beige mass (dashed line: junction tumor-spinal medulla). (B) Diffuse, basophilic cell infiltrates and edema within leptomeninges with compression of spinal cord. Spinal nerves (asterisks) are omitted by the tumor. HE stain. (C) Neoplastic infiltrates with junction to spinal medulla (bottom right) and necrosis (asterisk), HE stain. Scale bar: 50 μm. (D) High mitotic activity within the neoplasm (arrows), HE stain. Scale bar: 20 μm.
All other organs lacked significant macroscopic findings.
Histopathological examination of representative localizations of the CNS (cerebral cortex, hippocampus, cerebellum, mesencephalon, medulla oblongata, and cervical, thoracic, lumbar, and sacral spinal cord) revealed a restriction of the mass to the subarachnoidal space (Figure 1B). It was composed of closely packed, round, uninucleated cells arranged in sheets, accompanied by low amounts of fine fibrovascular stroma. The medium-sized cells possessed variably distinct cell borders and contained low amounts of finely granular eosinophilic cytoplasm. Nuclei measured 10–15 μm in diameter, were centrally to eccentrically located, round to oval and frequently hyperchromatic with one distinct small nucleolus. Multifocally, large necrotic areas were present (Figure 1C). There was mild anisocytosis and –karyosis with a mitotic count of 20 mitoses per 2.37 mm2 (Figure 1D). In two localizations, the tumor infiltrated superficially the adjacent neuroparenchyma of the dorsal medulla oblongata and the cerebral cortex.
The neuroparenchyma adjacent to the tumor multifocally showed compression and marked degenerative changes including spheroid formation and vacuolation of the white and gray matter.
Immunohistochemical Findings
In order to phenotype the tumor cells, selected representative paraffin-embedded samples of the brain and the spinal cord were subjected to immunohistochemistry. The respective primary antibodies and information referring to antigen-retrieval are listed in Table 1. Neoplastic cells showed a diffuse, intranuclear expression of oligodendrocyte transcription factor 2 (OLIG2) and doublecortin (Figures 2A,B). Moreover, tumor cells exhibited a diffuse cytoplasmic expression of microtubule-associated protein 2 (MAP2) and synaptophysin (Figures 2C,D). Some of the neoplastic cells showed a cytoplasmic vimentin expression (Figure 2E). Ki-67 as a proliferation marker protein was detected in more than 50% of the neoplastic cells (Figure 2F). Scattered ionized calcium-binding adapter molecule 1 (Iba1)-positive cells and few scattered glial fibrillary acid protein (GFAP)-positive cells were present within the neoplasm. Neoplastic cells lacked immunoreactivity for neurofilament (NF), S100 protein, neuron specific enolase (NSE), p75 neurotrophin receptor, neuronal nuclear protein (NeuN), periaxin, pan-cytokeratin, CD3, CD20, CD79a, paired box 5 transcription factor (PAX5), and multiple myeloma oncogene 1 (MUM1).

Figure 2. Spinal cord, cat. Tumor cells are diffusely immunopositive for OLIG2 (A), doublecortin (B), and MAP2 (C). The majority of tumor cells stained positive with synaptophysin (D). Positive vimentin reaction is mainly restricted to tumor—associated vasculature (E). High proliferation activity was demonstrated with positive nuclear staining for Ki-67 (F). Scale bars: 50 μm.
Discussion
The morphological findings in the present case, in particular the absence of a primary intraaxial tumor and the immunostaining profile, are consistent with primary diffuse leptomeningeal oligodendrogliomatosis, which is a tumor that is mainly composed of oligodendroglial neoplastic cells.
In general, tumors of oligodendroglial origin are histopathologically characterized by uniform, densely packed cells with vacuolated or eosinophilic cytoplasm, a round hyperchromatic nucleus and distinct cell borders with variable patterns of cell arrangement. Not infrequently, delayed formalin fixation causes a perinuclear halo, resulting in a “honeycomb pattern” appearance (23). However, tumor cell morphology and origin are variable and the tumor might resemble other tumor types, including neuronal tumors and lymphomas, which have to be excluded via immunohistochemistry.
