- 1Shelter Medicine Advancement, Toronto Humane Society, Toronto, ON, Canada
- 2Shelter and Veterinary Services, American Society for the Prevention of Cruelty to Animals, New York, NY, United States
Canine heartworm infection, caused by the filarial parasite Dirofilaria immitis, represents a serious and expanding animal welfare concern that is expected to increase due to the effects of climate change and the COVID-19 pandemic. A body of evidence has emerged to support the use of a non-arsenical adulticide treatment protocol, using moxidectin and doxycycline to kill adult heartworms over a prolonged period. While a three-dose protocol using the arsenical drug melarsomine is currently the safest and most effective treatment for heartworm infection, this drug is not available in some countries and is inaccessible for many owners and animal shelters. Moxidectin-doxycycline (moxi-doxy) provides a viable alternative to no treatment at all, in cases where arsenical treatment is not possible. Based on current evidence, the most effective non-arsenical treatment regimen is doxycycline 10 mg/kg PO q 12 or 24 h for 28 days, combined with topical moxidectin at label dose. Moxidectin is repeated monthly until no antigen detected (NAD) status is confirmed. Sustained release injectable moxidectin, in combination with doxycycline, may provide an alternative in remote regions or in settings where significant compliance or accessibility concerns exist, but more studies are needed. In moxi-doxy protocols, doxycycline should be repeated annually until NAD. This review summarizes the safety and efficacy of moxi-doxy, addresses controversies surrounding this treatment approach, and provides detailed recommendations for treatment regimens and post-treatment testing.
Introduction
Current guidelines for the treatment of adult heartworm (Dirofilaria immitis) infections in dogs recommend 3 doses of the arsenical drug, melarsomine dihydrochloride, accompanied by doxycycline and a macrocyclic lactone (ML) (1–3). This is considered to be the safest, most rapid and most efficacious treatment protocol. It is the only protocol recommended for treatment of severe heartworm disease (HWD) unless the disease is so severe that surgical intervention is warranted or life-threatening adverse effects to melarsomine and/or its parasiticidal results are anticipated (2, 4).
Although no longer considered best practice, melarsomine is labeled for 2 doses, 24 h apart, in dogs with asymptomatic, mild or moderate HWD (2). This protocol is commonly used in animal shelters, to reduce cost and length of stay (1, 5). The 2-dose protocol (with no adjunctive treatment) killed ~90% of adult worms, compared with 99% after 3 doses (6).
Despite the medical and scientific soundness of this approach, melarsomine treatment remains unattainable for many affected dogs and their caregivers (including many animal shelters) (5, 7–10). Due to increasing recognition of this problem, expanding pharmaceutical options, and a rapidly-growing scientific evidence base, practitioners have experimented with non-arsenical treatment protocols with little professional industry guidance.
The purpose of this review is to review the current literature on the use of non-arsenical protocols for HWD management, within an accessible care context and with an emphasis on the combination of moxidectin and doxycycline (moxi-doxy); and offer an evidence-based and informed “least harm” approach for practitioners to consider when necessary.
Is Perfect the Enemy of Good When it Comes to Heartworm Treatment?
Dirofilaria immitis is a significant emerging infection globally (10). The incidence is reported to be increasing in some endemic areas and spreading to regions in which it was not previously identified (11–13). This trend is expected to continue, exacerbated by expanding ranges resulting from climate change (13) and by the economic consequences of both climate change and the COVID-19 pandemic (14–16). These forces will deepen existing societal and economic inequities, which impact the social determinants of health for animals (7) as they do for humans (17). For these reasons, there is a growing imperative to specifically address the need for accessible and affordable treatment for heartworm infection (HWI), alongside efforts to more broadly remove barriers to veterinary care – a problem that affects over 27% of pet-owning households in the United States and has been called “the most significant animal welfare crisis affecting owned pets” (7). Reasons for pet owners' inability to access veterinary care—including heartworm prevention and treatment—include cost, not knowing where to seek care, lack of transportation, and lack of equipment such as carriers and leashes (7).
Heartworm has been described as a socioeconomic disease (18). Lower household income has been associated with higher prevalence, most likely because families were unable to purchase heartworm preventives (19). In most surveys, prevalence is markedly greater in dogs with limited or no access to veterinary care than owned dogs likely to have been tested at veterinary visits (12, 20–30) (Figure 1). Owners of heartworm-positive dogs are frequently unable to afford or otherwise access melarsomine treatment (8, 9, 18, 31, 32), which is expensive and requires multiple veterinary visits. The cost of treatment was reported by shelters to be the most important challenge for treating HWI (5). Concerns about pain at the injection site, drug toxicity, and the requirement for prolonged and strict exercise restriction are additional reasons that owners may elect alternative treatment protocols, even if melarsomine treatment is available to them.
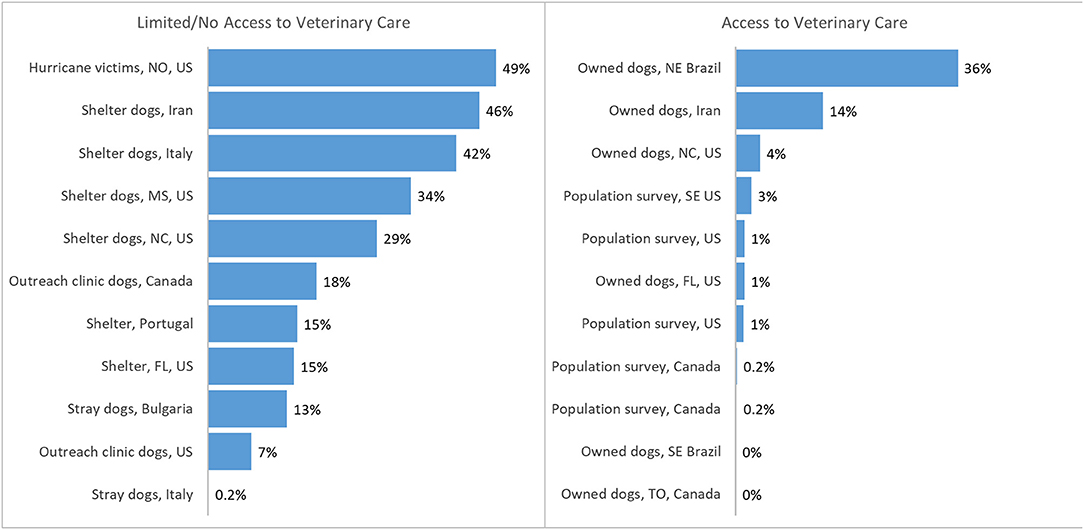
Figure 1. Prevalence or incidence of heartworm infection reported in populations of dogs with access (left) or with limited/no access (right) to veterinary care (20–30, 34–39). Population surveys = data from reference laboratories ± veterinary clinics. Portugal data averaged between 3 sites and over 3 years. TO, Toronto, Canada; US, United States; FL, Florida; NE, northeast; SE, southeast; NC, North Carolina; MS, Mississippi; NO, New Orleans.
Heartworm is a significant and growing concern for animal shelters in the US (1). When creating treatment protocols for affected populations of dogs in shelters, individual animal care must be balanced with limited resources, and division of those resources (including personnel to treat) to benefit as many animals as possible. The Association of Shelter Veterinarians' Position Statement on Heartworm Management acknowledges this balance, by encouraging shelters to treat affected dogs if possible, but to also ensure that “resources diverted toward heartworm management do not compromise the care of other shelter animals” (1). This Position Statement also provides background information, with a comprehensive tabulated comparison of a variety of arsenical and non-arsenical adulticidal protocols (1). The long duration of treatment coupled with the detrimental effects of increased length of stay in the shelter system, the cost of care throughout treatment, and the expense of melarsomine itself make this disease particularly challenging in shelter populations (40).
Even when resources are available, the lack of availability of melarsomine in some countries and the occurrence of multiple supply shortages in the past decade can leave large populations of dogs reliant on alternative treatments. Thus, while melarsomine treatment is acknowledged as best practice, safe and effective non-arsenical therapies are necessary to ensure that viable treatment options are available to all dogs with HWI.
In light of these concerns, many veterinarians have applied a “least harm principle” to heartworm management. This principle asserts that in the face of a situation in which both options may have negative consequences, the decision-maker should choose that which results in the least harm possible (41–43). In the case of treating a heartworm positive dog for which a melarsomine-based protocol is not feasible, the practitioner is faced with the option of not treating the dog or employing a non-arsenical approach. The authors contend that there is now sufficient evidence to support the use of non-arsenical treatment in many of these scenarios. Furthermore, such approaches can break the cycle of transmission, thereby benefiting not only the individual patient and owner but the larger population of susceptible animals.
Non-Arsenical Adulticide Treatment Protocols
Non-arsenical adulticide protocols that utilize a prolonged course of an ML have historically been labeled “slow-kill” or “soft-kill” (44, 45). Although the term “slow-kill” is still frequently used, it can refer to a variety of protocols with wide variations in evidence regarding the duration of use, safety, and efficacy. In the authors' experience, this term also often implies a safer option to many pet owners and carries a negative connotation with others. For all these reasons, we suggest that these terms should be avoided, and more objective and descriptive terminology used in their place.
Non-arsenical treatment is not recommended as a first-line treatment by the American Heartworm Society (AHS) and the Companion Animal Parasite Council (CAPC) (2, 46) and is not addressed in the European (ESCCAP) guidelines (3). The AHS Guidelines state that, in cases where melarsomine treatment is not possible or is contraindicated, treatment with doxycycline and an ML can be considered as a “salvage procedure” (2). This term generally refers to conditions that are refractory to other available treatment, or where the patient is unable to tolerate other treatments (47); thus excluding indications for use that lie outside of the individual patient's clinical condition, such as financial considerations, access to follow-up care and population-level impact. We contend that non-arsenical treatment is a reasonable alternative in specific circumstances and should be presented as such.
Outside of accessibility considerations, non-arsenical treatment is indicated if there is a history of a life-threatening adverse reaction to melarsomine; comorbidity conferring a guarded or grave prognosis; comorbidity making deep epaxial injection impracticable or during stabilization of severe heartworm-induced cor pulmonale (48). Despite recommendations against use in professional practice guidelines, non-arsenical protocols are widely used in certain circumstances and settings (5, 8–10, 32, 40, 49–51).
