- 1Department of Veterinary Sciences, University of Turin, Grugliasco, Italy
- 2Faculty of Veterinary Medicine, University of Teramo, Teramo, Italy
- 3Department of Animal Production, University of Murcia, Murcia, Spain
Growing attention is being directed toward insects as a novel and sustainable source of protein for pet food. The aim of the study was to evaluate nutrient digestibility of a diet containing black soldier fly larvae as its main protein source. Moreover, the purpose of the study was to compare the traditional in vivo total collection method with the in vivo marker method and in vitro digestibility method. Two isonitrogenous and isoenergetic dry diets containing either venison meal (CTRL diet) or black soldier fly larvae meal (BSF diet) as their primary sources of proteins were fed to six adult dogs, according to a Latin square design. The digestibility of nutrients was determined using both in vivo (“total collection” and “internal marker” approaches) and in vitro methods. The two diets showed similar nutrient digestibility values for dry matter, organic matter, ether extract, ash, and phosphorus. However, a statistical trend (p = 0.066) was observed indicating greater protein digestibility in the BSF diet compared with the CTRL diet. Calcium digestibility was higher in the BSF diet compared with the CTRL diet (p = 0.018). On the contrary, fiber digestibility was lower in the insect-based diet compared with the venison diet (p < 0.001). There was no difference between total collection and internal marker methods in the assessment of in vivo digestibility for any of the nutrients considered. The in vitro digestibility values for dry matter, organic matter, and crude protein, as well as the estimated in vivo digestibility of organic matter and crude protein by the means of the predictive equation, were aligned with the in vivo results, although in vitro estimations were consistently higher compared with those obtained by in vivo analysis. Digestibility analysis of a dog food containing insect meal as the sole source of protein (36.5% inclusion) showed promising results in terms of it presenting similar values as a meat-based diet, indicating its suitability as a sustainable protein source for pet food. Moreover, the study showed that both the in vivo marker method and the in vitro method could be possible alternatives to the traditional total collection method in digestibility trials.
Introduction
With the livestock industry at its limit in terms of sustainable production capacity, and the pet food business in constant growth, new sources of protein are being sought in order to meet the market's demand and the expectations of pet owners (1). Insects may provide a possible solution as an alternative feed, since they can partially replace traditional feed sources, while they also provide a means to bio-converting organic waste (2). Of the various insects being considered, the black soldier fly (Hermetia illucens) is showing particular promise due to its immediate potential for large-scale production (3).
The black soldier fly (BSF) has a balanced protein composition and one of the highest amino acid scores compared with other currently reared insects or traditional protein sources (such as fish meal) (4). Compared with crickets and mealworms, BSF boasts a more stable nitrogen and phosphorus composition and has a more advantageous feed conversion ratio (5). It can also be considered a possible sustainable solution due to the possibility of rearing the insects on materials deemed unsuitable for human nutrition, such as alimentary by-products and organic substrates (6).
As pointed out by Böhm et al. (7), insects may constitute an appropriate novel protein source for dogs, presenting cutaneous adverse food reactions. Nevertheless, societal negative opinions about the use of insect meal in pet nutrition have arisen, especially due to insect phobia and concerns about safety. Security aspects about insect consumption were also discussed critically in EFSA Scientific Opinion (8), where uncertainty regarding the risk of non-processed items, due to the lack of data, has been acknowledged. However, EFSA concluded that microbiological risks are expected to be comparable with other food raw materials, provided that insects are fed with allowed feedstuff. Consumers from Western countries still continue to have prejudices regarding the introduction of insects in their diet (9), and, due to the current “humanization trend” (10), this fact could be also translated to their pets. Notwithstanding, public opinion seems to be less concerned about the use of veterinary-prescribed diets based on insects (11). Indeed, veterinarians have expressed interest in hypoallergenic food alternatives prepared using insects (12). According to the Commission Regulation (EU) 2020/354 (March 4, 2020) (13), a product can be claimed to reduce ingredient and nutrient intolerances if it is composed of hydrolyzed proteins or selected and limited protein sources or selected carbohydrate sources. Therefore, according to the current European Regulations, a product composed only of insects as the main source of protein could be considered with the particular purpose of reduction of food intolerance. Concurrently, and reflecting the growing interest in this field of research (14), various recent studies have investigated the possibility of feeding BSF larvae to poultry (15–18), fish (19–21), and swine (22, 23). Recently, a thorough review from Bosch and Swanson (24) explored in depth the palatability, digestibility, and nutritional aspects of the inclusion of insects in dog and cat diet, showing the potential of insects as future pet food products.
The aim of the present study was to evaluate the inclusion of defatted BSF larvae meal in extruded dog food in terms of its in vivo and in vitro digestibility, in order to assess its suitability for the pet food market. Furthermore, the purpose of the study was to evaluate if the in vivo marker method and the in vitro digestibility method could be comparable to the traditional in vivo total collection method also in these particular diets. The estimated in vivo digestibility of organic matter and crude protein calculated by means of predictive equations utilizing data obtained by in vitro analysis was also assessed.
