- 1College of Life Science and Technology, Jining Normal University, Jining, China
- 2Inner Mongolia Jinyu Group Co., Ltd., Hohhot, China
- 3Shanxi Province Datong University, Datong, China
- 4Inner Mongolia Shuangqi Pharmaceutical Co., Ltd., Hohhot, China
- 5Hohhot Vocational College, Hohhot, China
- 6National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
Vaginal inflammation is a common disease of the dairy cows' reproductive tract. Lactic acid bacteria can combat purulent inflammation caused by pathogenic bacteria and regulate the NF-κB signaling pathway mediated by toll-like receptors (TLRs) in the inflammatory response. We studied the effect of Lactobacillus johnsonii SQ0048, an isolate with antibacterial activity, on the NF-κB signaling pathway in cow vaginal epithelial cells. The expression levels of serial effectors related to the TLRs-MyD88/NF-κB signaling pathway (TLR2, TLR4, MyD88, IKK, NF-κB, IL-1β, IL-6, TNF-α, and IL-10) were measured with real-time polymerase chain reaction (RT-PCR), ELISA, and Western blot analyses. TLR2 and TLR4 were activated by SQ0048 cells, as noted by increased mRNA expression levels of TLR2 and TLR4 in SQ0048-treated bovine vaginal epithelial cells relative to control cells (P <0.01). SQ0048 treatment also significantly increased MyD88 and IKK expression, and activated NF-κB in vaginal epithelial cells (P <0.01). In addition, SQ0048 treatment also significantly increased mRNA expression levels of IL-1β, IL-6, and TNF-α, but decreased IL-10 mRNA expression levels (P <0.01). These data indicate that strain SQ0048 presence can improve the immune functions of cow vaginal epithelial cells by activating TLRs-MyD88/NF-κB signaling pathways. However, further in vivo studies are required to confirm these findings.
Introduction
Lactic acid bacteria (LAB) are the main bacteria in the healthy female reproductive tract of humans and other mammals such as cows (1). Uterine inflammation can cause infertility in postpartum cows, and many pathogenic bacteria cause uterine inflammation (2). The production of cytokines related to inflammation is mediated by the NF-κB signaling pathway, which also plays an important role in infection by pathogenic bacteria (3). Some pathogen molecules, such as lipopolysaccharides, can be recognized by Toll-like receptors (TLRs) and they further activate NF-κB to stimulate the production of cytokines (4).
LAB control vaginal pathogenic bacteria in the female urogenital tracts by lowering pH and decreasing levels of hydrogen peroxide, and bacteriocins (5). Previous studies have demonstrated that NF-κB signaling is a key pathway involved in the anti-inflammatory role of Lactobacillus (6). The peptidoglycan of Lactobacillus can upregulate TLR2 receptors, participate in the innate immune response, and activate the TLR4 pathway (7); it also functions as a surveillance mechanism against pathogenic bacteria in the TLR4 pathway (8, 9). Extracellular polysaccharides produced by Lactobacillus can improve antiviral immunity, stimulate TLR2 and TLR4, and promote expression of IFN-α or IFN-β in cells (10). The IKK complex can inhibit NF-κB by controlling phosphorylation of the κB signaling pathway, which regulates cytokines to suppress inflammation under the stimulation of lactic acid bacteria (11). Another study confirmed that LAB could inhibit TLR-4-linked NF-κB activation (12).
Under normal conditions, LAB dominate the vaginal flora of dairy cows (1). LAB inhibit pathogenic microorganisms, reduce purulent vaginal secretions, and improve the environment of the bovine reproductive tract (13, 14). Further, LAB can change the intravaginal environment, limit the number of pathogenic bacteria reaching the uterus, and prevent or reduce the prevalence of uterine diseases in cows (13, 15). Most studies of Lactobacillus johnsonii have focused on the human relevance of these bacteria, and there are few reports of Lactobacillus johnsonii in cows. The lactobacillus strain SQ0048 isolated from bovine vagina has been shown to exhibit specific adherence to the epithelium and to produce inhibitory substances; however, the underlying mechanisms remain unclear (16). Therefore, we explored its regulatory effects and determined the probiotic mechanism of SQ0048 at the molecular level in bovine vaginal epithelial cells.
Materials and Methods
Lactobacillus johnsonii SQ0048 Source and Culture Conditions
The Lactobacillus johnsonii SQ0048 strain was isolated, identified, and preserved from the vaginas of healthy cows by Inner Mongolia Shuangqi Pharmaceutical Co., Ltd., as described in the patent certificate No: CN103070890B China. SQ0048 was grown anaerobically in 80 mL de Man, Rogosa, and Sharpe (MRS, Hopebio, Qingdao, China) at 37°C for 10 h. Bacteria were then collected by centrifugation at 5,000g for 10 min and washed three times with phosphate-buffered saline (PBS, Gibco/Thermo Fisher Scientific, Waltham, MA, USA). Finally, SQ0048 was resuspended in Dulbecco's modified Eagle medium (DMEM)/F-12 (DMEM/F12, Gibco, Grand Island, NY, USA) medium without additional reagents to the following concentrations: 108 CFU/mL, 109 CFU/mL, and 1010 CFU/mL.
Cell Treatment
Primary bovine vaginal epithelial cells (BVECs) were isolated, purified, cultured, and identified in accordance with the methods used previously (16). Briefly, bovine vaginal tissue fragments were washed with Dulbecco's PBS (DPBS, Gibco/Thermo Fisher Scientific, Waltham, MA, USA) containing penicillin (100 U/mL) and streptomycin (100 U/mL). The fragments were further digested using 0.1% chain protease at 4°C for 16 h, then scraped into DPBS, and centrifuged at 1,500 rpm for 5 min to harvest cells. The isolated cells were resuspended in DMEM/F-12 (Dulbecco's modified Eagle medium/Ham's F-12 nutrient medium, Gibco/Thermo Fisher Scientific, Waltham, MA, USA) containing 15% fetal bovine serum (ExCell Biology, Shanghai, China), penicillin (100 U/mL), and streptomycin (100 U/mL). The cells were then incubated in 5% CO2 at 37°C. The origin and purity of BVECs was verified using immunocytochemical staining. BVECs were passaged to the fourth generation. When the cells adhered to more than 90% of the bottle wall area and the density of cells was approximately 3 ×105 cells/mL, they were added to DPBS, washed, and added to DMEM/F-12 medium without additional reagents for 12 h. The cells subjected to different concentrations of SQ0048 (108 CFU/mL, 109 CFU/mL, and 1010 CFU/mL) were used to evaluate different parameters of the TLRs-MyD88/NF-κB signaling pathway, such as expression of TLR2, TLR4, MyD88, IKK, NF-κB, and inflammatory factors, to further determine the influence of SQ0048 on the TLRs-MyD88/NF-κB signaling pathway.
