- 1Indigenous Knowledge Systems Centre, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
- 2Food Security and Safety Niche Area, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
- 3Centre for Animal Health Studies, Faculty of Natural and Agricultural Sciences, North-West University, Mmabatho, South Africa
Cattle farming is a traditional agricultural system that contribute to the rural economic, social and cultural values of the communities. Cattle as common with other livestock, are affected by many diseases that cause mortality and economic losses. In many rural households, the use of plants and associated knowledge are popular for managing cattle diseases especially in areas experiencing challenges with conventional veterinary medicine. Evidence on the documentation of indigenous knowledge and biological evaluation of plants used against cattle diseases remain understudied and fragmented. The aim of the review is to collate and analyse the ethnoveterinary knowledge and biological evaluation of plants used against cattle diseases in South Africa. Different scientific databases were systematically explored to extract data from 37 eligible studies. A total of 310 medicinal plants from 81 families used to treat 10 categories of cattle diseases across seven (7) provinces in South Africa. Leguminosae (Fabaceae), Compositae (Astereceae), Asparagaceae, and Xanthorrhoeaceae were the most frequently used plant families. Common plant parts used were leaves and roots. Twenty-seven (27) combination remedies involving 2–6 plants were identified as treatment regimes against cattle diseases. Common preparation methods were infusion and decoction while the administration mode was predominantly unspecified (52%) while oral and topical contributed 26 and 22%, respectively. In terms of diseases, the most treated ones were general systems infection, reproduction disorders and gastrointestinal problems. Currently, an estimated 21% of the 310 plants have been evaluated for diverse biological activities using relevant bioassays related to cattle diseases. Antibacterial activity remained the most studied biological activity. Evidence from the review revealed the significance of ethnoveterinary medicine against cattle diseases especially in rural areas of South Africa. Nevertheless, the use of plants for cattle diseases among other ethnic groups, particularly in the Northern Cape and Western Cape, remain under-studied.
Introduction
Cattle farming is the backbone of the rural sector and contribute to social and cultural values such as ancestral rituals, lobola (bridal) payment, cleansing and sustainable rural livelihoods (1–3). Particularly, cattle are part of livestock farming and a catalyst to enhance household food security and alleviating poverty in small-scale cooperative farming areas. In South Africa, there are about 14 million cattle, which make up 1.6 million dairy cattle (604,781 cows in milk) and 12.5 million beef cattle. Furthermore, ~53 and 47% are in commercial and subsistence systems, respectively (4). However, cattle are often affected by many diseases that cause mortality and economic losses (5–7). Preventing and managing cattle diseases remain a major concern in South Africa as well as in other African countries (8). Therefore, ethnoveterinary medicine (EVM) has become a program that is used to protect and manage animal health and diseases (9–12).
Rural communities often utilize EVM and associated practices to maintain health of wide range of cattle populations (12–14). In South Africa, the use of medicinal plants for treating human diseases have been extensively documented in literature (15–18). However, the neglect relating to ethnoveterinary especially the botanical recording of medicinal plants used to treat animal diseases remain a major concern (19). The need for treatment possibilities is rapidly becoming a key aspect of basic health care within various communities (10, 20). The need to record indigenous knowledge of plants to mitigate their lost due to rapid urbanization and acculturation cannot be over-emphasized (21).
Global interest in EVM practices has increased in the last decade, leading to extensive work especially in Africa (10, 11, 22–24); Asia (25–30); North and South America (31, 32); as well as Europe (33–37). Interest in EVM research is due to readily availability, ease of preparation and administration as well as affordability (13, 38–40). Increasing evidence strongly suggests that EVM has the potential to improve agricultural productivity of local communities (11, 30, 41–43). The current review provides a critical appraisal on the trends and patterns for traditional knowledge and biological evaluation of plants used against cattle diseases in South Africa. It is anticipated that the review will identify existing knowledge gaps and may serve as a reference material for future research efforts in the field of EVM.
Materials and Methods
Selection of Scientific Publications
This review was based on the ethnoveterinary studies conducted in South Africa until May 2021. The information on traditional/indigenous knowledge on plants used against cattle diseases in South Africa was extracted from published scientific journals, books, reports from national, and regional, dissertation, theses, conference papers, and reports in South African universities websites/libraries (electronic data repositories), conference proceedings, regulatory and non-governmental organizations. Literature was searched using specific search terms in international online databases such as PubMed, JSTOR, Science Direct, Scopus, and Google Scholar. In the review process, the following search terms were included (singular or plural forms when necessary) in conjunction with South Africa: ethnoveterinary medicine, indigenous knowledge, cattle health care, local cattle husbandry, traditional cattle medicine, animal health anthropology, ethnomedicinal, plant, ethnopharmacology, folk medicine, herbal remedies for cattle diseases, and ethnobotanical papers containing information on plants which was unambiguously linked to a veterinary use. Research articles were also searched by examining bibliographies.
Selection Criteria
For any article/study to be included in the review, it must include and indicate details of a specific EVM plants relative to its use for treating cattle diseases within the research period (i.e., up to May 2021). For each study, the following information was collected: Latin name of plant used, plant parts, diseases or condition treating, dosage, preparation and mode of administration, the classification of cattle diseases or conditions or therapeutic use of plants. Articles that were excluded were review articles, those solely concerned with modern medicines, or those which cattle were not subject matter. Furthermore, letters, case-reports, manuals, and guidelines, and those reporting only human studies were excluded for this review (Figure 1). The selection of articles was done in four steps. Step one, the relevance of studies was checked based on their title. In the second step, abstracts were evaluated to match to the inclusion criteria. If primary inspection of an abstract of a paper did not give adequate information to make an informed judgment, the full paper was searched in the third step and reviewed by the authors prior to deciding on their inclusion in the review. Finally, those that met the inclusion criteria were retrieved for extra appraisal (Figure 1). All scientific plant names were cross-checked with The Plant List (www.theplantlist.org), while the common names were confirmed using PlantZAfrica (www.pza.sanbi.org).
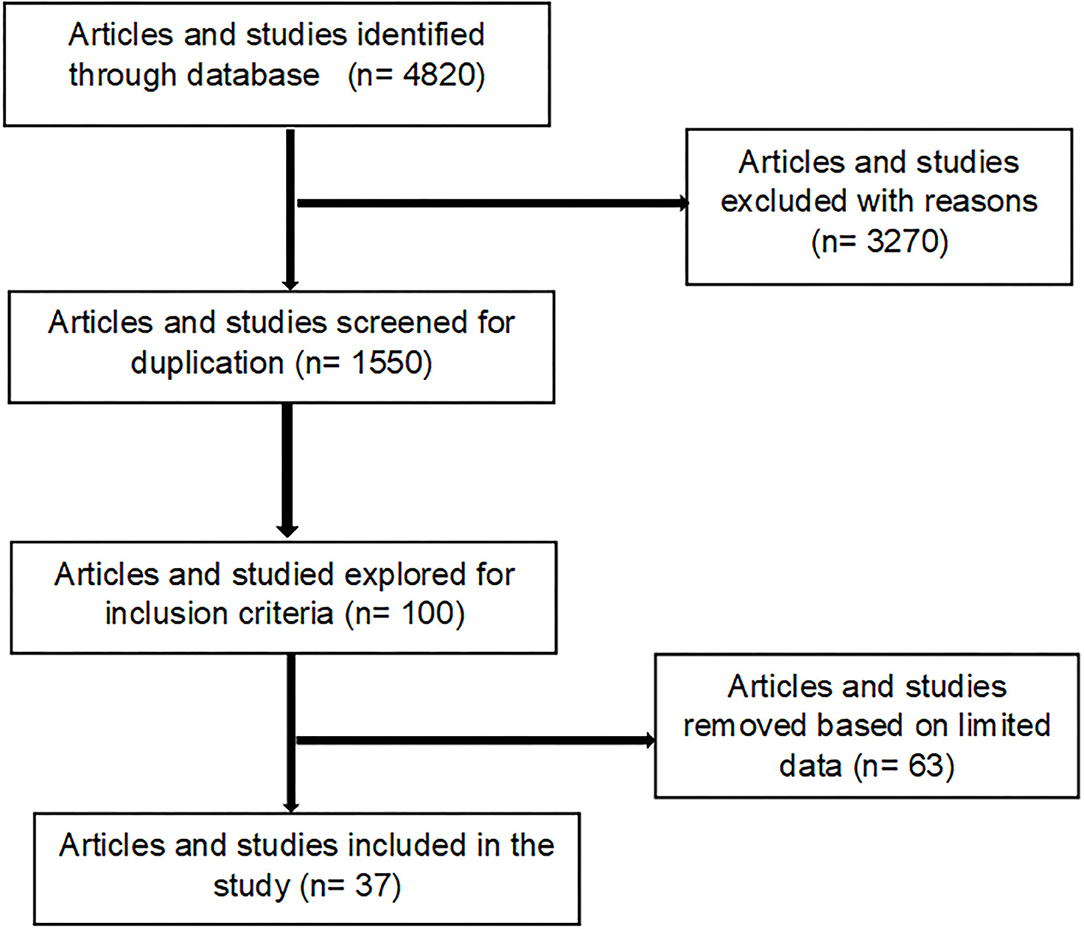
Figure 1. Process used for the selection of articles included in the generated inventory (Supplementary Table S1).
Results and Discussion
South African Ethnoveterinary Medicine Studies Based on Cattle Healthcare
In South Africa, most rural community farmers depend on conventional health practices to preserve and improve their livestock health by preventing and managing diseases (44). Cattle diseases have an influence on the economy and have an impact on cultural practices (45). Ethnoveterinary practices play a greater role in the welfare of cattle as an alternative or an integral part of traditional veterinary practices in rural communities. The use of medicinal plants and indigenous methods/practices for the treatment of diseases is not only limited to humans, but also applies to the treatment of different diseases in cattle (46). Farmers believe that indigenous practices and plants are easy to use/apply, affordable and have less side effects on their livestock. One of the earliest evidence on the use of the EVM was indicated in the work of Gerstner (47). Further studies have been undertaken toward increasing the database of therapies for animal diseases and conditions (Table 1). Studies have been undertaken throughout South Africa with a view to recording indigenous community knowledge of cattle health care, but many rural communities have limited documentation. This justifies the need for the continuation of work in the rest of the country in order to complete the documentation of the EVM used against diseases in cattle.
