- 1State Key Laboratory of Veterinary Etiological Biology, Key Laboratory of Veterinary Parasitology of Gansu Province, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China
- 2College of Veterinary Medicine, Northwest A&F University, Xianyang, China
- 3Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou, China
Antibiotic resistance is an increasingly prevalent problem worldwide. Probiotics are live microorganisms that provide health benefits to human beings and animals and also antimicrobial activity against pathogens and might be an antibiotic alternative. The gastrointestinal tract of animals can be a suitable source of finding novel antimicrobial agents, where the vast majority of gut microbes inhabit and a plurality of antimicrobial producers exhibit either a wide or narrow spectrum. Animals that live in Northwest China might possess a special commensal community in the gut. Therefore, the purpose of this study was to assess the effects of three probiotic strains (including Lactobacillus salivarius ZLP-4b from swine, Lactobacillus plantarum FBL-3a from beef cattle, and Bacillus velezensis JT3-1 from yak), which were isolated from livestock in this area, on the overall growth performance, immune function, and gut microbiota of mice. The results showed that the L. salivarius ZLP-4b group not only improved the growth performance but also amended the intestinal mucosa morphology of mice. Furthermore, the supplementation of L. plantarum FBL-3a and L. salivarius ZLP-4b strains significantly increased the content of anti-inflammatory cytokines IL-4 and IL-10 but decreased the pro-inflammatory factor IL-17A. The levels of pro-inflammatory factors IL-6, IL-17A, and TNF-α were also decreased by the B. velezensis JT3-1 group pretreatment. The 16S rDNA sequence results showed that the probiotic administration could increase the proportion of Firmicutes/Bacteroidetes intestinal microbes in mice. Furthermore, the relative abundance of Lactobacillus was boosted in the JT3-1- and ZLP-4b-treated groups, and that of opportunistic pathogens (including Proteobacteria and Spirochaetes) was diminished in all treated groups compared with the control group. In conclusion, B. velezensis JT3-1 and L. salivarius ZLP-4b supplementation enhanced the overall performance, intestinal epithelial mucosal integrity, and immune-related cytokines and regulated the intestinal microbiota in mice.
Introduction
Antibiotic has been applied for almost 100 years as a predominant strategy in controlling infectious diseases and improving the growth performance of animals (1). Along with the inappropriate and excessive use of antibiotics, antibiotic resistance issues have been gradually exposed (2). There is an increasing number of bacteria which have developed drug resistance, such as Salmonella with multidrug resistance (3). Besides this, antibiotic resistance is also found in association with gut microbiota disturbance. Furthermore, dysbiosis of microbiota might cause numerous diseases, including obesity, autoimmune diseases, allergy, and intestinal diseases such as irritable bowel syndrome (IBD) (4). Consequently, alternatives to antibiotic treatment were required to be explored (5).
Probiotics have been proven to confer benefits to human and animals within reasonable adoption (6). At present, major probiotics can be classified into Lactobacillus spp. (such as Lactobacillus rhamnosus and Lactobacillus acidophilus), Bacillus spp. (Bacillus subtilis), Bifidobacterium spp. (Bifidobacterium longum and Bifidobacterium animal), yeast (Saccharomyces cerevisiae) and Clostridium spp. (Clostridium butyricum), and so on (7). In addition, next-generation probiotics (Akkermansia muciniphila, Faecalibacterium prausnitzii, and Eubacterium hallii) and genetically modified probiotics (GM probiotics: mutation and overexpression) were vigorously researched (8, 9). The basic mechanism by which probiotics exert beneficial effects is explained as follows: (1) colonization and restoration of disordered intestinal microbiota in the host, (2) competitive exclusion of harmful microbes and antimicrobial molecule production, (3) cell antagonism, cell adhesion, and mucin expression, and (4) regulation of innate and acquired immunity of the host (10). Studies demonstrated that B. subtilis supplementation could ameliorate heat-induced behavioral and inflammatory reactions (11). Sun et al. (12) reported that Bifidobacterium administration altered the commensal community in the gut of mice and regulated the mucosal immunity as determined by Tregs in the colitis model under the cytotoxic T lymphocyte-associated protein 4 blockade conditions. Li et al. (13) also found that Bacillus spp. isolated from feces of yaks significantly impacts the growth performance, the action of intestinal digestive enzymes, immune responses, and antioxidative capacity in mice. In addition, Lactobacillus casei overproducing conjugated linoleic acids illustrated a significant protective effect on Salmonella enteric serovar Typhimurium challenge (14).