The present tumor was diffusely immunopositive for OLIG2, doublecortin, MAP2, synaptophysin, vimentin, and Ki-67 (24). Albeit being expressed in other gliomas, the proportion of OLIG2 positive cells in oligodendroglial tumors in humans and dogs is significantly higher compared to astrocytomas and oligoastrocytomas (24, 25). Positive reaction with OLIG2 has been demonstrated in oligodendrogliomatosis of other species and the first reported feline case of gliomatosis (16, 17, 19, 26, 27).
Doublecortin is expressed in invasive brain tumors in humans, in canine oligodendroglioma, anaplastic meningiomas, and leptomeningeal oligodendrogliomatosis (16, 28–30), which might give support to the theory that neuronal progenitors might contribute to the development of these tumors.
Microtubule-associated protein 2 (MAP2) immunopositivity was reported in canine oligodendroglioma, (31) in the previously described case of a cat with primary leptomeningeal gliomatosis, and an African hedgehog with an oligodendroglioma with neuronal differentiation (19, 31, 32).
Synaptophysin immunopositivity, as observed in the present case, has been reported in human and canine oligodendrogliomas and diffuse leptomeningeal glioneuronal tumors (8, 10, 33) as well as in an oligendroglioma with neuronal differentiation in an African hedgehog (32) and two dogs (34). However, immunopositivity seems to be variable, as there are also reports of oligodendroglial tumors and primary diffuse leptomeningeal oligodendroglial tumors which were immunonegative for synaptophysin (35, 36).
Vimentin expression in oligodendrogliomas is variable, but has been reported in the recently published feline case of primary leptomeningeal gliomatosis, a canine brain oligodendroglioma, a canine leptomeningeal spinal oligodendroglioma, and in human cases of PDLG (15, 16, 19, 20, 37). It was negative in a bovine and canine case of diffuse leptomeningeal oligodendrogliomatosis (26, 27). Predominantly, it stains vascular elements of the tumor stroma.
The present tumor showed a high labeling index for the proliferation marker Ki-67. Although it is often highly expressed in anaplastic glial tumors of cats, Ki-67 expression in feline gliomas does not always correlate with the grade of the tumor (12, 38). In the recently published case of feline primary diffuse leptomeningeal gliomatosis, approximately 5% of tumor cells were positive for Ki-67 (19).
The scattered GFAP- and Iba1-positive cells in our case were interpreted as reactive astrocytes and macrophages/microglia, respectively.
The present tumor was immunonegative for NF, NSE, NeuN, and S100. These markers are variably expressed in oligodendroglial tumors of domestic animals and humans (16, 26, 27, 33, 36, 37, 39, 40).
Immunonegativity of the present tumor for periaxin and p75NTR, pan-cytokeratin, and CD3, CD20, CD79a, PAX5, and MUM1 excluded nerval, epithelial, and lymphocytic origin, respectively.
At large, the variable and inconsistent immunohistochemical findings regarding neuronal markers in oligodendrogliomas and PDLG imply that oligodendrogliomas and oligodendroglioma-like tumors might arise from common glioneuronal progenitor cells (41, 42). This theory is supported by the fact that classic oligodendrogliomas may present with neurocytic rosettes and neurocytoma, a rare intracranial neuroepithelial tumor in humans, can show 1p/19q deletion, a mutation commonly found in human oligodendroglia-like tumors (33, 43).
It has been postulated that PDLG originates from heterotopic glial nests, which are small aggregates of glial cells within the subarachnoid space arising from protrusions of mature glia cells from the neuraxis. They are most frequently found at the level of the medulla oblongata and the lumbar spinal cord, in approximately 1% of random necropsies of humans. The pronounced manifestation of the tumor within the subarachnoidea of the medulla oblongata in the present case lends support to this theory. However, although glial heterotopias were detected in a human case of primary diffuse leptomeningeal oligodendroglioma at the brain base, these tumors can also manifest in other localizations along the entire CNS (20, 44).
In the current human WHO classification of tumors of the nervous system, various oligodendrogliomatous diffuse leptomeningeal masses either with or without primary intraaxial involvement and neuronal components, are categorized by specific genetic abnormalities and have recently been summarized under the term “primary diffuse glioneuronal tumor,” as the nosological position of these tumors remains controversial (8, 45).
