
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 20 September 2021
Sec. Animal Reproduction - Theriogenology
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.675669
This article is part of the Research Topic Immune Response Regulation and Ruminant Reproductive Diseases View all 7 articles
Endometritis is a common bacterial disease of dairy cows. Cathelicidins are host-defense peptides that play important roles in clearance of bacteria. However, the expression pattern of these peptides during endometritis is still unclear. We hypothesize that the levels of bovine cathelicidins increased during endometritis. This study was to investigate the changes of bovine cathelicidins during endometritis. Forty-four post-partum cows (28–35 days after calving) involved in this study were grouped according to the character of vaginal discharge (VD) into three groups. These were (1) cows with clear fluid (n = 8, healthy cows group, N); (2) cows with VD containing <50% off-white mucopurulent material (n = 20, moderate endometritis cows, M); (3) cows with VD containing > 50% yellow or white purulent material (n = 16, severe endometritis cows, S). The blood, VD, and endometrial biopsies samples were collected from each cow to assess the levels of cathelicidin 1–7. Furthermore, bovine endometrial epithelial cells (BEECs) were stimulated with different concentration of Escherichia coli (2 × 106 and 2 × 107 CFU/mL) to detect the cellular source of cathelicidins. Quantitative real-time PCR (RT-qPCR) was used to detect the relative mRNA expression of cathelicidins, and enzyme-linked immune sorbent assay (ELISA) method were used to measure the protein levels. The mRNA and protein levels of cathelicidin 1–7 significantly increased during bovine endometritis (both moderate and severe endometritis), while samples from severe cases showed lower levels of cathelicidins compared to moderate cases. BEECs can express cathelicidin 1–7, and E. coli triggered the release of these proteins. High concentration of E. coli decreased the mRNA and protein levels of cathelicidins. Taken together, our results supported that cathelicidins are released as host defense molecules against the bacteria during bovine endometritis, and BEECs play an active role in expression and production of cathelicidins.
Endometritis is one of the major bacterial diseases affecting reproductive performance of dairy cows by lengthening the calving interval, increasing number of services per conception and reducing calving rates (1). It also leads to significant economic loss to dairy industry due to reducing low milk production, increasing treatment cost, and poor fertility (2, 3). Endometritis is reported to mainly result from Gram-negative bacterial infection of the endometrium, such as Escherichia coli and Trueperella pyogenes (4, 5). The post-parturient invasion and growth of bacteria caused persistence inflammation of endometrium in dairy cows.
Cathelicidin is one of the major classes of mammalian host defense protein which is the crucial component of innate immune system (6). It exhibits multiple functions which includes direct anti-microbial activity, chemotaxis and regulatory function of inflammation (6–8). Ten cathelicidin genes including cathelicidin 1–7, Bac4, Bac2A, and IDR-1018 has been reported in cow (8), nevertheless, humans and mice only have one cathelicidin gene (9). Hence, these proteins are believed to play a crucial role in immunity of dairy cows. These proteins are confirmed to be released into milk during mastitis (10), and the expression of cathelicidin is also detected in mammary tissues (11). However, the expression pattern of cathelicidins during bovine endometritis still remains unknown.
It is well-known that cathelicidins are stored as intracellular granules in polymorphonuclear neutrophils (PMNs) which are recalled into the local tissues when infection occurs (12). In addition to PMNs, epithelial cell is also the important source of cathelicidins (13–15). In dairy cows, mammary epithelial cell is confirmed to produce and release cathelicidins (16). The role of bovine endometrial epithelial cells in expression and release of cathelicidins is not clearly defined. We assumed that cathelicidins also can be produced by endometrial epithelium cells to provide the first line of defense microbial invasion in bovine endometrium.
The objectives of this study were to investigate the role of bovine cathelicidins during endometritis, and detect the expression and production of cathelicidins in BEECs stimulated with E. coli.
The samples and clinical data were collected from Gansu Holstein Dairy Cow Breeding Center (Lanzhou, China). All cows were all housed, and the average annual temperature of Lanzhou city is −12–28°C. The inclusion criteria for the dairy cows in the present study were: (1) cows aged 3–5 years and 2–3 parities; (2) cows on 28–35 days post-partum; (3) cows without any signs of systemic symptoms during the post-partum period. The diagnostic criterion of endometritis was the presence of a purulent vaginal discharge, and the cows with clear vaginal discharge were considered as normal cows (1). Total of 125 post-partum cows were included in our screening scope. And forty-four post-parturient cows were adopted for further study based on the above standards. Vaginal discharge (VD) samples were collected using metricheck device (Simcro Limited, Mew Zealand). Briefly, the tail was lifted and perineum was cleaned with dry paper to remove fecal material and then wiped with 75% ethanol. Metricheck device was disinfected with 75% ethanol and then gently introduced into the vagina, the device was withdrawn and the discharge was collected in the cup. The cup was washed with saline and disinfected with 75% ethanol before the next collection. VD samples were transported to the laboratory on ice within 4–6 h of collection at the temperatures of 4°C.
Cows were grouped into normal cows group (N), moderate endometritis group (M), and severe endometritis group (S) according to the properties of vaginal discharge. The vaginal mucus was assessed by one person for color, proportion and volume of pus, and the character of the three groups assigned as follow: N, clear or translucent vaginal discharge; M, the vaginal discharge containing <50% off-white mucopurulent material; S, vaginal discharge containing > 50% yellow or white purulent material (17). The present study was approved by the Institutional Animal Care and Use Committee of Lanzhou Institute of Husbandry and Pharmaceutical Sciences of Chinese Academy of Agricultural Sciences (SYXK-2014-0002).
