- 1Faculty of Bioscience and Aquaculture, Nord University, Bodø, Norway
- 2Department of Pathology, School of Medicine, Case Western Reserve University, Cleveland, OH, United States
- 3Parasitology, Department of Paraclinical Sciences, Faculty of Veterinary Medicine, Norwegian University of Life Sciences, Oslo, Norway
- 4Department of Veterinary Basic and Diagnostic Sciences, College of Veterinary Medicine, Mekelle University, Mekelle, Ethiopia
Goats are a primary or additional income source for many families in resource-poor areas. Although often considered inferior to other livestock, the resilience of goats and their ability to thrive in a range of environments means that that they are of particular value. Furthermore, goats emit less methane than other livestock species. In these same areas, it is well-documented that cryptosporidiosis has a substantial impact on infant morbidity and mortality, as well as reducing child growth and development. As Cryptosporidium also causes diarrheal disease in goats, the question arises whether goats may represent a reservoir of infection to humans. Epidemiological studies regarding the potential for transmission of Cryptosporidium between goats and humans have largely concluded that Cryptosporidium species infecting goats are not zoonotic. However, these studies are mostly from developed countries, where goat husbandry is smaller, management routines differ greatly from those of developing countries, contact between goats and their owners is more limited, and cryptosporidiosis has less impact on human health. In this article, background information on goat husbandry in different countries is provided, along with information on Cryptosporidium prevalence among goats, at both the species and sub-species levels, and the potential for zoonotic transmission. The intention is to indicate data gaps that should be filled and to increase awareness of the role of goats as providers for low-income families, often living in areas where cryptosporidiosis is endemic and where appropriate baseline interventions could have a positive impact, regardless of species of goat or parasite.
Introduction
Goats are one of the species of livestock that were domesticated earliest, and are used worldwide for milk, meat, and hair/skin. Nowadays, goats are among the most popular and beneficial livestock for those with limited resources (1). Small-scale goat production is of considerable benefit to families and communities globally, in a variety of climates and conditions.
A landmark paper from 2005, “Goats – pathway out of poverty,” argued that goats are worthy of serious investment, with the potential for transforming the lives of some of the world's poorest people (2). Even under extreme climate conditions, goats have several characteristics that enable their capacity to convert feed into milk and meat (3).
In a world where our future is increasingly dominated by adaptation to climate change, goat-keeping is emerging as a truly important husbandry, not only for maintaining production levels, but also due to its relatively minor impact on climate as goats emit less methane than other livestock (4). There are about one billion goats worldwide, and the global goat population has more than doubled during the last four decades. According to the Food and Agriculture Organization, over 90% of goats are found in developing countries; Asia has the largest proportion of the world's goat population, followed by Africa (5).
Goats are traditionally managed differently to cattle, with flocks grazing in expansive enclosures or not enclosed at all, rather than being kept indoors. Goats are also popular as backyard livestock for hard-pressed families with few resources since livestock accounts for up to 60% of their income (1). In these settings, barriers against animal-human-animal transmission of zoonotic diseases are weakened. Thus, in promoting and supporting goat farming, it is important that efforts are also made to ensure that transfer of pathogens between goats and their owners is minimized.
Where Are the Goats, and Who Keeps Them?
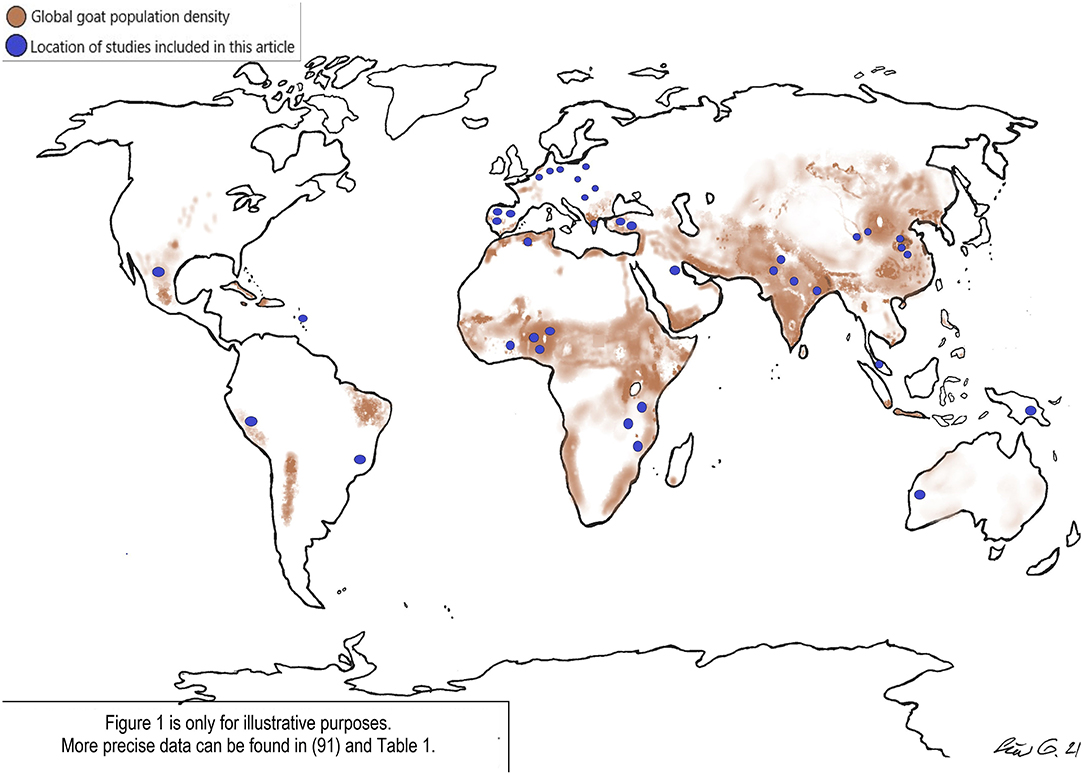
Over two-thirds of goats can be found in subtropical and tropical countries [(6); Figure 1].
In low-income countries of e.g., Asia, Africa, and Latin-America, locally adapted goat breeds are raised for milk and meat, and in dry and drought-prone areas, goat milk is often the only protein source in children's diets (7). In countries where the majority of goats are found, most goat owners belong to the lower socioeconomic strata (8–12), and, in rural areas, goats are largely managed by women and children (13, 14).
