- 1College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
- 2Department of Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, College of Veterinary Medicine, Northeast Agricultural University, Harbin, China
- 3Pharmacy Department, Harbin Medical University-Daqing, Daqing, China
- 4Chinese Center for Disease Control and Prevention, National Institute for Communicable Disease Control and Prevention, Beijing, China
Brucellosis is a common zoonosis in China, resulting in abortion in animals. Outbreaks of abortion in blue foxes caused by Brucella infection have rarely been reported. In the present study, 3–5 mL blood samples collected from the femoral veins of 10 abortuses of blue foxes were assessed by RBPT (Rose Bengal plate test) and SAT (serum tube agglutination test) to preliminarily investigate the source of infection for the clustering of abortion events at a blue fox farm in Heilongjiang Province. Screening experiments showed that all 10 blood samples were positive in the RBPT, while only eight blood samples out of the 10 were positive in the SAT. Subsequently, 10 tissue samples (spleen, lungs, stomach contents, and afterbirth) from the same 10 foxes were assessed using AMOS (acronym for B. abortus, melitensis, ovis, and suis)-PCR (polymerase chain reaction), and sequencing analysis was performed on amplification products to verify the results of the serology survey. Results showed a spectral band of ~731 bp in these samples. BLAST showed sequences of AMOS-PCR products in this study to be 100% similar (E = 0.0) to sequences in B. melitensis strain from GenBank. These data preliminarily indicated that the blue fox's outbreak of abortion events was caused by brucellosis via the B. melitensis strain. Then 726 serum samples were tested by RBPT and SAT to determine the prevalence of brucellosis on the farm. A comprehensive epidemiological and reproductive status survey of the infected blue fox population was performed. The seropositive rate was found to be 67.90% (493/726) by RBPT and 41.32% (300/726) by SAT. The technicians had stopped feeding the foxes with chicken carcasses and instead fed them raw ground sheep organs (lungs, tracheae, placentae, and dead sheep fetuses) infected by B. meliteneis strains, and that this change in diet caused the outbreak of abortion events. The high abortion rate (55%) and low cub survival rate (65%) were the most distinctive features of the outbreak; these factors led to severe economic losses. Feeding cooked sheep/goat offal and strict breeding management is necessary for disease prevention.
Introduction
Brucellosis is a widespread zoonotic disease that is caused by bacteria and is categorized as a bacterial human disease (1). The World Organization for Animal Health (OIE) lists brucellosis as a multi-animal comorbidity (2), and brucellosis is a second-category animal infectious disease in China (3). The disease mainly affects the reproductive systems of animals (4, 5). Although 12 Brucella species have been identified, B. melitensis, B. abortus, and B. suis are the most common pathogens occurring in human and animal infections (6). Among domestic animals, cattle, sheep, and pigs are infected most frequently, and the disease can be transmitted to bison, elk, wild boars, foxes, hares, African buffalo, and reindeer (7). Brucellosis has caused huge economic losses in the animal husbandry and economic animal breeding industries worldwide (8, 9). The highest and lowest prevalence rates of brucellosis among different fox species were found in red fox (Vulpes vulpes) (100%) and hoary fox (Lycalopex vetulus) (9%), respectively (10). A study showed Gardnerella vaginalis to be the main pathogen that causes miscarriage in foxes in China; the seropositivity rate range of fox population in China is 0.9–21.9%, and in some farms it exceeds 75% (11). Canine distemper virus, pseudorabies virus, and Staphylococcus aureus are common pathogenic agents in the fox population (12), but there is no report of fox abortion caused by Brucella spp. Moreover, the incidence of brucellosis in China has continued to rise in recent years. Heilongjiang Province was designated a Type I brucellosis severe epidemic region due to the ongoing high incidence rate of animal brucellosis (13, 14). The animal husbandry industry is a main economic pillar of this province, and fox and raccoon breeding are the main sources of income for many farmers in this region. In March 2017, an outbreak of abortion of unknown origin occurred at a blue fox breeding farm in Heilongjiang Province, resulting in a high rate of abortion in pregnant blue foxes and causing serious economic losses. At present, serological techniques remains the mainstay for brucellosis diagnosis (15). These include the Rose Bengal Plate Test (RBPT), serum agglutination test (SAT), and complement-fixation test (CFT) (16–18). However, CFT is a technically complex test, and it requires good laboratory facilities and well-trained personnel to perform it accurately and maintain its reagents (19). Moreover, identification of Brucella sp. by conventional tests involves considerable time, risk of human infection, and expert interpretation, whereas PCR is fast, safe, and easy to interpret (20, 21). Previous works described a Brucella PCR assay that can distinguish Brucella abortus (biovars 1, 2, and 4), Brucella melitensis (biovars 1, 2, and 3), Brucella ovis, and Brucella suis (biovar 1) from each other (22). In this study, RBPT, SAT, and AMOS (B. abortus, B. melitensis, Brucella ovis, and Brucella suis)—PCR were used to determine the cause of the outbreak of abortions at a blue fox farm. Our investigation will provide important data for technical guidance in the prevention of blue fox brucellosis as well as promote better management of blue foxes in Heilongjiang province, China.
