
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 28 January 2022
Sec. Animal Nutrition and Metabolism
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.791371
This article is part of the Research Topic The Interaction between Digestive Tract Microbes and Hosts in Poultry View all 25 articles
In modern intensive breeding system, broilers are exposed to various challenges, such as diet changes and pathological environment, which may cause the increase in the incidence rate and even death. It is necessary to take measures to prevent diseases and maintain optimal health and productivity of broilers. With the forbidden use of antibiotics in animal feed, polysaccharides from plants have attracted much attention owing to their lower toxicity, lower drug resistance, fewer side effects, and broad-spectrum antibacterial activity. It had been demonstrated that polysaccharides derived from plant exerted various functions, such as growth promotion, anti-inflammation, maintaining the integrity of intestinal mucosa, and regulation of intestinal microbiota. Therefore, the current review aimed to provide an overview of the recent advances in the impacts of plant-derived polysaccharides on anti-inflammation, gut health, and intestinal microbiota community of broilers in order to provide a reference for further study on maintaining the integrity of intestinal structure and function, and the related mechanism involved in the polysaccharide administration intervention.
In modern breeding system, broilers are often exposed to various external factors, such as diseases, nutritional, and environmental challenges, which lead to impaired growth, poor health, and even high mortality percentages owing to their imperfect intestinal function development and immature immunity (1). In addition, the pathogenic bacteria existing in the breeding environment can cause diseases influencing the health of broilers, which in turn results in high mortality rates and huge economic losses in the poultry industry (2). In the past, antibiotics were widely used as performance-enhancing feed additives in poultry production to promote productivity and prevent diseases (3). However, the extensive use of antibiotics not only caused the problems of antibiotic resistance but also induced the accumulation of antibiotic residues in animal products, and developed new strains of drug-resistant pathogenic bacteria (4). Therefore, the addition of antibiotics in animal feed was forbidden in most countries of the world. It has become imperative to find safe and effective alternatives to antibiotics that can improve chicken health by improving the gut health and maintaining the optimum growth of broilers.
Polysaccharides are large molecular weight polymers and bioactive macromolecules with complex molecular structures, which are usually composed of more than 10 monosaccharides linked by glycosidic linkages in linear or branched chains (5, 6). In general, polysaccharides are composed of complex sets of monosaccharides, such as mannose, galactose, glucose, and arabinose. Besides, it is known that the structural features of polysaccharides, the glycosidic bond of the main chain and molar mass, are related to their biological properties (7). Moreover, the bioactivities of polysaccharides could be enhanced by moderate structural modification (8, 9). Plants are an important source of natural polysaccharides. With regard to their different sources, plant polysaccharides are classified into dietary fiber (inulin, gums, pectin), algae, and traditional herbs (10). Numerous studies have been conducted to investigate the effects of bioactive ingredients derived from plant, such as polysaccharide, which was used as growth promoter feed additives to enhance the overall performance parameters as well as health conditions of broilers owing to their safety properties, including lower drug resistances and relatively fewer side effects (11, 12). It has been documented that polysaccharides extracted from plants exhibited promising effects on anti-inflammation, modulation of gut health, and intestinal microbiota of broilers (13–16). Therefore, in the current review, we attempted to summarize the various bioactive effects of plant polysaccharides on growth performance, immune status, gut health, and microbiota of broilers.
In previous studies, it has been demonstrated that polysaccharides had the potential to enhance the growth performance of broilers (17, 18) (Figure 1). It was reported that higher-dose polysaccharides supplemented in diets were beneficial to promote growth performance, while the effects of lower-dose polysaccharides were of no significance (19). Wu (17) suggested that a diet supplemented with 500, 1,000, and 2,000 mg/kg of Astragalus membranaceus polysaccharide (APS) effectively enhanced the body weight gain of broilers after a 6-week feeding trial. In contrast, Chen et al. (20) demonstrated that 200 mg/kg of APS polysaccharide supplementation did not affect the growth performance of broilers. The results mentioned above indicated that the effects of polysaccharides used as prebiotics and growth-promoter ingredients on the growth performance of broilers were in dose-dependent manners. The growth-promoting effects of polysaccharides may be due to its stimulating effects on the activities of digestive enzymes (17, 18), the promotion of nutrient digestibility (21), and the absorption of amino acids (22).
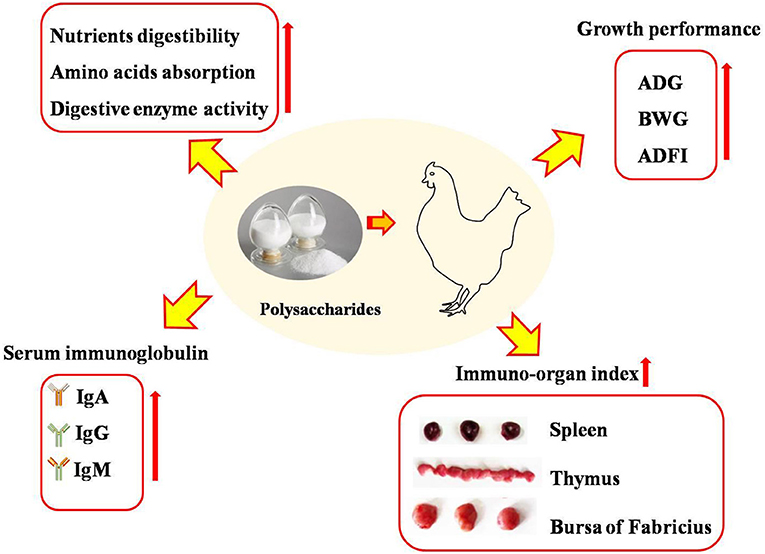
Figure 1. Dietary polysaccharides supplementation improved growth performance, increased nutrients digestibility and amino acids absorption, elevated the content of serum immunoglobulin and immune-ogran index of broilers. IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; ADG, average daily weight gain; BWG, body weight gain; ADFI, average daily feed intake.