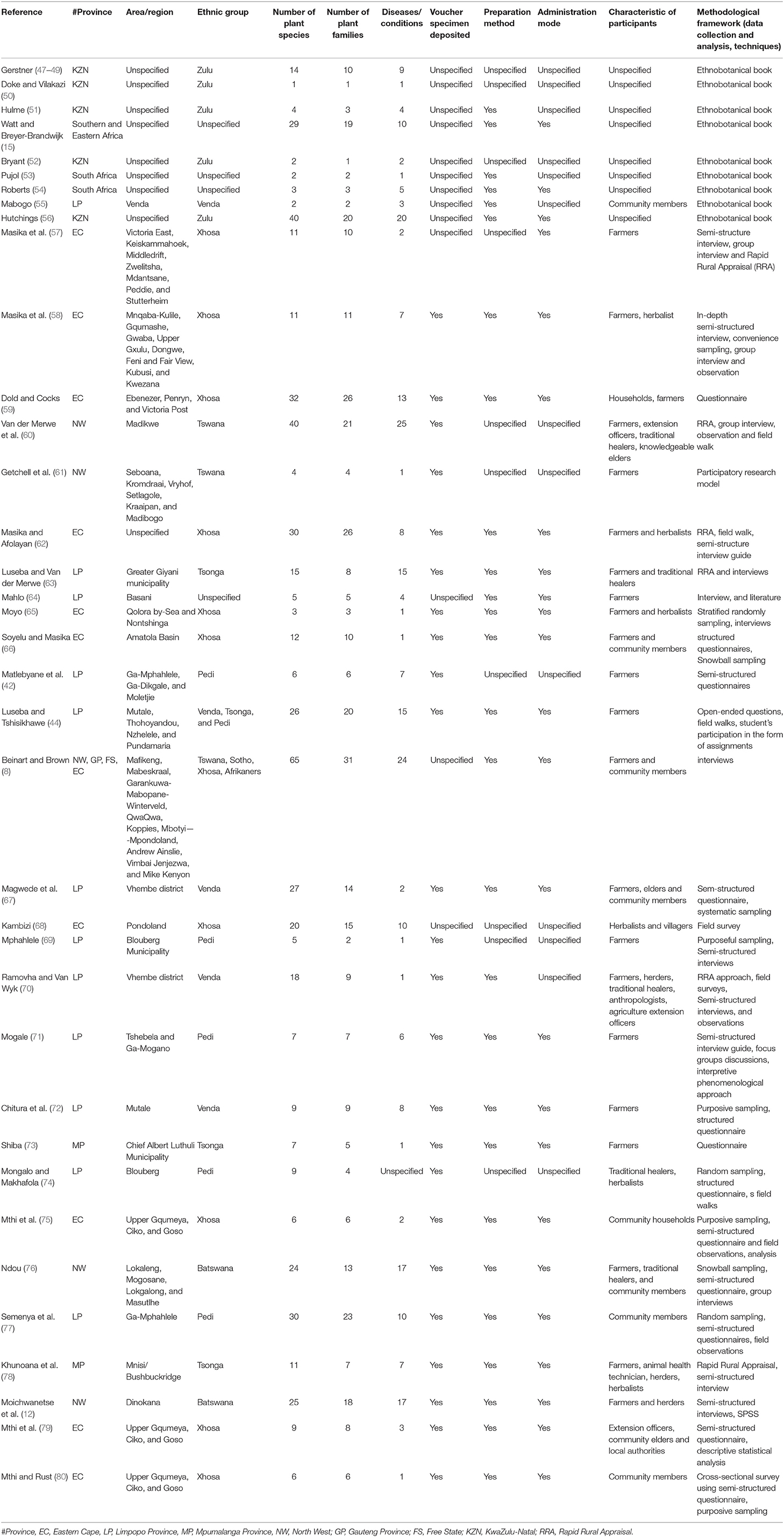
Table 1. An overview of reviewed literature on ethnoveterinary studies on plants used against cattle diseases in South Africa.
Based on inclusion and exclusion criteria, a total of 37 studies on EVM plants used against cattle disease conducted throughout South Africa were identified (Figure 1). In the last 10 years, we observed an increase in publications related to EVM plants used against cattle diseases, indicating an increasing interest in the field. In terms of the geographical distribution of the studies (Table 1), Limpopo province dominated accounting for 32% of the total number of articles. This is due to the province's rich plant diversity and its status as one of the country's hotspots (74). Other major contributions were the Eastern Cape (29.7%), North West and KwaZulu-Natal (13.5%), Mpumalanga (5.4%) while Gauteng and Free State province were the least (2.7%). The most studied ethnic groups were Xhosa (28.9%), baPedi (15.7%), Zulu, VhaVenda, and Batswana (13.2%) and Tsonga (10.5%) while the least responses were from the Basotho and Afrikaner (2.6%). A diverse range of participants involved in the studies were farmers, herbalists, traditional healers, community members (households), knowledge holder (elders), extension officers/animal health technicians, herders, and local authorities (Table 1).
There are numerous methods and approaches used for studying ethnoveterinary knowledge used to treat cattle diseases. Depending on the nature of the knowledge and the degree of certainty researchers had a variety of options. As a result, classification of such a range is critical in order to detect potential systematic patterns in the research literature. Twelve research methodologies were used to collect data, 5 sampling techniques, and 2 analysis methods used in South African EVM studies (Table 1). Semi-structured interview guides were the most commonly used data collection tool as demonstrated in 40% of the reviewed literature while Rapid Rural Appraisal was used in 16% of the articles. It is worth noting that some of the researchers used a variety of methodologies to conduct their research. The majority of studies did not demonstrate the use of approaches and theories to underpin the use of EVM in the treatment of cattle diseases (Table 1). The development of theories and approaches are necessary requirement for the proper development of any field (81). However, the process of developing theories is contentious. Some researchers believe that existing theories should be expanded upon (82) while others believe that new innovative theories should be encouraged in the spirit of plurality (83). Furthermore, none of the articles that took theoretical perspectives proposed a novel EVM theory but were all based on pre-existing theories (76).
Overview of Medicinal Plants and Families Used in Treating Cattle Diseases
An inventory of plants used against cattle diseases across seven (7) provinces of South Africa was generated (Supplementary Table 1). The plants are arranged in alphabetical order based on the botanical name (with synonyms in the brackets), as well as their families, local names (were available in Setswana/Tswana, Venda, English, Afrikaans, Tsonga, Zulu, and Xhosa), plant parts used, preparation and administration process, and diseases treated are provided. A total of 310 plant species (from 81 families) were used against different cattle diseases. The current review provides a strong indication that South Africa has rich diversity of EVM plants and associated indigenous knowledge. The most frequently mentioned plant which represents 5.5% of the inventory were Elephantorrhiza elephantina (Burch.) Skeels, Aloe ferox Mill., Dicerocaryum eriocarpum (Decne.) Abels, Senna italica Mill., Aloe marlothii A.Berger, Boophone disticha (L.f.) Herb., Solanum panduriforme E. Mey, Spirostachys africana Sond., Drimia sanguinea (Schinz) Jessop, Pappea capensis Eckl. & Zeyh, Calpurnia aurea (Aiton) Benth., Gunnera perpensa L., Carissa bispinosa (L.) Desf. ex Brenan, Clutia pulchella L., Gymnanthemum corymbosum (Thunb.) H.Rob., Volkameria glabra (E.Mey.) Mabb. & Y.W.Yuan, and Ximenia americana L. Their frequent use and higher number of mentions (3–4 times) in South Africa for diseases in cattle was established in the current review. The relatively high frequency of mentions for these plants is an indication of their effectiveness against diverse diseases in cattle.
In terms of diversity, 81 families were used as herbal medicine to treat and manage cattle diseases in South Africa (Figure 2 and Supplementary Tables 1, 2). Leguminosae/Fabaceae was the most dominant family and contributed 38 plants, followed by Compositae (24), Asparagaceae (17), Xanthorrhoeaceae (16), Lamiaceae and Solanaceae (13), Apocynaceae and Euphorbiaceae (11), Rubiaceae (10), Malvaceae (9) and Vitaceae (7). Leguminosae/Fabaceae had the highest number of plants used to treat cattle diseases which may be attributed to their higher abundance in the study area or due to high bioactivity (84). Similar studies have also been reported from other parts of world where participants mostly use the members of Leguminosae/Fabaceae for the preparation of EVM for the treatment of different livestock diseases (10, 85–87). However, the findings differ from those of other EVM studies in which the other families such as Apiaceae (88), Poaceae (30), Aloaceae (22), Asteraceae (89, 90) and Solanaceae (91) were ranked as the highest. The difference among these studies may be related to the dominant vegetation of the areas or cultural significance (30).