In the laboratory, pre-treatment of VD was modified according to method described for bovine vaginal discharge (18). Briefly, the discharge weighing ~1 g was taken from each sample and treated with sterile PBS (Gibco, Australia) containing 2 μg/mL protease inhibitor (Solarbio, China) at ratio 4:1 (v/w). Each sample was vortexed for 30 s and then rocked for 45 min at room temperature. Then the samples were centrifuged at 12,000 × g for 30 min at 4°C and the supernatant was collected for subsequent test. Bicinchoninic acid assay (BCA Protein Assay Kit Takara, Japan) was used to measure total protein level in the discharge samples, and the samples with higher total protein were diluted with PBS. Finally, total protein content in each sample is same. Then the working samples were stored at −80°C until further analysis.
Endometrial biopsies were performed using a biopsy forceps. Briefly, biopsy forceps (purchased and customized from online shops of the Taobao Mall, https://m.tb.cn/h.4zmpAhw?sm=fd2fa2, Supplementary Figure 1) was disinfected with 75% ethanol and introduced into the vagina. The tip of the forceps was guided into the horn of uterus through manipulation per rectum by an experienced veterinary. The biopsy was obtained approximately halfway between the cervix and utero-tubal junction carefully. A 3 × 3 mm tissue section was obtained using this forceps. Collected biopsy was placed into petri dish containing normal saline. Every tissue sample was divided into two pieces and immediately preserved in either liquid nitrogen for total RNA extraction or 10% formalin solution for histopathology examination using routine hematoxylin and eosin (HE) staining. Then, four-micrometer-thick sections cut from the fixed specimens were mounted on slides. Histologically, endometritis is characterized by some disruption of surface epithelium, infiltration with inflammatory cells, vascular congestion, and stromal edema and by varying degrees of lymphocyte and plasma cell accumulation in the superficial layers (19, 20). Slides were observed under microscope (Olympus, BX43), and assessed according to the histological features. The blood samples of tissues-collected cows were collected from caudal vein using vacuum tubes without anticoagulant and immediately stored on ice. Serum samples were harvested from the blood within 2 h, and were stored at −80°C until analysis.
Duplicate cytology smears were prepared immediately after the VD samples were collected. The slides were air-dried and stained using Diff-Quick staining protocol (Solarbio, China). Smears were evaluated microscopically and proportions of neutrophils were recorded. All cells were counted and the percentages of PMNs were calculated from average of 200 cells (18).
Bovine endometrial epithelial cells (BEECs) were isolated from a healthy uterus of a 6-month old dairy cow according to our previous study (19). Briefly, a healthy uterus was brought to laboratory within sterile PBS (pH 7.2) containing penicillin (100 μg/mL) and streptomycin (100 U/mL). Endometrium from uterine horn was cut off into 2–3 mm long pieces, washed in PBS (pH 7.2) twice, and then uterine tissue was digested with 1% collagenase I (Sigma, USA) diluted in DMEM/F12 (HyClone, USA) for 6 h. The digested endometrium was scraped using a sterile cell scraper, and scraped materials was collected and washed in PBS (pH 7.2). Then, the collected materials were centrifuged at 100 g for 5 min to collect cell suspension. Trypan Blue stain was used to estimate cells viability. Cells were cultured in DMEM/F12 with 10% fetal bovine serum (Gibco, Australia) and penicillin (100 μg/mL) and streptomycin (100 U/mL) at 37°C with a 5% CO2 and 95% sterile air when the viability >95%. The medium was changed every 3 days until the cells reached ~90% confluence.
Considering that the cultures were inevitably mixed by some stromal cells, which were removed according to the different sensitivity of epithelial cells and stromal cells to trypsin, and time-different digestion was conducted to obtain purified epithelial cells. The expression of epithelial-specific cytokeratin was adopted to estimate the purification of epithelial cells through using a mouse monoclonal antibody (Abcam, ab49779) and goat anti-mouse IgG-FITC (Abcam, ab6785).
BEECs growing in logarithmic phase were inoculated into a six-well plate at the concentration of 2 × 105 cells/well and incubated for 24 h, then treated with E. coli (ATCC 25922) at 2 × 106, 2 × 107, 2 × 108, 2 × 109, 2 × 1010 CFU/mL for 2 h, respectively. And the cell viability was estimated using a CCK-8 kit (Biosharp, China). Cell supernatant was collected for the detection of cathelicidin protein level.
Total RNA was extracted from BEECs and endometrial biopsy tissues using RNAiso Plus (Takara, Japan) according to the manufacture's protocol, then total of 1,000 ng RNA was used for reverse transcription by PrimeScript RT reagent Kit with gDNA Eraser (Takara, Japan). RT-qPCR was performed using SYBR Premix Ex TaqII (Takara, Japan) on an iQ5 thermal cycler (BioRad, US). Each 20 μL reaction was set up with 10 μL 2 × SYBR Premix Ex Taq, 0.4 μL of each primer (forward and reverse, 10 μM), 2 μL cDNA and 7.2 μL RNAse free water. All samples were run in duplicate. The reactions were incubated though an initial activation and denaturation step at 95°C for 30 s, followed by 40 cycle of 5 s at 95°C, 10 s at 60°C, and 5 s at 72°C. A melt curve analysis was performed between 60 and 95°C in 0.5°C increments to confirm the identity of the amplicons. The fold change of mRNA expression to β-actin was calculated using the 2−ΔΔCt method (21). The applied primers were detailed in Table 1.
The contents of cathelicidin 1–7 protein were detected using ELISA kit (MLBIO, China) according to the instruction of the manufacture. MULTISKAN MK3 (Thermo, USA) was used to read plate. The use of analyte optical densities and their corresponding expected concentrations to calculate the observed concentration using a 4-parameter logistic regression analysis.
