
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 04 August 2021
Sec. Veterinary Epidemiology and Economics
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.673820
This article is part of the Research Topic Socioeconomics of Vaccine Security for Transboundary Animal Diseases View all 6 articles
 Mi-Young Park
Mi-Young Park You Jin Han
You Jin Han Eun-Jin Choi
Eun-Jin Choi HeeYeon Kim
HeeYeon Kim Rokeya Pervin
Rokeya Pervin Wonseok Shin
Wonseok Shin Doheon Kwon
Doheon Kwon Jae Myoung Kim
Jae Myoung Kim Hyun Mi Pyo*
Hyun Mi Pyo*In South Korea, domestic cattle, pigs, and goats were subjected to mandatory foot-and-mouth disease (FMD) vaccination and year-round serosurveillance since 2011. In 2020, approximately USD 95 million was spent solely for FMD vaccine purchase for 59 million livestock, and 1.25 million samples were tested to estimate the population immunity and demonstrate the absence of virus circulation. As the FMD vaccination program was revised in 2018, the post-vaccination monitoring (PVM) was designed to evaluate the effectiveness of the vaccine program of three vaccines approved for routine use. To this end, monitoring post-vaccination immunity has been conducted by collecting 35,626 serum samples at 28 days post-vaccination following regular national vaccinations, which were carried out in April and in October in 2020. The design of the serological test for PVM was specially targeted at particular livestock groups, including dairy cattle, goats, and beef cattle aged 6–12 months, which were generally estimated to have a low expected seroprevalence. The risk factors had also been identified, considering the increased likelihood of infection in a particular location, herd size, and husbandry system applied in a targeted sample collection. Serum sample collection and SP-O and NSP antibody tests were performed by local veterinary laboratories using commercially available ELISAs. The current FMD vaccination program, which was performed twice a year following the regimen of primary vaccination and boost, resulted in over 80% population immunity. The seroprevalence monitored after the vaccination in fall was higher than the one studied in spring except in pigs. It was demonstrated that the seroprevalence of risk-based targeted samples ranged from 93.8 to 100% in cattle, 63.2 to 100% in pigs, and 20.0 to 100% in goats. Of note is the area near the North Korean borders which showed a relatively low seroprevalence among the targeted regions, and no NSP sero-positive reactor was detected in this region. When subpopulation immunity at the individual level was assessed, the seroprevalence in young cattle stock was slightly lower (95.8%) than that of adults (98.4%). In conclusion, the FMD vaccination campaign has been successfully implemented in Korea, and the PVM can be a supplementary program for massive routine surveillance in terms of providing timely information needed both to estimate population immunity and to properly target “risk-based surveillance.”
Post-vaccination monitoring (PVM) to evaluate the performance of vaccination regimens and program is essential for those countries embarking in vaccine-based foot-and-mouth disease (FMD) control policy (1–6). Especially in South Korea, 90% of the total budget, which was worth USD 98 million in 2020, assigned for FMD management is spent for vaccine purchase and vaccination in practice. PVM is important to evaluate the effectiveness of vaccines and vaccination program and plan for a future policy (7).
After experiencing a devastating FMD outbreak in 2010, a mandatory nationwide FMD vaccination for cattle, pigs, and goats was initiated to control the disease (8). Along with the implementation of FMD vaccines, a massive year-round serological surveillance program has been launched in 2011 to search the evidence of FMD virus (FMDV) circulation and evaluate population immunity. However, FMD was prevalent until 2016, and another large-scale FMD outbreak occurred in 2014–2015 (9–14) which prompted the introduction of diverse serotype O vaccine strains besides O1 Manisa. In 2017, a comprehensive biannual vaccination program for cattle and goats and post-vaccination sero-monitoring, in addition to year-round serosurveillance, were launched (10, 11, 15). The current vaccination regimen was adopted after the serotype A FMD outbreak in porcine in the spring of 2018. Presently, all susceptible livestock were vaccinated with oil-adjuvant inactivated vaccines, containing serotype O and A antigens, following a prime and boost inoculation schedule. As mentioned, the massive routine FMD serosurveillance, abiding by the national year-round surveillance program, provides valuable information on FMDV circulation in the field and population immunity by vaccination (16, 17). In this regard, 637,292 and 637,593 of serum samples were subjected to SP and NSP antibody ELISA, respectively, in 2020 (the national serosurveillance monthly report is available to the public at www.qia.go.kr). However, further information was required to evaluate the current vaccination regimens and program implemented at the end of 2018 as well as the success of the vaccination campaign. Hence, the sero-monitoring post-vaccination in 2020 was designed and applied to assess the impact of the current vaccination regimen and program by estimating vaccine-induced herd immunity at the population and subpopulation levels. In addition, the collected serosurveillance data were further analyzed at various subpopulation levels to evaluate the vaccine-induced immunity in high-risk groups.
