
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 17 February 2021
Sec. Comparative and Clinical Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.623800
This article is part of the Research Topic Precision Medicine in Veterinary Oncology View all 11 articles
Canine mammary tumors (CMTs) are the most common neoplasm in intact female dogs. Canine mammary cancer (CMC) represents 50% of CMTs, and besides surgery, which is the elective treatment, additional targeted and non-targeted therapies could offer benefits in terms of survival to these patients. Also, CMC is considered a good spontaneous intermediate animal model for the research of human breast cancer (HBC), and therefore, the study of new treatments for CMC is a promising field in comparative oncology. Dogs with CMC have a comparable disease, an intact immune system, and a much shorter life span, which allows the achievement of results in a relatively short time. Besides conventional chemotherapy, innovative therapies have a large niche of opportunities. In this article, a comprehensive review of the current research in adjuvant therapies for CMC is conducted to gather available information and evaluate the perspectives. Firstly, updates are provided on the clinical–pathological approach and the use of conventional therapies, to delve later into precision therapies against therapeutic targets such as hormone receptors, tyrosine kinase receptors, p53 tumor suppressor gene, cyclooxygenases, the signaling pathways involved in epithelial–mesenchymal transition, and immunotherapy in different approaches. A comparison of the different investigations on targeted therapies in HBC is also carried out. In the last years, the increasing number of basic research studies of new promising therapeutic agents on CMC cell lines and CMC mouse xenografts is outstanding. As the main conclusion of this review, the lack of effort to bring the in vitro studies into the field of applied clinical research emerges. There is a great need for well-planned large prospective randomized clinical trials in dogs with CMC to obtain valid results for both species, humans and dogs, on the use of new therapies. Following the One Health concept, human and veterinary oncology will have to join forces to take advantage of both the economic and technological resources that are invested in HBC research, together with the innumerable advantages of dogs with CMC as a spontaneous animal model.
Canine mammary tumors (CMTs) are a highly heterogeneous group of neoplasms that represent between 50 and 70% of all tumors in intact female dogs (1–4). The prevalence varies depending on the geographic location, being greater in countries where ovariectomy is not routinely performed (4). In these countries, the prevalence of mammary neoplasms in female dogs is three times higher than the prevalence in women (3). Historically, roughly 50% of CMTs are considered to be malignant (1, 2, 5, 6). However, recent studies have shown an increase in malignant vs. benign tumors over the last years, a similar trend that has been detected in human medicine (2). Canine mammary cancer (CMC) and human breast cancer (HBC) share not only the aforesaid trend but also many epidemiological, environmental, biological, clinical, genetic, and pathological features, including a remarkable histological and molecular heterogeneity. Many authors have claimed CMC as a good spontaneous model for the study of HBC, especially the inflammatory mammary cancer, the deadliest type (7–12). In female dogs and in women, mammary cancer is the most frequently diagnosed malignancy (4) and the leading cause of cancer-related death in women worldwide (13). For this reason, over the past two decades, innovative HBC treatments have incredibly evolved to place emphasis on more molecularly directed individual therapies while diminishing radio- and chemotherapy to reduce the adverse effects of treatment (14). Since the publication of the intrinsic molecular classification of Perou and Solie in 2000, which distinguished four subtypes of HBC (luminal A, luminal B, basal-like, and HER-2 enriched), the clinical management shifted to a biology-centered approach, based on the expressions of estrogen and progesterone receptors (luminal subtypes), the human epidermal growth factor receptor 2 (HER-2), and basal markers (positive for high-molecular-weight cytokeratins, as cytokeratins 5/6, 14, and 17) (15). Nowadays, the classification of five molecular subtypes (luminal A, luminal B HER-2–, luminal B HER-2+, HER-2 enriched, and triple negative) is the most widely utilized in human medicine to elect targeted therapy: anti-estrogenic drugs for the luminal subtypes and anti-HER-2 treatment for the HER-2-enriched tumors. Unfortunately, since triple-negative mammary cancer does not currently have a specific targeted therapy, its prognosis is poor (14). Surgery is the treatment of choice in both HBC and CMC, and adjuvant therapies are only given on a routine basis in HBC. On the contrary to HBC treatment, adjuvant chemotherapy has not been proven to have a clear benefit in dogs with CMC yet (16–19). In spite that the application of clinical staging of patients with CMC and the histological grading of the neoplasms have helped to standardize prognosis and treatments, no precision therapies are routinely administered to dogs bearing mammary neoplasms. For all these reasons, the CMC-related mortality is relatively high: over 40% of the patients die within a year after diagnosis (20). There is an urge to developing updated therapeutic protocols and targeted therapies. Therefore, a comprehensive review of the current research in adjuvant therapies for CMC is conducted here to gather available information and evaluate the perspectives.