Experimental Group Treatment
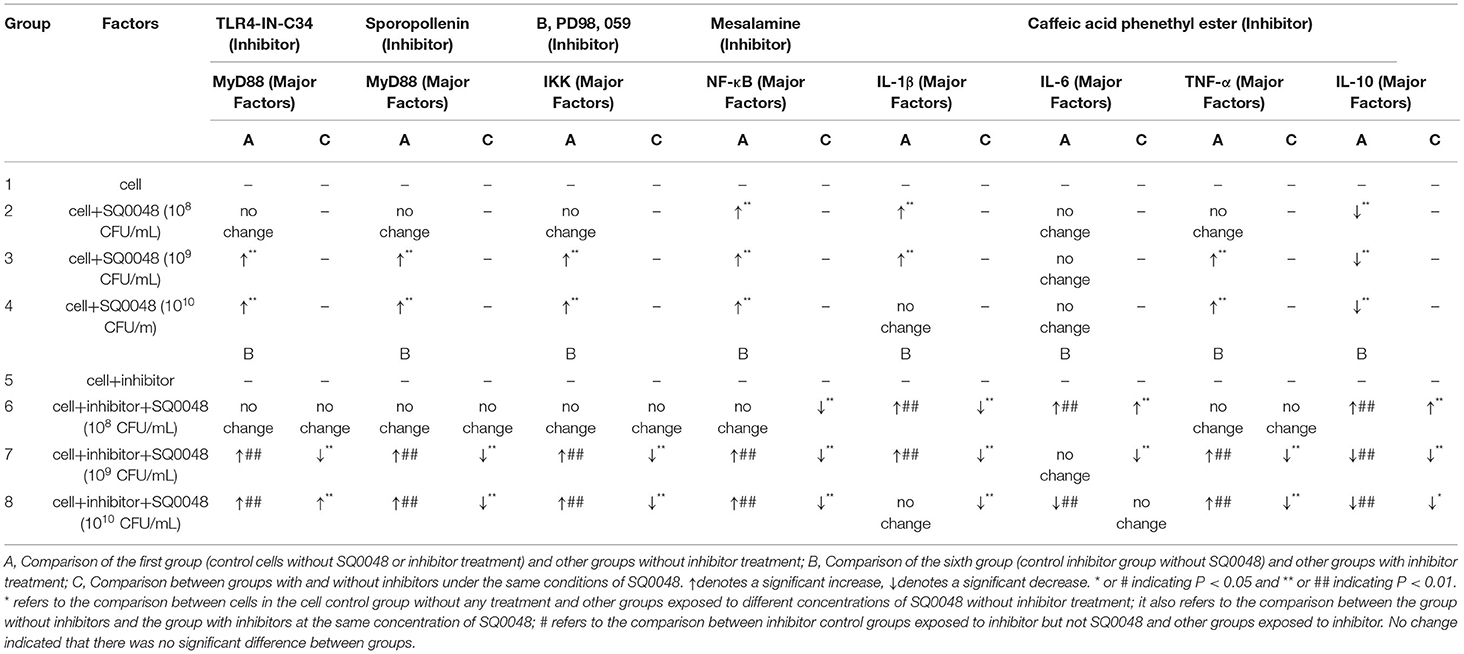
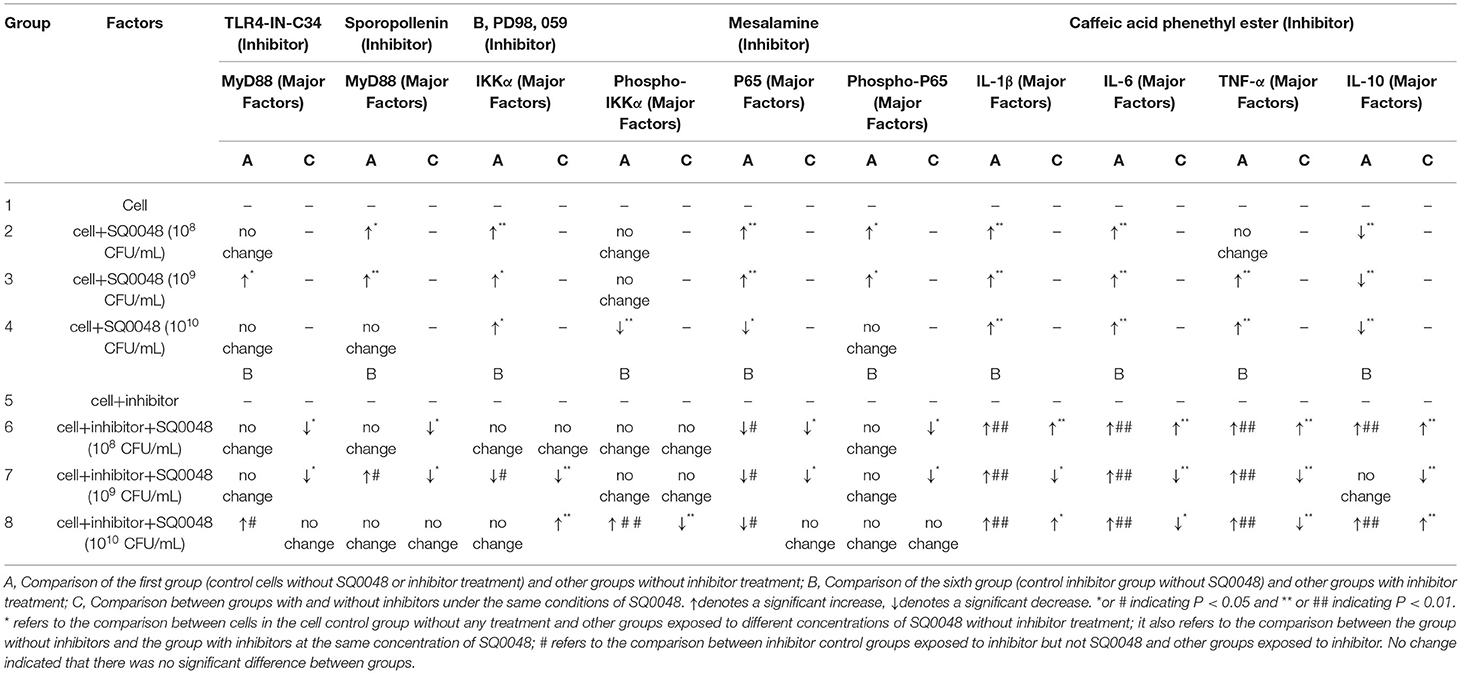
Our experiment was divided into eight groups, involving two experiment control groups (cell control group and inhibitor control group) and six testing groups (Table 1, factors section). A group of cells not subjected to any treatment was used as the cell control group, while the inhibitor control group had only inhibitors without SQ0048. The remaining six testing groups comprised SQ0048 with or without inhibitors at different concentrations (108 CFU/mL, 109 CFU/mL, and 1010 CFU/mL) (Table 1, factors section). The experiment of expression of TLR2 and TLR4 mRNA did not add inhibitors. At least three independent experiments were performed for each analysis in the same conditions.
Expression of TLR2 and TLR4 mRNA
Different concentrations of SQ0048 (108 CFU/mL, 109 CFU/mL, and 1010 CFU/mL) were added to cells treated and incubated for 4 h, as described previously (16). Real-time polymerase chain reaction (RT-PCR) was used to detect the expression of TLR2 and TLR4 mRNA (17). Total mRNA was extracted and reverse-transcribed to produce cDNA to conduct RT-PCR. AxyPrep Multisource Total mRNA Miniprep Kit (Axygen Scientific, Union City, CA, USA), PrimeScript II 1st Strand cDNA synthesis kit (Vazyme, Nanjing, China), and SYBR Green Master (Rox) (Roche, Mannheim, Germany) were used in this process in accordance with the manufacturers' instructions. The amplification program was as follows: 95°C for 5 min, 40 cycles of 95°C for 10 s, 58 to 60°C for 15 s, 72°C for 20 s. The primers were designed according to the bovine TLR2 and TLR4 sequences published in the GenBank database; the accession number, genome name, and sequences are shown in Supplementary Table 1. The results were analyzed using the 2−ΔΔCt (ΔΔCt = ΔCt – ΔCtcontrol and ΔCt = C ttarget – Ctβ−actin) method and normalized to the expression of β-actin (16).
