
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 25 June 2021
Sec. Veterinary Infectious Diseases
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.650942
This article is part of the Research TopicEmerging Zoonoses and Transboundary InfectionsView all 27 articles
 Jiali Sun1†
Jiali Sun1† Hao Dong2,3†
Hao Dong2,3† Xiaowei Peng1†
Xiaowei Peng1† Yufu Liu1†
Yufu Liu1† Hui Jiang1
Hui Jiang1 Yu Feng1
Yu Feng1 Qiaoling Li1
Qiaoling Li1 Liangquan Zhu1
Liangquan Zhu1 Yuming Qin1*
Yuming Qin1* Jiabo Ding1*
Jiabo Ding1*The transcriptional regulator MucR is related to normal growth, stress responses and Brucella virulence, and affects the expression of various virulence-related genes in smooth-type Brucella strains. However, the function of MucR in the rough-type Brucella canis remains unknown. In this study, we discovered that MucR protein was involved in resistance to heat stress, iron-limitation, and various antibiotics in B. canis. In addition, the expression level of various bacterial flagellum-related genes was altered in mucR mutant strain. Deletion of this transcriptional regulator in B. canis significantly affected Brucella virulence in RAW264.7 macrophage and mice infection model. To gain insight into the genetic basis for distinctive phenotypic properties exhibited by mucR mutant strain, RNA-seq was performed and the result showed that various genes involved in translation, ribosomal structure and biogenesis, signal transduction mechanisms, energy production, and conversion were significantly differently expressed in ΔmucR strain. Overall, these studies have not only discovered the phenotype of mucR mutant strain but also preliminarily uncovered the molecular mechanism between the transcriptional regulator MucR, stress response and bacterial virulence in B. canis.
In 1966, Brucella canis was first isolated from aborted tissues and vaginal discharge of beagles (1). In dogs, B. canis causes reproductive failure, while it causes fever, chills, malaise, peripheral lymphadenomegaly, and splenomegaly in humans (2). Recent studies have demonstrated that the intracellular trafficking route of B. canis was indistinguishable from that of B. abortus and a less robust response was observed in mice infected with B. canis compared with B. abortus in terms of proinflammatory cytokines, interferon-gamma levels, splenic inflammation, and hepatic granulomas (3). Another study indicated that only a dose of B. canis (up to 109 CFU) could induce splenomegaly in infected mice at 1 and 2 weeks post-inoculation and the suitable challenge dose (1 ×107 CFU) for investigating vaccine safety for B. canis seemed to be about 103-fold higher than that of smooth-type Brucella strains (4). It seems that B. canis is less pathogenic than other smooth-type Brucella species in this murine model.
Due to the low prevalence of brucellosis caused by B. canis, which is less pathogenic than other Brucella species, most countries have not established clear protocols for its prevention and the public risk of this pathogen is usually ignored. Additionally, comparatively little is known about the pathogenic mechanism and virulence factors of B. canis strains.
Brucella strains do not produce classical virulence factors, such as cytolisins, exotoxin, exoenzymes, fimbria, plasmids, and drug-resistant forms. Lipopolysaccharide (LPS), T4SS secretion system, and BvrR/BvrS system have been considered to be major virulence factors allowing interaction with the host cell surface, the formation of an early, late BCV (Brucella Containing Vacuole) and interaction with the endoplasmic reticulum (ER) when the bacteria multiply (5). Besides, some transcription regulators have been proved to be involved in Brucella virulence, such as the well-studied LuxR-type regulator VjbR (6).
The virulence-related transcriptional regulator MucR, which is a member of the Ros/MucR family, is considered to control the expression of various genes involved in the successful interaction of α-proteobacteria with their eukaryotic hosts (7–9). In B. melitensis and B. abortus, the transcriptional regulator MucR is an extensively studied virulence factor and mucR mutant strain has been considered to be a promising vaccine candidate in mice model against both intraperitoneal and aerosol challenge (10). In Brucella strains, previous studies have also demonstrated that MucR protein is involved in resistance to environmental stresses, such as acidic stress, oxidative stress, detergent, cationic peptide, and iron deficiency (9, 11). Additionally, previous transcriptomic and microarray analyses have revealed that MucR could directly and indirectly affect the expression of hundreds of genes involved in metabolism, cell wall/envelope biogenesis, replication, and translation (9, 12). In recent studies, various target genes, which were directly regulated by MucR protein, were also identified, such as babR, virB genes, bab1_0746, bab1_1035, bab1_1605, and bab1_1893 (12, 13). Compared to the well-studied MucR protein in smooth-type Brucella strains, no study has been carried out so far to demonstrate the function of MucR protein in B. canis strains.
Herein, we constructed mucR in-frame deleted mutant strain and complemented strain in B. canis. Then, we assessed the phenotypes of mucR mutant strain, such as growth characteristics and bacterial virulence in both macrophages and mice infection models. Moreover, the sensitivity of ΔmucR under various stress conditions was determined. Finally, RNA-seq analysis was performed to investigate the changes of the transcriptional profile of gene expression in mucR mutant strain compared with its parent strain RM6/66.
