
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 24 December 2021
Sec. Veterinary Pharmacology and Toxicology
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.773089
This article is part of the Research Topic Alternative and Complementary Methods for the Control of Infectious Diseases in Animals View all 11 articles
The rise of novel mcr mobile resistance genes seriously threatens the use of colistin as a last resort antibiotic for treatment of multidrug-resistant Gram-negative bacterial infections in humans. Large quantities of colistin are released annually into the environment through animal feces. This leads to environmental toxicity and promotes horizontal transmission of the mcr gene in aqueous environments. We examined colistin degradation catalyzed by the presence of strong oxidant Fe (VI). We found almost complete colistin degradation (>95%) by Fe (VI) at initial colistin levels of 30 μM at a molar ratio of Fe (VI): colistin of 30 using an initial pH 7.0 at 25°C for 60 min. The presence of humic acid did not alter the degradation rate and had no significant impact on the removal of colistin by Fe (VI). Quantitative microbiological assays of Fe (VI)-treated colistin solutions using Escherichia coli, Staphylococcus aureus, and Bacillus subtilis indicated that the residual antibacterial activity was effectively eliminated by Fe (VI) oxidation. Luminescent bacteria toxicity tests using Vibrio fischeri indicated that both colistin and its degradation products in water were of low toxicity and the products showed decreased toxicity compared to the parent drug. Therefore, Fe (VI) oxidation is a highly effective and environment-friendly strategy to degrade colistin in water.
Veterinary antibiotics are routinely used globally to control infectious diseases and to improve animal growth and feed efficiency (1). However, a large proportion of administered antibiotics are excreted in urine and feces as parent compounds due to poor gut absorption or incomplete metabolism (2, 3). China produces and consumes the most antibiotics of any country and 58% of the annual accumulation of 84,000 tons is excreted by animals (4, 5). This continued release into the environment combined with incomplete removal during wastewater treatment has resulted in the presence of these veterinary antibiotics in aquatic environments (6, 7). Antibiotic residues in water can alter ecosystems and even lead to the development of antimicrobial resistance and this is one of the most serious global threats to human and animal health (8–10). Therefore, measures to degrade or remove antibiotics during wastewater treatment are essential to reduce their harm to the environment, animals and humans.
Colistin (also known as polymyxin E) belongs to the polymyxin family of polypeptide antibiotics derived from Bacillus polymyxa var. colistinus and was introduced to clinical practice in the late 1950s (11). The bactericidal activity of colistin is essentially that of a cationic detergent that associates with Gram-negative bacterial cell membrane phospholipids and Lipid A. This association leads to rapid permeability changes in the membrane causing leakage of intracellular components, ultimately resulting in cell death (12). Most Gram-negative bacteria are susceptible to colistin and these include the most prevalent animal and human pathogens such as Enterobacter aerogenes, Escherichia coli, Haemophilus influenzae, Bordetella pertussis, Salmonella spp., Legionella pneumophila, Shigella spp., Klebsiella pneumoniae, and Pseudomonas aeruginosa (12). Colistin is widely used in animal farming and especially poultry and swine production because it is extremely effective for treating Gram-negative intestinal infections caused by E. coli, Salmonella spp., and Pseudomonas spp. Colistin is also used in Asia, Europe, and North America for animal growth promotion but its neuro- and nephrotoxicity restrict its use in humans (9, 12–14). The emergence of multidrug-resistant Gram-negative bacteria (e.g., Enterobacteriaceae, P. aeruginosa, and Acinetobacter baumannii) over the past decade has caused resurgence of colistin use as a last resort antibiotic for human infections (15).
When administered orally, colistin is very poorly absorbed by the gastrointestinal tract and is eliminated almost unchanged and thereby reaches water bodies or wastewater treatment facilities in its active form (12, 16). Conventional wastewater treatment methods do not adequately remove colistin. For instance, we have determined the colistin levels in swine wastewater collected from pig farms in central China and the results revealed colistin concentrations ranging from 145 to 10,628 ng L−1. A toxicity study on the earthworm Eisenia fetida revealed that colistin could up-regulate heat shock protein 70 and inhibit metallothionein gene expression while damaging mitochondria and the endoplasmic reticulum (17). In addition to these direct environmental effects of colistin, the development of resistance is increasing. It was formerly believed that bacterial resistance to polymyxins was very low and primarily the result of mutations in chromosomal genes. However, the discovery of a novel mobile colistin resistance mechanism encoded by mcr-1 on a mobilizable plasmid by has altered this notion (9, 18). Nine additional mcr genes (mcr-2-10) have since been discovered from animals and food isolates as well as human clinical strains (19–24). A wide range of human and animal pathogens have been identified that carry these plasmid-mediated resistance genes and this poses a huge threat to sustaining effectiveness of colistin against clinical infections caused by carbapenemase-producing carbapenem-resistant Enterobacteriaceae. This scenario heralds the destruction of the last family of the last resort antibiotics, the polymyxins (9, 18, 25).
Recent assessments of the risk of developing resistance when used as a feed additive has been raised by the European Union in June 2016 and China banned colistin as feed additive in April 2017 (26). Nonetheless, colistin still threatens ecosystems and human health thus efficient treatment strategies are needed to remove colistin from wastewater before environmental discharge.