Expression of Inflammatory Factors' mRNA
The suitable concentration of each inhibitor used in this study was added to the treated cells and incubated for suitable periods of time in the culture dishes. The suitable concentrations and times were optimized in the early stage of the experiment (Supplementary Table 2). The dishes were then washed three times with DPBS. Different concentrations of SQ0048 (108 CFU/mL, 109 CFU/mL, and 1010 CFU/mL) were added to the dishes and incubated for 4 h (16). mRNA expression levels of MyD88, IKK, NF-κB, IL-1β, IL-6, TNF-α, and IL-10 were detected under treatment with inhibitors using the RT-PCR method as described above. The primers were designed according to the bovine MyD88, IKK, NF-κB, IL-1β, IL-6, TNF-α, and IL-10 sequences published in the GenBank database (Supplementary Table 1). The names and manufacturers of each factor inhibitor used in this study are as follows: inhibitor of TLR4 is TLR4-IN-C34, co-inhibitor of TLR2 and TLR4 is Sparstolonin B, and inhibitor of NF-κB is Caffeic Acid Phenethyl Ester (Sigma-Aldrich, St. Louis, MO, USA); inhibitor of MEKK1 is B, PD98,059, and inhibitor of IKK is Mesalamine (Selleck Chemicals, Houston, Texas, USA).
Expression of Major Factors Protein
Same as the process described above, the treated cells were incubated separately with inhibitor, and then washed. After adding different concentrations of SQ0048, the expression levels of IL-1β, IL-6, TNF-α, and IL-10 proteins were detected in supernatants using ELISA kits [Bovine IL-6 DuoSet ELISA DY8190 and Bovine TNF-alpha DuoSet ELISA DY2279 (R&D Systems, Minneapolis, MN, USA); Bovine IL-1beta ELISA Kit ab273202 and Bovine IL-10 ELISA Kit ab277386 (Abcam, Cambridge, UK)]. Western blot analysis was used to measure MyD88, IKK, and NF-κB, protein levels, because relevant ELISA kits were not available for these proteins. Protein quantification was performed as previously described (18). Total protein was extracted using M-PER (Thermo Fisher, USA) with 1% Halt Protease Inhibitor from BVECs. Protein concentrations were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, USA). The extracted proteins were stored at −80°C. A protein sample (20 μg) was resolved on 12% SDS-PAGE gel, then blotted onto polyvinylidene fluoride membranes. The membranes were blocked with 3% BSA for 2 h at room temperature. The corresponding primary antibody was incubated overnight at 4°C. The primary antibody dilutions were as follows: MyD88 1:1000, IKK 1:1000, NF-κB 1:1000, and β-actin 1:3000. Proteins were visualized using a horse radish peroxidase (HRP)-conjugated secondary antibody for 1 h at 20–22°C. The membranes were exposed to Pierce Super signal West Femto Chemiluminescent Substrate and a high-performance chemiluminescence film. Band density was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The target protein band densities were normalized to β-actin. The primary antibodies used for western blot analysis were MyD88 (D80F5) Rabbit mAb (4283S) (004283S000307252017), IKK-alpha Rabbit Ab (2682S) (002682S000510132017), P-IKK-alpha/beta (S176/180) (16A6) Rabbit mAb (2697T) (002697T001910102017), P-NF-kappaB p65 (S536) (93H1) Rabbit mAb (3033T) (003033T001609082017), and NF-kappaB p65 (D14E12) XP (R) Rabbit mAb (8242T), (008242T000909152017) (Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies were goat anti-rabbit IgG HRP-linked and goat anti-mouse IgG HRP-linked (Cell Signaling Technology, Danvers, MA, USA). The primary antibodies and ELISA kits specific for bovine tissues were used.
Statistical Analyses
The experimental data were analyzed using analysis of variance (ANOVA) in SPSS and GraphPad Prism 6. Data significance was labeled with *&#, *or # indicating P < 0.05 and ** or ## indicating P < 0.01. * refers to the comparison between the cell control group with no SQ0048 and no inhibitor treatment, and other groups with different concentrations of SQ0048 and no inhibitor treatment, as well as the comparison between groups without inhibitors and the group with inhibitors with the same concentration of SQ0048. # refers to the comparison between the inhibitor control group with inhibitors but not SQ0048 and other groups with inhibitors.
Results
Expression of TLR2 and TLR4 mRNA
TLR2 and TLR4 mRNA levels in the 109 CFU/mL and 1010 CFU/mL SQ0048 groups were significantly higher than those in the cell control group (P <0.01). In contrast, the 108 CFU/mL SQ0048 group did not significantly affect mRNA levels relative to cell control (Figures 1A,B).
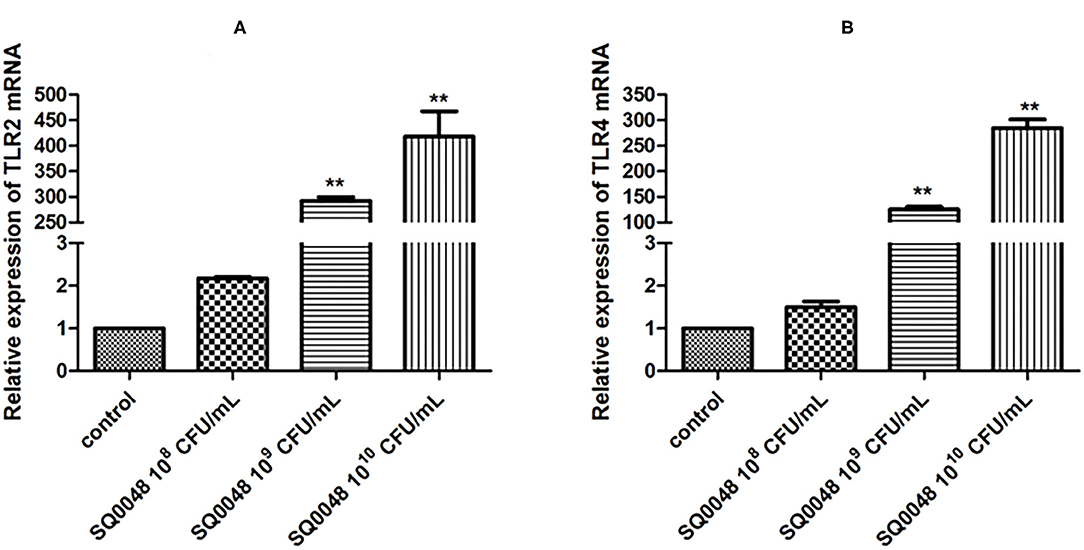
Figure 1. Effects of SQ0048 on mRNA expression levels of TLR2 (A) and TLR4 (B) in BVECs. All data are shown as means ± SD (n = 3). *P < 0.05 and **P < 0.01 relative to control.