Materials and Methods
All the experimental procedures were approved by the Bioethics Committee of the University of Turin (Italy) (prot. n. 336595).
Animals and Experimental Design
Six clinically healthy West Highland White Terrier adult dogs [three males and three females, 3 ± 1.8 years old, 7.2 ± 0.8 kg BW, BCS ranging between 4.5 and 5.5 on a nine-point scale (25)] were fed two isonitrogenous and isoenergetic dry extruded diets (control vs. insect diet) according to a Latin square design. During the digestibility experiment, the dogs were housed individually in 3 ×3-m kennels and had ad libitum access to fresh water. The dogs were allowed to walk freely for 1 h per day in a concrete outside the pen and play with toys during the adaptation periods.
Diets and Digestibility Protocol
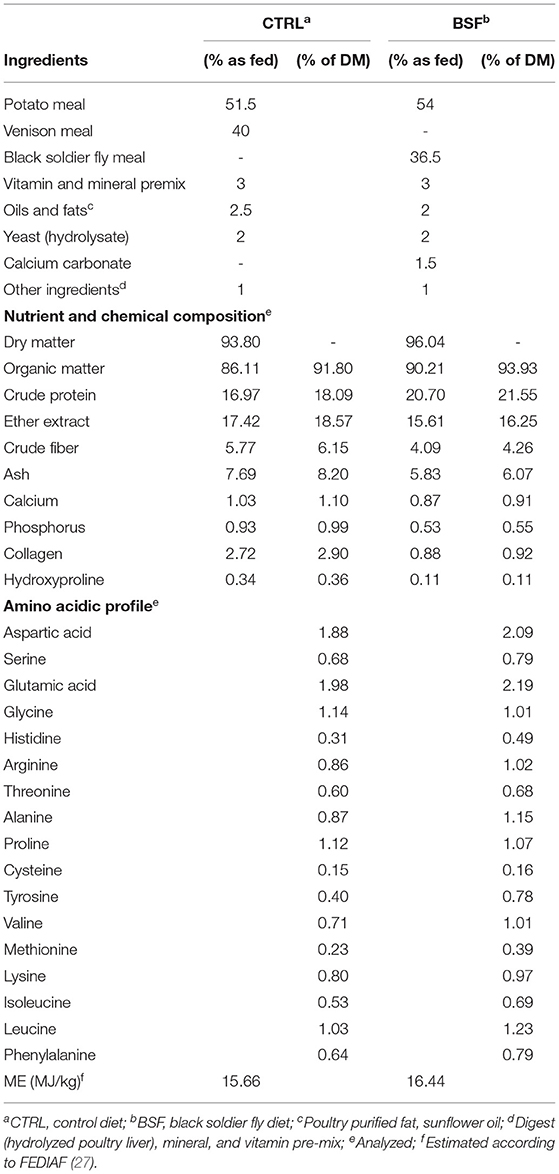
Two diets were tested during the trial. The diets were formulated to be isoenergetic and isonitrogenous. In the control diet (CTRL diet), the protein source was provided in the form of processed [rendering process, method III, according to the EU Reg. 142/2011 (26)] deer (Cervus elaphus) protein, whereas the insect diet (BSF diet) provided defatted BSF (H. illucens) larvae meal as its sole protein source (Hermetia Futtermittel GbR, Baruth/Mark, Germany). The chemical composition, amino acidic profile, and ingredient composition of both diets are shown in Table 1. Diets were formulated and balanced in order to meet nutrient requirements in accordance with the FEDIAF (27) nutrient guidelines for dogs.
Venison was chosen as the primary protein source for this trial since it is one of the protein sources usually incorporated in commercial foods for dogs which show adverse food reactions; similarly, insect meal showed a similar potential (7). Nevertheless, venison meal is more expensive than other common sources of proteins as well as insect meal so far and, for these reasons, was deemed eligible for the comparison of the diets.
The trial was conducted according to the guidelines of Carciofi et al. (28) regarding the use of a marker method and the total collection method for assessing in vivo total tract apparent digestibility. Chromium oxide (Cr2O3) was used as digestibility marker. It was added to a final concentration of 2.5 g/kg of diet. A 5-day test diet adaptation period preceded 5 days of feces collection during the experimental trial.
Food was weighed each day, divided into two equal portions, and given to the animals at 9 a.m. and 5 p.m. in stainless-steel bowls. Food quantity was administered considering maintenance energy requirements according to the FEDIAF equation (110 kcal × BW0.75) (27). Bowls were removed before the next meal, and any uneaten food was weighed and recorded. Feces were collected twice daily, weighed, and kept frozen at −20°C until analysis.