Construction of the suicide plasmid was performed as previously reported (14). Specifically, for B. canis RM6/66 mucR gene mutant, a 433-bp upstream fragment and a 500-bp downstream fragment of mucR gene were amplified using D-mucR-UF/UR and D-mucR-DF/DR primers, respectively (Supplementary Table 1). The two fragments were then used as templates for the second round of overlap polymerase chain reaction (PCR). The purified PCR product was digested with KpnI and HindIII restriction enzymes and cloned into the puc19-sacB plasmid, which was verified using PCR and sequencing.
The B. canis RM6/66 mucR gene unmarked in-frame deletion mutant was constructed with allelic replacement using a two-step method (14). The suicide plasmid was transformed into the wild-type B. canis RM6/66 strain by electro transformation. Single crossover integrates were then selected on tryptic soy agar (TSA) supplemented with ampicillin (100 μg/ml). The second crossover selection was conducted by plating the bacteria on TSA containing 5% sucrose. Ampicillin sensitive colonies were selected and verified by PCR and sequencing.
The mucR fragment, including the promoter sequence (500-bp of the intergenic region upstream of mucR start codon), was amplified with C-mucR-F/R primers (Supplementary Table 1). The PCR products were ligated to pMR11 plasmid, which was then referred to as the complementation plasmid pMR-mucR. To construct the complemented strain (CmucR), pMR-mucR was electroporated into mucR deletion mutant and then the cells were plated onto TSA containing ampicillin to detect the presence of pMR-mucR in the complemented strain. The resulting strains were verified by PCR and sequencing. For determination of minimum inhibitory concentrations (MICs) of antibiotics, the complemented strain (CmucR1) with chloramphenicol resistance was constructed using pBBR1MCS-1 plasmid using the same primers (C-mucR-F/R) as previously reported (15).
For RNA-seq assay, Brucella strains (RM6/66 and ΔmucR) were grown in three different tubes with TSB at 37°C from a single colony until the log phase was reached. Total RNA was isolated using TRIzol according to the manufacturer's instructions. Residual DNA in the samples was removed using DNase I. RNA concentration and purity were determined spectrophotometrically using an ND 1000 spectrophotometer (Thermo Scientific, Wilmington, USA).
The sequencing library of each RNA sample was prepared using NEB Next Ultra Directional RNA Library Prep Kit for Illumina as recommended by the manufacturer. Briefly, RNA fragments were reverse-transcribed and amplified to double-stranded cDNA and then ligated with an adaptor. The amplified cDNA was purified using the magnetic bead-based method and the molar concentration was determined for each cDNA library. The HiSeq X Ten platform was used to perform transcriptome sequencing. Sequencing and subsequent bioinformatics analysis were completed at Novel Bioinformatics Co., Ltd (Shanghai, China).
qRT-PCR was performed as previously described (16). Differentially expressed genes (DEGs) were verified by qRT-PCR using different samples like that in RNA-seq. Samples were amplified in a 20-μl reaction containing 10-μl 2 × SYBR® Premix Ex TaqTMII (TAKARA), 100 nM forward and reverse primers and 1-μl 10-fold diluted cDNA sample. 16S rRNA, which is constantly transcribed in bacteria, was chosen as the housekeeping gene. For each gene, qRT-PCR was performed in triplicate and relative transcription levels were determined using the 2−ΔΔCt method. Primers used for qRT-PCR are provided in Supplementary Table 1.
The multiplication of B. canis RM6/66 and its derived strains in RAW264.7 macrophages were evaluated to determine the ability of ΔmucR mutant strain to survive intracellularly. The assays were performed as previously described (14). Briefly, in a permissive culture condition, B. canis strains were cultured in a flask with 0.2 μM vent cap for 12 h. RAW264.7 cells were seeded in 24-well plates (Corning, NY, USA) and then infected with Brucella strains at a multiplicity of infection (MOI) of 100. After 1 h of incubation, cells were washed thrice with phosphate buffer saline (PBS) and incubated in Dulbecco's Modified Eagle Medium (DMEM) containing gentamycin (50 μg/ml) to eliminate extracellular bacteria. At 1, 24, and 48 h post-infection (hpi), cells were washed and lysed with 500 μl 0.1% (v/v) Triton X-100 water solution, and then the bacteria count was determined by plating on TSA. The assays were run in triplicate and repeated at least twice.
Virulence assay was performed using BALB/c mice as previously reported (14) with some modifications. Mice were inoculated intraperitoneally with 0.1 ml (1 ×107 CFU) of B. canis RM6/66 and its derived strains. On the 7th and 28th day post-infection (dpi), mice were euthanized via asphyxiation (n = 4 per group), after which the spleens were removed, weighed, and homogenized in 1 ml of PBS. The bacterial colonies were counted by the TSA plate method and CFUs per spleen were calculated.
To test the susceptibility of Brucella strains under different stress conditions, modified stress assays were performed as previously reported (9, 15). Brucella strains (with an initial density of 1 ×106 CFU/mL) were, respectively, cultured in an acidic tryptic soy broth (TSB) medium (pH 4.5) for 2 h. Afterward, the concentration of bacteria was measured by plate count and the survival rate (%) calculated as surviving bacteria relative to the TSB control.
To determine the sensitivity of ΔmucR mutant to heat shock stress and hypertonic environments, 5 μl gradient dilution of bacterial cultures (1 ×109 CFU/mL) were cultured on TSA medium at 42°C or TSA medium containing 200 mM sodium chloride (NaCl) at 37°C for 72 h, respectively.
