- 1Department of Medicine, Surgery and Reproduction, Hassan II Institute of Agronomy and Veterinary Medicine, Rabat, Morocco
- 2Department of Companion Animals, Faculty of Veterinary Medicine, Ghent University, Ghent, Belgium
- 3Department of Comparative Physiology and Biometrics, Faculty of Veterinary Medicine, Ghent University, Ghent, Belgium
- 4Department of Pathology and Veterinary Public Health, Unit of Parasitology, Hassan II Institute of Agronomy and Veterinary Medicine, Rabat, Morocco
- 5College of Veterinary Medicine, Western University of Health Sciences, Pomona, CA, United States
Anaplasma phagocytophilum is a worldwide emerging zoonotic tick-borne pathogen transmitted by Ixodid ticks and naturally maintained in complex and incompletely assessed enzootic cycles. Several studies have demonstrated an extensive genetic variability with variable host tropisms and pathogenicity. However, the relationship between genetic diversity and modified pathogenicity is not yet understood. Because of their proximity to humans, dogs are potential sentinels for the transmission of vector-borne pathogens. Furthermore, the strong molecular similarity between human and canine isolates of A. phagocytophilum in Europe and the USA and the positive association in the distribution of human and canine cases in the USA emphasizes the epidemiological role of dogs. Anaplasma phagocytophilum infects and survives within neutrophils by disregulating neutrophil functions and evading specific immune responses. Moreover, the complex interaction between the bacterium and the infected host immune system contribute to induce inflammatory injuries. Canine granulocytic anaplasmosis is an acute febrile illness characterized by lethargy, inappetence, weight loss and musculoskeletal pain. Hematological and biochemistry profile modifications associated with this disease are unspecific and include thrombocytopenia, anemia, morulae within neutrophils and increased liver enzymes activity. Coinfections with other tick-borne pathogens (TBPs) may occur, especially with Borrelia burgdorferi, complicating the clinical presentation, diagnosis and response to treatment. Although clinical studies have been published in dogs, it remains unclear if several clinical signs and clinicopathological abnormalities can be related to this infection.
Introduction
Canine granulocytic anaplasmosis (CGA) is an emerging zoonotic tick-borne disease that is distributed worldwide. The causative agent, Anaplasma phagocytophilum, is an obligate intracellular gram-negative alpha-proteobacterium that develops within granulocytic cells. It is usually transmitted by ticks belonging to the genus Ixodes and it causes disease in several mammalian species (1, 2). In the USA, both canine and human exposures have progressively increased from 2008 to 2010 with the number of reported human cases increasing by 53% during this period (3, 4). Data from the USA Center for Disease Control and Prevention (4) and Morbidity and Mortality Weekly Report (MMWR) reported 36,342 human cases between 2010 and 2018 and almost a 12-fold increase during this same period (4). Currently, human granulocytic anaplasmosis (HGA), is considered amongst the three most important vector-borne disease (VBD) in the USA with Lyme borreliosis and Zika virus (5, 6) and is increasingly being diagnosed in several European and Asian countries (7, 8).
The focus on canine VBDs has increased the past decade as they represent an important threat to both canine and human health (9). Because of their proximity to humans, dogs may serve as reservoirs of vector-borne pathogens, a source of infection for vectors, mechanical transporters of infected vectors, and as sentinel indicators of regional infection risk (2, 3, 10–15). Furthermore, the strong molecular similarity between human and canine isolates of A. phagocytophilum in Europe and the USA (16–21) and the positive association in the distributions of human and canine cases in the USA emphasizes the use for dogs as sentinels in epidemiological studies (3, 4, 9, 15, 22, 23).
The lack of specific clinicopathological signs, the frequent rapid evolution and positive prognosis even without treatment, the prompt response to a commonly used antibiotic and the possibility of coinfections (24–28) all make the diagnosis of CGA challenging for veterinarians. Description of signs and laboratory abnormalities associated with A. phagocytophilum infection in dogs is mostly available from Europe and North America. Although some studies have described the most common manifestations of CGA (13, 24–35), it remains unclear if some clinical signs and clinicopathological abnormalities are related to this infection. In this paper, we provide an overview of the current knowledge on the worldwide epidemiological features of A. phagocytophilum focusing on dogs, and describe the clinicopathological aspects of CGA with an emphasis on missing data.
Description of Anaplasma phagocytophilum
Classification
Anaplasma phagocytophilum is a bacterium belonging to the family of Anaplasmataceae in the order of Rickettsiales (36). The phylogenetic molecular analysis based on the 16S rRNA and the groEL genes sequencing in addition to morphologic and phenotypic characteristics have led to the reorganization of the family of Anaplasmataceae and the reclassification of some agents. Consequently, the name A. phagocytophilum was given in 2001 to three previously distinct agents, i.e., the agent that causes equine granulocytic anaplasmosis (Ehrlichia equi), the agent that causes tick-borne fever or pasture fever in sheep and cattle (Ehrlichia phagocytophila) and the agent that causes HGA [formerly human granulocytic ehrlichiosis (HGE)] (1). The renaming of these three agents as A. phagocytophilum has been controversial because of differences in their host tropism and cell target from other Anaplasma species, such as Anaplasma marginale (37). Additionally, although these three agents share genetic, antigenic and biological characteristics (1), they are considered phenotypic variants due to differences in their distribution, prevalence, virulence and target host species (38, 39).
Morphology and Genome
Anaplasma phagocytophilum typically exhibits coccoid to ellipsoid shapes measuring ~0.2–2.0 μm in diameter. The bacteria infect myeloid cells primarily neutrophils (and occasionally eosinophils), forming intracytoplasmic inclusions derived from the host cell membrane measuring 1.5–2.5 μm, called “morula” (from Latin “morum”: mulberry) (1, 40).
The A. phagocytophilum genome is composed of a single circular double-stranded chromosome. The complete genomic sequence is estimated at 1.47 megabases (Mb) and was published on GenBank in 2006 (NC007797) (19, 36). Despite its apparently simple genome, A. phagocytophilum exhibits an extensive genomic diversity (19, 41, 42). More than 500 partial A. phagocytophilum pseudogene sequences derived from human, ticks and animals from several US, European and Asian regions are available in GenBank (19). Moreover, twenty complete A. phagocytophilum genomes have been sequenced including 16 American and four European strains. However, genomes from only a few different strains per host species are available (aside from humans), underscoring the lack of information on strain diversity within different host species (19, 36).
Genetic Variability
Genetic variability between strains may explain the ecological complexity, the host tropism diversity, the differences in incidence and clinical presentation, severity and evolution of the disease documented in different countries (42–47). Many studies demonstrated different virulence and hosts tropism of specific A. phagocytophilum strains (17, 41–50). However, the host specificity of strains seems to be restricted and multiple infections with different strains are often observed. Farm and large wild animals, small mammals and ticks were especially prone to carrying multiple genetic variants. In humans and domestic animals double infections are not so frequent (51). The 16S rRNA gene nucleotide sequences analysis discriminated 15 worldwide variants differing in a variable fragment located near the 5' end of the gene. Among them, two are pathogenic for human and abundant all over the world (52). In the USA, several variants have been identified based on the sequencing of the 16S rRNA and the only pathogenic variant to humans (AP-ha) is also able to induce the disease in dogs, horses and mice but not in cattle. In Europe, other variants have been identified in humans and the AP-ha variant was also detected in wild ruminant species (41–43, 48, 49). Strains infecting domestic ruminants in Europe and white-tailed deer in the USA seem to genetically differ from those infecting humans, horses and dogs (44, 50). In Washington State, five different 16S rRNA variants (named WA1–5) that differed at four nucleotide positions were identified from dogs displaying clinical signs consistent with CGA. All WA variants were distinct from those identified in sheep in Norway and llama-associated ticks but one was identical to equine and human variants (24). In another European study, seven different 16S rRNA variants were identified from dogs, with the two most common variants showing statistically significant differences in the frequency of clinical signs and hematological abnormalities, which suggests possible differences in strain pathogenicity (45). Finally, a recent study showed that dogs can be naturally infected concurrently with A. phagocytophilum variant 1, variant 4, and HGE agent (53). The pathogenic role of the classic sheep variant, A. phagocytophilum variant 1, in the canine species is uncertain. Previous studies showed that the “HGA agent” appears to be more pathogenic for dogs than other variants (45).
The 16S rRNA gene was considered too conserved for use in the phylogenetic analysis of different strains of A. phagocytophilum. It has a poor resolution and failed to discriminate between ecotypes circulating in wild ruminants compared to other animals. Furthermore, the 16S rRNA sequence analysis could not categorize human-infective isolates in order to detect virulent strains and was unable to distinguish variants according to their geographic origin (43, 54–56). As such, other genes have been proposed to study the genetic variability of A. phagocytophilum including msp4, ankA, groEL operon, msp2/p44, pfam01617 superfamily, and drhm genes (19–21, 50, 56–59). Sequencing different genes revealed similarities between human and canine isolates, suggesting that dogs and humans may be infected by the same strains (16–21, 24, 45, 53, 60–62).
Vectors
Although several transmission modes have been reported (mostly in humans) (63–66), A. phagocytophilum is commonly transmitted to people and domestic animals through tick bites (67). It is naturally maintained in complex and poorly understood enzootic tick-wild animal cycles (55, 59, 68) and is transmitted most frequently by ticks of the Ixodes persulcatus complex. These ticks are commonly found in the northern hemisphere and their occurrence depends on climatic conditions (between 10 and 30°C, and >80% relative humidity) and the availability of hosts (49, 69).
In the USA, several ixodid ticks transmit this pathogen, depending on the geographic location. The main vector in the humid forests of the upper midwestern, north central and northeastern regions is Ixodes scapularis whereas Ixodes pacificus is located in shrub forests and deserts of the western USA (70–72). The prevalence of A. phagocytophilum DNA among ticks varies from <1% up to 50% throughout the country (73–76). Other tick species have been reported to be infected with A. phagocytophilum, such as Amblyomma americanum and Dermacentor spp., and Ixodes spinipalpis and Ixodes dentatus are recognized as competent vectors (77–81). Other Ixodes species including Ixodes angustus, Ixodes ochotonae, and Ixodes woodi are suggested to act as vectors for the bacterium (82, 83). In central and southern America, very few studies are published on the prevalence of A. phagocytophilum among ticks. However, among the three available studies, none have detected the DNA of this bacterium in Ixodes spp. ticks. In contrast, its DNA has been amplified from Rhipicephalus sanguineus, Amblyomma cajennense, Amblyomma dissimile, Amblyomma maculatum, Dermacentor variabilis (84–86). Amblyomma spp. and D. variabilis were positively correlated with A. phagocytophilum infection in Brazil and Mexico (84, 86).
In Europe, the most common vector is Ixodes ricinus (69), which is widely distributed from western Europe to central Asia. This tick lives mostly in humid wooded habitats and pastures and is rarely encountered in the Mediterranean region or in mixed or deciduous forests except at high altitudes (67). The prevalence of A. phagocytophilum DNA among I. ricinus ticks in Europe varies from <1 to 76.7% (87, 88). Other Ixodes spp. ticks seem to be involved in epidemiological cycles that are distinct from those involving I. ricinus (55, 89, 90). In addition, the DNA of this bacterium has been detected in several other tick species in Europe including Dermacentor reticulatus, Haemaphysalis concinna, Hyalomma marginatum, Ixodes ventaloi, and Ixodes trianguliceps (58, 91–95). Rhipicephalus species were also infected by A. phagocytophilum and could act as competent vectors in the eastern Mediterranean area (96–99). Ixodes persulcatus is another competent vector of A. phagocytophilum in eastern Europe and Asia, with rates of DNA detection up to 16.7 and 21.6%, respectively (100, 101).
Although I. persulcatus is considered the primary vector in Asia, A. phagocytophilum DNA has been detected in several other tick species including Ixodes nipponensis, Ixodes ovatus, Rhipicephalus turanicus, Rhipicephalus haemaphysaloides, H. marginatum, Boophilus kohlsi, Dermacentor silvarum, and several Haemaphysalis species (96, 102–106). Molecular investigations indicated that I. ovatus, Dermacentor silvarum, Hae. concinna, Haemaphysalis longicornis, Rhipicephalus microplus, R. sanguineus, and Dermacentor nuttalli might be involved in the transmission of A. phagocytophilum in China (8, 107–109).
In North Africa, one study in Morocco and Tunisia detected A. phagocytophilum DNA in 1 and 3% of I. ricinus and Hyalomma detritum, respectively (110). Two separate studies detected DNA in R. sanguineus from free-roaming dogs in Egypt and H. marginatum from horses in Tunisia with prevalence rates of 13.7 and 2.3%, respectively (111, 112). These studies indicate that A. phagocytophilum is likely to circulate in a wide variety of ticks, but their involvement in transmitting the bacterium to host has yet to be established (112).
Distribution and Prevalence of Anaplasma phagocytophilum
Anaplasma phagocytophilum has a worldwide distribution and endemic areas include some regions of the USA (northeastern and mid-Atlantic, Upper Midwest, and Pacific Northwest states), Europe and Asia (China, Siberian Russia, and Korea). These regions correspond to occurrence areas of I. persulcatus group ticks (12, 13, 24, 29, 113). Several prevalence studies in dogs have been conducted in various American, European, Asian and African countries (Table 1). However, data are lacking in large parts of Asia, Africa, South America and Australia. The geographic variation in tick exposure, the differences in inclusion criteria to select dog populations, and the use of different serologic tests [i.e., immunofluorescent antibody test (IFAT), enzyme-linked immunosorbent assay (ELISA) or Western blot] make comparison between studies difficult (234, 235). In addition, cross-reactivity with the most important other Anaplasma species infecting dogs, i.e., Anaplasma platys, is reported to occur for both IFA and ELISA (1, 9, 120, 121, 236–241). Therefore, in regions where both pathogens could be present (southern USA states, southern Europe, South America, Asia, and Africa), seropositivity may not necessarily reflect exposure to A. phagocytophilum and potential overestimation of the true prevalence and distribution can occur (9, 162, 189, 198, 234, 236, 238, 241). As a result, PCR-based assays are necessary to determine which of the two agents is responsible for positive serologic test results in regions where both bacteria are present (241). In areas where the Ixodes tick vector is less prevalent or absent, a positive Anaplasma spp. serologic result could be the result of A. platys exposure (164). Less frequent and minor serological cross-reactions were described at low titers between A. phagocytophilm and Ehrlichia species (i.e., Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia ewingii, and Neorickettsis sennetsu formerly Ehrlichia sonnetsu), especially with hyperimmune sera, when using IFA and immunoblot assay (1, 29, 39, 121, 127, 242, 243). However, it is not clear whether the cross-reactivity with E. canis was attributable, in part, to antibodies against A. platys because dogs are sometimes exposed to both E. canis and A. platys (164, 240). In contrast, no cross-reactivity has been documented between Anaplasma spp. and Ehrlichia spp. when using the point-of-care dot ELISA (234, 240).
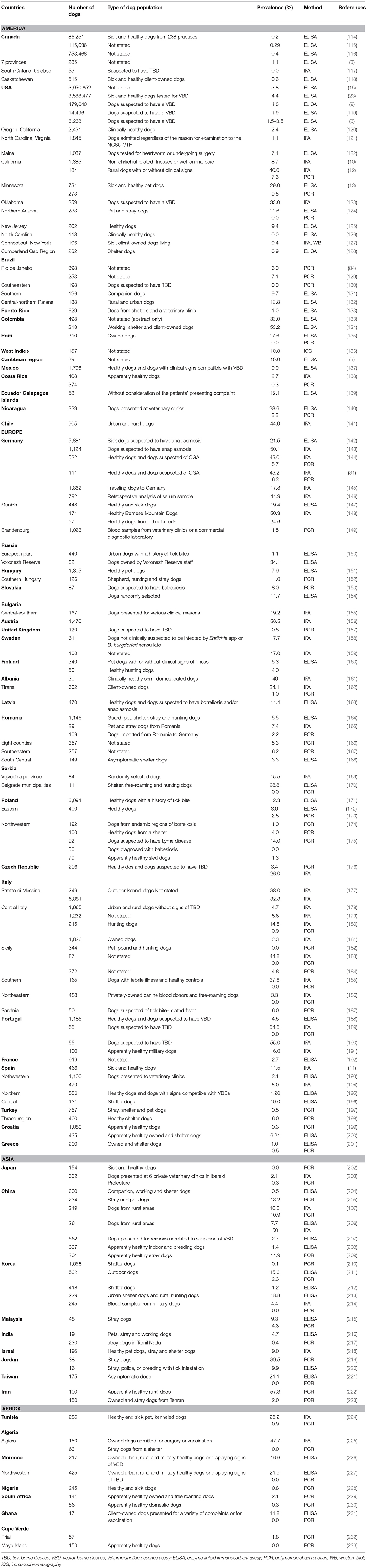
Table 1. Prevalence of Anaplasma spp. (A. phagocytophilum and A. platys) antibodies and/or DNA detection of A. phagocytophilum in blood samples from dogs in several countries.
The first CGA cases in the USA were detected in California; then, the exposure of dogs to this organism has been recorded in more than 39 USA states and highest rates were noted in the upper Midwestern, northeastern and western states. Serologic surveys revealed prevalence values of Anaplasma spp. antibodies ranging from 0.0 to 40.0% (3, 9, 10, 12, 13, 15, 23, 119, 120, 122–126, 236, 242, 244, 245). Five countrywide serologic studies showed an overall prevalence of Anaplasma spp. of 1.9 to 4.8% with the highest rates recorded in northeastern regions (3, 9, 15, 23, 119). The study that found a prevalence rate of 1.9%, used species-specific peptides to detect canine antibodies to A. phagocytophilum (3). In addition, cases confirmed by PCR were diagnosed in several USA states (12, 13, 24, 26, 27, 29, 124, 245–247). In the USA, over 100,000 and 220,000 dogs were seropositive to Anaplasma spp. in 2015 and 2019, respectively (248, 249). Two recent studies analyzing regional trends of Anaplasma spp. exposure in dogs showed that seroprevalence increased broadly in the northeastern, upper midwestern states, northern California, mid-atlantic coast and southern Oregon (249, 250). In Canada, six serologic surveys on Anaplasma spp. are available (Table 1) (3, 114–118), and six cases of CGA from Vancouver Island (251), Saskatoon (252) and Montreal (253) were confirmed by DNA detection. In Latin America and the Caribbean, the seroprevalence of Anaplasma spp. ranges from 1.0 to 53.2% (Table 1) (133, 134, 254). In addition, two studies and a case report have detected the DNA of A. phagocytophilum in Brazil (Table 1) (129, 255).
In Europe, Anaplasma spp. seroprevalence has been reported in almost all countries with rates ranging from 1.1 to 56.5% (143, 148, 150, 183, 190). The detection of A. phagocytophilum DNA has also been reported mostly from central and northern countries (Table 1) with prevalence rates up to 14.2% (174). Additionally, several cases of CGA have been described (25, 28, 30–32, 34, 256–262).
In Asia, Anaplasma spp. seroprevalence is available from China, Korea, Malaysia, Taiwan and Israel and range from 1.2 to 24.7% (Table 1) (212, 214). Anaplasma phagocytophilum DNA has also been detected in dogs with prevalence rates up to 39.5 and 57.3% in Jordan and Iran, respectively (219, 222).