Methods
Serological Testing
Blood samples were collected from the femoral vein, 3–5 mL per blue fox. A total of 10 serum samples (HBF001–010) were collected from 10 female foxes that had miscarried during 15–20 days in April 2017, and 726 serum samples [65 male foxes, 34 male cub foxes (<1 year old), 564 female foxes, and 61 female cub foxes (<1 year old)] from the blue fox farm were collected in October 2017 to implement the epidemiological survey. Both the Rose Bengal plate test (RBPT) and the Serum Agglutination Test (SAT) were performed according to standard serological procedures (23). RBPT and SAT were used to diagnose human brucellosis (23). RBPT antigen (production batch number: 201701) and SAT antigen (production batch number: 201702) were purchased from Qingdao Yibang Bioengineering Co., Ltd.; brucellosis positive control serum (production batch number: 201702) and negative control serum (production batch number: 201701) were purchased from China Veterinary Drug Supervision Institute. Sperm samples collected from male foxes were preliminarily screened for quality by microscopic examination. Some medicines, including oxytetracycline, astragalus polysaccharides, Vitamin E, and other herbs, were used to treat the blue foxes.
AMOS-PCR
The 10 tissue samples (liver, spleen, lungs, stomach contents, and afterbirth) from the same 10 aborted blue fox fetuses were collected following biosafety regulations. DNA of all samples was extracted using a Qiagen genome DNA prepare kit (Qiagen, Germany) according to the manufacturer's instructions. Subsequently, AMOS-PCR was employed to discriminate the species/biovar of Brucella strains. Amplification and detection procedures were as previously described (24). Briefly, the concentration of the four primer pairs was 25 μM/L, and primer A 1 μL, primer M 1.5 μL, primer O 1.5 μL, primer S 1 μL, primer IS711 2 μL, Taq DNA polymerase 1.25 U, and DNA template 2 μL. Finally, sterilized double distilled water was added to a final volume of 50 μL. Amplification parameters: 94°C pre-denaturation 5 min; 94°C 1 min, 60°C 1.5 min, 72°C 10 min, for 40 cycles; final extension at 72°C for 10 min. Five microliter products and 1 μL loading buffer were uploaded to agarose gels to determine the sizes of products. The target gene size was 498 bp for B. abortus (bv. 1, 2, and 4), 731 bp for B. melitensis, 976 bp for B. ovis, and 285 bp for B. suis (bv. 1). Then, 10 AMOS-PCR products were sequencing using M primer (F) and comparison was performed using the Basic Local Alignment Search Tool (BLAST).