Inflammatory Factor mRNA Expression
The mRNA expression level of MyD88 in the 109 CFU/mL and 1010 CFU/mL SQ0048 groups without inhibitors significantly increased compared with those in the cell control group (P <0.01) (Figures 2A,B; Table 1). The mRNA expression of MyD88 in the 109 CFU/mL SQ0048 group with inhibitors (TLR4-IN-C34 or Sporopollenin) was significantly lower than that in the same concentration SQ0048 group without inhibitors (P <0.01). The mRNA expression of MyD88 in the 1010 CFU/mL SQ0048 groups with inhibitor (Sporopollenin) was significantly lower than those in the same concentration SQ0048 groups without inhibitors (Sporopollenin) (P <0.01) (Figures 2A,B; Table 1). The expression of IKK mRNA in the 109 CFU/mL and 1010 CFU/mL SQ0048 groups without inhibitors was significantly higher than that of the cell control group (P <0.01) (Figure 2C; Table 1). The mRNA expression levels of IKK in the 109 CFU/mL and 1010 CFU/mL SQ0048 groups with inhibitors (B, PD98, 059) were significantly lower than those in the same concentration SQ0048 groups without inhibitors (P <0.01) (Figure 2C; Table 1). The mRNA expression levels of NF-κB in the 108 CFU/mL, 109 CFU/mL, and 1010CFU/mL SQ0048 groups without inhibitors were significantly greater than those in the cell control group (P <0.01) (Figure 2D; Table 1). The mRNA expression levels of NF-κB in all three concentrations of SQ0048 with the inhibitor Mesalamine were significantly lower than those in the same concentration SQ0048 groups without Mesalamine (P <0.01) (Figure 2D; Table 1). IL-1β mRNA levels in the 108 CFU/mL and 109 CFU/mL SQ0048 groups without inhibitors were significantly increased compared with those in the cell control group (P <0.01), but the mRNA expression levels of IL-1βin the three concentrations of SQ0048 with inhibitors (Caffeic acid phenethyl ester) were significantly lower than those in the same concentration SQ0048 groups without inhibitors (P <0.01) (Figure 2E; Table 1). IL-6 mRNA levels in all concentrations of SQ0048 without inhibitors were not significantly different from those in the cell control group (P > 0.05). The mRNA level of IL-6 in the 108 CFU/mL SQ0048 group with inhibitor (Caffeic acid phenethyl ester) was significantly higher than that in the same concentration SQ0048 group without inhibitor (P <0.01). The mRNA expression level of IL-6 in the 109 CFU/mL SQ0048 group with inhibitor (Caffeic acid phenethyl ester) was significantly lower than that in the same concentration of SQ0048 without inhibitor (P <0.01) (Figure 2F; Table 1). Expression of TNF-α mRNA had the same trend as IKK (Figure 2G; Table 1), while expression IL-10 mRNA had the opposite trend (Figure 2H; Table 1).
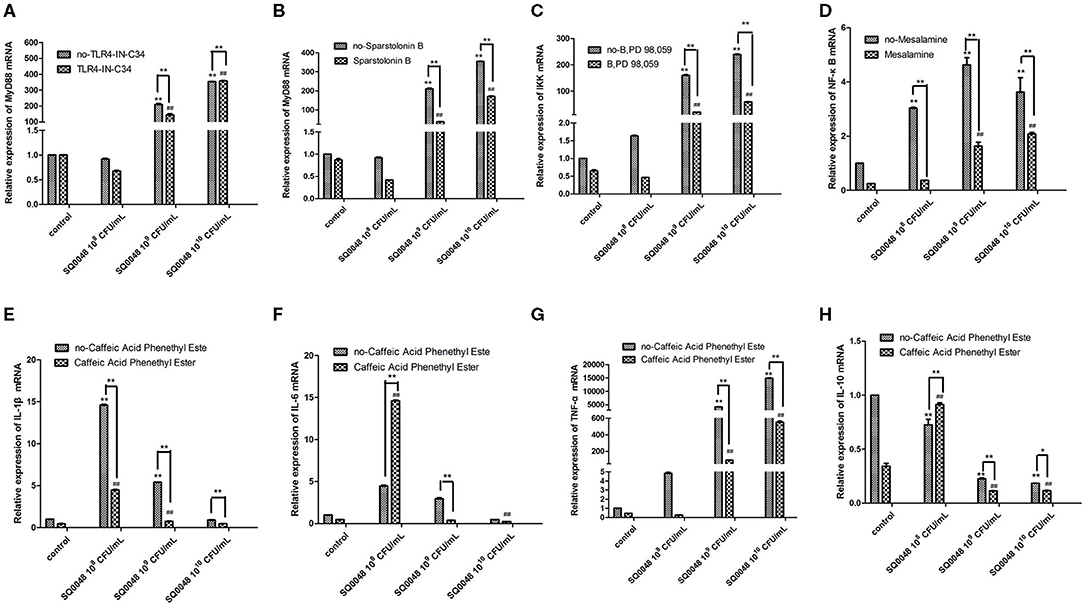
Figure 2. mRNA expression levels of MyD88 (A,B), IKK (C), NF-κB (D), IL-1β (E), IL-6 (F), TNF-α (G), and IL-10 (H) after treatment with inhibitors in BVECs. Note: (A) TLR4-IN-C34 (5 μm) for 30 min, (B) Sparstolonin B (10 μm) for 120 min; (C) B, PD98,059 (20 μm) for 90 min; (D) Mesalamine (1 mm) for 60 min; (E) Caffeic Acid Phenethyl Ester (20 μm) for 60 min; (F) Caffeic Acid Phenethyl Ester (20 μm) for 60 min; (G) Caffeic Acid Phenethyl Ester (20 μm) for 60 min; (H) Caffeic Acid Phenethyl Ester (20 μm) for 60 min. All data are shown as means ± SD (n = 3). * or # indicating P < 0.05 and ** or ## indicating P < 0.01. *refers to the comparison between cells in the cell control group not exposed to any treatment and other groups exposed to different concentrations of SQ0048 without inhibitor treatment; it also refers to the comparison between the group without inhibitors and the group with inhibitors at the same concentration of SQ0048; # refers to the comparison between the inhibitor control group with inhibitors but not SQ0048 and other groups with inhibitors.
Inflammatory Factor Protein Levels
The protein expression of MyD88 in the 109 CFU/mL SQ0048 group was consistent with the mRNA expression (Figures 3A, 4A,B; Table 2). The protein expression levels of NF-κB in the 108 CFU/mL and 109 CFU/mL SQ0048 groups were consistent with the mRNA expression (Figures 3C, 4E,F; Table 2). The protein expression level of IKKα was similar to mRNA expression in the 109 CFU/mL SQ0048 group (Figures 3B, 4C; Table 2). Phospho-IKKα levels in the 109 CFU/mL SQ0048 group were not affected by the presence or absence of inhibitors (Figures 3B, 4D; Table 2). IL-1β protein secretion and mRNA expression levels were consistent in the 109 CFU/mL SQ0048 groups (Figure 5A; Table 2). The protein secretion and mRNA expression of IL-6 and IL-10 were consistent in both the 108 CFU/mL and in 109 CFU/mL SQ0048 groups (Figures 5B,D; Table 2). TNF-α protein secretion levels and mRNA expression levels were consistent both in the 109 CFU/mL and 1010 CFU/mL SQ0048 groups (Figure 5C; Table 2). These data indicated that 109 CFU/mL SQ0048 was likely the most suitable incubation concentration for activation of the TLR-MyD88/NF-κB signaling pathway in bovine vaginal epithelial cells.