Xinjiang and Gansu are two provinces of Northwest China, which are characterized by a rough terrain and harsh weather. Consequently, livestock inhabiting in the alpine areas are very resilient to harsh ecological and climatic environmental changes. Thus, altitude hypoxia (low temperature and thin air) would put constant heat on the process of evolution (15). A previous study showed that environmental factors helped to shape the components and functions of the intestinal microbiota in people who lived at high altitudes (16). Fan et al. (17) demonstrated that altitude impacted on the diversity of microbes and herbage fermentation in the rumen of yaks. This hinted that the animals that lived in Northwest China might possess a special commensal community in the gut compared to those in the plain region. However, there are few research about the gut microbiota of these animals, and studies on probiotics existing in these animals are also seldom reported.
Therefore, this study set out to assess the effects of Bacillus spp. (Bacillus velezensis JT3-1 obtained from yak) and the effects of two strains of Lactobacillus (Lactobacillus plantarum FBL-3a sourced from beef cattle and Lactobacillus salivarius ZLP-4b isolated from swine) on the growth performance, intestinal morphology, immune-related cytokines, and gut microbiota of mice.
Materials and Methods
Probiotic Strains and Animal Experiments
The B. velezensis JT3-1 used in this experiment was obtained from feces of healthy domestic yak (Bos grunniens) in Gansu province of China. Two strains of Lactobacillus were also isolated from healthy animal feces in Northwest China and included L. plantarum FBL-3a isolated from feces of beef cattle in Xinjiang Uygur Autonomous Region and L. salivarius ZLP-4b obtained from feces of swine in Gansu province. Our previous results found that these probiotics exhibited good tolerance and antimicrobial activity. The antibiotic sensitivity experiments, resistance gene tests, and hemolytic experiments conducted showed the safety of the JT3-1, FBL-3a, and ZLP-4b strains. Based on this, the complete genome sequence of B. velezensis JT3-1 (GenBank: CP032506), L. plantarum FBL-3a (GenBank: CP034694), and L. salivarius ZLP-4b (GenBank: CP062071) was performed using a PacBio Sequel sequencing platform at Beijing Genewiz Bioinformatics Technology Co., Ltd. B. velezensis JT3-1 was cultured in nutrient agar medium at 37°C for 24 h aerobically. Lactobacillus was cultured at 37°C for 24 h under anaerobic environment on MRS agar medium. Then, the bacterial cells of these strains were evaluated by using the plate count method.
The 2-week-old female Kunming mice (n = 48) were obtained from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences and raised in specific pathogen-free conditions. They were randomly and equally assigned to four groups, including the control group and the JT3-1-, FBL-3a-, and ZLP-4b-treated groups. In the meantime, the mice in the experimental groups were orally gavaged JT3-1, FBL-3a, or ZLP-4b at 1 × 108 colony forming units per day for a continuous 2 weeks, respectively, while the control mice were given the same volume of phosphate-buffered saline. During the experiment, abnormal performance in mice was recorded, such as death, diarrhea, loss of appetite, weight loss, and so on.
Production Performance Analysis
In this test, the body weight of all mice were recorded at the same time every day and under identical conditions. Main organs such as the heart, lung, liver, kidney, thymus, and spleen were weighed by an electronic balance after euthanasia. The weight/body weight of an organ was expressed as organ index.
Histological Staining
The duodenum, jejunum, ileum, and colon tissues were dipped in 4% paraformaldehyde solution (Servicebio Co., Ltd., Wuhan, China) and stained using hematoxylin and eosin (H&E) solution. Images of morphological character were captured at ×40 and ×100 magnification by using a scanning electron microscope SU8100 (Hitachi Co., Ltd., Japan). The intestinal villus height, crypt depth, and the rate of villus height to crypt depth were calculated by CaseViewer 2.0 software.
Cytokine Profiling Assay
Essential cytokines in the serum of mice such as IL-4, IL-6, IL-10, IL-17A, and TNF-α were determined with Mouse ProcartaPlex Panel (Thermo Fisher Scientific, Waltham, USA) following the instructions from the manufacturer. The samples were read by Luminex TM 100/200TM instrument (Luminex Corp, Austin, TX, USA).
Fecal Extraction and 16S Sequence Analysis
Fresh feces in colon were collected under sterile conditions. The feces were promptly frozen in liquid nitrogen and then preserved at −80°C for the next step. The bacterial genomic DNA was extracted from the stored fecal pellets using QIAamp Fast DNA Stool Mini Kit (Qiagen, Germantown, MD) according to the manufacturer. The V3–V4 region of the bacterial 16S rRNA gene was PCR-amplified using the forward primer 5′-CCTACGGGNGGCWGCAG-3′ and reverse primer 5′- GACTACHVGGGTATCTAATCC-3′. The PCR product purification and amplicon library construction were performed by the Illumina NovaSeq PE250 platform at a commercial company (LC-Bio Technology Co., Ltd, Hang Zhou, China) according to the standard protocols.