In humans, there is an age predilection of diffuse leptomeningeal oligodendroglioma-like neoplasms toward children and young adults, although all ages are susceptible (45). Low-grade diffuse gliomas and oligodendrogliomas as well as diffuse leptomeningeal neuroepithelial tumors in children have been attributed to 1p and/or 19q loss with associated BRAF gain or mutation, resulting in an activated MAPK signaling pathway (46–48). In oligodendrogliomas, IDH1 or 2 mutations frequently occur (49, 50).
Regarding domestic animals, brachycephalic dog breeds are predisposed to develop oligodendroglioma with a suspected defect on chromosome 26 (2, 51). The exclusive representation of brachycephalic dogs (4 boxer dogs, 1 Staffordshire bull terrier, 1 Cane Corso) in published cases of canine diffuse leptomeningeal gliomatosis may suggest a similar breed disposition to PDLG (16, 17, 27, 52). However, further case data are required to confirm this assumption. For PDLG in domestic animals, no specific genetic alterations have been determined so far.
Clinical findings in humans and animals with leptomeningeal gliomatosis are relatively unspecific and are the sequel of impaired liquor circulation and compression of adjacent intraaxial structures (20, 39). In dogs and cats, progressive ataxia, decreased proprioception in all four limbs, tetraparesis and seizures are reported (16, 17, 19, 27, 52). CSF fluid in humans revealed elevated protein levels with low to moderate pleiocytosis and normal or low glucose levels (15, 53, 54), but neoplastic cells are not found in every CSF sample, probably due to their adhesion through cell processes, which makes them less prone to exfoliation (20, 54). This might also account for the present case, where CSF was lacking neoplastic cells. Neuroimaging techniques are a useful tool for intravital detection of CNS tumors with MRI being usually more sensitive than CT. The lower sensitivity of CT, which was used in the present case, compared to MRI might explain that the present tumor was not detected intravitally. Typical MRI findings in humans and animals in PDLG are diffuse leptomeningeal enhancement with no discernible intraaxial component (15, 17, 52). In a canine case of primary diffuse leptomeningeal oligodendrogliomatosis, a dural tail sign, which can also be found in various other masses, was detected in postcontrast T1W images (27). In addition, ventricular enlargement due to hydrocephalus and meningeal calcification might be observed (55, 56).
Histopathological examination of biopsy specimens is the only way to confirm a definite diagnosis intra vitam combined with MRI, however, repeated biopsies might be necessary to get a representative sample (15, 22, 57). PET-CT and intraoperative biopsy analysis can improve the effectivity of a representative sample in humans (58). Yet, to our knowledge, there are no reports of intravital histopathologic diagnosis of PDLG in domestic animals, which might be due to the clinical severity usually resulting in euthanasia.
Differentials in humans and domestic animals include other neoplasms, like secondary meningeal gliomatosis, ependymoma, pilocytic astrocytoma or multicentric neoplasia and meningitis of autoimmune or infectious etiology (15, 17, 20, 59). In cats, the most frequently reported extraparenchymal tumors of the spinal cord are lymphomas and osteosarcomas (5), of which especially lymphomas might share morphologic similarities with diffuse oligodendrogliomatosis. A possible infectious disease that needs to be ruled out in cats is feline infectious peritonitis (FIP) (60, 61).
Treatment is often difficult due to the lack of specific clinical, radiologic, and laboratory diagnostic criteria, confusion with more frequently occurring infectious, autoimmune or metabolic diseases, the progressive nature with a high mitotic rate, and widespread diffusion, frequently leading to secondary lesions like hydrocephalus (15, 20, 53). However, there are reports of combined radiation- and chemotherapy in humans that improve survival (62, 63). To date, there are no reports of specific PDLG treatment in domestic animals, but long-term remission of an anaplastic oligodendroglioma in a cat was achieved through combined radio- and chemotherapy (64).