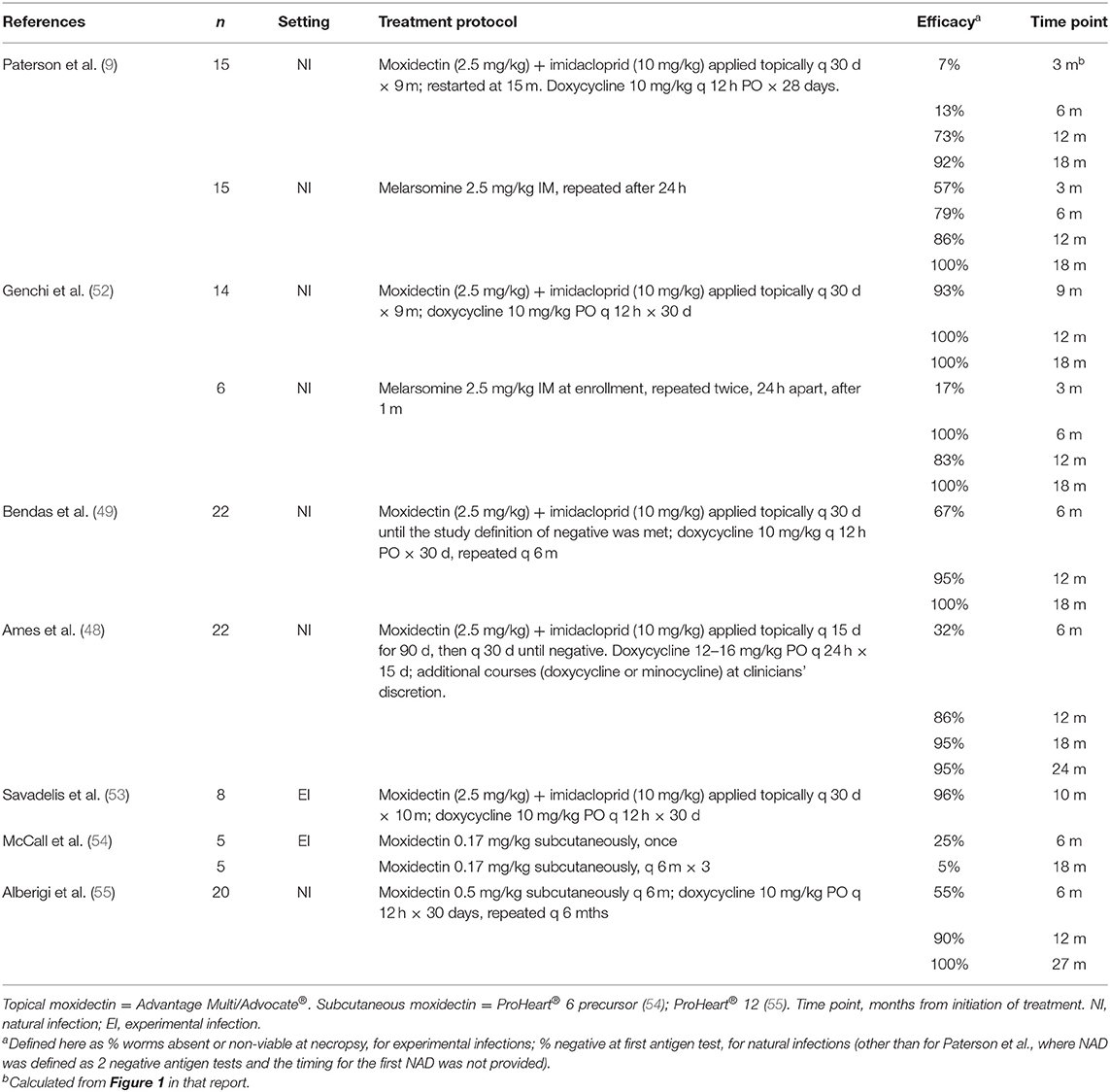
While numerous concerns exist regarding non-arsenical protocols, multiple studies have now demonstrated the safety and efficacy of moxi-doxy protocols (Table 1). These protocols tend to be used with variations, leading to a lack of uniformity in approach, and uncertainty on the part of practitioners and shelters faced with their implementation. A more standardized, evidence-based, approach is necessary to support practitioners utilizing these therapies.
Efficacy of Non-Arsenical Adulticide Protocols
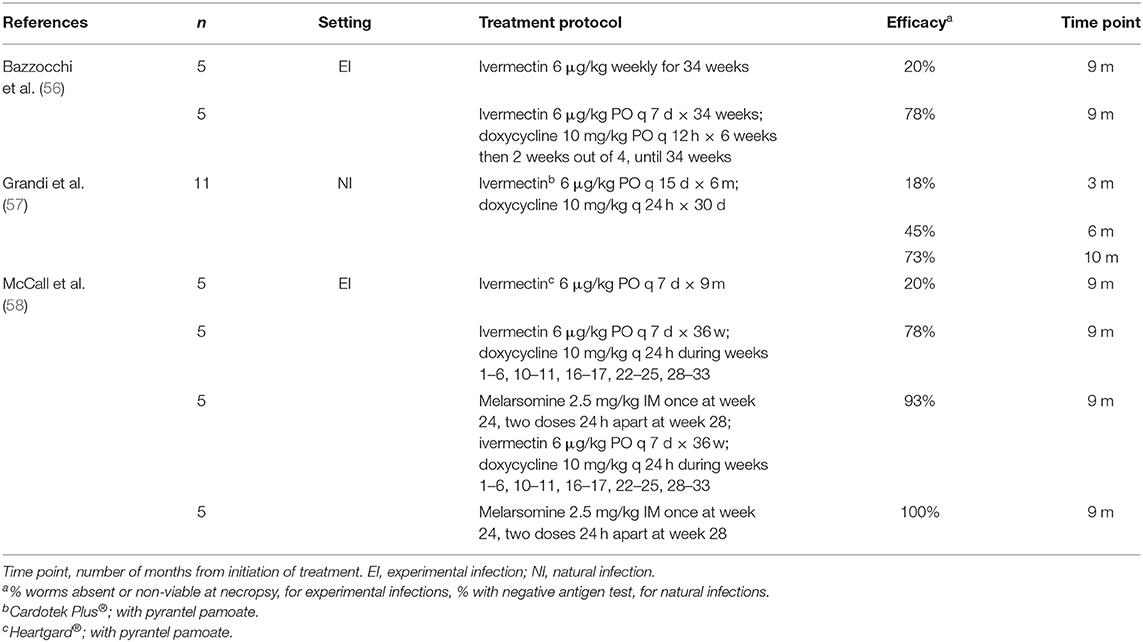
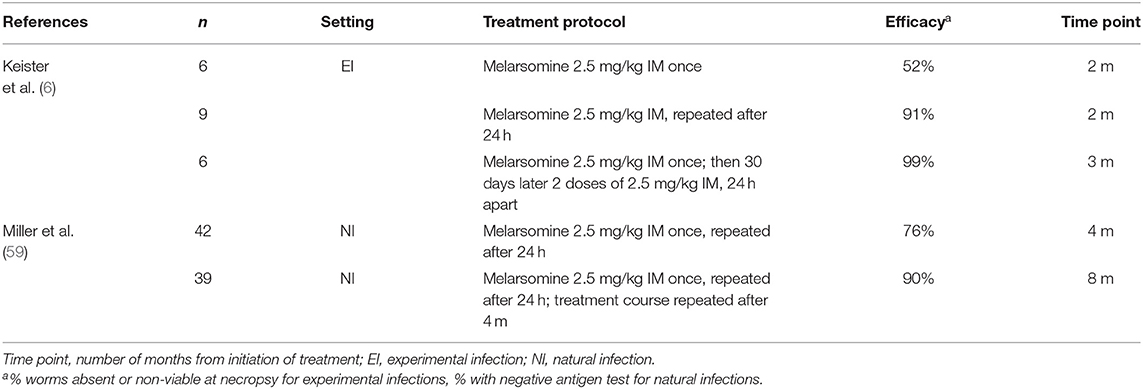
Studies showing the efficacy of moxi-doxy are summarized in Table 1, with selected studies of ivermectin (IVM) in Table 2 and melarsomine in Table 3, for comparison. This review will focus on moxidectin and IVM, as other MLs such as milbemycin and selamectin have poor efficacy for the elimination of adult worms (45).
Measures of efficacy depend on study type. Necropsy studies utilize counts of adult worms retrieved, while most clinical studies utilize antigen test results, sometimes in conjunction with echocardiography. Necropsy studies provide the most reliable measure of efficacy.
Efficacy of Ivermectin
A series of studies of the effects of IVM on different stages of D. immitis has been elegantly reviewed (45). Monthly IVM was highly effective against immature worms but required a longer duration of treatment to clear adult worms (45). The efficacy, at necropsy, for adult worms was 56% after 16 months (8-month-old worms) (60) and 95% after 29 months (7-month-old worms) (44). These studies used preventive dosage regimens, i.e., 6 μg/kg monthly.
The combination of IVM and doxycycline (IVM-doxy) demonstrated synergistic activity against adult worms compared with IVM alone (Table 2) (56, 58). Efficacy was 78% at 9 months (56), 73% at 10 months (57), and 78% at 9 months (58) after initiation of treatment. In these studies, IVM was used at the preventive dose of 6 μg/kg, but dosing frequency was increased to every 7 or 14 days (Table 2). Dogs with Class 3 HWD had higher worm burdens and poorer IVM-doxy adulticide efficacy, compared with Class 1 and Class 2 disease (61).
Ivermectin offers the advantage of being less expensive than moxidectin. However, despite improved adulticide efficacy when doxycycline is added, this combination is less effective than moxi-doxy and requires more frequent administration (Tables 1, 2). No published studies have directly compared IVM-doxy and moxi-doxy.
Efficacy of Moxidectin
Several studies have now evaluated moxi-doxy for adulticidal treatment, with encouraging results (Table 1) (9, 31, 48, 49, 52, 53, 55). As yet, no randomized, controlled trial (RCT) has directly compared the safety and efficacy of the current AHS-recommended melarsomine-based protocol and moxi-doxy.
The greater adulticide efficacy of moxidectin compared with IVM (Tables 1, 2) may be due to its unique pharmacokinetics and pharmacodynamics, which result in sustained exposure of the worms (62). Compared with IVM, moxidectin is more lipophilic, with a larger volume of distribution, longer half-life and slower elimination (62–64). Serum concentrations remain high for at least 28 days after administration of topical moxidectin at label dose, with steady state being reached after four monthly treatments (65). This is in contrast to other ML heartworm preventives, which have shorter half-lives and rely on reach-back effects to eliminate microfilariae acquired during the previous month (45, 65).
Moxidectin is available as a heartworm preventive in topical, sustained release (SR) injectable and oral formulations (64, 66–68). The labeled moxidectin dosage regimens are:
• Topical moxidectin (Advantage Multi®, Elanco; IMOXI, Vetoquinol; 100 mg/mL imidacloprid, 25 mg/mL moxidectin) – 2.5 mg/kg moxidectin applied to the skin once a month.
• SR moxidectin (ProHeart® 6; 3.4 mg/mL when constituted) – 0.17 mg/kg SQ once every 6 months;
• SR moxidectin (ProHeart® 12; 10 mg/mL when constituted) – 0.5 mg/kg SQ once every 12 months;
• Oral moxidectin (Simparica Trio; relative ratios 12 mg sarolaner: 240 μg moxidectin: 50 mg pyrantel; tablet sizes vary) - 24 μg/kg moxidectin orally once a month.
Topical moxidectin is the only ML formulation labeled for use in microfilaremic dogs (69) and clears microfilariae rapidly and effectively (70). Other MLs are relatively safe for microfilaremic dogs, at the prescribed dose and under veterinary supervision, but efficacy and clearance times are variable (71).
Topical Moxidectin
Five topical moxi-doxy studies have been published. Salient features are provided here, with further details summarized in Table 1.
All five studies used 10% moxidectin + 2.5% imidacloprid (Advantage Multi® or Advocate®, Elanco). Three of the study protocols administered the product every 30 days for 9 or 10 months (9, 52, 53), with resumption of monthly treatments at 15 months in one (9). In one study, monthly administration was continued until the study definition for NAD was met (49). In another, topical moxidectin was administered every 15 days for 90 days and every 30 days thereafter until NAD (48).
Doxycycline was administered at the recommended (2) dosage of 10 mg/kg q 12 h for 28–30 days in four studies (9, 49, 52, 53) and at 12–16 mg/kg q 24 h for 15 days in one (48). The course was repeated after 6 months (49); at varying intervals (doxycycline or minocycline) based on clinician discretion (48), or either not specified or not repeated in the other 3 studies.
• One RCT has been published, comparing moxi-doxy with melarsomine in 30 naturally infected dogs (15 per group) (9). Melarsomine was administered as a 2-dose protocol without doxycycline. Decline in antigenemia and NAD status was achieved more rapidly for melarsomine-treated dogs. Seven percent of the dogs in the moxi-doxy group had NAD at 3 months, 13% at 6 months, 73% at 12 months, and 92% at 18 months. Two dogs in the moxi-doxy group remained antigenemic, at 12 months and 18 months respectively, and were treated with melarsomine.
• One other study directly compared moxi-doxy and 3-dose melarsomine in naturally infected dogs (52). The study was not randomized and the melarsomine group did not receive doxycycline. Fourteen dogs received moxi-doxy and 6 received melarsomine. All dogs in the melarsomine group had NAD status at 6 months after administration, with one testing transiently positive at 12 months. Thirteen of 14 dogs in the moxi-doxy group (93%) had NAD at 9 months, and all tested negative at 12, 18 and 24 months. Moxi-doxy was statistically non-inferior to melarsomine at 12, 18, and 24 months.
• Another study reported results of moxi-doxy treatment in 22 naturally infected dogs (49). All dogs had NAD at 18 months, with 64% testing negative at 6 months and 96% at 12 months. Dogs with lower microfilaria counts tended to test negative sooner than those with higher counts, suggesting that efficacy may be negatively associated with worm burden and/or worm age.
• A study of 22 naturally infected dogs reported NAD status in 27% at 6 months, 86% at 12 months and 95% at 18 months, with one treatment failure as evidenced by a positive antigen test result at day 701 (48).