Dogs with CMC are staged according to the modified World Health Organization (WHO) TNM system (T, size of tumor; N, affectation of lymph nodes; M, distant metastasis) (21).
More recently, a pathological staging system inspired in human oncology, in which T is replaced by pathologic tumor size (pT) and N is replaced by the pathologic nodal status (pN), with the addition of lymphovascular invasion (LVI) has been proposed, the stages being 0, I, II, IIIA, and IIIB (22). The use of this system is still limited, the major criticism being its inability to discriminate different stages of malignancy in cases with no LVI or lymph node affectation, which are the vast majority.
The current staging system is not flexible enough to allow prognostic differences between specific tumor types and subtypes and does not consider tumor grades or lymphovascular invasion (21). Therefore, new “bio-score” systems combining the anatomical staging (TNM) with many histological and biological variables have been developed: multivariate scoring (scores from 0 to 40) and refined flexible scoring (scores from 0 to 6.5). Although both systems were accurate in predicting the survival, the refined flexible scoring was superior in differentiating dogs with a high or a low risk of metastasis (23). Greater efforts should be made to implement these bio-score systems in larger prospective studies, allowing for more widespread use and the refinement of these increasingly accurate prognostic systems.
The new classification of CMTs (24), which substitutes the WHO's 1974 and the last 2011 classification (5), together with the current grading system used worldwide for malignant CMC (9), as an adaptation of the Nottingham method utilized for HBC (25), have given tools to pathologists for accurate diagnosis and prognosis of CMTs since several studies have validated the prognostic significance of histopathological classification and grading (26, 27).
Normal mammary gland cells of both humans and dogs have distinct immunoprofiles that can be used for diagnosis and potentially for targeted therapies. Luminal epithelial cells are characterized by the expression of low-molecular-weight (LMW) type I acidic cytokeratins (CKs) 18 and 19 and type II basic CKs 7 and 8. Basal/myoepithelial cells express high-molecular-weight (HMW) type I acidic CKs 14 and 17 and type II basic CKs 5 and 6 (Figures 1, 2). Myoepithelial cells, much more proliferative in CMC than in HBC (5), also express other markers such as p63, vimentin, calponin, smooth muscle actin (SMA), P-cadherin, CD10, epidermal growth factor receptor (EGFR), maspin, and 14-3-3 sigma protein (28–31) (Figures 3–5). However, a study performed in 2014 showed that, as happens in human breast (32), the mammary gland subpopulations are more complex than this. Using single and double immunohistochemistry (IHC) on serial sections of normal canine mammary gland, five distinct subpopulations were identified: (1) progenitor cells (CK5+, CK14+, p63+, and VIM+); (2) intermediary myoepithelial cells (CK5+, CK14+, p63+, SMA+, CALP+, and VIM+); (3) terminally differentiated myoepithelial cells (CALP+, SMA+, and VIM+); (4) intermediary luminal glandular cells (CK5+, CK14+, and CK8/CK18+); and (5) terminally differentiated luminal glandular cells (CK8/CK18+). The ducts are considered regenerative niches as they contain progenitor cells and intermediary luminal glandular cells; however, these are located in the basal position (33) and not in the luminal position, as occurs in women (34).