In previous studies, it has been demonstrated that broilers in the status of immune suppression showed depressed growth performance (9, 23), as evidenced by lower weight gain and higher ratio of feed intake to weight gain, but dietary polysaccharide supplementation could be of benefit in reversing the negative effects on growth performance of broilers by inducing immune stimulation. Dietary Achyranthes bidentata polysaccharide supplementation increased body weight and average daily weight gain of broilers challenged with Escherichia coli K88 (14). It was also reported that Salmonella serotype Enteritidis-infected broilers exhibited impaired growth performance, but this was relieved by diets supplemented with polysaccharides isolated from alfalfa (24). Additionally, the biological activities of polysaccharides are closely related to their molecular weight and specific spatial structures (25). It has been demonstrated that, compared with untreated APS, administration of sulfated APS or γ-irradiated APS in diets was more effective in improving growth performance of broilers under the immunosuppression status (8, 9, 16). The results mentioned above indicated that polysaccharides or structurally modified polysaccharides were able to alleviate the negative effects on growth performance induced by bacterial infection.
Different from the beneficial effects of polysaccharides derived from traditional herbs and alfalfa on growth performance of broilers in normal or immunosuppression status, studies about diets supplemented with polysaccharides from dietary fiber (inulin, gum, and pectin) had no effects or even negative effects on growth performance of broilers. Ortiz et al. (26) suggested that the inclusion of inulin in diets exerted no growth-promoting effect at graded concentrations from 5 to 20 g/kg. Similarly, it was reported that incorporating inulin into a diet at 1, 2, and 4% dose did not improve the Overall growth performance of broilers (27). However, the inclusion of gum and pectin in diets had negative effects on growth performance of broilers, as evidenced by the depressed weight gain, lower feed intake, and higher ratio of daily weight gain to feed intake (28, 29), which maybe directly linked with the increased intestinal viscosity owing to the intake of indigestible polysaccharide such as pectin and gum (30).
The development of immune tissues is the basis of immune functionality, and the development and maturation of lymphocytes is often used as an indicator of immunity (Figure 1). Larger organ weight and index implies stronger humoral and cellular immune capacity (31). It was reported in a previous study that APS and γ-irradiated APS supplementation increased the relative weight of the thymus and spleen of broilers, as well as promoted the proliferation of T lymphocytes (9). Similarly, Chen et al. (20) suggested that APS had significant immune-stimulating effects on splenocyte proliferation. Besides, γ-irradiated APS, not APS, enhanced the proliferation of B lymphocytes, indicating that γ-irradiated APS was more effective than APS in immune modulation (9). The reason for the higher activity of γ-irradiated APS was that irradiation leads to the cleavage of glycosidic bonds by electromagnetic waves of gamma rays and resulted in low molecular weight polysaccharide products, which affected their biological activities (9, 32, 33). Enteromorpha polysaccharide administration increased the relative weight of the bursa of Fabricius in broilers (34) and improved the morphology of the bursa of Fabricius, including infiltration of inflammatory cells, destruction of the cell structure, and cell necrosis (35). It has been demonstrated that Camellia oleifera cake polysaccharides increased the weight or index of the spleen and thymus of broilers (19). Polysaccharides were also benefited to improve the T-lymphocyte percentage and enhance the development of immune organs, evidenced by the increased lymph follicle area in the bursa of Fabricius and white pulp area expansion, which suggested that polysaccharide treatment elicited lymphocyte formation in the bursa of Fabricius (36). Further study by transcriptome analysis revealed that dietary Enteromorpha polysaccharides regulated 20 differentially expressed genes of the bursa of Fabricius, which were mainly enriched in negative regulation of the Toll-like receptor signaling pathway and mainly enriched in phagosome, mitophagy-animal, Salmonella infection, and autophagy signaling pathways (34). Moreover, it was identified that algae-derived polysaccharides ameliorated the impairment of the bursa of Fabricius in heat-stressed broilers via modulation of the NF-κB signaling pathway (35). Guo et al. (37) demonstrated that dietary marine algal polysaccharide administration alleviated damage and apoptosis of the bursa of Fabricius in broilers induced by aflatoxin B1, which might be associated with p38MAPK-Nrf2/HO-1 and mitochondrial apoptotic signaling pathways.
Polysaccharides derived from plants could activate macrophages by recognizing and binding to specific receptors on the surfaces of macrophages, which is beneficial in improving immune function and exerting an immunomodulatory effect (38). Serum immunoglobulin (Ig), such as IgA, IgG, and IgM, play important roles in the immune system in poultry (39) and are related to the state of the immune function (Figure 1). It was reported that Lycium barbarum polysaccharide supplementation contributed to enhancing the concentrations of IgA, IgG, and IgM in the serum (18). Wu et al. (40) reported that inulin addition resulted in more IL-6 and tended to increase the concentration of IgA and IgM. Wu (17) and Long et al. (41) suggested that Astragalus membranaceus polysaccharides or Acanthopanax senticosus polysaccharide supplementation promoted the humoral immunity of broilers as evidenced by the increased concentration of IgA and IgG in the serum. The information mentioned above confirmed that polysaccharides could be beneficial in improving the humoral immunity of broilers.