• An experimental study used 16 experimentally infected dogs, 8 of which received moxi-doxy and 8 of which were untreated controls. Worm counts at necropsy were used to assess efficacy. The study reported 96% efficacy of moxi-doxy against adult worms after 10 months of treatment, with 5 dogs completely negative and 1–2 worms each recovered from the remaining three dogs (53). In untreated controls, adult worms were recovered from all dogs, with numbers ranging from 10 to 12 per dog.
In summary, topical moxidectin plus doxycycline has shown good efficacy across different study designs, dog populations and dosage regimens, with some dogs testing negative as early as 3–6 months after initiation of treatment and the majority reaching negative or NAD status by 10–18 months. A small number of treatment failures have been recorded.
Sustained Release Injectable Moxidectin
SR injectable moxidectin products (ProHeart® 6 and Proheart® 12, Zoetis) have been in use as heartworm preventives for many years (64, 68, 72) and have been widely adopted in Australia as well as marketed more recently in several Asian and Latin American countries. These products contain microspheres of moxidectin that are gradually released over a prolonged period of time, either 6 (ProHeart® 6) or 12 (ProHeart 12®) months depending on concentration and dose. SR formulations were designed to circumvent the poor compliance with monthly preventive administration that frequently occurs (29, 45, 73, 74).
The 6-month SR product was temporarily recalled in the US in 2004 due to concerns about severe anaphylactoid reactions within the first 48 h of treatment1. An extensive pharmacovigilance monitoring program and post-marketing surveillance showed allergic reactions of 1.26 per 10,000, with a similar rate (1.19) for non-allergic reactions (75). Recent studies of 12-month SR moxidectin demonstrated safety and efficacy as a preventive (64, 76). Reports of adverse events over an extended period were comparable to those reported for ivermectin/pyrantel (Heartgard® Plus, Boehringer Ingelheim) (64).
Two published studies have assessed the effect of SR moxidectin on adult heartworms (Table 1). Administration of 0.17 mg/kg of SR moxidectin alone (without doxycycline) had poor efficacy for clearing adult heartworms in experimental dogs; however, many worms were abnormal at necropsy (54). A recent study assessed the efficacy of SR moxidectin combined with doxycycline in 20 naturally infected dogs (55). Twelve-month SR moxidectin was given every 6 months (i.e., at twice the label frequency) and doxycycline was dosed at 10 mg/kg q 12 h for 30 days, repeated every 6 months. The injection frequency was selected to maintain higher serum concentrations. Efficacy was comparable to results from topical moxidectin, with 55% NAD at 6 months, 90% at 12 months and 100% at 27 months. In a related case report, a dog treated with this protocol was found to be pregnant before the second moxidectin injection and gave birth 1 week after the injection. Doxycycline was not repeated at the 6 month visit (77). She had NAD at 6 and 12 months, and there were no observed adverse effects for her or her puppies.
In a survey-based study examining veterinary practices at a clinic in Mississippi, United States, 6-month SR moxidectin was the most frequently prescribed non-arsenical protocol and was selected by clients who were unable to afford arsenical treatment (32). As this study was survey-based, no information about the success or outcomes of those cases was provided.
If supported by further studies, SR moxidectin would be a useful alternative to topical moxidectin for adulticide treatment in remote regions such as northern Canada, or in circumstances where a long-acting treatment is preferable due to accessibility and/or compliance issues.
Oral Moxidectin
To the authors' knowledge, oral moxidectin (Simparica Trio®, Zoetis) has not been evaluated for adulticidal efficacy.
Moxi-Doxy: How Long Should Treatment Continue?
Depending on the study purpose and design, the duration of treatment has varied (Table 1). Findings indicate that worm death rates approaching those achieved with melarsomine-based protocols should not be expected prior to ~10 months of continuous therapy. Given the unpredictable time to NAD status in an individual dog, moxidectin should be continued until NAD status has been confirmed. This could be defined as one or two negative antigen tests, as discussed below. Doxycycline should be repeated annually for dogs still testing positive, as mentioned below.
Doxycycline in Non-Arsenical Adulticide Protocols
Doxycycline has become a necessary component of heartworm treatment (2), because of its effects on the filarial endosymbiont bacteria, Wolbachia (78, 79). The addition of doxycycline markedly reduces the pulmonary pathology associated with dead and dying worms, reduces the risk of thromboembolism and disrupts heartworm development and transmission (33, 80–82). When given alone to dogs experimentally infected with third stage D. immitis larvae, doxycycline prevented the development of adult worms, with decreased effectiveness against older immature stages (83).
McCall et al. treated dogs experimentally infected with third-stage larvae (L3) with doxycycline for 1 month at 20 mg/kg/day and then verified the presence of adult worms at 8 months post-infection (83). When doxycycline was administered during the first month post-infection, no live worms were observed at necropsy, indicating 100% efficacy against infective larvae. When doxycycline was commenced 40 days post-infection, efficacy against developing worms was 98%. Finally, when treatment was initiated 65 days post-infection, efficacy against juvenile worms was 70%. Interestingly, none of the dogs that harbored live worms was microfilaremic.
The combination of doxycycline (20 mg/kg q 24 h ×30 d), IVM (6 μg/kg monthly) and melarsomine (2.5 mg/kg IM, followed by 2 injections 24 h apart 1 month later) resulted in dramatically reduced pulmonary perivascular inflammation and endothelial proliferation after treatment of HWI, compared with doxycycline alone or melarsomine alone (82). Doxycycline also accelerates adult worm clearance and improves adulticidal efficacy when used with MLs (56, 58, 84) (Table 2). Worms found at necropsy were abnormal on histology and electron microscopy, with marked alterations in embryogenesis (56) and it was suspected that most worms that persisted in dogs treated with IVM-doxy would not have survived (58).
Dosage and Duration of Doxycycline
In the typical 3-dose melarsomine protocol, doxycycline is started at the time of diagnosis and continued for 28–30 days (2, 3). The dosage recommended by the AHS is 10 mg/kg PO q 12 h (2), while the ESCCAP Guidelines recommend 10 mg/kg PO q 24 h (3) and the CAPC Guidelines do not specify a dosage (46). Both 10 and 20 mg/kg/day have been used successfully in both arsenical and non-arsenical protocols, but no RCT has compared their parasitological efficacy. Tables 1, 2 detail dosages of doxycycline utilized in non-arsenical adulticide studies.
Doxycycline or minocycline, at either 5 mg/kg or 10 mg/kg q 12 h for 28 days, were compared in a randomized study of 32 microfilaremic dogs with HWI (85). The 3-dose melarsomine protocol was used with IVM. There was no significant difference in the rate of microfilarial clearance between the groups. Microfilariae in all 8 dogs treated with 10 mg/kg doxycycline q 12 h were negative for Wolbachia DNA by day 28. Microfilariae in 2/8 dogs treated with 5 mg/kg doxycycline or 10 mg/kg minocycline and 3/8 dogs treated with 5 mg/kg of minocycline were still positive for Wolbachia DNA at day 28. The potential impact of the remaining Wolbachia DNA on pulmonary pathology was not evaluated. The frequency and severity of gastrointestinal side effects (vomiting, diarrhea, weight loss) were greater in dogs receiving 10 mg/kg of either medication compared to 5 mg/kg.
Results are available at the 21 day time point for dogs treated with different doses of doxycycline, allowing for some comparison between dosages, albeit from different studies (79, 85). In 17 naturally infected dogs treated with doxycycline 10 mg/kg q 24 h for 21 days, Wolbachia DNA was detected in blood samples from 15/17 (88%) on treatment day 0, with a similar proportion positive at day 21 (14/17, 82%) (79). This decreased to 2/17 (12%) on day 111 (~16 weeks). When different tetracycline dosages were compared, at the 21 day time point 3/8 dogs (38%) treated with doxycycline 10 mg/kg q 12 h had Wolbachia DNA, compared with 6/8 of dogs (75%) treated with doxycycline 5 mg/kg q 12 h and 5/8 (63%) treated with either 5 or 10 mg/kg minocycline q 12 h (85).
Wolbachia positivity and microfilaremia rebounded 10 months after cessation of doxycycline in heartworm-positive dogs (79). One of the 7 dogs was positive 13 weeks after the end of the third treatment cycle; this increased to 4/5 dogs that were sampled 10 months after the last treatment. Microfilaria counts had increased concomitantly and were not significantly different from day 0 at this time point.
A third report demonstrated significant decreases in anti-Wolbachia IgG antibodies in 49 naturally infected dogs treated with doxycycline at 5 mg/kg q 12 h, 10 mg/kg q 12 h, or 10 mg/kg q 24 h for 30 days; none of these dogs showed evidence of clinical illness after adulticide therapy (86).
Finally, although commonly reported anecdotally, shorter durations of doxycycline treatment have not been investigated to date and could be of value, given the cost of the treatment and supply disruptions in recent years. As there has been no RCT focusing on clinical outcomes, it is not known which dose and duration of doxycycline (or minocycline) offer the greatest clinical advantage to the patient. The increased cost and potential for adverse effects of higher doses raise concerns about owner compliance and drug absorption (in the face of gastrointestinal upset). In animals who have adverse effects at the higher dosage, it is a common clinical practice to decrease the dose to finish the full duration of doxycycline therapy.
A 28- or 30-day course of doxycycline has demonstrable effects on treatment outcome and either 10 or 20 mg/kg/day is acceptable. For dogs that remain antigen positive 12 months after completing a course of doxycycline, the course should be repeated (2, 80); there are no known clinical benefits to repeating the treatment prior to that time point.
Non-arsenical therapy should not be initiated without adjunctive doxycycline.
Moxi-Doxy: Testing to Confirm Treatment Efficacy
Treatment efficacy is considered to mean eradication of adult heartworms, which is clearly the ideal treatment endpoint. In endemic areas, however, particularly those where veterinary care is sporadic, inaccessible, or unaffordable, resolution of clinical signs (where present) should also be considered a valid treatment goal. While highly undesirable, reinfection is highly likely in many settings, and the resolution of clinical disease addresses immediate patient welfare concerns. While a discussion of immunity to complex multicellular parasites is well beyond the scope of this review, complex host immune responses occur (87, 88) and should not be entirely overlooked.
The preferred treatment goal is NAD status—the best proxy for parasite elimination in clinical patients—together with a negative microfilarial test (Figure 2). A single negative antigen test is recommended to confirm efficacy after melarsomine treatment, with the caveat that a negative test does not rule out the presence of larval or juvenile stages, or a small number of adult male worms (2, 89). Determining NAD status is more complex for ML-doxycycline treated animals. The issues center around:
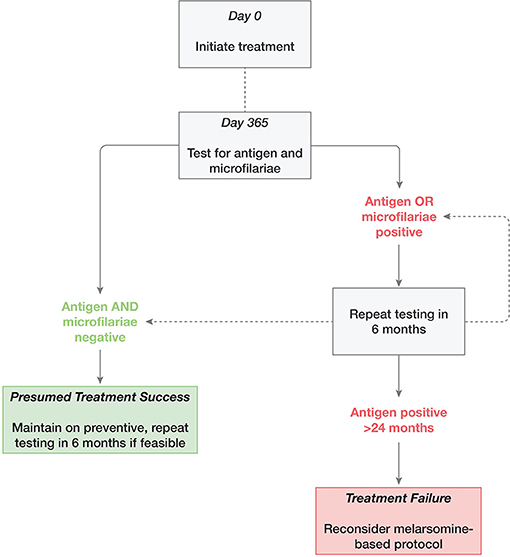
Figure 2. Suggested approach to retesting following adulticide treatment of heartworm infection with moxidectin and doxycycline.
• Whether a conventional antigen test is likely to be a false negative; and
• Whether a single negative antigen test is adequate to discontinue treatment.
Is Heat Treatment of Test Samples Required?
False negative antigen test results can be caused by antigen-blocking immune complexes (90). Immune complex dissociation (ICD), typically using heat, unmasks antigen that is blocked by antigen-antibody complexes. Heat treatment (HT) has demonstrated increased sensitivity in detecting early and male-only infections (89), but may also unmask residual antigen from dead and dying parasites (90). Results of the conventional, non-heat treated (NHT) antigen test and HT samples were compared in sera from shelter dogs, using necropsy counts of adult heartworms as the reference standard (89). Heat treatment increased the overall antigen test sensitivity from 87 to 95% (fewer false negatives), while also reducing specificity from 98 to 96% (more false positives). Heat treatment of sera from shelter dogs during screening for HWI modestly increased the proportion of positive results (91).