The Evaluation of Reproductive Performance in Female Blue Foxes
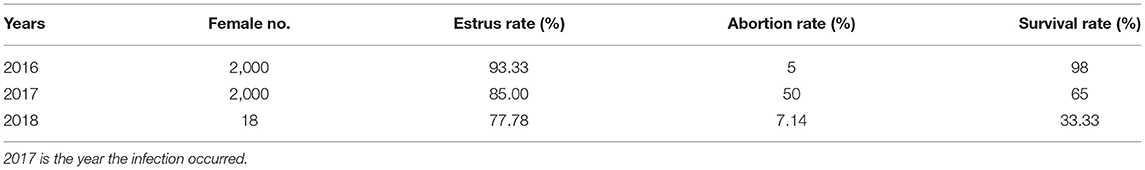
The breeding conditions, estrus rate, weak cub rate, abortion rate, disease occurrence, and medication use of the blue fox farm from 2017 to 2019 were investigated to determine the production performance impact of a female blue fox infected with B. melitensis.
Results
Serological Tests
In order to investigation the cause of the outbeak abortus event. First, ten samples from female foxes were collected and examined by RBPT and SAT. The RBPT results in all 10 serum samples from female foxes were positive. However, eight samples were positive for the SAT (titer 1:50, ++), while the two remaining samples were all suspect cases (titer 1:50, +) (Supplementary Table 1). A preliminary serological survey indicated that infection with Brucella spp. could be a cause of spontaneous abortion in blue foxes. Subsequently, for further survey the situation the infection in blue fax farming, a total of 726 serum samples were collected and detected by RBPT and SAT. The positive rate of the RBPT was 67.90% (493/726) (Table 1), and the positive rate of the SAT was 41.32% (300/726) (Table 1). The SAT titer in 125 samples was 1:25 + (Table 1). Finally, eight of the human staff of this far were screened for serum antibodies against Brucella infection in eight staff in this farming were performed, five staff members of the farm were diagnosed with brucellosis, while there were no brucellosis antibodies detected in the other three staff members. The obvious clinical symptoms (swollen testicles, bedridden, back pain, leg pain) were observed in five brucellosis patients. They frequently ground the raw internal organs of sheep/goat to feed the blue foxes.

Table 1. Brucellosis epidemic situation as detected by serological tests in 726 serum samples from blue fox breeding farm.
AMOS-PCR Amplification
The AMOS-PCR showed that the expected 731 bp size amplified result was observed in three positive controls (B. melitensis M5; 6. B. abortus A19, and B. suis S2), and there were no bands in the negative control E. coli strain. Moreover, an expected 731 bp band was detected among four different tissue types in the samples from aborted fetuses, including spleen, lung, stomach contents, and fetal coats, consistent with the target gene fragment of B. melitensis strains (Figure 1). PCR product sequencing showed that sequences ~700 bp in size were obtained from all 10 samples. Further BLAST showed that these sequences were 100% similar (E = 0.0, sort by percent identity as 100%) to sequences of B. melitensis strain hosted in GenBank (Supplementary Figure 1). This result further verified the results from serological tests as well as confirming that B. melitensis was the pathogen involved in the blue fox cluster of abortion events.
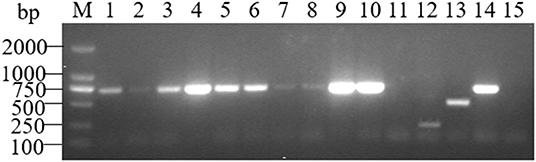
Figure 1. AMOS-PCR typing of the sample from three aborted fetuses of the blue fox. M, marker, DL2000 bp; lane 1–3, spleen, lungs and stomach contents samples from HBF001; lane 4–6, spleen, lungs and stomach contents samples from HBF002; lane 7–8, spleen and lungs samples from HBF003; lane 9–11, Fetal coats samples from three aborted fetus (HBF001-3); lane 12, B. suis S2; lane 13, B. abortus A19; lane 14, B. melitensis M5; lane 15. Negative control, E. coli.