In Africa, only a few prevalence studies have been published on Anaplasma spp. in dogs (Table 1). Seroprevalence rates recorded in African countries range from 11.8 to 47.7% (Table 1) (225, 231). Similarly, very limited studies have investigated A. phagocytophilum infection in dogs in this continent. The DNA of this bacterium has been detected in Tunisia, Nigeria, Cape Verde and South Africa (Table 1) (224, 228, 229, 232) but not in Algeria and Morocco (225, 227). In addition, an Anaplasma species closely related to A. phagocytophilum was detected in blood samples from South African dogs based on 16S rRNA gene sequencing (263) whereas all dogs from Algeria, Ghana and Maio Island tested negative by PCR (Table 1) (225, 231, 233).
Epidemiological Role of Dogs
Several wild and domestic animals are receptive to A. phagocytophilum infection. However, the disease has been reported only in a few species including domestic ruminants, horses, cats, dogs and humans (22, 24, 63, 264–269). Although dogs are susceptible to A. phagocytophilum infection, they are mostly recognized as incidental hosts and their role as potential reservoirs is still controversial (24, 270). As A. phagocytophilum is an obligate intracellular bacterium, its reservoirs should be animal hosts permitting its survival, particularly outside the activity period of its vectors (271). To be considered as a host reservoir, a host must be fed on by an infected vector tick at least occasionally, take up a critical number of the infectious agent during the bite by an infected tick, allow the pathogen to multiply and survive for a period in at least some parts of the body, and allow the pathogen to find its way into other feeding ticks (272, 273). Therefore, the detection of pathogens or their DNA in animal hosts is not enough to consider them as reservoir hosts (274).
Dogs are considered unlikely reservoir hosts due to the probable short duration of bacteremia (<28 days) and uncertainty regarding their ability to host enough nymphal tick stages to contribute to the spread of the bacterium (2, 67). In Austria, no significant difference in the seroprevalence of A. phagocytophilum among owners of seropositive pets and owners without pets was observed, suggesting that pets are not a source of infection for humans (275). However, wild and domestic carnivores are considered the primary source of tick-borne zoonotic agents to humans (276) and contact with pet cats and dogs has been proposed as a risk factor for tick exposure and tick-borne disease among humans (277, 278). Moreover, according to some authors, almost all studies investigating the role of dogs in the transmission of tick-borne diseases (TBDs) focused on companion dogs. These animals are usually treated for ectoparasites, have limited free access to the outdoors and host reservoir habitats, and are less exposed to ticks compared with hunting, stray or shelter dogs. Therefore, these studies may not accurately reflect the public health risk associated with dogs in endemic areas (152). Others suggested that domestic animals including dogs could be considered as reservoir hosts of A. phagocytophilum in Europe especially in urban areas (18, 270, 279–282). In a study from Hungary, the prevalence of A. phagocytophilum DNA in stray dogs was higher than in several studies from other European countries (152). In addition, two studies reported high prevalence rates of A. phagocytophilum DNA in dogs suspected to have Lyme disease and rural dogs from Poland and China, respectively (107, 174). Anaplasma phagocytophilum was also the most frequently detected bacterium by PCR in stray dogs that lived in close contact with domestic animals and humans in rural and peri-urban areas of the Mediterranean zone of Jordan (219). In addition, high prevalence rates of A. phagocytophilum DNA was found in I. ricinus collected from dogs in Belgium and Poland, and R. sanguineus (adult and nymphs) from free-roaming dogs in Egypt (111, 280, 283). Moreover, A. phagocytophilum DNA was detected in experimentally infected dogs during 60 days without immunosuppressive drug, and the canine immune response seems to have evolved to only partially control infection, suggesting a longer bacteremia possibly allowing timely transmission to the vector (18, 284). Based on these results, dogs could act as potential reservoir for the bacterium at least in some regions, but further studies are needed.
The geographical distribution of canine infection seems to parallel the distribution of HGA in the USA with a positive association of human and canine cases in many states (3, 23). Indeed, several studies found the highest prevalence rates of A. phagocytophilum antibodies in dogs from the upper midwest, northeast, and mid-atlantic, which correlate with areas where the highest incidence of human anaplasmosis were reported (3, 4, 9, 15, 22, 23). In addition, the estimated regression coefficient for the endemic risk factor in the contiguous USA model was positive and significant. This implies a higher prevalence among dogs living in areas where HGA is endemic (15). Furthermore, a study has evaluated regional and local temporal trends of canine Anaplasma spp. (A. phagocytophilum and A. platys) exposure using a Bayesian spatio-temporal binomial regression model for analyzing serologic test results. In this study, similarity was found between temporal trends in canine Anaplasma spp. seroprevalence and the reported incidence rate of HGA (249). Finally, human and canine strains of A. phagocytophilum were similar according to several gene sequencing studies, and human isolates have been reported to induce clinical disease in dogs in both Europe and the USA (16–21). Therefore, in addition to the possible role of dogs as potential reservoir hosts, the prevalence data of A. phagocytophilum infection in dogs provides important information on the incidence, risk factors, exposure sources, and real-time risk of exposure for human infection (3). More generally, several studies have documented the utility of using dogs as sentinels for human vector-borne diseases (VBDs) (14, 17, 18).
Pathogenesis of Anaplasma phagocytophilum Infection
Anaplasma phagocytophilum is transmitted by ticks to their hosts within 24–48 h of feeding time (285–288) but establishment of infections in dogs is apparently dependent on a minimum inoculation dose (288). Bacteremia, however, develops 4–7 days after the tick bite during natural infection or 3–4 days after experimental blood inoculation, suggesting that the bacterium remains at undetectable levels in the blood or replicates in other cells in the early stages of infection (69). Cell surface analysis suggested that the endothelial cells of the microvasculature provide an excellent site for A. phagocytophilum dissemination to peripheral blood granulocytes. Endothelial cells may play a crucial role in the development of persistent infections and are stimulated to express surface molecules and cytokines in a dose-dependent manner that may lead to inflammatory responses at the site of infection (289). After inoculation, A. phagocytophilum exhibits a biphasic developmental cycle in which the infectious small dense-cored cells bind to host cellular targets and enter the cytoplasm of neutrophils by endocytosis. After, the non-infectious reticulate cells multiply by binary fission within phagosomes until forming morulae. After 28–32 h, replication ceases and reticulate cells re-transition to dense-cored cells that are released after cell lysis to initiate the next wave of infection and possibly spread to multiple organs (40, 290, 291).
Anaplasma phagocytophilum has several strategies to dysregulate the bactericidal functions of neutrophils and ensure its survival and replication. This bacterium regulates host defense and antimicrobial mechanisms by a direct interaction with specific gene regulatory regions in the nucleus of the neutrophil, decreasing endothelial adherence, mobility, transmigration, phagocytic activity, and degranulation. It can also alter the respiratory and oxidative burst mechanism of neutrophils, delay apoptosis and increase the inflammatory recruitment of new neutrophils (289, 292–297). In addition, the antigenic variation of the immunodominant surface proteins msp2/p44 enables the bacterium to evade the specific immune response and to subvert the adaptive immune response (297). Neutrophils circulate for 10–12 h before they enter tissues and undergo apoptosis, which may lead to the destruction of the pathogens. Therefore, the decreased endothelial adherence and delayed apoptosis both enhance the bacterial survival and the replication to form morulae in a normally short-lived, terminally differentiated granulocytic cell. Furthermore, the impaired neutrophil function can result in an immune deficiency, predisposing patients to opportunistic infections (293–295). Anaplasma phagocytophilum was suggested to possibly manipulate the host endoplasmic reticulum stress signals to facilitate intracellular proliferation and infection of surrounding cells before or after host cell apoptosis (298).
The immune response induced by A. phagocytophilum is thought to play an important role in the initial control of the disease but may also induce inflammatory injuries associated with granulocytic anaplasmosis. Indeed, the absence of A. phagocytophilum control induces a clear rise in inflammatory lesions, which is considered the major pathogenic effect in humans and murine models (299–301). In dogs, the hematological modifications associated with A. phagocytophilum infection are similar to those induced by other members of Ehrlichia or Anaplasma genera, although they infect different blood cells, suggesting that the major mechanism of cytological injuries is related to an immunological response or to substances secreted from the bacteria (302, 303). Anaplasma phagocytophilum induces an upregulation of chemokine and pro-inflammatory cytokine [IL-8, macrophage inflammatory protein (MIP)-1a, MIP-1b, monocyte chemoattractant protein (MCP)-1] expression in vitro, which attracts leukocytes and inhibits hematopoiesis leading to myelosuppression (304, 305). In mice, several leukocyte populations expand during infection including NK and NKT cells followed later by CD4 and CD8 T lymphocytes and the immune response proceeds mostly through production of interferon gamma (IFN-γ), commonly produced by T lymphocytes (301–303, 306). In humans, the manifestation of severe disease is associated with hypercytokinemia and macrophage activation or hemophagocytic syndromes (MAS/HPS). The underlying pathogenesis of MAS/HPS is poorly understood; however, it is frequently associated with a defective function or depletion of cytotoxic cells and is driven mostly by the persistent stimulation of cytokine production, especially the macrophage-activating IFN-γ (307–309). The clear role of IFN-γ in the pathogenesis of the disease is demonstrated by the observation that the lack of this molecule in A. phagocytophilum-infected mice resolves inflammatory tissue injury (300).
Several studies have confirmed the role of the IFN-γ in mediating both the pathology and early control of bacteria, although it is not essential for bacterial clearance. Other protective mechanisms might be involved in the control of A. phagocytophilum infection, as some infected mice lacking IFN-γ are able to survive (292, 300–303, 306). Data suggest that the humoral immunity may also play an important role in the clearance of ehrlichial infections, as passive immunization has a moderately protective effect. Moreover, severely immunocompromised mice that lack both B and T cells remained persistently infected, as opposed to mice lacking only T cells, which were able to control the infection (301, 310). Experimentally infected dogs develop serologic responses (immunoglobulin G) 7 days after inoculation. However, positive A. phagocytophilum PCR assay results persist up to 42 days despite the high antibody response suggesting that the humoral response is not sufficient to clear the infection (284). It appears that the innate immune mediators used to activate phagocytes to kill other intracellular bacteria (reactive nitrogen intermediates, Toll-like receptor 2 and 4, MyD88, phagocyte NADPH oxidase) do not play a crucial role in A. phagocytophilum clearance and may contribute to the observed pathology (301, 303, 311).
Canine Granulocytic Anaplasmosis
Clinical Signs
The discrepancy between the high seroprevalence and the relatively low number of sick dogs in endemic areas suggests that most infected dogs remain apparently healthy or develop a mild self-limiting illness (9, 10, 13, 25). The severity of the disease varies from mild subclinical to severe acute forms (24, 34), with severe clinical presentation often associated with co-infections, the immune response of the host and the variability of strains pathogenicity (13, 18, 33, 45).
CGA is a multi-systemic unspecific acute illness characterized by many clinicopathological modifications due to the possible involvement of several body systems. After an incubation period of 1–2 weeks, the most frequently observed clinical signs include fever, lethargy, inappetence or anorexia, weight loss and musculoskeletal pain or discomfort (Table 2) (24, 25, 29, 30, 35, 53, 60, 312, 314). More than 75% of dogs display lethargy and inappetence or anorexia (24, 26, 29, 30, 33, 35). Lethargy has been reported in almost all infected dogs (13, 24–26, 29–31, 33, 35, 45) and was the most frequent clinical signs in several studies (24–27, 29, 33, 35). It was also reported to be disproportionately severe in comparison with the lack of other clinical abnormalities in a case report (247). Fever is both inconstant and variable with frequencies ranging from 46 to 100% (24, 33, 35) and values from 39.2 to 41.5°C (25– 27, 30, 35, 246, 251, 252, 257, 258, 261, 314). Fever generally coincides with the peak of bacteremia and lasts less than a week (33). Musculoskeletal pain or discomfort has been described in more than 50% of dogs and manifests in reluctance to move, weakness, stiffness, lameness, and myalgia. However, <10% of dogs have overt joint pain (29, 30). Lameness and joint swelling were reported in 11–34% (25, 34, 35) and 6–62% cases (24, 26, 35) respectively. They are more likely related to neutrophilic inflammation (25, 26, 244, 314, 315), but immune-mediated mechanisms also might be involved (244, 315). In a retrospective study, polyarthropathy (50%) was more frequently observed than monoarthropathy (5%) (27). In a report from California investigating the prevalence of tick-borne infections in dogs with polyarthritis and/or thrombocytopenia, A. phagocytophilum was the most frequently detected pathogen (244). Lymphadenopathy, splenomegaly and hepatomegaly were frequent findings in CGA (25, 26, 29, 35, 53, 314, 315). Splenomegaly was reported in 12–100% of naturally infected dogs (25, 26, 35). In canine and murine models of A. phagocytophilum infection, lymphadenopathy and splenomegaly are due to reactive lymphoid hyperplasia, with concurrent extra-medullary hematopoiesis in the spleen, enlarged activated lymph nodes and increased numbers of macrophages and plasma cells in the red pulp (284, 285, 314). In experimentally infected dogs, non-specific reactive hepatitis and mild periportal inflammatory lesions were also described (284, 314) and lesions tended to be more pronounced in dogs euthanized in the acute stage (314).
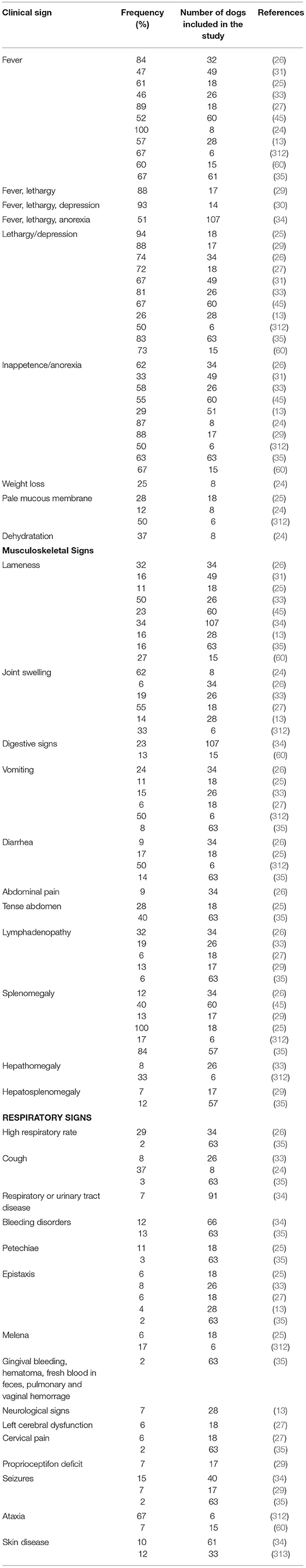
Table 2. Clinical signs associated with canine granulocytic anaplasmosis after natural infection and corresponding frequency recorded in several studies.
Other clinical signs include gastro-intestinal signs, polyuria, polydipsia, respiratory signs, pale mucous membranes, bleeding disorders, uveitis, scleral congestion, polymyositis, and neurological signs (Table 2) (13, 24, 26, 29, 30, 32, 33, 35, 53, 60, 256, 257, 312, 314, 316). Gastrointestinal signs include diarrhea, nausea, vomiting, and abdominal pain (25–27, 33–35, 312), but their origin is still unknown. In two cases of CGA displaying gastrointestinal signs, associated pancreatitis was suspected based on biochemistry and abdominal ultrasound abnormalities (246, 252). Respiratory signs include dyspnea, tachypnea, and coughing, which is usually infrequent, soft and non-productive (35, 238, 314). One patient displayed coughing and presented interstitial patterns on thoracic radiographs associated with focal alveolar patterns, and showed morulae within neutrophils upon microscopic examination of tracheal lavage specimen (238). Bleeding disorders including petechiae, gingival bleeding, melena, fresh blood in feces, epistaxis, pulmonary hemorrhage, vaginal hemorrhage or hematoma (35) are infrequent in dogs infected with A. phagocytophilum, unlike other rickettsial infections, such as E. canis, A. platys, and Rickettsia rickettsii infections or other infectious diseases, such as aspergillosis, bartonellosis, and leishmaniasis. Indeed, only 3–11% of CGA cases displayed epistaxis (25, 316). In two separate reports, dogs with CGA that presented with epistaxis had mild to moderate thrombocytopenia that could not explain the bleeding disorder. In addition, these dogs were seronegative to B. burgdorferi, E. canis, and Dirofilaria immitis, but other concurrent diseases were not ruled out. Therefore, other factors than thrombocytopenia may cause epistaxis, such as an infection-induced vasculitis (27, 33). Similarly, another report described two dogs with bleeding disorders associated with A. phagocytophilum infection that displayed platelet counts within the reference range (35). Although neurological signs were reported to occur in CGA (35), no studies investigating this association have confirmed the infection by PCR. Moreover, two studies failed to demonstrate an association between A. phagocytophilum infection and neurological signs (317, 318). Consequently, A. phagocytophilum seems to be a rare cause of neurological disease in dogs and other potential etiologies or concurrent diseases should be ruled out before a final diagnosis of CGA. Anaplasma phagocytophilum infection is also suspected to induce skin lesions in dogs (34, 313). In one study that investigated skin-associated lesions in seropositive dogs, four of 12 showed positive DNA amplification from skin lesions. The most frequent lesions identified in these dogs included erythema, papules and plaques that resolved after doxycycline therapy (239). Cutaneous lesions were also present in seropositive but PCR-negative dogs (313). In a previous case report, one dog positive to A. phagocytophilum by serologic tests, PCR from blood and post-mortem spleen samples, was presented first for skin problem including pruritus, hair loss and seborrhea in association with regenerative anemia, leukocytosis and thrombocytopenia. Ehrlichia canis and E. chaffeensis exposure were serologically excluded (256). The lack of typical clinical signs and thrombocytopenia in dogs with PCR-positive skin lesions could be suggestive of a persistent infection as reported in studies in sheep, suggesting that skin could be a site of persistence of A. phagocytophilum (313).
Evolution of the Disease
CGA is currently considered to be an acute disease. Clinical signs usually develop during the bacteremic phase (24, 25, 29, 30) and the duration of the disease is variable. In a retrospective study, the duration of illness ranged from 1 to 14 days with a median duration of 3 days (27). Two studies demonstrated that the majority of dogs were sick for <7 days prior to diagnosis (26, 35). However, the duration of clinical signs ranged from 1 day to 2 months (26). In another report, the duration of illness ranged from 1 to 8 days, but one dog remained infected for a month before the diagnosis was established (30).
Chronic or persistent A. phagocytophilum infection has not been demonstrated in naturally infected dogs and is still controversial (2, 24, 53, 319). In contrast, experimental studies showed a persistent infection in dogs for more than several months to almost a year (18, 284, 320–324). These studies support the findings of another report that demonstrated that dogs could have long-lasting infections with acute flare-up (30) whereas another one failed to demonstrate a chronic infection in experimentally infected dogs (324). The results of the latter study differ from those of three other reports in which repeated amplification of A. phagocytophilum DNA occurred in some dogs probably because of the differences in the way of inoculation. Indeed, in contrast to the other experimental studies in which the bacterium had been inoculated intravenously to the dogs (18, 284, 320–322), in Contreras et al. (324), dogs were infected through tick bites after Ixodes spp. infestation. A 1 year persistence of A. phagocytophilum infection has been described in a naturally infected Rhodesian ridgeback dog (53). In addition, some authors consider the possibility of a chronic phase characterized by more localized clinicopathological signs (such as lameness and proteinuria) that could be associated with immune-mediated mechanisms secondary to persistent antigen stimulation (34). Studies on E. canis infection in dogs showed that the spleen is probably the organ that harbors bacteria for the longest period and is the best source for the diagnosis of carrier state by PCR (325). Similarly, the spleen remained PCR-positive in monkeys and mice experimentally infected with human strains of A. phagocytophilum (299, 326).