Statistical analysis and graphs were performed using GraphPad prism 8 software (GraphPad Software, Inc. USA). One-way ANOVA analysis was used to compare the results between N group and M/S group, or between different treated cells. All data were presented as mean ± SD. Statistical significance was considered at P < 0.05.
Clinical examination of forty-four vaginal discharge samples revealed that 8 cows were healthy cows (normal group, N, n = 8), 20 cows were diagnosed with moderate endometritis (moderate endometritis group, M, n = 20), and 16 cows were diagnosed with severe endometritis (severe endometritis group, S, n = 16). The typical discharge characteristic in each group was shown in Figure 1.
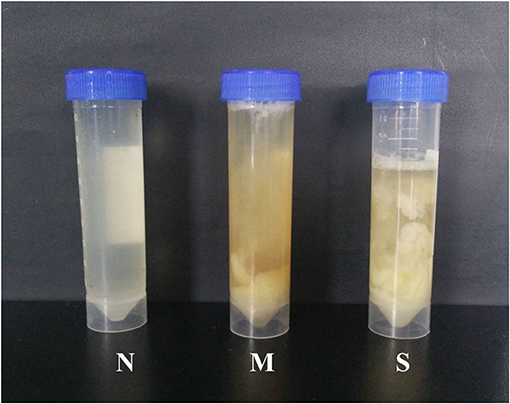
Figure 1. Typical samples of vaginal discharge character: N means clean vaginal discharge from normal cows; M means discharge containing <50% white or off-white pus from dairy cows with moderate endometritis; S means discharge containing > 50% white or yellow purulent material.
Cytological examination revealed recruited PMNs in VD collected 28–35 days post-partum (Figure 2). PMN counts of VD were <15% (13.76 ± 0.91%) in normal cows. In cows with endometritis, percentages of PMNs were significantly higher than in normal cows, the average PMNs% in cows with moderate endometritis and severe endometritis was 21.84 ± 0.69% and 27 ± 0.86%, respectively.
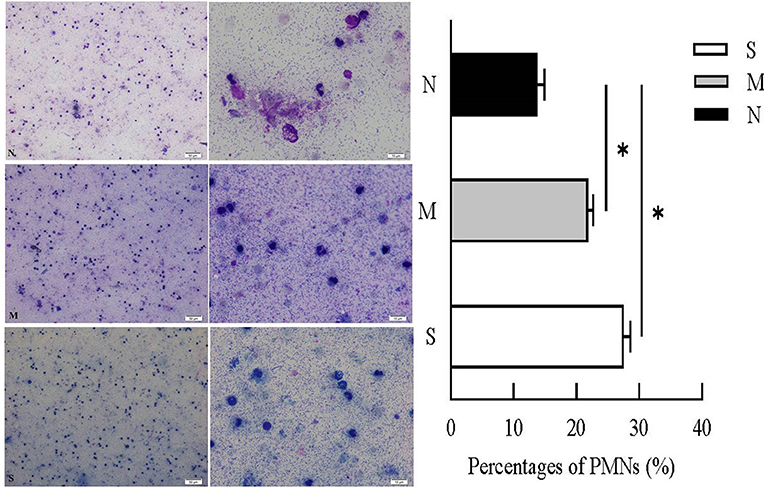
Figure 2. Percentages of PMNs were calculated from VD samples in N, M, and S group. N, M, and S represents the samples from normal cows, cows with mild endometritis and severe endometritis, respectively. All cells were counted and the percentages of PMNs were calculated from average of 200 cells. Representative image of cytological smears from N, M, and S group were presented. *P < 0.5, compared with the N group.
As shown in (Figure 3) the endometrial biopsy from healthy cows showed scanty presence of red blood cells (N); the biopsy from mild endometritis cows characterized by the abundant infiltration of lymphocytes, neutrophile granulocyte, and plasma cells, and also a few neutrophils and blood cells, and significant swelling of epithelial cells (M); severe endometritis (S) exhibited a degenerative necrosis of the epithelial cells with nuclear condensation, and damage of the mucosal layer; there was congestion of the capillaries with significant infiltration of red blood cells, granulocytes and lymphocytes, resulting in a general increase in cell density.
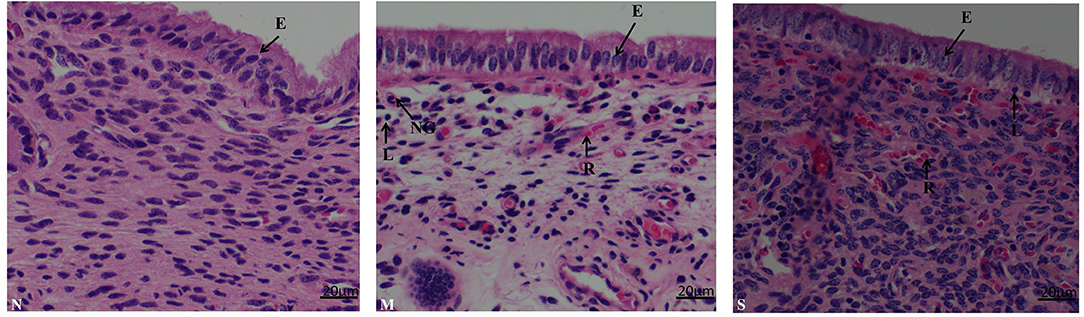
Figure 3. Histopathological characterization in endometrium biopsy samples from postpartum cows. N, M, and S represents the samples from normal cows, cows with moderate endometritis and severe endometritis, respectively. Cells labeled as E, epithelial cell; N, neutrophil; L, lymphocyte; P, plasmocyte; R, red blood cell, NG, neutrophile granulocyte.