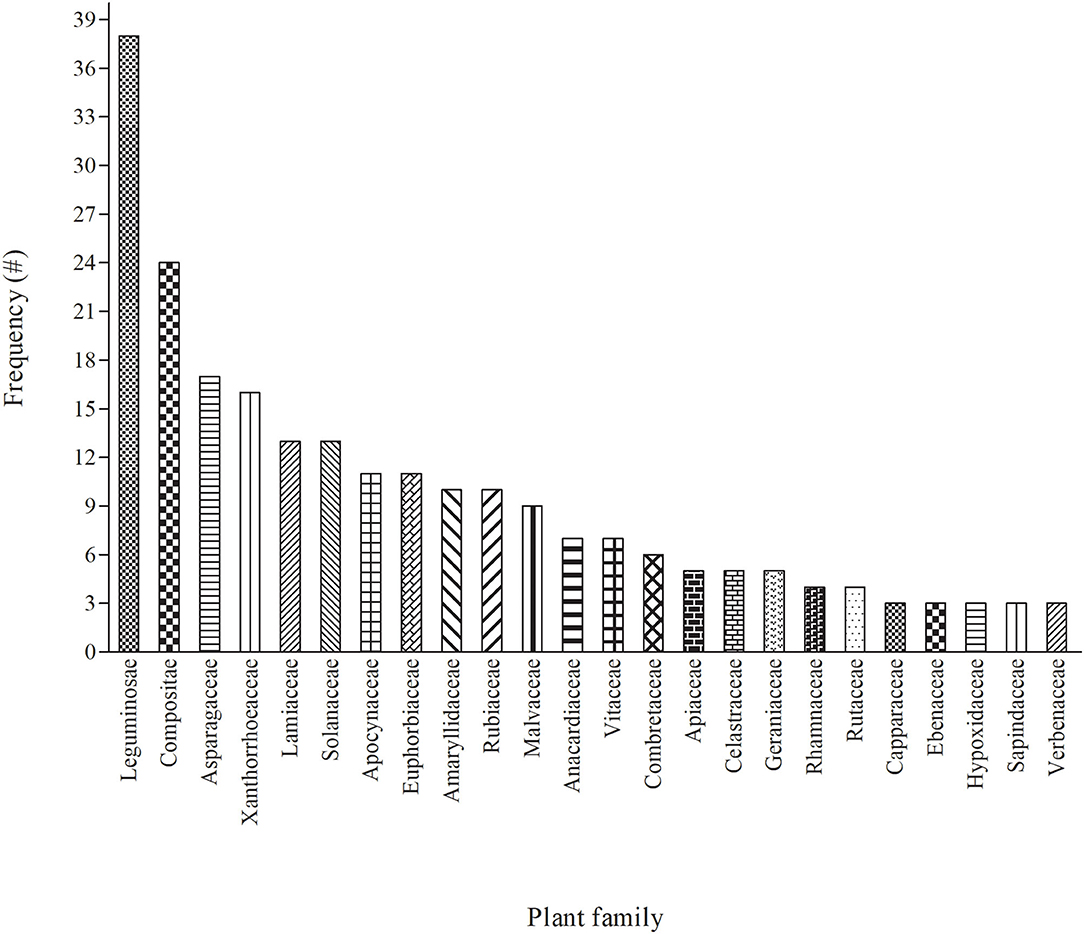
Figure 2. The 24 major (with ≥ 3 mentions) plant families used for treating cattle diseases in South Africa. Each of the remaining 57 plant families were mentioned once or twice each (see Supplementary Tables 1, 2 for details).
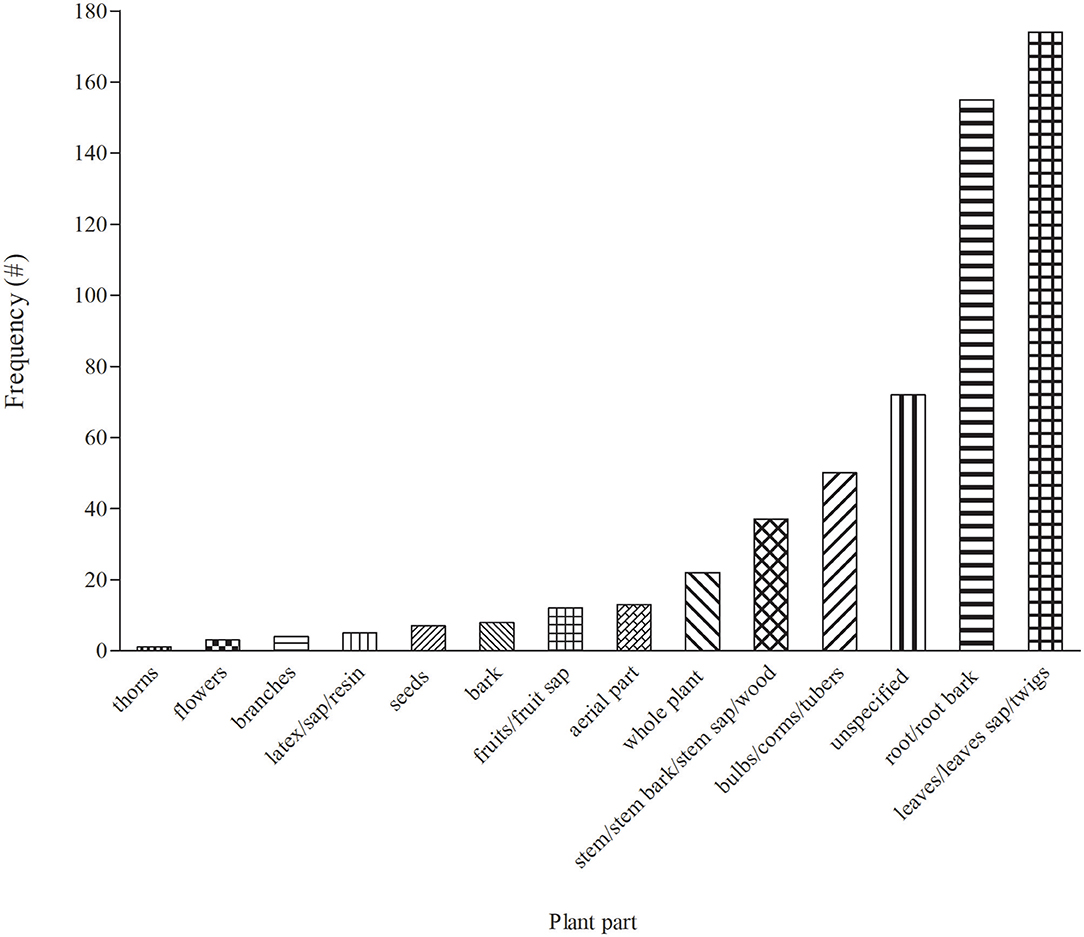
Plant Parts Used to Treat Cattle Diseases
In total, 14 plant parts/components were used for treating cattle diseases in South Africa (Figure 3). Leaves (30.7%) were the most widely used in EVM for treating cattle diseases. The popularity of leaves as one of the most preferred plant part has been a common pattern in South African EVM (46). Preference of leaves over other parts of plants remain common for various reasons including the relatively ease of access when compared to other plant parts. Furthermore, leaves are synthesizing organ for some important plant secondary metabolites that may exert medicinal properties (92–94). From a conservation perspective, individual plants are often not threatened by leaf harvesting for medicinal purpose. Roots constituted 27% and were the second most widely used plant parts, which may be due to rich pool of active compounds, especially terpenes (94). However, the selection of underground parts of the plant including the roots is not viable as it affects plant life and is considered to be highly detrimental to the survival of the whole plant if not done in a sustainable manner (95). As a result, proper harvesting strategies and conservation measures are required to ensure the long-term utilization of medicinal plant resources (95, 96).
Mono vs. Multi-Plants Application for the Treatment of Cattle Diseases
Even though monotherapy was the most common, the combination of two or more plants were evident in some instances as remedies for treating cattle diseases in South Africa (Table 2). In some instance, a combination of six (6) plants was indicated as treatment remedy for eradicating flea in cattle. Based on the findings by Moichwanetse et al. (12), these type of mixtures are often formulated with more than one plant in order to achieve synergistic or potentiating effects in cattle. Based on the study by Sarswat and Purohit (97), the use of plant mixtures is common for mitigating bovine infertility. Furthermore, the combination of various parts of a plant is commonly used to manufacture medicines for different health conditions in traditional medicine (98–100).
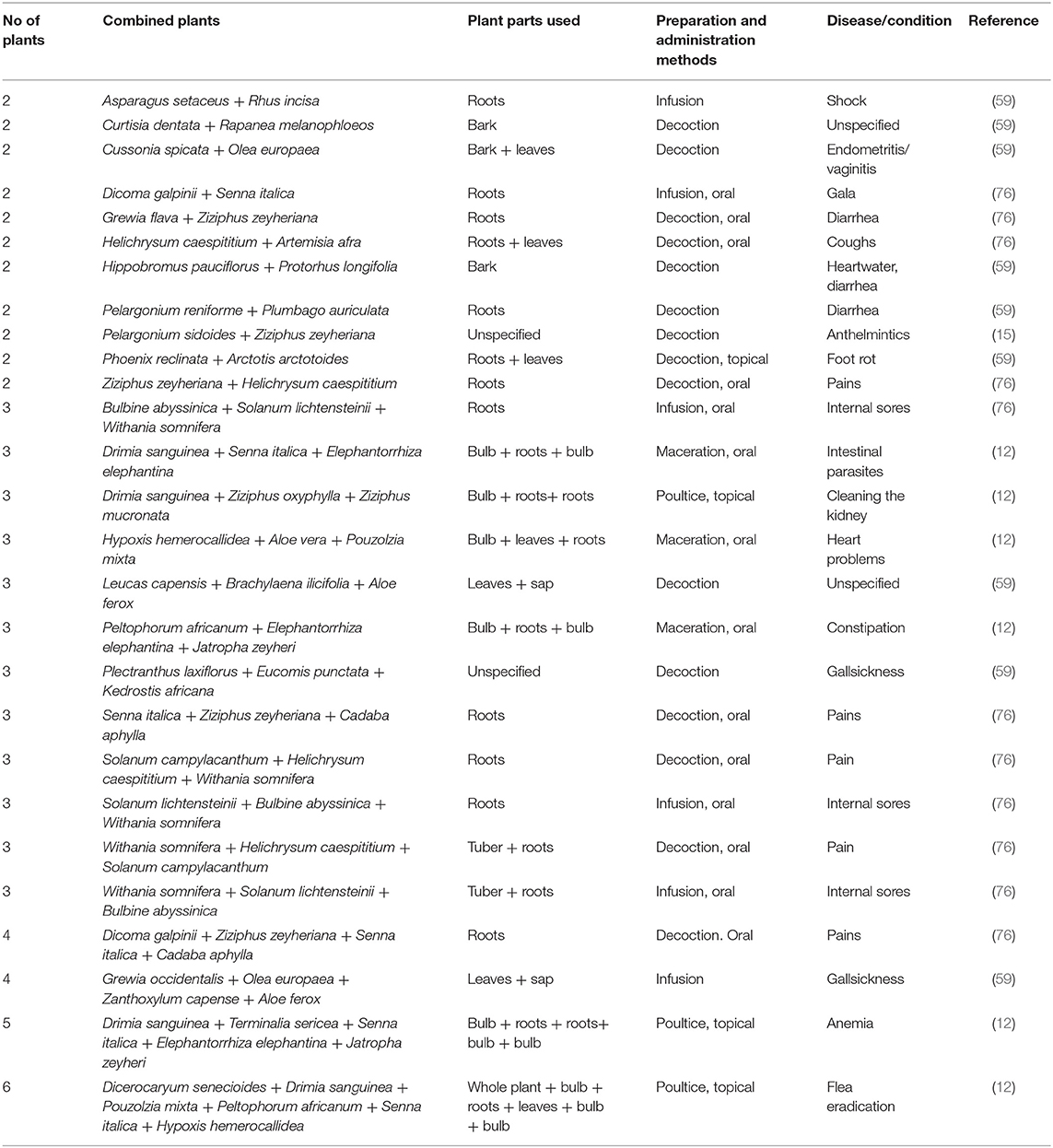
Table 2. Examples of plants used in combination therapy for treating cattle diseases in South Africa.