South Korea is comprised of eight cities and nine provinces. The demographics of cloven-hoofed livestock such as cattle, pigs, and goats are described in Supplementary Figure 1. The Ministry of Agriculture, Food, and Rural Affairs (MAFRA) supervises the national FMD vaccination and serosurveillance program, with the technical support of the Animal and Plant Quarantine Agency (APQA). The APQA plans for the national surveillance program and post-vaccination monitoring. Then, there are 46 regional veterinary services with trained veterinarians who conduct the sample collection and FMD diagnostic tests. An established vaccination registration system (Korea Animal Health Integrated system, KAHIS) to monitor vaccine distribution and administration regularly is operated by MAFRA, APQA, and the regional veterinary services.
All cattle, pigs, and goats are subjected to mandatory FMD vaccination by The Act on the Prevention of Contagious Animal Diseases, and the public is notified regarding FMD vaccination, clinical examination, and retention of immunization. Farm owners should also keep the record of FMD vaccination and carry the certificate of FMD vaccination to present on demand during the movement of domestic animals for trade and slaughter. There is a heavy fine to be imposed for non-compliance.
Since October 2018, three FMD vaccines, oil-adjuvanted and containing inactivated serotype O and A antigens, were used for immunization in the field. The vaccine strains varied by the manufacturer, yet all three vaccines were equal or greater than three protective doses of 50% (PD50). The calf and young goats receive the primary vaccination at 2–4 months old and the boost injection at 4 weeks later. The piglets were vaccinated at 8–12 weeks old and received a boost injection at 4 weeks later. After the first two-dose FMD vaccination, all animals were vaccinated every 6 months. The injection dose is 2 ml for cattle and pigs and 1 ml for small ruminants. The vaccines are inoculated intramuscularly, and the neck and ham are the recommended injection sites. Further details of the FMD vaccines mentioned in this article are presented in Supplementary Table 1.
Scheduled comprehensive national FMD vaccination campaigns are carried out, targeting all cattle and goats, in April and October considering the seasonal risk factor and 6-month interval of boost vaccination. Pig farms are generally excluded from the campaign schedule as they followed their own vaccination schedules optimized by the condition of each farm. In 2020, FMD vaccination was conducted from April 1 to May 28 (first round) and from October 5 to November 13 (second round). During this period, 3.5 million cattle and 525,926 goats were vaccinated (Table 1). The actual immunization of smallholdings with <50 cows was performed by a public veterinarian. In case of large-scale cattle farms, goat farms, and all pig operations, the farm owners were responsible for the vaccination on their own.
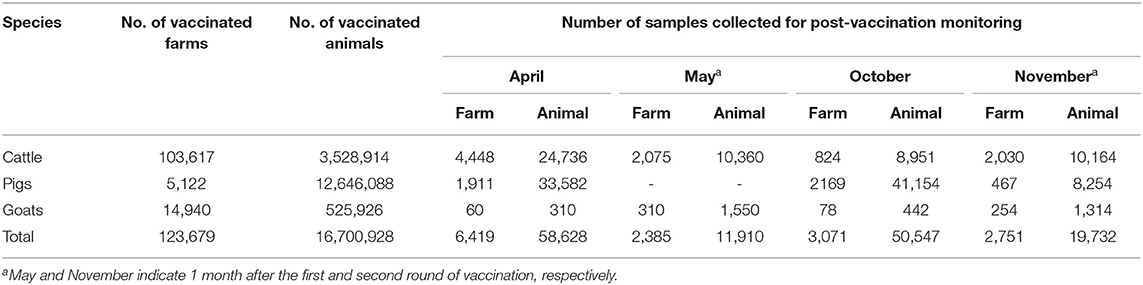
Table 1. Numbers of vaccinated animals and farms during the national vaccination in 2020 and the serum samples collected for post-vaccination monitoring.