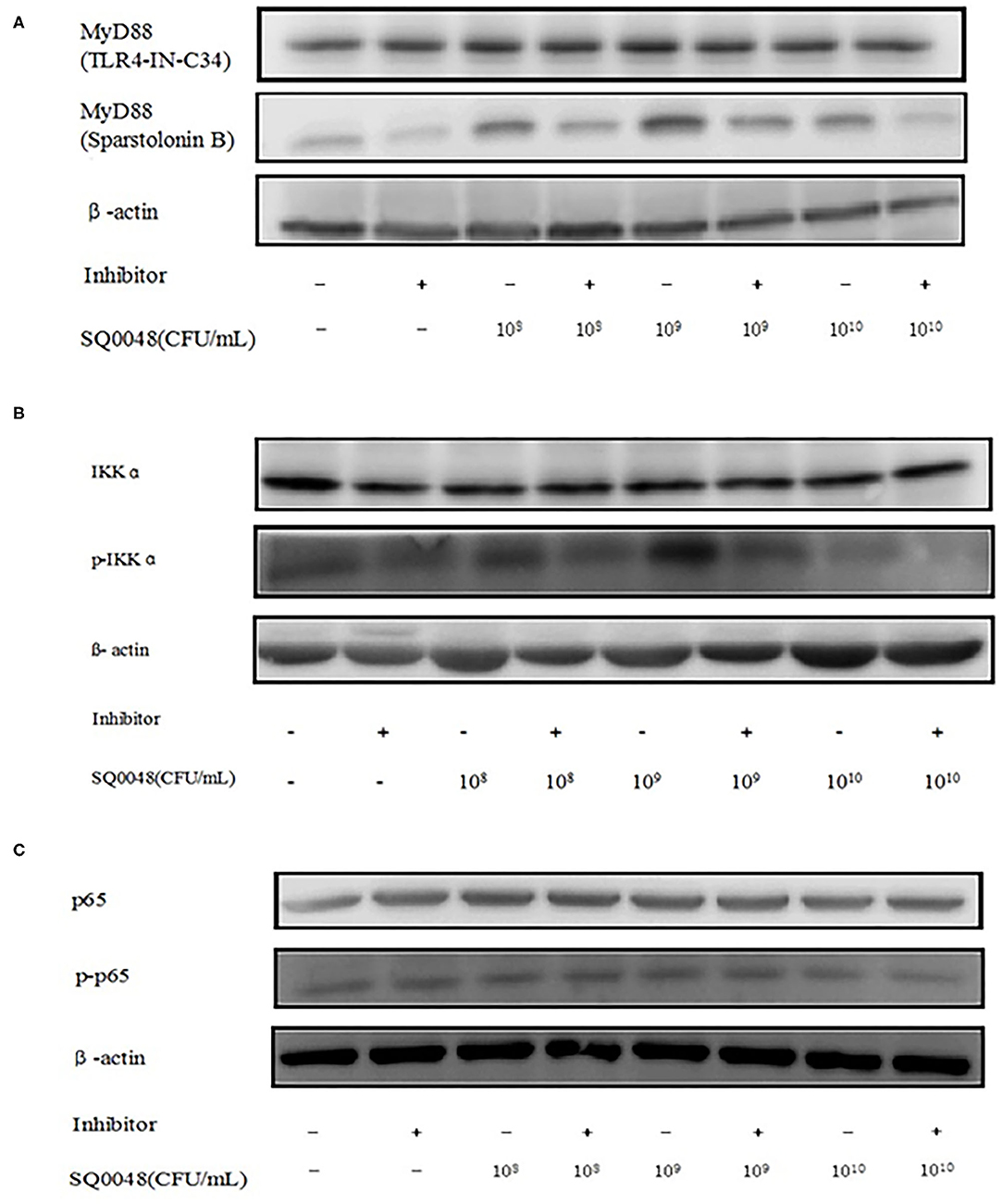
Figure 3. Protein expression levels of MyD88, IKK, and NF-κB after treatment with TLR4-IN-C34 and Sparstolonin (A), B,PD98,059 (B), and Mesalamine (C) in BVECs.
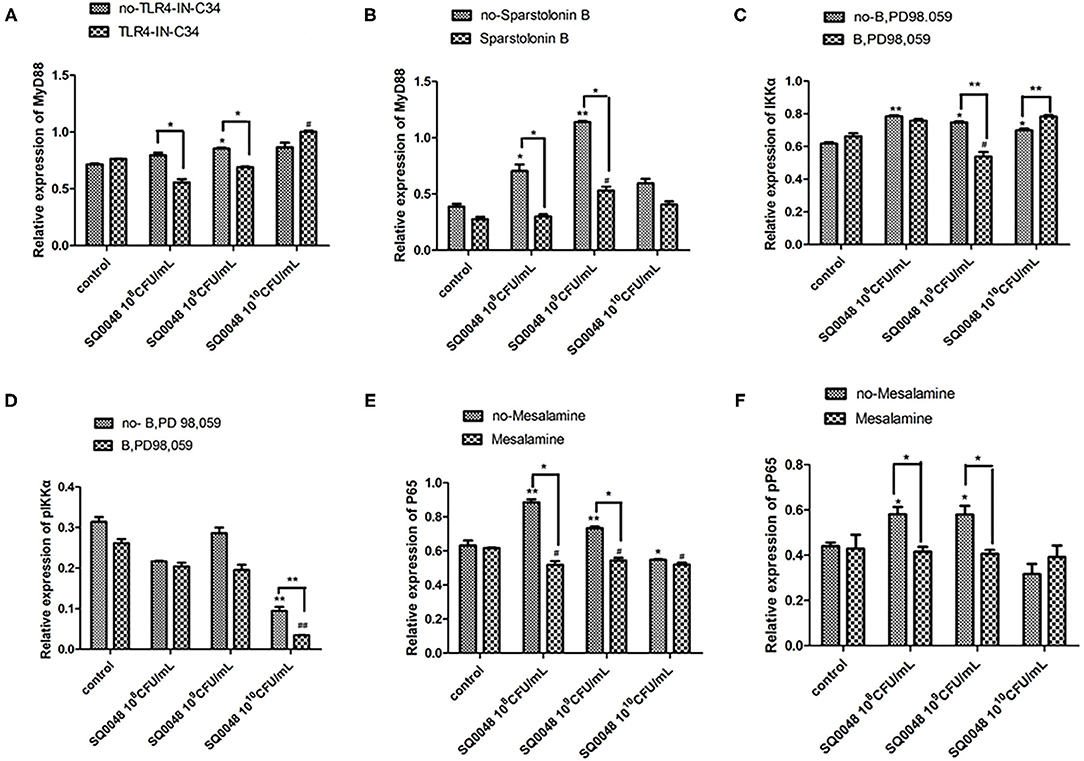
Figure 4. Protein expression levels of MyD88 (A,B), IKK (C,D), and NF-κB (E,F) after treatment with various inhibitors in BVECs using grayscale analysis. Note: TLR4-IN-C34 (A) and Sparstolonin B (B); phosphorylated IKKα (C) and non-phosphorylated IKKα (D) in treatment with B,PD98,059; phosphorylated NF-κB (E) and non-phosphorylated NF-κB (F) in the treatment by Mesalamine. All data are shown as means ± SD (n = 3). * or # indicating P < 0.05 and ** or ## indicating P < 0.01. *refers to the comparison between cells in the cell control group not exposed to any treatment and other groups exposed to different concentrations of SQ0048 without inhibitor treatment; it also refers to the comparison between groups without inhibitors and corresponding groups with inhibitors at the same concentration of SQ0048; # refers to the comparison between cells in the inhibitor control group with inhibitors but not SQ0048 and other groups with inhibitors.