Conclusion
The present report describes the first case of diffuse leptomeningeal oligodendrogliomatosis in a young adult cat including the evaluation of a broad immunohistochemical panel. This rare condition in humans and domestic animals should be considered as a differential diagnosis in animals with neurological symptoms, diffuse leptomeningeal enhancement on MRI and diffuse intrameningeal cell infiltration. Histopathological examination in combination with immunohistochemical staining are required to confirm the diagnosis and further clinical, pathological and molecular data are needed for a better understanding of this disease in domestic animals.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because the presented case derived from an animal which was submitted for routine diagnostic services in order to determine the cause of disease. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
JW conducted clinical and laboratory examinations and treatment. EC, MH-T, and CP conducted pathological and immunohistochemical examinations and case documentation. EC drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This publication was supported by Deutsche Forschungsgemeinschaft and University of Veterinary Medicine Hannover, Foundation within the funding programme Open Access Publishing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dunja Hoffmann, Siegfried Jelitto, Caroline Schütz, Julia Baskas, Christiane Namneck, Patrick John Simmons, and Petra Grünig for excellent technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.795126/full#supplementary-material
References
1. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. (2014) 16:896–913. doi: 10.1093/neuonc/nou087
2. Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C. Canine intracranial primary neoplasia: 173 cases (1986-2003). J Vet Intern Med. (2006) 20:669–75. doi: 10.1111/j.1939-1676.2006.tb02913.x
3. Troxel MT, Vite CH, Van Winkle TJ, Newton AL, Tiches D, Dayrell-Hart B, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985-2001). J Vet Intern Med. (2003) 17:850–9. doi: 10.1111/j.1939-1676.2003.tb02525.x
4. Zaki FA., Hurvitz AI, Spontaneous neoplasms of the central nervous system of the cat. J Small Anim Pract. (1976) 17:773–82. doi: 10.1111/j.1748-5827.1976.tb06943.x
5. Marioni-Henry K, Van Winkle TJ, Smith SH, Vite CH. Tumors affecting the spinal cord of cats: 85 cases (1980-2005). J Am Vet Med Assoc. (2008) 232:237–43. doi: 10.2460/javma.232.2.237
6. Dickinson PJ, Keel MK, Higgins RJ, Koblik PD, LeCouteur RA, Naydan DK, et al. Clinical and pathologic features of oligodendrogliomas in two cats. Vet Pathol. (2000) 37:160–7. doi: 10.1354/vp.37-2-160
7. Corbett JJ, and Newman NM, Symptomatic leptomeningeal metastases preceding other manifestations of occult primary brain tumors. Surg Neurol. (1981) 15:362–7. doi: 10.1016/0090-3019(81)90171-3
8. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. doi: 10.1007/s00401-016-1545-1
9. Higgins RJ, Bollen AW, Dickinson PJ, Siso-Llonch S. Tumors of the Nervous System. In: Meuten DJ, editor. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley-Blackwell (2017), p. 844–8.
10. Rissi DR, Levine JM, Eden KB, Watson VE, Griffin JFt, Edwards JF, et al. Cerebral oligodendroglioma mimicking intraventricular neoplasia in three dogs. J Vet Diagn Invest. (2015) 27:396–400. doi: 10.1177/1040638715584619
11. Young BD, Levine JM, Porter BF, Chen-Allen AV, Rossmeisl JH, Platt SR, et al. Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet Radiol Ultrasound. (2011) 52:132–41. doi: 10.1111/j.1740-8261.2010.01758.x
12. Rissi DR, and Miller AD. Feline glioma: a retrospective study and review of the literature. J Feline Med Surg. (2017) 19:1307–14. doi: 10.1177/1098612X16689506
13. Wesseling P, Capper D. WHO Classification of gliomas. Neuropathol Appl Neurobiol. (2018) 44:139–50. doi: 10.1111/nan.12432
14. Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. Histological Classification of Tumors of the Nervous System of Domestic Animals. 2nd series. Washington DC, USA: Armed Forces Institute of Pathology (1999), 17–21.
15. Yomo S, Tada T, Hirayama S, Tachibana N, Otani M, Tanaka Y, et al. A case report and review of the literature. J Neurooncol. (2007) 81:209–16. doi: 10.1007/s11060-006-9219-9
16. Kovi RC, Wünschmann A, Armién AG, Hall K, Carlson T, Shivers J, et al. Spinal meningeal oligodendrogliomatosis in two boxer dogs. Vet Pathol. (2013) 50:761–4. doi: 10.1177/0300985813476056
17. Canal S, Bernardini M, Pavone S, Mandara MT. Primary diffuse leptomeningeal gliomatosis in 2 dogs. Can Vet J. (2013) 54:1075–9.