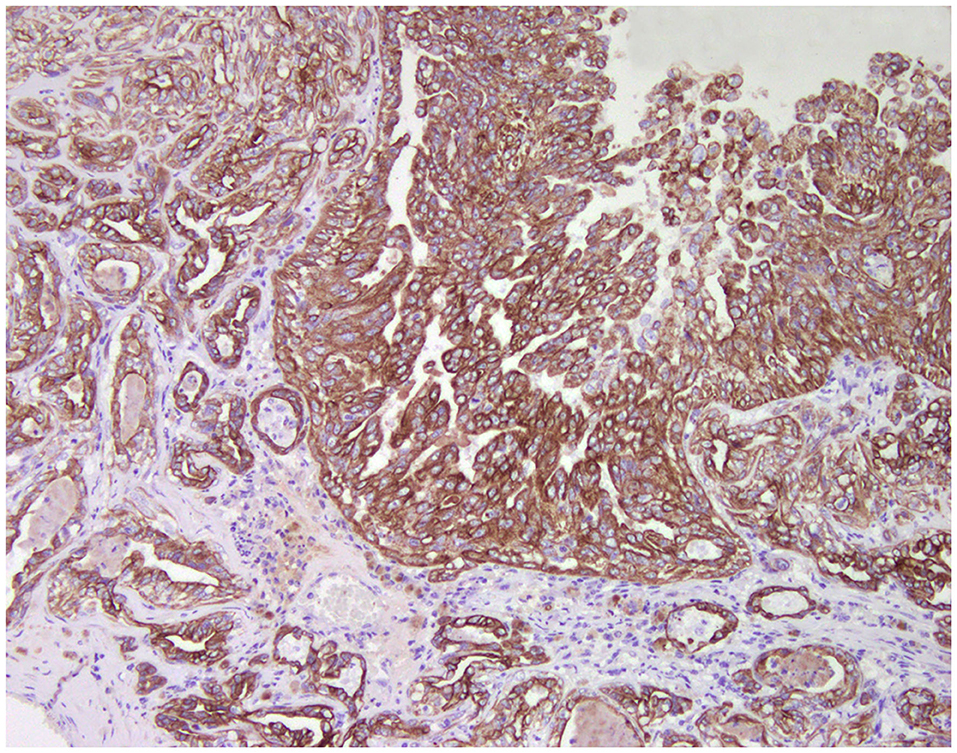
Figure 1. Tubulo-papillary carcinoma, mammary gland, dog. Immunohistochemical cytoplasmic staining of wide-spectrum cytokeratins (AE1/AE3). Luminal and basal/myoepithelial cells are positive (brown).
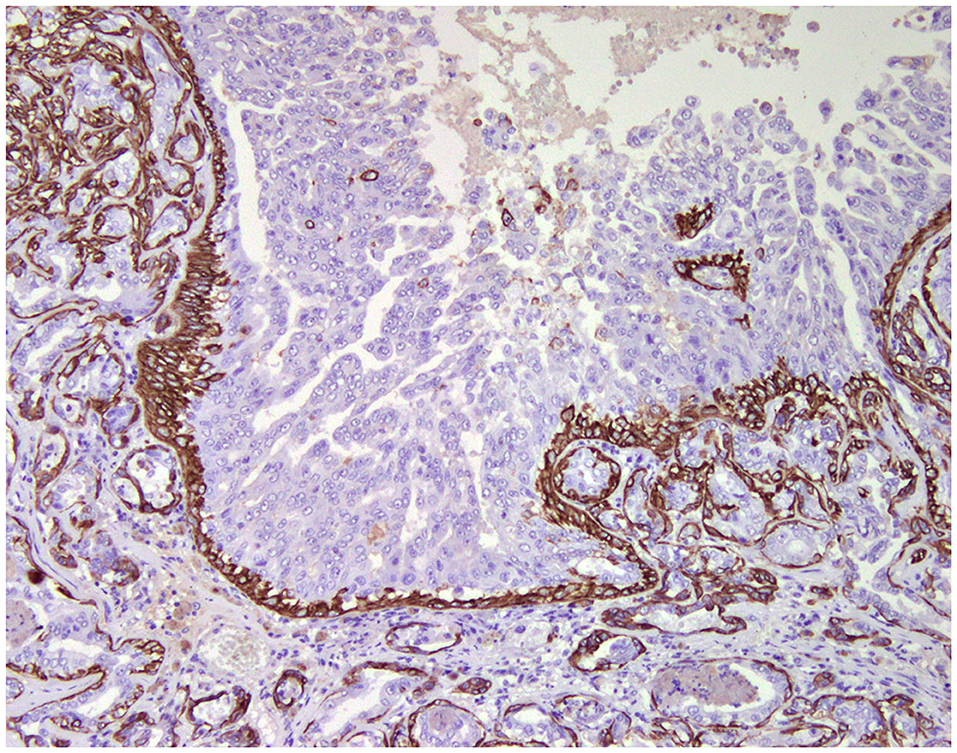
Figure 2. Tubulo-papillary carcinoma, mammary gland, dog. Immunohistochemical cytoplasmic staining of cytokeratin 14. Basal/myoepithelial cells are positive.