Previous treatment with MLs may exacerbate antigen blocking. Recent administration of a heartworm preventive was a significant risk factor for false negative results prior to HT (91). Heat treatment of sera after treatment of dogs with various ML-doxycycline combinations resulted in a high percentage of positive antigen tests after negative NHT results (92). In this small study, almost half of the dogs were inconsistently treated, with various protocols.
Dogs treated with either melarsomine or moxi-doxy had frequent conversions (>65%) from negative NHT to positive HT results (9), i.e., this phenomenon is not restricted to dogs treated with moxi-doxy. Three additional studies have compared HT and NHT antigen test results after moxi-doxy (48, 49, 93). In an experimental study, HT samples had higher optical density on the antigen test compared with NHT samples, even when both were positive (93). In a clinical study using samples taken every 6 months, HT samples initially disagreed with negative NHT samples in some dogs, with this discrepancy decreasing over time and no discrepancies at 18 and 24 months (49). All dogs that were NHT-negative/HT-positive were negative on both tests at the subsequent test 6 months later (49). In another clinical study, samples that were initially NHT-negative/HT-positive were all HT-negative 2–3 months later (48).
While HT is more sensitive for detection of residual HW antigen, this antigen may be released during and after HW death (90, 93) and the addition of this modification to the antigen test did not confer any diagnostic benefit following adulticide treatment with moxi-doxy (9, 48, 49, 93). Heat treatment is therefore not recommended when determining NAD status during moxi-doxy treatment.
Timing of Retesting
Based on findings to date (Table 1), approximately half of dogs may have NAD as early as 6 months after initiation of moxi-doxy treatment, with ~90% NAD by 12 months. Conversion to NAD status is expected to be slower in dogs with higher worm burdens (61). In one study, middle-aged dogs had higher worm burdens than younger and older dogs (94). Microfilaria count is not a reliable proxy for worm burden (94); however, NAD status was achieved earlier in dogs with lower microfilaria counts (49).
In general, retesting can reasonably be considered 6 months after initiation of treatment, but later testing (at 12 months) is a more appropriate use of limited resources (Figure 2). In addition to the severity of clinical signs, microfilaremia and age, timing should be based on practical considerations such as local experience, owner preference and resources, frequency of access to veterinary care, and adoption considerations (for shelters and rescues).
Should Negative Antigen Tests Be Repeated to Confirm NAD Status?
Two negative tests, 6 months apart, have been recommended to confirm NAD status after moxi-doxy treatment (2, 49). One study reported some variation in antigen test results and therefore defined NAD status as a minimum of two negative test results (9). Dogs were tested frequently in this study, initially monthly and then every 3 months from 9 to 18 months, compared with every 6 months in most other studies. There is no specific evidence that two tests are required at 6-month intervals. In studies where testing occurred at this frequency, no case was reported where a dog tested NAD at one time point and then positive at a subsequent time point (48, 49, 52).
The advantage of a second test is the reassurance of 6 months of additional treatment in the interim and the assurance that NAD status has persisted. Further, it would demonstrate that all stages of the life cycle have been accounted for. However, repeat testing and additional treatment may be impractical in remote communities or where other barriers to care exist.
Safety, Welfare, and Population Concerns
A number of safety, welfare, and population concerns have been raised regarding moxi-doxy treatment. These are addressed below and summarized in Table 4.
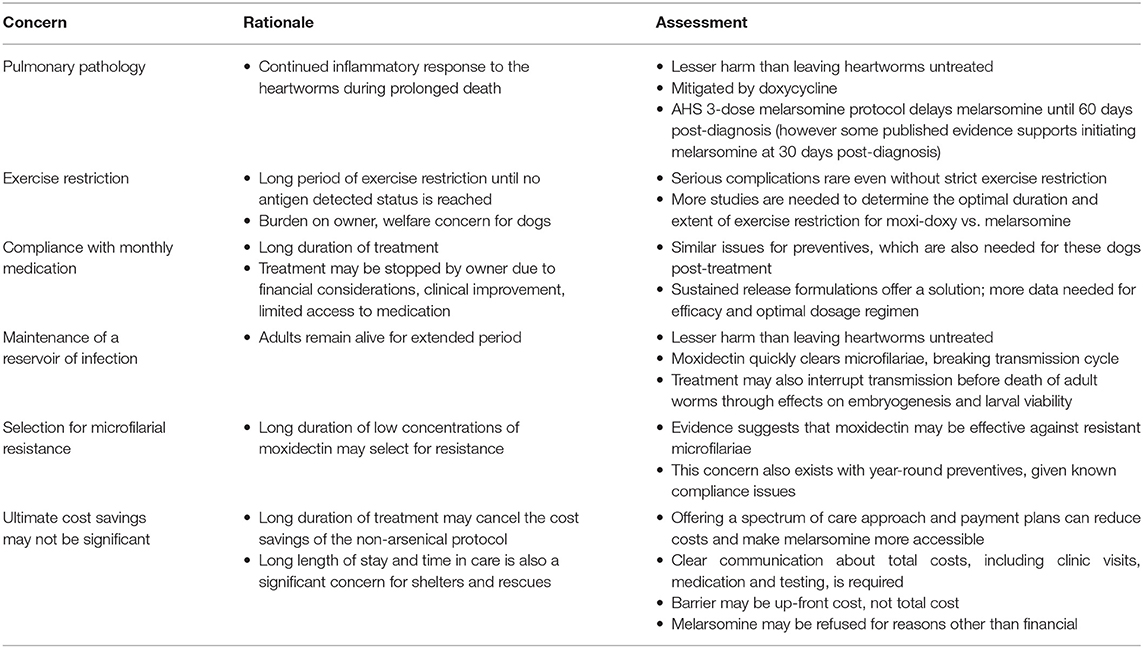
Table 4. Safety, welfare, and population concerns regarding heartworm adulticide treatment in dogs, using moxidectin and doxycycline.
Progression of Pulmonary Pathology
A primary objection to non-arsenical protocols, as regards patient safety and welfare, is that live adult worms remain in the pulmonary arteries for a prolonged period. This could result in progressive pulmonary endarteritis, pulmonary hypertension, perivascular inflammation, and potentially right heart failure (2). It is important to note that in the patient populations discussed here, the decision is not a choice between melarsomine and ML-doxycycline, but between a non-arsenical protocol and no treatment at all.
Measures of the extent and progression of pulmonary damage are difficult to assess accurately in clinical studies and without advanced imaging; this is further complicated by the fact that changes are not always reversible following treatment (95, 96).
When dogs with experimental adult HWI were treated with IVM, there was no difference in the progression of pulmonary disease (arterial and interstitial disease and pulmonary hypertension) when compared with milbemycin or no treatment (97). Both MLs were given according to label recommendations. When treatment was begun while the heartworms were immature, interstitial lung disease and increases in pulmonary arterial diameter were more severe in the IVM group at 9–11 months post-infection than in the milbemycin group and resembled changes in control dogs. Scores for IVM and milbemycin were similar beyond this time point. In a study of owned heartworm-positive dogs that were treated with monthly IVM for 24 months, 11/14 treated dogs (79%) and 3/3 untreated controls had normal echocardiographic and radiographic scores, that remained normal at the end of the treatment period (98). Two of 11 dogs treated with IVM (18%) had normal scores initially and developed abnormalities over the treatment period, and one had abnormal scores that worsened.
Both ML and doxycycline treatment are recommended prior to melarsomine, to reduce the worm burden and eliminate pro-inflammatory antigens, thereby reducing post-adulticide complications (33, 82). Doxycycline reduced the pulmonary inflammatory response to dead and dying worms when given prior to melarsomine, with a dramatically greater effect when IVM was added (82). This ameliorating effect could help offset the negative impact of slower worm death in ML-doxycycline protocols. MLs and doxycycline work synergistically to damage and eliminate pre-adult stages of D. immitis (83, 84). This prevents the development of additional adult worms during ML-doxycycline treatment and in this way may also help to slow disease progression, as compared with no treatment at all. This advantage may, however, be offset by the inflammatory response to dead and dying worms during treatment.
Clinical signs were not detected in dogs despite documented pulmonary changes during treatment with IVM (97). An asymptomatic presentation cannot be assumed to mean that pulmonary pathology is absent (96). However, in practical terms, clinical signs are of most concern to the owner and have the greatest welfare implications for the dog.
Five moxi-doxy studies have reported clinical, radiographic and/or echocardiographic findings (Table 5). New or worsening respiratory signs during moxi-doxy treatment have been absent or mild in the majority of dogs (9, 31, 48, 55) (Table 5), with only one dog in these studies requiring brief hospitalization (48). When moxi-doxy was compared with 2-dose melarsomine (without doxycycline), respiratory complications occurred in 4/15 dogs in the moxi-doxy group (27%) and 2/15 in the melarsomine group (13%) (9). Three of the 4 affected dogs in the moxi-doxy group required prednisone at ~2, 3, and 5 months, respectively. Another dog had parasitic pneumonitis and required intermittent prednisone (9). Hemoptysis and tachypnea were seen in the melarsomine group. In dogs treated with melarsomine, respiratory complications (including 2 deaths) decreased from 9/47 (19%) without doxycycline to 3/47 (7%) with doxycycline (81).
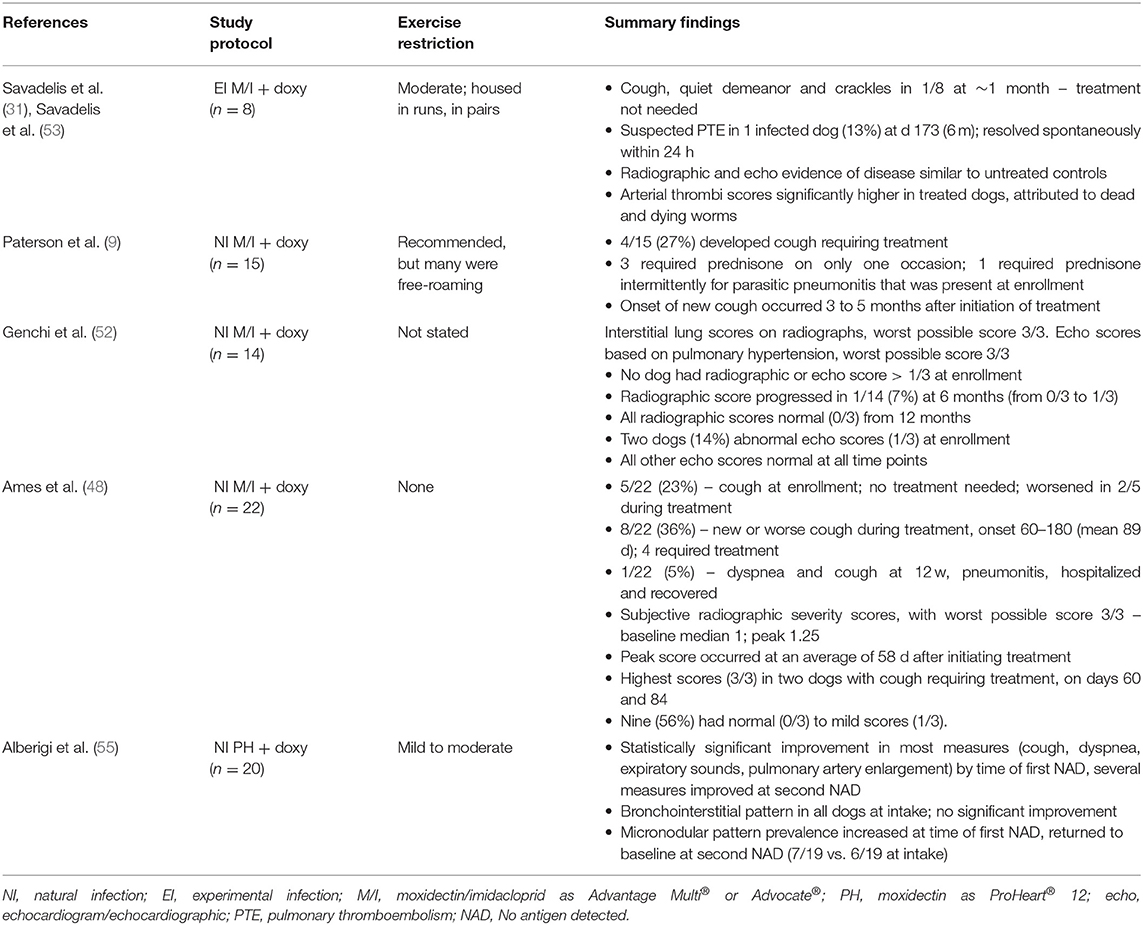
Table 5A. Cardiopulmonary changes during heartworm adulticide treatment in dogs, using moxidectin and doxycycline.