19. Zoll WM, Miller AD, Bandt C, Abbott JR. Primary leptomeningeal gliomatosis in a domestic shorthaired cat. J Vet Diagn Invest. (2019) 31:94–7. doi: 10.1177/1040638718822683
20. Debono B, Derrey S, Rabehenoina C, Proust F, Freger P, Laquerrière A. Primary diffuse multinodular leptomeningeal gliomatosis: case report and review of the literature. Surg Neurol. (2006). 65:273–82. doi: 10.1016/j.surneu.2005.06.038
21. Somja J, Boly M, Sadzot B, Moonen G, Deprez M. Primary diffuse leptomeningeal gliomatosis: an autopsy case and review of the literature. Acta Neurol Belg. (2010) 110:325–33.
22. Bailey P, and Robitaille Y. Primary diffuse leptomeningeal gliomatosis. Can J Neurol Sci. (1985) 12:278–81. doi: 10.1017/S031716710004717X
23. Vandevelde M, Higgins R, Oevermann A. Neoplasia. In: Vandevelde M, Higgins R, Oevermann A, editors. Veterinary Neuropathology: Essentials of Theory and Practice, 1st ed. Ames, IA: Wiley-Blackwell (2012) p. 137–9.
24. Mokhtari K, Paris S, Aguirre-Cruz L, Privat N, Crinière E, Marie Y, et al. Olig2 expression, GFAP, p53 and 1p loss analysis contribute to glioma subclassification. Neuropathol Appl Neurobiol. (2005) 31:62–9. doi: 10.1111/j.1365-2990.2004.00612.x
25. Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. (2004) 63:499–509. doi: 10.1093/jnen/63.5.499
26. Kauer RV, Bagatella S, Oevermann A. Diffuse Leptomeningeal Oligodendrogliomatosis in a Cow. Vet Pathol. (2020) 57:253–7. doi: 10.1177/0300985819887534
27. Lobacz MA, Serra F, Hammond G, Oevermann A, Haley AC. Imaging diagnosis-magnetic resonance imaging of diffuse leptomeningeal oligodendrogliomatosis in a dog with “dural tail sign”. Vet Radiol Ultrasound. (2018) 59:E1–e6. doi: 10.1111/vru.12441
28. Ide T, Uchida K, Kikuta F, Suzuki K, Nakayama H. Immunohistochemical characterization of canine neuroepithelial tumors. Vet Pathol. (2010) 47:741–50. doi: 10.1177/0300985810363486
29. Ide T, Uchida K, Suzuki K, Kagawa Y, Nakayama H. Expression of cell adhesion molecules and doublecortin in canine anaplastic meningiomas. Vet Pathol. (2011) 48:292–301. doi: 10.1177/0300985810389312
30. Daou MC, Smith TW, Litofsky NS, Hsieh CC, Ross AH. Doublecortin is preferentially expressed in invasive human brain tumors. Acta Neuropathol. (2005) 110:472–80. doi: 10.1007/s00401-005-1070-0
31. Demeter EA, Frank C, Rissi DR, Porter BF, Miller AD. Microtubule-Associated Protein 2 Expression in Canine Glioma. Front Vet Sci. (2019) 6:395. doi: 10.3389/fvets.2019.00395
32. Völker I, Schwarze I, Brezina TE, Köstlinger S, Hewicker-Trautwein M. [Oligodendroglioma with neuronal differentiation in an 8-month-old African hedgehog (Atelerix albiventris)]. Tierarztl Prax Ausg K Kleintiere Heimtiere. (2016) 44:348–54. doi: 10.15654/TPK-160112
33. Perry A, Scheithauer BW, Macaulay RJ, Raffel C, Roth KA, Kros JM. Oligodendrogliomas with neurocytic differentiation. A report of 4 cases with diagnostic and histogenetic implications. J Neuropathol Exp Neurol. (2002) 61:947–55. doi: 10.1093/jnen/61.11.947
34. Cornax I, Pluhar GE, Clark HB, O'Sullivan MG. Oligodendroglioma with neuronal differentiation in two boxer dogs. J Comp Pathol. (2019) 172:11–6. doi: 10.1016/j.jcpa.2019.08.003
35. Blümcke I, Becker AJ, Normann S, Hans V, Riederer BM, Krajewski S, et al. Distinct expression pattern of microtubule-associated protein-2 in human oligodendrogliomas and glial precursor cells. J Neuropathol Exp Neurol. (2001) 60:984–93. doi: 10.1093/jnen/60.10.984