Polysaccharides, a kind of natural macromolecular polymers, have attracted extensive attractions due to their diverse and important biological activities in immunomodulatory effects and anti-inflammation (6, 9, 13). Polysaccharides could be an immunopotentiator to promote the proliferation of B lymphocytes and T lymphocytes, the increase in serum antibody titer, and the reduction in the ratio of blood heterophil to lymphocyte (9, 12) (Figure 2). CD3+, CD4+, and CD8+ are vital T-lymphocyte markers and can be used as an indicator of the T-cell response (13). Astragalus polysaccharide increased CD3+ and CD8+ T-cell populations, as well as the numbers of CD4+ lymphocytes (13), indicating that APS administration benefited in cellular immunity.
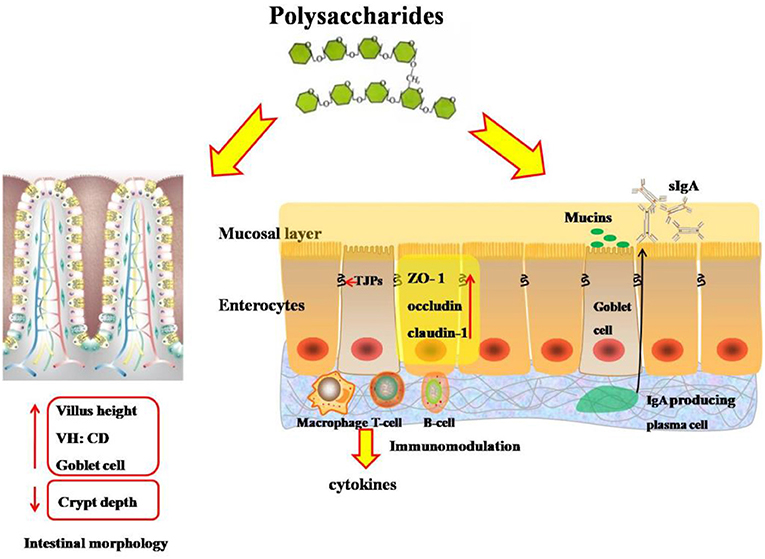
Figure 2. Effects of polysaccharides on intestinal morphology and intestinal mucosal immunity and barrier integrity of broilers. VH:CD, the ratio of villus height to crypt depth; TJPs, tight junction proteins; ZO-1, zonula-1; T-cell, T lymphocyte; B-cell, B lymphocyte; IgA, immunological A; sIgA, secretory immunological A.
In addition, cytokines are important mediators of immune responses. Intraepithelial lymphocytes, which are immunocompetent cells that first encounter enteric pathogens invading the mucosa and play an important role in gut mucosal immunity, can program cytokine production (such as IL-2 and IFN-γ) to protect the intestines from bacterial and viral invasion (8). Li et al. (42) found that γ-irradiated APS enhanced the numbers of intraepithelial lymphocytes, as well as increased mRNA expressions of IL-2 and IFN-γ. Moreover, it was demonstrated that dietary polysaccharide supplementation could modulate immune functions through regulating the production of cytokines and proinflammatory mediators. Zhang et al. (43) suggested that Glycyrrhiza polysaccharide mitigated the LPS-induced increase in IL-1β and IFN-γ in the liver. Liu et al. (44) found that algae-derived polysaccharide supplementation lowered the relative mRNA expression of TNF-α and IL-1β in the duodenum of broilers that suffered from heat stress. In addition, structural modification contributed to boost the activity of the polysaccharides. Wang et al. (12, 45) suggested that, in comparison with untreated APS, sulfated APS exhibited more effective anti-inflammatory effect in vitro and in vivo, evidenced by the downregulation of the increased expression of TNF-α and IL-1β induced by the LPS challenge. Similarly, Li et al. (9) and Ren et al. (32) demonstrated that γ-irradiated administration improved the immunomodulating activity of APS, which increased the intestinal intraepithelial lymphocytes and mRNA expressions of IL-2 and IL-10 in the jejunum of broilers. From the information mentioned above, it can be concluded that polysaccharides can promote the proliferation of immune cells and exert anti-inflammatory activity. Therefore, it is necessary to look for and evaluate polysaccharides with immunostimulating properties and further expand their application in the breeding of broilers.
The intestine is responsible for nutrient digestion and absorption, and the growth performance of broilers is associated with the morphological and functional integrity of the digestive system (46) (Figure 2). The values of villus height (VH) and the ratio of villus height to crypt depth (VH:CD) of the small intestine are useful indicators to estimate the absorption capacity of the small intestine (47). After 35 days of feeding supplemented with algae-derived polysaccharides from Enteromorpha, it was found that the VH and villus surface area of the duodenum and ileum were both increased (35). Previous studies also demonstrated that broilers fed with diets containing 600 mg/kg of Astragalus polysaccharides had higher VH and ratio of VH:CD (1, 42), suggesting that polysaccharides contributed to improve intestinal morphology associated with the prebiotic function of polysaccharides. Polysaccharides could work as a substrate on microflora population and bacterial metabolites, and aids in stimulating the fermentation rate and increasing the production of short-chain fatty acids, which benefits the differentiation and proliferation of intestinal epithelial cells (35, 48). Interestingly, APS supplementation on intestinal morphology showed transgenerational effect. The paternal dietary APS supplementation in a dose of 10 g/kg could promote transgenerational intestinal morphology of broiler chickens (49).