Method of Preparing Medicinal Plants for the Treatment of Cattle Diseases
Before administration of medicinal plants to treat cattle diseases, diverse methods of preparation are utilized, which may differ depending on the location and culture. Six (6) preparation methods (burnt, decoction, ground, infusion, maceration, and poultice) were used for treating diseases in cattle (Figure 4). Infusion (166 = 27.25%) was a popular method and it involves pouring cold/hot/warm water onto the plant material and allowing the mixture to cool. This was followed by decoction (149 = 24.46%), which involved boiling plant materials in a specific amount of water and allowing the mixture to cool before administration. However, the current observation differs from other countries whereby crushing and pounding were the most common used preparation methods for livestock diseases (101–103). Other methods of preparation such as maceration, grinding and poultice had low frequencies in the range of 4–7%. The methods of preparation differ depending on the type of disease being treated and the site of the ailment. The majority of the preparations were made using water.
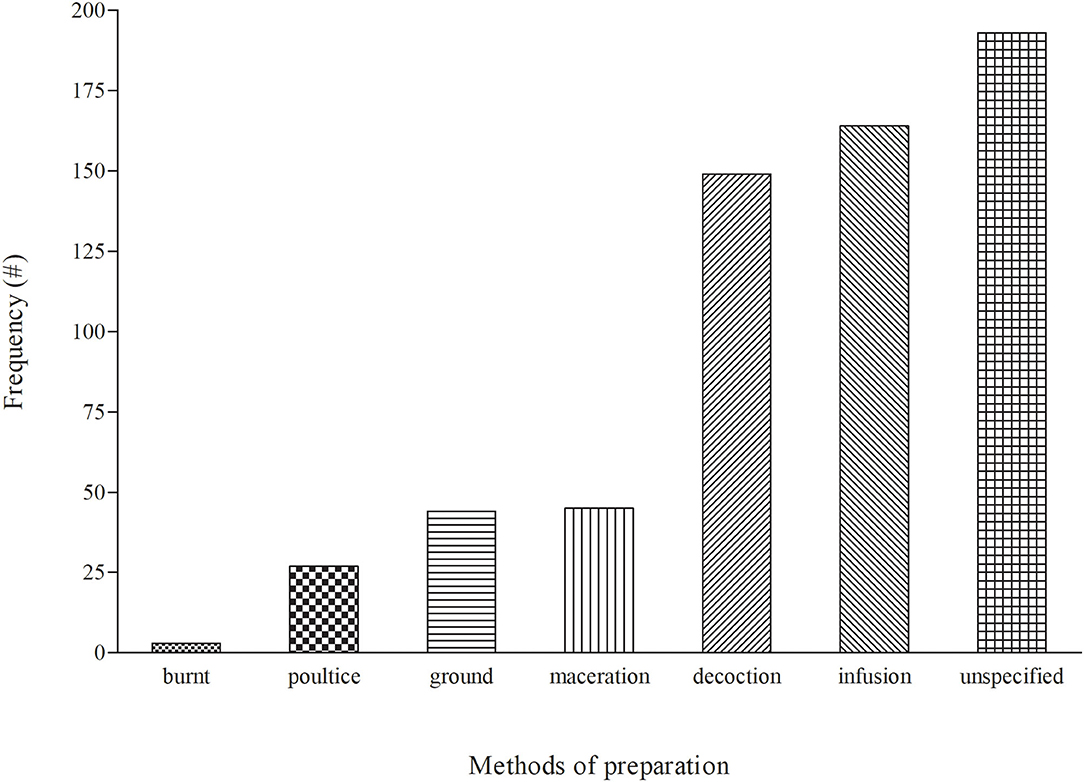
Figure 4. Distribution of the modes of preparation for medicinal plants used to treat cattle diseases in South Africa.
Mode of Administration/Application of Medicinal Plants for the Treatment of Cattle Diseases
The local communities use a variety of methods to administer EVM plants when treating diseases in cattle (Figure 5). The major route of administration for EVM plants was oral-based (157 = 26.5%). Oral administration is a simple and non-invasive form of systemic treatment. The route allows for the rapid absorption and distribution of the prepared medicines and allowing for sufficient curative power to be delivered (104). Topical which contributed 21.8% (105) was the second widely mode of application while 51.9% (308) of cases did not specify how herbal remedies should be administered. Across many African cultures, oral administration of medicinal plants is the most common route used to treat disease in cattle, as this ensures fast and direct interaction with different plant compounds at the site of action (101, 106, 107). The majority of the research documented in the current review omitted the dosage and vehicle usage. The dosage is important because it indicates how much should be used to treat the cattle and the units of measurement. However, EVM are generally known to have a significant flaw in terms of accuracy and standardization (102, 108).

Figure 5. Distribution of the mode of administration for medicinal plants used to treat cattle diseases in South Africa.
Common Diseases in Cattle Treated With Plants and Associated Indigenous Knowledge
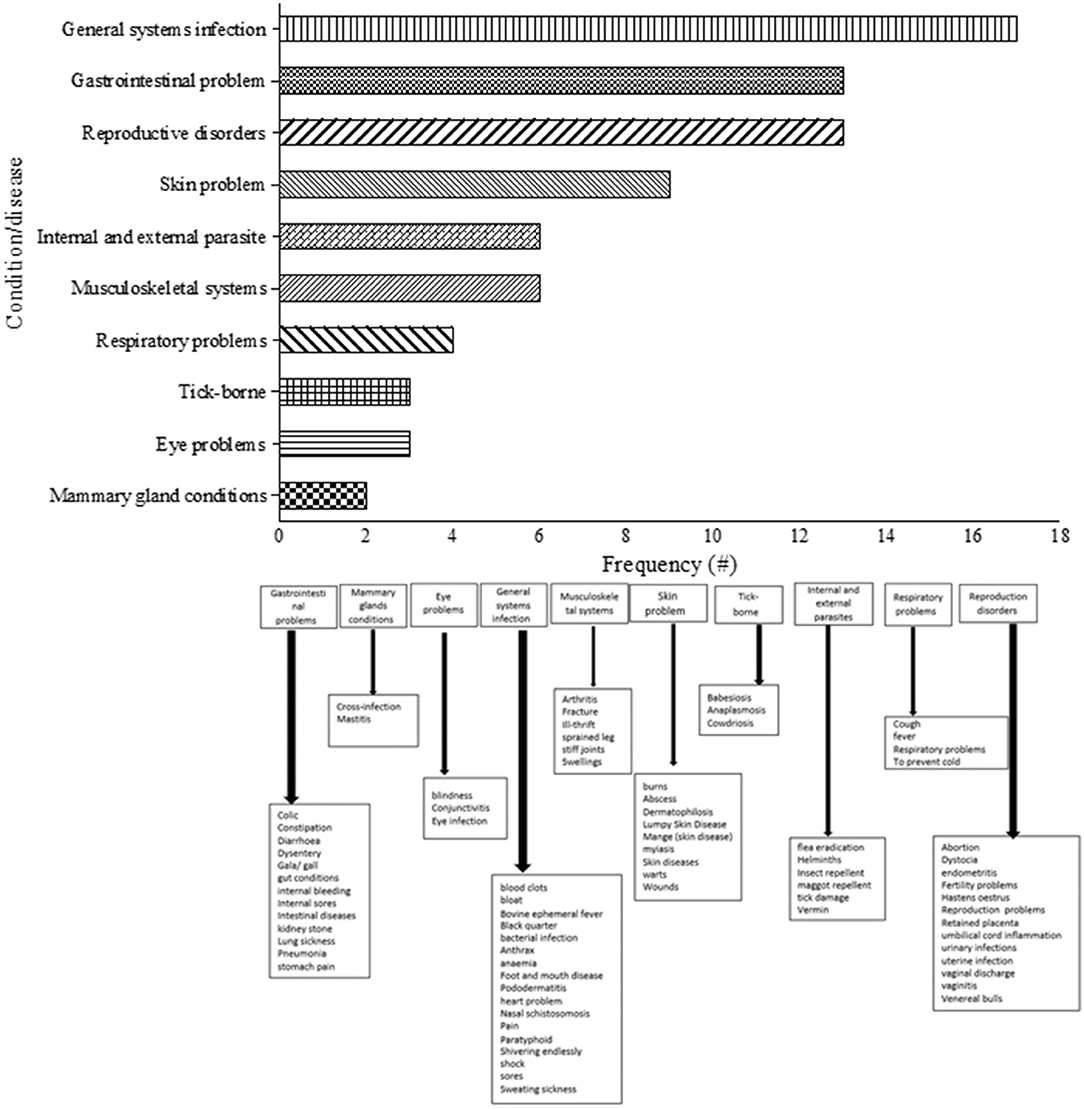
A total of 310 medicinal plants were used to treat several diseases in cattle which were categorized into 10 major groups (Figure 6). The classification of the different diseases was based on the study by Ndou (76), with slight modification. Some of the dominant categories included general systems infection, reproduction disorders, gastrointestinal problems, skin problem, internal/external parasites, musculoskeletal systems, and respiratory problems. On the other hand, treatment of conditions such as eye problems, tick-borne and mammary glands problem were relatively lower in terms of mentions in the reviewed literature. General systems infection was regarded as the most common disease category in cattle (Supplementary Table 3). The majority of these health challenges including digestive problems were easily diagnosed by participants through observation which may explain their high degree of mentions (30, 33–37). The current review identified that the 9 common conditions were anaplasmosis (treated with 69 plants), retained placenta and wounds (treated with 59 plants), diarrhea (treated with 50 plants), babesiosis (treated with 47 plants), helminths (treated with 46 plant) and constipation (treated with 25 plants). Plants such as Drimia sanguinea (Schinz) Jessop, Elephantorrhiza elephantina (Burch.) Skeels, Senna italica Mill., Boophone disticha (L.f.) Herb., Dicerocaryum eriocarpum (Decne.) Abels, Aloe ferox Mill., Cassia abbreviata Oliv., Cussonia spicata Thunb., and Cissus quadrangularis L. were recorded as the most frequently mentioned ones for treating cattle diseases (Supplementary Table 3).