To compare the growth of B. canis RM6/66, ΔmucR and the complemented strain in low iron medium, the TSB medium was limited in iron by adding different concentrations of the iron chelator, 2,2-dipyridyl (2.5, 5, and 10 mM). The bacteria were cultured in this medium at the same initial density (1 ×106 CFU/ml) and the CFUs were determined at 48 h for each strain.
MIC values of the antibiotics from both β-lactam (Ampicillin, Amoxycillin, and Carbenicillin) and non-β-lactam (Ciprofloxacin, Gentamicin, and Tetracycline) were determined as previously reported (16).
Differences between the means of the experimental and the control groups were analyzed using the independent-samples t-test using SPSS 17.0 program. The differences were considered to be significant at p < 0.05.
B. canis RM6/66 mucR deletion mutant was constructed via a double recombination event and confirmed by PCR (Figure 1A). mucR complement strain CmucR was constructed by restoring the mucR gene using the broad-range plasmid pMR11. The resultant strains were verified by PCR and sequencing.
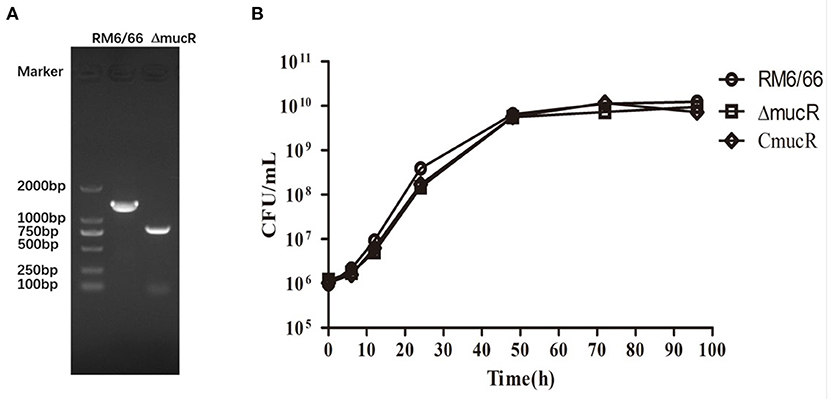
Figure 1. Characterization of mucR in-frame deleted mutant strain ΔmucR. (A) PCR amplification confirmed deletion of the mucR gene in ΔmucR. Lane M: DNA marker DL2000 (Takara, Dalian, China). B. canis RM6/66: a 1429-bp fragment was amplified using primer pair D-mucR-UF/D-mucR-DR. ΔmucR: an 846-bp fragment was amplified using primer pair D-mucR-UF/D-mucR-DR. Primer sequences are provided in Supplementary Table 1. (B) Deletion of mucR in B. canis RM6/66 did not affect bacterial growth. Brucella strains (initial density of 1 ×106 CFU/mL) were grown in TSB at 37°C with continuous shaking for 96 h. The CFUs of B. canis RM6/66 and ΔmucR were measured at different time intervals. Statistical significance was determined using the unpaired Student's t-test. ns, no significance.
To characterize the cell growth rate of ΔmucR strain, the bacterial strains were analyzed in TSB medium at 37°C with the same initial density of 1 ×106 CFU/ml. The result showed that the growth rate of ΔmucR was almost the same as its parent strain RM6/66 and the complementation strain CmucR at different time points (Figure 1B).
To assess the virulence of ΔmucR strain, the ability of ΔmucR to multiply within the cultured macrophage RAW264.7 was tested. As shown in Figure 2, the intracellular bacterial load of ΔmucR strain was almost the same as that of RM6/66 and CmucR at 1 h post-infection, while the bacterial load of ΔmucR was significantly reduced at 24 and 48 h (both with p < 0.05) in murine macrophage compared to RM6/66 and CmucR strains.
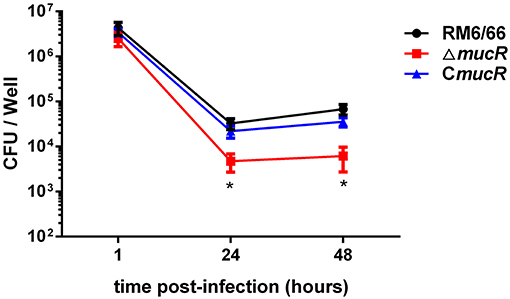
Figure 2. Role of mucR in the intracellular behavior of B. canis RM6/66 in RAW264.7 macrophages. Intracellular survival of B. canis RM6/66, ΔmucR, and CmucR strains in RAW264.7 macrophages. The MOI was 100:1. Data represent the means of three experiments performed in duplicate and error bars indicate the SD. *p < 0.05.
To assess the virulence of ΔmucR strain, the behaviors of ΔmucR and RM6/66 strains in an infected mouse model at 1 and 4 weeks were also analyzed. The spleen weight of the mice infected by the ΔmucR mutant infection group was significantly lighter than that of the RM6/66 infection group at both 1 (p < 0.05) and 4 (p < 0.001) weeks post-infection (Figure 3A). In addition, the CFU of ΔmucR mutant recovered from mice spleens was almost the same as that of RM6/66 at 1-week post-infection. However, the CFU number of mucR mutant recovered from the mice spleens was significantly lower at 4 weeks post-infection (p < 0.01) (Figure 3B).