The prognosis of the disease in dogs is usually favorable with a rapid remission after doxycycline therapy (24, 26–28, 35, 324). However, some fatal cases have been reported (33, 35, 256, 257). Among the 12 fatality cases reported, five died of immune-mediated hemolytic anemia (IMHA) complicated by disseminated intravascular coagulation (DIC) (33, 256, 257). Two of these dogs were seropositive for Neorickettsia risticii, R. rickettsii, and B. burgdorferi (33). One was euthanized after 14 days because of IMHA and another one died because of epileptic seizures after 3 days (35).
Coinfections
Coinfection with multiple VBPs in dogs appears more frequent in endemic areas (9, 13). In a large retrospective serologic study carried out in North America and the Caribbean, exposure to up to five vector-borne pathogens (VBPs) was detected in the same dogs (3). In a kennel of North Carolina, 40% of dogs had serologic evidence of exposure at the same time with Anaplasma spp., Babesia canis, Babesia vinsonii, E. canis, or R. rickettsii (236). In another study, 16.5% of USA dog samples were found to be seropositive for more than one pathogen (119). Two serologic surveys showed that 1.32 and 14.3% of dogs had antibodies against two pathogens in Italy and Morocco, respectively (178, 226). In Tunisia, 22.4% of dogs were seropositive for E. canis and A. phagocytophilum (224). In Algeria, coinfections by A. phagocytophilum and 1–3 other pathogens were higher in stray than client-owned dogs (225). Two studies investigated the association between co-infections with several VBPs and the occurrence on clinical canine leishmaniosis (327, 328) and one reported a statistical association between dogs with clinical leishmaniosis stages III and IV and the seroreactivity to A. phagocytophilum in Spain (327). Coinfection with B. burgdorferi and A. phagocytophilum is frequently described in dogs, probably because pathogens are transmitted by Ixodid ticks and maintained in sylvatic cycles with the same rodent reservoir (13, 33, 225, 329–331). In the USA, almost 22% of A. phagocytophilum-seropositive samples were also seropositive for B. burgdorferi (240). The prevalence of seropositive dogs to both pathogens was as high as 45% (12, 33). The ability of co-infected I. scapularis ticks to transmit B. burgdorferi and A. phagocytophilum was lower compared with transmission of either agent by singly infected ticks (331).
Experimental studies in mouse and human case reports of A. phagocytophilum and B. burgdorferi coinfection have described an enhanced severity and complexity of clinical signs along with an increased likelihood of disease compared with single infections (13, 329, 330, 332). Similarly, dogs seropositive for both agents (43%) were more likely to display clinical signs than those seroreactive to either A. phagocytophilum (25%) or B. burgdorferi (9%) (13). Experimental studies in rodents have demonstrated that coinfection modulates the host immune response to A. phagocytophilum and the production of interleukins (ILs), decreases IFN-γ levels and the number of CD8+ T cells which leads to more severe clinical signs, increases pathogen burdens in blood and tissues, and induces more persistent infections (13, 329, 330, 333). Furthermore, the interaction of both pathogens at the blood-endothelial cell interface seems to be a critical point in pathogenesis (332). Two in vitro studies on human blood-brain barrier models showed that A. phagocytophilum-infected neutrophils enhanced B. burgdorferi migration across both systemic and brain microvascular endothelial cells. Several mechanisms are thought to be involved including impaired phagocytic neutrophil function caused by A. phagocytophilum, increased production of vasoactive and pro-inflammatory molecules (IL-6, IL-8, IL-10, tumor necrosis factor alpha, and macrophage inflammatory protein 1α) and the release of matrix metalloproteinases (329, 332). These factors lead to enhanced vascular permeability and inflammatory response in tissues and promote B. burgdorferi migration, which results in worsened clinical manifestations (329, 330, 332, 333).
Laboratory Abnormalities
Hematological Modifications
Hematological modifications associated with CGA include thrombocytopenia, anemia, leukopenia, and lymphopenia, although variable white blood cells count (WBC) modifications have been described (Table 3) (13, 24–27, 29–33, 35, 45). In experimentally infected dogs, hematological changes usually occurred during the acute stage of infection and normalized a few days after morulae disappeared from blood (302, 314). Suggested mechanisms of cytopenia include cytokine myelosuppression, autoantibodies formation, infection of hematopoietic precursors, and blood cell consumption (especially platelets) (25, 304, 334). Bone marrow aspirates of infected dogs were hyper- or normocellular, with normal, increased, or decreased iron storage, a slight increase in immature erythroid cells, and megakaryocyte and myeloid hyperplasia associated with relative shift toward immature myeloid cells, suggesting impaired myelopoiesis (312, 314).
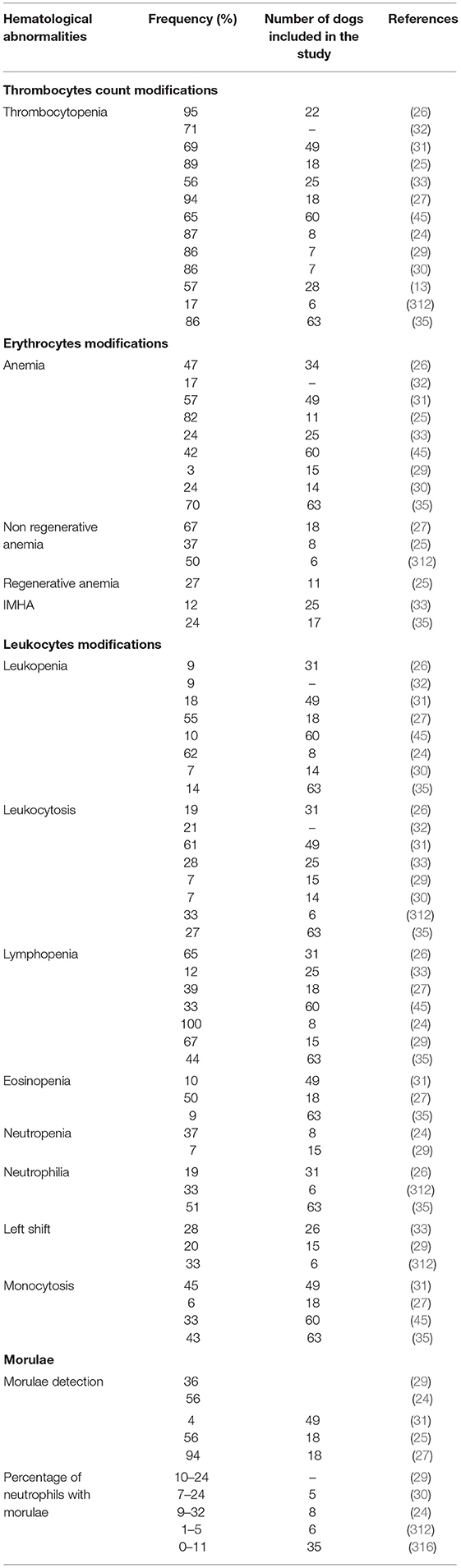
Table 3. Hematological abormalities associated with canine granulocytic anaplasmosis after natural infection and corresponding frequency recorded in several studies.
Thrombocytopenia is the most common disorder associated with CGA. It has been described in 16.7–95% of natural (13, 24–26, 29, 30, 35) and 100% of experimental infections (302, 314). According to some authors, thrombocytopenia reflects an ongoing immunological response in dogs even when associated with low antibody titers against A. phagocytophilum (34). A recent study showed a significant association between thrombocytopenia and high concentrations of circulating immune complexes (CIC), low albumin to globulin (A/G) ratios and an acute phase protein concentration. The importance of thrombocytopenia was emphasized as an indicator of acute anaplasmosis, regardless of antibody titer (28). Therefore, thrombocytopenia is considered the most relevant abnormality in the diagnosis of CGA after morulae detection (13, 24–26, 29, 30). The severity of thrombocytopenia varies from mild to severe and the platelet count has been reported to range from 5,000 to 164,000 cells/μl (24, 25, 29, 30, 35, 314). However, in a report, none of the 12 dogs seropositive to A. phagocytophilum had platelet counts lower than 105,000 cells/ml and dogs that were also seropositive to B. burgdorferi had a lower median platelet count of 51,000 cells/μl (33). In another study, five of the six CGA cases with significant thrombocytopenia had concurrent diseases (lymphoma and systemic lupus erythematosus) or were serologically positive to B. burgdorferi or E. canis (29). A prospective study aiming to investigate the presence of bacteria belonging to the genera Anaplasma and Ehrlichia in 159 blood samples from thrombocytopenic dogs, detected only two A. phagocytophilum-PCR positive dogs (335). As it has been described for a wide range of Ehrlichia species, CGA-associated thrombocytopenia may be related to platelet consumption due to DIC, immunological destruction, spleen sequestration or production of inhibitory factors (336–338). The organism seems to be able to enter megakaryocytes lineage but without impairment of their ability to produce platelets (339). The mechanism inducing thrombocytopenia seems to be more associated with an inflammatory process rather than with the direct action of A. phagocytophilum (34). Destruction of platelets has been suggested as a probable mechanism because of the increased number of both mature and immature megakaryocytes in the bone marrow (302). On the other hand, anti-platelet antibodies have been detected in both human and canine cases, with up to 60 and 80% of patients with CGA and HGA displaying anti-platelet antibodies, respectively (25, 35, 257, 336–338). However, thrombocytopenia usually occurs during the early stages of infection, before antibody detection and has also been described in severely immunocompromised mice due to B or T cell suppression, suggesting that mechanisms other than decreased hematopoietic production or immune-mediated destruction are involved. Increased platelet consumption is also suspected to play an important role (340, 341). In vitro, increased production of monocyte tissue pro-coagulant activity in peripheral blood mononuclear cells has been observed, supporting the platelet consumption hypothesis (27, 340).
Anemia is an inconstant hematological finding (34) described in 3–82% of dogs with clinical signs compatible with CGA either seropositive (33), PCR-positive (25, 27, 35, 45), displaying A. phagocytophilum-like morulae on fresh blood smear examination (24, 26) or being positive to two (29, 30) or three (31) aforementioned diagnostic methods (Table 3). In a retrospective study, no dogs were anemic, even during the bacteremic phase, but the mean values of hematocrit, hemoglobin concentration and red blood cell counts were significantly lower than in the control group (34). In contrast, three different studies described 63, 67, and 70% of anemic dogs (27, 35, 224). CGA-associated anemia is usually mild to moderate non-regenerative normocytic normochromic resembling anemia of inflammation (24, 27, 29, 31, 302, 312, 314). In nine dogs experimentally infected with A. phagocytophilum that developed mild normocytic normochromic anemia, decreased serum iron and total iron-binding capacity were recorded during bacteremia, but levels returned to reference ranges 1 week after the disappearance of morulae (302). In a report, most dogs had mild to moderate anemia with hematocrits ranging from 19 to 39%, but two had severe anemia with hematocrit levels <20% and three had signs of regeneration. Five were suspected to have hemolytic anemia based on increased serum levels of bilirubin but all had negative Coombs tests (25). Regenerative anemia has been less frequently reported, and severe IMHA is an unusual disorder associated with CGA (25, 33, 257, 258). One retrospective duty aiming to investigate infectious causes of lethal immune-mediated anemia in Croatian dogs, only two dogs were found positive to A. phagocytophilum DNA and one of these two dogs was also co-infected with B. canis (342). Six cases of IMHA in dogs with CGA have been reported in the UK, the USA and Denmark (33, 35, 257, 315). Authors from Germany described the possible occurrence of IMHA in a small number of dogs (25). Others from Belgium described IMHA in a dog with a positive titer to A. phagocytophilum and without other concomitant diseases (262). A previous case report described a dog with A. phagocytophilum infection (confirmed by positive PCR from blood and post-mortem spleen samples) with regenerative anemia, severe bilirubinuria, and positive test for osmotic resistance of red blood cells. This dog was serologically negative for babesiosis, leptospirosis, E. canis and E. chaffeensis infections (256). More recently, four dogs had a positive Coombs test among 17 ones that underwent this analysis in a case series on CGA (35). Only one case series has evaluated the prevalence of IMHA associated with CGA. In this study, three dogs had IMHA based on spherocytes in blood smears and/or positive Coombs test, without evidence of abdominal or thoracic neoplasia. However, two dogs had positive antibodies for at least one other TBP including Neorickettsia risticii (formerly Ehrlichia risticii), B. burgdorferi, and Rickettsia rickettsii. The authors emphasized that both R. rickettsii and B. burgdorferi are not commonly associated with IMHA and N. risticii is not yet associated with clinical disease in dogs as suggested by experimental studies (33). In addition, anti-erythrocyte antibodies have been detected in three dogs with CGA in the USA (312). Even if CGA has not yet been proven to be a common cause of IMHA, A. phagocytophilum should be included in the differential diagnosis, especially in endemic area (33).
The most diagnostically relevant hematological abnormality in CGA is the identification of A. phagocytophilum inclusions within neutrophils during blood smear examinations. Morulae appear classically as basophilic inclusions detectable by light microscopy of peripheral blood smears (41, 234). They are usually present transiently during the bacteremic phase (4–14 days after inoculation) and persist for 4–8 days in experimentally infected dogs (302, 314). Morulae can also be identified from cytocentrifuged synovial fluid, bone marrow aspirates, and they were also present in the abdominal fluid of an unusual CGA case and in the tracheal wash from a dog with respiratory signs (40, 238, 247, 302, 312, 315). The proportion of neutrophils containing morulae in blood smears varies from <1 to 34% (24, 29, 30, 312, 314, 316). In an experimentally study, the most severely affected dogs were those with higher percentage of neutrophils containing morulae and the lowest proportion was recorded in non-febrile dogs (314). In endemic areas, 38% of dogs displaying clinical signs compatible with CGA had morulae within neutrophils (13). Three studies reported that 56%, 94% (25, 27), and 88 to 93% (33) of dogs presented morulae while other reports failed to identify these inclusions (246, 257). It is important to mention that A. phagocytophilum morulae cannot be distinguished from those of E. ewingii, which can lead to misdiagnosis in the regions where both pathogens are present. Therefore, other methods, such as PCR are needed to confirm the diagnosis (2, 24, 302).
Experimentally infected dogs developed moderate leucopenia (314), but WBC count modifications in naturally infected dogs are considered non-specific and variable, and both decreased and increased WBC counts have been reported (24–27, 29–35, 45). Therefore, the use of the WBC count as a marker of the course of the disease is controversial (34). Lymphopenia is the most frequently reported WBC count abnormality in CGA (24–26, 29, 302, 314). Other reported modifications include leukocytosis, leukopenia, lymphocytosis, eosinopenia, monocytosis, monocytopenia and mild to moderate neutropenia or neutrophilia (24–27, 29–31, 35, 45, 53, 302, 312, 314). Left shift of neutrophils and toxic changes have also been reported to occur with A. phagocytophilum infection in dogs (26, 29, 33, 252, 257, 312).
Serum Biochemistry Profile Modification
Serum biochemistry profile modifications documented in CGA include increased liver enzyme activity, hyperbilirubinemia, hypophosphatemia, hyperproteinemia, hyperglobulinemia, and hypoalbuminemia (Table 4) (24–27, 29–31, 33, 35, 45, 314, 316). A moderate increase in alkaline phosphatase (ALP) was reported in 7–100% of CGA cases and mild to moderate hypoalbuminemia was present in 17–66% (25, 26, 29, 33). In a retrospective study, 30% of dogs displayed a slightly increased alanine aminotransferase (ALT) activity but concurrent diseases had not been ruled out (26). In another report, the most frequent findings in dogs with CGA were increased in liver enzymes and hyperbilirubinemia (35). According to some authors, hypoalbuminemia and hyperglobulinemia might be due to a decreased production of albumin in the liver associated with a rise in α- and β-globulin production (304). In a study investigating serum protein profiles of seropositive and PCR-positive dogs, the major modification was a low A/G ratio (84.4%), mostly in groups with antibody titers higher than 1:1,024. Hyperglobulinemia was due to an increase in the acute phase proteins (α2-, β1-, and β-2 globulin). In the same study, 62 and 71.8% of dogs in the group with lower A/G ratios had thrombocytopenia and clinical signs compatible with CGA, respectively, suggesting an acute infectious process. However, other diseases had not been excluded; hence dysproteinemia could possibly be the result of concurrent diseases (28). Others reported hypergammaglobulinemia as a prominent modification associated with CGA but without exclusion of concurrent diseases (316). Decreased serum levels of urea and hypokalemia have been recorded in 27% of dogs (25) and 27–37% of dogs were reported to have hyperbilirubinemia (25, 26, 35). An increase in serum amylase activity was described in 50% of CGA cases (29). Two case reports described dogs diagnosed with CGA with suspected pancreatitis on the basis of increased serum level of amylase and lipase and clinical signs suggesting pancreatitis (abdominal pain in the pancreatic region of one dog and abdominal ultrasound modifications in the pancreatic region of the other dog) (246, 252). In another previous report, two of seven dogs had increased serum lipase concentrations (24).
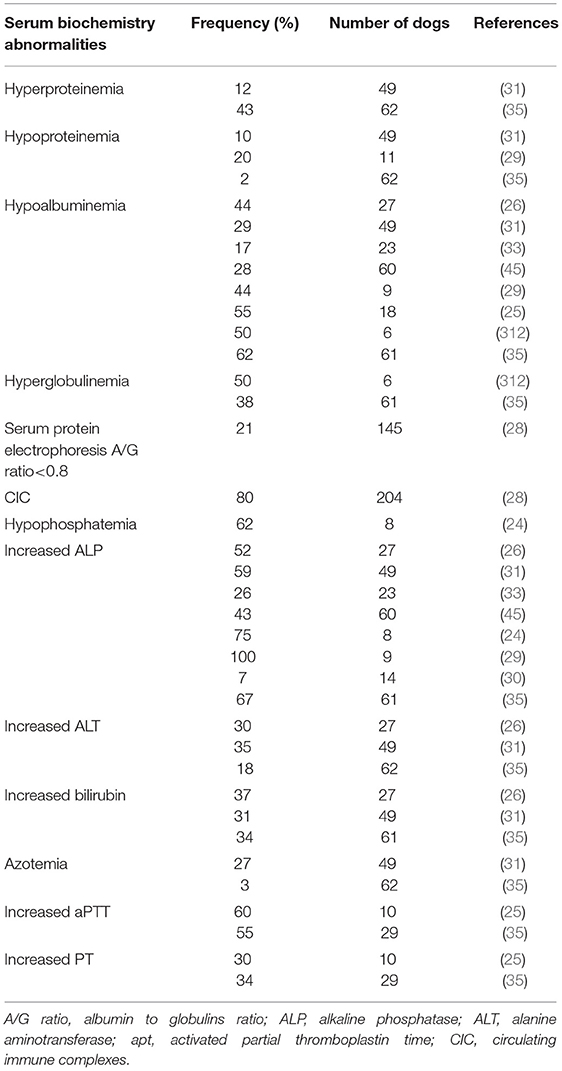
Table 4. Serum biochemistry abormalities associated with canine granulocytic anaplasmosis after natural infection and corresponding frequencies recorded in several studies.
Prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), along with increased fibrin-degradation product concentration and fibrinogen concentration have been reported in some CGA cases (Table 4) (25, 35, 252, 257, 314). DIC was suspected or diagnosed in four dogs; two of which had IMHA (25, 33, 257). Elevated aPTT was also described in one dog with SIRS secondary to A. phagocytophilum infection (26). In a recent study on portal vein thrombosis, four of 29 dogs had infectious diseases and one had A. phagocytophilum infection (343).