The protein levels of cathelicidins were estimated per mL of every discharge sample or serum sample. As shown in Figure 4, cathelicidin 1–7 protein in VD samples significantly increased in dairy cows with endometritis compared with normal cows regardless of the severity, however, those proteins were lower in S group than in M group. As shown in Figure 5, cathelicidin 1–7 can be detected in serum, and all measured cathelicidins showed similar alteration with VD samples.
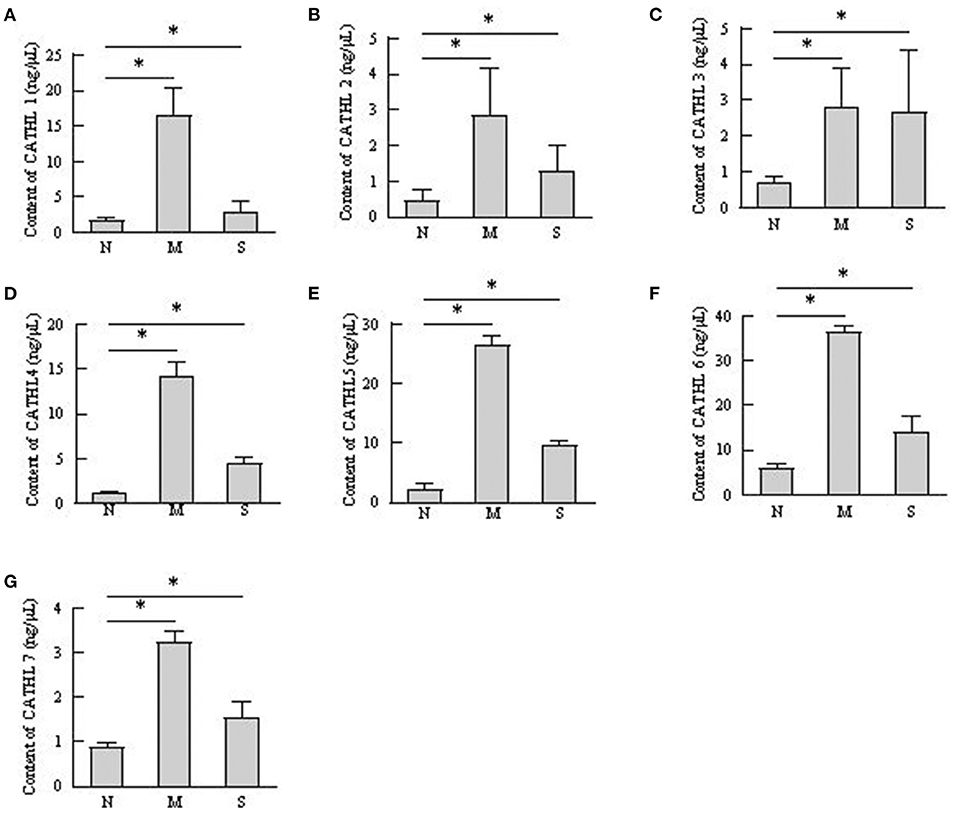
Figure 4. Contents of cathelicidin 1–7 in vaginal discharge detected by ELISA. Vaginal discharge samples were collected from N, M, and S represents the samples from normal cows (n = 8), cows with moderate endometritis (n = 20) and severe endometritis (n = 16), respectively. *P < 0.5, compared with the N group; (A–G) Cathelicidin 1–Cathelicidin 7.
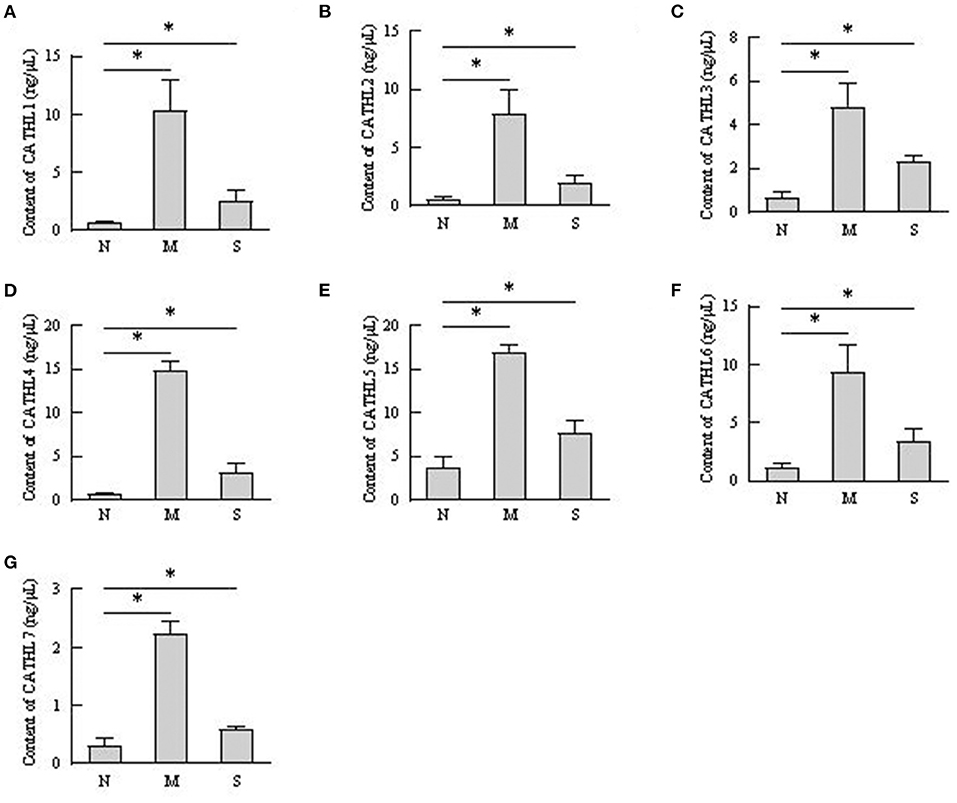
Figure 5. Contents of cathelicidin 1–7 in serum detected by ELISA. N, M, and S represents the samples from healthy cows, cows with moderate endometritis and severe endometritis, respectively. *P < 0.5, compared with the N group. (A–G) Cathelicidin 1–Cathelicidin 7.