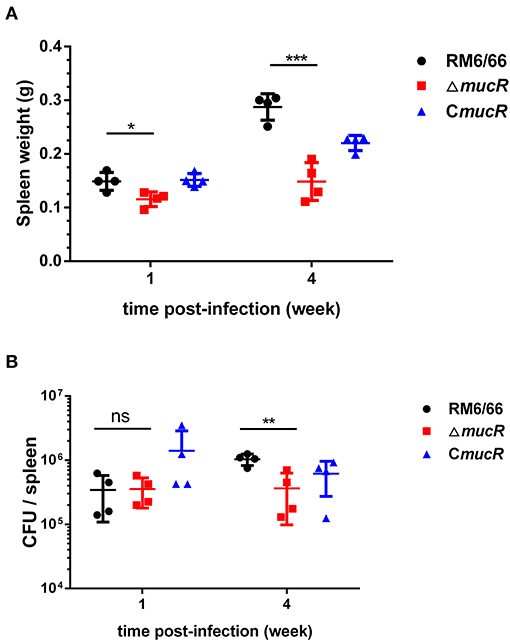
Figure 3. Evaluation of the residual virulence of B. canis RM6/66, ΔmucR and CmucR in BALB/c mice model. (A) Spleen weight of B. canis RM6/66, ΔmucR, and CmucR infected mice at 1 and 4 weeks post-infection. (B) Splenic CFUs of B. canis RM6/66, ΔmucR, and CmucR infected mice at 1 and 4 weeks post-infection. *p < 0.05, **p < 0.01, ***p < 0.001.
As shown in Figure 4A, the survival ratio of ΔmucR strain was not significantly affected in acidic TSB medium compared with RM6/66 strain and the complemented strain CmucR (Figure 4A). When exposed to a hypertonic environment, the survival ratio of ΔmucR was similar to that of the parental strain RM6/66 and CmucR (Figure 4B). However, when ΔmucR strain was cultured at 42°C for 72 h, the survival ratio of the mutant strain was significantly reduced than that of RM6/66 and CmucR (Figure 4B).
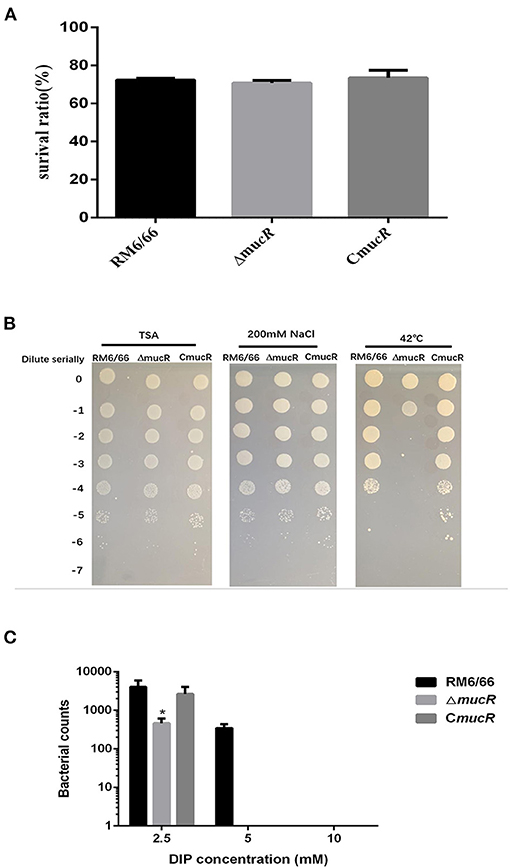
Figure 4. Growth behavior of B. canis RM6/66, ΔmucR, and CmucR strains under various stress conditions. (A) Brucella strains (initial density of 1 ×106 CFU/ml) were grown in TSB (pH 4.5) at 37°C for 2 h. Viable counts were taken 2 h after the challenge. Values represent the mean from three independent experiments performed in duplicate. (B) 5-μl gradient dilution of bacterial cultures were, respectively, cultured on TSA medium at 42°C or TSA medium containing 200 mM NaCl at 37°C for 72 h. These experiments were independently repeated thrice. (C) Survival of B. canis RM6/66, ΔmucR, and CmucR strains in TSB medium containing various concentrations of 2,2-dipyridyl (iron chelator) was determined. The graph represents the average data from at least three independent experiments. Statistical analysis was performed with a t-test.
The growth patterns of ΔmucR, B. canis RM6/66, and the complemented strain in the low iron medium were also measured. In the concentrations evaluated, the presence of 2,2-dipyridyl restricted the growth of all three strains. As compared with B. canis RM6/66, the growth of ΔmucR was significantly reduced at 2.5 mM 2,2-dipyridyl (p < 0.05) and abolished at a higher concentration (Figure 4C).
The susceptibilities of RM6/66, ΔmucR, and CmucR to β-lactam and non-β-lactam drugs were examined using standard procedures. The sensitivity of mucR mutant to Ciprofloxacin, Gentamicin, and Tetracycline was similar to that of the parental strain RM6/66 or CmucR. However, this mutant showed a higher sensitivity to all the three types of β-lactam agents tested. Such enhancement in susceptibility, estimated by MICs, was two-fold compared with its parent strain (Table 1).