Chemical Analyses
At the end of the collection period, pooled individual feces were thawed, homogenized, and freeze-dried. Feces samples were freeze-dried using a laboratory freeze dryer (5Pascal, Trezzano sul Naviglio, Italy). The process of lyophilization consisted of dry sublimation with water evaporation under low pressure (0.200 mbar) until the samples reached room temperature (25°C). Both the foods and freeze-dried feces were ground to pass through a 1-mm sieve and stored in airtight plastic containers for laboratory tests. The dry matter (DM) of the foods was determined by drying the samples at 103°C to constant weight. The foods and feces were analyzed according to the AOAC (29) standard procedures; thus, ash was determined by muffle furnace incineration (section 942.05), crude protein (CP) was ascertained using the Kjeldahl method (section 954.01), and ether extract (EE) was analyzed following acid hydrolysis (section 954.02). In addition, diet crude fiber (CF) was determined using the method described in section 962.09 (29), and amino acid content by HPLC (Waters Alliance System with a Waters 1525 Binary HPLC Pump, Waters 2707 Autosampler, and Waters 2475 Multi λ Fluorescence Detector, Milford, USA) after pre-column derivatization (30) in samples ground to pass a 0.5-mm sieve. The detection limit ranged from 2.9 to 20.1 pmol/μl depending on the amino acid. Tryptophan was not analyzed.
Samples of foods and feces were burnt to ashes and acid-digested in the microwave (31), prior to the determination of chromium concentrate by inductively coupled plasma optical emission spectrometry (ICP-OES). Calcium and phosphorus were also determined by ICP-OES in the absence of the previous incineration.
Hydroxyproline and the related collagen content were assessed according to the colorimetric method adapted by Kolar (32) and described in the AOAC (29) section 990.26. The acid hydrolysis of the sample was performed under heat; an oxidizing agent was added to the sample, and oxidized hydroxyproline was measured photometrically.
In vivo Digestibility Calculations
Apparent total tract digestibility coefficients (ATTDC) of the individual dietary elements of the two diets were calculated as follows:
a) Total fecal collection method (TFC):
where X is the total contents of DM, organic matter (OM), CP, EE, ash, calcium, or phosphorus in the consumed food or feces produced (Xdiet and Xfeces, respectively);
b) Marker method (Cr2O3):
where X represents the concentrations of DM, OM, CP, EE, ash, calcium, or phosphorus in the diet or feces;
Cr2O3 represents the chromium oxide concentration in the diet or feces;
(X/Cr2O3)diet = ratio between nutrient (X) and Cr2O3 concentration in the diet;
(X/Cr2O3)feces = ratio between nutrient (X) and Cr2O3 concentration in the feces.
In vitro Digestibility
The in vitro digestibility of DM, CP, and OM of the food was determined (in triplets) employing the methods described by Hervera et al. (33, 34). The methods involve two phases: the first entails incubation for 2 h under conditions simulating gastric digestion (pH 2, 39°C, and inclusion of pepsin), whereas the second phase simulates 4 h of post-gastric digestion (pH 6.8, 39°C, and inclusion of a pancreatin preparation for enzymatic digestion). The resulting residue was filtered, dried, and weighed to determine the remaining DM content and incinerated to determine the residual OM content. Residual CP was determined by ascertaining the nitrogen content of the residue (using the Kjeldahl method) and considering a N:P conversion factor of 6.25. The in vitro digestibility of DM, OM, and CP was calculated as the difference between the amount of each initial nutrient in the sample vs. the undigested residue, divided by the initial nutrient content of the sample.
Estimated Digestibility
Data from the in vitro digestibility analyses were also used to estimate in vivo OM and CP digestibility according to the regression equations reported by Hervera et al. (33, 34):
Estimated digestibility of OM (%) = −9.15 + 1.06 × in vitro OM digestibility (%) (33);
Estimated digestibility of CP (%) = 37.91 + 0.52 × in vitro CP digestibility (%) (34).
Statistical Analysis
The statistical unit was the individual dog for in vivo digestibility trials, and the diet for in vitro digestibility trials. The comparisons between diets (CTRL vs. BSF) and methods (in vivo TFC vs. Cr2O3) were analyzed using two-way ANOVA, considering the diet (D) and the method (M) of in vivo digestibility calculation as the source of variation, respectively. Before testing for group and method differences, the normality of the data distribution and the homogeneity of variance were assessed by the means of the Shapiro–Wilk test and Levene test, respectively. The significance level was set at p = 0.05. A statistical trend was considered for p ≤ 0.10. All statistical analyses were performed using R Software (version 3.6.1) (35).
Results
The foods were well-accepted during all the trial lengths, and no episode of nausea or vomiting has been reported. The in vivo ATTDC digestibility results are summarized in Table 2. The two methods used to estimate in vivo digestibility (TFC and Cr2O3) showed similar results between the CTRL and BSF groups in relation to DM, OM, EE, ash, and phosphorus. The ATTDC of CF was significantly lower (p < 0.001) in the BSF diet compared with the CTRL diet. On the contrary, the ATTDC of calcium was significantly higher (p < 0.05) in the BSF compared with the CTRL diet. A statistical trend (p = 0.066) was observed for the ATTDC of CP, being higher in the animals fed the BSF compared with the CTRL diet.