Statistical Analysis
Figures were generated using GraphPad Prism 6.0 software. The statistical significance in our study was determined by t-test and χ2 test by the SPSS statistical program, and P < 0.05 was considered statistically significant. The analysis was repeated for three times independently.
Results
Production Performance and Organ Index Analysis
During the experiment period, no aberrant behavior was found. The overall performance in all three groups of the mice that received probiotics was improved compared to the control group. As shown in Figure 1A, there was no difference in the body weight on days 7 and 14 in the experimental and control groups, except that the weight of the mice treated with ZLP-4b was significantly increased than that of the control group on day 14 (Figure 1A). No significant difference was observed in the weight of the heart, liver, spleen, lung, thymus, and kidney between the three probiotic-administrated groups and the control group (Figure 1B). The results indicated that probiotic JT3-1, FBL-3a, and ZLP-4b administration did not affect the growth performance of mice.
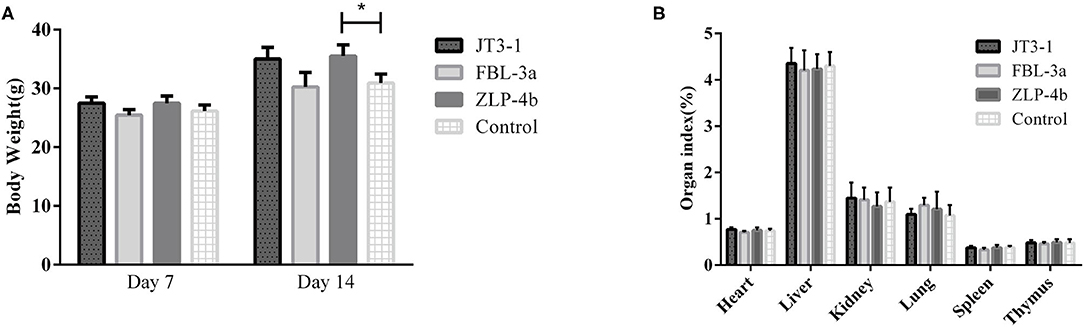
Figure 1. Probiotic supplementation improved the overall performance of mice. (A) The body weight of mice at days 7 and 14, respectively. (B) Organ indexes analysis of the heart, liver, and spleen of mice in all the groups. The data are presented as mean ± SD. The values indicate mean ± SD. *P < 0.05.
Probiotic Supplementations Improve the Intestinal Mucosa Morphology
This study was aimed to investigate whether these isolated strains contribute to the intestinal mucosa function of mice. It was found that the three probiotic administration groups significantly improved the intestinal epithelial mucosal integrity in the jejunum, ileum, and colon tissues but not the duodenum compared to the control mice (Figures 2A–D). Meanwhile, the villus heights and the ratios of villi heights to crypt depths in these tissues were also increased (Figures 2E–G). These results showed that the three probiotic-administrated groups presented an improved intestinal mucosal integrity.

Figure 2. The effects of probiotic pretreatment on the epithelial mucosa integrity of the intestine of mice. (A–D) Intestinal morphology shown by hematoxylin and eosin staining of the duodenum, jejunum, ileum, and colon tissues of mice. The images of intestinal morphology at ×40 and ×100 magnification are shown, respectively. (E) Statistical analysis of villus height (μm), (F) crypt depth (μm), and (G) the ratios of villus height (μm) to crypt depth (μm) in the duodenum, jejunum, and ileum of mice, respectively. (A) JT3-1 group, (B) FBL-3a group, (C) ZLP-4b group, and (D) control group. *P < 0.05; **P < 0.01.
Effects of Probiotics on Cytokine Modulation
The serum anti-inflammatory cytokines (IL-4 and IL-10) and pro-inflammatory cytokines (IL-6, IL-17A, and TNF-α) were measured to indicate the inflammatory levels in mice after probiotics were used. The IL-10 and IL-4 levels were dramatically enhanced in the FBL-3a and ZLP-4b groups, except in the JT3-1-treated group (P < 0.01 or P < 0.001, Figures 3D,E). The content of IL-6 and TNF-α showed a reduction in the JT3-1-treated group compared to the control mice; a similar decrease was also found in FBL-3a and ZLP-4b-mice, although the changes were not distinct (P < 0.01, P < 0.05, or P > 0.05, Figures 3A,B). In addition, Figure 3C revealed that the content of IL-17A in sera was reduced after the mice received probiotics compared to the control group (P < 0.05, Figure 3C). It seemed that B. velezensis JT3-1 preferred to inhibit pro-inflammatory cytokines, while L. plantarum FBL-3a and L. salivarius ZLP-4b were more inclined to stimulate the production of anti-inflammatory cytokines.