Previous studies on the role of MucR in B. melitensis 16M demonstrated that MucR protein could repress the expression of several flagellar genes (11). Thus, the expression of 15 flagellar genes in the mucR mutant strain was analyzed using qRT-PCR. Our results showed that the expression level of eight flagellar genes (ftcR, fliC, flgC, flhA, flgB, fliP, flgK, and fliF) was significantly increased in mucR mutant compared with its parent strain B. canis RM6/66 (Table 2).
To uncover the underlying mechanism of virulence attenuation of ΔmucR strain, changes of the transcriptional profile of gene expression of ΔmucR or RM6/66 were analyzed using transcriptomic analysis. The transcriptional data set was submitted to the Gene Expression Omnibus (GEO) repository (National Center for Biotechnology Information, NCBI) with access number GSE155748. To confirm the RNA-seq data, 12 genes were chosen for qRT-PCR. Transcriptional data of all selected genes gave identical tendencies by both methods RNA-seq and qRT-PCR (Table 3).
In total, 694 genes exhibited significant difference (log2 FC > 1 or < -1 and FDR <0.05). Among these genes, 409 genes were significantly up-regulated and 285 genes were significantly down-regulated in ΔmucR compared to its parent strain RM6/66 (Figures 5A,B). Genes, such as DK60_1986, DK60_2665, DK60_1987, K60_1068, DK60_1531, DK60_1985, DK60_2016, DK60_2832, DK60_1984, and aqpZ2, were among the top 10 up-regulated genes, while DK60_2150, DK60_1727, DK60_895, bfr, DK60_3051, DK60_2926, ffh, DK60_1851, atpC, and DK60_1852 were among the top 10 down-regulated genes.
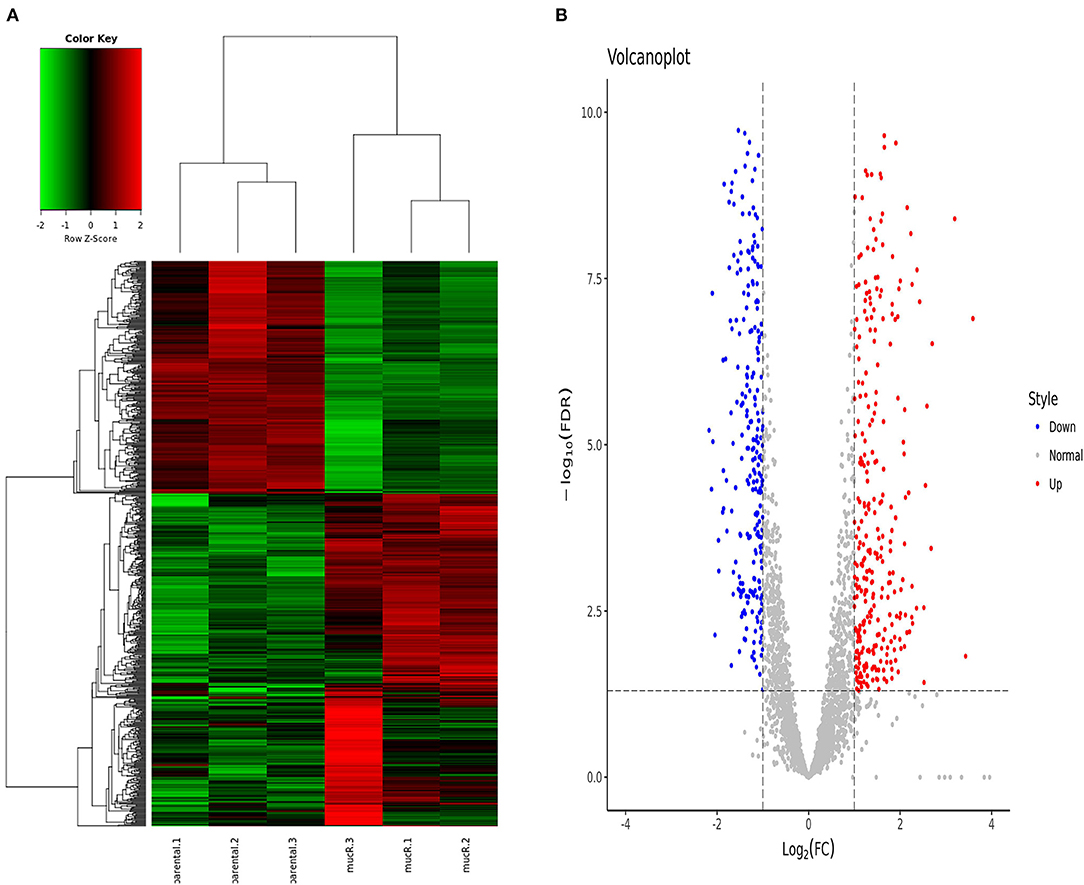
Figure 5. Differentially expressed genes identified by RNA-seq (n = 3 per group). (A) Heat map of the comparative transcriptome analysis of B. canis RM6/66 and ΔmucR. (B) Volcano plot of relative RNA expression from ΔmucR as compared to B. canis RM6/66. Genes in the upper left and right quadrants are significantly differentially expressed.