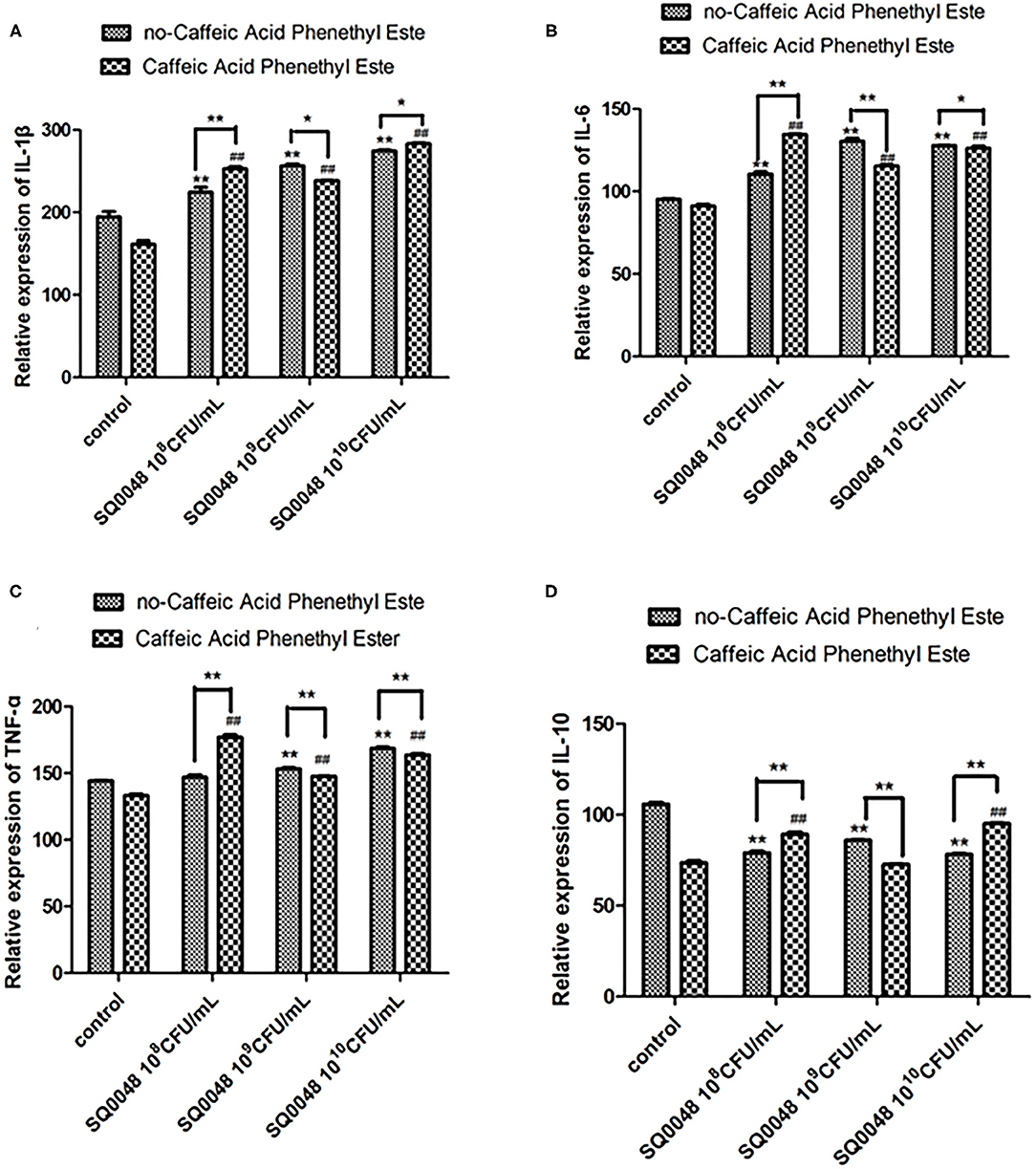
Figure 5. Protein expression levels of IL-1β (A), IL-6 (B), TNF-α (C), and IL-10 (D) in Caffeic Acid Phenethyl Ester-treated BVECs. All data are shown as means ± SD (n = 3). * or # indicating P < 0.05 and ** or ## indicating P < 0.01. * refers to the comparison between cells in the cell control group without any treatment and other groups exposed to different concentrations of SQ0048 without inhibitor treatment; it also refers to the comparison between the group without inhibitors and the group with inhibitors at the same concentration of SQ0048; # refers to the comparison between inhibitor control groups exposed to inhibitor but not SQ0048 and other groups exposed to inhibitor.
Discussion
Reproductive tract infections are often accompanied by systemic fluctuations of inflammatory cytokines (19). Vaginal epithelial cells are a part of the innate defense against vaginal inflammation. The inflammatory response of vaginal epithelial cells primarily involves the TLR and cytokine receptor pathways (20). The probiotic properties and antibacterial characteristics of LAB are closely related to the NF-κB signaling pathway (21). In the present study, 109 CFU/mL and 1010 CFU/mL SQ0048 stimulated TLR2 and TLR4 receptors. This suggested that SQ0048 could be recognized by TLR2/4, which is consistent with previous reports (22), The specificity of TLR2/TLR6 stimulation was confirmed with blocking antibodies. The MyD88-dependent pathway primarily mediates and activates NF-κB/AP-1. Treatment with MyD88 inhibitors decreased expression of NF-κB, but not all of the LAB decreased NF-κB activation (22). LAB or their extracts and metabolites trigger the innate immune response and activate NF-κB by stimulating TNF-α signaling in epithelial cells (23). LAB strains can also increase levels of proinflammatory cytokines, such as TNF-α, IL-12, and IL-8, thereby improving host immune function (24). Lactobacillus acidophilus can improve the phagocytic ability of macrophages in aged mice, activate the NF-κB pathway, and regulate cytokine levels (25). Activated and inactivated Lactobacillus rhamnosus GG can increase macrophage function by activating NF-κB (26). Our experimental data showed that 109 CFU/mL SQ0048 significantly increased expression of MyD88 and IKK and activated NF-κB in dairy cow vaginal epithelial cells. These results indicate that the biological function of SQ0048 is related to activation of the TLRs-MyD88/NF-κB signaling pathway in vaginal epithelial cells.