As shown in Figure 6, the results indicated that the mRNA expression of cathelicidin 1–7 can be detected in endometrial samples, and the mRNA level of cathelicidin 1–7 increased in tissues with endometritis regardless the severity. However, the expression of the detected cathelicidins decreased in S group than in M group.
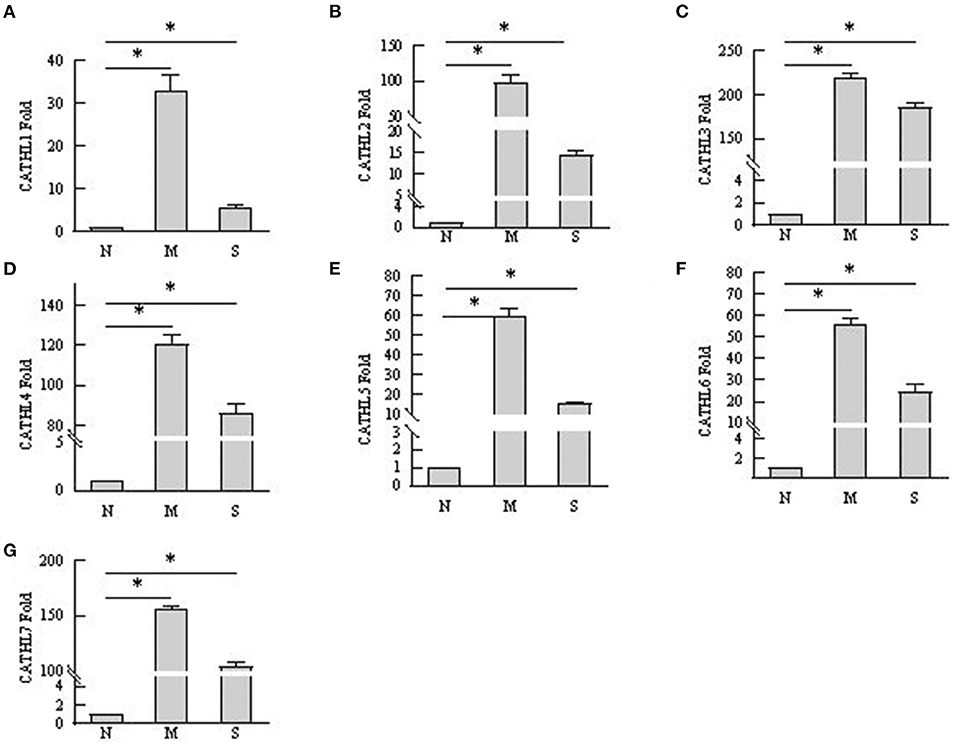
Figure 6. mRNA expression changes of cathelicidin 1–7 in endometrium tissues. N, M, and S represents the samples from normal cows, cows with mild endometritis and severe endometritis, respectively. *P < 0.5, compared with the N group. (A–G) Cathelicidin 1–Cathelicidin 7.
Apoptosis of more than 80% of BEECs were observed through the microscope treated with more than 2 × 108 CFU/mL E. coli. 2 × 106 CFU/mL and 2 × 107 CFU E. coli were used to stimulate BEECs as inflammatory model. Figure 7 showed that the expression of cathelicindin 1–7 significantly increased in BEECs treated with 2 × 106 CFU/mL E. coli, but 2 × 107 CFU E. coli leads to repression of the expression. Protein levels of cathelicidins were undetectable in the cell supernatant from cells without E. coli, and E. coli triggered the release of cathelicidns (Figure 8). Protein levels triggered by 2 × 106 CFU/mL E. coli were higher than 2 × 107 CFU/mL E. coli.
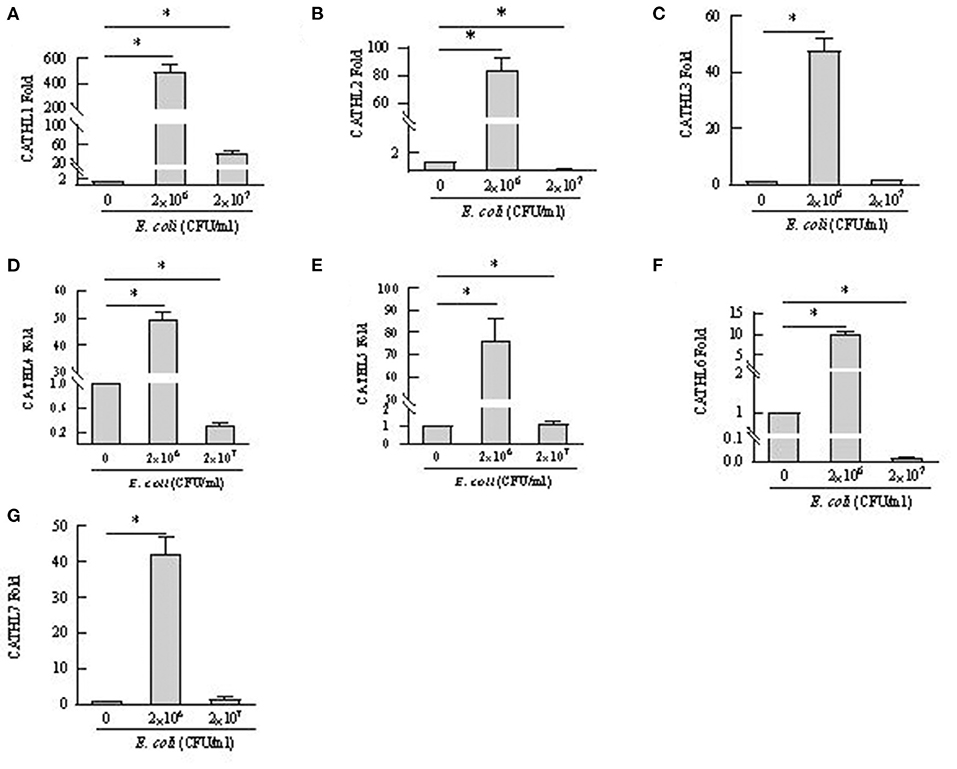
Figure 7. mRNA expression changes of cathelicidin 1–7 in bovine endometrial epithelial cells treated with E. coli. *P < 0.5, compared with the untreated cells. (A–G) Cathelicidin 1–Cathelicidin 7.