According to the result of clusters of orthologous genes (COG) analysis, DEGs were mainly involved in translation, ribosomal structure and biogenesis, signal transduction mechanisms, energy production and conversion, intracellular trafficking, secretion and vesicular transport, and extracellular structures (Figure 6A). The Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis demonstrated that the genes which exhibited significant difference were primarily enriched in the ribosome, oxidative phosphorylation, aminoacyl-tRNA biosynthesis, and protein export (Figure 6B).
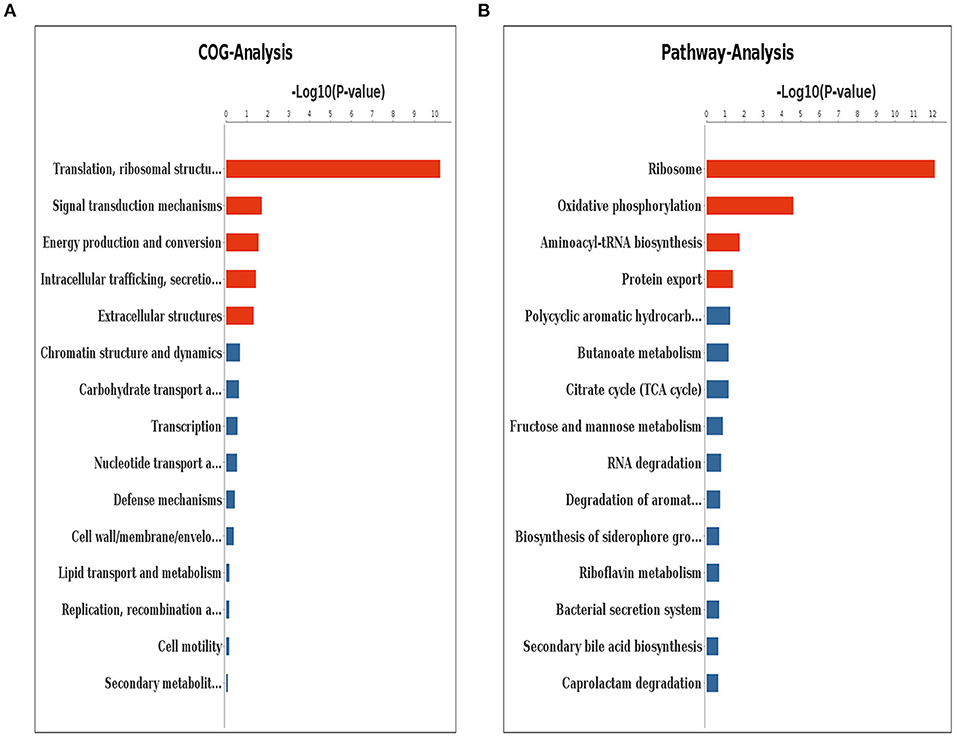
Figure 6. Clusters of orthologous genes (COG) analysis (A) and KEGG pathway analysis (B) for differentially expressed genes. The red columns indicate that these categories or pathways were significantly enriched (p < 0.05), while the blue columns imply that these categories or pathways were not significantly enriched.
In the present study, we report extensive characterization of an in-frame deletion mutant of B. canis mucR for the first time. This gene was previously found to regulate virulence in both cellular and murine models of infection in B. melitensis and B. abortus (11, 13). According to the result of the virulence assay, it was found that the mucR gene was also crucial for B. canis virulence in both the macrophage infection model and the murine infection model.
In previous studies, deletion of mucR gene in B. abortus 2308 and B. melitensis 16M resulted in a significant growth deficiency and mutant strains produced smaller-size colonies when grown on a solid medium (11, 13). However, we found that the growth curve of RM6/66, ΔmucR, and CmucR was almost the same when cultured in a TSB medium with the same initial concentration. Moreover, the sizes of the three B. canis colonies on blood agar had no significant difference.
In smooth-type Brucella strain, MucR protein contributed to the development of resistance to various stress conditions, such as acidic pH, iron-limitation, oxidative stress, cationic peptide, and detergents (9, 11, 13). Thus, we also comprehensively detected the stress response of mucR mutant strain in different conditions in this study. Our results indicated that the mucR mutant was more sensitive to heat stress and iron deficiency. In addition, reduced tolerance to these stress conditions, which might be encountered in the host macrophage, could be possible reasons for the attenuation of the mucR mutant strain.
In B. melitensis, MucR protein was found to be a repressor of flagellar gene expression (11). Based on the RNA-seq data, the expression of four flagellar genes was significantly up-regulated and the result of qRT-PCR also indicated that MucR could repress the transcription of flagellar genes in B. canis. Besides, it was reported that the expression of flagellar genes was tightly regulated by the QS regulator VjbR, RpoE1, and RpoH2 in B. melitensis (17–19). A recent study from our lab indicated that deletion of the QS regulator VjbR also affected the transcription level of the ftcR gene in B. canis (14). Whether, flagellar genes of B. canis were also regulated by RpoE1 or RpoH2 should be further explored.
To further uncover the mechanisms underlying virulence attenuation in the mucR mutant strain, we characterized the transcriptional profile of RM6/66 and ΔmucR by transcriptome analysis and up to 694 genes were found to be differentially expressed over two-fold in the mutant strain. The number of up-regulated genes was more than that of the down-regulated genes, which was consistent with the RNA-seq data in B. melitensis mucR strain (9). It seemed that MucR protein should be a global negative regulator in Brucella strains.