The International Livestock Research Institute recognized that goats are more important than cattle to the livelihoods of the rural poor, so investments in goat health, productivity, and sales may greatly assist with poverty alleviation.
The Cultures of Goat Keeping in Asia and Africa
Where extensive grazing is a main source of livelihood, goats have become an essential aspect of culture, social life, and even, in some places, religion, as goat meat is acceptable according to most scriptures.
Asia has identified the dairy-goat husbandry as especially sustainable in the face of climate change, and investments in several dairy-goat projects have been made during the past decade (7).
In India, for example, domesticated goats account for 20% of the global goat population (15) and goats remain a vital, but under-resourced and denigrated, part of the economy (7). Goats are an integral component in Indian livelihoods, contributing significantly to the income and socio-economic structure of rural farmers, and are often referred to as “the poor man's cow” (16). Goats are a reliable livelihood source in a range of Indian terrains, from deserts to coastal areas and high altitudes. However, unlike other sectors of Indian animal husbandry, the goat-meat industry is relatively disorganized, and abattoirs are usually unsuitable for goat slaughter.
Furthermore, goat husbandry in India takes place under federally unchecked conditions, particularly in rural areas. Regulatory bodies associated with commercial livestock rearing are lacking and most veterinary services inadequate, focusing on treatment rather than preventive measures (17), and gastrointestinal parasitism is prevalent in goats from all areas of India, representing a major health issue (18–20).
Africa holds over 40% of the global goat population, and over 60% are found in sub-Saharan countries. However, in contrast to Asia's relatively positive outlook on goat husbandry, goats are often associated with “backwardness” and “environmental destruction,” by government officials in Africa, making it difficult to gain their investment support (7).
Nevertheless, goats play a major role as a source of food and income, accounting for 30% of Africa's ruminant livestock and producing 17 and 12% of its meat and milk (13). Production systems vary, including smallholder mixed crop-livestock systems, smallholder intensive systems, extensive pastoral and transhumance systems, and large-scale ranching systems (14, 21). Goats in Africa usually graze freely, scavenging feed resources where available, and, during the cropping season, forage for crop residues. The limited management and reliance on children for care and welfare probably exacerbates the low meat and milk production per goat. In urban areas, goats may graze common ground, which is often contaminated and used as a communal latrine, or may be held in stalls and fed at home (14). However, in some parts of East-Africa, there are extensive pastoral and transhumance systems, where goats are reared in large numbers and occupy 50% of the region (22).
Cryptosporidium: an Overview
Cryptosporidium is an intestinal protozoan parasite with a worldwide distribution, a fecal-oral lifecycle, and is generally associated with diarrheal disease. It has a direct lifecycle in which the robust infectious oocyst stages are excreted with the feces into the environment and are immediately infectious for the next susceptible host.
Effects of Cryptosporidium on Goat Health
Cryptosporidium infection has an impact on growth and production in goats, and has been found to cause anorexia and diarrhea in goat kids, with morbidity and mortality reaching 50 and 100%, respectively (23–27), with accompanying economic consequences, impacting especially marginal farmers. Reduced growth, with and without diarrhea, has also been associated with Cryptosporidium infections in goats aged between 9 and 15 months, including in asymptomatic goats, raising further questions regarding long-term effects of apparently asymptomatic infections (28). Some studies have reported asymptomatic shedding of Cryptosporidium oocysts in adult goats (29, 30), but the long-term effects of chronic asymptomatic infections remain unclear, and goat health protocols recommended screening for Cryptosporidium infections after weaning, even in the absence of diarrhea (28).
Effects of Cryptosporidium on Human Health
Although Cryptosporidium has a global distribution, its impact on human health is greatest in developing countries where diarrheal disease exerts a huge health burden. Although global health is steadily improving, diarrheal disease remained the third most common cause of disability-adjusted life-years (DALYs) in the under-10 years age group in 2019 (31).
Given the high prevalence of cryptosporidiosis in people in resource-poor areas, this pathogen was included in the WHO “neglected disease initiative” in 2004 (32).
Cryptosporidium infection is particularly associated with pediatric diarrhea (33), but tends to be less important as a diarrheal pathogen in older age groups. A considerable mortality burden from cryptosporidiosis in children younger than 5-years (7.6 deaths per 100,000) has been reported (34), probably reflecting that cryptosporidiosis is acute and the explosive, voluminous diarrhea likely to have a major and immediate impact on infant survival. In addition, Cryptosporidium damages cells of the intestine and reduces absorption of nutrients. A meta-analysis suggested that the true burden of cryptosporidiosis was probably underestimated in previous reports, as effects subsequent to the acute phase of infection (decreased growth and enhanced risk of subsequent infections) were not included (35).
Diagnostic Methods
There are no techniques particularly for diagnosis of Cryptosporidium infection in goats, although various procedures are available. Staining techniques are often applied in studies investigating prevalence, and molecular techniques provide information regarding species and subtype. Choice of diagnostic technique depends on available equipment and reagents, analyst experience, and time and cost of analysis. Molecular methods are usually not a routine diagnostic in resource-poor settings, but sensitive and specific diagnostic methods are important everywhere, particularly when positive findings result in appropriate interventions such as improved hygiene and better farm management, both of which can be essential for disease control and prevention in both goats and humans. A recently published study indicated that auramine-phenol staining has high sensitivity and specificity for cryptosporidiosis and can be easily integrated with existing laboratory infrastructures in low-resource settings (36). Targeted sampling and preparation before diagnostics, along with dual application of staining and molecular techniques may provide the best possible results in terms of prevalence and epidemiology investigations.
Molecular aspects
Molecular tools have changed our understanding of Cryptosporidium spp. transmission. Genotyping and subtyping data have clearly demonstrated the presence of anthroponotic, as well as zoonotic, Cryptosporidium species in humans in industrialized nations. In contrast, transmission of cryptosporidiosis appears largely anthroponotic in some developing countries; for example, in Africa, despite frequent close contact between humans and animals, transmission appears to be mainly anthroponotic, and human Cryptosporidium infection is most often with C. hominis or C. parvum anthroponosum (37).