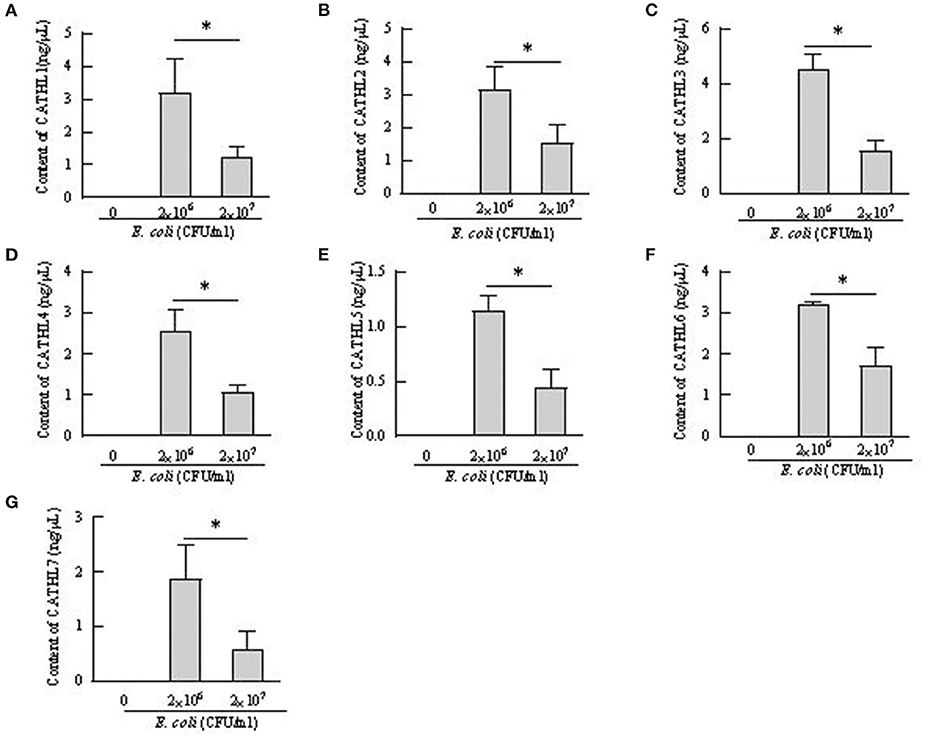
Figure 8. Contents of cathelicidin 1–7 in cell supernatant from bovine endometrial epithelial cells treated with E. coli. *P < 0.5, compared with the untreated cells. (A–G) Cathelicidin 1–Cathelicidin 7.
Uterus is normally sterile throughout pregnancy and exposed to many bacteria during post-partum period. Infection of endometrium with Gram-negative bacteria is the major pathogenesis of endometritis in cattle (22). Acting as parts of the innate host defense, cathelicidin is directly involved in pathogen clearing to providing a first line of defense against invading microbes (23). Markedly ascending cathelicidins in the milk of cows with mastitis has been observed (10), less is known about their roles during bovine endometritis. The present study firstly investigated the roles of the cathelicidins in cattle with endometritis. Levels of cathelicidins increased in vaginal discharge, serum, and endometrial biopsies samples from cows with endometritis, regardless of the severity of endometritis. These findings accords with the general reports that increased levels of cathelicidins are observed in a range of inflammatory conditions (8, 9, 24). The results demonstrated that cathelicidins play distinct roles in defensing against bacteria in uterus. Additionally, as shown in Figure 5, the concentrations of cathelicidins in serum of cows with endometritis were increased, which suggested that cows with endometritis showed systemic inflammatory response (24).
Several studies confirmed that cathelicidins increased in inflammatory disease caused by bacterial infection (25). Nevertheless, there was no report about whether the levels of cathelicidins increased in the rising severity of the disease. We observed that the levels of cathelicidins were higher in severe endometritis samples than in samples from normal cows, but their levels were lower than in moderate endometritis samples (Figures 4–6), thus indicating that the expression and production of cathelicidins were weakened in severe cases. We found for the first time that cathelicidins decreased in severe endometritis.