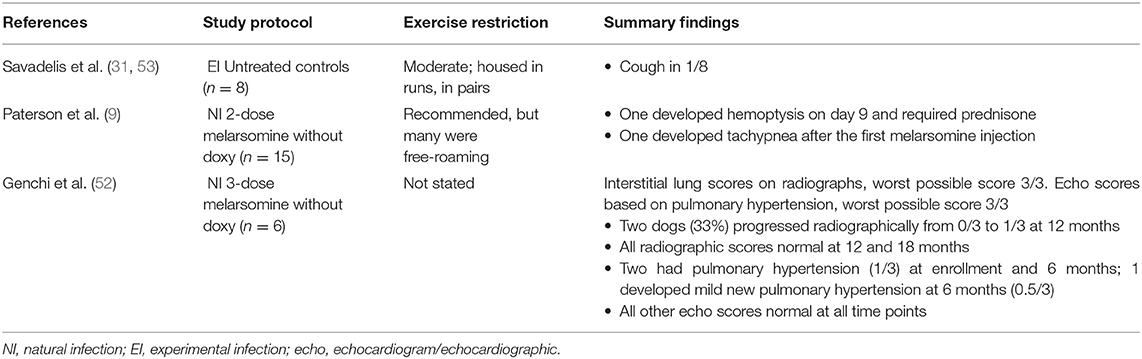
Table 5B. Cardiopulmonary changes during heartworm adulticide treatment in dogs, for control groups from studies of moxidectin and doxycycline.
The current AHS guidelines recommend prednisone as a routine adjunctive treatment to help reduce the risks of clinical pulmonary thromboembolism (2). This further complicates efforts to compare rates for respiratory signs and complications between arsenical and non-arsenical protocols, as studies evaluating moxi-doxy protocols have not included the routine use of glucocorticoids. While prednisone may not be required for all dogs receiving the 3-dose melarsomine protocol (99), the added anti-inflammatory effect may be of benefit as part of moxi-doxy protocols. Timing and duration are likely to be extremely difficult to ascertain, however, because of the unpredictable timing of worm death.
In an RCT involving experimentally infected dogs, clinical and necropsy findings were compared between those treated with moxi-doxy and untreated controls over 10 months (31) (Table 5). Radiographic abnormalities were not significantly different between the groups at any time point, but an interstitial or alveolar pattern score was higher than the baseline score on more study days for the treatment group. Echocardiographic scores did not differ between the groups. The histopathological arterial thrombus score was significantly higher for the treated group, an expected finding following worm death with any adulticidal treatment (31). The differences identified in this study were considered to have minimal or indeterminate clinical relevance during the time period examined (31); however, the study demonstrated that pulmonary changes did not improve during the early stages of moxi-doxy treatment.
In naturally infected dogs treated with moxi-doxy (n = 14) or 3 doses of melarsomine (without doxycycline) (n = 6), the majority of dogs in both groups had normal radiographic scores at all time points up to 24 months (52) (Table 5). One dog in the moxi-doxy group and 2 in the melarsomine group had mild new radiographic changes at 6 or 12 months, and all returned to normal 6 months later. Moxi-doxy was non-inferior to melarsomine for cardiac ultrasound scores, which were abnormal at baseline in 2 dogs in each group, became mildly abnormal at 6 months in one dog in the melarsomine group, and were normal in all dogs from the 12 month time point. In this study, therefore, there was mild, temporary progression of cardiac or pulmonary imaging scores in a small number of dogs, with the majority remaining normal throughout and the remainder improving over time. Similar trends were seen for dogs treated with IVM-doxy (100).
In 16 naturally-infected dogs treated with moxi-doxy that had radiographs taken, subjective grading showed mild average scores at enrollment (mean 1.04, median 1 of a possible worst score of 3), with slight worsening at 2 months (mean 1.44, median 1.25) (48) (Table 5). Nine of 16 dogs had normal to mildly abnormal radiographs. A dog that developed severe clinical signs was scored at 3/3 after 84 days of treatment and another, that developed a cough, had a score of 3/3 at 60 days.
Eight dogs had pulmonary arterial (PA) enlargement at the initiation of treatment with SR injectable moxidectin and doxycycline (55). This had resolved in several dogs (3/8 for caudal PA enlargement and 4/8 for main PA enlargement) at the first negative antigen test and in the majority (6/8 and 5/8, respectively) at the second negative antigen test. Bronchial and interstitial patterns were present in 19 dogs at enrollment and only resolved in 1/19. Micronodular pulmonary patterns became more common during treatment, being present in 6 dogs at enrollment, 9 at the first negative antigen test and 7 at the second negative antigen test.
Biomarkers have been used as a measure of the severity of cardiopulmonary changes in HWD (61, 101–104). These have not been measured during moxi-doxy treatment but were assessed in dogs given IVM-doxy (61). Concentrations of biomarkers decreased significantly by 10 months in all dogs treated with IVM-doxy but, in some dogs, some biomarkers remained elevated at the end of treatment (6 months) and/or at the end of the study. This was more frequently the case for dogs with Class 3 HWD (61). In both studies, dogs treated with melarsomine showed significant reductions in biomarkers over the course of treatment, with improvements considered to be markedly better than for IVM-doxy (a direct comparison was not performed) (61, 104). These findings support other evidence that less cardiopulmonary damage occurs in melarsomine-based treatment protocols.
Current AHS Guidelines recommend delaying treatment with melarsomine for 60 days after diagnosis of HWI (2), during which period MLs and doxycycline are administered. The reason for this delay was to overcome the so-called “susceptibility gap,” a period during which some stages of the parasite would not respond to either ML or adulticide treatment (105). However, this gap may not exist when 2- or 3-dose melarsomine protocols are used in conjunction with MLs, resulting in a recommendation to begin melarsomine as soon as possible after diagnosis (105).
A 60-day delay in melarsomine treatment also allows Wolbachia proteins and metabolites to be eliminated and weakens adult worms (2). During this period, the progression of pulmonary disease would be identical in the conventional and non-arsenical protocols. Parasite death is, however, much more rapid after initiation of melarsomine, with the elimination of adults within 2–3 months (without doxycycline) (Table 3) (6, 106).
In summary, the majority of dogs treated with moxi-doxy have shown no to mild respiratory complications, with only a few dogs requiring treatment and one needing brief hospitalization; no HW-associated deaths were reported (9, 31, 48, 52, 53, 55). This is despite the lack of strict exercise restriction in most cases. To date, there have been no RCTs directly comparing respiratory complications for 3-dose melarsomine protocols and moxi-doxy. Since clinical repiratory complications are rare and generally mild, the possibility of progression of pulmonary damage should not be a reason to withhold moxi-doxy from dogs with HWI when melarsomine is not a viable treatment option.
Exercise Restriction
Exercise restriction is recommended from the time of diagnosis of HWI until 6–8 weeks after the third melarsomine treatment (2) (i.e., ~5 months in total, including the 3-month higher risk period during and after rapid worm death). This is essential to help prevent cardiorespiratory complications (2).
The required stringency and duration of exercise restriction are unknown for ML-doxycycline protocols. In rural and remote communities in particular, many dogs roam outdoors and strict exercise restriction may be impracticable or require tethering. A prolonged period of exercise restriction without enrichment raises welfare concerns for dogs and may lead to behavioral problems that have implications for owners and the human-animal bond.
Of 79 dogs treated with moxi-doxy and not subjected to strict exercise restriction, the majority had an uncomplicated treatment course (9, 31, 48, 52, 55), with mild respiratory complications in some dogs (new or worsening cough) (Table 5). There were more serious, but short-lived, complications in two dogs (31, 48) and parasitic pneumonitis requiring intermittent treatment in one (9). No deaths were reported due to complications and only one dog required hospitalization for 24 h.
The potential for serious complications in some dogs nonetheless dictates that at least some degree of exercise restriction must be recommended (48), especially in the first several months of treatment. When treating HWI in any dog, the more severe the clinical signs, the more severe the exercise restriction should be (2). High worm burdens can be present in some dogs without proliferative lesions in the pulmonary arteries (107). Such dogs might not show clinical signs but could still be at high risk for pulmonary thromboembolism. A discussion of risk-benefit and the feasibility of strict exercise restriction should be held when dogs are treated with moxi-doxy; some level of clinician and owner discretion is required.
Prolonged length of stay in an animal shelter setting is a documented threat to both physical and behavioral health and welfare and must be avoided (108). Holding an animal in a shelter until they achieve NAD status is unacceptable and the capacity to provide for the needs of heartworm positive animals should be assessed prior to initiating treatment. In one survey, the majority of animal shelters treating heartworm positive dogs did so through the use of foster or foster-to-adopt programs (5). Regardless of the treatment protocol and operational programming in place, arrangements should be made for follow-up care and monitoring after adoption until such care can be transitioned to the new veterinarian.
Selection for Microfilarial Resistance
Resistance to HW preventives remains a significant concern (109–111), and concern about selection pressure for microfilarial resistance has been an important objection to non-arsenical treatment protocols. Small geographic areas of resistance have been documented in high-prevalence regions of the US (112). Resistance does not, however, appear to be common nor increasing in prevalence, with apparent lack of efficacy explained by other factors in most cases (73, 113). Identification and characterization of resistant isolates is challenging (112). It is unclear whether year-round prevention, as recommended for many regions, would necessarily result in less selection pressure than ML-doxycycline treatment of infected dogs.
Moxidectin has been reported to be more effective against resistant microfilariae than other MLs (62, 67, 114). This is thought to be due to its physicochemical and pharmacokinetic properties, in particular its high potency, distribution to fat and redistribution to plasma, resulting in longer persistence in the host and longer duration of action (62, 67).
The addition of doxycycline to treatment protocols has further reduced the risk of creating ML resistance, by interrupting embryogenesis and larval transmission (56, 58, 80, 115). Moxidectin is also directly microfilaricidal at the topical dose (69). Microfilariae are rapidly eliminated by moxi-doxy, frequently within 3–4 weeks (48, 49, 52, 53, 55). Elimination was more rapid than for IVM-doxycycline (56, 57), although direct comparisons are not available.
Based on these factors, the risk of selection for resistance might be lower for moxi-doxy than for previous ML treatment protocols, but more evidence is needed. Importantly, rapid clearance of microfilaremia in most cases (given that resistance remains uncommon) means that infected dogs treated with moxi-doxy will no longer be a reservoir of infection for at-risk animals in the community.
Conclusion
There is now an adequate body of evidence to demonstrate that moxi-doxy protocols are a viable option for adulticide treatment of HWI in dogs for which melarsomine treatment is not an option. In cases in which melarsomine is available, but the 3-dose protocol is not feasible for an owner or shelter, a modified melarsomine protocol (2 doses, beginning after a month of doxycycline) should first be considered before non-arsenical treatment is recommended. If non-arsenical adulticide treatment is the only viable treatment option, moxi-doxy protocols are preferred to other non-arsenical protocols. There is now adequate data to support recommendations for more uniform treatment and post-treatment testing protocols. Evidence-based recommendations for the use of moxi-doxy as an adulticide treatment are provided in Table 6.