Ferrate VI (FeO) [Fe (VI)] is an extremely effective oxidant that is in current use for bioremediation of wastewater (27–29). Under acidic conditions, the redox potential of Fe (VI) (2.2 V) is greater than ozone (2.07 V) and Fe (VI) is considered to be the strongest disinfectant and oxidant that are currently in use for wastewater and water treatment (28). This inorganic compound is environmentally friendly and its by-product is the non-toxic Fe (III) (30–32). Fe (VI) is a multipurpose treatment chemical and has been used to coagulant or precipitate arsenic (33) and heavy metals (34) and is also a powerful disinfectant for bacteria (31, 35) and viruses (36). Pre-treatment of an aqueous solution requires only a very small amount of Fe (VI) to improve the removal rate of natural organics including humic acid (HA) (37). Additionally, Fe (VI) was found to be effective in promoting the oxidative transformation of pharmaceuticals such as fluoroquinolones (38–40), sulfonamides (41), β-lactams (42) and β-blockers (43). Since the removal of colistin has not been explored, we examined whether Fe (VI) could promote colistin removal from wastewater.
This study aimed to (i) assess the influence of Fe (VI) level, solution pH and reaction duration on the removal of colistin to identify optimum conditions; (ii) evaluate whether humic acid in wastewater would interfere with colistin removal by Fe (VI); (iii) measure the antibacterial activity of reaction mixtures against E. coli, S. aureus, and B. subtilis and (iv) determine the toxicity of colistin before and after Fe (VI) oxidation using a Microtox bioassay testing system. As far as we know, this is the first paper to study the removal of colistin from water using Fe (VI) treatment.
Colistin sulfate (>66.7%) was obtained from China Institute of Veterinary Drug Control (Beijing, China). Peptone soy broth (TSB) and Mueller-Hinton broth (MHB) were purchased from Qingdao Hope (Qingdao, China). HPLC grade methanol, acetonitrile and formic acid purchased from Sigma-Aldrich (Munich, Germany). All other reagents were of analytical grade. Potassium ferrate solid [K2FeO4, Fe (VI)] of 99% purity was provided by Guangzhou Kexing Chemical (Guangzhou, China). Humic acid (HA) was obtained from Tianjin Berens Biotechnology (Tianjin, China). Ultrapure water was prepared using a Millipore Milli-Q system (Molsheim, France). Stock solutions of colistin (60 μM) were prepared by dissolving colistin sulfate in Milli Q water. Fe (VI) solution was prepared by adding solid Fe (VI) to 1 mM Na2B4O7/5 mM Na2HPO4 at pH 9.0.
Batch oxidation tests were conducted in 50 mL conical flasks equipped with magnetic stirrers and contained 10 mL of Fe (VI) and 10 mL colistin solution at the initial concentration of 30 μM followed by pH adjustment. The mixtures were stirred at 500 rpm at 25.0 ± 0.2°C and 0.8 mL samples were removed and immediately passed through a 0.22 mm nylon membrane filter into a 2.0 mL vial containing 0.2 mL of sodium thiosulfate (100 mM). The sample was then applied to an Oasis HLB solid phase extraction (SPE) cartridge (Waters, Milford, MA, USA) prior to LC-MS/MS analysis.
The amount of Fe (VI) that optimized colistin removal was examined by adding Fe (VI) into a colistin solution to maintain the molar ratios of Fe (VI) and colistin at 5:1, 10:1, 20:1, 30:1, and 40:1. The pH of the mixture was then adjusted to 9.0, stirred for 60 min at ambient temperature and then quenched with sodium thiosulfate followed by being applied to Oasis HLB SPE cartridges as per above.
The effects of pH on Fe (VI)-mediated colistin removal was examined in a Fe (VI): colistin 30:1 solution and the pH of the mixture was adjusted to 5.0, 6.0, 7.0, 8.0, 9.0, and 10. The samples were stirred for 60 min and then quenched and applied to SPE cartridges as per above. These initial conditions were also used to determine the optimal reaction durations for the Fe (VI)-mediated colistin removal at pH 7.0. The samples were stirred and samples were taken at 30 s, 1, 2, 5, 10, 15, 30, 45, and 60 min, quenched with sodium thiosulfate and then cleaned up by Oasis HLB SPE as per above. HA interference in the Fe (VI)-mediated colistin removal was examined using 10 mL colistin solutions individually mixed with HA at 0, 1.0, 5.0, 15, 30, and 60 mg L−1 followed by the addition of 10 mL Fe (VI) to maintain a molar ratio of Fe (VI): colistin at 30:1. The pH of the mixture was then adjusted to 7.0. The samples were reacted and quenched and SPE cleaned up as per above.
All experiments were performed in triplicate and the mean ± SD values were calculated. Analysis of variance was conducted with SPSS 17.0 software (IBM, Chicago, IL, USA) to elucidate statistical differences between groups.
Colistin levels in solutions were determined using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Chromatographic separation was performed with a TSKgel Amide-80 column (150 × 2.0 mm, 3.0 μm, Tosoh, Tokyo, Japan) in a column oven maintained at 40°C. Solvent A and B were 0.1% formic acid in water and acetonitrile, respectively. The flow rate was 0.6 mL min−1. Colistin was eluted with a linear gradient of 0–0.8 min, 5% A; 0.8–1.5 min, 5–80% A; 1.5–3.0 min, 80% A; 3.0–3.5 min, 80–5% A; 3.6–5 min, 5% A. The injection volume was 10 μL. An Acquity UPLC I-Class system coupled to a Xevo TQ-S mass spectrometer (UPLC-TQ-S/MS) was used for analyte determination (Waters). The conditions of instrumentation were set as follows: the electrospray ion source was used in positive ionization mode with multi-period multiple reaction monitoring (mpMRM) (Table 1). The operation conditions were as follows: source temperature, 120°C; capillary voltage, 3.0 kV; desolvation temperature, 400°C; desolvation gas flow rate, 950 L h−1; collision gas flow rate, 0.15 mL min−1, cone gas flow rate, 150 L h−1. The limit of detection (LOD) of colistin A and colistin B were 5 nM and limit of quantification (LOQ) of colistin A and colistin B were 15 nM.