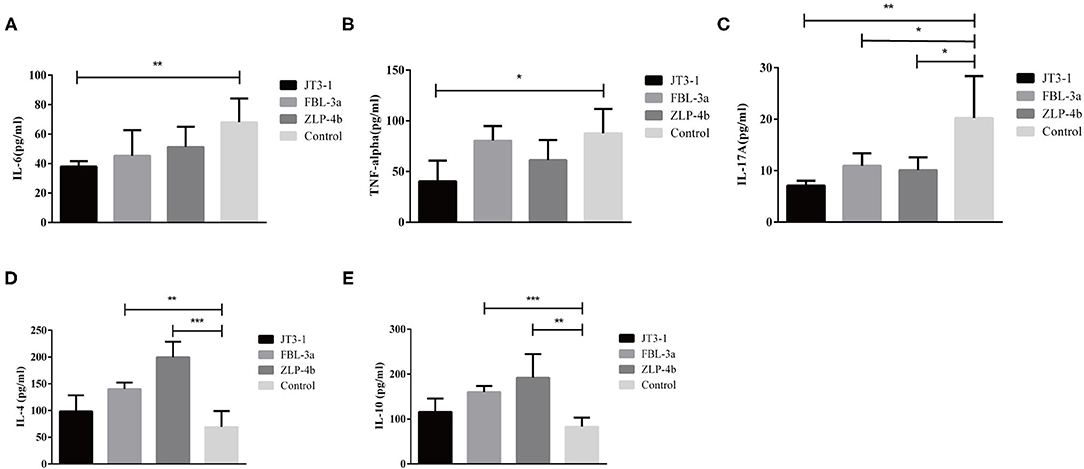
Figure 3. Effects of the oral administration of probiotics on cytokine modulation. (A–E) IL-6, TNF-α, IL-17A, IL-4, and IL-10 level in the serum of mice. Data are expressed as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Characterization of the Gut Microbiota in Mice After Probiotic Administration
The effects of probiotic administration on the intestinal microbial communities in mice were estimated by 16S rDNA sequence. The rarefaction curves showed that nearly all the bacteria species were sequenced in the feces of mice (Figure 4A). It was found that the mice possessed a lower bacterial species richness compared with the control group after the oral administration of probiotics (Figure 4B). Moreover, the detection of bacterial phyla displayed that the relative abundance of Firmicutes was significantly increased, while the relative abundance of Proteobacteria and Bacteroidetes markedly declined in the fecal microbiota from the probiotic-treated mice (Figure 4C). Besides this, some phyla such as Deferribacteres, Epsilonbacteraeota, Fusobacteria, and Actinbacteria showed a higher abundance, while lower Spirochaetes was observed in the probiotic-treated group (Figure 4D). At the genus level, the bacterial genera in the fecal microbiota showed that the relative abundance of Bilophila and Bacteroides was reduced, while that of Alistipes was increased in the probiotic supplementation group compared with that in the control group. Strangely, the Lactobacillus abundance in JT3-1 and ZLP-4b group was raised, but the FBL-3a-treated group was reduced (Figure 4E).
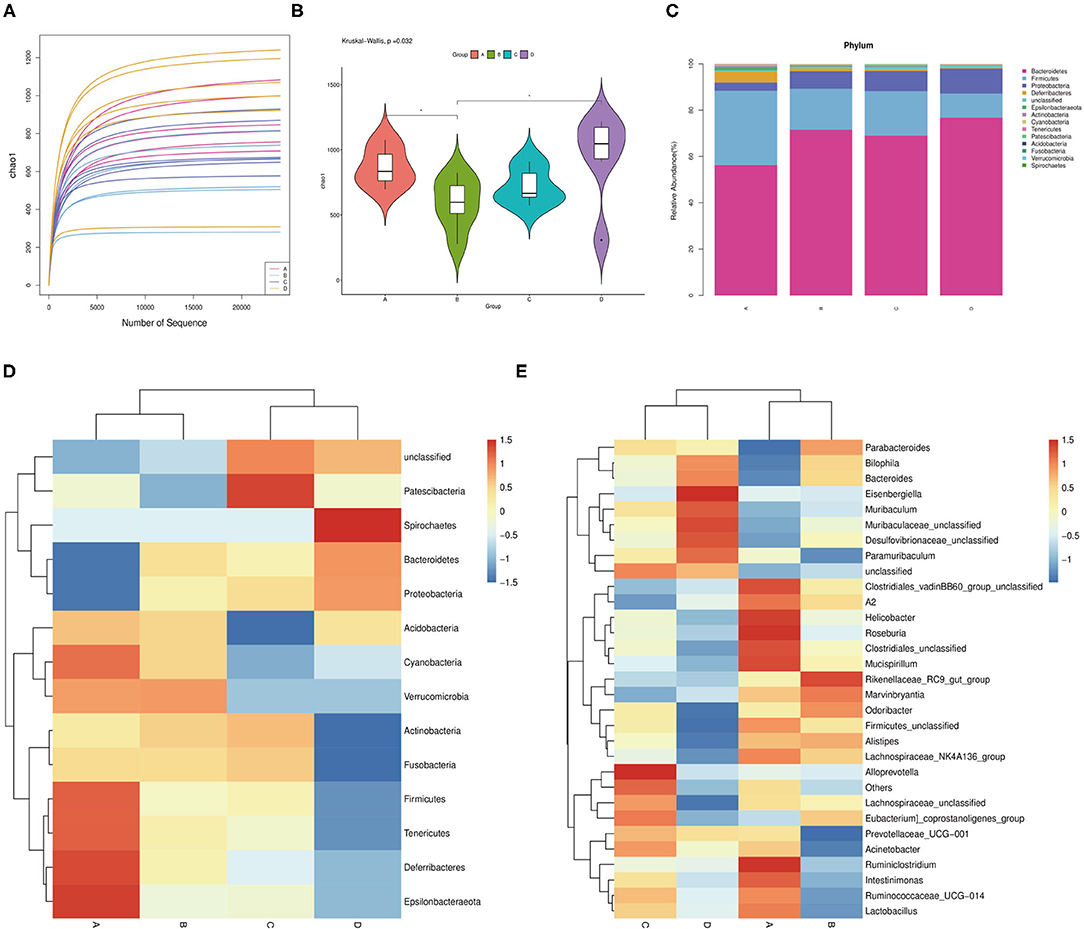
Figure 4. Microbial community changes in the colon content of mice after probiotic administration. (A) Bacterial rarefaction curves for assessing the sequencing depth of each sample. (B) Alpha diversities in bacterial communities as determined by Chao1 index. (C) Average relative abundances of taxa at the phylum level. (D) Heat map and hierarchical clustering of phylum in the intestinal bacterial communities of mice. (E) Clustering heat map analysis of bacterial genera at the genus level. (A) JT3-1 group, (B) FBL-3a group, (C) ZLP-4b group, and (D) control group.
Discussion