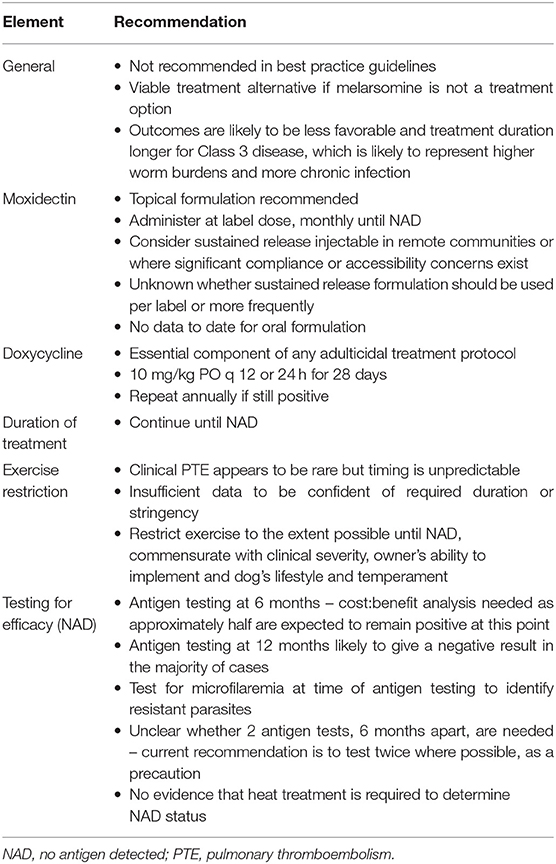
Table 6. Recommendations for moxidectin-doxycycline adulticidal treatment of asymptomatic and mild (Class 1 and 2) heartworm disease in dogs.
There is a need for RCTs that address several important concerns about moxi-doxy. For example, no RCT has compared respiratory complications in the same population of dogs treated with the currently recommended 3-dose melarsomine protocol and moxi-doxy, and no RCT has compared clinical outcomes for different degrees and durations of exercise restriction in dogs treated with moxi-doxy. Only one study has been published for SR injectable moxidectin as an adulticide treatment, and none for oral moxidectin. Such studies would be valuable additions to the literature.
Veterinarians using the “least harm” principle to expand treatment options for heartworm positive dogs should have a thorough understanding of the risks and benefits of non-arsenical treatment protocols, including moxi-doxy. Despite its limitations, the authors believe that the current evidence demonstrates that the benefits of moxi-doxy for affected individuals and at-risk populations outweigh the risks of untreated HWI. Moxi-doxy protocols can provide lifesaving relief for dogs and communities that lack access to melarsomine or routine veterinary care.
Author Contributions
All authors have made a substantial, direct and intellectual contribution to the work, and approved the final version.
Funding
This work and publication were funded by the Toronto Humane Society.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Toronto Humane Society Board and management for their support, and Drs. Uri Donnett and Andrew Moorhead for their constructive and insightful feedback. With grateful thanks to our friends and colleagues who work tirelessly to provide and promote accessible veterinary care.
Footnotes
1. ^https://www.avma.org/javma-news/2004-10-15/fort-dodge-recalls-proheart-6-citing-fda-safety-concerns.
References
1. Association of Shelter Veterinarians' Position Statement: Heartworm Management in Animal Shelters. (2019). p. 1–8. Available online at: https://asv.memberclicks.net/assets/docs/ASV.Heartworm~Management.pdf (accessed April 28, 2021).
2. Nelson CT, McCall JW, Jones S, Moorhead A. Current Canine Guidelines for the Prevention, Diagnosis, and Management of Heartworm (Dirofilaria immitis) Infection in Dogs. American Heartworm Society (2020). Available online at: https://d3ft8sckhnqim2.cloudfront.net/images/pdf/2020_AHS_Canine_Guidelines.pdf?1580934824 (accessed April 27, 2020).
3. Control of Vector-Borne Diseases in Dogs and Cats. (2019). Available online at: https://www.esccap.org/uploads/docs/t2kkcbgl_0775_ESCCAP_Guideline_GL5_v9_1p.pdf (accessed January 26, 2021).
4. Ames MK, Atkins CE. Treatment of dogs with severe heartworm disease. Vet Parasitol. (2020) 283:109131. doi: 10.1016/j.vetpar.2020.109131
5. DiGangi BA. The American Heartworm Society and Association of Shelter Veterinarians' 2019 shelter heartworm management practices survey. Vet Parasitol. (2020) 282:109130. doi: 10.1016/j.vetpar.2020.109130
6. Keister DM, Dzimianski MT, McTier TL. Dose selection and confirmation of RM 340, a new filaricide for the treatment of dogs with immature and mature Dirofilaria immitis. In: Conference Proceedings. AHS 1992 Symposium. Batavia, NY; Illinois: American Heartworm Society. p. 225–9.
7. Access to Veterinary Care: Barriers Current Practices and Public Policy. (2018). Available onine at: https://avcc.utk.edu/avcc-report.pdf (accessed September 26, 2019).
8. Dixon-Jimenez AC, Coleman AE, Rapoport GS, Creevy KE, Roth I, Correa M, et al. Approaches to canine heartworm disease treatment among alumni of a single College of Veterinary Medicine. J Am Anim Hosp Assoc. (2018) 54:246–56. doi: 10.5326/JAAHA-MS-6601
9. Paterson T, Fernandez C, Burnett PJ, Lessey L, Hockley T, Hagen R, et al. Heartworm control in Grenada, West Indies: results of a field study using imidacloprid 10% + moxidectin 2.5% and doxycycline for naturally-acquired Dirofilaria immitis infections. Vet Parasitol. (2020) 284:109194. doi: 10.1016/j.vetpar.2020.109194
10. Anvari D, Narouei E, Daryani A, Sarvi S, Moosazadeh M, Ziaei Hezarjaribi H, et al. The global status of Dirofilaria immitis in dogs: a systematic review and meta-analysis based on published articles. Res Vet Sci. (2020) 131:104–116. doi: 10.1016/j.rvsc.2020.04.002
11. Stoyanova H, Carreton E, Montoya-Alonso JA. Stray dogs of Sofia (Bulgaria) could be an important reservoir of heartworm (Dirofilaria immitis). Helminthologia. (2019) 56:329–33. doi: 10.2478/helm-2019-0033
12. Self SW, Pulaski CN, McMahan CS, Brown DA, Yabsley MJ, Gettings JR. Regional and local temporal trends in the prevalence of canine heartworm infection in the contiguous United States: 2012–2018. Parasit Vectors. (2019) 12:1–10. doi: 10.1186/s13071-019-3633-2
13. Morchón R, Carretón E, González-Miguel J, Mellado-Hernández I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe - new distribution trends. Front Physiol. (2012) 3:196. doi: 10.3389/fphys.2012.00196
14. Islam SN, Winkel J. Climate Change and Social Inequality. New York NY (2017). Available online at: http://www.ejnetindiaresource.org/ejissues/bali.pdfen- (accessed April 8, 2021).
15. Pereira M, Oliveira AM. Poverty and food insecurity may increase as the threat of COVID-19 spreads. Public Health Nutr. (2020) 23:3236–40. doi: 10.1017/S1368980020003493
16. Chudik A, Mohaddes K, Pesaran MH, Raissi M, Rebucci A. Economic Consequences of COVID-19: A Counterfactual Multi-Country Analysis. (2020). Available online at: https://theforum.erf.org.eg/2020/10/20/economic-consequences-covid-19-counterfactual-multi-country-analysis/ (accessed April 5, 2021).
17. Bambra C, Riordan R, Ford J. The COVID-19 pandemic and health inequalities. J Epidemiol Community Heal. (2020) 74:964–8. doi: 10.1136/jech-2020-214401
18. Hornak T. Melarsomine is expensive - what treatment options are successful or not in low income communities? In: Conference Proceedings. AHS 2019 Symposium. Batavia, NY; Illinois: American Hearworm Society. p. 76–77.
19. Wang D, Bowman DD, Brown HE, Harrington LC, Kaufman PE, McKay T, et al. Factors influencing U.S. canine heartworm (Dirofilaria immitis) prevalence. Parasit Vectors. (2014) 7:264. doi: 10.1186/1756-3305-7-264
20. Tzipory N, Crawford PC, Levy JK. Prevalence of Dirofilaria immitis, Ehrlichia canis, and Borrelia burgdorferi in pet dogs, racing greyhounds, and shelter dogs in Florida. Vet Parasitol. (2010) 171:136–9. doi: 10.1016/j.vetpar.2010.03.016
21. Donnett U, Hubbard K, Woodruff K, Varela-Stokes A. Prevalence of canine heartworm infection in Mississippi animal shelters. Vet Parasitol. (2018) 259:68–73. doi: 10.1016/j.vetpar.2018.07.007
22. Balakrishnan N, Musulin S, Varanat M, Bradley JM, Breitschwerdt EB. Serological and molecular prevalence of selected canine vector borne pathogens in blood donor candidates, clinically healthy volunteers, and stray dogs in North Carolina. Parasit Vectors. (2014) 7:116. doi: 10.1186/1756-3305-7-116
23. Jacobson LS, Ward KA, Lacaden AB, Hornak TA. Prevalence of heartworm in relocated, local and outreach clinic dogs: a Canadian sheltering perspective. Vet Parasitol. (2020) 283:109081. doi: 10.1016/j.vetpar.2020.109081
24. Alhoa AM, Landumb M, Ferreira C, Meirelesa J, Goncalves L, de Carvalhoa LM, et al. Prevalence and seasonal variations of dirofilariosis in Portugal. Vet Parasitol. (2014) 206:99–105. doi: 10.1016/j.vetpar.2014.08.014
25. Anvari D, Saadati D, Siyadatpanah A, Gholami S. Prevalence of dirofilariasis in shepherd and stray dogs in Iranshahr, southeast of Iran. J Parasit Dis. (2019) 43:319–23. doi: 10.1007/s12639-019-01096-5
26. Levy JK, Lappin MR, Glaser AL, Birkenheuer AJ, Anderson TC, Edinboro CH. Prevalence of infectious diseases in cats and dogs rescued following Hurricane Katrina. J Am Vet Med Assoc. (2011) 238:311–7. doi: 10.2460/javma.238.3.311
27. Evason M, Stull JW, Pearl DL, Peregrine AS, Jardine C, Buch JS, et al. Prevalence of Borrelia burgdorferi, Anaplasma spp., Ehrlichia spp. and Dirofilaria immitis in Canadian dogs, 2008 to 2015: A repeat cross-sectional study. Parasit Vectors. (2019) 12:1–11. doi: 10.1186/s13071-019-3299-9
28. McGill E, Berke O, Weese JS, Peregrine A. Heartworm infection in domestic dogs in Canada, 1977-2016: prevalence, time trend, and efficacy of prophylaxis. Can Vet J. (2019) 60:605–12.
29. Drake J, Wiseman S. Increasing incidence of Dirofilaria immitis in dogs in USA with focus on the southeast region 2013-2016. Parasit Vectors. (2018) 11:1–7. doi: 10.1186/s13071-018-2631-0
30. Theis JH, Kass PH, Davis E, Stevens F. Dirofilaria immitis infection in dogs from underserved, native American reservations in the United States. J Am Anim Hosp Assoc. (2011) 47:179–84. doi: 10.5326/JAAHA-MS-5598
31. Savadelis MD, Coleman AE, Rapoport GS, Sharma A, Sakamoto K, Keys DA, et al. Clinical assessment of heartworm-infected Beagles treated with a combination of imidacloprid/moxidectin and doxycycline, or untreated. J Vet Intern Med. (2020) 34:1734–45. doi: 10.1111/jvim.15853
32. Ku TN. Investigating management choices for canine heartworm disease in northern Mississippi. Parasit Vectors. (2017) 10:474. doi: 10.1186/s13071-017-2450-8
33. Kramer L, Crosara S, Gnudi G, Genchi M, Mangia C, Viglietti A, et al. Wolbachia, doxycycline and macrocyclic lactones: new prospects in the treatment of canine heartworm disease. Vet Parasitol. (2018) 254:95–97. doi: 10.1016/j.vetpar.2018.03.005
34. Dantas-Torres F, Figueredo LA, Sales KGDS, Miranda DEDO, Alexandre JLDA, Da Silva YY, et al. Prevalence and incidence of vector-borne pathogens in unprotected dogs in two Brazilian regions. Parasit Vectors. (2020) 13:1–7. doi: 10.1186/s13071-020-04056-8
35. Little S, Braff J, Place J, Buch J, Dewage BG, Knupp A, et al. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013–2019. Parasit Vectors. (2021) 14:1–16. doi: 10.1186/s13071-020-04514-3
36. Drake J, Parrish RS. Dog importation and changes in heartworm prevalence in Colorado 2013-2017. Parasit Vectors. (2019) 12:1–10. doi: 10.1186/s13071-019-3473-0
37. Petruccelli A, Ferrara G, Iovane G, Schettini R, Ciarcia R, Caputo V, et al. Seroprevalence of Ehrlichia spp., Anaplasma spp, Borrelia burgdorferi sensu lato, and Dirofilaria immitis in stray dogs, from 2016 to 2019, in southern Italy. Animals. (2021) 11:1–10. doi: 10.3390/ani11010009
38. Manev I. Serological survey of vector-borne pathogens in stray dogs from Sofia area, Bulgaria. Vet Parasitol Reg Stud Rep. (2020) 21:100441. doi: 10.1016/j.vprsr.2020.100441
39. Panarese R, Iatta R, Latrofa MS, Zatelli A, Ignjatović Cupina A, Montarsi F, et al. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: an expanding threat in the Mediterranean region. Int J Parasitol. (2020) 50:555–9. doi: 10.1016/j.ijpara.2020.04.002
40. Colby KN, Levy JK, Dunn KF, Michaud RI. Diagnostic, treatment, and prevention protocols for canine heartworm infection in animal sheltering agencies. Vet Parasitol. (2011) 176:333–41. doi: 10.1016/j.vetpar.2011.01.018
41. Rainbow C. Descriptions of Ethical Theories and Principles. (2002). Available online at: https://bio.davidson.edu/people/kabernd/indep/carainbow/Theories.htm (accessed March 23, 2021).