Colistin degradation was determined using a solution of 30 μM colistin and Fe (VI) 900 μM under the optimal reaction conditions of pH 7.0, stirring at 500 rpm at 25.0°C for 60 min. Blank reaction solutions were prepared in the same way except that colistin was replaced by water. The bacterial strains E. coli ATCC 25922, E. coli K88, S. aureus and B. subtilis were used at 1 × 106 CFU mL−1 and added to samples from the degradation assay employing broth microdilution assays to measure the antibacterial activities of the reaction solution. Briefly, colistin samples were 2-fold diluted in a 96-well microdilution plate in MHB. Inoculums (100 μL) prepared in MHB were added to each well and the plates were covered before incubated at 37°C for 16–20 h. The absorbance of the solutions at 600 nm was determined as a surrogate of cell density. The antibacterial activity of colistin at concentration of 30 μM was determined using the same method. Growth inhibition percentage was calculated from the corresponding absorbance value from the plate reader using Equation (1) (41), where the Amax represents the maximum value of absorbance which indicates no growth inhibition (0%). The inhibition growth I (%), therefore, varies from 0 to 100%.
OriginPro 9.1.0 statistical software (Origin Lab, Northampton, MA, USA) was employed to calculate the 50% growth inhibition (EC50) on basis of the dose-response curve prepared by each reaction solution. To assess the antibacterial activity of each sample, a potency equivalent quotient (PEQ) value was calculated from Equation (2) (44) where Colistin0 and colistint represents colistin solution before and after Fe (VI) treatment.
The ISO standard (45) with a Vibrio fischeri luminescence bioassay was employed to examine the toxicity of colistin and its degradation products. In brief, V. fischeri were exposed to the samples before and after Fe (VI) treatment under the optimal reaction conditions for 15 min at 15 ± 0.5°C, followed by determination of their bioluminescence intensities by a GloMax Multi Detection System (Promega, Madison, WI, USA). Then we computed the relative inhibitory rate (IR%) according to Equation (3) (46), in which E0 represents the normalized bioluminescence intensities of colistin solution before Fe (VI) treatment and E represents that of colistin solution after Fe (VI) treatment.
The emergence of mobile colistin resistance genes has threatened the role of colistin as the last line of defense against multidrug-resistant Gram-negative bacteria. Nonetheless, colistin is still allowed to be used as a feed additive in many countries and it is therefore necessary to find an effective removal procedure before it enters the aquatic environment. In this study we examined colistin degradation by Fe (VI) oxidization.
The initial dosage of Fe (VI) used is an important factor affecting its self-decomposition since it is proportional to its decomposition rate. In aqueous solution, this rate for Fe (VI) was previously determined to be 11% in solutions of 25 mM and complete decomposition occurred at levels of >30 mM within 60 min (47). We found that the molar ratio significantly affected colistin degradation (Figure 1). When the Fe (VI): colistin ration was increased from 5 to 30, colistin degradation rose from 8.3 to 96.9%. Degradation reached the level of statistical significance at molar ratios of 30 and 40 compared with the other ratios (P < 0.05; Table 2). The most likely reason for this was that at low K2FeO4 levels, the amount of Fe (VI) in solution available for reaction with colistin was insufficient and this was overcome at the higher K2FeO4 levels. Consistent with these interpretations, when the molar ratio increased from 30 to 40, colistin degradation increased slightly by only 0.8% and no significant difference between 30 and 40 was found (P > 0.05; Table 2). Results similar to these have been previously reported when Fe (VI) was employed to degrade bisphenol A (48). These reaction conditions provided sufficient Fe (VI) in solution to react with colistin and the further increase of potassium ferrate provided excess Fe (VI). The latter would however, accelerate its self-decomposition because elevated Fe (VI) concentrations are unstable (48) so that the effective concentration of Fe (VI) available for colistin degradation would be decreased. We therefore used a ratio of 30 for our remaining experiments since this level provided a high level of colistin degradation and was economical.
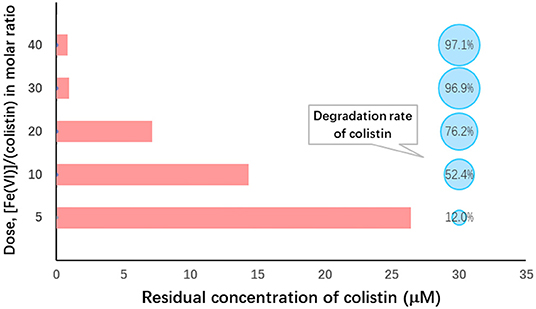
Figure 1. Ferrate (VI): colistin molar ratio dependence on colistin removal from solution. Initial colistin concentration, 30 μM; [Fe(VI)]/(colistin) molar ratio, 5–40; initial pH 9.0; temperature, 25°C; reaction duration, 60 min.
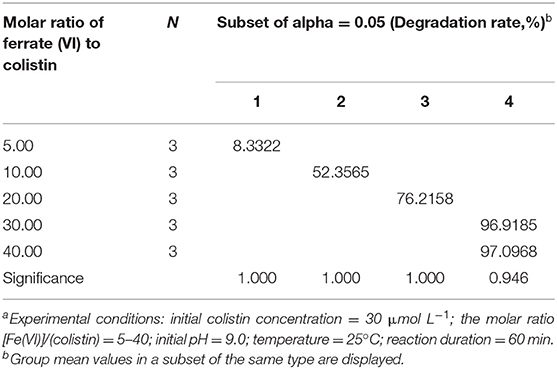
Table 2. Analysis of variance on degradation of colistin by different molar ratio of ferrate (VI) to colistina.