Iron is a necessary element for brucellosis survival (20). Bfr protein played an important role in controlling iron homeostasis, which accounted for about 70% of the intracellular iron content in B. abortus (21). Moreover, the heme transporter BhuA was reported to be related to Brucella virulence both in vitro and in vivo (22). In our study, we found that mucR mutant was more sensitive to iron-limitation stress and the result of RNA-seq in our study also showed that the expression of bfr, bhuA, and DK60_1666 (encoding ferric uptake regulator family protein) was affected. Thus, we speculated that the change in iron-limitation tolerance could also be a result of the abnormal expression of these iron metabolism-related genes.
During bacterial infection pathogen-host interactions expose bacteria to various physiological and biological stresses, including heat stress. To avoid degradation by the host cell defense systems, intracellular pathogens could adapt to changes in their environment by coordinated of gene expression (23). In previous study, the protein synthesis pattern in response to heat stresses were studied by two-dimensional polyacrylamide gel electrophoresis, and the expression of several proteins were increased, such as GroEL, DnaK, AapJ, and Fe and/or Mn SOD (24). In our study, the result of RNA-seq showed that the expression of aapJ, groEL, and DK60_400 (encoding hsp33 family protein) was affected. It seemed that the change in heat stress tolerance might be a result of the abnormal expression of these genes mentioned above.
Beta-lactam antibiotics can interfere with the final stage of cell wall synthesis by modifying activities of enzymes and penicillin-binding proteins (25). Based on RNA-seq results, up to 38 genes involved in cell wall/membrane/envelope biogenesis (COG: M) were found to be differently expressed in mucR deletion strain. Thus, it seemed that the reduced tolerance to β-lactam antibiotics in the mucR mutant should be due to changes in bacterial surface integrity.
The current study indicated that the transcriptional regulator MucR was essential for bacterial resistance to heat stress, iron limitation and antibiotics, as well as bacterial virulence in B. canis. In addition, various genes linked to the phenotype changes of mucR mutant were uncovered using RNA-seq assay. Our study not only reveals the pathogenic mechanism of B. canis but also provided insight into MucR regulon in different Brucella strains.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, GSE155748.
The animal study was reviewed and approved by the Animal Ethics Committee of China Institute of Veterinary Drug Control. The affiliation of this ethics committee was NO.33 Qingfeng Road, Daxing District, Beijing.
HD, YQ, and JD designed the experiments. JS, XP, YL, HJ, YF, and QL performed the experiments. JS and HD prepared the manuscript. YL and LZ analyzed the data. All authors have read and approved the final version of this manuscript.
This work was supported by the National Natural Science Foundation of China (No. 31602055) and the National Key Research and Development Program of China (NO. 2016YFD0500903).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Novel Bioinformatics Ltd., Co for the support of bioinformatics analysis with their Novel Bio Cloud Analysis Platform.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.650942/full#supplementary-material
1. Moore JA, Bennett M. A previously undescribed organism associated with canine abortion. Vet Rec. (1967) 80:604–5. doi: 10.1136/vr.80.20.604
2. Hensel ME, Negron M, Arenas-Gamboa AM. Brucellosis in dogs and public health risk. Emerg Infect Dis. (2018) 24:1401–6. doi: 10.3201/eid2408.171171
3. Chacon-Diaz C, Altamirano-Silva P, Gonzalez-Espinoza G, Medina MC, Alfaro-Alarcon A, Bouza-Mora L, et al. Brucella canis is an intracellular pathogen that induces a lower proinflammatory response than smooth zoonotic counterparts. Infect Immun. (2015) 83:4861–70. doi: 10.1128/IAI.00995-15
4. Stranahan LW, Khalaf OH, Garcia-Gonzalez DG, Arenas-Gamboa AM. Characterization of Brucella canis infection in mice. PLoS ONE. (2019) 14:e0218809. doi: 10.1371/journal.pone.0218809
5. Glowacka P, Zakowska D, Naylor K, Niemcewicz M, Bielawska-Drozd A. Brucella—virulence factors, pathogenesis and treatment. Pol J Microbiol. (2018) 67:151–61.doi: 10.21307/pjm-2018-029
6. Kleinman CL, Sycz G, Bonomi HR, Rodriguez RM, Zorreguieta A, Sieira R. ChIP-seq analysis of the LuxR-type regulator VjbR reveals novel insights into the Brucella virulence gene expression network. Nucleic Acids Res. (2017) 45:5757–69. doi: 10.1093/nar/gkx165