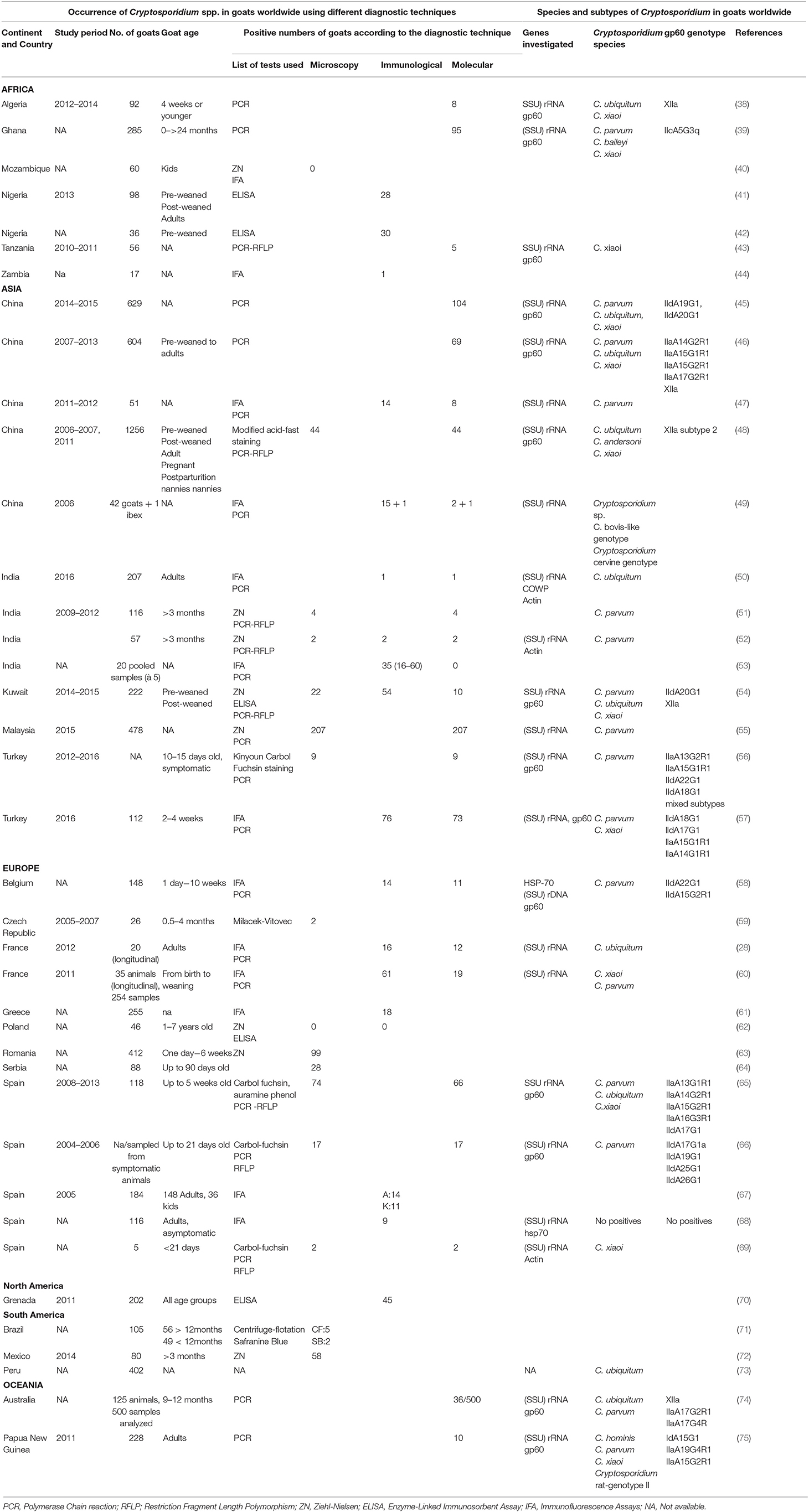
Nevertheless, as many Cryptosporidium species infect both humans and goats there is clearly the potential for transmission between the two host species. In the overview below, our focus remains on the most common zoonotic types. Details of studies are provided in Table 1, and the location of studies as related to goat distribution is shown in Figure 1.
C. parvum is perhaps the most studied zoonotic Cryptosporidium species. In studies from China in which C. parvum infectons from goats were diagnosed and the subtypes determined, the IId-subtype was found (not exclusively) in all investigations. C. parvum IId-subtypes seem to have a unique distribution in China, being predominant in C. parvum infections in humans, farm animals, and rodents (76–79). The IId-subtype has also been detected in goats in Europe, Asia, and Oceania (Table 1). However, the role of the rodent host, potentially an additional endemic amplifier, remains unknown in these areas.
In Africa, human C. parvum infections are dominated by the Iic-subtype, and the role of goats in transmission remains largely unknown. Although a study from Ghana reported finding the Iic-subtype in a goat, non-zoonotic, C. xiaoi dominated among goats kept in or around households (80). As far as we know, this is the only study where the Iic-subtype has been found in a goat.
The IIa-subtype seems to be present in C. parvum infections in goats in many parts of the world, having been reported from all continents except Africa, and, to date, publications investigating C. parvum subtypes in goats in North- and South-America are lacking.
C. ubiquitum has been detected in goats in studies from Europe, Asia, Africa, South America and Oceania (Table 1); in studies where subtyping has been conducted, only the subtype-XIIa was found. This subtype seems to predominate in ruminants, and humans are susceptible hosts for subtypes XIIa–XIId (81). C. ubiquitum is the most common species found in drinking water in rural USA, and human infections with this species has been detected mostly in developed countries, possibly due to the lower background of anthroponotic infections that predominate in developing countries (82), C. ubiquitum has been detected in feces from more animal species, and over a greater geographic range, than most Cryptosporidium species – with the exception of C. parvum (80). This distribution facilitates establishment of life cycles in extensive farming, where susceptible host animals are likely to be present and the infection barrier is weak. Data on clinical signs is scant, although this species has been identified in many cases of human cryptosporidiosis (81) and it has been isolated from diarrheic goat kids in Spain (65). A French study also found a periparturient rise in C. ubiquitum oocyst shedding from asymptomatic nanny goats (29). Although genotype analysis of C. ubiquitum has not been extensively performed, this species may represent a greater threat to both humans and animals given its ability to infect its next host, be it humans or their livestock.