The Epidemiology Investigation
The farm began breeding blue foxes in 2014. In 2016, there were 2,000 female foxes and 110 male foxes, and the abortion rate was 5%. Blue foxes started mating in March 2017, and miscarriages occurred 10–40 days after pregnancy [in general, around 53 days (49–56) for the entire pregnancy]. Although the female fox's estrus rate was 85% (1,700/2,000) in that year, the miscarriage rate was 50% (850/1,700); weak cubs accounted for 5% (250/5,000), and the mortality rate of foxes reached 35% (1,750/5,000) (Table 2). After the brucellosis was diagnosed, oxytetracycline, astragalus polysaccharides, Vitamin E, and other herbs were used for treatment, but these had no effect. Therefore, only 18 brucellosis-positive female foxes were kept for breeding in 2018, and the remainder were eliminated. The investigation found that from August 2016 to November 2016, previously used chicken carcasses were replaced by raw ground sheep internal organs (lungs, tracheae, placentae, and dead fetuses) to feed breeding foxes, and clustering of female fox abortions occurred a few months later. After being infected, female blue foxes were without any obvious manifestations; however, reduced sperm counts and deformed sperm in male foxes were observed in microscopic examinations (unpublished).
Discussion
Brucellosis is one of the most important infectious causes of reproductive disorders in various species of animals (25). Various Brucella species are well-known causes of contagious abortion in cattle, sheep, goats, swine, and other animals (26). In the present study, both serological and AMOS-PCR methods confirmed that a Brucella spp. strain was the cause of the outbreak of abortion among blue foxes on this farm. Similarly, a previous study reported that brucellosis was found in a fox farm (27). Molecular tools can support the results from serological tests to avoid cross-reaction with other pathogens (28). AMOS-PCR results showed the presence of this special 731 bp band in many aborted fetuses' samples. Moreover, sequences from PCR products have 100% similarity to B. melitensis sequences from GenBank. These data indicate that the outbreak at the blue fox farm was causing by the B. melitensis infected. A similar study showed that B. melitensis biovar 3 was the main pathogen responsible for cow and sheep abortion in China, and that this variant posed a human health risk (29). The seroprevalence of brucellosis in sheep and goat flocks was higher in eastern China, with 7.00% positive rate, than in any other region (30). Heilongjiang Province is one of the severe animal brucellosis epidemic regions in northern China (30). Moreover, ~9% (56/621) of the samples from yaks were seropositive for Brucella tested via SAT at the Qinghai-Tibet Plateau, China (31). Similarly, the individual yak seroprevalence of brucellosis was 2.8% and herd level seroprevalence was 18.2% (32). Also, Brucella strains were isolated from the wildlife in China, such as blue sheep (Pseudois nayaur), yaks (Bos mutus grunniens), and Tibetan gazelle (Procapra picticaudata) (33). B. melitensis biovar 3 from the spleen of an Asian badger (Meles leucurus) showed a MLVA-16 genotype similar to that of isolates from local aborted sheep fetuses (34).
Our surveys showed that sporadic abortion events occurred in 5% of pregnancies on this farm during 2016. However, a >50% abortion rate was observed in 2017. The blue fox farm did not introduce new foxes during the period 2014–2017, and the breeding environment had not changed. The only changed factor was the feed for the blue foxes, where raw ground offal of sheep from the local slaughterhouse was used to feed the breeding foxes instead of chicken carcasses as used previously. Subsequently, an outbreak abortion event occurred during March and April in 2017. Moreover, serological screening showed that the seropositive rate of brucellosis in the fox breeding farm was 41.32% (300/726), being 38.49% (40/64) in male foxes and 48.58% (274/564) in female foxes. Moreover, five out of eight staff in this farm were diagnosed with brucellosis. This evidence indirectly showed that feeding the raw viscera of sheep infected with Brucella spp. were the main cause for the outbreak of abortion events on the blue fox breeding farm. Due to the high abortion rate (55%), low cub survival rate (65%), and human infections, this farm was closed at the beginning of 2019. The study showed that the highest-threat organs of ruminants are the lungs, and the trend analysis also highlighted the cattle intestine as a potentially high-threat organ (35). Moreover, our previous study reported that B. melitensis was obtained from dogs that were often fed with sheep offal (36). Moreover, hares have been considered as a possible source of B. suis biovar 2 outbreaks in domestic pigs via swill feeding with offal from hunted infected hares (37).