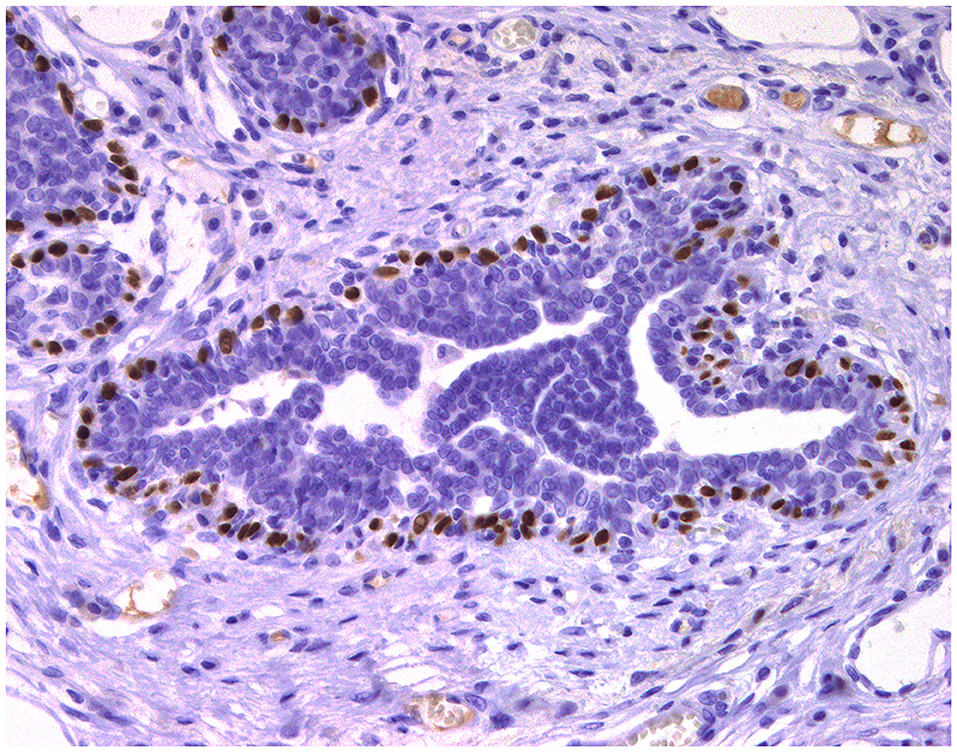
Figure 3. Epitheliosis, mammary gland, dog. Immunohistochemical nuclear staining of p63. Myoepithelial cells are positive.
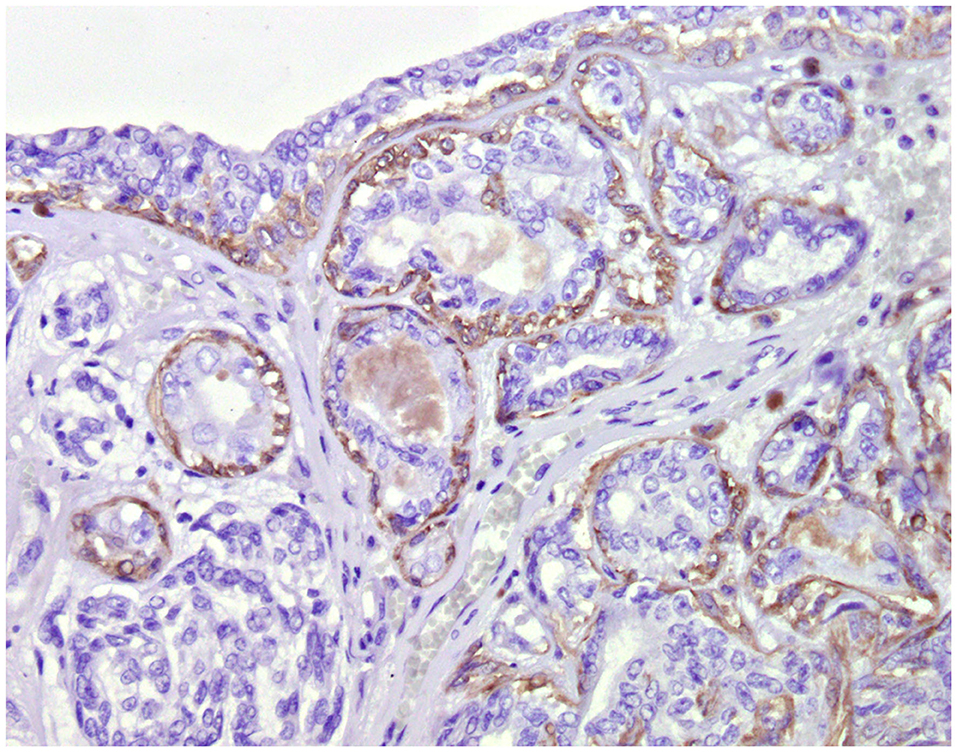
Figure 4. Tubular carcinoma, mammary gland, dog. Immunohistochemical cytoplasmic staining of calponin. Myoepithelial cells are positive.
