- Department of Veterinary Pathology, College of Veterinary Medicine, Seoul National University, Seoul, South Korea
The purpose of this study was to evaluate the efficacy of a new, single-dose bivalent vaccine containing porcine circovirus type 2b (PCV2b) and Mycoplasma hyopneumoniae against a dual PCV2b and M. hyopneumoniae challenge. At −25 days post challenge (dpc, 10 days of age), one pig group (designated as the vaccinated/challenged group) received a single, 1.0 ml dose of bivalent vaccine. Pigs in both the vaccinated/challenged and unvaccinated/challenged groups were then inoculated intranasally with PCV2b and M. hyopneumoniae at 0 dpc (35 days of age). Pigs in vaccinated/challenged group induced significantly higher levels of neutralizing antibodies against PCV2b and cell-mediated immunity against PCV2b and M. hyopneumonia when compared with pigs in unvaccinated/challenged group. The vaccination of pigs with a bivalent vaccine also reduced PCV2b viremia, reduced mycoplasmal nasal shedding, and decreased the severity of both lung and lymphoid lesions for PCV2b and M. hyopneumoniae infection, respectively. The results of this study demonstrated that the evaluated bivalent vaccine was effective in protecting pigs against PCV2b and M. hyopneumoniae infection.
Introduction
Pneumonia caused by multiple infectious agents has been described with the term “Porcine Respiratory Disease Complex (PRDC).” PRDC infection is categorized by reduced pig performance, increased medication costs to the producer, and an increased mortality rate during the finishing process (15 to 20 weeks of age) (1). The etiology of PRDC is extremely diverse and occurs in both all-in-all-out as well as in continuous production systems. The main three causative agents of PRDC are porcine circovirus type 2 (PCV2), porcine reproductive and respiratory syndrome virus (PRRSV), and Mycoplasma hyopneumoniae, which are known to be responsible for serious economic damages within the global pig industry (1). Among the three, co-infection of pigs with PCV2 and M. hyopneumoniae is the most frequent combination in field PRDC cases and is the most rapidly increasing within the Asian pork industry (2). Vaccination is routinely implemented to control PRDC in relation to PCV2 and M. hyopneumoniae infection (3). This increase in vaccination numbers has led to a demand for single-dose bivalent vaccines containing PCV2 and M. hyopneumoniae. This experimental challenge study was designed to help meet this demand by evaluating the efficacy of a new bivalent PCV2b and M. hyopneumoniae vaccine (Circo/MycoGard, Pharmgate Animal Health, Wilmington, NC, USA) containing killed Baculovirus vector and M. hyopneumoniae bacterin vaccine with a trivalent-adjuvanted formulation against an experimental challenge of PCV2b and M. hyopneumoniae.
Materials and Methods
A total of 24 colostrum-fed, cross-bred, conventional piglets were purchased at 7 days of age from a PRRSV- and M. hyopneumoniae-free commercial farm. The negative status of the farm was based on serological testing of the breeding herd, and long term clinical and slaughter history. Sows residing on the commercial farm were naïve to vaccination against PCV2 and M. hyopneumoniae, while all piglets were vaccinated against both pathogens at 21 days of age. The pigs were weaned early at 7 days of age before vaccination of PCV2 and M. hyopneumoniae and selected for this study based on their seronegative results for PRRSV (IDEXX PRRS X3 Ab test, IDEXX Laboratories Inc., Westbrook, Maine, USA), M. hyopneumoniae (M. hyo. Ab test, IDEXX Laboratories Inc.), and PCV2 (PCV2 Ab Mono Blocking, Synbiotics, Lyon, France). In addition, negative results for viral and mycoplasmal infections were also obtained for PCV2 and PRRSV from sera samples and for M. hyopneumoniae from nasal swabs as tested by real-time polymerase chain reaction (PCR) (4–6).
A total of 24 pigs were randomly allocated into three groups that contained eight piglets per groups (4 = male and 4 = female). Three rooms, uniform in design that allowed free access to feed and water troughs each contained ten pens in facility of Seoul National University. Four pigs were randomly assigned to the pens from each of the three groups. All randomizations were performed using the random number generator function (Excel, Microsoft Corporation, Redmond, WA, USA).