In order to identify the causative pathogen of blue fox abortion, we tried to isolate and cultivate Gardnerella vaginalis and other common abortion-related pathogens, but only a few Staphylococcus and Streptococcus strains were detected in abortion afterbirth. What we particularly regret is that our laboratory (Heilongjiang Bayi Agricultural University) did not meet the expected biosafety requirements necessary for bacteriological experiments, so Brucella strains isolation were not performed. Isolated Brucella from the (wild) red fox (Vulpes vulpes) (38, 39), gray fox (40), and tundra wolf (41) have been reported. Therefore, our conclusion is a reasonable explanation for this outbreak of abortion events. In addition, blue foxes infected by Brucella strains were without any obvious symptoms except the abortion after pregnancy at 10–40 days. This observation agrees with a previous report that B. melitensis in the adult ewe is generally asymptomatic and self-limiting within about 3 months. However, because the bacteria may enter and cause necrosis of the chorionic villi and fetal organs, abortion or stillbirths may occur (42, 43). Another study showed that brucellosis is essentially a disease of sexually mature animals, the preferred site being the reproductive tracts of males and females. If the animal is not pregnant, the infected animal may be without clinical symptoms and may have a negative serological reaction. However, if such an animal becomes pregnant, the production of the simple carbohydrate erythritol in the fetus and its membranes causes rapid multiplication of bacteria in the uterus, and this is likely to end in abortion (44). In this study, a 77.78% (14/18) estrus rate was recorded in blue foxes after infection by B. melitensis. In comparison with 2016, the estrus rate had declined; the abortion rate was 10 times higher than previously, and the survival rate of the pups dropped significantly. B. melitensis primarily affects the reproductive tracts of sheep and goats, and the infection is characterized by late abortion, stillbirth, a weakened fetus, and to a lesser extent orchitis and infection of the accessory sex glands and impaired fertility in males (45). The stillbirths and weakened fetuses in this case resulted in economic losses. The infected staff member often participated in the offal grinding, and thus the specific source of infection needs further investigation. B. melitensis infects mainly sheep and goats and other animals, resulting in an important zoonosis that has a significant effect on the husbandry economy and the public health of many developing countries.
Our study has several limitations. Due to restrictions by the limited lab facilities, the isolation and culture of Brucella from abortus samples were not carried out. Moreover, a tracing-back survey of the source of sheep (goats) offal is lacking. Animal offal samples have been collected from the local slaughterhouse for further bacteriological experiments, and genetic phylogenetic analysis will provide the available information to reveal the complete transmission chain of events.
Conclusion
In the present study, we combined RBPT, SAT, and AMOS-PCR to investigate the cause of an abortion outbreak event in a blue fox farm in Heilongjiang province. Our experiments showed that blue foxes ingesting sheep offal infected with B. melitensis was the main cause of the outbreak. These data indirectly verified the severe animal brucellosis epidemic trend in this region, where B. melitensis infection was a spillover from the main host to the blue fox. These events pose a public health risk to people in the fur and catering industries and to workers in other breeding industries that provide animal feed. It is thus time to launch an animal brucellosis prevention program against the spread of Brucella.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Ethics Statement
The animal study was reviewed and approved by Ethics Committee of the Heilongjiang Bayi Agricultural University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
YZ, YM, and YR collected the samples and performed the serology and AMOS-PCR amplifications. ZLiu performed data analysis and drafted the manuscript. YZ and ZLiu conducted epidemiological investigations. YZ and ZLi participated in the design of the study, critically reviewed the manuscript, and managed the project. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by Heilongjiang Bayi Agricultural University Funds (No. XDB201820) and Natural Science Talent Support Program (No. ZRCPY201807), the Natural Science Foundation Project of Heilongjiang Province (LH2020C083), the National Natural Science Foundation of China (No. 81703426), and the China Postdoctoral Science Foundation (No. 2019M651312), and National Key R&D Program of China, Grant Numbers 2019YFC1200700, 2019YFC1200601-6; and the National Natural Science Foundation of China (No. 82073624). The funding agencies had no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the staff of the blue fox breeding farm for assistance with the sample collection and for their cooperation with the epidemiological investigation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.666254/full#supplementary-material
Abbreviations
RBPT, Rose-Bengal plate test; SAT, serum tube agglutination test; AMOS-PCR, B. abortus-melitensis-ovis-suis polymerase chain reaction.