Epidemiological Evidence for Sharing of Cryptosporidium Between Goats and Their Keepers
It is well known that younger animals, and people, are at greatest risk of Cryptosporidium infection, and are most likely to develop symptomatic disease if infected. Other epidemiological aspects are concerned with routes of exposure, and geographical, meteorological, cultural, and other environmental factors that may affect transmission patterns. Of interest regarding epidemiological pressures for interspecies transmission between goats and people, is looking at where zoonotic transmission from goats to humans has been documented. Although we know that the brunt of the global cryptosporidiosis burdens is borne by populations in Africa, Asia, and Latin America, it is difficult to recognize specific transmission occasions or outbreaks in these countries due to the high prevalence of infections. In other countries, however, outbreaks can be recognized, and some have been associated with direct or indirect contact with goats and their products. For example, an outbreak of cryptosporidiosis in USA was associated with consumption of unpasteurized goat milk (83) and an outbreak of cryptosporidiosis among school children in Norway was associated with contact with lambs and goat kids at a holiday farm, where the same sub-type of C. parvum (IIaA19G1R1) was found in both children, lambs, and goat kids (84). It is also noteworthy that in all studies from Table 1 where the species of Cryptosporidium was identified, zoonotic species were detected in all investigations except two.
Of particular relevance regarding goats and Cryptosporidium regarding human health, is that in those countries where cryptosporidiosis exerts a particular burden, it is, as previously outlined, children who are most affected; and it is also children who most often have the job of looking after goats in these same regions of the world. The grazing habits of goats, generally browsing on woody shrubs and weeds rather than grazing grass, may indicate that they are less likely to ingest parasites (85). However, in many settings, particularly poor urban or peri-urban areas, where shrubs are scant, they will be forced to search for nutrients closer to the ground. When foraging these scarce food resources on the ground, goats may be more likely to ingest Cryptosporidium spp. oocysts contaminating the environment, possibly shed by the human kid tending the goats, or from the goat kid foraging beside it. Similarly, children tending a flock of goat kids are likely to be exposed to parasite transmission stages in goat feces. In the cooler climates of temperate regions bovines, particularly calves, are often considered a source of zoonotic transmission of Cryptosporidium; in other global regions, it seems possible that goats may be an even more likely source.
Prevention and Control
Persistent diarrhea seriously affects nutritional status, growth, and intellectual function. Meeting these challenges is profoundly important, particularly in developing countries. Cryptosporidium oocysts have high infectivity, robustness, and resistance to disinfectants, which underscores the need for improved treatment options. No safe and effective treatment for cryptosporidiosis has been identified to date, although efforts to direct resources toward this objective continue to be made (86). Although C. hominis apparently still predominates in many settings, zoonotic transmission should not be neglected. In line with the One Health initiative, general rules of hygiene barriers between and among humans and animals in any setting should be implemented and thus reduce infection risks, not only of Cryptosporidium, but other zoonotic pathogens as well. As children and women are often responsible for tending backyard livestock, and also usually prepare food and/or fetch water, focusing on this group in hygiene training and information dissemination could improve the wellbeing of both them and their goats beyond their backyard. Studies that focus particularly on the likelihood of transmission of Cryptosporidium between goats and their keepers may provide more specific information on where interventions should be targeted, without losing the value from goat-keeping as an important resource for lifting families and communities out of poverty.
Goats Are Saving the World
Organizations like Heifer International have helped small-scale farmers to obtain and benefit from goats in widely ranging situations, including in the dry forest areas of Peru, landless women in India, tropical forest areas of West-Africa, farmers in peri-urban areas St. Petersburg, the densely populated highlands of East-Africa, as well as the Sichuan province in China. Most of these goats are kept in small flocks of 3–10 animals, and are mainly cared for by children and women. Women have a significant role in goat-keeping in rural areas, enabling them to contribute substantially to the household economy (87).
In a resource-poor region of northern India, goat prices almost doubled when low-cost shelters, feeders, and water sheds were provided, in addition to improved breeding practices and prophylactic measures (7). Other development projects with goat interventions have given a positive return rate for both small- and large-scale goat-keepers in both Africa and South America, which, in turn, increased their income substantially (88). A zero-grazing management practice has often been introduced, which involves keeping goats in pens with limited outdoor space for exercise and all feed being brought to them. Manure is collected and either composted or applied to crops (89). This system has proven very successful in disease control, breeding management, and goat-rearing integration, including better protection of natural resources (90). However, the application of manure to crops might impose potential health risks and appropriate measures to protect both farmers and ensure safe produce should be taken into consideration.
The socio-economic status of farmers plays a major role in flock size and adoption of scientific management practices for goat rearing, which thereby raises income and socio-economic level of the owner, and particularly benefits socio-economically deprived women.
Conclusion
Cryptosporidiosis is an important diarrheal illness; in people in developing countries it exerts a substantial burden on child health, growth, and development (35) and in ruminant livestock, including goats, it affects growth and production (28). With goats an important livestock species for under-resourced communities, it is important to ensure that this potential reservoir of zoonotic Cryptosporidium is addressed and managed, and research needs to be conducted in the relevant regions.
The One Health initiative, focusing on reducing disease interface between humans and animals in areas where infection risk is greatest, could be harnessed to reduce health burdens and economic challenges where most needed. This depends largely on local endemic status and appropriate interventions. Studies on prevalence and species/genotypes of Cryptosporidium infecting people in developing countries are extensive, but there are considerably fewer of such investigations among domestic livestock. More information provided through further epidemiological studies on the species of Cryptosporidium infecting livestock and humans in these regions will fill data gaps and may assist in pinpointing relevant approaches to minimizing transmission. Goat-keeping is often a trade for the poorest in society, and awareness of proper hygienic routines and appropriate animal management strategies could benefit both human and animal health, as well as improving the economy and welfare of the goat-keepers and their herds.
Author Contributions
KU conceived the study and wrote the main bulk of the manuscript. SC and TK contributed significantly with local knowledge regarding both epidemiology and animal husbandry in the manuscript. LJR structured and contributed to all parts of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Line Kristin Grendal (@linemakesart) for her artistic contributions to the figure in this article.