The paracellular permeability of the intestinal barrier is regulated by a complex protein system called tight junctions (50) (Figure 2). Tight junctions, the multiprotein complexes, are composed of transmembrane proteins, peripheral membrane proteins, and regulatory molecules including kinases, among which the claudin family proteins are the most important of the transmembrane proteins, whereas the ZO family proteins are the peripheral membrane proteins and are crucial to tight junction assembly (51). D-lactic acid and diamine oxidase are generally used as sensitive biomarkers for intestinal permeability to reflect the extent of damage in the intestine tract (52). Polysaccharide supplementation decreased the content of D-lactic acid and the activity of diamine oxidase in the serum, implying that polysaccharides improved the intestinal permeability and protected the intestinal barrier function (35). Accordingly, accumulated evidences showed that polysaccharides improved the intestinal integrity, maintained the integrity of intestinal epithelium, and upregulated mRNA expressions or protein abundances of tight junction proteins such as ZO-1, occludin, and claudin-1 in the jejunum and ileum of broilers in normal or stressed conditions (12, 24, 44). It was generally reported that polysaccharides could not be absorbed through the intestinal mucosa into the body, so the health benefits of polysaccharides were rooted in their effects on the intestinal mucosal immune system (49). Therefore, the reasons for maintaining the integrity of intestinal mucosa by polysaccharides could be speculated as follows: First, polysaccharides improved the proliferation and maturation of intestinal epithelial cells to regulate the intestinal immune functions by synthesizing and releasing mucins, and enhancing the integrity of intestinal mucosa (42, 53). Second, a diet supplemented with polysaccharides is beneficial in promoting the development of IgA-producing cells in the intestinal mucosa and increasing the production of secretory immunoglobulin A, which is a major antibody isotype in the intestinal mucosal immunity that can prevent pathogens and toxins from invading the epithelial surface, and maintain the homeostasis of the intestine (24, 42, 54). Additionally, the protective effects of polysaccharides on the intestinal mucosa barrier were partly associated with its anti-inflammatory effects (12, 55).
The intestines are populated by a complex and dynamic microbial community, which contributes to the health status of the host animals and is the first barrier against pathogens (41, 56). The cecum is often the focus for chicken gut microbial studies because almost microbial populations are colonizations in this site and also due to their role in carbohydrate fermentation, which is considered to contribute significantly to general intestinal “health” (57). Through 12 phyla of bacteria, represented analysis by high-quality 16S rRNA gene sequences of chicken bacteria, the predominant bacteria were Firmicutes (almost 70% of sequences) and Bacteroidetes (12.3% of sequences) phyla, with cell densities exceeding 1011 per g of digesta (58, 59). Within the phylum Firmicutes, Clostridium, Ruminococcus, Lactobacillus, Eubacterium, and Fecalibacterium are the domain bacteria (60), while genera Bacteroides, Prevotella, Parabacteroides, and Alistipes are the domain bacteria in phylum Bacteroidetes (58). However, the composition of the intestinal microbiota can be altered by dietary manipulations and pathological environment (61).
It has been demonstrated that plant bioactive substances can be fermented to produce metabolites by the gut microbiota in the distal gastrointestine, and further change and reshape the intestinal microbial community via fermentation (62) (Figures 3, 4). Polysaccharides, serving as unique carbon sources for intestinal bacteria, have a beneficial microbial modulation effect, which demonstrated that broilers receiving polysaccharides have a higher abundance of beneficial bacteria and a lower abundance of harmful bacteria (36). Camellia oleifera cake polysaccharide supplementation could promote the growth of probiotics and had the potential to inhibit the number of pathogenic bacteria (19). Numerous studies proved that polysaccharide (such as Achyranthes bidentata polysaccharides, Camellia oleifera cake polysaccharides, APS, and Acanthopanax senticosus polysaccharides) treatment increased the abundances of Proteobacteria, Bacteroides, Lactobacillus, and Ruminococcaceae, and decreased the abundances of Escherichia coli and Salmonella (14, 19, 36, 41). It was reported that inulin supplementation decreased the cecal concentrations of Escherichia coli, Salmonella, and Campylobacter in broilers (63), and increased Bifidobacterium (64). Similarly, Xia et al. (27) found that there was a decrease in Firmicutes and Actinobacteria, and an increase in Bacteroidetes and Proteobacteria in response to inulin inclusion. Wu et al. (40) suggested that inulin supplementation decreased the numbers of Escherichia coli and pH in the cecum of broilers. The changes in these bacteria contributed to reduce intestinal pH, improve the intestinal microbial balance via reducing the population of pathogenic species, and thus improves the health of the host (65). However, pectin and gum addition significantly increased intestinal viscosity, which further influenced microbial activity. It has been demonstrated that pectin administration significantly increased the contribution of anaerobic bacteria, affected the microbial composition in the small intestine, particularly that of Enterococci, Clostridia, and Escherichia coli, which may be responsible for the lower antinutritive properties induced by pectin supplementation (28).
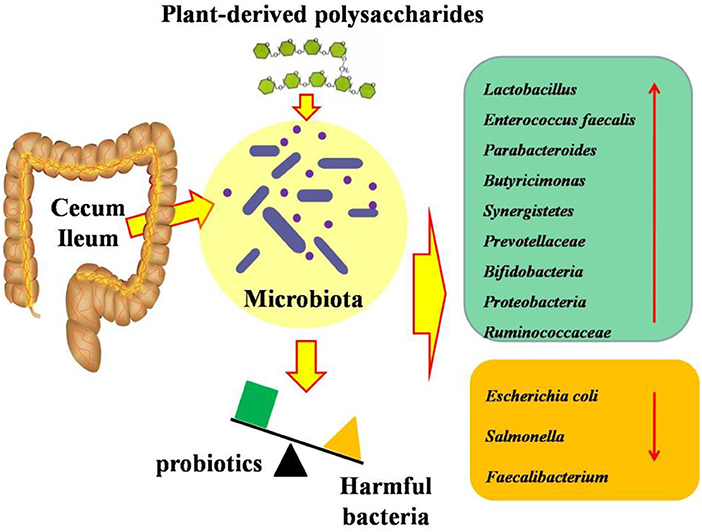
Figure 3. Effects of plant-derived polysaccharides on intestinal microbiota in ileum and cecum of broilers.