In addition to the nationwide systemic mass vaccination, supplementary vaccination to beef up the population immunity in high-risk regions was given in late September till early October of 2020. Indeed these high-risk regions or farms fall in one of the following categories: (i) regions where the NSP antibody-positive reactors in 2019 were detected, (ii) regions where the high-density pig operation complex with a previous history of FMD outbreak were located, (iii) farms located near the border to North Korea, and (iv) farms with a recent history of penalty imposition due to low herd immunity. The geographical distribution of these high-risk regions or farms was depicted in Supplementary Figure 2. A total of 1.02 million pigs from 530 farms, 204,844 cattle from 3,681 farms, and 20,396 goats from 493 farms were vaccinated in these regions. The trivalent vaccine, containing O, A, and Asia 1 antigens, was used in high-risk areas (Supplementary Table 1).
In May and November, a total of 31,642 blood samples were collected from cattle, goat, and pig farms by the 46 regional veterinary staff in cities and provinces. These samples were collected 1 month after the biannual vaccinations, which allowed us to evaluate the comprehensive national FMD vaccination (Supplementary Table 2).
The size of the farms for sample collection was estimated using a two-stage cluster sampling design based on the following parameters as described elsewhere (18): 95% confidence, 5% precision, and 80% expected sero-prevalence. Then, individual farms were selected randomly using simple random sampling. In addition, the sample size for sero-monitoring post-vaccination was determined by applying a weighting factor to the target subpopulation, which was 6–12-month-old beef cattle, dairy cattle, and animals in large-scale farms showing a low herd immunity. A total of 3,984 samples from the targeted subpopulation were also collected at 1 month post-supplementary vaccination in the high-risk areas mentioned above. The actual blood collection and visual inspection were conducted by the local veterinary staff and Livestock Health Control Association (LHCA), a public organization funded by the government. APQA releases the guidelines for the number of blood samples per farm and the age criteria for animal selection. In case of cattle and goat farms, five animals from a farm were randomly selected for sampling. For pig farms, 16 animals from a farm were subjected to blood sampling. Age-stratified sampling scheme was applied to cattle farms, with at least two samples from beef cattle aged 6–12 months that must be included among five samples. During blood sampling, the veterinary staff conducted a clinical examination concomitantly.
Serological tests were performed by the 46 regional veterinary laboratories in cities and provinces using commercially available ELISAs under the supervision of the central laboratory, APQA. Sera were assessed for vaccine-induced antibodies using three commercial type O SP antibody ELISAs: PrioCHECKTM FMDV Type O Ab strip kit (Thermo Fisher Scientific), VDPro® FMDV Type O Ab b-ELISA (Median Diagnostics, South Korea), and BIONOTE FMD Type O Ab ELISA (BIONOTE Inc., South Korea). To identify the FMD virus infection, two commercial NSP antibody ELISAs—VDPro® FMDV NSP Ab ELISA (Median Diagnostics, South Korea) and BIONOTE FMD NSP Ab ELISA (BIONOTE Inc., South Korea)—were used according to the manufacturer's instruction. To increase the diagnostic specificity, a positive result in both NSP ELISAs was considered as NSP antibody-positive (19). Further details on the ELISA kits used in this study are presented in Supplementary Tables 3, 4.
R software (www.r-project.org) and EXCEL were used to compile the ELISA results. To evaluate the effectiveness of the vaccine program, population immunity surveyed by sero-monitoring post-vaccination was compared to the national serosurveillance data collected in April and October, which were considered to reflect population immunity before the regular biannual national vaccination (the national surveillance data is open to the public at www.qia.go.kr). A comparison by breeds (dairy and beef cattle), herd size (large-scale and smallholdings), and age criteria (6–12 months and over 1 year old in cattle, fattening, and breeding in pigs) was further analyzed to identify the risk factors. Paired t-test was performed, and p < 0.05 was considered statistically significant. For the purpose of this study, herd immunity was defined as seroprevalence with 95% confidence interval (CI) estimated for FMDV serotype O.
According to MAFRA, 59.4 million doses of FMD vaccines, worth USD 95 million, were released in 2020 to vaccinate domestic animals. The nationwide FMD vaccination was executed following the national FMD vaccination program in April and October. There was a total of 31,642 serum samples collected from 2,385 farms in nine provinces across the country at 1 month after the first round and second round of vaccination in May and November, respectively. The results of the clinical examination of the presence of FMD symptoms, conducted during blood collection, and NSP antibody ELISA indicated that there was no infection in the field (unpublished data). The species-dependent seroprevalence against serotype O was higher than 80% in all species tested after the mandatory scheduled vaccination (Figure 1). These results demonstrated that the scheduled systemic vaccination was effective to build up herd immunity. In detail, the seroprevalence by species studied after the second round of vaccination in November ranged from 90.2 to 98.0%, while that after the first round of vaccination in May was 88.6 to 97.8%. The seroprevalence of cattle was over 97.0% at all times, while those in pigs and goats were enhanced from 87.6 to 92.8% and 85.3 to 90.2%, respectively. These results suggested that the biannual vaccination practice was effective to improve the vaccine-induced immunity in pig and goat population.