Given that the incidence and severity of various cattle diseases are widespread in rural areas (109–112), the detrimental effect on meat and milk production are often enormous on small-holder livestock farmers (11, 30, 42, 46). As a result, indigenous communities extensively depend on the use of EVM and associated indigenous knowledge to understand the cause, clinical signs and transmission mode of disease occurrence (39, 113). The ability of the community members to understand the diseases is achieved through experiences. They use techniques such as observing the breathing and vocalization, urine and dung, tasting milk, behavioral change, knowledge of vectors and social interaction (76, 114).
Overview of Biological Evaluation of Plants Used to Manage Cattle Diseases
Out of the 310 plants, ~21% (66 plants) have been screened for biological activity in targeted assays relating to EVM used against cattle diseases (Table 3). Plants were tested for biological activities including antibacterial, antifungal, anti-ticks, antioxidant, antimycobacterial, anti-inflammatory and cytotoxicity. An estimated 70% of the plants (46 of the 66) were screened for antibacterial activity which make it the most studied biological activity. In addition, 51% of the plants (34 of the 66) were evaluated for anthelmintic property while 38% (25 of the 66 plants) have been tested for safety based on cytotoxicity effect. The most frequently screened plant was Aloe marlothii that have been screened in 11 bioassays. Other plants that have been subjected to multiple bioassays were Cissus quadrangularis, Dicerocaryum eriocarpum, Schkuhria pinnata, and Volkameria glabra (7 bioassays), Ricinus communis and Schotia brachypetala (6 bioassays), Aloe ferox, Apodytes dimidiata, Clausena anisata, Cussonia spicata, Elephantorrhiza elephantina, Pterocarpus angolensis, Sclerocarya birrea, Zanthoxylum capense, and Ziziphus mucronata (5 bioassays). Plants used for therapeutic purposes are normally assumed to be safe. This is mainly due to the long-term use of medicinal plants for the treatment of diseases based on basic knowledge accumulated and shared from generation to generation over many centuries (136).

Table 3. Overview of the biological evaluation of plants used to manage cattle diseases in South Africa.
Concluding Remarks and Future Perspective
Based on this extensive review, South Africa has a diverse range of plants used for mitigating diseases affecting cattle. The distribution and utilization pattern of EVM reveals a significant variation across a range of geographical settings for 7 out of the 9 provinces in South Africa. Despite the gradual socio-cultural transformation over the years, the inhabitants have retained remarkable knowledge of the plants and their uses up to present days. This suggests that the use of plants for the management of cattle diseases remain culturally rooted among South Africans. The leaves were the most commonly used plant part while the most common methods of preparation were infusions and decoctions. Even though we successfully generated an inventory of 310 medicinal plants used to treat cattle diseases, significant knowledge gaps such as the absence of diagnostic methods for the diseases, preparation methods, administration route and plant parts existed for a number of the plants. This fragmented information emphasizes the need for a well-planned and holistic approach when conducting EVM surveys. The need to adhere to good practices and guidelines particularly “The recommended standards for conducting and reporting ethnopharmacological field studies” (137) and “The need for accurate scientific nomenclature for plants” cannot be overemphasized (138). Furthermore, documenting the use of plants in EVM among South African ethnic groups should embrace indigenous research methodologies in order to gain more cultural insight from the participants. South Africa's unique heritage, both in terms of its rich plant diversity and its cultural traditions, need to be studied, and developed for the benefit of all its people and animals. Furthermore, pharmacological properties studies of EVM plants are a worthwhile endeavor that can contribute to the discovery of new entity to existing drug pools. Establishment of the mechanisms of action remain pertinent to mitigate the drug resistance issues that is increasingly encountered among disease-causing organisms. Toxicology studies must also be strongly incorporated so that potential toxic effects of plants can be identified at early stage of bio-prospecting. In addition, the study of the synergistic effects of plants used in combination would also be beneficial in the development of potent extracts or herbal mixture for resource-poor livestock farmers.
Author Contributions
The project was conceptualized by MVC with guidance from AOA and MM. MVC prepared the draft manuscript under the supervision of AOA and MM. All authors contributed to the article and approved the submitted version.
Funding
MVC received financial support from the North-West University (NWU) Grow Our Timber scholarship, NWU Faculty of Natural and Agricultural Sciences postgraduate bursary and Kopano Youth Club. AOA acknowledge the financial support from the National Research Foundation (NRF Indigenous Knowledge Systems Grant no: 118585) and the North-West University UCDG: Staff Development—Advancement of Research Profiles: Mobility Grant (NW 1EU0130) for outgoing academic visits.
Author Disclaimer
Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF (Funder) does not accept any liability in this regard.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Faculty of Natural and Agricultural Sciences, North-West University for institutional support and payment of the article processing cost. We are grateful to Ms. Banele Khoza of the North-West University's library for assistance with literature search.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.710884/full#supplementary-material
References
1. Maikhuri R, Nautiyal S, Rao K, Saxena K. Role of medicinal plants in the traditional health care system: a case study from Nanda Devi Biosphere Reserve. Curr Sci. (1998) 75:152–7. Available online at: http://www.jstor.org/stable/24100539
2. Shackleton CM, Shackleton SE, Cousins B. The role of land-based strategies in rural livelihoods: the contribution of arable production, animal husbandry and natural resource harvesting in communal areas in South Africa. Dev Southern Afr. (2001) 18:581–604. doi: 10.1080/03768350120097441
3. Dovie DB, Shackleton CM, Witkowski E. Valuation of communal area livestock benefits, rural livelihoods and related policy issues. Land Use Policy. (2006) 23:260–71. doi: 10.1016/j.landusepol.2004.08.004
4. Department of Agriculture FF. Department of Agriculture, Forestry & Fisheries of the Republic of South Africa “Animal Production”. Pretoria: Department of Agriculture, Forestry & Fisheries (2019).
5. Van Veen TS. Sense or nonsense? Traditional methods of animal parasitic disease control. Vet Parasitol. (1997) 71:177–94. doi: 10.1016/S0304-4017(97)00031-9
6. Molla W, de Jong MC, Gari G, Frankena K. Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Prev Vet Med. (2017) 147:100–7. doi: 10.1016/j.prevetmed.2017.09.003
7. Rashid M, Rashid MI, Akbar H, Ahmad L, Hassan MA, Ashraf K, et al. A systematic review on modelling approaches for economic losses studies caused by parasites and their associated diseases in cattle. Parasitology. (2019) 146:129–41. doi: 10.1017/S0031182018001282
8. Beinart W, Brown K. African Local Knowledge & Livestock Health: Diseases & Treatments in South Africa. Woodbridge, DE: Boydell & Brewer Ltd. (2013).
9. Mathias-Mundy E, McCorkle CM. Ethnoveterinary Medicine: An Annotated Bibliography. Iowa: Iowa State University Research Foundation Ames (1989).
10. Maroyi A. Use of traditional veterinary medicine in Nhema communal area of the Midlands province, Zimbabwe. Afr J Tradit Complement Alternative Med. (2012) 9:315–22. doi: 10.4314/ajtcam.v9i3.3
11. Gabalebatse M, Ngwenya BN, Teketay D, Kolawole OD. Ethno-veterinary practices amongst livestock farmers in Ngamiland District, Botswana. Afr J Tradit Complement Alternative Med. (2013) 10:490–502. doi: 10.4314/ajtcam.v10i3.16
12. Moichwanetse BI, Ndhlovu PT, Sedupane G, Aremu AO. Ethno-veterinary plants used for the treatment of retained placenta and associated diseases in cattle among Dinokana communities, North West Province, South Africa. South Afr J Botany. (2020) 132:108–16. doi: 10.1016/j.sajb.2020.04.005
13. McCorkle CM, Mathias-Mundy E. Ethnoveterinary medicine in Africa. S Afr J Psychol. (1992) 62:59–93. doi: 10.2307/1160064
14. Palni L, Maikhuri R, Rao K. Conservation of the Himalayan Agroecosystems: Issues and Priorities. Eco-Regional Cooperation for Biodiversity Conservation in the Himalaya. New York, NY: UNDP (1998).