Antibiotics had a profound impact on bacterial infection in the therapeutic treatment of diseases (18). However, a number of serious problems also appeared subsequently as the synthesis of antibiotics in large quantity, such as the threat of bacterial resistance, antibiotic-associated diarrhea, and super-infection. In this study, we identified that three candidate probiotic strains from Northwest China improved the immune function and gastrointestinal health of mice. Firstly, no exceptional changes of body weight and organ index both in the control group and the treated groups demonstrated the safety of these three probiotics during the oral administration period. Secondly, the length of the villi and the villus length/crypt depth ratio were improved, and the integrity of the intestinal epithelial mucosa in the ileum and colon tissues was ameliorated, except the duodenum and jejunum segments, compared to the control mice after probiotic gavage. A similar result reported that probiotic supplementation enhanced the height of villus and the depth of crypt (19). Maybe it is just because microorganisms mainly colonize the back part of the intestine, particularly in the colon where microbes act as a major modulator of the immune system of the mucosa (20).
Cytokines play a considerable part in the immune modulatory and defense system of the host (21). However, not all probiotics could suppress pro-inflammatory cytokines and enhance anti-inflammatory factors—for instance, Escherichia coli strain Nissle 1917 had both pro- and anti-inflammatory effects because of the fact that E. coli Nissle 1917 also benefits from the inflamed environment, and it could account for why its anti-inflammatory effects are mild to moderate (22, 23). IL-4 and IL-10 play an important role in the immune modulatory pathway of the host as acknowledged inflammatory suppressor, and IL-10 production depends on IL-4 (24, 25). The cooperation between IL-10 and IL-4 suppressed Th1-related parameters (26). IL-4 is required for the induction of the class switch to IgG1 antibodies in cardiolipin-specific B cells (27). A previous study reported that the level of IL-4 was significantly evaluated after supplementation of a probiotic mixture of L. paracasei and L. fermentum (28). Some research data confirmed that IL-10 had a protective role in epithelial cells (29). In addition, IL-10 plays an effective role in inhibiting inflammation and pathogen clearance during Borrelia recurrentis infection (30), and L. lactis producing IL-10 could be a therapy of murine colitis (31). Similar effects were also found in our research that probiotic administration significantly enhanced IL-4 and IL-10 content in the FBL-3a and ZLP-4b groups. It suggested that FBL-3a and ZLP-4b have anti-inflammatory potential to some extent.