Urinalysis Modifications
Acute renal failure (ARF) is a complication described in some HGA cases (344, 345). In a recent study, 30.6% of human patients with confirmed A. phagocytophilum infection by PCR had abnormalities on urinalysis including hemoglobinuria or myoglobinuria (not distinguished by further analysis). Hemoglobinuria/myoglobinuria could be the precursor of ARF described in severe human cases (346). Experimental studies revealed evidence of A. phagocytophilum DNA in the kidneys of three persistently infected lambs and lesions of vasculitis and thrombosis in the kidney of a horse (347, 348). Similarly, one study amplified A. phagocytophilum DNA in the kidney of one dog after necropsy (342). CGA is suspected to induce immune-mediated glomerulonephritis (IMGN) likely by vasculitis (349). In contrast to blood modification, urinary abnormalities have not been fully assessed in dogs and only a few reports have described abnormalities in urinalysis (Table 5) (25, 26, 28–30, 33, 312). One such study described the presence of mild to moderate proteinuria, glucosuria, bilirubinuria, hematuria, and epithelial cells in urine sediments. In the same report, only three of eight dogs in which urinalysis was performed were also measured for urine protein to creatinine (UPC) ratios, and one displayed a mild increase (0.88) (25). Another report showed a significant difference in proteinuria between A. phagocytophilum seropositive and seronegative dogs (34). In a retrospective study, two dogs displayed proteinuria with UPC ratios of 1.5 and 2.2 (26) and 17% of dogs had proteinuria in another report. In this study the only dog with a UPC ratio higher than one had antibodies against both A. phagocytophilum and B. burgdorferi (33). More recently, 3% of CGA cases included retrospectively displayed signs of azotemia (35), however other concurrent diseases causing azotemia have not been ruled out. In most studies on CGA, proteinuric dogs were identified mainly on the basis of dipstick and only a few of them underwent UPC measurement. Moreover, urinary tract infection (UTI) was not excluded in all dogs. However, another study demonstrated that 38% of dogs had proteinuria without signs of UTI, which could be compatible with kidney injury (29). Proteinuria due to middle and high molecular weight proteins was found exclusively in 30.5% of A. phagocytophilum-seropositive dogs. The authors indicated that proteinuria might be the result of chronic antigenic stimulation and suggested that persistent infection can lead to the development of IMGN (34). In one case of CGA, a persistent proteinuria after 28 days of doxycycline therapy was reported. The dog remained asymptomatic during a 305-day follow up; however, mild proteinuria was still present even with a renin-angiotensin-aldosterone system inhibitor (261). More recently, a case report described a dog with IMGN complicated with systemic hypertension and chronic kidney disease without any identified etiology except an active A. phagocytophilum infection on the basis of a very high antibody (1:20,480) titer at first consultation and more than a 4-fold decrease in antibody titer several weeks after (262). Finally, the consensus statement of the American College of Veterinary Internal Medicine (ACVIM) for dogs with suspected glomerular disease recommends serologic screening for anaplasmosis of patients with renal proteinuria in addition to other infectious diseases known to induce proteinuria (350).
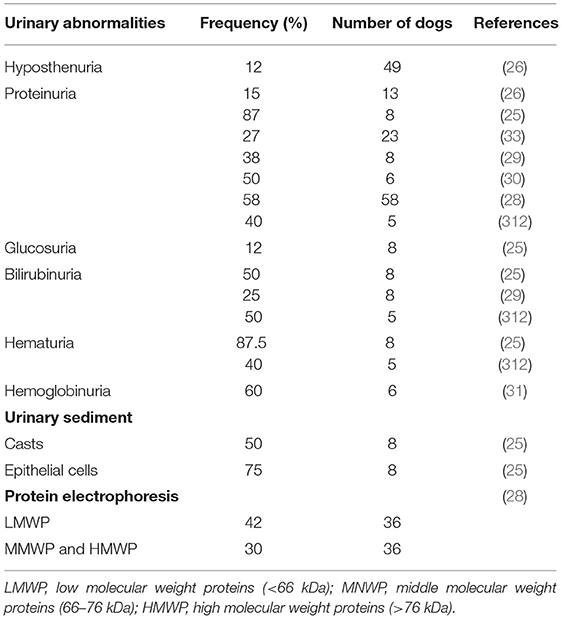
Table 5. Urinary abormalities associated with granulocytic anaplasmosis after natural infection and corresponding frequency recorded in several studies.
Conclusion and Futures Perspectives
Understanding granulocytic anaplasmosis is important due to its zoonotic aspect, potential severe outcomes in both dogs and humans, and the possibility of using epidemiological data in canine species as a good estimation of risk for human exposure. The aims of this review were to summarize the wide epidemiological data published on A. phagocytophilum in canine species and to describe the clinicopathological aspects of CGA that are available in the few case series and reports. In this manuscript, the authors wanted to gather together all data on A. phagocytophilum in dogs that can be valuable for researchers and to highlight the fields where important information is still missing and toward which future research should be focused. Indeed, information regarding the prevalence of A. phagocytophilum in some parts of the world, the potential role of dogs as competent reservoir hosts, the possibility of tick species other than Ixodes spp. acting as vectors of A. phagocytophilum and the implication of the genetic variability in the pathogenesis of the disease with some strains being potentially more virulent for humans is still incomplete or lacking. The pathogenesis of CGA is not fully elucidated too. Finally, some publications on CGA discussed the possibility of a chronic evolution and the association of this disease with serious clinicopathological manifestations with a crucial impact on the prognosis and management, such as immune-mediated hemolytic anemia, glomerulonephritis, and neurological signs that are still incomplete and thus need further investigations.
Author Contributions
SE wrote the manuscript. SD, LD, LE, MK, and HS drafted and revised the manuscript. All authors have made a substantial, intellectual contribution to the work, and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Pr. Pedro Diniz for the reviewing of this manuscript.
References
1. Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst and Evol Microbiol. (2001) 51:2145–65. doi: 10.1099/00207713-51-6-2145
2. Carrade DD, Foley JE, Borjesson DL, Sykes JE. Canine granulocytic anaplasmosis: a review. J Vet Intern Med. (2009) 23:1129–41. doi: 10.1111/j.1939-1676.2009.0384.x
3. Qurollo AB, Chandrashekar R, Hegarty BC, Beall MJ, Stillman BA, Liu J, et al. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species-specific peptides. Infect Ecol Epidemiol. (2014) 4:24699. doi: 10.3402/iee.v4.24699
4. Centers for Disease Control and Prevention (CDC). Statistics and Epidemiology: Annual Cases of Anaplasmosis in the United States. (2020). Available online at: https://www.cdc.gov/anaplasmosis/stats/index.html (accessed March 10, 2021).
5. Dumler JS. The biological basis of severe outcomes in Anaplasma phagocytophilum infection. FEMS Immunol Med Microbiol. (2012) 64:13–20. doi: 10.1111/j.1574-695X.2011.00909.x
6. Center for Disease Control and Prevention. Reportable Vector-Borne Disease Cases by State. (2016). Available online at: https://www.cdc.gov/ncezid/dvbd/vital-signs/2016-data.html (accessed May 03, 2021).
7. Matei IA, Estrada-Peña A, Cutler SJ, Vayssier-Taussat M, Varela-Castro L, Potkonjak A, et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors. (2019) 12:599. doi: 10.1186/s13071-019-3852-6
8. Zhang L, Cui F, Wang L, Zhang L, Zhang J, Wang S, et al. Investigation of anaplasmosis in Yiyuan County, Shandong Province, China. Asian Pac J Tro Med. (2011) 4:568–72. doi: 10.1016/S1995-7645(11)60148-X
9. Bowman D, Little SE, Lorentzen L, Shields J, Sullivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet Parasitol. (2009) 160:138–48. doi: 10.1016/j.vetpar.2008.10.093
10. Foley JE, Foley P, Madigan JE. Spatial distribution of seropositivity to the causative agent of granulocytic ehrlichiosis in dogs in California. Am J Vet Res. (2001) 62:1599–605. doi: 10.2460/ajvr.2001.62.1599
11. Solano-Gallego L, Llull J, Osso M, Hegarty B, Breitschwerdt E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet Res. (2006) 37:231–44. doi: 10.1051/vetres:2005054
12. Henn JB, Gabriel MW, Kasten RW, Brown RN, Theis JH, Foley JE, et al. Gray foxes (Urocyon cinereoargenteus) as a potential reservoir of a Bartonella clarridgeiae-like bacterium and domestic dogs as sentinels for zoonotic arthropod-borne pathogens in northern California. J Clin Microbiol. (2007) 45:2411–8. doi: 10.1128/JCM.02539-06
13. Beall MJ, Chandrashekar R, Eberts MD, Cyr KE, Diniz PPVP, Mainville C, et al. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis. (2008) 8:455–64. doi: 10.1089/vbz.2007.0236
14. Hamer SA, Tsao JI, Walker ED, Mansfield L, Foster ES, Graham HJ. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Res. (2009) 70:49–56. doi: 10.2460/ajvr.70.1.49
15. McMahan CS, Wang D, Beall MJ, Bowman DD, Little SE, Pithua PO, et al. Factors associated with Anaplasma spp. seroprevalence among dogs in the United States. Parasit Vectors. (2016) 9:169. doi: 10.1186/s13071-016-1431-7
16. Pusterla N, Huder J, Wolfensburger C, Litschi B, Parvis A, Lutz H. Granulocytic ehrlichiosis in two dogs in Switzerland. J Clin Microbiol. (1997) 35:2307–9. doi: 10.1128/JCM.35.9.2307-2309.1997
17. Morissette E, Massung RF, Foley JE, Alleman AR, Foley P, Barbet AF. Diversity of Anaplasma phagocytophilum strains, USA. Emerg Infect Dis. (2009) 15:928–31. doi: 10.3201/eid1506.081610
18. Scorpio DG, Dumler JS, Barat NC, Cook JA, Barat CE, Stillman BA, et al. Comparative strain analysis of Anaplasma phagocytophilum infection and clinical outcomes in a canine model of granulocytic anaplasmosis. Vector Borne Zoonotic Dis. (2011) 11:223–9. doi: 10.1089/vbz.2009.0262
19. Barbet AF, Al-Khedery B, Stuen S, Granquist EG, Felsheim RF, Munderloh UG. An emerging tick-borne disease of humans is caused by a subset of strains with conserved genome structure. Pathogens. (2013) 2:544–55. doi: 10.3390/pathogens2030544
20. Al-Khedery B, Barbet AF. Comparative genomics identifies a potential marker of human-virulent Anaplasma phagocytophilum. Pathogens. (2014) 3:25–35. doi: 10.3390/pathogens3010025
21. Strasek Smrdel K, von Loewenich FD, Petrovec M, Avsic Zupanc T. Diversity of ankA and msp4 genes of Anaplasma phagocytophilum in Slovenia. Ticks Tick Borne Dis. (2015) 6:164–6. doi: 10.1016/j.ttbdis.2014.11.008
22. Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuinston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. (2011) 85:124–31. doi: 10.4269/ajtmh.2011.10-0613
23. Little SE, Beall MJ, Bowman DD, Chandrashekar R, Stamaris J. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. In the United States, 2010–2012. Parasit Vectors. (2014) 7:257. doi: 10.1186/1756-3305-7-257
24. Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla SK. Genetic variants of Anaplasma phagocytophilum infecting dogs in Western Washington State. J Clin Microbiol. (2005) 43:796–801. doi: 10.1128/JCM.43.2.796-801.2005
25. Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J Vet Intern Med. (2008) 22:1289–95. doi: 10.1111/j.1939-1676.2008.0180.x
26. Granick JL, Armstrong PJ, Bender JB. Anaplasma phagocytophilum infection in dogs: 34 cases (2000–2007). J Am Vet Med Assoc. (2009) 234:1559–65. doi: 10.2460/javma.234.12.1559
27. Eberts MD, Diniz PP, Beall MJ, Stillman BA, Chandrashekar R, Breitschwerdt EB. Typical and atypical manifestations of Anaplasma phagocytophilum infection in dogs. J Am Anim Hosp Assoc. (2011) 47:88–94. doi: 10.5326/JAAHA-MS-5578
28. Ravnik U, Bajuk BP, Lusa L, Tozon N. Serum protein profiles, circulating immune complexes and proteinuria in dogs naturally infected with Anaplasma phagocytophilum. Vet Microbiol. (2014) 173:160–5. doi: 10.1016/j.vetmic.2014.07.007
29. Greig B, Asanovich KM, Armstrong PJ, Dumler JS. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. (1996) 34:44–8. doi: 10.1128/JCM.34.1.44-48.1996
30. Egenvall AE, Hedhammar AA, Bjoersdorff AI. Clinical features and serology of 14 dogs affected by granulocytic ehrlichiosis in Sweden. Vet Rec. (1997) 140:222–6. doi: 10.1136/vr.140.9.222
31. Jensen J, Simon D, Escobar HM, Soller JT, Bullerdiek J, Beelitz P, et al. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health. (2007) 57:94–101. doi: 10.1111/j.1863-2378.2007.01028.x
32. Ravnik U, Tozon N, Strasek K, Avsic Zupanc T. Clinical and haematological features in Anaplasma phagocytophilum seropositive dogs. Clin Microbiol Infect. (2009) 15:39–40. doi: 10.1111/j.1469-0691.2008.02167
33. Mazepa AW, Kidd LB, Young KM, Trepanier L. Clinical presentation of 26 Anaplasma phagocytophilum-seropositive dogs residing in an endemic area. J Am Animal Hosp Assoc. (2010) 46:405–12. doi: 10.5326/0460405
34. Ravnik U, Tozon N, Strasek Smrdel K, Avsic Zupanc T. Anaplasmosis in dogs: the relation of haematological, biochemical and clinical alterations to antibody titre and PCR confirmed infection. Vet Microbiol. (2011) 149:172–6. doi: 10.1016/j.vetmic.2010.10.005
35. Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. (2018) 59:112–20. doi: 10.1111/jsap.12787
36. Dunning Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen JA, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. (2006) 2:e21. doi: 10.1371/journal.pgen.0020021
37. Uilenberg G, Thiaucourt F, Jongejan F. On molecular taxonomy: What is in a name? Exp Appl Acarol. (2004) 32:301–12. doi: 10.1023/b:appa.0000023235.23090.a7
38. Barlough JE, Madigan JE, DeRock E, Dumler JS, Bakken JS. Protection against Ehrlichia equi is conferred by prior infection with the human granulocytic Ehrlichia species (HGE agent). J Clin Microbiol. (1995) 33:3333–4. doi: 10.1128/JCM.33.12.3333-3334.1995
39. Dumler JS, Asavovich KM, Bakken JS, Richter P, Kimsey R, Madigan JE. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic Ehrlichia. J Clin Microbiol. (1995) 33:1098–103. doi: 10.1128/JCM.33.5.1098-1103.1995
40. Popov VL, Han VC, Chen SM, Dumler JS, Feng HM, Andreadis TG, et al. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J Med Microbiol. (1998) 47:235–51. doi: 10.1099/00222615-47-3-235
41. Massung RF, Mather TN, Priestley RA, Levin ML. Transmission efficiency of the AP-variant 1 strain of Anaplasma phagocytophila. Ann N Y Acad Sci. (2003) 990:75–9. doi: 10.1111/j.1749-6632.2003.tb07340.x
42. Massung RF, Priestley RA, Miller NJ, Mather TN, Levin ML. Inability of a variant strain of Anaplasma phagocytophilum to infect mice. J Infect Dis. (2003) 188:1757–63. doi: 10.1086/379725
43. Massung RF, Mauel RJ, Owens JH, Allan N, Courtney JW, Stafford KC III, Mather TN. Genetic variants of Ehrlichia phagocytophila, Rhode Island and Connecticut. Emerg Infect Dis. (2002) 8:467–72. doi: 10.3201/eid0805.010251
44. Massung RF, Courtney JW, Hiratzka SL, Pitzer VE, Smith G, Dryden RL. Anaplasma phagocytophilum in white-tailed deer. Emerg Infect Dis. (2005) 11:1604–6. doi: 10.3201/eid1110.041329
45. Silaghi C, Kohn B, Chirek A, Thiel C, Nolte I, Liebisch, et al. Relationship of molecular and clinical findings on Anaplasma phagocytophilum involved in natural infections of dogs. J Clin Microbiol. (2011) 49:4413–4. doi: 10.1128/JCM.06041-11
46. Foley J, Nieto NC, Madigan J, Sykes J. Possible differential host tropism in Anaplasma phagocytophilum strains in the western United States. Ann NY Acad Sci. (2008) 1149:94–7. doi: 10.1196/annals.1428.066
47. Hoar BR, Nieto NC, Rhodes DM, Foley JE. Evaluation of sequential coinfection with Anaplasma phagocytophilum and Anaplasma marginale in cattle. Am J Vet Res. (2008) 69:1171–8. doi: 10.2460/ajvr.69.9.1171
48. Massung RF, Lee K, Mauel MJ, Gusa A. Characterization of the rRNA genes of Ehrlichia chaffeensis and Anaplasma phagocytophila. DNA Cell Biol. (2002) 21:587–96. doi: 10.1089/104454902320308960
49. Dugat T, Lagrée AC, Maillard R, Boulouis HJ, Haddad N. Opening the black box of Anaplasma phagocytophilum diversity: current situation and future perspectives. Front Cell Infect Microbiol. (2015) 5:61. doi: 10.3389/fcimb.2015.00061
50. de la Fuente J, Massung RF, Wong SJ, Chu FK, Lutz H, Meli M, et al. Sequence analysis of the msp4 gene of Anaplasma phagocytophilum strains. J Clin Microbiol. (2005) 43:1309–17. doi: 10.1128/JCM.43.3.1309-1317.2005
51. Huhn C, Winter C, Wolfsperger T, Wüppenhorst N, Strašek Smrdel K, Skuballa J, et al. Analysis of the population structure of Anaplasma phagocytophilum using multilocus sequence typing. PLoS ONE. (2014) 9:e93725. doi: 10.1371/journal.pone.0093725
52. Rar V, Golovljova I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol. (2011) 11:1842–61. doi: 10.1016/j.meegid.2011.09.019
53. Hovius E, de Bruin A, Schouls L, Hovius J, Dekker N, Sprong H. A lifelong study of a pack Rhodesian ridgeback dogs reveals subclinical and clinical tick-borne Anaplasma phagocytophilum infections with possible reinfection or persistence. Parasit Vectors. (2018) 11:238. doi: 10.1186/s13071-018-2806-8
54. Bown KJ, Lambin X, Ogden NH, Petrovec M, Shaw SE, Woldehiwet Z, Birtles RJ. High-resolution genetic fingerprinting of European strains of Anaplasma phagocytophilum by use of multilocus variable-number tandem-repeat analysis. J Clin Microbiol. (2007) 45:1771–6. doi: 10.1128/JCM.00365-07
55. Bown KJ, Lambin X, Ogden NH, Begon M, Telford G, Woldehiwet Z, Birtles RJ. Delineating Anaplasma phagocytophilum ecotypes in coexisting discrete enzootic cycles. Emerg Infect Dis. (2009) 15:1948–54. doi: 10.3201/eid1512.090178
56. Scharf W, Schauer S, Freyburger F, Petrovec M, Schaarschmidt-Kiener D, Liebisch G, et al. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J Clin Microbiol. (2011) 49:790–6. doi: 10.1128/JCM.02051-10
57. Barbet AF, Meeus PFM, Belanger M, Bowie MV, Yi J, Lundgren AM, et al. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect Immun. (2003) 71:1706–18. doi: 10.1128/iai.71.4.1706-1718.2003
58. Paulauskas A, Radzijevskaja J, Rosef O. Molecular detection and characterization of Anaplasma phagocytophilum strains. Comp Immunol Microbiol Infect Dis. (2012) 35:187–95. doi: 10.1016/j.cimid.2012.01.001
59. Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Dieter H, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. (2014) 7:365. doi: 10.1186/1756-3305-7-365
60. Engvall EO, Pettersson B, Persson M, Artursson K, Johansson KE. A 16S rRNA- based PCR assay for detection and identification of granulocytic Ehrlichia species in dogs, horses, and cattle. J Clin Microbiol. (1996) 34:2170–4. doi: 10.1128/JCM.34.9.2170-2174.1996
61. von Stedingk LV, Gürtelschmid M, Hanson HS, Gustafson R, Dotevall L, Engvall EO, et al. The human granulocytic ehrlichiosis (HGE) agent in Swedish ticks. Clin Microbiol Infect. (1997) 3:573–574. doi: 10.1111/j.1469-0691.1997.tb00311.x
62. Pusterla N, Pusterla JB, Deplazes P, Wolfensberger C, Muller W, Horauf A, et al. Seroprevalence of Ehrlichia canis and of canine granulocytic Ehrlichia infection in dogs in Switzerland. J Clin Microbiol. (1998) 36:3460–2. doi: 10.1128/JCM.36.12.3460-3462.1998
63. Zhang L, Liu Y, Ni D, Li Q, Yu Y, Yu XJ, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. J Am Med Assoc. (2008) 300:2263–70. doi: 10.1001/jama.2008.626
64. Horowitz HW, Kilchevsky E, Haber S, Aguero-Rosenfeld M, Kranwinkel R, James EK, et al. Perinatal transmission of the agent of human granulocytic ehrlichiosis. N Engl J Med. (1998) 339:375–8. doi: 10.1056/NEJM199808063390604
65. Bakken JS, Krueth JK, Lund T, Malkovitch D, Asanovich K, Dumler JS. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin Infect Dis. (1996) 23:198. doi: 10.1093/clinids/23.1.198
66. Fine AB, Sweeney JD, Nixon CP, Knoll BM. Transfusion-transmitted anaplasmosis from a leukoreduced platelet pool. Transfusion. (2016) 56:699–704. doi: 10.1111/trf.13392
67. Beugnet F, Marie JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. (2009) 163:298–305. doi: 10.1016/j.vetpar.2009.03.028
68. Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum — a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. (2013) 3:31. doi: 10.3389/fcimb.2013.00031
69. Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol. (2010) 167:108–22. doi: 10.1016/j.vetpar.2009.09.013
70. Keirans JE, Hutcheson HJ, Durden LA, Klompen JS. Ixodes scapularis (Acari: Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol. (1996) 33:297–318. doi: 10.1093/jmedent/33.3.297
71. Richter PJ Jr, Kimsey RB, Madigan JE, Barlough JE, Dumler JS, Brooks DL. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae). J Med Entomol. (1996) 33:1–5. doi: 10.1093/jmedent/33.1.1
72. des Vignes F, Levin ML, Fish D. Comparative vector competence of Dermacentor variabilis and Ixodes scapularis (Acari: Ixodidae) for the agent of human granulocytic ehrlichiosis. J Med Entomol. (1999) 36:182–5. doi: 10.1093/jmedent/36.2.182
73. Magnarelli LA, Stafford KC III, Mather TN, Yeh MT, Horn KD, Dumler JS. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to Ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. (1995) 33:2710–4. doi: 10.1128/JCM.33.10.2710-2714.1995
74. Moreno CX, Moy F, Daniels TJ, Godfrey HP, Cabello FC. Molecular analysis of microbial communities identified in different developmental stages of Ixodes scapularis ticks from Westchester and Dutchess Counties, New York. Environ Microbiol. (2006) 8:761–72. doi: 10.1111/j.1462-2920.2005.00955.x
75. Swanson KI, Norris DE. Co-circulating microorganisms in questing Ixodes scapularis nymphs in Maryland. J Vector Ecol. (2007) 32:243–51. doi: 10.3376/1081-1710(2007)32[243:cmiqis]2.0.co;2
76. Walk ST, Xu G, Stull JW, Rich SM. Correlation between tick density and pathogen endemicity, New Hampshire. Emerg Infect Dis. (2009) 15:585–7. doi: 10.3201/eid1504.080940
77. Goethert HK, Telford SR. Enzootic transmission of the agent of human granulocytic ehrlichiosis among cottontail rabbits. Am J Trop Med Hyg. (2003) 68:633–7.