42. Davis SL. The least harm principle may require that humans consume a diet containing large herbivores, not a vegan diet. J Agric Environ Ethics. (2003) 16:387–94. doi: 10.1023/A:1025638030686
43. Chonko L. Ethical Theories. Available online at: https://www.dsef.org/wp-content/uploads/2012/07/EthicalTheories.pdf (accessed March 23, 2021).
44. McCall JW, Guerrero J, Roberts RE, Supakornej N, Mansour AE, Dzimianski MT, et al. Further evidence of clinical prophylactic, retroactive (reach-back) and adulticidal activity of monthly administrations of ivermectin (Heartgard PlusTM) in dogs experimentally infected with heartworms. In: Seward RI, editor. Conference Proceedings. AHS 2001 Symposium San Antonio, Texas, US: American Heartworm Society. p. 189–200.
45. McCall JW. The safety-net story about macrocyclic lactone heartworm preventives: a review, an update, and recommendations. Vet Parasitol. (2005) 133:197–206. doi: 10.1016/j.vetpar.2005.04.005
46. Companion Animal Parasite Council Guidelines: Heartworm. (2020). Available online at: https://capcvet.org/guidelines/heartworm/ (accessed April 15, 2021).
47. Shiel WC. Definition of Salvage Therapy. MedicineNet Available online at: https://www.medicinenet.com/salvage_therapy/definition.htm (accessed March 23, 2021).
48. Ames MK, Vanvranken P, Evans C, Atkins CE. Non-arsenical heartworm adulticidal therapy using topical moxidectin-imidacloprid and doxycycline: a prospective case series. Vet Parasitol. (2020) 282:109099. doi: 10.1016/j.vetpar.2020.109099
49. Bendas AJR, Mendes-De-Almeida F, Von Simson C, Labarthe N. Heat pretreatment of canine samples to evaluate efficacy of imidacloprid + moxidectin and doxycycline in heartworm treatment. Parasit Vectors. (2017) 10:6–10. doi: 10.1186/s13071-017-2189-2
50. Murphy KR, Chung I, Zweifel L, Rubin S, Diggs TJ, Torres T, et al. Implementation of a novel heartworm prevention strategy through community engagement in low-income urban neighborhoods (poster presentation). In: Conference Presentation. AHS 2019 Symposium. New Orleans, LA, p. 77–79.
51. Genchi M, Rinaldi L, Venco L, Cringoli G, Vismarra A, Kramer L. Dirofilaria immitis and D. repens in dog and cat: A questionnaire study in Italy. Vet Parasitol. (2019) 267:26–31. doi: 10.1016/j.vetpar.2019.01.014
52. Genchi M, Vismarra A, Lucchetti C, Viglietti A, Crosara S, Gnudi G, et al. Efficacy of imidacloprid 10%/moxidectin 2.5% spot on (Advocate®, Advantage Multi®) and doxycycline for the treatment of natural Dirofilaria immitis infections in dogs. Vet Parasitol. (2019) 273:11–6. doi: 10.1016/j.vetpar.2019.07.011
53. Savadelis MD, Ohmes CM, Hostetler JA, Settje TL, Zolynas R, Dzimianski MT, et al. Assessment of parasitological findings in heartworm-infected beagles treated with Advantage Multi® for dogs (10% imidacloprid + 2.5% moxidectin) and doxycycline. Parasit Vectors. (2017) 10:1–7. doi: 10.1186/s13071-017-2190-9
54. McCall JW, Supakorndej P, Dzimianski MT, Supakorndej N, Mansour AE, Jun JJ, et al. Evaluation of retroactive and adulticidal activity of moxidectin canine SR (sustained-release) injectable formulation against Dirofilaria immitis infection in dogs. In: Seward RL, editor. Conference Proceedings. AHS 2001 Symposium. Batavia, NY; Illinois.
55. Alberigi B, Fernandes JI, Paiva JP, Mendes-De-Almeida F, Knackfuss F, Merlo A, et al. Efficacy of semi-annual therapy of an extended-release injectable moxidectin suspension and oral doxycycline in Dirofilaria immitis naturally infected dogs. Parasit Vectors. (2020) 13:1–8. doi: 10.1186/s13071-020-04380-z
56. Bazzocchi C, Mortarino M, Grandi G, Kramer LH, Genchi C, Bandi C, et al. Combined ivermectin and doxycycline treatment has microfilaricidal and adulticidal activity against Dirofilaria immitis in experimentally infected dogs. Int J Parasitol. (2008) 38:1401–10. doi: 10.1016/j.ijpara.2008.03.002
57. Grandi G, Quintavalla C, Mavropoulou A, Genchi M, Gnudi G, Bertoni G, et al. A combination of doxycycline and ivermectin is adulticidal in dogs with naturally acquired heartworm disease (Dirofilaria immitis). Vet Parasitol. (2010) 169:347–51. doi: 10.1016/j.vetpar.2010.01.025
58. McCall JW, Genchi C, Kramer L, Guerrero J, Dzimianski MT, Supakorndej P, et al. Heartworm and Wolbachia: therapeutic implications. Vet Parasitol. (2008) 158:204–14. doi: 10.1016/j.vetpar.2008.09.008
59. Miller M, Keister D, Tanner P, Meo N. Clinical efficacy of melarsomine dihydrochloride (RM340) and thiacetarsamide in dogs with moderate (Class 2) heartworm disese. In: Soll M, Knight D, editors. Conference Proceedings. AHS 1995 Symposium. Batavia, NY; Illinois: American Heartworm Society. p. 233–41.
60. McCall J, Ryan W, Roberts R, Dzimianski M. Heartworm adulticidal activity of prophylactic doses of ivermectin (6μg/kg) plus pyrantel administered monthly to dogs. In: Seward L, editor. Conference Proceedings. AHS 1998 Symposium. Batavia, NY; Illinois: American Hearworm Society (1998). p. 209–15.
61. Yoon WK, Kim YW, Suh SI, Hyun C. Evaluation of cardiopulmonary and inflammatory markers in dogs with heartworm infection treated using the slow kill method. Vet Parasitol. (2017) 244:35–8. doi: 10.1016/j.vetpar.2017.07.015
62. Prichard RK, Geary TG. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int J Parasitol Drugs Drug Resist. (2019) 10:69–83. doi: 10.1016/j.ijpddr.2019.06.002
63. Prichard R, Ménez C, Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol Drugs Drug Resist. (2012) 2:134–53. doi: 10.1016/j.ijpddr.2012.04.001
64. McTier TL, Kryda K, Wachowski M, Mahabir S, Ramsey D, Rugg D, et al. ProHeart® 12, a moxidectin extended-release injectable formulation for prevention of heartworm (Dirofilaria immitis) disease in dogs in the USA for 12 months. Parasit Vectors. (2019) 12:369. doi: 10.1186/s13071-019-3632-3
65. Bowman DD, Grazette AR, Basel C, Wang Y, Hostetler JA. Protection of dogs against canine heartworm infection 28 days after four monthly treatments with Advantage Multi® for Dogs. Parasit Vectors. (2016) 9:1–7. doi: 10.1186/s13071-016-1293-z
66. Genchi C, Rossi L, Cardini G, Kramer LH, Venco L, Casiraghi M, et al. Full season efficacy of moxidectin microsphere sustained release formulation for the prevention of heartworm (Dirofilaria immitis) infection in dogs. Vet Parasitol. (2002) 110:85–91. doi: 10.1016/S0304-4017(02)00325-4
67. McTier TL, Six RH, Pullins A, Chapin S, Kryda K, Mahabir SP, et al. Preventive efficacy of oral moxidectin at various doses and dosage regimens against macrocyclic lactone-resistant heartworm (Dirofilaria immitis) strains in dogs. Parasit Vectors. (2019) 12:1–11. doi: 10.1186/s13071-019-3685-3
68. Holm-Martin M, Atwell R. Evaluation of a single injection of a sustained-release formulation of moxidectin for prevention of experimental heartworm infection after 12 months in dogs. Am J Vet Res. (2004) 65:1596–99. doi: 10.2460/ajvr.2004.65.1596
69. Bowman DD, Charles SD, Arther RG, Settje T. Laboratory evaluation of the efficacy of 10 % imidacloprid + 2.5 % moxidectin topical solution (Advantage® Multi, Advocate®) for the treatment of Dirofilaria immitis circulating microfilariae in dogs. Parasitol Res. (2015) 114:165–74. doi: 10.1007/s00436-015-4522-z
70. Frangipane di Regalbono A, Di Cesare A, Traversa D, Simonato G, Poser H, Danesi P, et al. Microfilaricidal efficacy of a single administration of Advocate® (Bayer Animal Health) in dogs naturally infected with Dirofilaria immitis or Dirofilaria repens. Vet Parasitol. (2016) 226:30–4. doi: 10.1016/j.vetpar.2016.06.024
71. Bowman DD, Mannella C. Macrocyclic lactones and Dirofilaria immitis microfilariae. Top Companion Anim Med. (2011) 26:160–72. doi: 10.1053/j.tcam.2011.07.001
72. Bowman DD, McTier TL, Adams EL, Mahabir SP, Login JA, Bidgood T, et al. Evaluation of the efficacy of ProHeart® 6 (moxidectin) against a resistant isolate of Dirofilaria immitis (JYD-34) in dogs. Parasit Vectors. (2017) 10:502. doi: 10.1186/s13071-017-2431-y
73. Atkins CE, Murray MJ, Olavessen LJ, Wade Burton K, Marshall JW, Brooks CC, et al. Heartworm ‘lack of effectiveness' claims in the Mississippi delta: computerized analysis of owner compliance – 2004–2011. Vet Parasitol. (2014) 206:106–113. doi: 10.1016/j.vetpar.2014.08.013
74. Varloud M, Padgett K, Johnson C, Fairchild S. Heartworm prevention: the dog owner perspective. In: Conference Proceedings. AHS 2019 Symposium. Batavia, NY; Illinois: American Heartworm Society. p. 69.
75. Health FDA. ProHeart 6 (moxidectin). (2005). Available online at: https://www.who.int/tdr/publications/documents/moxidectin-12-fort-dodge-briefing.pdf?ua=1 (accessed April 28, 2021).