IL-6, TNF-α, and IL-17A are important pro-inflammatory mediators that play a crucial role in the processes of inflammation (32). IL-6 and TNF-α gene expression was upregulated in most intestinal inflammations such as IBD, colitis, and necrotizing enterocolitis (33–35). In addition, IL-6 exerts an anti-inflammatory role in a pancreatitis model by regulating the generation of cytokines, expression of adhesion agents, and activation of neutrophils (36). Two B. subtilis strains (BS1 and BS2) could decrease the content of TNF-α and IL-6 in a mice model (13). IL-23 promoted the pro-inflammatory cytokines IL-6 and IL-17 in autoimmune inflammatory disease models (37). IL-17A played a pivotal part in the development of dextran sodium sulfate-induced colitis by regulating the balance of Th17 and Treg cells (38). A recent research revealed that L. helveticus and L. rhamnosus suppressed interleukin 17 transcription in Citrobacter rodentium-induced colitis in mice (39). In this study, we found that B. velezensis JT3-1 could diminish the content of TNF-α, IL-6, and IL-17A, while L. plantarum FBL-3a and L. salivarius ZLP-4b merely restrained the IL-17A level. Such results are in accordance with the previous studies, implying that probiotics obtained from livestock in Northwest China exerted a protective and salutary function in mice via modulating cytokine secretion.
Gut microbes play a substantial role in the maintenance of intestinal barrier function and modulation of the immune pathway (21), and the diversity of the gut microbiota is significant to the health of the hosts. We found that the species of bacteria in the gut decreased to a certain extent after probiotic administration, but some positive changes also emerged. The increase of Firmicutes/Bacteroidetes ratio contributes to nutrient intake and energy transformation (40). Proteobacteria include a lot of pathogenic germs such as Salmonella, E. coli, Vibrio cholera, and Helicobacter bacteria which can cause many infectious diseases (41). In Spirochaetes, many pathogens were identified as causes of many illnesses—for instance, Leptospira (leptospirosis), Borrelia burgdorferi (Lyme disease), Treponema pallidum (syphilis), Treponema carateum (pinta), Borrelia recurrentis (relapsing fever), and Treponema pertenue (yaws) (42–44). In this study, we found that the relative abundance of these two bacteria (Proteobacteria and Spirochaetes) was reduced by isolated probiotic pretreatment. Bilophila can activate Th1 cells to promote the production of IFN-γ, but it causes appendicitis as opportunistic pathogens (45). In our study, the abundance of Bilophila is reduced after probiotic supplementation. Lactobacillus is negatively correlated with TNF-α; maybe it is why the FBL-3a group with few Lactobacillus appeared to have higher levels of TNF-α than the other groups (46).
In our laboratory, before the animal experiment, many in vitro tests were conducted to assess the antibacterial activity and safety of JT3-1, FBL-3a, and ZLP-4b, including bile salt acid and tolerance tests, antibacterial tests, hemolytic activity tests, and antibiotic susceptibility assay (47–49). Our results demonstrated that Bacillus and Lactobacillus isolated from livestock in Northwest China can confer benefits to mice by improving the intestinal mucosa integrity and modulating the cytokines linked to inflammation and immunity. Moreover, probiotic administration exerted positive effects on the gut microbiome of mice. This work supported the potential for B. velezensis JT3-1 and L. salivarius ZLP-4b to be functional probiotics based on their capacity of beneficial modulation of the intestinal morphology and immune-related cytokines. Further research should be performed before these strains are served in clinical practice.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, CP032506; https://www.ncbi.nlm.nih.gov/genbank/, CP034694; https://www.ncbi.nlm.nih.gov/genbank/, CP062071.
Ethics Statement
The animal study was reviewed and approved by Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
YiL and YoL designed this study and critically revised the manuscript. DJ, JiaW, HL, and XY performed the experiments and data analysis. JLi, JinW, GG, JLu, SX, and HY participated in the coordination and manuscript revision. All authors read and approved the final manuscript.