36. Bourne TD, Mandell JW, Matsumoto JA, Jane JA, Lopes MB. Primary disseminated leptomeningeal oligodendroglioma with 1p deletion. case report. J Neurosurg. (2006) 105:465-9. doi: 10.3171/ped.2006.105.6.465
37. Park CH. Oligodendroglioma in a French bulldog. J Vet Sci. (2003) 4:195–7. doi: 10.4142/jvs.2003.4.2.195
38. Hammond JJ. deLahunta A, Glass EN, Kent M, Summers BA, and Miller AD. Feline spinal cord gliomas. J Vet Diagn Invest. (2014) 26:513–20. doi: 10.1177/1040638714533118
39. Ozkul A, Meteoglu I, Tataroglu C, Akyol A. Primary diffuse leptomeningeal oligodendrogliomatosis causing sudden death. J Neurooncol. (2007) 81:75–9. doi: 10.1007/s11060-006-9208-z
40. Cho HJ, Myung JK, Kim H, Park CK, Kim SK, Chung CK, et al. Primary diffuse leptomeningeal glioneuronal tumors. Brain Tumor Pathol. (2015) 32:49–55. doi: 10.1007/s10014-014-0187-z
41. Gardiman MP, Fassan M, Orvieto E, D'Avella D, Denaro L, Calderone M, et al. Diffuse leptomeningeal glioneuronal tumors: a new entity? Brain Pathol. (2010) 20:361–6. doi: 10.1111/j.1750-3639.2009.00285.x
42. Williams BP, Read J, Price J. The generation of neurons and oligodendrocytes from a common precursor cell. Neuron. (1991) 7:685–93. doi: 10.1016/0896-6273(91)90381-9
43. Perry A, Fuller CE, Banerjee R, Brat DJ, Scheithauer BW. Ancillary FISH analysis for 1p and 19q status: preliminary observations in 287 gliomas and oligodendroglioma mimics. Front Biosci. (2003) 8:a1–9. doi: 10.2741/896
44. Riva M, Bacigaluppi S, Galli C, Citterio A, Collice M. Primary leptomeningeal gliomatosis: case report and review of the literature. Neurol Sci. (2005) 26:129–34. doi: 10.1007/s10072-005-0446-1
45. Rodriguez FJ, Perry A, Rosenblum MK, Krawitz S, Cohen KJ, Lin D, et al. Disseminated oligodendroglial-like leptomeningeal tumor of childhood: a distinctive clinicopathologic entity. Acta Neuropathol. (2012) 124:627–41. doi: 10.1007/s00401-012-1037-x
46. Kim YH, Nonoguchi N, Paulus W, Brokinkel B, Keyvani K, Sure U, et al. Frequent BRAF gain in low-grade diffuse gliomas with 1p/19q loss. Brain Pathol. (2012) 22:834–40. doi: 10.1111/j.1750-3639.2012.00601.x
47. Schniederjan MJ, Alghamdi S, Castellano-Sanchez A, Mazewski C, Brahma B, Brat DJ, et al. Diffuse leptomeningeal neuroepithelial tumor: 9 pediatric cases with chromosome 1p/19q deletion status and IDH1 (R132H) immunohistochemistry. Am J Surg Pathol. (2013) 37:763–71. doi: 10.1097/PAS.0b013e31827bf4cc
48. Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. (2013) 45:602–12. doi: 10.1038/ng.2611
49. Capper D, Reuss D, Schittenhelm J, Hartmann C, Bremer J, Sahm F, et al. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. (2011) 121:241–52. doi: 10.1007/s00401-010-0770-2
50. Wesseling P, van den Bent M, Perry A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol. (2015) 129:809–27. doi: 10.1007/s00401-015-1424-1
51. Truvé K, Dickinson P, Xiong A, York D, Jayashankar K, Pielberg G, et al. Utilizing the dog genome in the search for novel candidate genes involved in glioma development-genome wide association mapping followed by targeted massive parallel sequencing identifies a strongly associated locus. PLoS Genet. (2016) 12:e1006000. doi: 10.1371/journal.pgen.1006000
52. Giron C, Paquette D, Culang D, Doré M, Masseau I. Diffuse meningeal oligodendrogliomatosis characterized by spinal intra-parenchymal nodules on magnetic resonance imaging in a dog. Can Vet J. (2020) 61:1312–8.