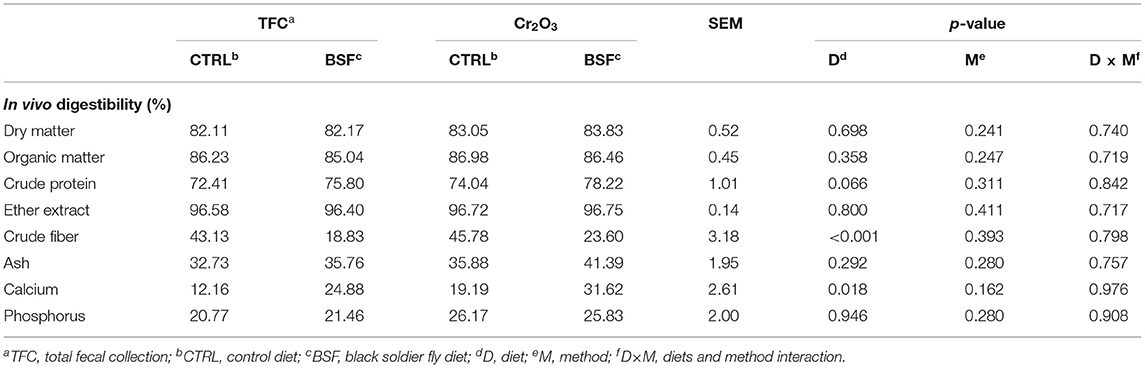
Table 2. Comparison of the in vivo digestibility using the total fecal collection method (TFC) and in vivo digestibility with marker (Cr2O3) in six dogs (mean values are presented).
No statistical differences were observed between the two ATTDC methods (TFC vs. Cr2O3). Furthermore, no statistical interaction between diets and methods was found.
The in vitro digestibility data and estimated in vivo digestibility results, obtained utilizing the regression equations described in Hervera et al. (33, 34), are reported in Table 3. The digestibility values for DM, OM, and CP obtained using the in vitro method were higher for both the CTRL and the BSF diet (by an average of +8.43, +5.25, and +6.08%, respectively) compared with those obtained using in vivo methods. The estimations of in vivo digestibility of OM and CP (based on in vitro data) were consistently higher than the data obtained using in vivo ATTDC methods: in vitro estimation of in vivo digestibility overestimated OM and CP digestibility by up to 4.0% and 9.8%, respectively, compared with the in vivo methods.
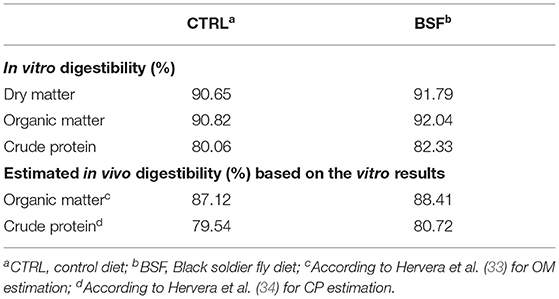
Table 3. Comparison of the in vitro digestibility of the two diets (CTRL vs. BSF) and estimated in vivo digestibility based on the in vitro results.
Discussion
This study evaluated the nutritional quality of defatted BSF larvae meal as a potential sustainable novel raw material for pet food, to be integrated into extruded diets as a protein source. In addition, it explored the suitability of the in vivo marker method and the in vitro digestibility method with the traditional in vivo total collection method.
Although the control (containing venison meal) and insect-based diets were formulated to be isonitrogenous, our analysis showed CP content to be almost 4% lower in the former (16.97 vs. 20.70%, respectively); the discrepancy between the diets was nevertheless within the limits stipulated in the EU regulation 2017/2279 regarding “Tolerances for analytical constituents” (36). It is also important to remember that since chitin is a nitrogen-containing polysaccharide, this could also have led to a mild overestimation of the protein content in the BSF diet (6, 37).
We must also acknowledge that the higher crude protein content of the BSF diet compared with the CTRL diet could be an overestimation due to our use of a nitrogen to protein (N:P) conversion factor of 6.25. In fact, several authors recently pointed out that this conventionally used conversion factor may lead to the overestimation of protein content in a variety of feedstuffs (38, 39), including insect meals (40, 41). Furthermore, although Finke et al. (42) estimated that the amount of nitrogen in insect chitin would not significantly affect the total amount of nitrogen, other authors support the hypothesis that the presence of non-protein nitrogen (NPN) in insect CP could cause the overestimation of CP (40, 41).