The seroprevalence by provincial level is presented in Table 2. There was no difference in seroprevalence between the provinces, except the seroprevalence of goats in Gyeongsangnam-do (GN) (72.5–73.0%), ChungcheongNam-do (75.7%), and GyeoungGi-do (78.8%) provinces, where the seroprevalence was relatively low compared to the seroprevalence of the goat population in other provinces (85.3–90.2%). According to individual farm data, such low seroprevalence in those three provinces was due to the negligence of vaccination of a few goat farms in the region. However, this unreliable immune status was improved at 1 month after the second round of vaccination in November.
Provided that vaccine is effective to circulating virus, a farm-based herd immunity of over 80% is expected to stop viral transmission (1, 2, 20, 21). The majority of cattle farms maintained adequate immune status as presented in Figure 2, and the farms with <80% herd immunity were <1% of the total cattle farms (Table 3). Such results implied that FMD vaccination was well-conducted in cattle compared to that in other livestock. In the case of pig farm, the proportion of farms with <80% immunity steadily decreased from 24.0 to 13.1% (Table 3). Similarly, the proportion of goat farms with <80% immunity rapidly dropped from 25.3 to 10.2% after the second round of vaccination (Table 3). These results suggest that the current biannual vaccination program was effective to increase and maintain the level of herd immunity in these species.
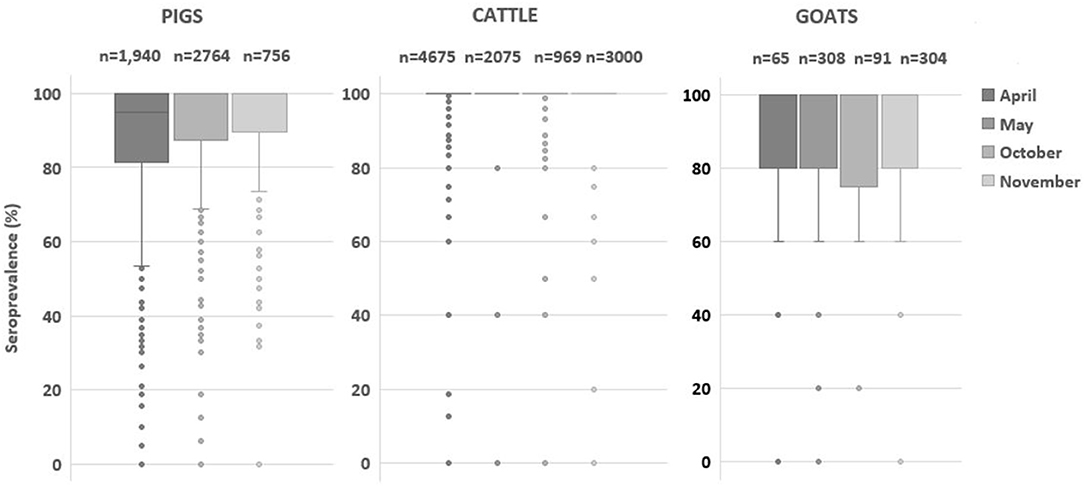
Figure 2. Comparison of herd immunity (%) estimated at the farm level by species. The box plot graph presents foot-and-mouse disease serotype O seroprevalence (%) of cattle, pigs and, goats in April and October (pre-biannual vaccination) and May and November (post-biannual vaccination). The box plot represents inter-quartile range, and the horizontal line is the median value.
The subpopulation immunity of cattle and pigs in different age groups is presented in Table 4. The seroprevalence of young calves was lower (88.8–96.2%) than that of cattle (97.6–98.7%) in the course of time (p < 0.05). A similar finding was observed in pigs as the population immunity of fattening pigs (86.7–91.7%) was significantly lower (p < 0.01) than that of the breeding pigs (94.2–97.0%). Nevertheless, the proportion of sero-positive cattle aged 6–12 months increased after the second round of vaccination from 88.8 to 95.5%. Similarly, in fattening pigs, the population immunity was considerably enhanced from 86.7 to 91.7% in November.