7. Mueller K, Gonzalez JE. Complex regulation of symbiotic functions is coordinated by MucR and quorum sensing in Sinorhizobium meliloti. J Bacteriol. (2011) 193:485–96. doi: 10.1128/JB.01129-10
8. Janczarek M, Kutkowska J, Piersiak T, Skorupska A. Rhizobium leguminosarum bv. trifolii rosR is required for interaction with clover, biofilm formation and adaptation to the environment. BMC Microbiol. (2010) 10:284. doi: 10.1186/1471-2180-10-284
9. Dong H, Liu W, Peng X, Jing Z, Wu Q. The effects of MucR on expression of type IV secretion system, quorum sensing system and stress responses in Brucella melitensis. Vet Microbiol. (2013) 166:535–42. doi: 10.1016/j.vetmic.2013.06.023
10. Arenas-Gamboa AM, Rice-Ficht AC, Kahl-McDonagh MM, Ficht TA. Protective efficacy and safety of Brucella melitensis 16MDeltamucR against intraperitoneal and aerosol challenge in BALB/c mice. Infect Immun. (2011) 79:3653–8. doi: 10.1128/IAI.05330-11
11. Mirabella A, Terwagne M, Zygmunt MS, Cloeckaert A, De Bolle X, Letesson JJ. Brucella melitensis MucR, an orthologue of Sinorhizobium meliloti MucR, is involved in resistance to oxidative, detergent, and saline stresses and cell envelope modifications. J Bacteriol. (2013) 195:453–65. doi: 10.1128/JB.01336-12
12. Borriello G, Russo V, Paradiso R, Riccardi MG, Criscuolo D, Verde G, et al. Different Impacts of MucR binding to the babR and virB promoters on gene expression in Brucella abortus 2308. Biomolecules. (2020) 10:788. doi: 10.3390/biom10050788
13. Caswell CC, Elhassanny AE, Planchin EE, Roux CM, Weeks-Gorospe JN, Ficht TA, et al. Diverse genetic regulon of the virulence-associated transcriptional regulator MucR in Brucella abortus 2308. Infect Immun. (2013) 81:1040–51. doi: 10.1128/IAI.01097-12
14. Liu Y, Sun J, Peng X, Dong H, Qin Y, Shen Q, et al. Deletion of the LuxR-type regulator VjbR in Brucella canis affects expression of type IV secretion system and bacterial virulence, and the mutant strain confers protection against Brucella canis challenge in mice. Microb Pathog. (2020) 139:103865. doi: 10.1016/j.micpath.2019.103865
15. Liu Y, Dong H, Peng X, Gao Q, Jiang H, Xu G, et al. RNA-seq reveals the critical role of Lon protease in stress response and Brucella virulence. Microb Pathog. (2019) 130:112–9. doi: 10.1016/j.micpath.2019.01.010
16. Liu W, Dong H, Liu W, Gao X, Zhang C, Wu Q. OtpR regulated the growth, cell morphology of B. melitensis and tolerance to beta-lactam agents. Vet Microbiol. (2012) 159:90–8. doi: 10.1016/j.vetmic.2012.03.022
17. Delory M, Hallez R, Letesson JJ, De Bolle X. An RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M. J Bacteriol. (2006) 188:7707–10. doi: 10.1128/JB.00644-06
18. Ferooz J, Lemaire J, Delory M, De Bolle X, Letesson JJ. RpoE1, an extracytoplasmic function sigma factor, is a repressor of the flagellar system in Brucella melitensis. Microbiology (Reading). (2011) 157:1263–8. doi: 10.1099/mic.0.044875-0
19. Delrue RM, Deschamps C, Leonard S, Nijskens C, Danese I, Schaus JM, et al. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell Microbiol. (2005) 7:1151–61. doi: 10.1111/j.1462-5822.2005.00543.x
20. Roop RN. Metal acquisition and virulence in Brucella. Anim Health Res Rev. (2012) 13:10–20. doi: 10.1017/S1466252312000047
21. Almiron MA, Ugalde RA. Iron homeostasis in Brucella abortus: the role of bacterioferritin. J Microbiol. (2010) 48:668–73. doi: 10.1007/s12275-010-0145-3
22. Paulley JT, Anderson ES, Roop RN. Brucella abortus requires the heme transporter BhuA for maintenance of chronic infection in BALB/c mice. Infect Immun. (2007) 75:5248–54. doi: 10.1128/IAI.00460-07
23. Murray PJ, Young RA. Stress and immunological recognition in host-pathogen interactions. J Bacteriol. (1992) 174:4193–6. doi: 10.1128/jb.174.13.4193-4196.1992
24. Teixeira-Gomes AP, Cloeckaert A, Zygmunt MS. Characterization of heat, oxidative, and acid stress responses in Brucella melitensis. Infect Immun. (2000) 68:2954. doi: 10.1128/IAI.68.5.2954-2961.2000
Keywords: Brucella canis, MucR, virulence, RNA-Seq, stress responses
Citation: Sun J, Dong H, Peng X, Liu Y, Jiang H, Feng Y, Li Q, Zhu L, Qin Y and Ding J (2021) Deletion of the Transcriptional Regulator MucR in Brucella canis Affects Stress Responses and Bacterial Virulence. Front. Vet. Sci. 8:650942. doi: 10.3389/fvets.2021.650942
Received: 08 January 2021; Accepted: 31 May 2021;
Published: 25 June 2021.
Edited by:
Lester J. Perez, University of Illinois at Urbana–Champaign, United StatesReviewed by:
Huanying Pang, Guangdong Ocean University, ChinaCopyright © 2021 Sun, Dong, Peng, Liu, Jiang, Feng, Li, Zhu, Qin and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuming Qin, cWlueXVtaW5nNzNAMTYzLmNvbQ==; Jiabo Ding, ZGluZ2ppYWJvQDEyNi5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.