References
1. Devendra C. Small ruminants in Asia; contribution to food security, poverty alleviation and opportunities for productivity enhancement. In: Proceeding of International Workshop on Small Ruminant Production and Development in South East Asia. Vietnam: MEKARN, Nong Lam, HCMC (2005). p. 19–32.
2. Peacock C. Goats—A pathway out of poverty. Small Rumin Res. (2005) 60:179–86. doi: 10.1016/j.smallrumres.2005.06.011
3. Silanikove N. The physiological basis of adaptation in goats to harsh environments. Small Rumin Res. (2000) 35:181–93. doi: 10.1016/S0921-4488(99)00096-6
4. Darcan NK, Silanikove N. The advantages of goats for future adaptation to climate change: a conceptual overview. Small Ruminant Research. (2018) 163:34–8. doi: 10.1016/j.smallrumres.2017.04.013
5. FAO Live Animals Goats World Total Africa Total America Total Asia Total Europe Total Oceania Total Year 1980 1990 2000 2010 and 2018 QA “Live Animals” “5000” “World” “5111” “Stocks” “1016” “Goats” “1990” “1990” “Head” ‘ ‘588817562” “A” “Aggregate may include official semi-official estimated or calculated data”. Available online at: http://www.fao.org/faostat/en/#data/QA (accessed December 31, 2020).
7. Miller BA, Lu CD. Current status of global dairy goat production: an overview. Asian Austr J Anim Sci. (2019) 32:1219–32. doi: 10.5713/ajas.19.0253
8. Singh M, Dixit A, Roy A, Singh S. Goat rearing: a pathway for sustainable livelihood security in Bundelkhand region. Agric Econo Res Rev. (2013) 26:79–88. doi: 10.22004/ag.econ.158491
9. Neupane N, Neupane H, Dhital B. A socioeconomic view of status and prospects of goat farming in rural areas of Nepal. J Inst Agric Anim Sci. (2018) 35:1–8. doi: 10.3126/jiaas.v35i1.22508
10. Ngambi JW, Alabi QJ, Norris D. Role of goats in food security; poverty alleviation and prosperity with special reference to sub-saharan africa: a review. Indian J Anim Res. (2013) 47:1–9.
11. Islam MA, Hossain MN, Chokraborti SS, Rahman S, Tasnim A, Al Zabir A. Socio-economic profile of goat rearing farmers and their management practices in Sylhet, Bangladesh. J Agric Ecol Res Int. (2018) 15:1–10. doi: 10.9734/JAERI/2018/43818
12. Byaruhanga C, Oluka J, Olinga S. Socio-economic aspects of goat production in a rural agro-pastoral system of Uganda. Crops. (2015) 114:105. doi: 10.13189/ujar.2015.030604
13. Lebbie SHB. Goats under household conditions. Small Rumin Res. (2004) 51:131–6. doi: 10.1016/j.smallrumres.2003.08.015
14. Muigai AWT, Okeyo AM, Ojango JMK. Goat production in eastern Africa: practices, breed characteristics, and opportunities for their sustainability. In: Sustainable Goat Production in Adverse Environments: Volume I. Cham: Springer International Publishing (2017). p. 31–57.
15. Joshi MB, Rout PK, Mandal AK, Tyler-Smith C, Singh L, Thangaraj K. Phylogeography and origin of Indian domestic goats. Mol Biol Evol. (2004) 21:454–62. doi: 10.1093/molbev/msh038
16. MacHugh DE, Bradley DG. Livestock genetic origins: goats buck the trend. Proc Natl Acad Sci USA. (2001) 98:5382–4. doi: 10.1073/pnas.111163198
17. Kumar S, Vihan VS, Deoghare PR. Economic implication of diseases in goats in India with reference to implementation of a health plan calendar. Small Rumin Res. (2003) 47:159–64. doi: 10.1016/S0921-4488(02)00237-7
18. Jithendran KP, Bhat TK. Epidemiology of parasitoses in dairy animals in the North West Humid Himalayan Region of India with particular reference to gastrointestinal nematodes. Trop Anim Health Prod. (1999) 31:205–14. doi: 10.1023/A:1005263009921
19. Choubisa SL, Jaroli VJ. Gastrointestinal parasitic infection in diverse species of domestic ruminants inhabiting tribal rural areas of southern Rajasthan, India. J Parasit Dis. (2013) 37:271–5. doi: 10.1007/s12639-012-0178-0
20. Singh AK, Das G, Roy B, Nath S, Naresh R, Kumar S. Prevalence of gastro-intestinal parasitic infections in goat of Madhya Pradesh, India. J Parasit Dis. (2015) 39:716–9. doi: 10.1007/s12639-014-0420-z
21. Pamo TE, Boukila B, Tendonkeng F. Goat production research in Africa: a sign post review for research in the new millennium. Int J Biol Sci. (2007) 1:76–89. doi: 10.4314/ijbcs.v1i1.39702
22. Muigai AW, Okeyo AM, Ojango JM. Goat production in Eastern Africa: Practices, Breed Characteristics, and Opportunities for their Sustainability. Cham: Springer (2017). p. 31–57.
23. Santín M. Clinical and subclinical infections with Cryptosporidium in animals. N Z Vet J. (2013) 61:1–10. doi: 10.1080/00480169.2012.731681
24. Thompson RCA, Palmer CS, O'Handley R. The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Vet J. (2008) 177:18–25. doi: 10.1016/j.tvjl.2007.09.022