At −25 days post challenge (dpc, 10 days of age), pigs in the Vaccinated/Challenged (Vac/Ch) group were vaccinated intramuscularly on the right side of the neck with 1.0 ml of a bivalent vaccine containing PCV2b and M. hyopneumoniae (Circo/MycoGard, Serial No: CMG-18007, Expiration date: 02.28.2020). Pigs in the Unvaccinated/Challenged (UnVac/Ch) and Unvaccinated/Unchallenged (UnVac/UnCh) groups received a 1.0 ml injection of phosphate buffered saline (PBS, 0.01 M, pH 7.4) in the same anatomical location as the Vac/Ch group.
At 0 dpc (35 days old), pigs in the Vac/Ch and UnVac/Ch groups were challenged by inoculation with PCV2b (strain SNUVR000463, GenBank no. KF871068). Five hours later, an M. hyopneumoniae (strain SNU98703) challenge was administered. Co-infection with PCV2b (strain SNUVR000463) and M. hyopneumoniae (strain SNU98703) induced severe pneumonia in lungs and lymphoid depletion in the lymph node in infected pigs (7). The wait interval was performed to avoid the mixture of two pathogens which could have resulted in an infectivity decrease. During the PCV2b challenge, a 3 ml inoculation containing 1.2 × 105 (50% tissue culture infective dose (TCID50)/ml) was administered intranasally. Five hours post-PCV2 inoculation, pigs were intramuscularly anesthetized with a mixture of 2.2 mg/kg xylazine hydrochloride (Rompun, Bayer), 2.2 mg/kg tiletamine hydrochloride, and 2.2 mg/kg zolazepam hydrochloride (Zoletil 50, Virbac). Then, pigs were inoculated intratracheally with 7 ml of M. hyopneumoniae (strain SNU98703) culture medium containing 107 color changing units (CCU)/ml. All study methods were approved previously by the Seoul National University Institutional Animal Care and Use, and Ethics Committee (SNU-181018-8-2).
At 21 dpc (56 day old), all pigs were sedated with an intravenous injection of sodium pentobarbital prior to euthanasia by electrocution as previously described (8). Tissues were collected from each pig at necropsy. Tissue preparation included fixation in a 10% neutral buffered formalin solution for 24 h before they were routinely processed and embedded in paraffin.
Pigs were monitored daily and scored weekly for clinical signs as previously described (9). Scores ranged from 0 to 6: 0 = normal; 1 = rough haircoat; 2 = rough haircoat and dyspnea; 3 = mild dyspnea and abdominal breathing; 4 = moderate dyspnea and abdominal breathing; 5 = severe dyspnea and abdominal breathing; 6 = death.
Pigs were weighed at 10 (−25 dpc) and 56 (21 dpc) days of age. Average daily gain (ADG) was calculated as the difference between the starting and final weight divided by the number of days spanning the duration of the stage, and included data for pigs that died or were removed from the study.
Blood and nasal swabs were collected from all pigs at −25, −14, 0, 7, 14, and 21 dpc. A commercial kit (QIAamp DNA Mini Kit, QIAGEN, Valencia, CA, USA) was use to extract DNA from serum samples and nasal swabs. The number of genomic DNA copies for M. hyopneumoniae was quantified by real-time PCR (4). To construct a standard curve, real-time PCR was performed in quadruplicate in 10-fold serial dilution of chromosomal DNA from M. hyopneumoniaes strain SNU98703, with concentrations ranging from 10 ng/μl to 1 fg/μl. One fetogram of chromosomal DNA from M. hyopneumoniae is considered to be ~one genome equivalent (10). A negative control was included in each run using double distilled water as the template.
The number of genomic DNA copies for PCV2b was quantified by real-time PCR (3). To construct a standard curve, real-time PCR was performed in quadruplicate in two different assays: (i) 10-fold serial dilutions of the PCV2b plasmid were used as the standard, with concentrations ranging from 1010 to 102 copies/ml, and (ii) 10-fold serial dilutions of PCV2b cultured in PCV1-free PK-15 cells were used at concentrations ranging from 104.5 TCID50/ml to 10−3.5 TCID50/ml. The PCV2b plasmid was prepared as described previously (3). Culture supernatants of PCV1-free PK-15 cells were used as negative control.