IL-1β is either not expressed or only weakly expressed in vaginal epithelial cells under normal physiological conditions. However, under pathological conditions, IL-1β expression increases, activating the inflammatory response. Locally high concentrations of IL-1β also contribute to killing pathogenic microorganisms and reducing their pervasion. IL-1β-mediated inflammation could have evolved to fight microorganisms and support tissue repair (27). IL-6 induces inflammation by stimulating the secretion of IL-1β. During infection, increased IL-6 levels promote migration of neutrophils to the site of inflammation and reduce leukocyte apoptosis.
Probiotic bacteria can trigger production of multiple cytokines at the systemic level, such as IL-6, IL-12, TNF-α, and IFN-γ (28). IL-6 can stimulate antibody synthesis through B cell activation and promote lymphocyte proliferation, both of which are beneficial to Th2 differentiation (29). Th2 cytokines can affect IgA, IgG, and natural killer cells, and can also identify and kill infected cells, stimulate epithelial cell proliferation, regulate intestinal and lung wound repair and healing, and inhibit mucosal fungi and bacterial infection (30).
Supernatant cultures of Lactobacillus kefiri BCRC14011, Lactobacillus kefiranofaciens BCRC16059, L. kefiranofaciens M1, L. kefiri M2, Leuconostoc mesenteroides M3, and Lactococcus lactis M4 can increase macrophage expression of IL-12, IL-6, IL-1β, and TNF-α in macrophages in vitro, and have strong antibacterial activity (31). L. crispatus and L. iners can increase expression of IL-6, IL-8, and TNF-α (32). TNF-α is an important cytokine involved in inflammation and immune responses. When TNF-α expression increases, it can lead to disturbances in the immune regulation of the body and contribute to pathological immune damage (33). However, an appropriate increase in TNF-α can also prevent infection. IL-10 inhibits proinflammatory responses and limits pathological inflammatory tissue damage, and is the most important anti-inflammatory cytokine (34).
Both proinflammatory cytokines and anti-inflammatory cytokines have a dual role. Proinflammatory cytokines are essential for initiation of the inflammatory response. Although an excessive inflammatory response could lead to sepsis and other related diseases, the cytokine cascade could be beneficial to the host by initiating an appropriate inflammatory response to infection or injury. This most likely occurs in humans with weakened immune systems, such as in newborns and in the elderly. Our results showed that 109 CFU/mL SQ0048 significantly increased expression of IL-1β, IL-6, and TNF-α and decreased expression of IL-10. This might indicate that the anti-inflammatory mechanism of SQ0048 involves the high concentrations of IL-1β, IL-6, and TNF-α, and the low concentration of IL-10 contributes to killing pathogenic microorganisms.
We showed that 109 CFU/mL SQ0048 upregulated expression levels of MyD88, IKK, and NF-κB factors by activating TLR2 and TLR4, thereby activating the TLR2/TLR4-mediated NF-κB signaling pathway. SQ0048 also increased IL-1β, IL-6, and TNF-α expression and decreased expression of IL-10. Based on our findings, we suggest that the SQ0048 strain is involved in regulating the immune function of cow vaginal epithelial cells. Activation of TLR2 and TLR4 could control the replication of pathogenic bacteria (9), and NF-κB might be related to the immunoregulatory characteristics of LAB as well as the species and source of the strain in epithelial cells (35). As described above, these data suggest that strain SQ0048 may be important for improving the immune function of cow vaginal epithelial cells, but this study has some limitations. Specifically, a mechanism for how SQ0048 improves immune function in vaginal epithelial cells is not currently apparent from these results. Consequently, in vivo experiments are needed to support the observations reported here. Nevertheless, this study provides new insights into the regulatory effects of LAB on diseases of the reproductive tracts of cows.
Data Availability Statement
The original contributions generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
All animal experiments were carried out according to the Animal Welfare and Research Ethics Committee of Jining Normal University (Inner Mongolia, China) (Approval ID: 41, 010, 620–1), and all efforts were made to minimize animal suffering. All animals were owned by private farms and we also obtained informed consent to use the animals in our study from the owner (s) of the animals. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
CC performed experiments research and participated in the design of the study. ZLi and XM managed the study. CC, LZ, JM, QT, and YF conducted data processing and analyses. CC wrote the paper, ZLiu and YL critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Key R&D Program of China (Grant Numbers. 2019YFC1200700 and 2019YFC1200601-6), the National Natural Science Foundation of China (No. 82073624), and the Doctoral Fund of Jining Teachers College (jsbsjj2101). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
LZ is employed by the company Inner Mongolia Jinyu Group Co., Ltd. YL and XM are employed by the company Inner Mongolia Shuangqi Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We were grateful to the staff in Inner Mongolia Jinyu Group Co., Ltd and Inner Mongolia Shuangqi Pharmaceutical Co, Ltd for assistance with strain identification and collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.670949/full#supplementary-material
Abbreviations
BV, Bacterial vaginosis; VVC, Vulvovaginal candidiasis; HPV, Human papillomavirus; HIV, Human immunodeficiency virus; LAB, Lactic acid bacteria; PCR, Polymerase chain reaction; RT-PCR, Real-time polymerase chain reaction; ECL, Enhanced chemiluminescence; TLR, Toll-like receptor.
References
1. Genís S, Bach À, Arís A. Effects of intravaginal lactic acid bacteria on bovine endometrium: implications in uterine health. Vet Microbiol. (2017) 204:174–9. doi: 10.1016/j.vetmic.2017.04.025
2. Sheldon IM, Owens SE. Postpartum uterine infection and endometritis in dairy cattle. Anim Reprod. (2017) 14:622–9. doi: 10.21451/1984-3143-AR1006
3. Ramakrishnan SK, Zhang H, Ma X, Jung I, Schwartz AJ, Triner D, et al. Intestinal non-canonical NFκB signaling shapes the local and systemic immune response. Nat Commun. (2019) 10:660. doi: 10.1038/s41467-019-08581-8
4. Yu J, Zhu YH, Yang GY, Zhang W, Zhou D, Su JH, et al. Anti-inflammatory capacity of Lactobacillus rhamnosus GG in monophasic variant Salmonella infected piglets is correlated with impeding NLRP6-mediated host inflammatory responses. Vet Microbiol. (2017) 210:91–100. doi: 10.1016/j.vetmic.2017.08.008
5. Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. (2004) 28:405–40. doi: 10.1016/j.femsre.2004.01.003
6. Rottenberg S, Schmuckli-Maurer J, Grimm S, Heussler VT, Dobbelaere DA. Characterization of the bovine IkappaB kinases (IKK)alpha and IKKbeta, the regulatory subunit NEMO and their substrate IkappaBalpha. Gene. (2002) 299:293–300. doi: 10.1016/S0378-1119(02)01011-9