15. Watt JM, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. London: Livingstone (1962).
17. Van Wyk B-E, Heerden FV, Oudtshoorn BV. Poisonous Plants of South Africa. Pretoria: Briza Publications (2002).
18. Van Wyk B-E. The potential of South African plants in the development of new medicinal products. South Afr J Botany. (2011) 77:812–29. doi: 10.1016/j.sajb.2011.08.011
19. Viljoen A, Sandasi M, Vermaak I. The role of the South African Journal of Botany as a vehicle to promote medicinal plant research-A bibliometric appraisal. South Afr J Botany. (2019) 122:3–10. doi: 10.1016/j.sajb.2018.07.024
20. Njoroge GN, Bussmann RW. Ethnotherapeautic management of skin diseases among the Kikuyus of Central Kenya. J Ethnopharmacol. (2007) 111:303–7. doi: 10.1016/j.jep.2006.11.025
21. Van Wyk B-E. A review of ethnobotanical research in southern Africa. South Afr J Botany. (2002) 68:1–13. doi: 10.1016/S0254-6299(16)30447-1
22. Chinsembu K, Negumbo J, Likando M, Mbangu A. An ethnobotanical study of medicinal plants used to treat livestock diseases in Onayena and Katima Mulilo, Namibia. South Afr J Botany. (2014) 94:101–7. doi: 10.1016/j.sajb.2014.06.007
23. Nyahangare ET, Mvumi BM, Mutibvu T. Ethnoveterinary plants and practices used for ecto-parasite control in semi-arid smallholder farming areas of Zimbabwe. J Ethnobiol Ethnomed. (2015) 11:30. doi: 10.1186/s13002-015-0006-6
24. Dzoyem JP, Tchuenteu RT, Mbarawa K, Keza A, Roland A, Njouendou AJ, et al. Ethnoveterinary Medicine and Medicinal Plants Used in the Treatment of Livestock Diseases in Cameroon. In: McGaw LJ, Abdalla MA editors. Ethnoveterinary Medicine: Present and Future Concepts. Cham: Springer (2020). doi: 10.1007/978-3-030-32270-0_9
25. Shen S, Qian J, Ren J. Ethnoveterinary plant remedies used by Nu people in NW Yunnan of China. J Ethnobiol Ethnomed. (2010) 6:24. doi: 10.1186/1746-4269-6-24
26. Shang X, Tao C, Miao X, Wang D, Wang Y, Yang Y, et al. Ethno-veterinary survey of medicinal plants in Ruoergai region, Sichuan province, China. J Ethnopharmacol. (2012) 142:390–400. doi: 10.1016/j.jep.2012.05.006
27. Venugopalan V, Kumaravelayutham P, Krishnamurthy Y. Ethnoveterinary uses of medicinal plants among the Lambani community in Chitradurga district, Karnataka, India. Asian Pac J Trop Biomed. (2012) 2:S470–6. doi: 10.1016/S2221-1691(12)60256-1
28. Souto WMS, Pinto LC, Mendonça LET, Mourão JS, Vieira WLS, Montenegro PFGP, et al. Medicinal Animals in Ethnoveterinary Practices: A World Overview. In: Alves RRN, Rosa IL, editors. Animals in Traditional Folk Medicine: Implications for Conservation. Berlin; Heidelberg: Springer-Verlag (2013). doi: 10.1007/978-3-642-29026-8_4
29. Suroowan S, Javeed F, Ahmad M, Zafar M, Noor MJ, Kayani S, et al. Ethnoveterinary health management practices using medicinal plants in South Asia-a review. Vet Res Commun. (2017) 41:147–68. doi: 10.1007/s11259-017-9683-z
30. Aziz MA, Khan AH, Pieroni A. Ethnoveterinary plants of Pakistan: a review. J Ethnobiol Ethnomed. (2020) 16:25. doi: 10.1186/s13002-020-00369-1
31. Lans C, Turner N, Brauer G, Grant Lourenco G, Georges K. Ethnoveterinary medicines used for horses in Trinidad and in British Columbia, Canada. J Ethnobiol Ethnomed. (2006) 2:31. doi: 10.1186/1746-4269-2-31
32. Ritter RA, Monteiro MVB, Monteiro FOB, Rodrigues ST, Soares ML, Silva JCR, et al. Ethnoveterinary knowledge and practices at Colares island, Pará state, eastern Amazon, Brazil. J Ethnopharmacol. (2012) 144:346–52. doi: 10.1016/j.jep.2012.09.018
33. Mayer M, Vogl CR, Amorena M, Hamburger M, Walkenhorst M. Treatment of organic livestock with medicinal plants: a systematic review of European ethnoveterinary research. Complement Med Res. (2014) 21:375–86. doi: 10.1159/000370216
34. Bartha SG, Quave CL, Balogh L, Papp N. Ethnoveterinary practices of Covasna County, Transylvania, Romania. J Ethnobiol Ethnomed. (2015) 11. doi: 10.1186/s13002-015-0020-8
35. Mayer M, Zbinden M, Vogl CR, Ivemeyer S, Meier B, Amorena M, et al. Swiss ethnoveterinary knowledge on medicinal plants-a within-country comparison of Italian speaking regions with north-western German speaking regions. J Ethnobiol Ethnomed. (2017) 13:1. doi: 10.1186/s13002-016-0106-y
36. Stucki K, Dal Cero M, Vogl CR, Ivemeyer S, Meier B, Maeschli A, et al. Ethnoveterinary contemporary knowledge of farmers in pre-alpine and alpine regions of the Swiss cantons of Bern and Lucerne compared to ancient and recent literature-is there a tradition? J Ethnopharmacol. (2019) 234:225–44. doi: 10.1016/j.jep.2018.12.022
37. Mertenat D, Dal Cero M, Vogl CR, Ivemeyer S, Meier B, Maeschli A, et al. Ethnoveterinary knowledge of farmers in bilingual regions of Switzerland-is there potential to extend veterinary options to reduce antimicrobial use? J Ethnopharmacol. (2020) 246:112184. doi: 10.1016/j.jep.2019.112184
38. Adekunle O, Oladele O, Olukaiyeja T. Indigenous control methods for pests and diseases of cattle in Northern Nigeria. Livestock Res Rural Dev. (2002) 14. Available online at: http://www.lrrd.org/lrrd14/2/adek142.htm
39. Atawodi S, Ameh D, Ibrahim S, Andrew J, Nzelibe H, Onyike E, et al. Indigenous knowledge system for treatment of trypanosomiasis in Kaduna State of Nigeria. J Ethnopharmacol. (2002) 79:279–82. doi: 10.1016/S0378-8741(01)00351-8
40. Ahmed MJ, Murtaza G. A study of medicinal plants used as ethnoveterinary: harnessing potential phytotherapy in Bheri, district Muzaffarabad (Pakistan). J Ethnopharmacol. (2015) 159:209–14. doi: 10.1016/j.jep.2014.11.016
41. Iqbal Z, Lateef M, Jabbar A, Muhammad G, Khan MN. Anthelmintic activity of Calotropis procera (Ait) Ait F flowers in sheep. J Ethnopharmacol. (2005) 102:256–61. doi: 10.1016/j.jep.2005.06.022
42. Matlebyane M, Ng'ambi J, Aregheore E. Indigenous knowledge (IK) ranking of available browse and grass species and some shrubs used in medicinal and ethno-veterinary practices in ruminant livestock production in Limpopo province, South Africa. Livestock Res Rural Dev. (2010) 22:54. Available online at: http://www.lrrd.org/lrrd22/3/matl22054.htm
43. Okoli C, Tamboura H, Hounzangbe-Adote S. Ethnoveterinary medicine and management sustainable livestock in West Africa. In: David R, Dibongi K, editors. Ethnoveterinary Botanical Medicine: Herbal Medicine for Animal Health. Luseba: CRC Press Taylor & Francis Group (2010).
44. Luseba D, Tshisikhawe M. Medicinal plants used in the treatment of livestock diseases in Vhembe region. Limpopo province, South Africa. J Med Plants Res. (2013) 7:593–601. doi: 10.5897/JMPR12.1213
45. Comaroff JL, Comaroff J. Goodly beasts, beastly goods: cattle and commodities in a South African context. Am Ethnol. (1990) 17:195–216. doi: 10.1525/ae.1990.17.2.02a00010
46. McGaw LJ, Famuyide IM, Khunoana ET, Aremu AO. Ethnoveterinary botanical medicine in South Africa: A review of research from the last decade (2009 to 2019). J Ethnopharmacol. (2020) 257:112864. doi: 10.1007/978-3-030-32270-0
47. Gerstner J. A preliminary checklist of Zulu names of plants with short notes. Bantu Stud. (1938) 12:215–36. doi: 10.1080/02561751.1938.9676078
48. Gerstner J. A Preliminary checklist of Zulu names of plants. Part Four Bantu Stud. (1939) 13:131–50. doi: 10.1080/02561751.1939.9676095
49. Gerstner J. A preliminary check list of Zulu names of plants: with short notes. Bantu Stud. (1941) 15:277–301. doi: 10.1080/02561751.1941.9676141
50. Doke C, Vilakazi B. Zulu-English Dictionary. Johannesburg: Witwatersrand University Press (1948).
56. Hutchings A, Scott AH, Lewis G, Cunningham A. Zulu Medicinal Plants: An Inventory. Pietermaritzburg: University of Natal press (1996).