The presence of M. hyopneumoniae and PCV2 antibodies were evaluated in serum samples by use of commercially available enzyme-linked immunosorbent assay (ELISA) kits (M. hyo Ab test, IDEXX Laboratories Inc and SERELISA PCV2 Ab Mono Blocking, Synbiotics). Testing was conducted in accordance with each manufacturer's kit instructions, where samples were considered as positive for M. hyopneumoniae antibody if the sample-to-positive (S/P) ratio was ≥ 0.4 and as positive for PCV2 antibodies if the reciprocal ELISA titer was > 350. Serum samples were also tested for serum virus neutralization against PCV2b (11).
Inactivated M. hyopneumoniae and PCV2b antigens using challenge strains for M. hyopneumoniae and PCV2b were prepared for enzyme-linked immunospot (ELISpot) assay as previously described (12, 13). An ELISpot assay was conducted to measure the numbers of M. hyopneumoniae- and PCV2b-specific interferon-γ secreting cells (IFN-γ-SC). Peripheral blood mononuclear cells (PBMC) were stimulated with inactivated M. hyopneumoniae and PCV2b antigens and results reported as the number of IFN-γ-SC per million PBMC (12, 14). Phytohemagglutinin (Roche Diagnostics GmbH, Mannheim, Germany) and PBS used as a positive and negative control, respectively.
Lung lesion scoring was performed for M. hyopneumoniae infection and lymphoid lesion for PCV2b infection by two veterinary pathologists. Severity of lung lesion was scored (0 to 6) based on peribronchiolar and perivascular lymphoid tissue hyperplasia (15). Severity of lymphoid lesion was scored (0 to 5) based on lymphoid depletion and granulomatous inflammation (16).
All real-time PCR data was transformed to log10 values prior to statistical analysis. The Shapiro-Wilk test evaluated data for normal distribution. One-way analysis of variance (ANOVA) was used to examine whether there were statistically significant differences at each time point within the three groups. If a one-way ANOVA test resulted in a statistical significance, data was further evaluated by conducting a post-hoc test for a pairwise comparison with Tukey's adjustment. The Kruskal-Wallis test was performed if the normality assumption was not met. Kruskal Wallis test results which showed a statistical significance were further evaluated with the Mann-Whitney test to include Tukey's adjustment to compare the differences among the groups. The Pearson's correlation coefficient was used to assess the correlation of PCV2b viremia with neutralizing antibody titers against PCV2b, PCV2b viremia with PCV2b-specific IFN-γ-SC, and nasal shedding of M. hyopneumoniae and M. hyopneumoniae-specific IFN-γ-SC. Results were reported in P-value where a value of P < 0.05 was considered to be significant.
Results
The mean scores for respiratory disease were significantly lower (P < 0.05) in pigs from the Vac/Ch group (mean ± standard deviation, 0.38 ± 0.52 for 7 dpc, 0.75 ± 0.71 for 14 dpc, and 0.63 ± 0.52 for 21 dpc) when compared with the UnVac/Ch group (mean ± standard deviation, 1.00 ± 0.53 for 7 dpc, 2.13 ± 0.83 for 14 dpc, and 2.13 ± 0.83 for 21 dpc) at 7, 14, and 21 dpc. Pigs from the Group 3 remained normal throughout the experiment.
The body weight of the pigs did not differ significantly among three groups at study day 0 (the time of vaccination, 10 days of age). Pigs from the Vac/Ch (mean ± standard deviation, 301.1 ± 11.3) and UnVac/UnCh (mean ± standard deviation, 309.2 ± 4.8) groups had significantly higher (P < 0.05) ADG (unit = gram/pig/day) between 10 and 56 days of age when compared with those from UnVac/Ch (mean ± standard deviation, 277.5 ± 16.4) group.
Nasal swabs evaluation between 7 and 21 dpc reported significantly less (P < 0.05) M. hyopneumoniae genomic copies in pigs from the Vac/Ch group when compared with the UnVac/Ch group (Figure 1A). Blood sample evaluation from the same timeframe (7 to 21 dpc) also reported significantly less (P < 0.05) PCV2d genomic copies in the Vac/Ch group when compared with the UnVac/Ch group (Figure 1B). No M. hyopneumoniae or PCV2 were detected in the pigs from the UnVac/UnCh group.