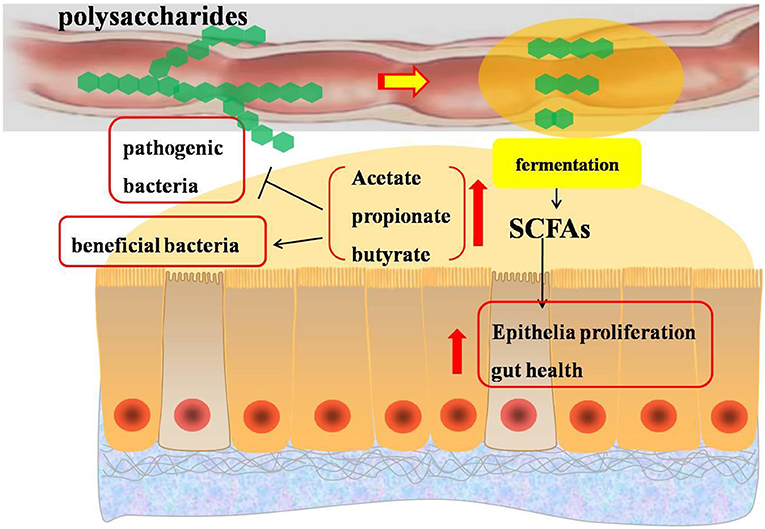
Figure 4. Effects of polysaccharides on short-chain fatty acids in the intestine of broilers. SCFAs, short-chain fatty acids.
Previous studies suggested that certain gut bacteria were responsible for the breakdown of polysaccharides not metabolized during transit through the small intestine into short-chain fatty acids, mainly including acetate, propionate, and butyrate, which contributed to promote epithelial proliferation and affect the intestinal ecology of the host animals (62, 66). The increased concentration of short-chain fatty acids in the gastrointestine is associated with an increase in the population of beneficial bacteria and a decrease in pathogenic bacteria (67, 68). The phylum Firmicutes (genus Faecalibacterium, Shuttleworthia, and Ruminococcaceae) and Bacteroidetes were known for their fermentative end products in the form of different types of short-chain fatty acids, such as acetate, butyrate, and lactate (69). Parabacteroides distasonis, a beneficial bacterium in the bowel, could regulate host metabolism and ameliorate metabolic dysfunction by producing succinate (70). Similarly, Butyricimonas bacteria could improve intestinal barrier functions by producing short-chain fatty acids from the fermentation of polysaccharides such as alfalfa polysaccharide (71). The information mentioned above indicated that polysaccharides could influence gut microbial community composition and contribute to produce short-chain fatty acids to provide energy for enterocytes and improve gut health (72). Besides, short-chain fatty acids might inhibit Salmonella growth and invasion (73) and reduce the abundance of Escherichia coli, which might be associated with the increased acidity in the cecum induced by the production of short-chain fatty acids (74).
Plant-derived polysaccharides are vital ingredients with multifunctions. The application of polysaccharides in poultry breeding exhibited more advantages, such as low drug resistance, low side effects, and broad-spectrum antibacterial effect. Polysaccharides from plants are beneficial for growth promotion and proliferation of intestinal epithelial cells, activating immune system, regulating the abundance of intestinal microbiota, and protecting the immune barrier of the intestinal mucosa in broilers. However, there are still some questions about polysaccharides applied in broiler diets to be clarified. First, plant polysaccharides are a class of polymeric molecules composed of long chains of monosaccharide units bound together by glycosidic linkages. Therefore, some attention should be paid to ascertain the exact effective ingredients of polysaccharides. Second, the relationship of the structure and activity of polysaccharides needs further study. Third, the evaluation criteria for the efficiency of polysaccharides are insufficient. Fourth, the regulation mechanism of polysaccharides on intestinal microbiota is still completely unknown and the combined strategies are needed to clarify it. In addition, the combined application of plant polysaccharides with some natural active substances to maintain gut health and the optimal growth performance of broilers may be in the right direction and can contribute to explore more biologically active plant-derived polysaccharides.