References
1. Ackelsberg J, Liddicoat A, Burke T, Szymczak WA, Levi MH, Ostrowsky B, et al. Brucella exposure risk events in 10 clinical laboratories, New York City, USA, 2015 to 2017. J Clin Microbiol. (2020) 58:e01096-19. doi: 10.1128/JCM.01096-19
2. Zhou K, Wu B, Pan H, Paudyal N, Jiang J, Zhang L, et al. ONE Health approach to address zoonotic brucellosis: a spatiotemporal associations study between animals and humans. Front Vet Sci. (2020) 7:521. doi: 10.3389/fvets.2020.00521
3. Shang D, Xiao D, Yin J. Epidemiology and control of brucellosis in China. Vet Microbiol. (2002) 90:165–82. doi: 10.1016/S0378-1135(02)00252-3
4. Silbereisen A, Tamborrini M, Wittwer M, Schürch N, Pluschke G. Development of a bead-based luminex assay using lipopolysaccharide specific monoclonal antibodies to detect biological threats from Brucella species. BMC Microbiol. (2015) 15:198. doi: 10.1186/s12866-015-0534-1
5. Tadesse G. Brucellosis seropositivity in animals and humans in ethiopia: a meta-analysis. PLoS Negl Trop Dis. (2016) 10:e0005006. doi: 10.1371/journal.pntd.0005006
6. Hull NC, Schumaker BA. Comparisons of brucellosis between human and veterinary medicine. Infect Ecol Epidemiol. (2018) 8:1500846. doi: 10.1080/20008686.2018.1500846
7. Godfroid J, Nielsen K, Saegerman C. Diagnosis of brucellosis in livestock and wildlife. Croat Med J. (2010) 51:296. doi: 10.3325/cmj.2010.51.296
8. Charypkhan D, Sultanov AA, Ivanov NP, Baramova SA, Taitubayev MK, Torgerson PR. Economic and health burden of brucellosis in Kazakhstan. Zoo Public Health. (2019) 66:487–94. doi: 10.1111/zph.12582
9. Dadar M, Alamian S, Behrozikhah AM, Yazdani F, Kalantari A, Etemadi A, et al. Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. Vet Res Forum. (2019) 10:315–21. doi: 10.30466/vrf.2018.89680.2171
10. Dadar M, Shahali Y, Fakhri Y, Godfroid J. The global epidemiology of Brucella infections in terrestrial wildlife: a meta-analysis. Transbound Emerg Dis. (2020) 1–15. doi: 10.1111/tbed.13735
11. Li ZY, Chen ZG. Research progress of Gardnerella vaginalis in fox. Agric Sci Res. (2006) 27:65–7. doi: 10.3969/j.issn.1673-0747.2006.04.019
13. Jiang W, Chen J, Li Q, Jiang L, Huang Y, Lan Y, et al. Epidemiological characteristics, clinical manifestations and laboratory findings in 850 patients with brucellosis in Heilongjiang Province, China. BMC Infect Dis. (2019) 19:439. doi: 10.1186/s12879-019-4081-5
14. Liang PF, Zhao Y, Zhao JH, Pan DF, Guo ZQ. Human distribution and spatial-temporal clustering analysis of human brucellosis in China from 2012 to 2016. Infect Dis Poverty. (2020) 9:142. doi: 10.1186/s40249-020-00754-8
15. Sathyanarayan MS, Suresh DR, Sonth SB, Krishna S, Surekha YA. A comparative study of agglutination tests, blood culture & Elisa in the laboratory diagnosis of human brucellosis. Int J Biol Med Res. (2011) 2:569–72.