Funding
The authors would like to acknowledge the National Key Research and Development Program of China (2018YFD0501804, 2018YFD0502305, and 2017YFD0501200), the Agriculture Research System of MOF and MARA (CARS-37), and the Jiangsu Co-innovation Center program for the Prevention and Control of Important Animal Infectious Diseases and Zoonosis for the funding support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Davies J. Microbes have the last word. A drastic re-evaluation of antimicrobial treatment is needed to overcome the threat of antibiotic-resistant bacteria. EMBO Rep. (2007) 8:616–21. doi: 10.1038/sj.embor.7401022
2. Davison HC, Low JC, Woolhouse ME. What is antibiotic resistance and how can we measure it? Trends Microbiol. (2000) 8:554–9. doi: 10.1016/S0966-842X(00)01873-4
3. Obaidat MM, Stringer AP. Prevalence, molecular characterization, and antimicrobial resistance profiles of Listeria monocytogenes, Salmonella enterica, and Escherichia coli O157:H7 on dairy cattle farms in Jordan. J Dairy Sci. (2019) 102:8710–20. doi: 10.3168/jds.2019-16461
4. Schulfer AF, Battaglia T, Alvarez Y, Bijnens L, Ruiz VE, Ho M, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol. (2018) 3:234–42. doi: 10.1038/s41564-017-0075-5
5. Gungor OE, Kirzioglu Z, Kivanc M. Probiotics: can they be used to improve oral health? Benef Microbes. (2015) 6:647–56. doi: 10.3920/BM2014.0167
6. Sanders ME, Akkermans LM, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. (2010) 1:164–85. doi: 10.4161/gmic.1.3.12127
7. Holzapfel WH, Haberer P, Geisen R, Björkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. (2001) 73:365S−73S. doi: 10.1093/ajcn/73.2.365s
8. Almeida D, Machado D, Andrade JC, Mendo S, Gomes AM, Freitas AC. Evolving trends in next-generation probiotics: a 5W1H perspective. Crit Rev Food Sci Nutr. (2020) 60:1783–96. doi: 10.1080/10408398.2019.1599812
9. Barra M, Danino T, Garrido D. Engineered probiotics for detection and treatment of inflammatory intestinal diseases. Front Bioeng Biotechnol. (2020) 8:265. doi: 10.3389/fbioe.2020.00265
10. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. (2019) 10:S49–66. doi: 10.1093/advances/nmy063
11. Wang WC, Yan FF, Hu JY, Amen OA, Cheng HW. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci. (2018) 96:1654–66. doi: 10.1093/jas/sky092
12. Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci USA. (2020) 117:27509–15. doi: 10.1073/pnas.1921223117
13. Li A, Wang Y, Li Z, Qamar H, Mehmood K, Zhang L, et al. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Fact. (2019) 18:112. doi: 10.1186/s12934-019-1161-6
14. Peng M, Tabashsum Z, Patel P, Bernhardt C, Biswas C, Meng J, et al. Prevention of enteric bacterial infections and modulation of gut microbiota with conjugated linoleic acids producing Lactobacillus in mice. Gut Microbes. (2020) 11:433–52. doi: 10.1080/19490976.2019.1638724
15. Zhang C, Lu Y, Feng Q, Wang X, Lou H, Liu J, et al. Differentiated demographic histories and local adaptations between Sherpas and Tibetans. Genome Biol. (2017) 18:115. doi: 10.1186/s13059-017-1242-y
16. Li L, Zhao X. Comparative analyses of fecal microbiota in Tibetan and Chinese Han living at low or high altitude by barcoded 454 pyrosequencing. Sci Rep. (2015) 5:14682. doi: 10.1038/srep14682
17. Fan Q, Wanapat M, Yan T, Hou F. Altitude influences microbial diversity and herbage fermentation in the rumen of yaks. BMC Microbiol. (2020) 20:370. doi: 10.1186/s12866-020-02054-5
18. Van Giau V, An SSA, Hulme J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des Devel Ther. (2019) 13:327–43. doi: 10.2147/DDDT.S190577
19. Liu L, Zeng D, Yang M, Wen B, Lai J, Zhou Y, et al. Probiotic Clostridium butyricum improves the growth performance, immune function, and gut microbiota of weaning rex rabbits. Probiotics Antimicrob Proteins. (2019) 11:1278–92. doi: 10.1007/s12602-018-9476-x
20. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. (2003) 361:512–9. doi: 10.1016/S0140-6736(03)12489-0
21. Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. (2018) 10. doi: 10.3390/nu10080988
22. Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, et al. Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. (2013) 14:26–37. doi: 10.1016/j.chom.2013.06.007
23. Weiss G. Intestinal irony: how probiotic bacteria outcompete bad bugs. Cell Host Microbe. (2013) 14:3–4. doi: 10.1016/j.chom.2013.07.003
24. Zhao Y, Zhao H, Sun Y, Hao J, Qi X, Zhou X, et al. IL-4 induces a suppressive IL-10-producing CD8+ T cell population via a Cdkn2a-dependent mechanism. J Leukoc Biol. (2013) 94:1103–12. doi: 10.1189/jlb.0213064
25. Makita N, Hizukuri Y, Yamashiro K, Murakawa M, Hayashi Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int Immunol. (2015) 27:131–41. doi: 10.1093/intimm/dxu090