78. Baldridge GD, Scoles GA, Burkhardt NY, Schloeder B, Kurtti TJ, Munderloh UG. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae). J Med Entomol. (2009) 46:625–32. doi: 10.1603/033.046.0330
79. Zeidner NS, Burkot TR, Massung R, Nicholson WL, Dolan MC, Rutherford JS, et al. Transmission of the agent of human granulocytic ehrlichiosis by Ixodes spinipalpis ticks: evidence of an enzootic cycle of dual infection with Borrelia burgdorferi in Northern Colorado. J Infect Dis. (2000) 182:616–9. doi: 10.1086/315715
80. Holden K, Boothby JT, Anand S, Massung RF. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J Med Entomol. (2003) 40:534–9. doi: 10.1603/0022-2585-40.4.534
81. Clark L. Anaplasma phagocytophilum in small mammals and ticks in northeast Florida. J Vector Ecol. (2012) 37:262–8. doi: 10.1111/j.1948-7134.2012.00226.x
82. Foley J, Rejmanek D, Fleer K, Nieto N. Nidicolous ticks of small mammals in Anaplasma phagocytophilum-enzootic sites in northern California. Ticks Tick Borne Dis. (2011) 2:75–80. doi: 10.1016/j.ttbdis.2011.03.003
83. Rejmanek D, Freycon P, Bradburd G, Dinstell J, Foley J. Unique strains of Anaplasma phagocytophilum segregate among diverse questing and non-questing Ixodes tick species in the western United States. Ticks Tick Borne Dis. (2013) 4:482–7. doi: 10.1016/j.ttbdis.2013.06.003
84. Santos HA, Thomé SM, Baldani CD, Silva CB, Peixoto MP, Pires MS, et al. Molecular epidemiology of the emerging zoonosis agent Anaplasma phagocytophilum (Foggie, 1949) in dogs and Ixodid ticks in Brazil. Parasit Vectors. (2013) 6:348. doi: 10.1186/1756-3305-6-348
85. Campos-Calderón L, Ábrego-Sánchez L, Solórzano-Morales A, Alberti A, Tore G, Zobba R, et al. Molecular detection and identification of Rickettsiales pathogens in dog ticks from Costa Rica. Ticks Tick Borne Dis. (2016) 7:1198–202. doi: 10.1016/j.ttbdis.2016.07.015
86. Sosa-Gutierrez CG, Vargas-Sandoval M, Torres J, Gordillo-Pérez G. Tick-borne rickettsial pathogens in questing ticks, removed from humans and animals in Mexico. J Vet Sci. (2016) 17:353–60. doi: 10.4142/jvs.2016.17.3.353
87. Asman M, Nowak M, Cuber P, Strzelczyk J, Szilman E, Szilman P, et al. The risk of exposure to Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, Babesia sp. and co-infections in Ixodes ricinus ticks on the territory of Niepołomice Forest (southern Poland). Ann Parasitol. (2013)59:13–19.
88. Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, et al. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol. (2014) 4:103. doi: 10.3389/fcimb.2014.00103
89. Bown KJ, Lambin X, Telford GR, Ogden NH, Telfer S, Woldehiwet Z, et al. Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl Environ Microbiol. (2008) 74:7118–25. doi: 10.1128/AEM.00625-08
90. Silaghi C, Skuballa J, Thiel C, Pfister K, Petney T, Pfäffle M, et al. The European hedgehog (Erinaceus europaeus) — a suitable reservoir forvariants of Anaplasma phagocytophilum? Ticks Tick Borne Dis. (2012) 3:49–54. doi: 10.1016/j.ttbdis.2011.11.005
91. Santos AS, Santos-Silva MM, Almeida VC, Bacellar F, Dumler JS. Detection of Anaplasma phagocytophilum DNA in Ixodes ticks (Acari: Ixodidae) from Madeira island and Setubal district, mainland Portugal. Emerg Infect Dis. (2004) 10:1643–8. doi: 10.3201/eid1009.040276
92. Tomanovic S, Chochlakis D, Radulovic Z, Milutinovic M, Cakic S, Mihaljica D, et al. Analysis of pathogen co-occurrence in host-seeking adult hard ticks from Serbia. Exp Appl Acarol. (2013) 59:367–76. doi: 10.1007/s10493-012-9597-y
93. Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis. (2003) 9:63–70. doi: 10.3201/eid0901.020169
94. Blanarová L, Stanko M, Carpi G, Miklisová D, Víchová B, Mošanský L, et al. Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in Central Europe. Ticks Tick Borne Dis. (2014) 5:928–38. doi: 10.1016/j.ttbdis.2014.07.012
95. Cicculli V, DeCarreaux D, Ayhan N, Casabianca F, de Lamballerie X, Charrel R, et al. Molecular screening of Anaplasmataceae in ticks collected from cattle in Corsica, France. Exp App Acarol. (2020) 81:561–74. doi: 10.1007/s10493-020-00527-w
96. Keysary A, Massung RF, Inbar M, Wallach AD, Shanas U, Mumcuoglu K, et al. Molecular evidence for Anaplasma phagocytophilum in Israel. Emerg Infect Dis. (2007) 13:1411–2. doi: 10.3201/eid1309.070455
97. Psaroulaki A, Chochlakis D, Ioannou I, Florentia A, Gikas A, Tselentis Y. Acute anaplasmosis in human in Cyprus. Clin Microbiol Infect. (2008) 15:10–1. doi: 10.1111/j.1469-0691.2008.02646.x
98. Chastagner A, Bailly X, Leblond A, Pradier S, Vourc'h G. Single Genotype of Anaplasma phagocytophilum identified from ticks, Camargue, France. Emerg Infect Dis. (2013) 19:825–6. doi: 10.3201/eid1905.121003
99. Dugat T, Chastagner A, Lagrée AC, Petit E, Durand B, Thierry S, et al. A new multiple-locus variable-number tandem repeat analysis reveals different clusters for Anaplasma phagocytophilum circulating in domestic and wild ruminants. Parasit Vectors. (2014) 7:439. doi: 10.1186/1756-3305-7-439
100. Eremeeva ME, Oliveira A, Robinson JB, Ribakova N, Tokarevich NK, Dasch GA. Prevalence of bacterial agents in Ixodes persulcatus ticks from the Vologda Province of Russia. Ann NY Acad Sci. (2006) 1078:291–8. doi: 10.1196/annals.1374.054
101. Ybañez AP, Matsumoto K, Kishimoto T, Yokoyama N, Inokuma H. Dual presence of Anaplasma phagocytophilum and its closely related Anaplasma sp. in ixodid ticks in Hokkaido, Japan, and their specific molecular detection. J Vet Med Sci. (2012) 74:1551–60. doi: 10.1292/jvms.12-0197
102. Ohashi N, Inayoshi M, Kitamura K, Kawamori F, Kawaguchi D, Nishimura Y, et al. Anaplasma phagocytophilum-infected ticks, Japan. Emerg Infect Dis. (2005) 11:1780–3. doi: 10.3201/eid1111.050407
103. Cao WC, Zhan L, He J, Foley JE, DE Vlas SJ, Wu XM, et al. Natural Anaplasma phagocytophilum infection of ticks and rodents from a forest area of Jilin Province, China. Am J Trop Med Hyg. (2006) 75:664–8.
104. Chae JS, Yudo H, Shringi S, Klein TA, Kim HC, Chong ST, et al. Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J Vet Sci. (2008) 9:285–93. doi: 10.4142/jvs.2008.9.3.285
105. Yoshimoto K, Matsuyama Y, Matsuda H, Sakamoto L, Matsumoto K, Yokoyama N, et al. Detection of Anaplasma bovis and Anaplasma phagocytophilum DNA from Haemaphysalis megaspinosa in Hokkaido, Japan. Vet Parasitol. (2010) 168:170–2. doi: 10.1016/j.vetpar.2009.10.008
106. Kuo CC, Huang JL, Chien CH, Shih HC, Wang HC. First molecular detection of Anaplasma phagocytophilum in the hard tick Rhipicephalus haemaphysaloides in Taiwan. Exp Appl Acarol. (2018) 75:437–43. doi: 10.1007/s10493-018-0283-6
107. Zhang L, Liu H, Xu B, Lu Q, Li L, Chang L, et al. Anaplasma phagocytophilum infection in domestic animals in ten provinces/cities of China. Am J Trop Med Hyg. (2012) 87:185–9. doi: 10.4269/ajtmh.2012.12-0005
108. Jiang BG, Cao WC, Niu JJ, Wang JX, Li HM, Sun Y, et al. Detection and identification of Ehrlichia species in Rhipicephalus (Boophilus) microplus ticks in cattle from Xiamen, China. Vector Borne Zoonotic Dis. (2011) 11:325. doi: 10.1089/vbz.2009.0219
109. Wang S, Kou ZQ, Wang M, Ren YY, Hu B, Fang M, Bi ZW. A survey and identification of Ehrlichia chaffeensis and Anaplasma phagocytophilum in Shandong. Dis Surv. (2012) 27:642–3. doi: 10.3784/j.issn.1003-9961.2012.8.018
110. Sarih M, M'Ghirbi Y, Bouattour A, Gern L, Baranton G, Pontic D. Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. J Clin Microbiol. (2005) 43:1127–32. doi: 10.1128/JCM.43.3.1127-1132.2005
111. Ghafar MW, Amer SA. Prevalence and first molecular characterization of Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis, in Rhipicephalus sanguineus ticks attached to dogs from Egypt. J Adv Res. (2012) 3:189–94. doi: 10.1016/j.jare.2011.08.002
112. M'ghirbi Y, Yaïch H, Ghorbel A, Bouattour A. Anaplasma phagocytophilum in horses and ticks in Tunisia. Parasit Vectors. (2012) 5:180. doi: 10.1186/1756-3305-5-180
113. Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. (2007) 45:S45–51. doi: 10.1086/518146
114. Villeneuve A, Goring J, Marcotte L, Overvelde S. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia canis, and Dirofilaria immitis among dogs in Canada. Can Vet J. (2011) 52:527–30.
115. Herrin BH, Peregrine AS, Goring J, Beall MJ, Little SE. Canine infection with Borrelia burgdorferi, Dirofilaria immitis, Anaplasma spp. and Ehrlichia spp. in Canada, 2013–2014. Parasit Vectors. (2017) 10:244. doi: 10.1186/s13071-017-2184-7
116. Evason M, Stull JW, Pearl DL, Peregrine AS, Jardine C, Buch JS, et al. Prevalence of Borrelia burgdorferi, Anaplasma spp., Ehrlichia spp. and Dirofilaria immitis in Canadian dogs, 2008 to 2015: a repeat cross-sectional study. Parasit Vectors. (2019) 12:64. doi: 10.1186/s13071-019-3299-9
117. Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J. (2006) 47:1194–200.
118. Gaunt MC, Carr AP, Taylor SM. Serological survey of canine vector-borne diseases in Saskatchewan, Canada. Can Vet J. (2018) 59:1109–11.
119. Yancey CB, Hegarty BC, Qurollo BA, Levy MG, Birkenheuer AJ, Weber DJ, et al. Regional seroreactivity and vector-borne disease co-exposures in dogs in the United States from 2004–2010: utility of canine surveillance. Vector Borne Zoonotic Dis. (2014) 14:724–32. doi: 10.1089/vbz.2014.1592
120. Carrade D, Foley J, Sullivan M, Foley CW, Sykes JE. Spatial distribution of seroprevalence for Anaplasma phagocytophilum, Borrelia burgdorferi, Ehrlichia canis, and Dirofilaria immitis in dogs in Washington, Oregon, and California. Vet Clin Pathol. (2011) 40:293–302. doi: 10.1111/j.1939-165X.2011.00334.x
121. Suksawat J, Hegarty BC, Breitschwerdt EB. Seroprevalence of Ehrlichia canis, Ehrlichia equi, and Ehrlichia risticii in sick dogs from North Carolina and Virginia. J Vet Intern Med. (2000) 14:50–5. doi: 10.1892/0891-6640(2000)014<0050:socear>2.3.co;2
122. Rand PW, Lacombe EH, Elias SP, Cahill BK, Lubelczyk CB, Smith RP Jr. Multitarget test for emerging Lyme disease and anaplasmosis in a serosurvey of dogs, Maine, USA. Emerg Infect Dis. (2011) 17:899–902. doi: 10.3201/eid1705.100408
123. Rodgers SJ, Morton RJ, Baldwin CA. A serological survey of Ehrlichia canis, Ehrlichia equi, Rickettsia rickettsii, and Borrelia burgdorferi in dogs in Oklahoma. J Vet Diagn Invest. (1989) 1:154–9. doi: 10.1177/104063878900100212
124. Diniz P, Beall MJ, Omark K, Chandrashekar R, Baniluk DA, Cyr KE, et al. High prevalence of tick-borne pathogens in dogs from an Indian Reservation in northeastern Arizona. Vector Borne Zoonotic Dis. (2010) 10:117–23. doi: 10.1089/vbz.2008.0184
125. Gaito A, Gjivoje V, Lutz S, Baxter B. Comparative analysis of the infectivity rate of both Borrelia burgdorferi and Anaplasma phagocytophilum in humans and dogs in a New Jersey community. Infect Drug Resist. (2014) 7:199–201. doi: 10.2147/IDR.S68742
126. Balakrishnan N, Musulin S, Varanat M, Bradley JM, Breitschwerdt EB. Serological and molecular prevalence of selected canine vector borne pathogens in blood donor candidates, clinically healthy volunteers, and stray dogs in North Carolina. Parasit Vectors. (2014) 7:116. doi: 10.1186/1756-3305-7-116
127. Magnarelli L, Ijdo J, Anderson K, Madigan JE, Dumler JS, Fikrig E. Antibodies to Ehrlichia equi in dogs from the northeastern United States. J Am Vet Med Assoc. (1997) 211:1134–7.
128. Patterson G, Tanhauser M, Schmidt P, Spangler D, Faulkner C, Faulkner V, et al. Serosurvey of arthropod-borne diseases among shelter dogs in the Cumberland Gap Region of the United States. BMC Vet Res. (2020) 16:221. doi: 10.1186/s12917-020-02440-1
129. Santos HA, Pires MS, Vilela JAR, Santos TM, Faccini JLH, Baldani CD, et al. Detection of Anaplasma phagocytophilum in Brazilian dogs by real-time polymerase chain reaction. J Vet Diagn Invest. (2011) 23:770–4. doi: 10.1177/1040638711406974
130. Diniz PP, Schwartz DS, de Morais HS, Breitschwerdt EB. Surveillance for zoonotic vector-borne infections using sick dogs from southeastern Brazil. Vector Borne Zoonotic Dis. (2007) 7:689–97. doi: 10.1089/vbz.2007.0129
131. Lasta CS, dos Santos AP, Messick JB, Oliveira ST, Biondo AW, da Costa Vieira RF, et al. Molecular detection of Ehrlichia canis and Anaplasma platys in dogs in Southern Brazil. Rev Bras Parasitol Vet. (2013) 2:360–6. doi: 10.1590/S1984-29612013000300007
132. Vieira TSWJ, da Costa Vieira RF, Gomes do Nascimento AA, Tamekuni K, Dos Santos Toledo R, Chandrashekar R, et al. Serosurvey of tick-borne pathogens in dogs from urban and rural areas from Parana State, Brazil. Rev Bras Parasitol Vet. (2013) 22:104–9. doi: 10.1590/s1984-29612013000100019
133. McCown ME, Opel T, Grzeszak B. Vector-borne disease surveillance in Puerto rico: pathogen prevalence rates in canines? Implications for public health and the US Military? Applying the one health concept. J Spec Oper Med. (2013) 13:59–63.