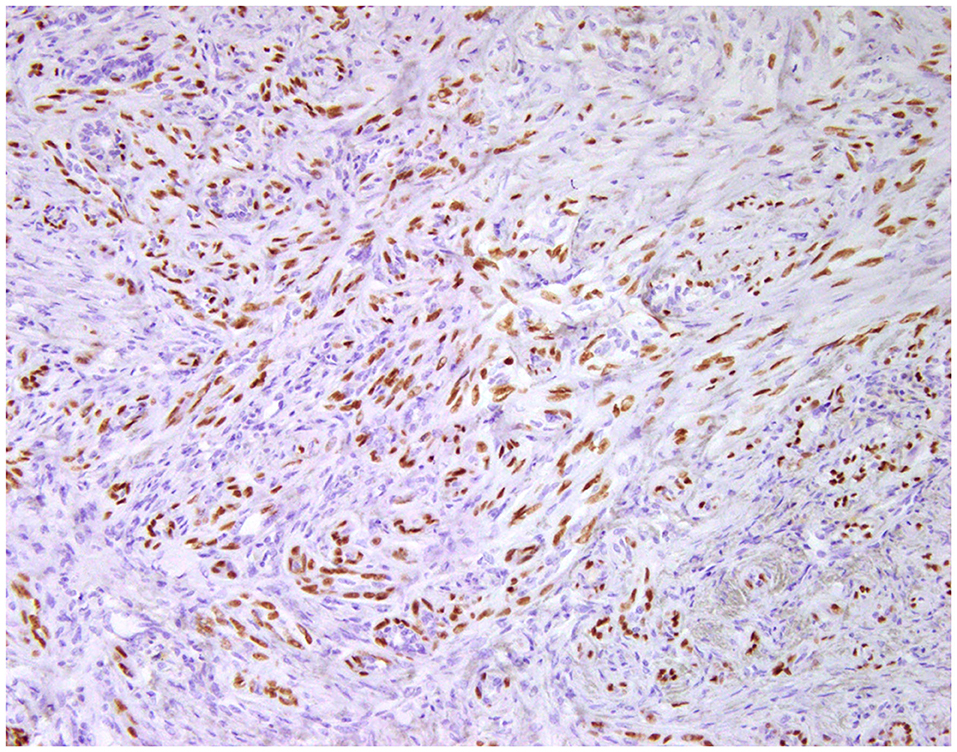
Figure 5. Carcinoma and malignant myoepithelioma, mammary gland, dog. Immunohistochemical nuclear staining of p63. Interstitial proliferated myoepithelial cells are positive.
In human breast pathology, IHC is routinely used to assist with the prognosis and to determine the specific treatment for patients (35). Over the past years, there has been considerable efforts to characterize and classify HBC at the molecular level to establish effective individual treatments. However, due to time and cost constraints, the surrogate molecular breast cancer classification is still largely based on IHC assessment of biomarkers: estrogen receptor (ER), progesterone receptor (PR), HER-2, and Ki-67, among others (36). Nowadays, HBC is classified on the following molecular subtypes by IHC (Table 1): luminal A (ER/PR+, HER-2–, and Ki-67 low), luminal B HER-2 negative (ER+, PR– or low, HER-2–, and Ki-67 high), luminal B HER-2 positive (ER+, PR+/–, HER-2+, and Ki-67 high), HER-2 enriched or overexpressed (ER/PR–, HER-2+, and Ki-67 high), and triple negative (ER–, PR–, HER-2–, and Ki-67 high). Triple-negative breast cancer (TNBC) can be further subdivided, according to genetic signatures, into luminal androgen receptor (LAR), mesenchymal (MES), and two basal-like subtypes (positive for high-molecular-weight cytokeratins): immunosuppressed (basal-like immunosuppressed, BLIS) and immune-activated (basal-like immune-activated, BLIA), depending on the upregulation or downregulation of genes associated with T, B, and natural killer (NK) cells (14, 36, 37).