The subpopulation immunity of cattle categorized by herd size and breed is presented in Table 5. There was no difference in herd immunity by farm size (P > 0.05). The herd immunity of large-scale cattle farms was between 96.7 and 98.9%. The herd immunity of smallholdings was similar to that of large-scale cattle farms (96.5 and 98.4%). However, herd immunity was different by breed. Compared to the herd immunity of beef farms, those of dairy farms were higher throughout the year except after the second round of vaccination in November (p = 0.4118). Data on seroprevalence after the supplementary vaccination in high-risk regions are summarized in Table 6. The population immunity of cattle in high-risk areas was 96.2–98.7%, that of pig was 63.2–99.0%, and that of goats was 72.2–85.3%. However, the herd immunity of districts where the high-risk regions belonged to was 93.8–100% in cattle, 63.2–100% in pigs, and 20.0–100% in goats (Figure 3). In addition, we noted that those located near the North Korea border showed a low seroprevalence among the high-risk areas.
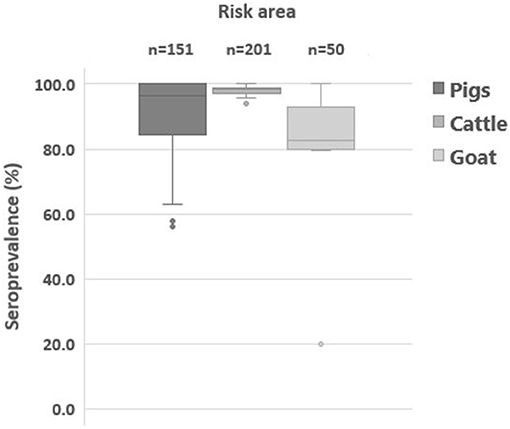
Figure 3. Distribution of herd immunity (%) estimated at a targeted farm in high-risk areas. Box plot graph presents foot-and-mouse disease serotype O seroprevalence (%) of cattle, pigs, and goats at 1 month after the supplementary vaccination in high-risk areas. The box plot represents inter-quartile range, and the horizontal line is the median value.
The population immunity in pigs progressively increased from 87.6 to 92.8% in 2020. Fattening pigs particularly showed a rapid increase of subpopulation immunity from 86.7% in May to 91.7% in November (Table 4). In addition, the proportion of farms with herd immunity below 80% steadily decreased from 24.0% in May to 13.1% in November (Table 3 and Figure 3). These results suggested that the current vaccination regimen, which is comprised of prime and boost immunization, is effective to induce a vaccine-derived antibody response in pigs. Similarly, a high level of seroprevalence of over 88% in cattle aged 6–12 months demonstrated the effectiveness of the current biannual vaccination program.
Massive vaccination, monitoring of post-vaccination immunity, and active and passive serosurveillance are important measures for the control of FMD (1–6, 24). Experiences from South America and Europe suggested that the implementation of a systematic vaccine policy can successfully eradicate FMDV (3, 22, 25). After the massive FMD outbreaks in 2010, South Korea initiated a mandatory nationwide vaccination and serosurveillance to estimate the overall population immunity either by previous infection or vaccination. In addition, post-vaccination monitoring was implemented to help in the impact assessment of the current vaccination program and in identifying the weakness of the vaccination campaign by estimation of population immunity at various categories in 2017 (1, 2, 22–26).
In the present study, data on post-vaccination sero-monitoring conducted in 2020 after two rounds of vaccination in May and November to monitor the immune status at population, district, farms, and subpopulation levels in Korea are presented. During the post-vaccination sero-monitoring, all serum samples were NSP antibody-negative in two NSP Ab ELISAs such that it substantiated the absence of virus circulation and transmission (27–29). In addition, this result implied that the population immunity was sorely derived from the vaccination.
The overall population immunity from April to November in 2020 was consistently higher than 80% in all the targeted species including pigs. To achieve over 80% herd immunity in pig and in under-1-year-old population, prime and boost vaccination regimens were implemented in late 2018. The sero-monitoring and national serosurveillance results showed that the seroprevalence in the pig population was over 80% regardless of time, and the proportion of farms with herd immunity under 80% was decreased in 2020. Such high population immunity in pig has significance considering that most of the pigs are subjected to FMD vaccination once during their lifetime, and the immune response to FMD vaccines was relatively weak and readily waned in porcine (30–33). Therefore, the results of our study suggest that the current vaccination regimen, which adopted priming at 8–12 weeks old and boosting in a month, was effective in the young age group of pigs as well as in other species. In many Asian countries where a major target for vaccination to FMDV is pigs, they strive to achieve a herd immunity of 80% or more (30–32). However, the evaluation of FMD vaccine performance is mainly emphasized on their use in cattle, so there are difficulties to collate data for optimizing FMD vaccination regimens in pigs. Therefore, it was very encouraging to reach almost 90% of immunity in pigs, an important domesticated livestock species in Korea.