BZ wrote this manuscript. NL, YX, and ZH helped in literature search. MH, NL, and JZ assisted in revising the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by tBasic Project of Guizhou Provincial Natural Science Foundation [Qian Kehe (2017) 1205], Zunyi 15851 Talent Project (2050020213#), Zunyi City- School Joint Fund (Zunshi Kehe No. 277) and Zunshi Kehe (2018) No. 08], Rural Industrial Revolution Project of Guizhou Province (Zunshi He Rural Industry 201905), Zunyi City-School Joint Fund (Zunshi Kehe HZ 277), and Characteristic Laboratory of Animal Resources Conservation and Utilization of Chishui River Basin(Qianjiaohe KY [2013]111-03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang Q, Wang XF, Xing T, Li JL, Zhu XD, Zhang L, et al. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult Sci. (2021) 100:100909. doi: 10.1016/j.psj.2020.11.075
2. He CL, Fu BD, Shen HQ, Jiang XL, Zhang CS, Wu SC, et al. Xiang-qi-tang increases avian pathogenic Escherichia coli-induced survival rate and regulates serum levels of tumor necrosis factor alpha, interleukin-1 and soluble endothelial protein C receptor in chicken. Biol Pharm Bull. (2011) 34:379–82. doi: 10.1248/bpb.34.379
3. Lin J, Hunkapiller AA, Layton AC, Chang YJ, Robbins KR. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog Dis. (2013) 10:331–7. doi: 10.1089/fpd.2012.1348
4. Suresh G, Das RK, Kaur Brar S, Rouissi T, Avalos Ramirez A, Chorfi Y, et al. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit Rev Microbiol. (2018) 44:318–35. doi: 10.1080/1040841X.2017.1373062
5. Hou C, Chen L, Yang L, Ji X. An insight into anti-inflammatory effects of natural polysaccharides. Int J Biol Macromole. (2020) 153:248–55. doi: 10.1016/j.ijbiomac.2020.02.315
6. Yu Y, Shen M, Song Q, Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohyd Polym. (2018) 183:91–101. doi: 10.1016/j.carbpol.2017.12.009
7. Dai-Hung N, Se-Kwon K. Sulfated polysaccharides as bioactive agents from marine algae. Int J Biol Macromole. (2013) 62:70–75. doi: 10.1016/j.ijbiomac.2013.08.036
8. Wang X, Li Y, Shen J, Wang S, Yao J, Yang X. Effect of Astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int J Biol Macromole. (2015) 76:188–94. doi: 10.1016/j.ijbiomac.2015.02.040
9. Li S, Ren L, Zhu X, Li J, Zhang L, Wang X, et al. Immunomodulatory effect of γ-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim Sci J. (2019) 90:117–27. doi: 10.1111/asj.13133
10. Jun L, Stefan W, Chun Lin X. A review of bioactive plant polysaccharides: biological activities, functionalization, and biomedical applications. Bioact Carbohydrates Dietary Fibre. (2015) 5:31–61. doi: 10.1016/j.bcdf.2014.12.001
11. Yasodha T, Jeevitha S, Yogesh S, Ramanan M. Growth performance parameters of broiler chickens as influenced by herbal poultry feed. Int Res J Anim Vet Sci. (2019) 1:26–30. doi: 10.18689/mjaes-1000105
12. Wang X, Wang S, Li Y, Wang F, Yang X, Yao J. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int J Biol Macromol. (2013) 60:248–52. doi: 10.1016/j.ijbiomac.2013.05.037
13. Kallon S, Li X, Ji J, Chen C, Xi Q, Chang S, et al. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J Anim Sci Biotech. (2013) 4:22. doi: 10.1186/2049-1891-4-22
14. Liu Z, Wang X, Ou S, Arowolo MA, Hou DX, He J. Effects of polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with K88. Polymers. (2018) 10:1233. doi: 10.3390/polym10111233
15. Zhang S, Ou J, Luo Z, Kim IH. Effect of dietary β-1,3-glucan supplementation and heat stress on growth performance, nutrient digestibility, meat quality, organ weight, ileum microbiota, and immunity in broilers. Poult Sci. (2020) 99:4969–77. doi: 10.1016/j.psj.2020.06.036
16. Liu YS, Li S, Wang XF, Xing T, Li JL, Zhu XD, et al. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult Sci. (2020) 100:273–82. doi: 10.1016/j.psj.2020.09.089
17. Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult Sci. (2018) 97:3489–93. doi: 10.3382/ps/pey220
18. Long LN, Kang BJ, Jiang Q, Chen JS. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult Sci. (2020) 99:744–51. doi: 10.1016/j.psj.2019.10.043
19. Wang J, Zhang MY, Gou ZY, Jiang SQ, Zhang YZ, Wang MH, et al. The effect of Camellia oleifera cake polysaccharides on growth performance, carcass traits, meat quality, blood profile, and caecum microorganisms in yellow broilers. Animals. (2020) 10:266. doi: 10.3390/ani10020266
20. Chen HL, Li DF, Chang BY, Gong LM, Dai JG, Yi GF. Effects of Chinese herbal polysaccharides on the immunity and growth performance of young broilers. Poult Sci. (2003) 82:364–70. doi: 10.1093/ps/82.3.364
21. Du HY, Liu HM, Yang GY, Yu C, Wang SB. Effects of Enteromorpha prolifera polysaccharides on intestinal digestive enzyme activity, microbial number and nutrient apparent utilization of broilers. Chinese J Anim Nutr. (2019) 31:956–61. doi: 10.3969/j.issn.1006-267x.2019.02.053
22. Kong XF, Yin FG, He QH, Liu HJ, Li TJ, Huang RL, et al. Acanthopanax senticosus extract as a dietary additive enhances the apparent ileal digestibility of amino acids in weaned piglets. Livest Sci. (2009) 123:261–7. doi: 10.1016/j.livsci.2008.11.015
23. Yang XJ, Li WL, Feng Y, Yao JH. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult Sci. (2011) 90:2740–6. doi: 10.3382/ps.2011-01591
24. Li Z, Zhang C, Li B, Zhang S, Haj FG, Zhang G, et al. The modulatory effects of alfalfa polysaccharide on intestinal microbiota and systemic health of Salmonella serotype (ser.) Enteritidis-challenged broilers. Sci Rep. (2021) 11:10910. doi: 10.1038/s41598-021-90060-6
25. Choi JI, Kim HJ. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohyd Polym. (2013) 97:358–62. doi: 10.1016/j.carbpol.2013.05.002