In our trial, the ATTDC of DM, OM, and EE were similar in both BSF and CTRL groups, whereas the ATTDC of CP were higher in the BSF vs. CTRL group. A similar result was obtained by Lei et al. (43), where increasing levels of BSF meal inclusion (at 0, 1, and 2%) in Beagle dog rations raised nitrogen digestibility, whereas EE digestibility remained similar to that of the control diet. However, Gariglio et al. (18) observed that up to 9% BSF meal inclusion in the diet of growing Muscovy ducks did not change diet digestibility, with the exception of the ATTDC of EE, which was improved in BSF groups. In line with these data, Biasato et al. (23) observed no change in the ATTDC of BSF diets (up to 10% inclusion) in growing piglets. Similarly, Freel et al. (44) did not notice any difference in ATTDC of DM, CP, and EE in a trial involving 56 Beagle dogs fed with diets containing graded levels of BSF meal (5.0, 10.0, and 20.0%) and BSF oil (1.0, 2.5, 5.0%). Furthermore, in a study where BSF meal completely replaced soybean meal in the diet of laying hens, Cutrignelli et al. (45) found BSF to correlate with lower crude protein digestibility, whereas lipid digestibility remained unaffected. Likewise, Kröger et al. (46), in a study involving 12 Beagles, observed a decrease in ATTDC of CP in the BSF group compared to the control group, while the ATTDC of DM was increased when dogs were fed the diet containing the BSF meal (at 20.0% of inclusion). This result could be explained by differing levels of chitin, which can negatively affect protein digestibility (47). Indeed, the reported difference in fiber digestibility between the diets supports this result and explanation, since chitin gets recognized as part of the crude fiber fraction during the analysis (48). Furthermore, the mean values of crude protein ATTDC (for BSF-based diets) observed in our study were in line with those found in Kröger et al. (46) but below those recovered in Freel et al. (44).
Hydroxyproline can be used as an index of protein quality (49), due to its being a marker of collagen content (50). The levels of collagen and of hydroxyproline were higher in the control diet compared with the BSF diet, probably due to the fact that collagen is limited in insect meal compared to that in vertebrate protein meal. This could also explain the higher level of digestibility of the BSF diet compared with the control diet, at least with regard to crude protein digestibility, since the net protein utilization of collagen is zero (51). Collagen content also influences the N:P ratio of protein sources, and consequently the real CP content of the diets, in particular that of the control diet (39). It may also be speculated that the control diet had a decreased crude protein digestibility due to the higher ash content; however, high levels of crude ash did not appear to decrease protein digestibility, as previously reported by Bockskopf and Kamphues (52).
The difference in calcium digestibility could be due to the use of different ingredients to adjust the calcium level of the diets. Indeed, calcium carbonate was added to the BSF diet to obtain the minimum requirements for dogs, whereas in the CTRL diet the calcium requirements were satisfied by the presence of ground bone in the venison meal (thus avoiding the need for any calcium salt addition), and this could have led to the discrepancy. Interestingly, Lei et al. (43) noticed significant increases in the level of calcium in the blood of beagles as the BSF larvae meal content of their food was increased. This result points toward a potential increase in the bioavailability of this macro-element that depends on the inclusion of BSF larvae meal in the diet; however, further investigations are required to confirm and understand the basis of any possible relationship.
It is important to note that no statistical differences were observed between the ATTDC values determined using the marker method and the total collection method for both CTRL and BSF diets, confirming the validity of the marker method as an alternative to the total collection method (28). The values of in vitro DM, OM, and CP digestibility were also similar to the results obtained with the two in vivo methods, despite being, in line with the previous literature (33, 34), slightly overestimated in the former. We also evaluated whether the equations for the estimation of in vivo crude protein and OM digestibility, utilizing in vitro digestibility data, as described in Hervera et al. (33, 34), fitted with the results obtained in this study (shown in Table 3). Since the predictive equations proposed were only used to assess feedstuff based on vertebrates and, to our knowledge, no other study inspected if they could be applicable to invertebrates, we decided to include these findings. For both the venison and insect diet, the predictive equations gave slightly overestimated values compared with the mean of the in vivo digestibility results, even though they were substantially similar from a nutritional perspective. Indeed, the discrepancy between the crude protein digestibility estimated using the equation and the in vivo crude protein digestibility results ranged from 3.2 to 9.8%, whereas the overestimation of the OM digestibility ranged from 0.2 to 4.0%, with lower deviations and a narrower range. According to these results, predictive equations utilizing in vitro digestibility values appear to constitute a valid tool for the analysis of feedstuff digestibility and therefore offer a means to reduce, if not avoid, the use of live animals.