134. McCown ME, Alleman A, Sayler KA, Chandrashekar R, Thatcher B, Tyrrell P, et al. Point prevalence survey for tick-borne pathogens in military working dogs, shelter animals, and pet populations in northern Colombia. J Spec Oper Med. (2014) 14:81–5.
135. Starkey LA, Newton K, Brunker J, Crowdis K, Edourad EJP, Meneus P, et al. Prevalence of vector-borne pathogens in dogs from Haiti. Vet Parasitol. (2016) 224:7–12. doi: 10.1016/j.vetpar.2016.04.017
136. Kelly PJ, Xu C, Lucas H, Loftis A, Abete J, Zeoli F, et al. Ehrlichiosis, babesiosis, anaplasmosis and hepatozoonosis in dogs from St. Kitts, West Indies. PLoS ONE. (2013) 8:e53450. doi: 10.1371/journal.pone.0053450
137. Movilla R, García C, Siebert S, Roura X. Countrywide serological evaluation of canine prevalence for Anaplasma spp., Borrelia burgdorferi (sensu lato), Dirofilaria immitis and Ehrlichia canis in Mexico. Parasit Vectors. (2016) 9:421. doi: 10.1186/s13071-016-1686-z
138. Bonilla MC, Campos-Calderón L, Jiménez-Rocha AE, Romero-Zúñiga JJ, Alberti A, Zobba R, et al. Characterization of Anaplasma spp. infection in dogs from Costa Rica. Vet Parasitol Reg Stud Rep. (2017) 8:60–5. doi: 10.1016/j.vprsr.2017.02.003
139. Jimenez IA, Vega Mariño PA, Stapleton GS, Prieto JB, Bowman DD. Canine vector-borne disease in domestic dogs on Isla Santa Cruz, Galápagos. Vet Parasitol Reg Stud Rep. (2020) 19:100373. doi: 10.1016/j.vprsr.2020.100373
140. Springer A, Montenegro VM, Schicht S, Pantchev N, Strube C. Seroprevalence and current infections of canine vector-borne diseases in Nicaragua. Parasit Vectors. (2018) 11:585. doi: 10.1186/s13071-018-3173-1
141. Acosta-Jamett G, Weitzel T, López J, Alvarado D, Abarca K. Prevalence and risk factors of antibodies to Anaplasma spp. in Chile: a household-based cross-sectional study in healthy adults and domestic dogs. Vector Borne Zoonotic Dis. (2020) 20:572–9. doi: 10.1089/vbz.2019.2587
142. Krupka I, Pantchev N, Lorentzen L, Weise M, Straubinger RK. Tick-transmitted, bacterial infections in dogs: seroprevalences of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in Germany. Prakt Tierarzt. (2007) 88:776–88.
143. Barutzki D, De Nicola A, Zeziola M, Reule M. Seroprevalence of Anaplasma phagocytophilum infection in dogs in Germany. Berl Munch Tierarztl Wochenschr. (2006) 119:342–7.
144. Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. (2011) 91:71–6. doi: 10.1016/j.rvsc.2010.08.008
145. Menn B, Lorentz S, Naucke TJ. Imported and travelling dogs as carriers of canine vector-borne pathogens in Germany. Parasit Vectors. (2010) 3:34. doi: 10.1186/1756-3305-3-34
146. Globokar Vrhovec M, Pantchev N, Failing K, Bauer C, Travers-Martin N, Zahner H. Retrospective analysis of canine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of leishmaniosis. Parasitol Res. (2017) 116:S131–44. doi: 10.1007/s00436-017-5499-6
147. Barth C, Straubinger RK, Sauter-Louis C, Hartmann K. Prevalence of antibodies against Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum and their clinical relevance in dogs in Munich, Germany. Berl Münch Tierärztl Wochenschr. (2012) 125:337–44.
148. Preyß-Jägeler C, Müller E, Straubinger RK, Hartmann K. Prevalence of antibodies against Borrelia burgdorferi, Anaplasma phagocytophilum, and Leptospira interrogans serovars in Bernese mountain dogs. Tierarztl Prax Ausg K Kleintiere Heimtiere. (2016) 44:77–85. doi: 10.15654/TPK-140962
149. Liesner JM, Krücken J, Schaper R, Pachnicke S, Kohn B, Müller E, et al. Vector-borne pathogens in dogs and red foxes from the federal state of Brandenburg, Germany. Vet Parasitol. (2016) 224:44–51. doi: 10.1016/j.vetpar.2016.05.012
150. Volgina NS, Romashov BV, Romashova NB, Shtannikov AV. Prevalence of Borreliosis, Anaplasmosis, Ehrlichiosis and Dirofilaria immitis in dogs and vectors in Voronezh Reserve (Russia). Comp Immunol Microbiol Infect Dis. (2013) 36:567–74. doi: 10.1016/j.cimid.2013.08.003
151. Farkas R, Gyurkovszky M, Lukàcs Z, Aladics B, Solymosi N. Seroprevalence of some vector-borne infections of dogs in Hungary. Vector Borne Zoonotic Dis. (2014) 14:256–60. doi: 10.1089/vbz.2013.1469
152. Hornok S, Dénes B, Meli ML, Tanczos B, Fekete L, Gyuranecz M, et al. Non-pet dogs as sentinels and potential synanthropic reservoirs of tick-borne and zoonotic bacteria. Vet Microbiol. (2013) 167:700–3. doi: 10.1016/j.vetmic.2013.08.011
153. Majlathova V, Majlath I, Vichova B, Gul'ová I, Derdáková M, Sesztáková E, et al. Polymerase chain reaction confirmation of Babesia canis canis and Anaplasma phagocytophilum in dogs suspected of babesiosis in Slovakia. Vector Borne Zoonotic Dis. (2011) 11:1447–51. doi: 10.1089/vbz.2010.0276
154. Cabanová V, Pantchev N, Hurníková Z, Miterpáková M. Recent study on canine vector-borne zoonoses in southern Slovakia — serologic survey. Acta Parasitol. (2015) 60:749–58. doi: 10.1515/ap-2015-0107
155. Pantchev N, Schnyder M, Globokar Vrhovec M, Schaper R, Tsachev I. Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol Res. (2015) 114:S117–30. doi: 10.1007/s00436-015-4518-8
156. Kirtz B, Czettel B, Thum D, Leidinger E. Anaplasma phagozytophilum in einer österreichischen Hundepopulation: eine Prävalenz-Studie (2001–2006). Kleintierpraxis. (2007) 52:562–8.
157. Shaw SE, Binns SH, Birtles RJ, Day MJ, Smithson R, Kenny MJ. Molecular evidence of tick-transmitted infections in dogs and cats in the United Kingdom. Vet Rec. (2005) 157:645–8. doi: 10.1136/vr.157.21.645
158. Egenvall A, Bonnett BN, Gunnarsson A, Hedhammar A, Shoukri M, Bornstein S, et al. Seroprevalence of granulocytic Ehrlichia spp. and Borrelia burgdorferi sensu lato in Swedish dogs 1991–1994. Scand J Infect Dis. (2000) 32:19–25. doi: 10.1080/00365540050164164
159. Elfving K, Malmsten J, Dalin AM, Nilsson K. Serologic and molecular prevalence of Rickettsia helvetica and Anaplasma phagocytophilum in wild cervids and domestic mammals in the central parts of Sweden. Vector Borne Zoonotic Dis. (2015) 15:529–34. doi: 10.1089/vbz.2015.1768
160. Pérez Vera C, Kapiainen S, Junnikkala S, Aaltonen K, Spillmann T, Vapalahti O. Survey of selected tick-borne diseases in dogs in Finland. Parasit Vectors. (2014) 7:285. doi: 10.1186/1756-3305-7-285
161. Hamel D, Silaghi C, Knaus M, Visser M, Kusi I, Rapti D, et al. Detection of Babesia canis subspecies and other arthropod-borne diseases in dogs from Tirana, Albania. Wien Klin Wochenschr. (2009) 121:42–5. doi: 10.1007/s00508-009-1234-3
162. Hamel D, Shukullari E, Rapti D, Silaghi C, Pfister K, Rehbein S. Parasites and vector-borne pathogens in client-owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitol Res. (2016) 115:489–99. doi: 10.1007/s00436-015-4765-8
163. Berzina I, Capligina V, Bormane A, Pavulina A, Baumanis V, Ranka R, et al. Association between Anaplasma phagocytophilum seroprevalence in dogs and distribution of Ixodes ricinus and Ixodes persulcatus ticks in Latvia. Ticks Tick Borne Dis. (2013) 4:83–8. doi: 10.1016/j.ttbdis.2012.08.003
164. Mircean V, Dumitrache MO, Gyöke A, Pantchev N, Jodies R, Mihalca D, et al. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector Borne Zoonotic Dis. (2012) 12:595–604. doi: 10.1089/vbz.2011.0915
165. Hamel D, Silaghi C, Lescai D, Pfister K. Epidemiological aspects on vector-borne infections in stray and pet dogs from Romania and Hungary with focus on Babesia spp. Parasitol Res. (2012) 110:1537–45. doi: 10.1007/s00436-011-2659-y
166. Matei IA, Ionică AM, D'Amico G, Corduneanu A, Daskalaki AA, Lefkaditis M, et al. Altitude-dependent prevalence of canine granulocytic anaplasmosis in Romania. Vector Borne Zoonotic Dis. (2017) 17:147–51. doi: 10.1089/vbz.2016.1998
167. Enache D, Imre M, Ilie MS, Coprean D. Seroprevalence of Anaplasma phagocytophilum in dogs from Constanta county. J Biotechnol. (2015) 208:S95. doi: 10.1016/j.jbiotec.2015.06.298
168. Cazan CD, Ionică AM, Matei IA, D'Amico G, Muñoz C, Berriatua E, et al. Detection of Leishmania infantum DNA and antibodies against Anaplasma spp., Borrelia burgdorferi s.l. and Ehrlichia canis in a dog kennel in South-Central Romania. Acta Vet Scand. (2020) 62:42. doi: 10.1186/s13028-020-00540-4
169. Potkonjak A, Gutiérrez R, Savic S, Vračar V, Nachum-Biala Y, Jurišić A, et al. Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick Borne Dis. (2016) 7:199–203. doi: 10.1016/j.ttbdis.2015.10.007
170. Kovačević Filipović MM, Beletić AD, Ilić BoŽović AV, Milanović Z, Tyrrell P, Buch J, et al. Molecular and Serological Prevalence of Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeenses, E. ewingii, Borrelia burgdorferi, Babesia canis, B. gibsoni and B. vogeli among clinically healthy outdoor dogs in Serbia. Vet Parasitol Reg Stud Reports. (2018) 14:117-122. doi: 10.1016/j.vprsr.2018.10.001
171. Krämer F„ Schaper R, Schunack B, Polozowski A, Piekarska J, Szwedko A, et al. Serological detection of Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis antibodies and Dirofilaria immitis antigen in a countrywide survey in dogs in Poland. Parasitol Res. (2014) 113:3229–39. doi: 10.1007/s00436-014-3985-7
172. Dziegiel B, Adaszek L, Carbonero A, Łyp P, Winiarczyk M, Debiak P, et al. Detection of canine vector-borne diseases in eastern Poland by ELISA and PCR. Parasitol Res. (2016) 115:1039–44. doi: 10.1007/s00436-015-4832-1
173. Skotarczak B, Adamska M, Rymaszewska A, Supron M, Sawczuk M, Maciejewska A. Anaplasma phagocytophila and protozoans of Babesia genus in dogs from endemic areas of Lyme disease in north-western Poland. Wiad Parazytol. (2004) 50:555–61.
174. Rymaszewska A, Adamska M. Molecular evidence of vector-borne pathogens coinfecting dogs from Poland. Acta Vet Hung. (2011) 59:215–23. doi: 10.1556/AVet.2011.008
175. Welc-Faleciak R, Rodo A, Siński E, Bajer A. Babesia canis and other tick-borne infections in dogs in Central Poland. Vet Parasitol. (2009) 166:191–8. doi: 10.1016/j.vetpar.2009.09.038
176. Kybicová K, Schánilec P, Hulínská D, Uherková L, Kurzová Z, Spejchalová S. Detection of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in dogs in the Czech Republic. Vector Borne Zoonotic Dis. (2009) 9:655–62. doi: 10.1089/vbz.2008.0127
177. Pennisi MG, Caprì A, Solano-Gallego L, Lombardo G, Torina A, Masucci M. Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis, and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area (Italy). Ticks Tick Borne Dis. (2012) 3:315–8. doi: 10.1016/j.ttbdis.2012.10.026
178. Ebani VV, Bertelloni F, Torracca B, Cerri D. Serological survey of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann Agric Environ Med. (2014) 21:671–5. doi: 10.5604/12321966.1129912
179. Ebani VV, Cerri D, Fratini F, Ampola M, Andreani E. Seroprevalence of Anaplasma phagocytophilum in domestic and wild animals from central Italy. New Microbiol. (2008) 31:371–5.
180. Ebani VV, Bertelloni F, Turchi B, Cerri D. Serological and molecular survey of Anaplasma phagocytophilum in Italian hunting dogs. Ann Agric Environ Med. (2013) 20:289–92.