26. Ortiz L, Rodríguez M, Alzueta C, Rebolé A, Treviño J. Effect of inulin on growth performance, intestinal tract sizes, mineral retention and tibial bone mineralisation in broiler chickens. Brit Poult Sci. (2009) 50:325–32. doi: 10.1080/00071660902806962
27. Xia Y, Kong J, Zhang G, Zhang X, Seviour R, Kong Y. Effects of dietary inulin supplementation on the composition and dynamics of cecal microbiota and growth-related parameters in broiler chickens. Poult Sci. (2019) 98:6942–53. doi: 10.3382/ps/pez483
28. Langhout D, Schutte J, Van Leeuwen P, Wiebenga J, Tamminga S. Effect of dietary high- and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Brit Poult Sci. (1999) 40:340–7. doi: 10.1080/00071669987421
29. Maisonnier S, Gomez J, Brée A, Berri C, Baéza E, Carré B. Effects of microflora status, dietary bile salts and guar gum on lipid digestibility, intestinal bile salts, and histomorphology in broiler chickens. Poult Sci. (2003) 82:805–14. doi: 10.1093/ps/82.5.805
30. Lee JT, Bailey CA, Cartwright AL. β-Mannanase ameliorates viscosity-associated depression of growth in broiler chickens fed guar germ and hull fractions. Poult Sci. (2003) 82:1925–31. doi: 10.1093/ps/82.12.1925
31. Zuidhof MJ, Schneider BL, Carney VL, Korver DR, Robinson FE. Growth, efficiency, and index of commercial broilers from 1957, 1978, and 2005. Poult Sci. (2014) 93:2970–82. doi: 10.3382/ps.2014-04291
32. Ren LN, Wang XF, Li S, Li JL, Zhu XD, Zhang L, et al. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int J Biol Macromole. (2018) 120:641–9. doi: 10.1016/j.ijbiomac.2018.08.138
33. Cho M, Yang C, Kim SM, You SG. Molecular characterization and biological activities of watersoluble sulfated polysaccharides from Enteromorpha prolifera. Food Sci Biotech. (2010) 19:525–33. doi: 10.1007/s10068-010-0073-3
34. Qiu SJ, Zhang R, Guo Y, Zhao Y, Zhao ZH, Liu WC. Transcriptome analysis reveals potential mechanisms of the effects of dietary Enteromorpha polysaccharides on bursa of Fabricius in broilers. Vet Med Sci. (2021) 7:1881–9. doi: 10.1002/vms3.573
35. Liu WC, Ou BH, Liang ZL, Zhang R, Zhao ZH. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult Sci. (2021) 100:101139. doi: 10.1016/j.psj.2021.101139
36. Li SP, Zhao XJ, Wang JY. Synergy of Astragalus polysaccharides and probiotics (Lactobacillus and Bacillus cereus) on immunity and intestinal microbiota in chicks. Poult Sci. (2009) 88:519–25. doi: 10.3382/ps.2008-00365
37. Guo Y, Balasubramanian B, Zhao ZH, Liu WC. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult Sci. (2021) 100:844–57. doi: 10.1016/j.psj.2020.10.050
38. Liao XD, Ma G, Cai J, Fu Y, Yan XY, Wei XB, et al. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult Sci. (2015) 94:662–7. doi: 10.3382/ps/pev038
39. Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW, Yoon WK, et al. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int Immunopharmacol. (2003) 3:1301–12. doi: 10.1016/S1567-5769(03)00118-8
40. Wu X, Wen Z, Hua J. Effects of dietary inclusion of Lactobacillus and inulin on growth performance, gut microbiota, nutrient utilization, and immune parameters in broilers. Poult Sci. (2019) 98:4656–63. doi: 10.3382/ps/pez166
41. Long LN, Zhang HH, Wang F, Yin YX, Yang LY, Chen JS. Research note: effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult Sci. (2021) 100:101028. doi: 10.1016/j.psj.2021.101028
42. Li S, Wang XF, Ren LN, Li JL, Zhu XD, Xing T, et al. Protective effects γ-irradiated Astragalus polysaccharides and mucosal immune function of immunosuppressed broilers. Poult Sci. (2019) 98:6400–10. doi: 10.3382/ps/pez478
43. Zhang C, Li CX, Shao Q, Chen WB, Ma L, Xu WH, et al. Effects of Glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult Sci. (2021) 100:100927. doi: 10.1016/j.psj.2020.12.025
44. Liu WC, Zhu YR, Zhao ZH, Jiang P, Yin FQ. Effects of dietary supplementation of algae-derived polysaccharides on morphology, tight junctions, antioxidant capacity and immune response of duodenum in broilers under heat stress. Animals. (2021) 11:2279. doi: 10.3390/ani11082279
45. Wang XF, Shen J, Li SZ, Zhi LH, Yang XJ, Yao JH. Sulfated Astragalus polysaccharide regulates the inflammatory reaction in LPS-infected broiler chicks. Int J Bio Macromole. (2014) 69:146–50. doi: 10.1016/j.ijbiomac.2014.05.004
46. Gopinger E, Xavier EG, Elias MC, Catalan AAS, Castro MLS, Nunes AP.F.B., et al. The effect of different dietary levels of canola meal on growth performance, nutrient digestibility, and gut morphology of broiler chickens. Poult Sci. (2014) 93:1130–6. doi: 10.3382/ps.2013-03426
47. Chiang G, Lu WQ, Piao XS, Hu JK, Gong LM, Thacker PA. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Aust J Anim Sci. (2010) 23:263–71. doi: 10.5713/ajas.2010.90145
48. Rémésy C, Levrat MA, Gamet L, Demigné C. Cecal fermentations in rats fed oligosaccharides (inulin) are modulated by dietary calcium level. Am J Physiol. (1993) 264:G855–62. doi: 10.1152/ajpgi.1993.264.5.G855
49. Li YL, Lei XY, Guo WR, Wu SG, Duan YL, Yang X, et al. Transgenerational endotoxin tolerance-like effect caused by paternal dietary Astragalus polysaccharides in broilers' jejunum. Int J Biol Macromol. (2018) 111:769–79. doi: 10.1016/j.ijbiomac.2018.01.095
50. Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. (2014) 36:204–12. doi: 10.1016/j.semcdb.2014.09.022
51. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. (2009) 9:799–809. doi: 10.1038/nri2653
52. Gilani S, Gordon SH, Soressa MK, Rebecca EAF, Cuong DT, Rober JH. New biomarkers for intestinal permeability induced by lipopolysaccharide in chickens. Anim Prod Sci. (2016) 56:1984–97. doi: 10.1071/AN15725
53. Chen Z, Xie J, Hu MY, Tang J, Shao ZF, Li MH. Protective effects of γ-aminobutyric acid (GABA) on the small intestinal mucosa in heat-stressed Wenchang chicken. J Anim Plant Sci. (2015) 25:78–87.
54. Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. (2011) 4:603–11. doi: 10.1038/mi.2011.41
55. Ji XL, Peng Q, Yuan YP, Shen J, Xie XY, Wang MH. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Food Chem. (2017) 227:349–57. doi: 10.1016/j.foodchem.2017.01.074
56. Zhu XY, Zhong T, Pandya Y, Joerger RD. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl Environ Microb. (2002) 68:124–37. doi: 10.1128/AEM.68.1.124-137.2002
57. Broom LJ, Kogut MH. The role of the gut microbiome in shaping the immune system of chickens. Vet Immunol Immunop. (2018) 204:44–51. doi: 10.1016/j.vetimm.2018.10.002
58. Wei S, Morrison M, Yu Z. Bacterial census of poultry intestinal microbiome. Poult Sci. (2013) 92:671–83. doi: 10.3382/ps.2012-02822
59. Sun BS, Hou LY, Yang Y. The development of the gut microbiota and short-chain fatty acids of layer chickens in different growth periods. Front Vet Sci. (2021) 8:666535. doi: 10.3389/fvets.2021.666535
60. Kang K, Hu Y, Wu S, Shi S. Comparative metagenomic analysis of chicken gut microbial community, function, and resistome to evaluate noninvasive and cecal sampling resources. Animals. (2021) 11:1718. doi: 10.3390/ani11061718
61. Guo FC, Williams BA, Kwakkel RP, Li HS, Li XP, Luo JY, et al. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult Sci. (2004) 83:175–82. doi: 10.1093/ps/83.2.175
62. Zhang TH, Yang Y, Liang Y, Jiao X, Zhao CH. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients. (2018) 10:1055. doi: 10.3390/nu10081055
63. Yusrizal Y, Chen TC. Effect of adding chicory fructans in feed on faecal and intestinal microflora and excretory volatile ammonia. Int J Poult Sci. (2003) 2:188–94. doi: 10.3923/ijps.2003.188.194
64. Rada V, Dusková D, Marounek M, Petr J. Enrichment of bifidobacteria in the hen caeca by dietary inulin. Folia microbiol. (2001) 46:73–5. doi: 10.1007/BF02825891
65. Bucław M. The use of inulin in poultry feeding: a review. J Anim Physiol Anim N. (2016) 100:1015–22. doi: 10.1111/jpn.12484
66. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharm Ther. (2008) 27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x
67. Engberg RM, Hedemann MS, Steenfeldt S, Jensen BB. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult Sci. (2004) 83:925–38. doi: 10.1093/ps/83.6.925
68. Zhang S, Zhong G, Shao D, Wang Q, Hu Y, Wu T, et al. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult Sci. (2021) 100:100935. doi: 10.1016/j.psj.2020.12.032
69. Comstock LE. Importance of glycans to the host-bacteroides mutualism in the mammalian intestine. Cell Host Microbe. (2009) 5:522–6. doi: 10.1016/j.chom.2009.05.010
70. Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. (2019) 26:222–35.e225. doi: 10.1016/j.celrep.2018.12.028
71. Ulger Toprak N, Bozan T, Birkan Y, Isbir S, Soyletir G. Butyricimonas virosa: the first clinical case of bacteraemia. New Microbes New Infect. (2015) 4:7–8. doi: 10.1016/j.nmni.2014.12.004
72. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nat Commun. (2012) 489:220–30. doi: 10.1038/nature11550
73. Liao XD, Shao YX, Sun GM, Yang YF, Zhang LY, Guo YL, et al. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult Sci. (2020) 99:5883–95. doi: 10.1016/j.psj.2020.08.033
74. Shang HM, Song H, Wang LN, Wu B, Ding GD, Jiang YY, et al. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on performance, gut microflora, and cholesterol metabolism in broiler chickens. Livest Sci. (2014) 167:276–85. doi: 10.1016/j.livsci.2014.07.004
Keywords: polysaccharide, intestinal morphology, immunity, gut microbiota, broilers
Citation: Zhang B, Liu N, Hao M, Zhou J, Xie Y and He Z (2022) Plant-Derived Polysaccharides Regulated Immune Status, Gut Health and Microbiota of Broilers: A Review. Front. Vet. Sci. 8:791371. doi: 10.3389/fvets.2021.791371
Received: 08 October 2021; Accepted: 06 December 2021;
Published: 28 January 2022.
Edited by:
Shourong Shi, Poultry Institute, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Qingzhen Zhong, Jilin Agricultural University, ChinaCopyright © 2022 Zhang, Liu, Hao, Zhou, Xie and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bolin Zhang, Ym9saW4temhhbmdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.