Conclusions
The present study suggests that the inclusion of BSF in extruded diets for dogs (at 36.5%) offers a promising alternative source of dietary protein for this species, in particular in relation to the digestibility profile of crude protein, crude fat, and OM. Our findings also highlight the need for further studies in order to understand the effect of chitin on fiber digestibility and mineral absorption in a BSF-based diet.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Ethic Committee of Turin University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
AS, EP, LPe, and LPr conceived and designed the experiment. EP and NR collected the experimental data. EV, FH, JM, JN, and SM carried out the chemical analyses. AS, LPe, and UA performed the statistical analysis. All the authors interpreted the data. AS, LPe, and LPr wrote the first draft of the manuscript. All the authors reviewed the manuscript for intellectual content and gave approval for the final version to be published.
Funding
This study was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR) under the program Dipartimenti di Eccellenza ex L.232/2016 awarded to the Department of Veterinary Science, University of Turin (Italy).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was the result of the author's visit (21142/IV/19) funded by the Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia (Spain) in connection with the Jiménez De La Espada Regional Programme for Mobility, Collaboration and Knowledge Exchange (Spain) (to AS). The authors are grateful to Q.vet Srl (Faule, CN, Italy), which provided the experimental foods.
Abbreviations
CTRL diet, venison meal-based diet/control diet; BSF diet, black soldier fly larvae-based diet/insect diet; BSF, black soldier fly; ME, metabolizable energy; DM, dry matter; OM, organic matter; CP, crude protein; EE, ether extract/crude fat; CF, crude fiber; HPLC, high-performance liquid chromatography; ATTDC, apparent total tract digestibility coefficients; TFC, total fecal collection method; SEM, standard error of the mean; D, diet; M, method; D × M, interaction between diets and methods.
References
1. Bosch G, Vervoort JJM, Hendriks WH. In vitro digestibility and fermentability of selected insects for dog foods. Anim Feed Sci Technol. (2016) 221:174–84. doi: 10.1016/j.anifeedsci.2016.08.018
2. Food and Argriculture Organization of the United Nations. Edible Insects. Future Prospects for Food and Feed Security. Food and Argriculture Organization of the United Nations (2013).
3. Veldkamp T, van Duinkerken G, van Huis A, Lakemond C, Ottevanger E, Bosch G, et al. Insects as a sustainable feed ingredient in pig and poultry diets - a feasibility study. Wageningen UR Livestock Res. (2012) 1–62.
4. Bosch G, Zhang S, Oonincx DGAB, Hendriks WH. Protein quality of insects as potential ingredients for dog and cat foods. J Nutr Sci. (2014) 3:1–4. doi: 10.1017/jns.2014.23
5. Oonincx DGAB, Van Broekhoven S, Van Huis A, Van Loon JJA. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE. (2015) 10:e0144601. doi: 10.1371/journal.pone.0144601
6. Spranghers T, Ottoboni M, Klootwijk C, Ovyn A, Deboosere S, De Meulenaer B, et al. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J Sci Food Agric. (2017) 97:2594–600. doi: 10.1002/jsfa.8081
7. Böhm TMSA, Klinger CJ, Gedon N, Udraite L, Hiltenkamp K, Mueller RS. Effekt eines Insektenprotein-basierten futters auf die symptomatik von futtermittelallergischen Hunden. Tierarztliche Praxis Ausgabe K Kleintiere Heimtiere. (2018) 46:297–302. doi: 10.15654/TPK-170833
8. EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. (2015) 13:4257. doi: 10.2903/j.efsa.2015.4257
9. Moruzzo R, Mancini S, Boncinelli F, Riccioli F. Exploring the acceptance of entomophagy: a survey of Italian consumers. Insects. (2021) 12:123. doi: 10.3390/insects12020123
10. Okin GS. Environmental impacts of food consumption by dogs and cats. PLoS ONE. (2017) 12:e0181301. doi: 10.1371/journal.pone.0181301
11. Leriche I, Chala V, Ereau C. Pet owners' perception of insects as a protein source for cats and dogs. In: Proceedings XI Southern European Veterinary Conference. Barcelona (2014).
12. Pagani E, Russo N, Schiavone A, Prola L. Veterinary practitioners perception of insects as protein source for pets. In: Proceedings 20th European Society of Veterinary and Comparative Nutrition Congress. Berlin (2016).
13. European Commission. Commission regulation (EU) 2020/354 of 4 March 2020 establishing a list of intended uses of feed intended for particular nutritional purposes repealing Directive 2008/38/EC. Off J Eur Union. (2020). Available online at: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX%3A32020R0354 (accessed December 14, 2020).