16. Mcgiven JA, Tucker JD, Perrett LL, Stack JA, Brew SD, Macmillan AP. Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT. CFT, and iELISA. J Immunol Methods. (2003) 278:171–8. doi: 10.1016/S0022-1759(03)00201-1
17. Salisu US, Kudi CA, Bale JOO, Babashani M, Kaltungo BY, Saidu SNA, et al. Seroprevalence of Brucella antibodies in camels in Katsina State, Nigeria. Trop Anim Health Prod. (2017) 49:1041–6. doi: 10.1007/s11250-017-1297-5
18. Shome R, Kalleshamurthy T, Natesan K, Jayaprakash KR, Byrareddy K, Mohandoss N, et al. Serological and molecular analysis for brucellosis in selected swine herds from Southern India. J Infect Public Health. (2019) 12:247–51. doi: 10.1016/j.jiph.2018.10.013
19. Erdenlig Gürbilek S, Tel OY, Keskin O. Comparative evaluation of three serological tests for the detection of Brucella antibodies from infected cattle herds. J Appl Anim Res. (2017) 45:557–9. doi: 10.1080/09712119.2016.1222942
21. Al-Garadi MA, Khairani-Bejo S, Zunita Z, Omar AR. Isolation and identification of Brucella melitensis in goats. J Animal Vet Adv. (2011) 10:972–9. doi: 10.3923/javaa.2011.972.979
22. Bricker BJ, Halling SM. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J Clin Microbiol. (1995) 33:1640–2. doi: 10.1128/JCM.33.6.1640-1642.1995
23. Yagupsky P, Morata P, Colmenero JD. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. (2019) 33:e00073-19. doi: 10.1128/CMR.00073-19
24. Du SN, Wang ZJ, Yu GW, Cui YL, Chen JJ, Hu N, et al. Epidemiological characteristics of human brucellosis in Tongliao city of Inner Mongolia Autonomous Region, 2004-2018. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:1063–7. doi: 10.3760/cma.j.cn112338-20190901-00642
25. Woldemeskel M. Zoonosis due to Bruella suis with special reference to infection in dogs (Carnivores): a brief review. Open J Vet Med. (2013) 3:213–21. doi: 10.4236/ojvm.2013.33034
26. Puri M, Patel N, Gaikwad V, Despande H, Pandey P. A study of prevalence of brucellosis in cases of spontaneous abortions. Res J Pharm Biol Chem Sci. (2015) 6:312–20.
27. Tworek R, Serokowa D, Machnicka B. Brucellosis on a fox farm. Przegl Epidemiol. (1957) 11:307–8.
28. Ntirandekura JB, Matemba LE, Kimera SI, Muma JB, Karimuribo ED. Association of brucellosis to abortions in humans and domestic ruminants in Kagera ecosystem, Tanzania. Transbound Emerg Dis. (2020) 67:1879–87. doi: 10.1111/tbed.13516
29. Zhang H, Deng X, Cui B, Shao Z, Zhao X, Yang Q, et al. Abortion and various associated risk factors in dairy cow and sheep in Ili, China. PLoS ONE. (2020) 15:e0232568. doi: 10.1371/journal.pone.0232568
30. Ran X, Chen X, Wang M, Cheng J, Ni H, Zhang XX, et al. Brucellosis seroprevalence in ovine and caprine flocks in China during 2000-2018: a systematic review and meta-analysis. BMC Vet Res. (2018) 14:393. doi: 10.1186/s12917-018-1715-6
31. Xulong L, Hailong Q, Zhaoyang B, Yanling Y, Chunhui S, Xiaoyan L, et al. Seroprevalence of Brucella infection in yaks (Bos grunniens) on the Qinghai-Tibet plateau of China. Trop Anim Health Prod. (2011) 43:305–6. doi: 10.1007/s11250-010-9726-8
32. Zeng J. Epidemiology of Brucellosis in Yaks in the Tibet Autonomous Region of China. Perth, WA: Murdoch University (2017).