7. Genís S, Bach À, Fàbregas F, Arís A. Potential of lactic acid bacteria at regulating Escherichia coli infection and inflammation of bovine endometrium. Theriogenology. (2016) 85:625–37. doi: 10.1016/j.theriogenology.2015.09.054
8. Shida K, Makino K, Morishita A, Takamizawa K, Hachimura S, Ametani A, et al. Lactobacillus casei inhibits antigen-induced ige secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. (1998) 115:278–87. doi: 10.1159/000069458
9. Vinderola G, Matar C, Perdigon G. Role of the epithelial cells in the immune effects mediated by gram-positive probiotic bacteria. Involvement of Toll-like receotors. Clin Diagn Lab Immunol. (2005) 12:1075–84. doi: 10.1128/CDLI.12.9.1075-1084.2005
10. Kanmani P, Albarracin L, Kobayashi H, Iida H, Komatsu R, Humayun Kober AKM, et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol. (2018) 93:253–65. doi: 10.1016/j.molimm.2017.07.009
11. Pendharkar S, Magopane T, Larsson P-G, Bruyn GD, Marcotte H. Identification and characterisation of vaginal lactobacilli from South African women. BMC Infect Dis. (2013) 13:43. doi: 10.1186/1471-2334-13-43
12. Lee JH, Lee B, Lee HS, Bae EA, Lee H, Ahn YT, et al. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-kappaB activation in experimental colitis. Int J Colorectal Dis. (2009) 24:231–7. doi: 10.1007/s00384-008-0618-6
13. Wang Y, Ametaj BN, Ambrose DJ, Gänzle MG. Characterisation of the bacterial microbiota of the vagina of dairy cows and isolation of pediocin-producing Pediococcus acidilactici. BMC Microbiol. (2013) 13:19. doi: 10.1186/1471-2180-13-19
14. Miranda-Casoluengo R, Lu J, Williams EJ, Miranda-Casoluengo AA, Carrington SD, Evans ACO, et al. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE. (2019) 14:e0200974. doi: 10.1371/journal.pone.0200974
15. Sandra G, Alejandro S-C, Àlex B, Francesc F, Anna A. A combination of lactic acid bacteria regulates Escherichia coli infection and inflammation of the bovine endometrium. J Dairy Sci. (2017) 100:479–92. doi: 10.3168/jds.2016-11671
16. Niu C, Cheng C, Liu Y, Huang S, Fu Y, Li P. Transcriptome profiling analysis of bovine vaginal epithelial cell response to an isolated lactobacillus strain. mSystems. (2019) 4:e00268–19. doi: 10.1128/mSystems.00268-19
17. Günther J, Czabanska A, Bauer I, Leigh JA, Holst O, Seyfert HM. Streptococcus uberis strains isolated from the bovine mammary gland evade immune recognition by mammary epithelial cells, but not of macrophages. Vet Res. (2016) 47:13. doi: 10.1186/s13567-015-0287-8
18. Luo Q, Yan X, Bobrovskaya L, Ji M, Yuan H, Lou H, et al. Anti-neuroinflammatory effects of grossamide from hemp seed via suppression of TLR-4-mediated NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. Mol Cell Biochem. (2017) 428:129–37. doi: 10.1007/s11010-016-2923-7
19. Chan JP, Chang CC, Hsu WL, Liu WB, Chen TH. Association of increased serum acute-phase protein concentrations with reproductive performance in dairy cows with postpartum metritis. Vet Clin Pathol. (2010) 39:72–8. doi: 10.1111/j.1939-165X.2009.00182.x
20. Javaid N, Yasmeen F, Choi S. Toll-like receptors and relevant emerging therapeutics with reference to delivery methods. Pharmaceutics. (2019) 11:441. doi: 10.3390/pharmaceutics11090441
21. Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, et al. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microb Infect. (2005) 7:1117–27. doi: 10.1016/j.micinf.2005.03.016
22. Ren C, Zhang Q, De Haan BJ, Zhang H, Faas MM, De Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Rep. (2016) 6:34561. doi: 10.1038/srep34561
23. Laiño J, Villena J, Kanmani P, Kitazawa H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: new insights into molecular interactions with host cells. Microorganisms. (2016) 4:27. doi: 10.3390/microorganisms4030027
24. Belhadj H, Harzallah D, Bouamra D, Khennouf S, Dahamna S, Ghadbane M. Phenotypic and genotypic characterization of some lactic Acid bacteria isolated from bee pollen: a preliminary study. Biosci Microbiota Food Health. (2014) 33:11–23. doi: 10.12938/bmfh.33.11
25. Kim YG, Ohta T, Takahashi T, Kushiro A, Nomoto K, Yokokura T, et al. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes Infect. (2006) 8:994–1005. doi: 10.1016/j.micinf.2005.10.019
26. Miettinen M, Lehtonen A, Julkunen I, Matikainen S. Lactobacilli and Streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J Immunol. (2000) 164:3733–40. doi: 10.4049/jimmunol.164.7.3733
27. Dinarello Charles A. Immunological and inflammatory functions of the interleukin- 1 family. Annu Rev Immunol. (2009) 27:519–50. doi: 10.1146/annurev.immunol.021908.132612
28. Esvaran M, Conway PL. Strain dependent protection conferred by Lactobacillus spp. administered orally with a Salmonella Typhimurium vaccine in a murine challenge model. Vaccine. (2012) 30:2654–61. doi: 10.1016/j.vaccine.2012.02.011
29. Su Z, Fang H, Hong H, Shi L, Zhang W, Zhang W, et al. An investigation of biomarkers derived from legacy microarray data for their utility in the RNA-seq era. Genome Biol. (2014) 15:523. doi: 10.1186/s13059-014-0523-y
30. Kim SI, Choi ME. TGF-β-activated kinase-1: new insights into the mechanism of TGF-β signaling and kidney disease. Kidney Res Clin Pract. (2012) 31:94–105. doi: 10.1016/j.krcp.2012.04.322
31. Jeong EJ, Kim NH, Heo JD, Lee KY, Rho JR, Kim YC, et al. Antifibrotic compounds from Liriodendron tulipifera attenuating HSC-T6 proliferation and TNF-α production in RAW264.7 cells. Biol Pharm Bull. (2015) 38:228–34. doi: 10.1248/bpb.b14-00583
32. Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis. (2014) 209:1989–99. doi: 10.1093/infdis/jiu004
33. Muoz-Carrillo JL, Contreras-Cordero JF, Gutiérrez-Coronado O, Villalobos-Gutiérrez PT, Hernández-Reyes VE. Cytokine Profiling Plays a Crucial Role in Activating Immune System to Clear Infectious Pathogens. London: IntechOpen (2018). doi: 10.5772/intechopen.80843
34. De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, et al. Serum interleukin10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. (2000) 117:365–73. doi: 10.1378/chest.117.2.365
Keywords: Lactobacillus johnsonii, bovine vagina epithelial cells, TLRs-MyD88/NF- κB, TLR2 and TLR4, SQ0048
Citation: Cheng C, Zhang L, Mu J, Tian Q, Liu Y, Ma X, Fu Y, Liu Z and Li Z (2021) Effect of Lactobacillus johnsonii Strain SQ0048 on the TLRs-MyD88/NF-κB Signaling Pathway in Bovine Vaginal Epithelial Cells. Front. Vet. Sci. 8:670949. doi: 10.3389/fvets.2021.670949
Received: 15 March 2021; Accepted: 06 July 2021;
Published: 10 August 2021.
Edited by:
Nick Wheelhouse, Edinburgh Napier University, United KingdomReviewed by:
Francesca Mercati, University of Perugia, ItalyMallikarjun Bidarimath, Cornell University, United States
Copyright © 2021 Cheng, Zhang, Mu, Tian, Liu, Ma, Fu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Liu, bGl1emhpZ3VvQGljZGMuY24=; Zhenjun Li, bGl6aGVuanVuQGljZGMuY24=
 Chao Cheng1
Chao Cheng1 Zhiguo Liu
Zhiguo Liu Zhenjun Li
Zhenjun Li