Extensive PMNs were recruited into uterus from the circulation to respond against the invading pathogens (26), and these immune cells can secrete cytokines, chemokines, and antimicrobial peptides at the site of infection as part of the inflammatory responses to assist in clearance of the infection (27). Cathelicidins can be stored in polymorphonuclear neutrophils (PMNs) as secondary granules and then released upon bacterial infection (28), and they always show good correlation with PMN (8). However, in our study, vaginal discharge samples with high percentage of PMN showed lower concentration of cathelicidins (Figures 2–4). We speculated that PMN is not the only cellular resource of cathelicidins in uterus. With the exception of PMN, cathelicidin can also be expressed and produced by epithelial cells (29). Cubeddu et al. (16) found that in areas showing inflammatory lesions, only residual intact mastitis epithelial cells can express cathelicidin. Tomasinsig et al. (25) reported that the protein levels of cathelicidins showed good positive correlation with the percentage of PMN in uterine cyto-brush samples of post-partum cows, which is contradictory to our findings. In this study, we selected the cows at 28–35 day post-partum, whereas Ledgard et al. (27) chose the cows at 15 day post-partum. Persistent infection could cause endometrial damage. Histopathological signs of severe endometritis (Figure 3) showed epithelial necrosis of endometrial tissue. Based on these findings, we assumed that dairy cows can produce endometrial cathelicidins.
BEECs can express and produce chemokines and acute phase proteins to defense the invading pathogens (30, 31), while the role of BEECs in the expression and production of cathelicidins is still unclear. Our results indicted that BEECs can express cathelicidin, but the proteins were undetectable in the cell supernatant samples without bacteria. Moreover, low concentration of E. coli (2 × 106 CFU/mL) enhanced their mRNA expression and triggered the release of cathelicidins (Figures 7, 8). Similarly, Chromek et al. (32) detected the mRNA expression of cathelicidin in renal epithelial cells, and the release of cathelicidin was detected upon contact with bacteria. Nevertheless, we observed a decrease in the mRNA expression and protein levels of cathelicidins in BEECs upon high concentration of E. coli (2 × 107 CFU/mL) (Figures 7, 8). And BEECs treated with 2 × 107 E. coli showed significant decreased cell viability (Figure 9). In the in vivo mastitis model, cathelicidin only can be expressed in residual epithelial cells of heavily inflamed area (32). The damage of cells may restrain the expression and release of cathelicidins.
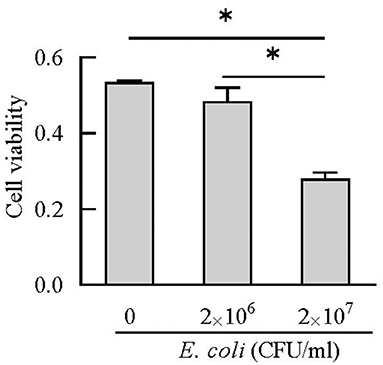
Figure 9. Cell viability was estimated using CCK-8 kit. *P < 0.5, compared with the untreated cells.
In conclusion, dairy cows can produce endometrial cathelicidins, and the expression and production of these cathelicidins increased during endometritis. Cathelicidins production decrease in sever endometritis. BEECs play an active role in expression and production of cathelicidins to defend against bacterial infection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Lanzhou Institute of Husbandry and Pharmaceutical Sciences of Chinese Academy of Agricultural Sciences (SYXK-2014-0002).
ZY and BH: conceptualization. YL and XM: methodology. YL and JY: data curation. YL, XW, and JY: validation. YL: writing—original draft. ZY and BH: writing—review and editing and funding acquisition. All authors have read and agreed to the published version of the manuscript.
This research was funded by National Key R&D Program of China (No. 2017YFD0502201), Science and Technology Innovation Project (No. CAAS-ASTIP-2014-LIHPS-03), and Key research and development plan of Gansu province (20YF8NA029).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.675669/full#supplementary-material
1. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, et al. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci. (2002) 85:2223–36. doi: 10.3168/jds.S0022-0302(02)74302-6
2. Sheldon IM, Price SB, Cronin J, Gilbert RO, Gadsby JE. Mechanisms of infertility associated with clinical and subclinical endometritis in high producing dairy cattle. Reprod Domest Anim. (2009) 44:1–9. doi: 10.1111/j.1439-0531.2009.01465.x
3. Manimaran A, Kumaresan A, Jeyakumar S, Mohanty TK, Sejian V, Kumar N, et al. Potential of acute phase proteins as predictor of postpartum uterine infections during transition period and its regulatory mechanism in dairy cattle. Vet World. (2016) 9:91–100. doi: 10.14202/vetworld.2016.91-100
4. Bicalho ML, Machado VS, Oikonomou G, Gilbert RO, Bicalho RC. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet Microbiol. (2012) 157:125–31. doi: 10.1016/j.vetmic.2011.11.034
5. Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. (2002) 123:837–45. doi: 10.1530/reprod/123.6.837
6. Pinheiro DSF, Gallo RL, Nizet V. Differing effects of exogenous or endogenous cathelicidin on macrophage toll-like receptor signaling. Immunol Cell Biol. (2009) 87:496–500. doi: 10.1038/icb.2009.19
7. Wieczorek M, Jenssen H, Kindrachuk J, Scott WR, Elliott M, Hilpert K, et al. Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem Biol. (2010) 17:970–80. doi: 10.1016/j.chembiol.2010.07.007