76. Krautmann MJ, Mahabir S, Fielder A, Collard W, Wolthuis TL, Esch K, et al. Safety of an extended-release injectable moxidectin suspension formulation (ProHeart® 12) in dogs. Parasit Vectors. (2019) 12:433. doi: 10.1186/s13071-019-3690-6
77. Alberigi B, Souza C da SF de, Fernandes JI, Merlo A, Labarthe N. Use of slow-release injectable moxidectin for treatment of Dirofilaria immitis infection during pregnancy. Front Vet Sci. (2020) 6:1–4. doi: 10.3389/fvets.2019.00440
78. Menozzi A, Bertini S, Turin L, Serventi P, Kramer L, Bazzocchi C. Doxycycline levels and anti-Wolbachia antibodies in sera from dogs experimentally infected with Dirofilaria immitis and treated with a combination of ivermectin/doxycycline. Vet Parasitol. (2015) 209:281–84. doi: 10.1016/j.vetpar.2015.02.023
79. Rossi MID, Paiva J, Bendas A, Mendes-de-Almeida F, Knackfuss F, Miranda M, et al. Effects of doxycycline on the endosymbiont Wolbachia in Dirofilaria immitis (Leidy, 1856)-naturally infected dogs. Vet Parasitol. (2010) 174:119–23. doi: 10.1016/j.vetpar.2010.07.019
80. McCall JW, Kramer L, Genchi C, Guerrero J, Dzimianski MT, Mansour A, et al. Effects of doxycycline on heartworm embryogenesis, transmission, circulating microfilaria, and adult worms in microfilaremic dogs. Vet Parasitol. (2014) 206:5–13. doi: 10.1016/j.vetpar.2014.09.023
81. Nelson CT, Myrick ES, Nelson TA. Clinical benefits of incorporating doxycycline into a canine heartworm treatment protocol. Parasit Vectors. (2017) 10:1–4. doi: 10.1186/s13071-017-2446-4
82. Kramer L, Grandi G, Passeri B, Gianelli P, Genchi M, Dzimianski MT, et al. Evaluation of lung pathology in Dirofilaria immitis-experimentally infected dogs treated with doxycycline or a combination of doxycycline and ivermectin before administration of melarsomine dihydrochloride. Vet Parasitol. (2011) 176:357–60. doi: 10.1016/j.vetpar.2011.01.021
83. McCall JW, Kramer L, Genchi C, Guerrero J, Dzimianski MT, Supakorndej P, et al. Effects of doxycycline on early infections of Dirofilaria immitis in dogs. Vet Parasitol. (2011) 176:361–7. doi: 10.1016/j.vetpar.2011.01.022
84. Chandrashekar R, Beall MJ, Saucier J, O'Connor T, McCall JW, McCall SD, et al. Experimental Dirofilaria immitis infection in dogs: effects of doxycycline and Advantage Multi® administration on immature adult parasites. Vet Parasitol. (2014) 206:93–8. doi: 10.1016/j.vetpar.2014.08.011
85. Savadelis MD, Day KM, Bradner JL, Wolstenholme AJ, Dzimianski MT, Moorhead AR. Efficacy and side effects of doxycycline versus minocycline in the three-dose melarsomine canine adulticidal heartworm treatment protocol. Parasit Vectors. (2018) 11:1–7. doi: 10.1186/s13071-018-3264-z
86. Carretón E, Morchón R, Falcón-Cordón Y, Falcón-Cordón S, Matos JI, Montoya-Alonso JA. Evaluation of different dosages of doxycycline during the adulticide treatment of heartworm (Dirofilaria immitis) in dogs. Vet Parasitol. (2020) 283:109141. doi: 10.1016/j.vetpar.2020.109141
87. Yasuda K, Nakanishi K. Host responses to intestinal nematodes. Int Immunol. (2018) 30:93–102. doi: 10.1093/intimm/dxy002
88. Kwarteng A, Ahuno ST, Akoto FO. Killing filarial nematode parasites: role of treatment options and host immune response. Infect Dis Poverty. (2016) 5:4–9. doi: 10.1186/s40249-016-0183-0
89. Gruntmeir JM, Long MT, Blagburn BL, Walden HS. Canine heartworm and heat treatment: an evaluation using a well based enzyme-linked immunosorbent assay (ELISA) and canine sera with confirmed heartworm infection status. Vet Parasitol. (2020) 283:109169. doi: 10.1016/j.vetpar.2020.109169
90. Little S, Saleh M, Wohltjen M, Nagamori Y. Prime detection of Dirofilaria immitis: understanding the influence of blocked antigen on heartworm test performance. Parasit Vectors. (2018) 11:1–10. doi: 10.1186/s13071-018-2736-5
91. DiGangi BA, Dworkin C, Stull JW, O'Quin J, Elser M, Marsh AE, et al. Impact of heat treatment on Dirofilaria immitis antigen detection in shelter dogs. Parasit Vectors. (2017) 10:124–8. doi: 10.1186/s13071-017-2443-7
92. Drake J, Gruntmeir J, Merritt H, Allen L, Little SE. False negative antigen tests in dogs infected with heartworm and placed on macrocyclic lactone preventives. Parasit Vectors. (2015) 8:1–5. doi: 10.1186/s13071-015-0698-4
93. Savadelis MD, Roveto JL, Ohmes CM, Hostetler JA, Settje TL, Dzimianski MT, et al. Evaluation of heat-treating heartworm-positive canine serum samples during treatment with Advantage Multi® for Dogs and doxycycline. Parasit Vectors. (2018) 11:1–8. doi: 10.1186/s13071-018-2685-z
94. Venco L, Genchi C, Colson PV, Kramer L. Relative utility of echocardiography, radiography, serologic testing and microfilariae counts to predict adult worm burden in dogs naturally infected with heartworms. In: Seward RL, editor. Conference Proceedings. AHS 2001 Symposium. Batavia, NY; Illinois: American Heartworm Society. p. 111–24.
95. Falcón-Cordón Y, Montoya-Alonso JA, Caro-Vadillo A, Matos-Rivero JI, Carretón E. Persistence of pulmonary endarteritis in canine heartworm infection 10 months after the eradication of adult parasites of Dirofilaria immitis. Vet Parasitol. (2019) 273:1–4. doi: 10.1016/j.vetpar.2019.07.008
96. Maerz I. Clinical and diagnostic imaging findings in 37 rescued dogs with heartworm disease in Germany. Vet Parasitol. (2020) 283:109156. doi: 10.1016/j.vetpar.2020.109156
97. Rawlings CA, Bowman DD, Howerth EW, Stansfield DG, Legg W, Luempert LG. Response of dogs treated with ivermectin or milbemycin starting at various intervals after Dirofilaria immitis infection. Vet Ther. (2001) 2:193–207.
98. Venco L, McCall JW, Guerrero J, Genchi C. Efficacy of long-term monthly administration of ivermectin on the progress of naturally acquired heartworm infections in dogs. Vet Parasitol. (2004) 124:259–68. doi: 10.1016/j.vetpar.2004.06.024
99. Atkins C. Treating heartworm infection. Ancillary corticosteroid therapy in dogs. Todays Vet Pract. (2012) 2:85–8. Available online at: https://todaysveterinarypractice.com/heartworm-hotline-treating-heartworm-disease-ancillary-corticosteroid-therapy/
100. Mavropoulou A, Gnudi G, Grandi G, Volta A, Kramer LH, Quintavalla C. Clinical assessment of post-adulticide complications in Dirofilaria immitis-naturally infected dogs treated with doxycycline and ivermectin. Vet Parasitol. (2014) 205:211–5. doi: 10.1016/j.vetpar.2014.06.014
101. Carretón E, Morchón R, Montoya-Alonso JA. Cardiopulmonary and inflammatory biomarkers in heartworm disease. Parasit Vectors. (2017) 10:534. doi: 10.1186/s13071-017-2448-2
102. Carretón E, Morchón R, Simon F, Juste MC, Gonzalez-Miguel J, Montoya-Alonso JA. Evaluation of cardiopulmonary biomarkers during classic adulticide treatment versus the American Heartworm Society recommended treatment protocol in dogs infected by Dirofilaria immitis. Vet Parasitol. (2014) 206:55–9. doi: 10.1016/j.vetpar.2014.08.015
103. Carretón E, Morchón R, Simón F, Juste MC, Méndez JC, Montoya-Alonso JA. Cardiopulmonary and inflammatory biomarkers in the assessment of the severity of canine dirofilariosis. Vet Parasitol. (2014) 206:43–7. doi: 10.1016/j.vetpar.2014.08.019
104. Yoon WK, Kim YW, Suh SIL, Choi R, Lee SG, Hyun C. Evaluation of cardiopulmonary and inflammatory markers in dogs with heartworm infection during treatment with the 2014 American Heartworm Society recommended treatment protocol. Parasit Vectors. (2017) 10:535. doi: 10.1186/s13071-017-2427-7
105. Bowman DD, Drake J. Examination of the “susceptibility gap” in the treatment of canine heartworm infection. Parasit Vectors. (2017) 10:513. doi: 10.1186/s13071-017-2433-9
106. Vezzoni A, Genchi C, Raynaud JP. Adulticide efficacy of RM 340 in dogs with mild and severe natural infections. In: Conference Proceedings. AHS 1992 Symposium. Batavia, NY; Illinois: American Heartworm Society. p. 231–40.
107. Kaiser L, Williams JF. Dirofilaria immitis: worm burden and pulmonary artery proliferation in dogs from Michigan (United States). Vet Parasitol. (2004) 124:125–9. doi: 10.1016/j.vetpar.2004.06.015
108. Newbury S, Blinn MK, Bushby PA, Cox CB, Dinnage JD, Griffin B, et al. Guidelines for Standards of Care in Animal Shelters. Association of Shelter Veterinarians (2010). Available online at: https://www.sheltervet.org/assets/docs/shelter-standards-oct2011-wforward.pdf (accessed June 8, 2021).
109. Bourguinat C, Lee ACY, Lizundia R, Blagburn BL, Liotta JL, Kraus MS, et al. Macrocyclic lactone resistance in Dirofilaria immitis: failure of heartworm preventives and investigation of genetic markers for resistance. Vet Parasitol. (2015) 210:167–78. doi: 10.1016/j.vetpar.2015.04.002
110. Wolstenholme AJ, Evans CC, Jimenez PD, Moorhead AR. The emergence of macrocyclic lactone resistance in the canine heartworm, Dirofilaria immitis. Parasitology. (2015) 142:1249–59. doi: 10.1017/S003118201500061X
111. Bourguinat C, Keller K, Bhan A, Peregrine A, Geary T, Prichard R. Macrocyclic lactone resistance in Dirofilaria immitis. Vet Parasitol. (2011) 181:388–92. doi: 10.1016/j.vetpar.2011.04.012
112. Sanchez J, Dharmarajan G, George MM, Pulaski C, Wolstenholme AJ, Gilleard JS, et al. Using population genetics to examine relationships of Dirofilaria immitis based on both macrocyclic lactone-resistance status and geography. Vet Parasitol. (2020) 283:109125. doi: 10.1016/j.vetpar.2020.109125
113. Rohrbach BW, Patton S. Effects of diagnostic test accuracy and treatment efficacy on the occurrence of suspected failure of heartworm prophylaxis in dogs. J Vet Intern Med. (2013) 27:791–7. doi: 10.1111/jvim.12092
114. McTier TL, Pullins A, Inskeep GA, Gagnon G, Fan H, Schoell A, et al. Microfilarial reduction following ProHeart® 6 and ProHeart® SR-12 treatment in dogs experimentally inoculated with a resistant isolate of Dirofilaria immitis. Parasit Vectors. (2017) 10:1–6. doi: 10.1186/s13071-017-2430-z
Keywords: heartworm, Dirofilaria immitis, slow kill, moxidectin, doxycycline, accessible veterinary care, adulticide treatment, melarsomine
Citation: Jacobson LS and DiGangi BA (2021) An Accessible Alternative to Melarsomine: “Moxi-Doxy” for Treatment of Adult Heartworm Infection in Dogs. Front. Vet. Sci. 8:702018. doi: 10.3389/fvets.2021.702018
Received: 28 April 2021; Accepted: 30 June 2021;
Published: 27 July 2021.
Edited by:
Donato Traversa, University of Teramo, ItalyReviewed by:
Viktor Dyachenko, Veterinary Laboratory Freiburg, GermanyAlexandre Bendas, Universidade Federal Rural Do Rio de Janeiro, Brazil
J. Alberto Montoya-Alonso, University of Las Palmas de Gran Canaria, Spain
Copyright © 2021 Jacobson and DiGangi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda S. Jacobson, bGphY29ic29uQHRvcm9udG9odW1hbmVzb2NpZXR5LmNvbQ==
 Linda S. Jacobson
Linda S. Jacobson Brian A. DiGangi
Brian A. DiGangi