33. Ma JY, Wang H, Zhang XF, Xu LQ, Hu GY, Jiang H, et al. MLVA and MLST typing of Brucella from Qinghai, China. Infect Dis Poverty. (2016) 5:26. doi: 10.1186/s40249-016-0123-z
34. Liu X, Yang M, Song S, Liu G, Zhao S, Liu G, et al. Brucella melitensis in Asian badgers, Northwestern China. Emerging Infect Dis. (2020) 26:804–6. doi: 10.3201/eid2604.190833
35. Večerek V, Kozak A, Malena M, Chloupek P, Pištěková V. Viscera of slaughtered ruminants and potential threats to human health. Acta Vet Brno. (2003) 72:631–8. doi: 10.2754/avb200372040631
36. Wang H, Xu WM, Zhu KJ, Zhu SJ, Zhang HF, Wang J, et al. Molecular investigation of infection sources and transmission chains of brucellosis in Zhejiang, China. Emerg Microbes Infect. (2020) 9:889–99. doi: 10.1080/22221751.2020.1754137
37. Godfroid J, Garin-Bastuji B, Saegerman C, Blasco JM. Brucellosis in terrestrial wildlife. Rev Sci Tech. (2013) 32:27–42. doi: 10.20506/rst.32.1.2180
38. Scholz HC, Hofer E, Vergnaud G, Le Fleche P, Whatmore AM, Al Dahouk S, et al. Isolation of Brucella microti from mandibular lymph nodes of red foxes, Vulpes vulpes, in lower Austria. Vect Borne Zoo Dis. (2009) 9:153–6. doi: 10.1089/vbz.2008.0036
39. Hofer E, Revilla-Fernández S, Al Dahouk S, Riehm JM, Nöckler K, Zygmunt MS, et al. A potential novel Brucella species isolated from mandibular lymph nodes of red foxes in Austria. Vet Microbiol. (2012) 155:93–9. doi: 10.1016/j.vetmic.2011.08.009
40. Szyfres B, Tomé JG. Natural Brucella infection in Argentine wild foxes. Bull World Health Organ. (1965) 34:919–23.
41. Tessaro SV, Forbes LB. Experimental Brucella abortus infection in wolves. J Wildl Dis. (2004) 40:60–5. doi: 10.7589/0090-3558-40.1.60
42. Olsen SC, Palmer MV. Advancement of knowledge of Brucella over the past 50 years. Vet Pathol. (2014) 51:1076. doi: 10.1177/0300985814540545
43. Djangwani J, Ooko Abong G, Gicuku Njue L, Kaindi DWM. Brucellosis: prevalence with reference to East African community countries - a rapid review. Vet Med Sci. (2021) 1–17. doi: 10.1002/vms3.425
44. Tesfaye G, Wondimu A, Asebe G, Regasa F, Mamo G. Sero-prevalence of bovine brucellosis in and Around Kombolcha, Amhara Regional State, Ethiopia. Mycobact Dis. (2017) 7:2. doi: 10.4172/2161-1068.1000242
Keywords: Brucella melitensis, abortion, reproductive, blue fox, goats (sheep) offal
Citation: Zhou Y, Meng Y, Ren Y, Liu Z and Li Z (2021) A Retrospective Survey of the Abortion Outbreak Event Caused by Brucellosis at a Blue Fox Breeding Farm in Heilongjiang Province, China. Front. Vet. Sci. 8:666254. doi: 10.3389/fvets.2021.666254
Received: 09 February 2021; Accepted: 06 April 2021;
Published: 15 June 2021.
Edited by:
Anuwat Wiratsudakul, Mahidol University, ThailandReviewed by:
Maryam Dadar, Razi Vaccine and Serum Research Institute, IranKumaragurubaran Karthik, Tamil Nadu Veterinary and Animal Sciences University, India
Copyright © 2021 Zhou, Meng, Ren, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Liu, d2xjYmx6Z0AxMjYuY29t; Zhenjun Li, bGl6aGVuanVuQGljZGMuY24=
†These authors have contributed equally to this work
 Yulong Zhou1†
Yulong Zhou1† Zhiguo Liu
Zhiguo Liu Zhenjun Li
Zhenjun Li