Population immunity was further assessed in various subpopulations to identify the potential risk factors. There was no significant difference of immunity in cattle by herd size. However, there were slight differences in age group as adult cattle over 12 months old showed a higher seroprevalence compared to young stocks under 12 months old. Such observation may be due to the cumulative vaccination effect in adult cattle population. Similarly, except after the second round of vaccination in November, there was a slight difference in immunity between dairy and beef cattle. Nevertheless, the systematic biannual vaccination adhering to a synchronized schedule of vaccine administration led to improved vaccine coverage and resulted in a positive impact of vaccination campaigns. The Korean government also provides support, including subsidies for vaccine purchase, public veterinarian for vaccine inoculation, and educational campaign for farmers and stakeholders, which encouraged FMD vaccination.
In order to determine a high-risk region for additional supplementary vaccination, previous epidemiological and serosurveillance results were used (19). The high-risk regions were locations such as those where previous FMD outbreaks had occurred, the ones near the North Korea borders, areas where the farms have NSP sero-positive reactor or the ones with a record of such, and locations of farms with a recent history of penalty imposition due to non-compliance with the vaccination program. The seroprevalence of pigs and goats was slightly low in these regions. It was especially noted that few farms with low herd immunity were located near the North Korea border. However, there was no NSP antibody-positive case found in all the samples tested, indicating no circulation or transmission of FMDV. Supplementary vaccination and sero-monitoring post-vaccination in high-risk regions allowed the detection of farms and subpopulations with inadequate immunity and subsequent corrective actions by the government.
If properly planned and conducted, sero-monitoring post-vaccination could replace mass serosurveillance at a fraction of the resources and cost. In 2020, approximately USD 5 million was spent to purchase serological test kits, and 1.3 million serological samples were tested for routine serosurveillance. In addition, such a mass sampling requires the cooperation of farm owners, and it can be a necessary burden. As described, sero-monitoring post-vaccination was able to provide sufficient information for estimating herd immunity at various population levels and identifying risk factors. The generated data can also help to refine the vaccination regimens and programs and evaluate the vaccination campaign. Hence, post-vaccination sero-monitoring could substitute mass serosurveillance if budget and resources are scarce (7).
Publicly available datasets were analyzed in this study. This data can be found here: http://www.qia.go.kr/animal/prevent/listwebQiaCom.do?type=2_8ktyzl&.
M-YP conceived and designed the study and drafted the manuscript. YH, HK, and RP performed the serological tests and contributed to data arrangement. E-JC, WS, and DK collected and analyzed the serosurveillance data. E-JC and JK directed the project. HP reviewed and revised the manuscript. All authors approved the final version of the manuscript for publication.
This study was a part of the research project Application of Validation and Development of a confirmatory test for detection of non-structural protein antibodies against foot-and-mouth disease virus in pigs (no. M-1543082-2021-23) funded by the Korean Ministry of Agriculture, Food, and Rural Affairs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the staffs in the local veterinary laboratory for conducting serological tests and entering data to the KAHIS management system for national serosurveillance and post-vaccination monitoring. We also thank the farm owners and LHCA staffs for their cooperation in the blood sample collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.673820/full#supplementary-material
1. Giancarlo F, Paton D, Duffy S, Chris B, Knight-Jones T. Foot and Mouth Disease Vaccination and Post-Vaccination Monitoring Guidelines: Food and Agriculture Organization of the United Nations and World Organization for Animal Health (2016). Available online at: http://www.fao.org/e/i5975e/i5975e.pdf (accessed at: Feb 25, 2021).
2. World Organization for Animal Health. Manual 3. Foot and Mouth Disease Vaccination and Post-Vaccination Monitoring. (2018). Available online at: http://rr-asia.oie.int/projects/fmd/seacfmd-manual/ (accessed at: Feb 25, 2021).