14. Gasco L, Biasato I, Dabbou S, Schiavone A, Gai F. Animals fed insect-based diets: state-of-the-art on digestibility, performance and product quality. Animals. (2019) 9:1–32. doi: 10.3390/ani9040170
15. Biasato I, Ferrocino I, Dabbou S, Evangelista R, Gai F, Gasco L, et al. Black soldier fly and gut health in broiler chickens: insights into the relationship between cecal microbiota and intestinal mucin composition. J Anim Sci Biotechnol. (2020) 11:11. doi: 10.1186/s40104-019-0413-y
16. Marono S, Loponte R, Lombardi P, Vassalotti G, Pero ME, Russo F, et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult Sci. (2017) 96:1783–90. doi: 10.3382/ps/pew461
17. Schiavone A, De Marco M, Martínez S, Dabbou S, Renna M, Madrid J, et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J Anim Sci Biotechnol. (2017) 8:1–9. doi: 10.1186/s40104-017-0181-5
18. Gariglio M, Dabbou S, Biasato I, Capucchio MT, Colombino E, Hernández F, et al. Nutritional effects of the dietary inclusion of partially defatted Hermetia illucens larva meal in Muscovy duck. J Anim Sci Biotechnol. (2019) 10:37. doi: 10.1186/s40104-019-0344-7
19. Belghit I, Liland NS, Gjesdal P, Biancarosa I, Menchetti E, Li Y, et al. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture. (2019) 503:609–19. doi: 10.1016/j.aquaculture.2018.12.032
20. Renna M, Schiavone A, Gai F, Dabbou S, Lussiana C, Malfatto V, et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J Anim Sci Biotechnol. (2017) 8:57. doi: 10.1186/s40104-017-0191-3
21. Shakil Rana KM, Abdus Salam M, Hashem S, Ariful Islam M, Salam MA. Development of black soldier fly larvae production technique as an alternate fish feed aquaponics wheatgrass view project investigations of heavy metal concentration in poultry feed, bangladesh view project development of black soldier fly larvae production technique as an alternate fish feed. Int J Res Fish Aquaculture. (2015) 5:41–7.
22. van Heugten E, Martinez G, McComb A, Koutsos E. 285 Black soldier fly (Hermetia illucens) larvae oil improves growth performance of nursery pigs. J Anim Sci. (2019) 97:118. doi: 10.1093/jas/skz258.244
23. Biasato I, Renna M, Gai F, Dabbou S, Meneguz M, Perona G, et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J Anim Sci Biotechnol. (2019) 10:1. doi: 10.1186/s40104-019-0325-x
24. Bosch G, Swanson KS. Effect of using insects as feed on animals: pet dogs and cats. J Insects Food Feed. (2020) 1–12. doi: 10.3920/JIFF2020.0084
25. Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. (1997) 22:10–15.
26. European Commission. Commission regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European parliament and of the council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that directive. Off J Eur Union. (2011) 54:1–254. Available online at: http://data.europa.eu/eli/reg/2011/142/oj
27. FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs. FEDIAF (2018).
28. Carciofi AC, Vasconcellos RS, de Oliveira LD, Brunetto MA, Valério AG, Bazolli RS, et al. Chromic oxide as a digestibility marker for dogs—a comparison of methods of analysis. Anim Feed Sci Technol. (2007) 134:273–82. doi: 10.1016/j.anifeedsci.2006.12.005
29. Association of Official Analysis Chemists International (AOAC). Official Methods of Analysis of AOAC International. Association of Official Analysis Chemists International (2000).
30. Madrid J, Villodre C, Valera L, Orengo J, Martínez S, López MJ, et al. Effect of crude glycerin on feed manufacturing, growth performance, plasma metabolites, and nutrient digestibility of growing-finishing pigs. J Anim Sci. (2013) 91:3788–95. doi: 10.2527/jas.2013-5684
31. García-Rico L, Ramos Ruiz RE, Gutiérrez Coronado L. Use of microwave digestion and atomic absorption spectrophotometry to determine chromic oxide as a digestibility marker in feed, feces, and ileal content. J AOAC Int. (1999) 82:575–8. doi: 10.1093/jaoac/82.3.575
32. Kolar K. Colorimetric determination of hydroxyproline as measure of collagen content in meat and meat products: NMKL collaborative study. J Assoc Offic Analyt Chemists. (1990) 73:54–57. doi: 10.1093/jaoac/73.1.54
33. Hervera M, Baucells MD, Blanch F, Castrillo C. Prediction of digestible energy content of extruded dog food by in vitro analyses. J Anim Physiol Anim Nutr. (2007) 91:205–9. doi: 10.1111/j.1439-0396.2007.00693.x
34. Hervera M, Baucells MD, González G, Pérez E, Castrillo C. Prediction of digestible protein content of dry extruded dog foods: comparison of methods. J Anim Physiol Anim Nutr. (2009) 93:366–72. doi: 10.1111/j.1439-0396.2008.00870.x
35. R Core Team 2019. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2019). Available online at: http://wwwR-project.org/ (accessed December 10, 2019).