The stability of ferrate is enhanced at higher pH (47) according to the following formula (49):
The production of OH− from the reaction would lead to pH elevation that would also serve to inhibit Fe (VI) self-decomposition, thereby improving the stability of Fe (VI) in solution. In contrast, the presence of elevated H+ in solution as the pH falls would neutralize the generated OH− and increase the reaction rate but also accelerate Fe (VI) decomposition. Therefore, Fe (VI) is more stable under alkaline conditions than under neutral and acidic conditions.
In this study, however, we found that the highest level of colistin degradation (96.6%) occurred under neutral conditions (pH 7.0) and the lowest (89.7%) was observed at pH 10 (Figure 2). Analysis of variance indicated that colistin degradation at pH 7 was significantly greater than those at all other pH values we examined (P < 0.05) and that at pH 10 was significantly lower than those at other pH values (P < 0.05; Table 3). Similar results were obtained when Fe (VI) was used to oxidize chloramphenicol (50). The initial pH of the solution most likely influences not only Fe (VI) stability which decreases as the pH declines, but also the oxidation capacity of Fe (VI) that decreases at elevated pH. Although Fe (VI) is stable under alkaline conditions, its low redox potential (0.7 V) restricts the reaction and colistin removal. Under acidic conditions, although Fe (VI) shows a high redox potential (2.2 V), its self-decomposition hindered the colistin reaction and Fe (VI) self-decay occurred rapidly within minutes at pH <6.0 (50, 51). Therefore, extremes of pH were not conducive to colistin removal and we selected pH 7.0 as the optimal initial pH for further study.
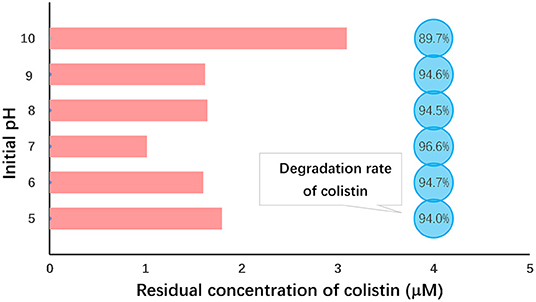
Figure 2. Initial pH dependence of colistin removal. Initial colistin concentration, 30 μM; [Fe (VI)]/(colistin) molar ration, 30; initial pH 5.0–10.0; temperature, 25°C; reaction duration, 60 min.
In order to achieve better degradation of colistin by Fe (VI), the effect of reaction duration was studied using the optimized level of Fe (VI) at pH 7.0. Colistin was degraded rapidly in the first 10 min and then the rate slowed (Figure 3). The degradation rate was significantly increased from 94.6 to 98.9% from 30 s to 10 min (P < 0.05) and remained at a plateau until 45 min and then increased slightly (Table 4). Due to the strong oxidizing power of Fe (VI), the majority of colistin (98.9%) was removed in the first 10 min. Following this, Fe (VI) consumption in the oxidation reaction as well as self-decomposition slowed the degradation rate. A time limit for the reaction was therefore set at 60 min to maximize colistin removal and was used in the remainder of this study.
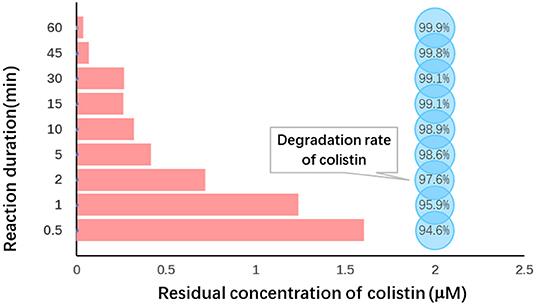
Figure 3. Reaction duration dependence of colistin removal. Initial colistin concentration, 30 μM; [F (VI)]/(colistin) molar ration, 30; initial pH, 7.0; temperature, 25°C, reaction duration, 0.5–60 min.
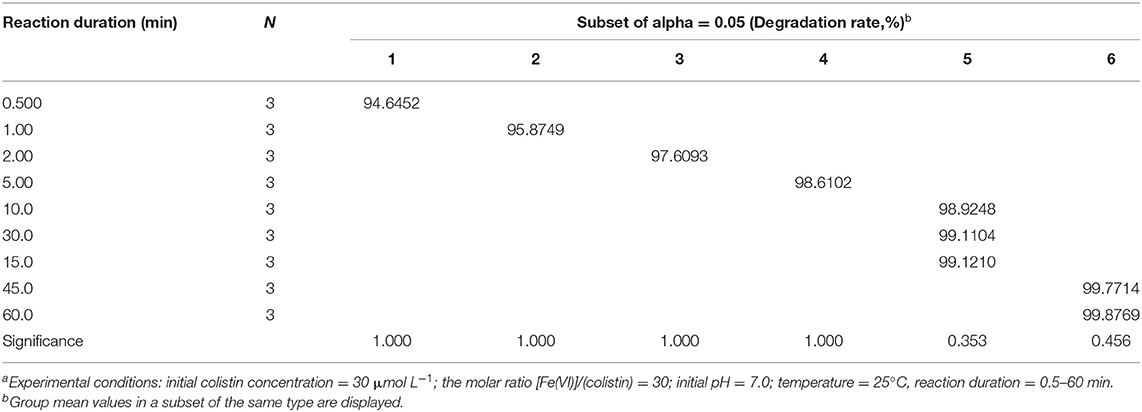
Table 4. Analysis of variance on degradation of colistin by ferrate (VI) at different reaction durationa.