26. Oh SJ, Chung DH. Invariant NKT cells producing IL-4 or IL-10, but not IFN-gamma, inhibit the Th1 response in experimental autoimmune encephalomyelitis, whereas none of these cells inhibits the Th17 response. J Immunol. (2011) 186:6815–21. doi: 10.4049/jimmunol.1003916
27. Hewitson JP, Filbey KJ, Esser-Von Bieren J, Camberis M, Schwartz C, Murray J, et al. Concerted activity of IgG1 antibodies and IL-4/IL-25-dependent effector cells trap helminth larvae in the tissues following vaccination with defined secreted antigens, providing sterile immunity to challenge infection. PLoS Pathog. (2015) 11:e1004676. doi: 10.1371/journal.ppat.1004676
28. Wang IJ, Wang JY. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin Exp Allergy. (2015) 45:779–87. doi: 10.1111/cea.12489
29. Werner T, Shkoda A, Haller D. Intestinal epithelial cell proteome in IL-10 deficient mice and IL-10 receptor reconstituted epithelial cells: impact on chronic inflammation. J Proteome Res. (2007) 6:3691–704. doi: 10.1021/pr070222x
30. Cadavid D, Londoño D. Understanding tropism and immunopathological mechanisms of relapsing fever spirochaetes. Clin Microbiol Infect. (2009) 15:415–21. doi: 10.1111/j.1469-0691.2009.02785.x
31. Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. (2000) 289:1352–5. doi: 10.1126/science.289.5483.1352
32. De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. (2015) 34:3493–503. doi: 10.1038/onc.2014.286
33. Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. (2020) 10:5225–41. doi: 10.7150/thno.43716
34. Gupta RA, Motiwala MN, Mahajan UN, Sabre SG. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J Ethnopharmacol. (2018) 219:222–32. doi: 10.1016/j.jep.2018.02.043
35. Yang G, Jin T, Yin S, Guo D, Zhang C, Xia X, et al. Trans-Cinnamaldehyde mitigated intestinal inflammation induced by Cronobacter sakazakii in newborn mice. Food Funct. (2019) 10:2986–96. doi: 10.1039/C9FO00410F
36. Vonlaufen A, Aurrand-Lions M, Pastor CM, Lamagna C, Hadengue A, Imhof BA, et al. The role of junctional adhesion molecule C (JAM-C) in acute pancreatitis. J Pathol. (2006) 209:540–8. doi: 10.1002/path.2007
37. Yen D, Cheung J, Scheerens H, Poulet F, Mcclanahan T, Mckenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. (2006) 116:1310–6. doi: 10.1172/JCI21404
38. Xu M, Duan XY, Chen QY, Fan H, Hong ZC, Deng SJ, et al. Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. Biomed Pharmacother. (2019) 109:2396–408. doi: 10.1016/j.biopha.2018.11.087
39. Rodrigues DM, Sousa AJ, Johnson-Henry KC, Sherman PM, Gareau MG. Probiotics are effective for the prevention and treatment of Citrobacter rodentium-induced colitis in mice. J Infect Dis. (2012) 206:99–109. doi: 10.1093/infdis/jis177
40. Grigor'eva IN. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. J Pers Med. (2020) 11:13. doi: 10.3390/jpm11010013
41. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. (2015) 33:496–503. doi: 10.1016/j.tibtech.2015.06.011
42. Vedantam G, Viswanathan VK. Spirochaetes and their twisted ways. Gut Microbes. (2012) 3:399–400. doi: 10.4161/gmic.22051
43. Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. (2016) 2:16090. doi: 10.1038/nrdp.2016.90
44. Hu W, Lin X, Yan J. Leptospira and leptospirosis in China. Curr Opin Infect Dis. (2014) 27:432–6. doi: 10.1097/QCO.0000000000000097
45. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. (2012) 487:104–8. doi: 10.1038/nature11225
46. Lammers A, Wieland WH, Kruijt L, Jansma A, Straetemans T, Schots A, et al. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev Comp Immunol. (2010) 34:1254–62. doi: 10.1016/j.dci.2010.07.001
47. Li Y, Li X, Jia D, Liu J, Wang J, Liu A, et al. Complete genome sequence and antimicrobial activity of Bacillus velezensis JT3-1, a microbial germicide isolated from yak feces. 3 Biotech. (2020) 10:231. doi: 10.1007/s13205-020-02235-z
Keywords: Bacillus velezensis, Lactobacillus plantarum, Lactobacillus salivarius, immunity, intestinal flora
Citation: Li Y, Jia D, Wang J, Li H, Yin X, Liu J, Wang J, Guan G, Luo J, Yin H, Xiao S and Li Y (2021) Probiotics Isolated From Animals in Northwest China Improve the Intestinal Performance of Mice. Front. Vet. Sci. 8:750895. doi: 10.3389/fvets.2021.750895
Received: 31 July 2021; Accepted: 24 August 2021;
Published: 27 September 2021.
Edited by:
Guillermo Tellez, University of Arkansas, United StatesReviewed by:
Jesús Adonai Maguey González, National Autonomous University of Mexico, MexicoYordan Martinez Aguilar, Zamorano, Honduras
Copyright © 2021 Li, Jia, Wang, Li, Yin, Liu, Wang, Guan, Luo, Yin, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youquan Li, eW91cXVhbi1saUAxNjMuY29t
 Yingying Li1,2
Yingying Li1,2 Junlong Liu
Junlong Liu Jinming Wang
Jinming Wang Guiquan Guan
Guiquan Guan Youquan Li
Youquan Li