181. Ebani VV. Serological survey of Ehrlichia canis and Anaplasma phagocytophilum in dogs from central Italy: an update (2013–2017). Pathogens. (2019) 8:3. doi: 10.3390/pathogens8010003
182. de la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, et al. Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet Res. (2006) 2:24. doi: 10.1186/1746-6148-2-24
183. Torina A, Vicente J, Alongi A, Scimeca S, Turla R, Nicosia S, et al. Observed prevalence of tick-borne pathogens in domestic animals in Sicily, Italy during 2003–2005. Zoonoses Public Health. (2007) 54:8–15. doi: 10.1111/j.1863-2378.2007.00989.x
184. Torina A, Alongi A, Naranjo V, Scimeca S, Nicosia S, Di Marco V, et al. Characterization of Anaplasma infections in Sicily, Italy. Ann N Y Acad Sci. (2008) 1149:90–3. doi: 10.1196/annals.1428.065
185. Solano-Gallego L, Caprì A, Pennisi MG, Caldin M, Furlanello T, Trotta M. Acute febrile illness is associated with Rickettsia spp. infection in dogs. Parasit Vectors. (2015) 8:216. doi: 10.1186/s13071-015-0824-3
186. Vascellari M, Ravagnan S, Carminato A, Cazzin S, Carli E, Da Rold G, et al. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasit Vectors. (2016) 9:369. doi: 10.1186/s13071-016-1639-6
187. Alberti A, Addis MF, Sparagano O, Zobba R, Chessa B, Cubeddu T, et al. Anaplasma phagocytophilum, Sardinia, Italy. Emerg Infect Dis. (2005) 11:1322–4. doi: 10.3201/eid1108.050085
188. Cardoso L, Mendão C, Madeira de Carvalho L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal — a national serological study. Parasit Vectors. (2012) 5:62. doi: 10.1186/1756-3305-5-62
189. Santos AS, Alexandre N, Sousa R, Núncio MS, Bacellar F, Dumler JS. Serological and molecular survey of Anaplasma species infection in dogs with suspected tick-borne disease in Portugal. Vet Rec. (2009) 164:168–71. doi: 10.1136/vr.164.6.168
190. Santos AS, Bacellar F, Dumler JS. A 4-year study of Anaplasma phagocytophilum in Portugal. Clin Microbiol Infect. (2009) 15:46–7. doi: 10.1111/j.1469-0691.2008.02172
191. Alho AM, Pita J, Amaro A, Amaro F, Schnyder M, Grimm F, et al. Seroprevalence of vector-borne pathogens and molecular detection of Borrelia afzelii in military dogs from Portugal. Parasit Vectors. (2016) 9:225. doi: 10.1186/s13071-016-1509-2
192. Pantchev N, Schaper R, Limousin S, Norden N, Weise M, Lorentzen L. Occurrence of Dirofilaria immitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in domestic dogs in France: results of a countrywide serologic survey. Parasitol Res. (2009) 105:S101–14. doi: 10.1007/s00436-009-1501-2
193. Miró G, Montoya A, Roura X, Gálvez R, Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: a multicentre study. Parasit Vectors. (2013) 6:117. doi: 10.1186/1756-3305-6-117
194. Amusategui I, Tesouro MA, Kakoma I, Sainz A. Serological reactivity to Ehrlichia canis, Anaplasma phagocytophilum, Neorickettsia risticii, Borrelia burgdorferi and Rickettsia conorii in dogs from northwestern Spain. Vector Borne Zoonotic Dis. (2008) 8:797–803. doi: 10.1089/vbz.2007.0277
195. Díaz-Regañón D, Roura X, Suárez ML, León M, Sainz A. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasit Vectors. (2020) 13:301. doi: 10.1186/s13071-020-04172-5
196. Couto CC, Lorentzen L, Beall MJ, Shields J, Bertolone N, Couto JI, et al. Serological study of selected vector-borne diseases in shelter dogs in central Spain using point-of-care assays. Vector Borne Zoonotic Dis. (2010) 10:885–8. doi: 10.1089/vbz.2009.0063
197. Aktas M, Özübek S, Altay K, Ipek NDS, Balkaya I, Armagan Erdem Utuk AE, et al. Molecular detection of tick-borne rickettsial and protozoan pathogens in domestic dogs from Turkey. Parasit Vectors. (2015) 8:157. doi: 10.1186/s13071-015-0763-z
198. Cetinkaya H, Matur E, Akyazi I, Ekiz E, Aydin L, Toparlak M. Serological and molecular investigation of Ehrlichia spp and Anaplasma spp in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick Borne Dis. (2016) 7:706–14. doi: 10.1016/j.ttbdis.2016.02.021
199. Huber D, Reil I, Duvnjak S, Jurkovic D, Lukacevic D, Pilat M, et al. Molecular detection of Anaplasma platys, Anaplasma phagocytophilum and Wolbachia sp. but not Ehrlichia canis in Croatian dogs. Parasitol Res. (2017) 116:3019–26. doi: 10.1007/s00436-017-5611-y
200. Mrljak V, Kuleš J, Mihaljević Z, Torti M, Gotić J, Crnogaj M, et al. Prevalence and geographic distribution of vector-borne pathogens in apparently healthy dogs in Croatia. Vector Borne Zoonotic Dis. (2017) 17:398–408. doi: 10.1089/vbz.2016.1990
201. Diakou A, Di Cesare A, Morelli S, Colombo M, Halos L, Simonato G, et al. Endoparasites and vector-borne pathogens in dogs from Greek islands: pathogen distribution and zoonotic implications. PLoS Negl Trop Dis. (2019) 13:e0007003. doi: 10.1371/journal.pntd.0007003
202. Inokuma H, Ohno K, Onishi T, Raoult D, Brouqui P. Detection of ehrlichial infection by PCR in dogs from Yamaguchi and Okinawa Prefectures, Japan. J Vet Med Sci. (2001) 63:815–7. doi: 10.1292/jvms.63.815
203. Fukui Y, Inokuma H. Subclinical infections of Anaplasma phagocytophilum and Anaplasma bovis in dogs from Ibaraki, Japan. Jpn J Infect Dis. (2019) 72:168–72. doi: 10.7883/yoken.JJID.2018.470
204. Xia Z, Yu D, Mao J, Zhang Z, Yu J. The occurrence of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis and Anaplasma phagocytophium in dogs in China. J Helminthol. (2012) 86:185–9. doi: 10.1017/S0022149X11000198
205. Cui Y, Yan Y, Wang X, Cao S, Zhang Y, Jian F, et al. First molecular evidence of mixed infections of Anaplasma species in dogs in Henan, China. Ticks Tick Borne Dis. (2017) 8:283–9. doi: 10.1016/j.ttbdis.2016.12.001
206. Wang S, He J, Zhang L. Serological investigation of vector-borne disease in dogs from rural areas of China. Asian Pac J Trop Biomed. (2012) 2:102–3. doi: 10.1016/S2221-1691(11)60201-3
207. Zhang J, Liu Q, Wang D, Li W, Beugnet F, Zhou J. Epidemiological survey of ticks and tick-borne pathogens in pet dogs in south-eastern China. Parasite. (2017) 24:35. doi: 10.1051/parasite/2017036
208. Wang J, Kelly P, Zhang J, Shi Z, Song C, Zheng X, et al. Detection of Dirofilaria immitis antigen and antibodies against Anaplasma phagocytophilum, Borrelia burgdorferi and Ehrlichia canis in dogs from ten provinces of China. Acta Parasitol. (2018) 63:412–5. doi: 10.1515/ap-2018-0047
209. Yang B, Ye C, Sun E, Wen Y, Qian D, Sun H. First molecular evidence of Anaplasma spp. co-infection in stray dogs from Anhui, China. Acta Trop. (2020) 206:105453. doi: 10.1016/j.actatropica.2020.105453
210. Lee S, Lee SH, VanBik D, Kim NH, Kim KT, Goo YK, et al. First molecular detection and phylogenetic analysis of Anaplasma phagocytophilum in shelter dogs in Seoul, Korea. Ticks Tick Borne Dis. (2016) 7:945–50. doi: 10.1016/j.ttbdis.2016.04.011
211. Suh GH, Ahn KS, Ahn JH, Kim HJ, Leutenegger C, Shin SS. Serological and molecular prevalence of canine vector-borne diseases (CVBDs) in Korea. Parasit Vectors. (2017) 10:146. doi: 10.1186/s13071-017-2076-x
212. Jung BY, Gebeyehu EB, Seo MG, Byun JW, Kim HY, Kwak D. Prevalence of vector-borne diseases in shelter dogs in Korea. Vet Rec. (2012) 171:249. doi: 10.1136/vr.100650
213. Lim S, Irwin PJ, Lee S, Oh M, Ahn K, Myung B, Shin S. Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasit Vectors. (2010) 3:32. doi: 10.1186/1756-3305-3-32
214. Bell DR, Berghaus RD, Patel S, Beavers S, Fernandez I, Sanchez S. Seroprevalence of tick-borne infections in military working dogs in the Republic of Korea. Vector Borne Zoonotic Dis. (2012) 12:1023–30. doi: 10.1089/vbz.2011.0864
215. Koh FX, Panchadcharam C, Tay ST. Vector-borne diseases in stray dogs in Peninsular Malaysia and molecular detection of Anaplasma and Ehrlichia spp. from Rhipicephalus sanguineus (Acari: Ixodidae) ticks. J Med Entomol. (2016) 53:183–7. doi: 10.1093/jme/tjv153
216. Borthakur SK, Deka DK, Bhattacharjee K, Sarmah PC. Seroprevalence of canine dirofilariosis, granulocytic anaplasmosis and lyme borreliosis of public health importance in dogs from India's North East. Vet World. (2014) 7:665–7. doi: 10.14202/vetworld.2014.665-667
217. Manoj RRS, Iatta R, Latrofa MS, Capozzi L, Raman M, Colella V, et al. Canine vector-borne pathogens from dogs and ticks from Tamil Nadu, India. Acta Trop. (2020) 203:105308. doi: 10.1016/j.actatropica.2019.105308
218. Levi O, Waner T, Baneth G, Keysary A, Bruchim Y, Silverman J, et al. Seroprevalence of Anaplasma phagocytophilum among healthy dogs and horses in Israel. J Vet Med B Infect diseases Vet Public Health. (2006) 53:78–80. doi: 10.1111/j.1439-0450.2006.00911.x
219. Qablan MA, Kubelová M, Siroký P, Modry D, Amr ZS. Stray dogs of northern Jordan as reservoirs of ticks and tick-borne hemopathogens. Parasitol Res. (2012) 111:301–7. doi: 10.1007/s00436-012-2839-4
220. Obaidat MM, Alshehabat MA. Zoonotic Anaplasma phagocytophilum, Ehrlichia canis, Dirofilaria immitis, Borrelia burgdorferi, and spotted fever group rickettsiae (SFGR) in different types of dogs. Parasitol Res. (2018) 117:3407–12. doi: 10.1007/s00436-018-6033-1
221. Yuasa Y, Tsai YL, Chang CC, Hsu TH, Chou CC. The prevalence of Anaplasma platys and a potential novel Anaplasma species exceed that of Ehrlichia canis in asymptomatic dogs and Rhipicephalus sanguineus in Taiwan. J Vet Med Sci. (2017) 79:1494–502. doi: 10.1292/jvms.17-0224
222. Hamidinejat TH, Bahrami S, Mosalanejad B, Pahlavan S. First molecular survey on Anaplasma phagocytophilum revealed high prevalence in rural dogs from Khuzestan Province, Iran. Iran J Parasitol. (2019) 14:297–302. doi: 10.18502/ijpa.v14i2.1142
223. Yousefi A, Chaechi Nosrati MR, Golmohammadi A, Azami S. Molecular detection of Anaplasma phagocytophilum as a zoonotic agent in owned and stray dogs in Tehran, Iran. Archiv Razi Instit. (2019) 74:33–8. doi: 10.22092/ari.2018.114893.1142
224. M'ghirbi Y, Ghorbel A, Amouri M, Nebaoui A, Haddad S, Bouattour A. Clinical, serological, and molecular evidence of ehrlichiosis and anaplasmosis in dogs in Tunisia. Parasitol Res. (2009) 104:767–74. doi: 10.1007/s00436-008-1253-4
225. Azzag N, Petit E, Gandoin C, Bouillin C, Ghalmi F, Haddad N, et al. Prevalence of select vector-borne pathogens in stray and client-owned dogs from Algiers. Comp Immunol Microbiol Infect Dis. (2015) 38:1–7. doi: 10.1016/j.cimid.2015.01.001
226. Elhamiani Khatat S, Khallaayoune K, Errafyk N, Van Gool F, Duchateau L, Daminet S, et al. Detection of Anaplasma spp. and Ehrlichia spp. antibodies, and Dirofilaria immitis antigens in dogs from seven locations of Morocco. Vet Parasitol. (2017) 239:86–9. doi: 10.1016/j.vetpar.2017.04.004
227. Elhamiani Khatat S, Daminet S, Kachani M, Leutenegger C, Duchateau L, El Amri H, et al. Anaplasma spp. in dogs and owners in northwestern Morocco. Parasit Vectors. (2017) 10:202. doi: 10.1186/s13071-017-2148-y
228. Aquino LC, Kamani J, Haruna JM, Paludo GR, Hicks CA, Helps CR, et al. Analysis of risk factors and prevalence of haemoplasma infection in dogs. Vet Parasitol. (2016) 221:111–7. doi: 10.1016/j.vetpar.2016.03.014
229. Kolo AO, Sibeko-Matjila KP, Maina AN, Richards AL, Knobel DL, Majtila PT. Molecular detection of zoonotic Rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector Borne Zoonotic Dis. (2016) 16:245–52. doi: 10.1089/vbz.2015.1849
230. Kolo AO, Collins NE, Brayton KE, Chaisi M, Blumberg L, Frean J, et al. Anaplasma phagocytophilum and other Anaplasma spp. in various hosts in the Mnisi Community, Mpumalanga Province, South Africa. Microorganisms. (2020) 8:1812. doi: 10.3390/microorganisms8111812
231. Clarke LL, Ballweber LR, Little SE, Lapplin MR. Prevalence of select vector-borne disease agents in owned dogs of Ghana. J S Afr Vet Assoc. (2014) 85:996. doi: 10.4102/jsava.v85i1.996
232. Kirchner M, Brunner A, Edelhofer R, Joachim A. Vector-borne parasites of dogs on the Islands of Cabo Verde. Wien Klin Wochenschr. (2008) 120:49–53. doi: 10.1007/s00508-008-1075-5
233. Lauzi S, Maia JP, Epis S, Marcos R, Pereira C, Luzzago C, et al. Molecular detection of Anaplasma platys, Ehrlichia canis, Hepatozoon canis and Rickettsia monacensis in dogs from Maio Island of Cape Verde archipelago. Ticks Tick Borne Dis. (2016) 7:964–9. doi: 10.1016/j.ttbdis.2016.05.001
234. Diniz PP, Breitschwerdt EB. Anaplasma phagocytophilum infection (canine granulocytic anaplasmosis). In: Greene GE, editor. Infectious Diseases of the Dog and Cat. St. Louis, MO: Elsevier Sauders (2012). p. 244–54.
235. Sainz A, Roura X, Miró G, Estrada-Pena A, Kohn B, Harrus S, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors. (2015) 8:75. doi: 10.1186/s13071-015-0649-0
236. Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, et al. Coinfection with multiple tick-borne pathogens in a Walker hound kennel in North Carolina. J Clin Microbiol. (1999) 37:2631–8. doi: 10.1128/JCM.37.8.2631-2638.1999
237. Inokuma H, Fujii K, Matsumoto K, Okuda M, Nakagome K, Kosugi R, et al. Demonstration of Anaplasma (Ehrlichia) platys inclusions in peripheral blood platelets of a dog in Japan. Vet Parasitol. (2002) 110:145–52. doi: 10.1016/s0304-4017(02)00289-3
238. Plier ML, Breitschwerdt EB, Hegarty BC, Kidd LB. Lack of evidence for perinatal transmission of canine granulocytic anaplasmosis from a bitch to her offspring. J Am Anim Hosp Assoc. (2009) 45:232–8. doi: 10.5326/0450232
239. Santos F, Coppede JS, Pereira AL, Oliveira LP, Roberto PG, Benedetti RBR, et al. Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirao Preto, Brazil. Vet J. (2009) 179:145–8. doi: 10.1016/j.tvjl.2007.08.017
240. Chandrashekar R, Mainville CA, Beall MJ O, Connor T, Eberts MD, Alleman AR, et al. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res. (2010) 71:1443–50. doi: 10.2460/ajvr.71.12.1443
241. Harvey JW. Anaplasma platys infection (thrombocytotropic anaplasmosis). In: Greene GE, editor. Infectious Diseases of the Dog and Cat. St. Louis, MO: Elsevier Saunders (2012). p. 241–4.
242. Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. (1998) 36:2645–51. doi: 10.1128/JCM.36.9.2645-2651.1998
243. Waner T, Harrus S, Jongejan F, Bark H, Keysary A, Cornelissen AW. Significance of serological testing for ehrlichial diseases in dogs with special emphasis on the diagnosis of canine monocytic ehrlichiosis caused by Ehrlichia canis. Vet Parasitol. (2001) 95:1–15. doi: 10.1016/s0304-4017(00)00407-6
244. Foley J, Drazenovich N, Leutenegger CM, Chomel BB. Association between polyarthritis and thrombocytopenia and increased prevalence of vector-borne pathogens in Californian dogs. Vet Rec. (2007) 160:159–62. doi: 10.1136/vr.160.5.159
245. Liddell AM, Stockham SL, Scott MA, Summer JW, Paddock CD, Gaudreault-Keener M, et al. Predominance of Ehrlichia ewingii in Missouri dogs. J Clin Microbiol. (2003) 41:4617–22. doi: 10.1128/jcm.41.10.4617-4622.2003
246. Arsenault WG, Messick JB. Acute granulocytic ehrlichiosis in a Rottweiler. J Am Anim Hosp Assoc. (2005) 41:323–6. doi: 10.5326/0410323
247. Kane A, Bloc G, Heeb LA. An unusual presentation of granulocytic anaplasmosis in a young dog. J Am Anim Hosp Assoc. (2011) 47:276–9. doi: 10.5326/JAAHA-MS-5652
248. Liu Y, Watson SC, Gettings JR, Lund RB, Nordone SK, Yabsley MJ, et al. A bayesian spatio-temporal model for forecasting Anaplasma species seroprevalence in domestic dogs within the contiguous United States. PLoS ONE. (2017) 12:e0182028. doi: 10.1371/journal.pone.0182028
249. Gettings JR, Self SCW, McMahan CS, Brown DA, Nordone SK, Yabsley MJ. Regional and local temporal trends of Borrelia burgdorferi and Anaplasma spp. seroprevalence in domestic dogs: contiguous United States 2013–2019. Front Vet Sci. (2020) 7:561592. doi: 10.3389/fvets.2020.561592
250. Dewage BG, Little S, Payton M, Beall M, Braff J, Szlosek D, et al. Trends in canine seroprevalence to Borrelia burgdorferi and Anaplasma spp. in the eastern USA, 2010–2017. Parasit Vectors. (2019) 12:476. doi: 10.1186/s13071-019-3735-x
251. Lester SJ, Breitschwerdt EB, Collis CD, Hegarty BC. Anaplasma phagocytophilum infection (granulocytic anaplasmosis) in a dog from Vancouver Island. Can Vet J. (2005) 46:825–7.
252. Cockwill KR, Taylor SM, Snead ECR, Dickinson R, Cosford K, Malek S, et al. Granulocytic anaplasmosis in three dogs from Saskatoon, Saskatchewan. Can Vet J. (2009) 50:835–40.
253. Elhamiani Khatat S, Culang D, Gara-Boivin C. Granulocytic anaplasmosis in two dogs from Quebec. Can Vet J. (2018) 9:663–7.
254. McCown ME, Monterroso VH, Cardona W. Surveillance for Ehrlichia canis, Anaplasma phagocytophilum, Borrelia burgdorferi, and Dirofilaria immitis in dogs from three cities in Colombia. J Spec Oper Med. (2014) 14:86–90.
255. Silveira JA, Valente PC, Paes PR, Vasconcelos AV, Silvestre BT, Ribeiro MFB, et al. The first clinical and laboratory evidence of co-infection by Anaplasma phagocytophilum and Ehrlichia canis in a Brazilian dog. Ticks Tick Borne Dis. (2015) 6:242–5. doi: 10.1016/j.ttbdis.2015.01.003
256. Tozon N, Petrovec M, Avsic-Zupanc T. Clinical and laboratory features of the first detected cases of A. phagocytophila infections in dogs from Slovenia. Ann N Y Acad Sci. (2003) 990:424–8. doi: 10.1111/j.1749-6632.2003.tb07405.x
257. Bexfield NH, Villiers EJ, Herrtage ME. Immune-mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract. (2005) 46:543–8. doi: 10.1111/j.1748-5827.2005.tb00284.x
258. Melter O, Stehlik I, Kinska H, Volfova I. Infection with Anaplasma phagocytophilum in a young dog: a case report. Vet Med. (2007) 52:207–12. doi: 10.17221/2001-VETMED
259. Domingos MC, Trotta M, Briend-Marchal A, Medaille C. Anaplasmosis in two dogs in France and molecular and phylogenetic characterization of Anaplasma phagocytophilum. Vet Clin Pathol. (2011) 40:215–21. doi: 10.1111/j.1939-165X.2011.00321.x
260. Dewree R, Coquereaux G, Heymann P, Cochez C. Anaplasma phagocytophilum in a dog in Belgium. Acta Clin Belg. (2014) 69:S4–5.
261. Dondi F, Russo S, Agnoli C, Mengoli N, Balboni A, Alberti A, et al. Clinicopathological and molecular findings in a case of canine Anaplasma phagocytophilum infection in Northern Italy. ScientificWorldJournal. (2014) 2014:810587. doi: 10.1155/2014/810587
262. Elhamiani Khatat S, Defauw P, Marynissen S, Van de Maele I. Exposure to Anaplasma phagocytophilum in two dogs in Belgium. Vlaams Diergen Tijds. (2015) 84:39–46. doi: 10.21825/vdt.v84i1.16621
263. Inokuma H, Oyamada M, Kelly PJ, Jacobson LA, Fournier PE, Itamoto K, et al. Molecular detection of a new Anaplasma species closely related to Anaplasma phagocytophilum in canine blood from South Africa. J Clin Microbiol. (2005) 43:2934–7. doi: 10.1128/JCM.43.6.2934-2937.2005
264. Barlough JE, Madigan JE, Turoff DR, Clover JR. An Ehrlichia strain from a llama (Lama glama) and llama-associated ticks (Ixodes pacificus). J Clin Microbiol. (1997) 35:1005–7. doi: 10.1128/JCM.35.4.1005-1007.1997
265. Burgess H, Chilton NB, Krakowetz CN, Williams C, Lohmann K. Granulocytic anaplasmosis in a horse from Saskatchewan. Can Vet J. (2012) 53:886–8.
266. Gordon WS, Brownlee A, Wilson DR, Macleod J. Tick-borne fever (a hitherto undescribed disease of sheep). J Comp Pathol Ther. (1932) 65:301–7. doi: 10.1016/S0368-1742(32)80025-1
267. Tinkler SH, Firshman AM, Sharkey LC. Premature parturition, edema, and ascites in an alpaca infected with Anaplasma phagocytophilum. Can Vet J. (2012) 53:1199–202.
268. Aktas M, SÖzübek S. Bovine anaplasmosis in Turkey: First laboratory confirmed clinical cases caused by Anaplasma phagocytophilum. Vet Microbiol. (2015) 178:246–51. doi: 10.1016/j.vetmic.2015.05.021
269. Savidge C, Ewing P, Andrews J, Aucoin D, Lapplin JR, Moroff S. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J Feline Med Surg. (2016) 18:85–91. doi: 10.1177/1098612X15571148
270. Schorn S, Pfister K, Reulen H, Mahling M, Manitz J, Thiel C, et al. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus in Bavarian public parks, Germany. Ticks Tick Borne Dis. (2011) 2:196–203. doi: 10.1016/j.ttbdis.2011.09.009
271. Nuttall PA, Labuda M. Dynamics of infection in tick vectors and at the tick-host interface. Adv Virus Res. (2003) 60:233–72. doi: 10.1016/s0065-3527(03)60007-2
272. Gray J, Kahl O, Lane RS, StanekG. Lyme Borreliosis Biology, Epidemiology and Control. Wallingford: CABI (2002).
273. Pichon B, Estrada-Pena A, Kahl, Manneli A. Detection of animal reservoirs of tick-borne zoonoses in Europe. Int J Med Microbiol. (2006) 296:129–30.
274. Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hhbalek Z, Földvari G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. (2014) 2:251. doi: 10.3389/fpubh.2014.00251
275. Skerget M, Wenisch C, Daxboeck F, Krause R, Haberl R, Stuenzner D. Cat or dog ownership and seroprevalence of ehrlichiosis, Q fever, and cat-scratch disease. Emerg Infect Dis. (2003) 9:1337–9. doi: 10.3201/eid0910.030206
276. André MR. Diversity of Anaplasma and Ehrlichia/Neoehrlichia agents in terrestrial wild carnivores worldwide: implications for human and domestic animal health and wildlife conservation. Front Vet Sci. (2020) 5:293. doi: 10.3389/fvets.2018.00293
277. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. (2007) 76:1314–22.
278. Jones EH, Hinckley AF, Hook SA, Meek JI, Backenson B, Kugeler KJ, et al. Pet ownership increases human risk of encountering ticks. Zoonoses Public Health. (2018) 65:74–9. doi: 10.1111/zph.12369
279. Silaghi C, Gilles J, Höhle M, Fingerle V, Just FT, Pfister K. Anaplasma phagocytophilum infection in Ixodes ricinus, Bavaria, Germany. Emerg Infect Dis. (2008) 14:972–4. doi: 10.3201/eid1406.071095
280. Claerebout E, Losson B, Cochez C, Casaert S, Dalemans AC, de Cat A, et al. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit Vectors. (2013) 6:183. doi: 10.1186/1756-3305-6-183
281. Coipan EC, Fonville M, Tijsse-Klasen E, van der Giessen JW, Takken W, Sprong H, et al. Geodemographic analysis of Borrelia burgdorferi sensu lato using the 5S-23S rDNA spacer region. Infect Genet Evol. (2013) 17:216–22. doi: 10.1016/j.meegid.2013.04.009
282. Overzier E, Pfister K, Thiel C, Herb I, Mahling M, Silaghi C. Anaplasma phagocytophilum in questing Ixodes ricinus ticks: comparison of prevalences and partial 16S rRNA gene variants in urban, pasture, and natural habitats. Appl Environ Microbiol. (2013) 79:1730–4. doi: 10.1128/AEM.03300-12
283. Król N, Obiegala A, Pfeffer M, Lonc E, Kiewra D. Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration, South-West Poland. Parasit Vectors. (2016) 9:351. doi: 10.1186/s13071-016-1632-0
284. Nair ADS, Cheng C, Ganta CK, Sanderson MW, Alleman AR, Munderloh UG, et al. Comparative experimental infection study in dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum. PLoS ONE. (2016) 11:e0148239. doi: 10.1371/journal.pone.0148239
285. Hodzic E, Fish D, Maretzki CM, De Silva AM, Feng S, Barthold SW. Acquisition and transmission of the agent of granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol. (1998) 36:3574–8. doi: 10.1128/JCM.36.12.3574-3578.1998
286. des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC III, Fish D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis. (2001) 183:773–8. doi: 10.1086/318818
287. Katavolos P, Armstrong PM, Dawson JE, Telford SR III. Duration of tick attachment required for transmission of granulocytic ehrlichiosis. J Infect Dis. (1998) 177:1422–5. doi: 10.1086/517829
288. Fourie JJ, Evans A, Labuschagne M, Crafford D, Madder M, Pollmeier M, Schunack B. Transmission of Anaplasma phagocytophilum (Foggie, 1949) by Ixodes ricinus (Linnaeus, 1758) ticks feeding on dogs and artificial membranes. Parasit Vectors. (2019) 12:136. doi: 10.1186/s13071-019-3396-9
289. Wang J, Dyachenko V, Munderloh UG, Straubinger RK. Transmission of Anaplasma phagocytophilum from endothelial cells to peripheral granulocytes in vitro under shear flow conditions. Med Microbiol Immunol. (2015) 204:593–603. doi: 10.1007/s00430-015-0387-0
290. Lai TH, Kumagai Y, Hyodo M, Hayakawa Y, Rikihisa Y. The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two-component system and role of cyclic Di-GMP in host cell infection. J Bacteriol. (2009) 191:693–700. doi: 10.1128/JB.01218-08
291. Troese MJ, Kahlon A, Ragland SA, Ottens AK, Ojogun N, Nelson KT, et al. Proteomic analysis of Anaplasma phagocytophilum during infection of human myeloid cells identifies a protein that is pronouncedly upregulated on the infectious dense-cored cell. Infect Immun. (2011) 79:4696–707. doi: 10.1128/IAI.05658-11
292. Martin ME, Bunnell JE, Dumler JS. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J Infect Dis. (2000) 181:374–8. doi: 10.1086/315206
293. Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. (2005) 174:6364–72. doi: 10.4049/jimmunol.174.10.6364
294. Carlyon JA, Fikrig E. Mechanism of evasion of neutrophil killing by Anaplasma phagocytophilum. Curr Opin Hematol. (2006) 13:28–33. doi: 10.1097/01.moh.0000190109.00532.56
295. Rikihisa Y. Ehrlichia subversion of host innate responses. Curr Opin Microbiol. (2006) 9:95–101. doi: 10.1016/j.mib.2005.12.003
296. Garcia-Garcia JC, Rennoll-Bankert KE, Pelly S, Milstone AM, Dumler JS. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect Immun. (2009) 77:2385–91. doi: 10.1128/IAI.00023-09
297. Palmer GH, Bankhead T, Lukehart SA. ‘Nothing is permanent but change’-antigenic variation in persistent bacterial pathogens. Cell Microbiol. (2009) 11:1697–705. doi: 10.1111/j.1462-5822.2009.01366.x
298. Yoshikawa Y, Sugimoto K, Ochiai Y, Ohashi N. Intracellular proliferation of Anaplasma phagocytophilum is promoted via modulation of endoplasmic reticulum stress signaling in host cells. Microbiol Immunol. (2020) 64:270–9. doi: 10.1111/1348-0421.12770
299. Bunnell JE, Trigiani ER, Srinivas SR, Dumler JS. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J Infect Dis. (1999) 180:546–50. doi: 10.1086/314902
300. Martin ME, Caspersen K, Dumler JS. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am J Pathol. (2001) 158:1881–8. doi: 10.1016/s0002-9440(10)64145-4
301. Birkner K, Steiner B, Rinkler C, Kern Y, Aichele P, Bogdan C, et al. The elimination of Anaplasma phagocytophilum requires CD41 T cells, but is independent of Th1 cytokines and a wide spectrum of effector mechanisms. Eur J Immunol. (2008) 38:3395–410. doi: 10.1002/eji.200838615
302. Lilliehöök I, Egenvall A, Tvedten HW. Hematopathology in dogs experimentally infected with a Swedish granulocytic Ehrlichia species. Vet Clin Pathol. (1998) 27:116–22. doi: 10.1111/j.1939-165x.1998.tb01030.x
303. Scorpio DG, von Loewenich FD, Goebel H, Bogdan C, Dumler JS. Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clin Vaccine Immunol. (2006) 13:806–9. doi: 10.1128/CVI.00092-06
304. Eckersall PD. Acute phase proteins as markers of inflammatory lesions. Comp Hematol Int. (1995) 5:93–7.
305. Klein MB, Hu S, Chao CC, Goodman JL. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without the induction of proinflammatory cytokines. J Infect Dis. (2000) 182:200–5. doi: 10.1086/315641
306. Akkoyunlu M, Fikrig E. Gamma interferon dominates the murine cytokine response to the agent of human granulocytic ehrlichiosis and helps to control the degree of early rickettsemia. Infect Immun. (2000) 68:1827–33. doi: 10.1128/iai.68.4.1827-1833.2000
307. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. (2000) 6:601–8. doi: 10.3201/eid0606.000608
308. Dumler JS, Barat NC, Barat CE, Bakken JS. Human granulocytic anaplasmosis and macrophage activation. Clin Infect Dis. (2007) 45:199–204. doi: 10.1086/518834
309. Janka GE, Lehmberg K. Hemophagocytic syndromes — an update. Blood Rev. (2014) 28:135–42. doi: 10.1016/j.blre.2014.03.002
310. Sun W, Ijdo JW, Telford SR III, Hodzic E, Zhang Y, Barthold SW, Fikrig E. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J Clin Invest. (1997) 100:3014–8. doi: 10.1172/JCI119855
311. von Loewenich FD, Scorpio DG, Reischl U, Dumler JS, Bogdan C. Frontline: control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR)2 and TLR4, or the TLR adaptor molecule MyD88. Eur J Immunol. (2004) 34:1789–97. doi: 10.1002/eji.200425029
312. Goldman EE, Breitschwerdt EB, Grindem CB, Hegarty BC, Walls JJ, Dumler JS. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J Vet Intern Med. (1998) 12:61–70. doi: 10.1111/j.1939-1676.1998.tb02096.x
313. Berzina I, Krudewig C, Silaghi C, Matise I, Ranka R, Müller N, et al. Anaplasma phagocytophilum DNA amplified from lesional skin of seropositive dogs. Ticks Tick Borne Dis. (2014) 5:329–35. doi: 10.1016/j.ttbdis.2013.12.010
314. Egenvall A, Bjoersdorff A, Lilliehook I, Engvall EO, Karlstam E, Artursson K, et al. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet Rec. (1998) 143:412–7. doi: 10.1136/vr.143.15.412
315. Kjelgaard-Hansen M, Lundorff Jensen A, Houser GA, Jessen LR, Kristensen AT. Use of serum C-reactive protein as an early marker of inflammatory activity in canine type II immune-mediated polyarthritis: case report. Acta Vet Scand. (2006) 48:9. doi: 10.1186/1751-0147-48-9
316. Tarello W. Canine granulocytic ehrlichiosis (CGE) in Italy. Acta Vet Hung. (2003) 51:73–90. doi: 10.1556/avet.51.2003.1.7
317. Jäderlund KH, Egenvall A, Bergström K, Hedhammar A. Seroprevalence of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in dogs with neurological signs. Vet Rec. (2007) 160:825–31. doi: 10.1136/vr.160.24.825
318. Jäderlund KH, Bergström K, Egenvall A, Hedhammar A. Cerebrospinal fluid PCR and antibody concentrations against Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in dogs with neurological signs. J Vet Intern Med. (2009) 23:669–72. doi: 10.1111/j.1939-1676.2009.0313.x
319. Brown WC. Adaptive immunity to Anaplasma pathogens and immune dysregulation: implications for bacterial persistence. Comp Immunol Microbiol Infect Dis. (2012) 35:241–52. doi: 10.1016/j.cimid.2011.12.002
320. Egenvall A, Lilliehöök I, Bjöersdorff A, Engvall EO, Karlstam E, Artursson K, et al. Detection of granulocytic Ehrlichia species DNA by PCR in persistently infected dogs. Vet Rec. (2000) 146:186–90. doi: 10.1136/vr.146.7.186
321. Alleman A, Chandrashekar R, Beall M, Cyr K, Barbet A, Lundgren A, et al. Experimental inoculation of dogs with human isolates (NY18) of Anaplasma phagocytophilum and demonstration of persistent infection following doxycycline therapy. J Vet Intern Med. (2006) 20:763.
322. Wamsley H. Mammalian host cell reservoirs during Anaplasma infection (dissertation), University of Florida, Gainesville, FL, United States (2009).
323. Granquist EG, Stuen S, Crosby L, Lundgren AM AR, Barbet AF. Variant-specific and diminishing immune responses towards the highly variable MSP2 (P44) outer membrane protein of Anaplasma phagocytophilum during persistent infection in lambs. Vet Immunol Immunopathol. (2010) 133:117–24. doi: 10.1016/j.vetimm.2009.07.009
324. Contreras ET, Dowers KL, Moroff S, Lappin MR. Clinical and laboratory effects of doxycycline and prednisolone in Ixodes scapularis-exposed dogs with chronic Anaplasma phagocytophilum infection. Top Companion Anim Med. (2018) 33:147–9. doi: 10.1053/j.tcam.2018.08.001
325. Harrus S, Waner T, Aizenberg I, Foley JE, Poland AM, Bark H. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol. (1998) 36:73–6. doi: 10.1128/JCM.36.1.73-76.1998
326. Foley JE, Lerche NW, Dumler JS, Madigan JE. A simian model of human granulocytic ehrlichiosis. Am J Trop Med Hyg. (1999) 60:987–93. doi: 10.4269/ajtmh.1999.60.987
327. Baxarias M, Álvarez-Fernández A, Martínez-Orellana P, Montserrat-Sangrà S, Ordeix L, Rojas A, et al. Does co-infection with vector-borne pathogens play a role in clinical canine leishmaniosis? Parasit Vectors. (2018) 11:135. doi: 10.1186/s13071-018-2724-9
328. Attipa C, Solano-Gallego L, Leutenegger CM, Papasouliotis K, Soutter F, Balzer J, et al. Associations between clinical canine leishmaniosis and multiple vector-borne co-infections: a case-control serological study. BMC Vet Res. (2019) 15:331. doi: 10.1186/s12917-019-2083-6
329. Nyarko E, Grab DJ, Dumler JS. Anaplasma phagocytophilum-infected neutrophils enhance transmigration of Borrelia burgdorferi across the human blood brain barrier in vitro. Int J Parasitol. (2006) 36:601–5. doi: 10.1016/j.ijpara.2006.01.014
330. Nieto NC, Foley JE. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife, and Ixodes ricinus-complex ticks. Vector Borne Zoonotic Dis. (2009) 9:93–102. doi: 10.1089/vbz.2008.0072
331. Levin ML, Fish D. Acquisition of coinfection and simultaneous transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis ticks. Infect Immun. (2000) 68:2183–6. doi: 10.1128/iai.68.4.2183-2186.2000
332. Grab DJ, Nyarko E, Barat NC, Nikolskaia OV, Dumler JS. Anaplasma phagocytophilum-Borrelia burgdorferi coinfection enhances chemokine, cytokine, and matrix metalloprotease expression by human brain microvascular endothelial cells. Clin Vaccine Immunol. (2007) 14:1420–4. doi: 10.1128/CVI.00308-07
333. Holden H, Hodzic E, Feng S, Freet KJ, Lefebvre RB, Barthold SW. Coinfection with Anaplasma phagocytophilum alters Borrelia burgdorferi population distribution in C3H/HeN mice. Infect Immun. (2005) 73:3440–4. doi: 10.1128/IAI.73.6.3440-3444.2005
334. Klein MB, Miller JS, Nelson CM, Goodman JL. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J Infect Dis. (1997) 176:1405–9. doi: 10.1086/517332
335. De Arcangeli S, Balboni A, Serafini F, Battilani M, Dondi F. Anaplasma phagocytophilum infection in thrombocytopenic dogs. Vet Ital. (2018) 54:73–8. doi: 10.12834/VetIt.1070.5796.2
336. Smith RD, Ristic M, Huxsoll DL, Baylor RA. Platelet kinetics in canine ehrlichiosis: evidence for increased platelet destruction as the cause of thrombocytopenia. Infect Immun. (1975) 11:1216–21. doi: 10.1128/IAI.11.6.1216-1221.1975
337. Waner T, Harrus S, Weiss DJ, Bark H, Keysary A. Demonstration of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol. (1995) 48:177–82. doi: 10.1016/0165-2427(95)05420-b
338. Wong SJ, Thomas JA. Cytoplasmic, nuclear, and platelet autoantibodies in human granulocytic anaplasmosis patients. J Clin Microbiol. (1998) 36:1959–63. doi: 10.1128/JCM.36.7.1959-1963.1998
339. Granick JL, Reneer DV, Carlyon JA, Borjesson DL. Anaplasma phagocytophilum infects cells of the megakaryocyte lineage through sialyated ligands but fails to alter platelet production. J Med Microbiol. (2008) 57:416–23. doi: 10.1099/jmm.0.47551-0
340. Behl R, Klein MB, Dandelet L, Bach RR, Goodman JL, Key NS. Induction of tissue factor procoagulant activity in myelomonocytic cells inoculated by the agent of human granulocytic ehrlichiosis. Thromb Haemost. (2000)83:114–8.
341. Borjesson DL, Simon SI, Tablin F, Barthold SW. Thrombocytopenia in a mouse model of human granulocytic ehrlichiosis. J Infect Dis. (2001) 184:1475–9. doi: 10.1086/324518
342. Beck A, Huber D, Antolić M, Anzulović Z, Reil I, Polkinghorne A, et al. Retrospective study of canine infectious haemolytic anaemia cases reveals the importance of molecular investigation in accurate postmortal diagnostic protocols. Comp Immunol Microbiol Infect Dis. (2019) 65:81–7. doi: 10.1016/j.cimid.2019.05.006
343. Respess M, O'Toole TE, Taeymans O, Rogers CL, Johnston A, Webster CRL. Portal vein thrombosis in 33 dogs: 1998–2011. J Vet Intern Med. (2012) 26:230–7. doi: 10.1111/j.1939-1676.2012.00893.x
344. Li H, Zhou Y, Wang W, Guo D, Huang S, Jie S. The clinical characteristics and outcomes of patients with human granulocytic anaplasmosis in China. Int J Infect Dis. (2011) 15:e859–66. doi: 10.1016/j.ijid.2011.09.008
345. Zhuo M, Calev H, Saunders SJ, Li J, Stillman IE, Danziger J. Acute kidney injury associated with human granulocytic anaplasmosis: a case report. Am J Kidney Dis. (2019) 74:696–9. doi: 10.1053/j.ajkd.2019.03.428
346. Schotthoefer AM, Meece JK, Ivacic LC, Bertz PD, Zhang K, Weiler T, et al. Comparison of a real-time PCR method with serology and blood smear analysis for diagnosis of human anaplasmosis: importance of infection time course for optimal test utilization. J Clin Microbiol. (2013) 51:2147–53. doi: 10.1128/JCM.00347-13
347. Stuen S, Casey ANJ, Woldehiwet Z, French NP, Ogden NH. Detection by the polymerase chain reaction of Anaplasma phagocytophilum in tissues of persistently infected sheep. J Comp Pathol. (2006) 134:101–4. doi: 10.1016/j.jcpa.2005.06.006
348. Franzen P, Aspan A, Egenvall A, Gunnarsson A, Karlstam E, Pringle J. Molecular evidence for persistence of Anaplasma phagocytophilum from acute experimental infection. J Vet Intern Med. (2009) 23:636–42. doi: 10.1111/j.1939-1676.2009.0317.x
349. Littman MP. Protein-losing nephropathy in small animals. Vet Clin North Am Small Anim Pract. (2011) 41:31–62. doi: 10.1016/j.cvsm.2010.09.006
Keywords: canine granulocytic anaplasmosis, Anaplasma phagocytophilum, dogs, epidemiology, tick-borne disease, zoonosis
Citation: El Hamiani Khatat S, Daminet S, Duchateau L, Elhachimi L, Kachani M and Sahibi H (2021) Epidemiological and Clinicopathological Features of Anaplasma phagocytophilum Infection in Dogs: A Systematic Review. Front. Vet. Sci. 8:686644. doi: 10.3389/fvets.2021.686644
Received: 27 March 2021; Accepted: 17 May 2021;
Published: 23 June 2021.
Edited by:
Sandra Diaz Sanchez, University of Castilla-La Mancha, SpainReviewed by:
Simone Morelli, University of Teramo, ItalyDiana Scorpio, Texas Biomedical Research Institute, United States
Copyright © 2021 El Hamiani Khatat, Daminet, Duchateau, Elhachimi, Kachani and Sahibi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah El Hamiani Khatat, ZWxoYW1pYW5pc0B5YWhvby5mcg==; cy5lbGhhbWlhbmlAaWF2LmFjLm1h
 Sarah El Hamiani Khatat
Sarah El Hamiani Khatat Sylvie Daminet
Sylvie Daminet Luc Duchateau3
Luc Duchateau3 Latifa Elhachimi
Latifa Elhachimi Malika Kachani
Malika Kachani