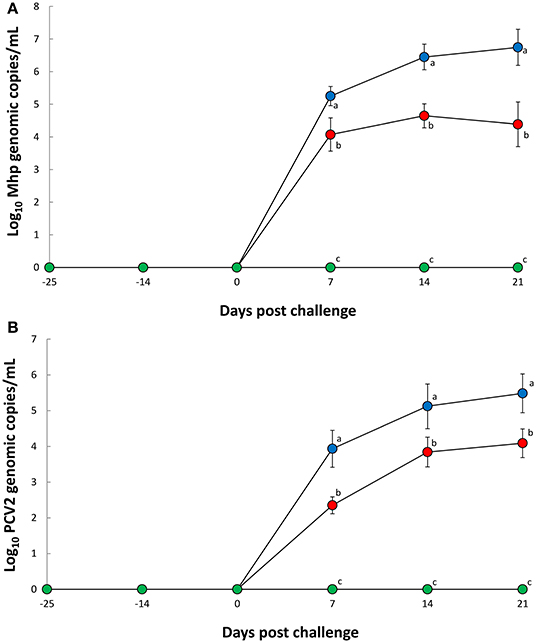
Figure 1. Mean values of the genomic copy number of Mycoplasma hyopneumoniae DNA in nasal swabs (A) and porcine circovirus type 2b in blood (B) from Vac/Ch ( ), UnVac/Ch (
), UnVac/Ch ( ), and UnVac/UnCh (
), and UnVac/UnCh ( ) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among three groups.
) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among three groups.
Pigs in the Vac/Ch group had a significantly higher (P < 0.05) M. hyopneumoniae ELISA S/P ratio in their −14 to 21 dpc serum samples when compared with the UnVac/Ch group (Figure 2A). Pigs in the Vac/Ch group had a significantly higher (P < 0.05) PCV2 ELISA titer from −14, to 21 dpc in their serum samples when compared with the UnVac/Ch group (Figure 2B). Pigs in the Vac/Ch group had significantly higher (P < 0.05) neutralizing antibody titers against PCV2b from −14 to 21 dpc when compared with the UnVac/Ch group (Figure 2C). There was a correlation between number of genomic copies of PCV2b in the blood and neutralizing antibody titers against PCV2b (r = −0.810, P = 0.015). M. hyopneumoniae and PCV2 antibodies were not detected in pigs from the UnVac/UnCh group.
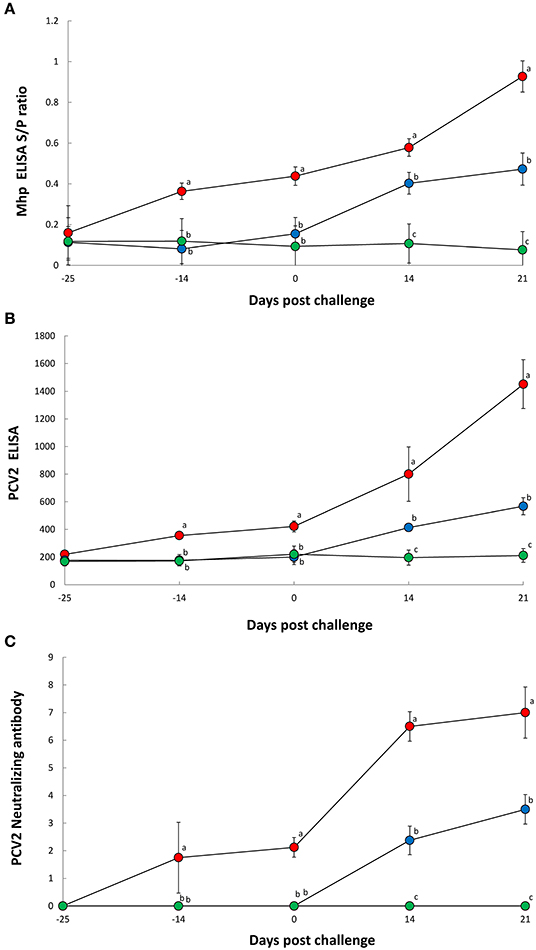
Figure 2. ELISA antibody levels of Mycoplasma hyopneumoniae (A), ELISA antibody levels of porcine circovirus type 2 (B), and neutralizing antibody titers against porcine circovirus type 2b (C) in serum from Vac/Ch ( ), UnVac/Ch (
), UnVac/Ch ( ), and UnVac/UnCh (
), and UnVac/UnCh ( ) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among three groups.
) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among three groups.
Pigs in the Vac/Ch group had a significantly higher (P < 0.05) number of M. hyopneumoniae- (Figure 3A) and PCV2b- (Figure 3B) specific IFN-γ-SC in their PBMC from −14, 0, 14, and 21 dpc when compared with the UnVac/Ch group. There was a correlation between the number of genomic copies of M. hyopneumoniae in the nasal swabs and numbers of M. hyopneumoniae-specific IFN-γ-SC (r = −0.758, P = 0.029). M. hyopneumoniae and PCV2b-specific IFN-γ-SC were not detected in pigs from the UnVac/UnCh group.
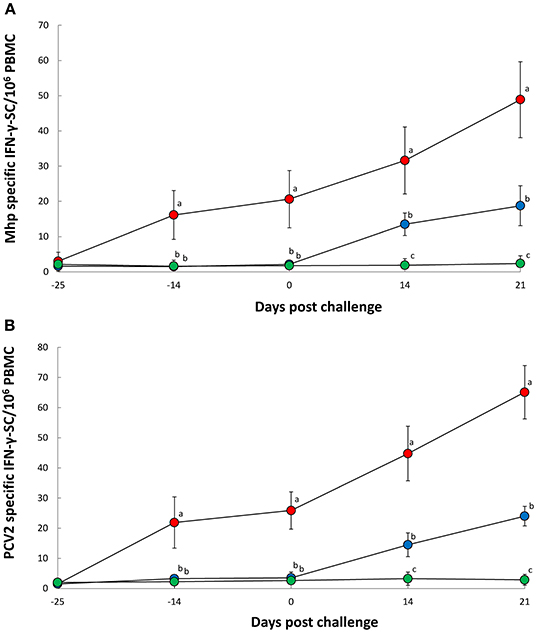
Figure 3. Frequency of Mycoplasma hyopneumoniae-specific interferon-γ secreting cells (IFN-γ-SC) (A) and porcine circovirus type 2b-specific IFN-γ-SC (B) in peripheral blood mononuclear cells (PBMC) from Vac/Ch ( ), UnVac/Ch (
), UnVac/Ch ( ), and UnVac/UnCh (
), and UnVac/UnCh ( ) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among three groups.
) groups. Variation is expressed as the standard deviation. Different superscripts (a, b, and c) indicate significant (P < 0.05) difference among three groups.
Pigs in the Vac/Ch group had significantly lower (P < 0.05) macroscopic (mean ± standard deviation, 17.4 ± 5.3) and microscopic (mean ± standard deviation, 0.9 ± 0.34) lung lesion scores for M. hyopneumoniae infection at 21 dpc when compared with the UnVac/Ch group with macroscopic (mean ± standard deviation, 45.8 ± 3.2) and microscopic (mean ± standard deviation, 3.5 ± 0.4) lung lesion scores. Pigs in the Vac/Ch group also had significantly lower (P < 0.05) microscopic lymphoid lesions scores (mean ± standard deviation, 0.7 ± 0.2) for PCV2b infection at 21 dpc when compared with the UnVac/Ch group with microscopic lymphoid lesions scores (mean ± standard deviation, 3.5 ± 0.5). Macroscopic and microscopic lung lesions, and microscopic lymphoid lesions were not observed in pigs from the UnVac/UnCh group.
Discussion
The study results demonstrated that the evaluated bivalent vaccine containing PCV2b and M. hyopneumoniae against a dual challenge of PCV2b and M. hyopneumoniae is efficacious in protecting pigs. The evaluation of growth performance was identified as the critical factor in determining the efficacy of this bivalent vaccine as PCV2 and M. hyopneumoniae co-infection is mainly characterized by poor growth performance. Vaccination of pigs with the evaluated bivalent vaccine resulted in improved growth performance when compared with unvaccinated pigs.