25. Thamsborg SM, Jørgensen RJ, Henriksen SA. Cryptosporidiosis in kids of dairy goats. Vet Rec. (1990) 127:627–8.
26. Sevinç F, Simşek ATILLA, Uslu U. Massive Cryptosporidium parvum infection associated with an outbreak of diarrhoea in neonatal goat kids. Turkish J Vet Anim Sci. (2006) 29. 6:1317–20. Available online at: https://dergipark.org.tr/en/pub/tbtkveterinary
27. Vieira LS, Silva MB, Tolentino AC, Lima JD, Silva AC. Outbreak of cryptosporidiosis in dairy goats in Brazil. Vet Rec. (1997) 140:427–8. doi: 10.1136/vr.140.16.427
28. Jacobson C, Al-Habsi K, Ryan U, Williams A, Anderson F, Yang R, et al. Cryptosporidium infection is associated with reduced growth and diarrhoea in goats beyond weaning. Vet Parasitol. (2018) 260:30–7. doi: 10.1016/j.vetpar.2018.07.005
29. Paraud C, Pors I, Rieux A, Brunet S. High excretion of Cryptosporidium ubiquitum by peri-parturient goats in one flock in western France. Vet Parasitol. (2014) 202:301–4. doi: 10.1016/j.vetpar.2014.03.024
30. Castro-Hermida JA, Delafosse A, Pors I, Ares-Mazás E, Chartier C. Giardia duodenalis and Cryptosporidium parvum infections in adult goats and their implications for neonatal kids. Vet Rec. (2005) 157:623–7. doi: 10.1136/vr.157.20.623
31. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
32. Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the “Neglected diseases initiative.” Trends Parasitol. (2006) 22:203–8. doi: 10.1016/j.pt.2006.02.015
33. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet. (2013) 382:209–22. doi: 10.1016/S0140-6736(13)60844-2
34. GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. (2018) 18:1211–28. doi: 10.1016/S1473-3099(18)30362-1
35. Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health. (2018) 6:e758–68. doi: 10.1016/S2214-109X(18)30283-3
36. Johansen ØH, Abdissa A, Zangenberg M, Mekonnen Z, Eshetu B, Bjørang O, et al. Performance and operational feasibility of two diagnostic tests for cryptosporidiosis in children (CRYPTO-POC): a clinical, prospective, diagnostic accuracy study. Lancet Infect Dis. (2020). doi: 10.1016/S1473-3099(20)30556-9. [Epub ahead of print].
37. Robertson LJ, Johansen ØH, Kifleyohannes T, Efunshile AM, Terefe G. Cryptosporidium infections in Africa—how important is zoonotic transmission? A review of the evidence. Front Vet Sci. (2020) 7:575881. doi: 10.3389/fvets.2020.575881
38. Baroudi D, Hakem A, Adamu H, Amer S, Khelef D, Adjou K, et al. Zoonotic Cryptosporidium species and subtypes in lambs and goat kids in Algeria. Parasit Vectors. (2018) 11:582. doi: 10.1186/s13071-018-3172-2
39. Squire SA, Yang R, Robertson I, Ayi I, Ryan U. Molecular characterization of Cryptosporidium and Giardia in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Infect Genet Evol. (2017) 55:236–43. doi: 10.1016/j.meegid.2017.09.025
40. Miambo RD, Laitela B, Malatji MP, De Santana Afonso SM, Junior AP, Lindh J, et al. Prevalence of Giardia and Cryptosporidium in young livestock and dogs in Magude District of Maputo Province, Mozambique. Onderstepoort J Vet Res. (2019) 86:e1–6. doi: 10.4102/ojvr.v86i1.1709
41. Akinkuotu OA, Okwelum N, Famakinde SA, Akinkuotu AC. Prevalence of Cryptosporidium infection in recently acclimatized Kalahari red goats in Nigeria. Vom J Vet Sci. (2016) 11:112–116. Available online at: https://www.ejmanager.com/my/vjvs/submit.php?lng=
42. Ambrose AO, Olakunle FB. Cryptosporidium Infection in Pre-Weaned Ruminants and Pigs in Southwestern Nigeria (2014). Framingham, MA: Global Journal of Medical Research G: Veterinary Science & Veterinary Medicine.
43. PLOS Neglected Tropical Diseases Staff. Correction: epidemiology and molecular characterization of Cryptosporidium spp. in humans, wild primates, and domesticated animals in the Greater Gombe Ecosystem, Tanzania. PLoS Negl Trop Dis. (2015) 9:e0003650. doi: 10.1371/journal.pntd.0003650
44. Siwila J, Phiri IGK, Enemark HI, Nchito M, Olsen A. Occurrence of Cryptosporidium and Giardia in domestic animals in peri-urban communities of Kafue district, Zambia. Tanzania Vet J. (2013) 28:49–59. doi: 10.4314/tvj.v28i0
45. Peng X-Q, Tian G-R, Ren G-J, Yu Z-Q, Lok JB, Zhang L-X, et al. Infection rate of Giardia duodenalis, Cryptosporidium spp. and Enterocytozoon bieneusi in cashmere, dairy and meat goats in China. Infect Genet Evol. (2016) 41:26–31. doi: 10.1016/j.meegid.2016.03.021
46. Mi R, Wang X, Huang Y, Zhou P, Liu Y, Chen Y, et al. Prevalence and molecular characterization of Cryptosporidium in goats across four provincial level areas in China. PLoS ONE. (2014) 9:e111164. doi: 10.1371/journal.pone.0111164
47. Ma L, Sotiriadou I, Cai Q, Karanis G, Wang G, Wang G, et al. Detection of Cryptosporidium and Giardia in agricultural and water environments in the Qinghai area of China by IFT and PCR. Parasitol Res. (2014) 113:3177–84. doi: 10.1007/s00436-014-3979-5
48. Wang R, Li G, Cui B, Huang J, Cui Z, Zhang S, et al. Prevalence, molecular characterization and zoonotic potential of Cryptosporidium spp. in goats in Henan and Chongqing, China. Exp Parasitol. (2014) 142:11–6. doi: 10.1016/j.exppara.2014.04.001
49. Karanis P, Plutzer J, Halim NA, Igori K, Nagasawa H, Ongerth J, et al. Molecular characterization of Cryptosporidium from animal sources in Qinghai province of China. Parasitol Res. (2007) 101:1575–80. doi: 10.1007/s00436-007-0681-x
50. Utaaker KS, Myhr N, Bajwa RS, Joshi H, Kumar A, Robertson LJ. Correction to: goats in the city: prevalence of Giardia duodenalis and Cryptosporidium spp. in extensively reared goats in northern India. Acta Vet Scand. (2018) 60:52. doi: 10.1186/s13028-018-0402-8
51. Maurya PS, Rakesh RL, Pradeep B, Kumar S, Kundu K, Garg R, et al. Prevalence and risk factors associated with Cryptosporidium spp. infection in young domestic livestock in India. Trop Anim Health Prod. (2013) 45:941–6. doi: 10.1007/s11250-012-0311-1
52. Rakesh RL, Banerjee PS, Garg R, Maurya PS, Kundu K, Jacob SS, et al. Genotyping of Cryptosporidium spp. isolated from young domestic ruminants in some targeted areas of India. Indian J Ani Sci. (2014) 84:819–23.