3. Leon EA, Perez AM, Stevenson MA, Robiolo B, Mattion N, Seki C, et al. Effectiveness of systematic foot and mouth disease mass vaccination campaigns in Argentina. Rev Sci Tech. (2014) 33:917–26. doi: 10.20506/rst.33.3.2329
4. Sharma GK, Mahajan S, Matura R, Biswal JK, Ranjan R, Subramaniam S, et al. Herd immunity against foot-and-mouth disease under different vaccination practices in india. Transbound Emerg Dis. (2017) 64:1133–47. doi: 10.1111/tbed.12478
5. Knight-Jones TJ, Gubbins S, Bulut AN, Stark KD, Pfeiffer DU, Sumption KJ, et al. Mass vaccination, immunity and coverage: modelling population protection against foot-and-mouth disease in Turkish cattle. Sci Rep. (2016) 6:22121. doi: 10.1038/srep22121
6. Singh RK, Sharma GK, Mahajan S, Dhama K, Basagoudanavar SH, Hosamani M, et al. Foot-and-mouth disease virus: immunobiology, advances in vaccines and vaccination strategies addressing vaccine failures-an indian perspective. Vaccines. (2019) 7:90. doi: 10.3390/vaccines7030090
7. Knight-Jones TJ, Rushton J. The economic impacts of foot and mouth disease - what are they, how big are they and where do they occur? Prev Vet Med. (2013) 112:161–73. doi: 10.1016/j.prevetmed.2013.07.013
8. Park JH, Lee KN, Ko YJ, Kim SM, Lee HS, Shin YK, et al. Control of foot-and-mouth disease during 2010–2011 epidemic, South Korea. Emerg Infect Dis. (2013) 19:655–9. doi: 10.3201/eid1904.121320
9. Park JH, Tark D, Lee KN, Chun JE, Lee HS, Ko YJ, et al. Control of type O foot-and-mouth disease by vaccination in Korea, 2014–2015. J Vet Sci. (2018) 19:271–9. doi: 10.4142/jvs.2018.19.2.271
10. Jo HE, Ko MK, Choi JH, Shin SH, Jo H, You SH, et al. New foot-and-mouth disease vaccine, O JC-R, induce complete protection to pigs against SEA topotype viruses occurred in South Korea, 2014–2015. J Vet Sci. (2019) 20:e42. doi: 10.4142/jvs.2019.20.e42
11. Park SY, Lee JM, Kim AY, Park SH, Lee SI, Kim H, et al. Efficient removal of non-structural protein using chloroform for foot-and-mouth disease vaccine production. Vaccines. (2020) 8:483. doi: 10.3390/vaccines8030483
12. Park JH, Tark D, Lee KN, Lee SY, Ko MK, Lee HS, et al. Novel foot-and-mouth disease virus in Korea, July-August 2014. Clin Exp Vaccine Res. (2016) 5:83–7. doi: 10.7774/cevr.2016.5.1.83
13. Kim T, Ryoo S, Nah JJ, Sagong MG, Lee S, Lee KN, et al. Complete genome sequence of a foot-and-mouth disease virus of serotype O, isolated from Gochang, Republic of Korea, in 2016. Genome Announc. (2017) 5:e01671–16. doi: 10.1128/genomeA.01671-16
14. Ryoo S, Kim T, Nah JJ, Sagong MG, Lee S, Lee KN, et al. Complete genome sequence of a foot-and-mouth disease virus of serotype O isolated from Gimje, Republic of Korea, in 2016. Genome Announc. (2017) 5:e01694–16. doi: 10.1128/genomeA.01694-16
15. Galdo Novo S, Malirat V, Maradei ED, Pedemonte AR, Espinoza AM, Smitsaart E, et al. Efficacy of a high quality O1/Campos foot-and-mouth disease vaccine upon challenge with a heterologous Korean O Mya98 lineage virus in pigs. Vaccine. (2018) 36:1570–6. doi: 10.1016/j.vaccine.2018.02.015
16. World Organization for Animal Health. Terrestrial Animal Health Code. (2019). Available online at: https://www.oie.int/en/standard-setting/terrestrial-code/access-online/ (accessed at: Feb 25, 2021).
17. Food and Agriculture Organization. The Progressive Control Pathway for Foot and Mouth Disease control (PCP-FMD), Stage Descriptions and Standards, 2nd Edn (2018). Available online at: http://www.fao.org/3/CA1331EN/ca1331en.pdf (accessed at: Feb 25, 2021).
18. Thrusfield M. Veterinary Epidemiology. 3rd Edn. Oxford: Blackwell Publishing Ltd (2007). p. 610.
19. Caporale V, Giovannini A, Zepeda C. Surveillance strategies for foot and mouth disease to prove absence of disease and absence of viral circulation. Rev Sci Tech. (2012) 31:747–59. doi: 10.20506/rst.31.3.2156
20. Balakrishnan S, Bhanu Rekha V. Herd immunity: an epidemiological concept to eradicate infectious diseases. J Entomol Zool Stud. (2018) 6:8.