57. Masika P, Sonandi A, Van Averbeke W. Perceived causes, diagnosis and treatment of babesiosis and anaplasmosis in cattle by livestock farmers in communal areas of the central Eastern Cape Province, South Africa. J S Afr Vet Assoc. (1997) 68:40–4. doi: 10.4102/jsava.v68i2.867
58. Masika P, Van Averbeke W, Sonandi A. Use of herbal remedies by small-scale farmers to treat livestock diseases in central Eastern Cape Province, South Africa. J S Afr Vet Assoc. (2000) 71:87–91. doi: 10.4102/jsava.v71i2.685
59. Dold AP, Cocks ML. Traditional veterinary medicine in the Alice district of the Eastern Cape Province, South Africa. S Afr J Sci. (2001) 97:375–9. Available online at: https://hdl.handle.net/10520/EJC97371
60. Van der Merwe D, Swan G, Botha C. Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa. J S Afr Vet Assoc. (2001) 72:189–96. doi: 10.4102/jsava.v72i4.651
61. Getchell J, Vatta A, Motswatswe P, Krecek R, Moerane R, Pell A, et al. Raising livestock in resource-poor communities of the North West Province of South Africa-a participatory rural appraisal study. J S Afr Vet Assoc. (2002) 73:177–84. doi: 10.4102/jsava.v73i4.583
62. Masika P, Afolayan A. An ethnobotanical study of plants used for the treatment of livestock diseases in the Eastern Cape Province, South Africa. Pharm Biol. (2003) 41:16–21. doi: 10.1076/phbi.41.1.16.14694
63. Luseba D, Van der Merwe D. Ethnoveterinary medicine practices among Tsonga speaking people of South Africa. Onderstepoort J Vet Res. (2006) 73:115–22. doi: 10.4102/ojvr.v73i2.156
64. Mahlo SM. Antibacterial Activity of Selected Plants Used in Ethnoveterinary Medicine. Turfloop: University of Limpopo (Turfloop Campus) (2006).
65. Moyo B. Determination and Validation of Ethno-veterinary Practices Used as Alternatives in Controlling Cattle Ticks by Resource-Limited Farmers in the Eastern Cape Province, South Africa. Alice: University of Fort Hares (2008).
66. Soyelu O, Masika P. Traditional remedies used for the treatment of cattle wounds and myiasis in Amatola Basin, Eastern Cape Province, South Africa. Onderstepoort J Vet Res. (2009) 76:393–7. doi: 10.4102/ojvr.v76i4.23
67. Magwede K, Tshisikhawe M, Luseba D, Bhat R. Ethnobotanical survey of medicinal plants used in treatment of ticks. Phyton Int J Exp Botany. (2014) 83:155–65. doi: 10.32604/phyton.2014.83.155
68. Kambizi L. (2014). Indigenous plants for ethnoveterinary uses in the Pondoland, South Africa. In: XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): V World (1125) Brisbane, QLD. doi: 10.17660/ActaHortic.2016.1125.40
69. Mphahlele M. The in vitro anthelmintic effects of ethnoveterinary medicinal plant extracts used in Blouberg District, Limpopo Province, South Africa (Master's dissertation). Polokwane, South Africa (2016).
70. Ramovha LI, Van Wyk AE. Ethnoveterinary practices of the Vhavenḑa, South Africa, in the treatment of redwater (mali) in cattle. Indilinga Afr J Indig Knowl Syst. (2016) 15:314–27. Available online at: https://hdl.handle.net/10520/EJC-533e3c84d
71. Mogale MS. The use of indigenous knowledge in beef cattle husbandry in Tshebela Village, Limpopo Province (Master's dissertation). Polokwane, South Africa (2017).
72. Chitura T, Muvhali P, Shai K, Mushonga B, Kandiwa E. Use of medicinal plants by livestock farmers in a local municipality in Vhembe district, South Africa. Appl Ecol Environ Res. (2018) 16:6589–605. doi: 10.15666/aeer/1605_65896605
73. Shiba MR. In vitro determination of efficacy of indigenous plant extracts used for internal parasites control by small-holder livestock farmers in chief Albert Luthuli Municipality, Mpumalanga Province, South Africa (Master's dissertation). Polokwane, South Africa (2018).
74. Mongalo NI, Makhafola TJ. Ethnobotanical knowledge of the lay people of Blouberg area (Pedi tribe), Limpopo Province, South Africa. J Ethnobiol Ethnomed. (2018) 14:46. doi: 10.1186/s13002-018-0245-4
75. Mthi S, Rust J, Morgenthal T, Moyo B. An ethno-veterinary survey of medicinal plants used to treat bacterial diseases of livestock in three geographical areas of the Eastern Cape Province, South Africa. J Med Plants Res. (2018) 12:240–7. doi: 10.5897/JMPR2017.6444
76. Ndou RV. A Study of Ethnoveterinary Medicine in the North West Province (Master's dissertation). North West University, Mafikeng, South Africa (2018).
77. Semenya SS, Mokgoatšana S, Maroyi A. Exploration of plant species used by bapedi ethnic group for ethnoveterinary purposes: a case study of Ga-Mphahlele region in the Limpopo Province, South Africa. J Pharmacy Nutr Sci. (2019) 9:167–74.
78. Khunoana ET, Madikizela B, Erhabor JO, Nkadimeng SM, Arnot L, Van Wyk I, et al. A survey of plants used to treat livestock diseases in the Mnisi community, Mpumalanga, South Africa, and investigation of their antimicrobial activity. South Afr J Botany. (2019) 126:21–9. doi: 10.1016/j.sajb.2019.07.026
79. Mthi S, Rust J, Yawa M, Tyasi L. Ethnoveterinary medicinal plants application for the treatment of tick-borne diseases in cattle around the Eastern Cape Province of South Africa. J Med Plants Econ Dev. (2020) 4:7. doi: 10.4102/jomped.v4i1.100
80. Mthi S, Rust J. Application of indigenous knowledge in treating retained placenta in cattle. E-Cronicon. (2020) 5:1–5. Available online at: https://www.ecronicon.com/ecve/ECVE-05-00208.php
81. Wacker JG. A definition of theory: research guidelines for different theory-building research methods in operations management. J Operat Manage. (1998) 16:361–85. doi: 10.1016/S0272-6963(98)00019-9
82. Pfeffer J. Mortality, reproducibility, and the persistence of styles of theory. Organization Sci. (1995) 6:681–6. doi: 10.1287/orsc.6.6.681
83. Van Maanen J. Fear and loathing in organization studies. Organization Sci. (1995) 6:687–92. doi: 10.1287/orsc.6.6.687
84. Van Wyk B-E. A family-level floristic inventory and analysis of medicinal plants used in traditional African medicine. J Ethnopharmacol. (2020) 249:112351. doi: 10.1016/j.jep.2019.112351
85. Tabuti JR, Dhillion SS, Lye KA. Ethnoveterinary medicines for cattle (Bos indicus) in Bulamogi county, Uganda: plant species and mode of use. J Ethnopharmacol. (2003) 88:279–86. doi: 10.1016/S0378-8741(03)00265-4
86. Okello J, Ssegawa P. Medicinal plants used by communities of Ngai Subcounty, Apac District, Northern Uganda. Afr J Ecol. (2007) 45:76–83. doi: 10.1111/j.1365-2028.2007.00742.x
87. Tabuti JR. Herbal medicines used in the treatment of malaria in Budiope county, Uganda. J Ethnopharmacol. (2008) 116:33–42. doi: 10.1016/j.jep.2007.10.036
88. Aziz MA, Khan AH, Adnan M, Ullah H. Traditional uses of medicinal plants used by indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J Ethnobiol Ethnomed. (2018) 14:11. doi: 10.1186/s13002-018-0212-0
89. Tariq A, Mussarat S, Adnan M, AbdElsalam NM, Ullah R, Khan AL. Ethnoveterinary study of medicinal plants in a tribal society of Sulaiman range. Sci World J. (2014) 2014:127526. doi: 10.1155/2014/127526
90. Yigezu Y, Haile DB, Ayen WY. Ethnoveterinary medicines in four districts of Jimma zone, Ethiopia: cross sectional survey for plant species and mode of use. BMC Vet Res. (2014) 10:76. doi: 10.1186/1746-6148-10-76
91. ul Hassan H, Murad W, Tariq A, Ahmad A. Ethnoveterinary study of medicinal plants in Malakand Valley, district Dir (lower), Khyber Pakhtunkhwa, Pakistan. Irish Vet J. (2014) 67:6. doi: 10.1186/2046-0481-67-6
92. Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran:(Part 1): General results. J Ethnopharmacol. (2005) 102:58–68. doi: 10.1016/j.jep.2005.05.035
93. Adeyemi MH. The potential of secondary metabolites in plant material as deterents against insect pests: A review. Afr J Pure Appl Chem. (2010) 4:243–6. doi: 10.5897/AJPAC.9000168
94. da Silva JJM, Campanharo SC, Paschoal JAR. Ethnoveterinary for food-producing animals and related food safety issues: A comprehensive overview about terpenes. Comprehen Rev Food Sci Food Safety. (2021) 20:48–90. doi: 10.1111/1541-4337.12673
95. Moyo M, Aremu AO, Van Staden J. Medicinal plants: An invaluable, dwindling resource in sub-Saharan Africa. J Ethnopharmacol. (2015) 174:595–606. doi: 10.1016/j.jep.2015.04.034
96. Alebie G, Urga B, Worku A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malar J. (2017) 16:307. doi: 10.1186/s12936-017-1953-2
97. Sarswat CS, Purohit GN. Use of ethno-veterinary medicine for therapy of reproductive disorders in cattle. J Entomol Zool Stud. (2020) 8:1006–16.