53. Daniels ME, Shrivastava A, Smith WA, Sahu P, Odagiri M, Misra PR, et al. Cryptosporidium and Giardia in humans, domestic animals, and village water sources in rural India. Am J Trop Med Hyg. (2015) 93:596–600. doi: 10.4269/ajtmh.15-0111
54. Majeed QAH, El-Azazy OME, Abdou N-EMI, Al-Aal ZA, El-Kabbany AI, Tahrani LMA, et al. Epidemiological observations on cryptosporidiosis and molecular characterization of Cryptosporidium spp. in sheep and goats in Kuwait. Parasitol Res. (2018) 117:1631–6. doi: 10.1007/s00436-018-5847-1
55. Mat Yusof A, Hashim N, Md Isa ML. First molecular identification of Cryptosporidium by 18S rRNA in goats and association with farm management in Terengganu. Asian Pac J Trop Biomed. (2017) 7:385–8. doi: 10.1016/j.apjtb.2017.01.008
56. Taylan-Ozkan A, Yasa-Duru S, Usluca S, Lysen C, Ye J, Roellig DM, et al. Cryptosporidium species and Cryptosporidium parvum subtypes in dairy calves and goat kids reared under traditional farming systems in Turkey. Exp Parasitol. (2016) 170:16–20. doi: 10.1016/j.exppara.2016.06.014
57. Ipek DS. Prevalence and molecular characterisation of Cryptosporidium spp. in diarrhoeic pre-weaned goat kids reared under traditional farming system in Diyarbakir, Southeastern Anatolia City, Turkey. Rev Med Vet. (2017) 168:229–34. Available online at: https://www.scimagojr.com/journalsearch.php?tip=sid&q=18865
58. Geurden T, Thomas P, Casaert S, Vercruysse J, Claerebout E. Prevalence and molecular characterisation of Cryptosporidium and Giardia in lambs and goat kids in Belgium. Vet Parasitol. (2008) 155:142–5. doi: 10.1016/j.vetpar.2008.05.002
59. Strnadová P, Svobodová V, Vernerova E. Protozoální infekce jehnat a kuzlat na farmách v Ceské republice P, Protozoal infections occuring in farms in the Czech Republic (in Czech). Veterinárství. (2008) 58:451–8. Available online at: http://vetweb.cz/protozoalni-infekce-jehnat-a-kuzlat-na-farmach-v-ceske-republice/
60. Rieux A, Paraud C, Pors I, Chartier C. Molecular characterization of Cryptosporidium spp. in pre-weaned kids in a dairy goat farm in western France. Vet Parasitol. (2013) 192:268–72. doi: 10.1016/j.vetpar.2012.11.008
61. Tzanidakis N, Sotiraki S, Claerebout E, Ehsan A, Voutzourakis N, Kostopoulou D, et al. Occurrence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in sheep and goats reared under dairy husbandry systems in Greece. Parasite. (2014) 21:45. doi: 10.1051/parasite/2014048
62. Majewska AC, Werner A, Sulima P, Luty T. Prevalence of Cryptosporidium in sheep and goats bred on five farms in west-central region of Poland. Vet Parasitol. (2000) 89:269–75. doi: 10.1016/S0304-4017(00)00212-0
63. Bejan AL. Criptosporidioza viteilor si izeilor: cercetari privind diagnosticul, epidemiologia si etiopatogeneza, Cryptosporidiosis in calves and goat kids: research concerning diagnosis, epidemiology, and etiopathogenesis (In Romanian). Dissertation. Universitatea de Ştiinţe Agricole i Medicină Veterinară Cluj-Napoca (2009). Available online at: http://usamvcluj.ro/files/teze/bejan.pdf (accessed February 18, 2021).
64. Mišić ZB, Katić-Radivojević SP, Kulišić Z. Cryptosporidium infection in lambs and goat kids in Serbia. Acta Vet. (2006) 56:49–54. doi: 10.2298/AVB0601049M
65. Díaz P, Quílez J, Prieto A, Navarro E, Pérez-Creo A, Fernández G, et al. Cryptosporidium species and subtype analysis in diarrhoeic pre-weaned lambs and goat kids from north-western Spain. Parasitol Res. (2015) 114:4099–105. doi: 10.1007/s00436-015-4639-0
66. Quílez J, Torres E, Chalmers RM, Hadfield SJ, Del Cacho E, Sánchez-Acedo C. Cryptosporidium genotypes and subtypes in lambs and goat kids in Spain. Appl Environ Microbiol. (2008) 74:6026–31. doi: 10.1128/AEM.00606-08
67. Castro-Hermida JA, González-Warleta M, Mezo M. Natural infection by Cryptosporidium parvum and Giardia duodenalis in sheep and goats in Galicia (NW Spain). Small Rumin Res. (2007) 72:96–100. doi: 10.1016/j.smallrumres.2006.08.008
68. Castro-Hermida JA, Almeida A, González-Warleta M, Correia da Costa JM, Rumbo-Lorenzo C, Mezo M. Occurrence of Cryptosporidium parvum and Giardia duodenalis in healthy adult domestic ruminants. Parasitol Res. (2007) 101:1443–8. doi: 10.1007/s00436-007-0624-6
69. Díaz P, Quílez J, Robinson G, Chalmers RM, Díez-Baños P, Morrondo P. Identification of Cryptosporidium xiaoi in diarrhoeic goat kids (Capra hircus) in Spain. Vet Parasitol. (2010) 172:132–4. doi: 10.1016/j.vetpar.2010.04.029
70. Chikweto A, Veytsman S, Tiwari K, Cash K, Stratton G, Thomas D, et al. Prevalence of Cryptosporidium spp. in asymptomatic small ruminants in Grenada, West Indies. Vet Parasitol. (2019) 15:100262. doi: 10.1016/j.vprsr.2019.100262
71. Bomfim TCB, Huber F, Gomes RS, Alves LL. Natural infection by Giardia sp. and Cryptosporidium sp. in dairy goats, associated with possible risk factors of the studied properties. Vet Parasitol. (2005) 134:9–13. doi: 10.1016/j.vetpar.2005.05.067