8. Young-Speirs M, Drouin D, Cavalcante PA, Barkema HW, Cobo ER. Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int J Antimicrob Agents. (2018) 51:813–21. doi: 10.1016/j.ijantimicag.2018.02.006
9. Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. (2005) 7:179–96. doi: 10.21775/cimb.007.179
10. Addis MF, Tedde V, Dore S, Pisanu S, Puggioni G, Roggio AM. Evaluation of milk cathelicidin for detection of dairy sheep mastitis. J Dairy Sc. (2016) 99:6446–56. doi: 10.3168/jds.2015-10293
11. Whelehan CJ, Barry-Reidy A, Meade KG, Eckersall PD, Chapwanya A, Narciandi F, et al. Characterisation and expression profile of the bovine cathelicidin gene repertoire in mammary tissue. BMC Genomics. (2014) 15:128. doi: 10.1186/1471-2164-15-128
12. Pisanu S, Cubeddu T, Pagnozzi D, Rocca S, Cacciotto C, Alberti A, et al. Neutrophil extracellular traps in sheep mastitis. Vet Res. (2015) 46:59. doi: 10.1186/s13567-015-0196-x
13. Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immu. (2000) 68:4297–302. doi: 10.1128/IAI.68.7.4297-4302.2000
14. Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. (2002) 70:953–63. doi: 10.1128/IAI.70.2.953-963.2002
15. Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I, et al. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. (2011) 141:1852–63. doi: 10.1053/j.gastro.2011.06.079
16. Cubeddu T, Cacciotto C, Pisanu S, Tedde V, Alberti A, Pittau M, et al. Cathelicidin production and release by mammary epithelial cells during infectious mastitis. Vet Immunol Immunopathol. (2017) 189:66–70. doi: 10.1016/j.vetimm.2017.06.002
17. Williams EJ, Fischer DP, Pfeiffer DU, England GC, Noakes DE, Dobson H, et al. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. (2005) 63:102–17. doi: 10.1016/j.theriogenology.2004.03.017
18. Adnane M, Chapwanya A, Kaidi R, Meade KG, O'Farrelly C. Profiling inflammatory biomarkers in cervico-vaginal mucus (CVM) postpartum: potential early indicators of bovine clinical endometritis? Theriogenology. (2017) 103:117–22. doi: 10.1016/j.theriogenology.2017.07.039
19. Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology. (2006) 65:1516–30. doi: 10.1016/j.theriogenology.2005.08.021
20. Bondurant RH. Inflammation in the bovine female reproductive tract. J Anim Sci. (1999) 77(Suppl. 2):101–10. doi: 10.2527/1999.77suppl_2101x
21. Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. (2006) 1:1559–82. doi: 10.1038/nprot.2006.236
22. Li YJ, Wang DS, Wu XH, He BX, Cheng ZR, Szenci O, et al. Decreasing of S100A4 in bovine endometritis in vivo and in vitro. Theriogenology. (2020) 153:68–73. doi: 10.1016/j.theriogenology.2020.05.015
23. Dohmen MJ, Joop K, Sturk A, Bols PE, Lohuis JA. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology. (2000) 54:1019–32. doi: 10.1016/S0093-691X(00)00410-6
24. Siqueira LC, Favaretto B, Moraes BT, de Freitas VO, Bicalho RC, Horn RC„, et al. Bovine endometritis and the inflammatory peripheral cholinergic system. Appl Biochem Biotechnol. (2020) 190:1242–56. doi: 10.1007/s12010-019-03157-0
25. Tomasinsig L, Benincasa M, Scocchi M, Skerlavaj B, Tossi A, Zanetti M, et al. Role of cathelicidin peptides in bovine host defense and healing. Probiotics Antimicrob Proteins. (2010) 2:12–20. doi: 10.1007/s12602-010-9035-6
26. Addis MF, Pisanu S, Ghisaura S, Pagnozzi D, Marogna G, Tanca A, et al. Proteomics and pathway analyses of the milk fat globule in sheep naturally infected by Mycoplasma agalactiae provide indications of the in vivo response of the mammary epithelium to bacterial infection. Infect Immun. (2011) 79:3833–45. doi: 10.1128/IAI.00040-11
27. Ledgard AM, Smolenski GA, Henderson H, Lee RS. Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reprod Fertil Dev. (2015) 27:395–406. doi: 10.1071/RD13144
28. Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. (2006) 306:199–230. doi: 10.1007/3-540-29916-5_8
29. Addis MF, Pisanu S, Marogna G, Cubeddu T, Pagnozzi D, Cacciotto C, et al. Production and release of antimicrobial and immune defense proteins by mammary epithelial cells following Streptococcus uberis infection of sheep. Infect Immun. (2013) 81:3182–97. doi: 10.1128/IAI.00291-13
30. Andoh K, Nishimori A, Sakumoto R, Hayashi KG, Hatama S. The chemokines CCL2 and CXCL10 produced by bovine endometrial epithelial cells induce migration of bovine B lymphocytes, contributing to transuterine transmission of BLV infection. Vet Microbiol. (2020) 242:108598. doi: 10.1016/j.vetmic.2020.108598
31. Davies D, Meade KG, Herath S, Eckersall PD, Gonzalez D, White JO, et al. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod Biol Endocrinol. (2008) 6:53. doi: 10.1186/1477-7827-6-53
Keywords: dairy cow, endometritis, cathelicidin, bovine endometrial epithelial cell, E. coli
Citation: Li Y, Ma X, Yang J, Wu X, Yan Z and He B (2021) Expression Pattern of Cathelicidins in Dairy Cows During Endometritis and Role of Bovine Endometrial Epithelial Cells in Production of Cathelicidins. Front. Vet. Sci. 8:675669. doi: 10.3389/fvets.2021.675669
Received: 03 March 2021; Accepted: 16 August 2021;
Published: 20 September 2021.
Edited by:
Kangfeng Jiang, Yunnan Agricultural University, ChinaReviewed by:
Izhar Hyder Qazi, Shaheed Benazir Bhutto University of Veterinary and Animal Sciences, PakistanCopyright © 2021 Li, Ma, Yang, Wu, Yan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuoting Yan, eWFuenVvdGluZ0BjYWFzLmNu; Baoxiang He, aGViYW94QGd4dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.