Colistin removal at 60 min was 99.9% and exceeded the level in the molar ratio and pH experiments where the maximum values were each 96.9 and 96.6%, respectively. When we examined the influence of Fe (VI) dosage on degradation, we set the initial pH at 9.0. In those experiments, the highest degradation rate was obtained at an Fe (VI): colistin molar ratio of 30 and pH 7.0 for 60 min. These slight discrepancies can be accounted for by differences in reaction batches of Fe (VI) due to (1) moisture, gas and atmospheric reducing substances that would interact with solid K2FeO4 and reduce the stability of Fe (VI) and (2) the rapid degradation of Fe (VI) under suboptimal conditions such that occur during pH adjustments that could alter the amount of active Fe (VI) present in the K2FeO4. However, since excess Fe (VI) was used in each reaction, trace amounts of colistin in the final reaction mixture were at the level of experimental error and not reaction condition. Therefore, Fe (VI) could readily and effectively remove colistin under the optimal conditions used in this study.
Dissolved organic matter such as HA are common in wastewaters and natural water (52). We therefore examined whether HA at levels of 0–60 mg L−1 inhibited colistin removal by Fe (VI) under optimal conditions. We found that compared to the control, the presence of HA had no apparent impact on the degradation efficiency of colistin (P > 0.05; Table 5). Similar results were obtained in removal of polychlorinated diphenyl sulfides by Fe (VI) (52). The rapid reaction times for Fe (VI) and colistin most likely allowed this reaction to outcompete reactions with HA.
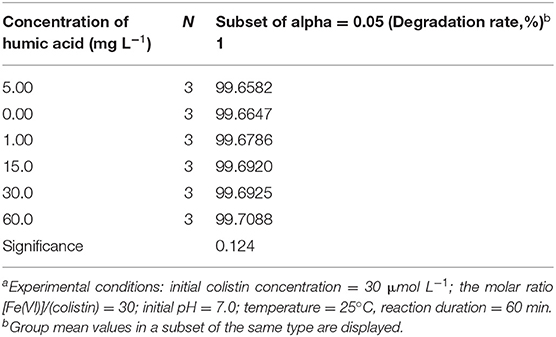
Table 5. Analysis of variance on degradation of colistin by ferrate (VI) in the presence of humic acida.
In order to find out if the colistin degradation products disturb the microecological balance in water and if they still pose selective pressure on generation and spread of mcr-1 in the environment, the antibacterial activities of colistin and its degradation products were determined with E. coli, S. aureus, and B. subtilis and we used PEQ analysis as a quantitative test of the dose-response curves. A PEQ value of 1 indicated that the EC50 of colistin before and after treatment were not different. A reduction in the antibacterial activity of colistin results in an increase in the EC50 of (colistint) and a PEQ <1. Similarly, PEQ > 1 means that treatment enhances the antibacterial activity of colistin (53). All our experiments resulted in PEQ <1 (Figure 4), indicating decreases in antibacterial activity. The E. coli strains ATCC 25922 and K88 generated PEQ < 0.02 and S. aureus gave PEQ = 0.15, implying that the antibacterial activity of colistin against them was nearly eliminated by Fe (VI) oxidation. The B. subtilis PEQ was 0.88 and close to 1 and was the result of high EC50 (colistin0) and EC50 (colistint) values. B. subtilis is a Gram-positive probiotic bacterium and is widely distributed in the environment. Since colistin is a cationic polypeptide that targets lipid A that is absent in Gram-positive bacteria (14, 54), the high value of EC50 (colistin0) against B. subtilis was expected. The value of PEQ close to 1 indicated that similar to colistin, the degradation products showed no obvious antibacterial activity to B. subtilis as well. However, it has been previously reported that colistin can destabilize the biofilm matrix structure even in species with intrinsic colistin resistance such as S. aureus, resulting in the release of planktonic cells that are more susceptible to antibiotics (55). In order to examine the change of antibacterial activity of colistin against both Gram-negative and Gram-positive bacteria, we included E. coli, the most common Gram-negative bacterium carrying plasmid-mediated resistance mcr genes as well as S. aureus, the representative Gram-positive bacteria in this study. The PEQ for S. aureus was higher than that for E. coli because the EC50 (colistin0) against S. aureus was ~10-fold greater than that against E. coli. Nonetheless, the EC50 (colistint) against E. coli and S. aureus were similar. Fe (VI) has been proven effective in eliminating the activity of other antibiotics including trimethoprim (41), β-lactams (44) and fluoroquinolone (53). In conclusion, the colistin degradation products showed almost no antibacterial activity against representative Gram-negative and Gram-negative bacteria demonstrating that the method for colistin degradation was environmentally friendly. They would not affect the microecological balance in water and posed no selective pressure on generation and spread of mcr-1 in the environment.
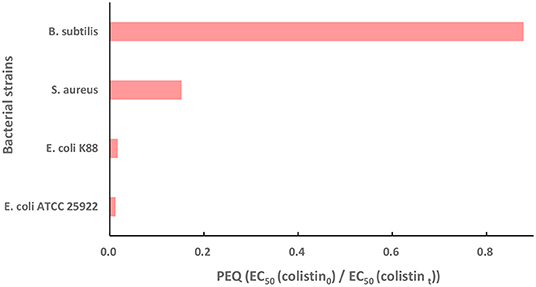
Figure 4. The potency equivalent quotient (PEQ) of colistin against E. coli ATCC 25922, wild type strain E. coli K88, S. aureus and B. subtilis. Initial colistin concentration, 30 μM; [Fe(VI)]/(colistin) molar ratio, 30; initial pH, 7.0; temperature, 25°C, reaction duration, 60 min. PEQ = EC50 (colistin0)/EC50 (colistint) where colistin0 and colistint represent colistin before and after Fe (VI) treatment.