53. Bilic M, Welsh CT, Rumboldt Z, Hoda RS. Disseminated primary diffuse leptomeningeal gliomatosis: a case report with liquid based and conventional smear cytology. Cytojournal. (2005) 2:16. doi: 10.1186/1742-6413-2-16
54. Dietrich PY, Aapro MS, Rieder A, Pizzolato GP. Primary diffuse leptomeningeal gliomatosis (PDLG): a neoplastic cause of chronic meningitis. J Neurooncol. (1993) 15:275–83. doi: 10.1007/BF01050075
55. Chen R, Macdonald DR, Ramsay DA. Primary diffuse leptomeningeal oligodendroglioma. case report. J Neurosurg. (1995) 83:724–8. doi: 10.3171/jns.1995.83.4.0724
56. Rogers LR, Estes ML, Rosenbloom SA, Harrold L. Primary leptomeningeal oligodendroglioma: case report. Neurosurgery. (1995) 36:166–8. doi: 10.1227/00006123-199501000-00021
57. Korein J, Feigin I, Shapiro MF. Oligodendrogliomatosis with intracranial hypertension. Neurology. (1957) 7:589–94. doi: 10.1212/WNL.7.8.589
58. Sáez-Alegre M, Saceda Gutiérrez JM, Utrilla Contreras C, Aracil Santos FJ, García-Feijoo P, Carceller Benito F. Diffuse leptomeningeal glioneuronal tumour: where to biopsy? case report and literature review. Childs Nerv Syst. (2020). doi: 10.1007/s00381-020-04955-2
59. Lee JK, Ko HC, Choi JG, Lee YS, Son BC. A case of diffuse leptomeningeal glioneuronal tumor misdiagnosed as chronic tuberculous meningitis without brain biopsy. Case Rep Neurol Med. (2018) 2018:1391943. doi: 10.1155/2018/1391943
60. Fondevila D, Vilafranca M, Pumarola M. Primary central nervous system T-cell lymphoma in a cat. Vet Pathol. (1998) 35:550–3. doi: 10.1177/030098589803500613
61. Shrader S, Lai S, Cline K, Moon R. Gliomatosis cerebri in the brain of a cat. Vet Sci. (2016) 3:3. doi: 10.3390/vetsci3030013
62. Beauchesne P, Pialat J, Duthel R, Barral FG, Clavreul G, Schmitt T, et al. Aggressive treatment with complete remission in primary diffuse leptomeningeal gliomatosis–a case report. J Neurooncol. (1998) 37:161–7. doi: 10.1023/A:1005888319228
63. Michotte A, Chaskis C, Sadones J, Veld PI, Neyns B. Primary leptomeningeal anaplastic oligodendroglioma with a 1p36-19q13 deletion: report of a unique case successfully treated with Temozolomide. J Neurol Sci. (2009) 287:267–70. doi: 10.1016/j.jns.2009.08.047
Keywords: CNS, feline, glioneuronal tumor, meninges, OLIG2, oligodendroglioma, PDLG
Citation: Chludzinski E, Puff C, Weber J and Hewicker-Trautwein M (2021) Case Report: Primary Diffuse Leptomeningeal Oligodendrogliomatosis in a Young Adult Cat. Front. Vet. Sci. 8:795126. doi: 10.3389/fvets.2021.795126
Received: 14 October 2021; Accepted: 22 November 2021;
Published: 15 December 2021.
Edited by:
Anna Oevermann, University of Bern, SwitzerlandReviewed by:
Maria Teresa Mandara, University of Perugia, ItalyDan R. Rissi, University of Georgia, United States
Copyright © 2021 Chludzinski, Puff, Weber and Hewicker-Trautwein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marion Hewicker-Trautwein, TWFyaW9uLkhld2lja2VyLVRyYXV0d2VpbkB0aWhvLWhhbm5vdmVyLmRl
 Elisa Chludzinski
Elisa Chludzinski Christina Puff
Christina Puff Jürgen Weber2
Jürgen Weber2 Marion Hewicker-Trautwein
Marion Hewicker-Trautwein