The bivalent vaccine tested in this study was administered to piglets at 10 days of age. There was potential, therefore, for interference with maternally derived antibodies. In this study, interference by maternally derived antibodies was not evaluated as pigs tested as seronegative against PCV2 prior to study initiation. It can be deduced, however, maternally derived antibodies did not significantly interfere with the induction of both humoral and cell-mediated immunity in piglets post PCV2 vaccination (17).
M. hyopneumoniae is known to have a potentiating effect on the level of PCV2 viremia in pigs co-infected with M. hyopneumoniae and PCV2 (15). The reduction of PCV2b viremia was therefore an effect of bivalent vaccination but not contribute to an immunosuppressive effect of M. hyopneumoniae infection in vaccinated pigs challenged with PCV2b and M. hyopneumoniae.
Neutralizing antibodies and IFN-γ-SC are responsible for the reduction of PCV2 viremia (18–20), where these levels are considered measurements of protective immunity (19, 20). The amount of neutralizing antibodies present correlated with the PCV2b viremia reduction in the present study, while frequency of IFN-γ-SC did not. On other hands, it should be noted that the protective immunity mechanism against M. hyopneumoniae is not fully understood, and therefore caution should always be exercised with evaluating it against study conclusions. Cell-mediated immunity has proven to play an important role in controlling M. hyopneumoniae infection (21). In the present study, cell-mediated immunity as measured by IFN-γ-SC correlated with a significant reduction in the amount of M. hyopneumoniae loads through nasal shedding. This study demonstrated that pig vaccination and challenge induced high levels of protective immunity, reduced the amount of PCV2b viremia and severity of lymphoid lesions, and reduced the amount of M. hyopneumoniae nasal shedding and severity of lung lesions.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by Seoul National University Institutional Animal Care and Use Committee (SNU-181018-8-2).
Author Contributions
YA performance of the experimental trials and data analysis and writing of the manuscript. SY, TO, and KP preparation of the inoculum and lab analysis. HC immune analysis. JS pathological analysis. CC development of protocol, design of the study, review of the final manuscript, and approval for publication. All authors read and approved the final manuscript.
Funding
This work was supported by contract research funds (Grant no. 550-20190078) of the Research Institute for Veterinary Science (RIVS) from the College of Veterinary Medicine and by the BK 21 FOUR Future Veterinary Medicine Leading Education and Research Center (Grant no. A0449-20200100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chae C. Porcine respiratory disease complex: interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet J. (2016) 212:1–6. doi: 10.1016/j.tvjl.2015.10.030
2. Kim J, Chung H-K, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. (2003) 166:251–6. doi: 10.1016/S1090-0233(02)00257-5
3. Park C, Jeong J, Choi K, Chae C. Efficacy of a new bivalent vaccine of porcine circovirus type 2 and Mycoplasma hyopneumoniae (FosteraTMPCV MH) under experimental conditions. Vaccine. (2016) 34:270–5. doi: 10.1016/j.vaccine.2015.11.034
4. Dubosson C R, Conzelmann C, Miserez R, Boerlin P, Frey J, Zimmermann W, et al. Development of two real-time PCR assays for the detection of Mycoplasma hyopneumoniae in clinical samples. Vet Microbiol. (2004) 102:55–65. doi: 10.1016/j.vetmic.2004.05.007
5. Gagnon CA, del Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J Vet Diagn Invest. (2008) 20:545–58. doi: 10.1177/104063870802000503
6. Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, et al. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. (2004) 42: 4453–61. doi: 10.1128/JCM.42.10.4453-4461.2004
7. Jeong J, Kang I, Kim S, Park KH, Park C, Chae C. Comparison of 3 vaccination strategies against porcine reproductive and respiratory syndrome virus, Mycoplasma hyopneumoniae, and porcine circovirus type 2 on 3 pathogen challenge model. Can J Vet Res. (2018) 82:39–47.
8. Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, et al. Report of the AVMA panel on euthanasia. J Am Vet Med Assoc. (2001) 218:669–696. doi: 10.2460/javma.2001.218.669
9. Seo HW, Park S-J, Park C, Chae C. Interaction of porcine circovirus type 2 and Mycoplasma hyopneumoniae vaccines on dually infected pigs. Vaccine. (2014) 32:2480–6. doi: 10.1016/j.vaccine.2014.02.088
10. Kurth KT, Hsu T, Snook ER, Thacker EL, Thacker BJ, Minion FC. Use of a Mycoplasma hyopneumoniae nested polymerase chain reaction test to determine the optimal sampling sites in swine. J Vet Diagn Invest. (2002) 14:463–9. doi: 10.1177/104063870201400603
11. Pogranichnyy RM, Yoon KJ, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. (2000) 13:143–53. doi: 10.1089/vim.2000.13.143
12. Bandrick M, Pieters M, Pijoan C, Molitor TW. Passive transfer of maternal Mycoplasma hyopneumoniae-specific cellular immunity to piglet. Clin Vaccine Immunol. (2008) 15:540–3. doi: 10.1128/CVI.00466-07
13. Rodriguez-Arrioja GM, Segalés J, Balasch M, Rosell C, Quintant J, Folch JM, Plana-Duran J, Mankertz A, Domingo M. Serum antibodies to porcine circovirus type 1 and type 2 in pigs with and without PMWS. Vet Rec. (2000) 146:762–4. doi: 10.1136/vr.146.26.762
14. Seo HW, Han K, Oh Y, Park C, Chae C. Efficacy of a reformulated inactivated chimeric PCV1-2 vaccine based on clinical, virological, pathological and immunological examination under field conditions. Vaccine. (2000) 30: 6671–7. doi: 10.1016/j.vaccine.2012.08.065
15. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng X-J, Halbur PG. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. (2004) 41:624–40. doi: 10.1354/vp.41-6-624
16. Kim J, Chae C. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J Comp Pathol. (2004) 131:121–6. doi: 10.1016/j.jcpa.2004.02.001
17. Martelli P, Saleri R, Ferrarini G, De Angelis E, Cavalli V, Benetti M, et al. Impact of maternally derived immunity on piglets' immune response and protection against porcine circovirus type 2 (PCV2) after vaccination against PCv2 at different age. BMC Vet Res. (2016) 12:77. doi: 10.1186/s12917-016-0700-1
18. Fort M, Fernandes LT, Nofrarias M, Díaz I, Sibila M, Pujols J, et al. Development of cell-mediated immunity to porcine circovirus type 2 (PCV2) in caesarean-derived, colostrum-deprived piglets. Vet Immunol Immunopathol. (2009) 129:101–7. doi: 10.1016/j.vetimm.2008.12.024
19. Meerts P, Misinzo G, Lefebvre D, Nielsen J, Bøtner A, Kristensen CS, et al. Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res. (2006) 2:6–16. doi: 10.1186/1746-6148-2-6
20. Meerts P, Van-Gucht S, Cox E, Vandebosch A, Nauwynck HJ. Correlation between type of adaptive immune response against porcine circovirus type 2 and level of virus replication. Viral Immunol. (2005) 18:333–41. doi: 10.1089/vim.2005.18.333
Keywords: mycoplasma hyopneumoniae, porcine circovirus type 2, porcine respiratory disease complex, bivalent vaccine, experimental challenge
Citation: Ahn Y, Yang S, Oh T, Park KH, Cho H, Suh J and Chae C (2021) Efficacy Evaluation of a Bivalent Vaccine Containing Porcine Circovirus Type 2b and Mycoplasma hyopneumoniae Against an Experimental Dual Challenge. Front. Vet. Sci. 8:652313. doi: 10.3389/fvets.2021.652313
Received: 12 January 2021; Accepted: 07 April 2021;
Published: 30 April 2021.
Edited by:
Massimo Amadori, Istituto Zooprofilattico Sperimentale Lombardia ed Emilia Romagna (IZSLER), ItalyReviewed by:
Meera Surendran Nair, Pennsylvania State University (PSU), United StatesAneesh Thakur, University of Copenhagen, Denmark
Copyright © 2021 Ahn, Yang, Oh, Park, Cho, Suh and Chae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanhee Chae, swine@snu.ac.kr
 Yongjun Ahn
Yongjun Ahn Taehwan Oh
Taehwan Oh Chanhee Chae
Chanhee Chae