Colistin has significant side effects when used in humans and animals including nephrotoxicity that results in hematuria, proteinuria, urinary casts and increased blood urea and creatinine levels (54). In addition, this antibiotic is also neurotoxic with symptoms including paresthesia, peripheral neuropathy, muscle weakness and neuromuscular blockade resulting in respiratory paralysis (56). Colistin is also an exotoxin and is toxic to the earthworm E. fetida (17). However, there are no studies that assess the biological toxicity of colistin and its degradation products in water. We therefore wanted to determine whether colistin and its degradation products from Fe (VI) oxidation are non-toxic. For these experiments we measured alterations in the luminescence of V. fischeri with exposure to colistin and the degradation products. In order to remove the interference of Fe (VI) on the growth of V. fischeri, Fe (VI) activity at the end of the test periods were quenched using Na2S2O3 prior to bacterial exposure in the Microtox test.
Our results indicated that the IR% of colistin degradation products (2.99%) to V. fischeri was lower than that of colistin (7.38%), although these differences were not significant (P > 0.05). This was consistent with previous studies for the Fe (VI) oxidation of indomethacin (57), bisphenol A (48) and tetrabromobisphenol A (46). In the latter studies, the degradation products exhibited less toxicity to the V. fischeri bacteria compared to the parent drug. Our results also indicated that colistin and its degradation products were of low toxicity to the water environment because their IR% value were <30 (58).
One limitation of the current study is that we did not describe the degradation kinetics and identify the degradation products. Due to the large excess of Fe (VI) involved in the reactions, we found that >90% of colistin was degraded after reaction for 5 s (data not shown), indicating that most of degradation was completed nearly in an instant. The purpose of this study was to find an effective way to eliminate colistin in a short time and in turn, to reduce its risk of promoting the generation and spread of plasmid-mediated resistance mcr genes in the environment. The latter could cause failure as rescue treatments for human infections with multidrug-resistant bacilli rather than to compare subtle differences under different conditions. Therefore, we did not further study the degradation kinetics of colistin by Fe (VI) oxidation.
Since colistin sulfate is actually a mixture of colistin A and B as well as minor components, we were unable to identify all the components in the reaction mixtures using LC-MS/MS (data not shown). However, the final degradation products possessed minimal antibacterial activity against sensitive and non-sensitive bacteria and most had no toxicity to the luminescent bacteria. Hence, a failure to identify the products would not affect the application of Fe (VI) to remove colistin in practice under the optimal conditions proposed in this study.
The degradation of colistin by Fe (VI) was investigated and we found that (1) colistin can be effectively degraded by Fe (VI) oxidation (99.9%) using an Fe (VI): colistin molar ratio of 30 with an initial colistin concentration of 30 μM at pH 7.0, 25°C for 60 min. (2) The presence of humic acid had no obvious impact on the removal of colistin by Fe (VI). (3) The products of colistin produced by Fe (VI) degradation possessed no antibacterial activity against E. coli, S. aureus and B. subtilis, indicating that Fe (VI) would be effective at reducing selective pressure of colistin on environmental bacteria for generation and spread of plasmid-mediated colistin resistance genes. (4) Both colistin and its degradation products in water were of low toxicity to Vibrio fischeri.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
LW contributed to research, wrote the first draft of the manuscript, revision, read, and approved the submitted version. SL, XW, and BL contributed to data analysis, revision, read, and approved the submitted version. ZW contributed conception, revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
This work was financially supported by National Natural Science Foundation of China (31760750, 32160853), Key Research and Development Project of Jiangxi Province (20171BBF60054), Science and Technology Projects of Jiangxi Provincial Education Department (GJJ180184), and Key Research and Development Project of Jiangxi Province (20161BBF60088).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge and appreciate the information that was provided by the referenced authors for this study. We thank S. H. Fang and H. J. Huang for assistance with EC50 analysis and suggestions for kinetics and products experiments.
1. Sarmah AK, Meyer MT, Boxall ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. (2006) 65:725–59. doi: 10.1016/j.chemosphere.2006.03.026
2. Ben W, Qiang Z, Adams C, Zhang H, Chen L. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatographytographyraphyhromatoJ Chromatogr A (2008) 1202:173–80. doi: 10.1016/j.chroma.2008.07.014
3. Chen Q, Guo X, Hua G, Li G, Feng R, Liu X. Migration and degradation of swine farm tetracyclines at the river catchment scale: can the multi-pond system mitigate pollution risk to receiving rivers? Environ Pollut. (2017) 220:1301–10. doi: 10.1016/j.envpol.2016.11.004
4. Liu X, Lu S, Guo W, Xi B, Wang W. Antibiotics in the aquatic environments: a review of lakes, China. Sci Total Environ. (2018) 627:1195–208. doi: 10.1016/j.scitotenv.2018.01.271
5. Xie WY, Shen Q, Zhao FJ. Antibiotics and antibiotic resistance from animal manures to soil: a review. Eur J Soil Sci. (2018) 69:181–95. doi: 10.1111/ejss.12494
6. Kummerer K. Antibiotics in the aquatic environment–a review–part II. Chemosphere. (2009) 75:43–41. doi: 10.1016/j.chemosphere.2008.12.006
7. Ma R, Wang B, Lu S, Zhang Y, Yin L, Huang J, et al. Characterization of pharmaceutically active compounds in Dongting Lake, China: occurrence, chiral profiling and environmental risk. Sci Total Environ. (2016) 557–558:268–75. doi: 10.1016/j.scitotenv.2016.03.053
8. Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol R. (2010) 74:417–33. doi: 10.1128/MMBR.00016-10
9. Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. (2016) 16:161–8. doi: 10.1016/S1473-3099(15)00424-7
10. Gonz4lez-Pleiter M, Gonzalo S, Rodea-Palomares I, Leganeg F, Rosal R, Boltes K, et al. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: implications for environmental risk assessment. Water Res. (2013) 47:2050–64. doi: 10.1016/j.watres.2013.01.020
11. Kasiakou SK, Michalopoulos A, Soteriades ES, Samonis G, Sermaides GJ, Falagas ME. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother. (2005) 49:3136–46. doi: 10.1128/AAC.49.8.3136-3146.2005
12. Sarkar S, DeSantis ERH, Kuper J. Resurgence of colistin use. Am J Health-Syst Ph. (2007) 64:2462–4. doi: 10.2146/ajhp060501
13. Catry B, Cavaleri M, Baptiste K, Grave K, Grein K, Holm A, et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): development of resistance in animals and possible impact on human and animal health. Int J Antimicrob Agents. (2015) 46:297–306. doi: 10.1016/j.ijantimicag.2015.06.005
14. Rhouma M, Beaudry F, Theriault W, Letellier A. Colistin in pig production: chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front Microbiol. (2016) 7:1789. doi: 10.3389/fmicb.2016.01789
15. EMA. Updated Advice on the Use of Colistin Products in Animals Within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. London: EMA (2016).