98. Quinlan MB, Quinlan RJ. Modernization and medicinal plant knowledge in a Caribbean horticultural village. Med Anthropol Q. (2007) 21:169–92. doi: 10.1525/maq.2007.21.2.169
99. Nanyingi MO, Mbaria JM, Lanyasunya AL, Wagate CG, Koros KB, Kaburia HF, et al. Ethnopharmacological survey of Samburu district, Kenya. J Ethnobiol Ethnomed. (2008) 4:14. doi: 10.1186/1746-4269-4-14
100. Belayneh A, Asfaw Z, Demissew S, Bussa NF. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J Ethnobiol Ethnomed. (2012) 8:42. doi: 10.1186/1746-4269-8-42
101. Gradé JT, Tabuti JRS, Van Damme P. Ethnoveterinary knowledge in pastoral Karamoja, Uganda. J Ethnopharmacol. (2009) 122:273–93. doi: 10.1016/j.jep.2009.01.005
102. Lulekal E, Kelbessa E, Bekele T, Yineger H. An ethnobotanical study of medicinal plants in Mana Angetu District, southeastern Ethiopia. J Ethnobiol Ethnomed. (2008) 4:10. doi: 10.1186/1746-4269-4-10
103. Teklehaymanot T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J Ethnobiol Ethnomed. (2017) 13:40. doi: 10.1186/s13002-017-0166-7
104. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. (2013) 9:65. doi: 10.1186/1746-4269-9-65
105. Fouche G, Adenubi OT, Leboho T, McGaw LJ, Naidoo V, Wellington KW, et al. Acaricidal activity of the aqueous and hydroethanolic extracts of 15 South African plants against Rhipicephalus turanicus and their toxicity on human liver and kidney cells. Onderstepoort J Vet Res. (2019) 86:1–7. doi: 10.4102/ojvr.v86i1.1665
106. Bussmann RW. Ethnobotany of the Samburu of Mt. Nyiru, South Turkana, Kenya. J Ethnobiol Ethnomed. (2006) 2:35. doi: 10.1186/1746-4269-2-35
107. Yineger H, Kelbessa E, Bekele T, Lulekal E. Ethnoveterinary medicinal plants at bale mountains national park, Ethiopia. J Ethnopharmacol. (2007) 112:55–70. doi: 10.1016/j.jep.2007.02.001
108. Givens D, Owen E, Adesogan A, Axford R, Omed H. Current procedures, future requirements and the need for standardization. In: Givens DI, Owen E., Axford RFE, Omed HM, editors. Forage Evaluation in Ruminant Nutrition. Wallingford: Cabi Publishing (2000). doi: 10.1079/9780851993447.0449
109. Bahaman A, Ibrahim A, Adam H. Serological prevalence of leptospiral infection in domestic animals in West Malaysia. Epidemiol Infect. (1987) 99:379–92. doi: 10.1017/S0950268800067868
110. Tomley FM, Shirley MW. Livestock infectious diseases and zoonoses. Philos Trans R Soc Lond B Biol Sci. (2009) 364:2637–42. doi: 10.1098/rstb.2009.0133
111. Carslake D, Grant W, Green LE, Cave J, Greaves J, Keeling M, et al. Endemic cattle diseases: comparative epidemiology and governance. Philos Trans R Soc Lond B Biol Sci. (2011) 366:1975–86. doi: 10.1098/rstb.2010.0396
112. Reid R. Pastoral People, Livestock, and Wildlife. California, NY: University of California Press (2012). doi: 10.1525/9780520954076
113. Mudzengi CP. Promoting the use of ethnoveterinary practices in livestock health management in Masvingo Province, Zimbabwe. Ethnobotany Res Appl. (2014) 12:397–405. doi: 10.17348/era.12.0.397-405
114. Jacob MO, Farah KO, Ekaya WN. Indigenous knowledge: the basis of the Maasai Ethnoveterinary diagnostic skills. J Human Ecol. (2004) 16:43–8. doi: 10.1080/09709274.2004.11905714
115. Luseba D, Elgorashi E, Ntloedibe D, Van Staden J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. South Afr J Botany. (2007) 73:378–83. doi: 10.1016/j.sajb.2007.03.003
116. Naidoo V. Screening of Four Plants Commonly Used in Ethnoveterinary Medicine for Antimicrobial, Antiprotozoal and Anti-Oxidant Activity. Masters degree, University of Pretoria, Pretoria, South Africa (2006).
117. Mawela KG. The Toxicity and Repellent Properties of Plant Extracts Used in Ethnoveterinary Medicine to Control Ticks. Masters degree University of Pretoria, Pretoria, South Africa. (2008).
118. Sserunkuma P, McGaw L, Nsahlai I, Van Staden J. Selected southern African medicinal plants with low cytotoxicity and good activity against bovine mastitis pathogens. South Afr J Botany. (2017) 111:242–7. doi: 10.1016/j.sajb.2017.03.032
119. Maphosa V, Masika PJ, Bizimenyera ES, Eloff J. In vitro anthelminthic activity of crude aqueous extracts of Aloe ferox, Leonotis leonurus and Elephantorrhiza elephantina against Haemonchus contortus. Trop Anim Health Prod. (2010) 42:301–7. doi: 10.1007/s11250-009-9421-9
120. Chitura T, Shiba MR, Afful DB, Shai K, Muvhali PT, Tsotetsi-Khambule AM. In vitro anthelmintic activity of seven medicinal plants used to control livestock internal parasites in chief Albert Luthuli municipality, South Africa. Livestock Res Rural Dev. (2019) 31. Available online at: http://www.lrrd.org/lrrd31/2/teedz31014.html
121. Theron EMC. In vitro studies on the anti-tick properties of selected plant species against veterinary and economically important ticks in South Africa (Ph.D. Thesis). University of South Africa, Pretoria, South Africa (2019).
122. Adamu M, Naidoo V, Eloff JN. Some southern African plant species used to treat helminth infections in ethnoveterinary medicine have excellent antifungal activities. BMC Complement Altern Med. (2012) 12:213. doi: 10.1186/1472-6882-12-213
123. Elisha IL, Botha FS, McGaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern Med. (2017) 17:133. doi: 10.1186/s12906-017-1645-z
124. Fouche G, Leboho T, Wellington KW, Sakong BM, Adenubi OT, Pauw E, et al. Anthelmintic activity of acetone extracts from South African plants used on egg hatching of Haemonchus contortus. Onderstepoort J Vet Res. (2016) 83:a1164. doi: 10.4102/ojvr.v83i1.1164
125. Fouché G, Eloff JN, Wellington KW. Evaluation of South African plants with acaricide activity against ticks. Int J Pharmacol Pharm Sci. (2017) 11:386–90. doi: 10.5281/zenodo.1131796
126. Fouche G, Sakong BM, Adenubi OT, Dzoyem JP, Naidoo V, Leboho T, et al. Investigation of the acaricidal activity of the acetone and ethanol extracts of 12 South African plants against the adult ticks of Rhipicephalus turanicus. Onderstepoort J Vet Res. (2017) 84:1523. doi: 10.4102/ojvr.v84i1.1523
127. Wellington KW, Leboho T, Sakong BM, Adenubi OT, Eloff JN, Fouche G. Further studies on South African plants: Acaricidal activity of organic plant extracts against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet Parasitol. (2017) 234:10–2. doi: 10.1016/j.vetpar.2016.12.014
128. McGaw LJ, Van der Merwe D, Eloff J. In vitro anthelmintic, antibacterial and cytotoxic effects of extracts from plants used in South African ethnoveterinary medicine. Vet J. (2007) 173:366–72. doi: 10.1016/j.tvjl.2005.09.004
129. Masika P, Afolayan A. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. J Ethnopharmacol. (2002) 83:129–34. doi: 10.1016/S0378-8741(02)00242-8
130. Shai LJ, Bizimenyera E, Bagla V, McGaw LJ, Eloff JN. Curtisia dentata (Cornaceae) leaf extracts and isolated compounds inhibit motility of parasitic and free-living nematodes. Onderstepoort J Vet Res. (2009) 76:249–56. doi: 10.4102/ojvr.v76i2.49
131. Mphahlele M, Tsotetsi-Khambule AM, Shai LJ, Luseba D. In vitro anthelmintic activity of aqueous extracts of five medicinal plant against eggs and the infective stage of Haemonchus contortus. Livestock Res Rural Dev. (2016) 28. Available online at: http://www.lrrd.org/lrrd28/12/luse28225.html
132. Bizimenyera E, Githiori J, Eloff J, Swan G. In vitro activity of Peltophorum africanum Sond. (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubriformis. Vet Parasitol. (2006) 142:336–43. doi: 10.1016/j.vetpar.2006.06.013
133. Bagla VP, McGaw LJ, Eloff JN. The antiviral activity of six South African plants traditionally used against infections in ethnoveterinary medicine. Vet Microbiol. (2012) 155:198–206. doi: 10.1016/j.vetmic.2011.09.015
134. Magano S, Thembo K, Ndlovu S, Makhubela NFH. The anti-tick properties of the root extracts of Senna italica subsp. arachoides. Afr J Biotechnol. (2008) 7:4. Available online at: https://www.ajol.info/index.php/ajb/article/view/58457
135. Nchu F, Magano SR, Eloff JN. In vitro anti-tick properties of the essential oil of Tagetes minuta L. (Asteraceae) on Hyalomma rufipes (Acari: Ixodidae). Onderstepoort J Vet Res. (2012) 79:1–5. doi: 10.4102/ojvr.v79i1.358
136. Mensah M, Komlaga G, Forkuo AD, Firempong C, Anning AK, Dickson RA. Toxicity and safety implications of herbal medicines used in Africa. Herbal Med. (2019) 63:1992–849. doi: 10.5772/intechopen.72437
137. Weckerle CS, de Boer HJ, Puri RK, van Andel T, Bussmann RW, Leonti M. Recommended standards for conducting and reporting ethnopharmacological field studies. J Ethnopharmacol. (2018) 210:125–32. doi: 10.1016/j.jep.2017.08.018
Keywords: animal health, ethnobotany, food security, livestock, antibacterial, retained placenta
Citation: Chakale MV, Mwanza M and Aremu AO (2021) Ethnoveterinary Knowledge and Biological Evaluation of Plants Used for Mitigating Cattle Diseases: A Critical Insight Into the Trends and Patterns in South Africa. Front. Vet. Sci. 8:710884. doi: 10.3389/fvets.2021.710884
Received: 17 May 2021; Accepted: 12 July 2021;
Published: 19 August 2021.
Edited by:
Nora Mestorino, National University of La Plata, ArgentinaReviewed by:
José Antonio González, University of Salamanca, SpainHector Sumano, Universidad Nacional Autónoma de México, Mexico
Adrian Luis Lifschitz, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2021 Chakale, Mwanza and Aremu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adeyemi O. Aremu, b2xhZGFwby5hcmVtdUBud3UuYWMuemE=
 Mompati V. Chakale
Mompati V. Chakale Mulunda Mwanza2,3
Mulunda Mwanza2,3 Adeyemi O. Aremu
Adeyemi O. Aremu