22. León EA, Stevenson MA, Fernández D, Robiolo B, Aznar MN, Duffy SJ, et al., editors. Serological evaluation of a foot-and-mouth disease vaccination campaign in young cattle in Buenos Aires province, Argentina. 12th Symposium of the International Society for Veterinary Epidemiology and Economics (ISVEE). Durban, South Africa (2009).
23. Knight-Jones TJD, McLaws M, Rushton J. Foot-and-mouth disease impact on smallholders - what do we know, what don't we know and how can we find out more? Transbound Emerg Dis. (2017) 64:1079–94. doi: 10.1111/tbed.12507
24. Knight-Jones TJ, Bulut AN, Gubbins S, Stark KD, Pfeiffer DU, Sumption KJ, et al. Randomised field trial to evaluate serological response after foot-and-mouth disease vaccination in Turkey. Vaccine. (2015) 33:805–11. doi: 10.1016/j.vaccine.2014.12.010
25. Naranjo J, Cosivi O. Elimination of foot-and-mouth disease in South America: lessons and challenges. Philos Trans R Soc Lond B Biol Sci. (2013) 368:20120381. doi: 10.1098/rstb.2012.0381
26. Rweyemamu M, Roeder P, MacKay D, Sumption K, Brownlie J, Leforban Y. Planning for the progressive control of foot-and-mouth disease worldwide. Transbound Emerg Dis. (2008) 55:73–87. doi: 10.1111/j.1865-1682.2007.01016.x
27. Chen SP, Lee MC, Sun YF, Yang PC. Application of non-structural protein ELISA kits in nationwide FMD surveillance in pigs to demonstrate virus circulation in Taiwan. Vet Microbiol. (2011) 152:266–9. doi: 10.1016/j.vetmic.2011.05.011
28. Paton DJ, Fussel AE, Vosloo W, Dekker A, De Clercq K. The use of serosurveys following emergency vaccination, to recover the status of "foot-and-mouth disease free where vaccination is not practised. Vaccine. (2014) 32:7050–6. doi: 10.1016/j.vaccine.2014.10.064
29. Paton DJ, de Clercq K, Greiner M, Dekker A, Brocchi E, Bergmann I, et al. Application of non-structural protein antibody tests in substantiating freedom from foot-and-mouth disease virus infection after emergency vaccination of cattle. Vaccine. (2006) 24:6503–12. doi: 10.1016/j.vaccine.2006.06.032
30. Lyons NA, Lyoo YS, King DP, Paton DJ. Challenges of generating and maintaining protective vaccine-induced immune responses for foot-and-mouth disease virus in pigs. Front Vet Sci. (2016) 3:102. doi: 10.3389/fvets.2016.00102
31. Dekker A, Chenard G, Stockhofe N, Eble PL. Proper timing of foot-and-mouth disease vaccination of piglets with maternally derived antibodies will maximize expected protection levels. Front Vet Sci. (2016) 3:52. doi: 10.3389/fvets.2016.00052
32. Lee HS, Lee NH, Seo MG, Ko YJ, Kim B, Lee JB, et al. Serological responses after vaccination of growing pigs with foot-and-mouth disease trivalent (type O, A and Asia1) vaccine. Vet Microbiol. (2013) 164:239–45. doi: 10.1016/j.vetmic.2013.02.012
Keywords: foot-and-mouth disease virus, population immunity, mass vaccination, vaccination campaign, post-vaccination monitoring
Citation: Park M-Y, Han YJ, Choi E-J, Kim H, Pervin R, Shin W, Kwon D, Kim JM and Pyo HM (2021) Post-vaccination Monitoring to Assess Foot-and-Mouth Disease Immunity at Population Level in Korea. Front. Vet. Sci. 8:673820. doi: 10.3389/fvets.2021.673820
Received: 28 February 2021; Accepted: 22 June 2021;
Published: 04 August 2021.
Edited by:
Bouda Vosough Ahmadi, European Commission for the Control of Foot and Mouth Disease (EuFMD), ItalyReviewed by:
Carolina Stenfeldt, United States Department of Agriculture, United StatesCopyright © 2021 Park, Han, Choi, Kim, Pervin, Shin, Kwon, Kim and Pyo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyun Mi Pyo, aG1weW9Aa29yZWEua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.