16. Bressan CR, Kunz A, Schmidell W, Soares HM. Toxicity of the colistin sulfate antibiotic used in animal farming to mixed cultures of nitrifying organisms. Water Air Soil Pollut. (2013) 224:1441. doi: 10.1007/s11270-013-1441-4
17. Guo R, Ding X, Zhong X, Gao S, Sun Y. Molecular and ultrastructural insights into the earthworm Eisenia fetida of the assessment of ecotoxicity during colistin exposure. Environ Sci Pollut R. (2014) 21:13405–11. doi: 10.1007/s11356-014-3256-2
18. Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. (2017) 30:557–96. doi: 10.1128/CMR.00064-16
19. Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surv. (2016) 21:1. doi: 10.2807/1560-7917.ES.2016.21.27.30280
20. Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar. Paratyphi B J Antimicrob Chemother. (2017) 72:3317–24. doi: 10.1093/jac/dkx327
21. Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. (2017) 22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589
22. Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. (2017) 8:e5178:17ichia col1128/mBio.00543-17
23. Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. (2018) 23:672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672
24. Hu S, Lv Z, Wang Y, Shen J, Ke Y. Rapid detection of human origin colistin-resistance genes mcr-1, mcr-3, mcr-8, mcr-10 in clinical fecal samples. Arch Microbiol. (2021) 203:4405–17. doi: 10.1007/s00203-021-02407-2
25. Liu Z, Han Z, Yu X, Wen G, Zeng C. Crystal structure of the catalytic domain of MCR-1 (cMCR-1) in complex with d-xylose. Crystals. (2018) 8:172. doi: 10.3390/cryst8040172
26. The Ministry of Agriculture of the People's Republic of China. Announcement No. 2428 of the Ministry of Agriculture of the People's Republic of China. (2016). p. 1–2.
27. Yang B, Ying G, Zhao J, Liu S, Zhou L, Chen F. Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate(VI) treatment of secondary wastewater effluents. Water Res. (2012) 46:2194–204. doi: 10.1016/j.watres.2012.01.047
28. Jiang J. Advances in the development and application of ferrate (VI) for water and wastewater treatment. J. Chem. Technol. Biotechnol. (2014) 89:165–77. doi: 10.1002/jctb.4214
29. Sharma VK, Zboril R, Varma RS. Ferrates: greener oxidants with multimodal action in water treatment technologies. Accounts Chem Res. (2015) 48:182–91. doi: 10.1021/ar5004219
30. Jiang JQ, Lloyd B. Progress in the development and use of ferrate (VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res. (2002) 36:1397–408. 10.1016/S0043-1354(01)00358-X
31. Cho M, Lee Y, Choi W, Chung H, Yoon J. Study on Fe (VI) species as a disinfectant: quantitative evaluation and modeling for inactivating Escherichia coli. Water Res. (2006) 40:3580–5. doi: 10.1016/j.watres.2006.05.043
32. Jiang JQ, Panagoulopoulos A, Bauer M, Pearce P. The application of potassium ferrate for sewage treatment. J Environ Manage. (2006) 79:215–20. doi: 10.1016/j.jenvman.2005.06.009
33. Prucek R, Tucek J, Kolarik J, Filip J, Marusak Z, Sharma VK, et al. Ferrate (VI)-induced arsenite and arsenate removal by in situ structural incorporation into magnetic iron (III) oxide nanoparticles. Environ Sci Technol. (2013) 47:3283–92. doi: 10.1021/es3042719
34. Prucek R, Tuček J, Kolačík J, Hušková I, Filip J, Varma RS, et al. Ferrate(VI)-prompted removal of metals in aqueous media: mechanistic delineation of enhanced efficiency via metal entrenchment in magnetic oxides. Environ Sci Technol. (2015) 49:2319–27. doi: 10.1021/es5048683
35. Jiang J, Wang S, Panagoulopoulos A. The exploration of potassium ferrate (VI) as a disinfectant/coagulant in water and wastewater treatment. Chemosphere. (2006) 63:212–9. doi: 10.1016/j.chemosphere.2005.08.020
36. Hu L, Page MA, Sigstam T, Kohn T, MariKoh BJ, Strathmann TJ. Inactivation of bacteriophage MS2 with potassium ferrate (VI). Environ Sci Technol. (2012) 46:12079–87. doi: 10.1021/es3031962
37. Lim M, Kim M. Removal of natural organic matter from river water using potassium ferrate (VI). Water Air Soil Pollut. (2009) 200:181–9. doi: 10.1007/s11270-008-9902-x
38. Jiang J, Zhou Z, Pahl O. Preliminary study of ciprofloxacin (cip) removal by potassium ferrate (VI). Sep Purif Technol. (2012) 88:95–8. doi: 10.1016/j.seppur.2011.12.021
39. Feng M, Wang X, Chen J, Qu R, Sui Y, Cizmas L, et al. Degradation of fluoroquinolone antibiotics by ferrate (VI): effects of water constituents and oxidized products. Water Res. (2016) 103:48–57. doi: 10.1016/j.watres.2016.07.014
40. Zhou Z, Jiang J. Reaction kinetics and oxidation products formation in the degradation of ciprofloxacin and ibuprofen by ferrate (VI). Chemosphere. (2015) 119:S95–100. doi: 10.1016/j.chemosphere.2014.04.006
41. Anquandah GAK, Sharma VK, Knight DA, Batchu SR, Gardinali PR. Oxidation of trimethoprim by ferrate (VI): kinetics, products, and antibacterial activity. Environ Sci Technol. (2011) 45:10575–81. doi: 10.1021/es202237g
42. Barişçi S, Ulu F, SillanpääM, Dimoglo A. The usage of different forms of ferrate (VI) ion for amoxicillin and ciprofloxacin removal: density functional theory based modelling of redox decomposition. J Chem Technol Biotechnol. (2016) 91:257–66. doi: 10.1002/jctb.4625
43. Anquandah GAK, Sharma VK, Panditi VR, Gardinali PR, Kim H, Oturan MA. Ferrate (VI) oxidation of propranolol: kinetics and products. Chemosphere. (2013) 91:105–9. doi: 10.1016/j.chemosphere.2012.12.001
44. Karlesa A, De Vera GA, Dodd MC, Park J, Espino MP, Lee Y. Ferrate(VI) oxidation of beta-lactam antibiotics: reaction kinetics, antibacterial activity changes, and transformation products. Environ Sci Technol. (2014) 48:10380–9. doi: 10.1021/es5028426
45. Water Quality Determination Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent bacteria test)-Part 3: Method Using Freeze-Dried Bacteria; ISO 11348-3. International Organization for Standardization: Geneva (2007).