72. Romero-Salas D, Alvarado-Esquivel C, Cruz-Romero A, Aguilar-Domínguez M, Ibarra-Priego N, Merino-Charrez JO, et al. Prevalence of Cryptosporidium in small ruminants from Veracruz, Mexico. BMC Vet Res. (2016) 12:14. doi: 10.1186/s12917-016-0638-3
73. Xiao L. Overview of Cryptosporidium presentations at the 10th international workshops on opportunistic protists. Eukaryot Cell. (2009) 8:429–36. doi: 10.1128/EC.00295-08
74. Al-Habsi K, Yang R, Williams A, Miller D, Ryan U, Jacobson C. Zoonotic Cryptosporidium and Giardia shedding by captured rangeland goats. Vet Parasitol. (2017) 7:32–5. doi: 10.1016/j.vprsr.2016.11.006
75. Koinari M, Lymbery AJ, Ryan UM. Cryptosporidium species in sheep and goats from Papua New Guinea. Exp Parasitol. (2014) 141:134–7. doi: 10.1016/j.exppara.2014.03.021
76. Zhao GH, Du SZ, Wang HB, Hu XF, Deng MJ, Yu SK, et al. First report of zoonotic Cryptosporidium spp. Giardia intestinalis and Enterocytozoon bieneusi in golden takins (Budorcas taxicolor bedfordi. Infect Genet Evol. (2015) 34:394–401. doi: 10.1016/j.meegid.2015.07.016
77. Jian F, Liu A, Wang R, Zhang S, Qi M, Zhao W, et al. Common occurrence of Cryptosporidium hominis in horses and donkeys. Infect Genet Evol. (2016) 43:261–6. doi: 10.1016/j.meegid.2016.06.004
78. Zhao Z, Wang R, Zhao W, Qi M, Zhao J, Zhang L, et al. Genotyping and subtyping of Giardia and Cryptosporidium isolates from commensal rodents in China. Parasitology. (2015) 142:800–6. doi: 10.1017/S0031182014001929
79. Lv C, Zhang L, Wang R, Jian F, Zhang S, Ning C, et al. Cryptosporidium spp. in wild, laboratory, and pet rodents in china: prevalence and molecular characterization. Appl Environ Microbiol. (2009) 75:7692–9. doi: 10.1128/AEM.01386-09
80. Li N, Xiao L, Alderisio K, Elwin K, Cebelinski E, Chalmers R, et al. Subtyping Cryptosporidium ubiquitum,a zoonotic pathogen emerging in humans. Emerg Infect Dis. (2014) 20:217–24. doi: 10.3201/eid2002.121797
81. Fayer R, Santín M, Macarisin D. Cryptosporidium ubiquitum n. sp. in animals and humans. Vet Parasitol. (2010) 172:23–32. doi: 10.1016/j.vetpar.2010.04.028
82. Feng Y, Ryan UM, Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. (2018) 34:997–1011. doi: 10.1016/j.pt.2018.07.009
83. Rosenthal M, Pedersen R, Leibsle S, Hill V, Carter K, Roellig DM. Cryptosporidiosis associated with consumption of unpasteurized goat milk—Idaho. Morb Mortal Wkly Rep. (2015) 64:194. Available online at: https://www.jstor.org/stable/24856365
84. Lange H, Johansen OH, Vold L, Robertson LJ, Anthonisen IL, Nygard K. Second outbreak of infection with a rare Cryptosporidium parvum genotype in schoolchildren associated with contact with lambs/goat kids at a holiday farm in Norway. Epidemiol Infect. (2014) 142:2105–13. doi: 10.1017/S0950268813003002
85. Robertson LJ. Giardia and Cryptosporidium infections in sheep and goats: a review of the potential for transmission to humans via environmental contamination. Epidemiol Infect. (2009) 137:913–21. doi: 10.1017/S0950268809002295
86. Choy RKM, Huston CD. Cryptosporidiosis should be designated as a tropical disease by the US Food and Drug Administration. PLoS Negl Trop Dis. (2020) 14:e0008252. doi: 10.1371/journal.pntd.0008252
87. Tudu NK, Roy DC. Socio-economic profile of women goat keepers and rearing challanges in goat in nadia district of West Bengal. Int J Sci Environ Technol. (2015) 4:331–6.
88. Skapetas B, Bampidis V. Goat production in the world: present situation and trends. Livest Res Rural Dev. (2016) 28:200. Available online at: http://www.lrrd.org/lrrd28/11/skap28200.html
89. De Vries J. Goats for the poor: some keys to successful promotion of goat production among the poor. Small Rumin Res. (2008) 77:221–4. doi: 10.1016/j.smallrumres.2008.03.006
90. Shirima E. Benefits from dual purpose goats for crop and livestock production under small-scale peasant systems in Kondoa eroded areas, Tanzania. Livestock Res Rural Dev. (2005) 17:138. Available online at: http://www.lrrd.org/lrrd17/12/shir17138.htm
Keywords: Cryptosporidium, goats (Capra aegagrus hircus), genotypes, One Health, zoonosis
Citation: Utaaker KS, Chaudhary S, Kifleyohannes T and Robertson LJ (2021) Global Goat! Is the Expanding Goat Population an Important Reservoir of Cryptosporidium? Front. Vet. Sci. 8:648500. doi: 10.3389/fvets.2021.648500
Received: 31 December 2020; Accepted: 08 February 2021;
Published: 05 March 2021.
Edited by:
Viliam Šnábel, Institute of Parasitology (SAS), SlovakiaReviewed by:
Arwid Daugschies, Leipzig University, GermanyMartha Betson, University of Surrey, United Kingdom
Copyright © 2021 Utaaker, Chaudhary, Kifleyohannes and Robertson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kjersti Selstad Utaaker, a2plcnN0aS5zLnV0YWFrZXJAbm9yZC5ubw==
 Kjersti Selstad Utaaker
Kjersti Selstad Utaaker Suman Chaudhary2
Suman Chaudhary2 Tsegabirhan Kifleyohannes
Tsegabirhan Kifleyohannes Lucy Jane Robertson
Lucy Jane Robertson