36. European Commission. Commission regulation (EU) 2017/2279 of 11 December 2017 amending annexes II, IV, VI, VII and VIII to regulation (EC) No 767/2009 of the European parliament and of the council on the placing on the market and use of feed (text with EEA relevance.) C/2017. Off J Eur Union. (2017) 328:3–11. Available online at: http://data.europa.eu/eli/reg/2017/2279/oj
37. Diener S, Zurbrügg C, Tockner K. Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Manag Res. (2009) 27:603–10. doi: 10.1177/0734242X09103838
38. Sriperm N, Pesti GM, Tillman PB. Evaluation of the fixed nitrogen-to-protein (N:P) conversion factor (6.25) versus ingredient specific N:P conversion factors in feedstuffs. J Sci Food Agric. (2011) 91:1182–6. doi: 10.1002/jsfa.4292
39. Mariotti F, Tomé D, Mirand PP. Converting nitrogen into protein - beyond 6.25 and Jones' factors. Crit Rev Food Sci Nutr. (2008) 48:177–84. doi: 10.1080/10408390701279749
40. Nery J, Gasco L, Dabbou S, Schiavone A. Protein composition and digestibility of black soldier fly larvae in broiler chickens revisited according to the recent nitrogen-protein conversion ratio. J Insects Food Feed. (2018) 4:171–7. doi: 10.3920/JIFF2018.0006
41. Janssen RH, Vincken JP, Van Den Broek LAM, Fogliano V, Lakemond CMM. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J Agric Food Chem. (2017) 65:2275–8. doi: 10.1021/acs.jafc.7b00471
42. Finke MD. Estimate of chitin in raw whole insects. Zoo Biol. (2007) 26:105–15. doi: 10.1002/zoo.20123
43. Lei XJ, Kim TH, Park JH, Kim IH. Evaluation of supplementation of defatted black soldier fly (Hermetia illucens) larvae meal in beagle dogs. Ann Anim Sci. (2019) 19:767–77. doi: 10.2478/aoas-2019-0021
44. Freel TA, McComb A, Koutsos EA. Digestibility and safety of dry black soldier fly larvae (BSFL) meal and BSFL oil in dogs. J Anim Sci. (2021) 99:skab047. doi: 10.1093/jas/skab047
45. Cutrignelli MI, Messina M, Tulli F, Randazzi B, Olivotto I, Gasco L, et al. Evaluation of an insect meal of the black soldier Fly (Hermetia illucens) as soybean substitute: intestinal morphometry, enzymatic and microbial activity in laying hens. Res Vet Sci. (2018) 117:209–15. doi: 10.1016/j.rvsc.2017.12.020
46. Kröger S, Heide C, Zentek J. Evaluation of an extruded diet for adult dogs containing larvae meal from the black soldier fly (Hermetia illucens). Anim Feed Sci Technol. (2020) 270:114699. doi: 10.1016/j.anifeedsci.2020.114699
47. Longvah T, Mangthya K, Ramulu P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. (2011) 128:400–3. doi: 10.1016/j.foodchem.2011.03.041
48. Nafisah A, Nahrowi, Mutia R, Jayanegara A. Chemical composition, chitin and cell wall nitrogen content of black soldier fly (Hermetia illucens) larvae after physical and biological treatment. In: IOP Conference Series: Materials Science and Engineering. Kazimierz Dolny (2019).
49. Messia MC, Marconi E. Innovative and rapid procedure for 4-hydroxyproline determination in meat-based foods. Methods Mol Biol. (2012) 828:281–9. doi: 10.1007/978-1-61779-445-2_22
50. Colgrave ML, Allingham PG, Tyrrell K, Jones A. Multiple reaction monitoring for the accurate quantification of amino acids: using hydroxyproline to estimate collagen content. Methods Mol Biol. (2012) 828:291–303. doi: 10.1007/978-1-61779-445-2_23
Keywords: sustainability, pet food, digestibility, protein, novel feed materials, insect meal
Citation: Penazzi L, Schiavone A, Russo N, Nery J, Valle E, Madrid J, Martinez S, Hernandez F, Pagani E, Ala U and Prola L (2021) In vivo and in vitro Digestibility of an Extruded Complete Dog Food Containing Black Soldier Fly (Hermetia illucens) Larvae Meal as Protein Source. Front. Vet. Sci. 8:653411. doi: 10.3389/fvets.2021.653411
Received: 14 January 2021; Accepted: 29 April 2021;
Published: 11 June 2021.
Edited by:
Simone Mancini, University of Pisa, ItalyReviewed by:
Monica Isabella Cutrignelli, University of Naples Federico II, ItalyCharles Gregory Aldrich, Kansas State University, United States
Camilla Mariane Menezes Souza, Federal University of Paraná, Brazil
Copyright © 2021 Penazzi, Schiavone, Russo, Nery, Valle, Madrid, Martinez, Hernandez, Pagani, Ala and Prola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Achille Schiavone, YWNoaWxsZS5zY2hpYXZvbmVAdW5pdG8uaXQ=
 Livio Penazzi1
Livio Penazzi1 Joana Nery
Joana Nery Emanuela Valle
Emanuela Valle Silvia Martinez
Silvia Martinez Elena Pagani
Elena Pagani Ugo Ala
Ugo Ala Liviana Prola
Liviana Prola