46. Han Q, Dong W, Wang H, Liu T, Tian Y, Song X. Degradation of tetrabromobisphenol A by ferrate (VI) oxidation: performance, inorganic and organic products, pathway and toxicity control. Chemosphere. (2018) 198:92–102. doi: 10.1016/j.chemosphere.2018.01.117
47. Schreyer JM, Ockerman LT. Stability of the ferrate (V1) ion in aqueous solution. Anal Chem. (1951) 23:1312–4. doi: 10.1021/ac60057a028
48. Han Q, Wang H, Dong W, Liu T, Yin Y, Fan H. Degradation of bisphenol A by ferrate (VI) oxidation: kinetics, products and toxicity assessment. Chem Eng J. (2015) 262:34–40. doi: 10.1016/j.cej.2014.09.071
49. Sharma VK. Potassium ferrate VI: an environmentally friendly oxidant. Adv Environ Res. (2002) 6:143–56. doi: 10.1016/S1093-0191(01)00119-8
50. Zhou J, Chen K, Hong Q, Zeng F, Wang H. Degradation of chloramphenicol by potassium ferrate (VI) oxidation: kinetics and products. Environ Sci Pollut R. (2017) 24:10166–71. doi: 10.1007/s11356-017-8656-7
51. Graham N, Jiang C, Li X, Jiang J, Ma J. The influence of pH on the degradation of phenol and chlorophenols by potassium ferrate. Chemosphere. (2004) 56:949–56. doi: 10.1016/j.chemosphere.2004.04.060
52. Chen J, Xu X, Zeng X, Feng M, Qu R, Wang Z, et al. Ferrate (VI) oxidation of polychlorinated diphenyl sulfides: kinetics, degradation, and oxidized products. Water Res. (2018) 143:1–9. doi: 10.1016/j.watres.2018.06.023
53. Feng M, Cizmas L, Wang Z, Sharma VK. Activation of ferrate (VI) by ammonia in oxidation of flumequine Kinetics, transformation products, and antibacterial activity assessment. Chem Eng J. (2017) 323:584–91. doi: 10.1016/j.cej.2017.04.123
54. Dijkmans AC, Wilms EB, Kamerling IMC, Birkhoff W, Ortiz-Zacarias NV, van Nieuwkoop C, et al. Colistin: revival of an old polymyxin antibiotic. Ther Drug Monit. (2015) 37:419–27. doi: 10.1097/FTD.0000000000000172
55. Klinger-Strobel M, Stein C, Forstner C, Makarewicz O, Pletz MW. Effects of colistin on biofilm matrices of Escherichia coli and staphylococcus aureus. Int J Antimicrob Ag. (2017) 49:472–9. doi: 10.1016/j.ijantimicag.2017.01.005
56. Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. (1970) 72:857–68. doi: 10.7326/0003-4819-72-6-857
57. Huang J, Wang Y, Liu G, Chen P, Wang F, Ma J, et al. Oxidation of indometacin by ferrate (VI): kinetics, degradation pathways, and toxicity assessment. Environ Sci Pollut R. (2017) 24:10786–95. doi: 10.1007/s11356-017-8750-x
58. Ministry of Natural Resources of the People's Republic of China. Technical Regulations for Monitoring Biological Toxicity of Wastewater–Accute Toxicity Assessment by Luminescent Bacteria -Method Using Vibrio fischeri (Huanhaizi 2015 No. 29). (In Chinese). Beijing: Department of Ecological Environment Protection SOAM (2015). p. 6.
Keywords: degradation, colistin sulfate, humic acid, antibacterial activity, toxicity, water
Citation: Wang L, Lv S, Wang X, Liu B and Wang Z (2021) Ferrate (VI) Oxidation Is an Effective and Safe Way to Degrade Residual Colistin - a Last Resort Antibiotic - in Wastewater. Front. Vet. Sci. 8:773089. doi: 10.3389/fvets.2021.773089
Received: 09 September 2021; Accepted: 06 December 2021;
Published: 24 December 2021.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaCopyright © 2021 Wang, Lv, Wang, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Wang, MTAxNjMxMjEzOEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.