Despite the relevance of molecular subtyping in HBC, highly variable and even contradictory results have been obtained in CMC (10, 38–43). Regardless of the established guidelines for immunohistochemical assessment (31), variable application of the criteria has been utilized, and the percentages of the molecular subtypes differ enormously among investigations.
HER-2 immunodetection in CMTs has always remained controversial. In a study performed by Abadie et al. (10), following appropriate standardized intrinsic and extrinsic controls (31), there were no HER-2-enriched tumors, in contrast to other studies in which between 5 and 15% of the mammary neoplasms were classified as HER-2 enriched (38, 42, 43). Previous studies identified HER-2 in normal, hyperplastic, and dysplastic mammary tissues and found no relation with prognostic parameters such as disease-free interval (DFI), overall survival (OS), and lymphovascular invasion, suggesting that HER-2 may play a role in the proliferation of mammary tissue in female dogs, but not conclusively in its malignant transformation (40). Furthermore, in a recent publication (44), HER-2 messenger RNA (mRNA) expression was observed in neoplastic and non-neoplastic mammary tissues using a novel quantitative RNA in situ hybridization assay, which correlates with the immunohistochemistry score. Among the non-neoplastic mammary tissues (hyperplasia), all cases showed HER-2: 21.4% were classified as 1+, while 78.6% were positive (2+ and 3+) (Figure 6). Moreover, within neoplastic tissues, no significant associations between HER-2 expression and clinical parameters were found.
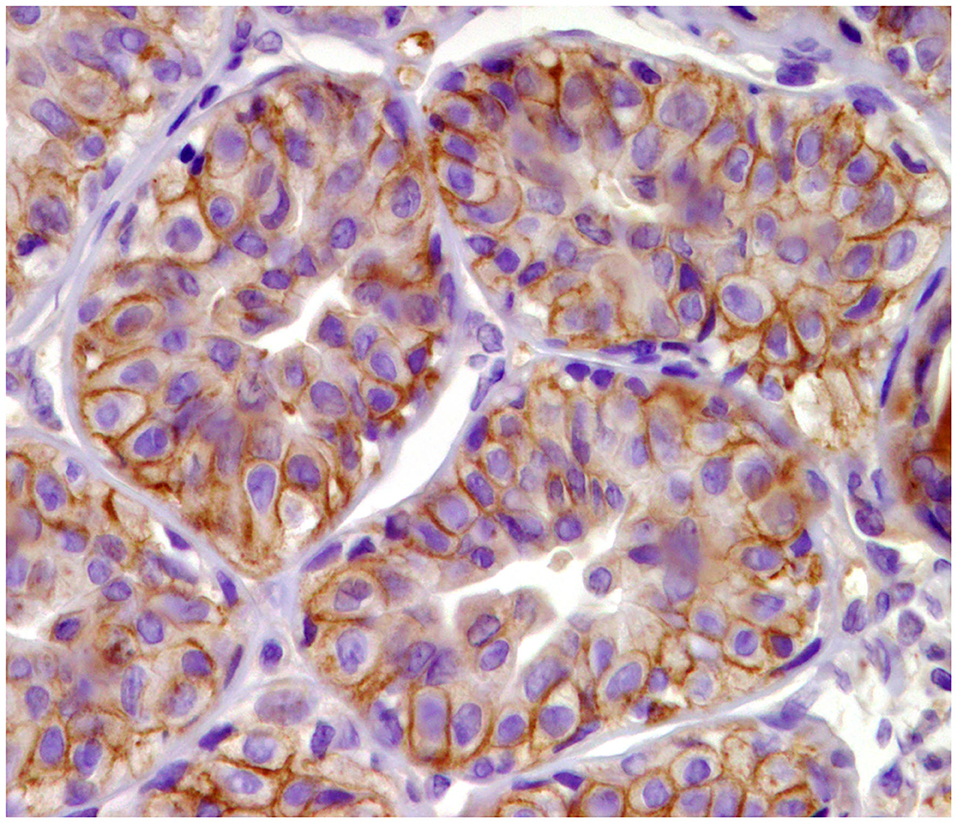
Figure 6. Tubular carcinoma, mammary gland, dog. Immunohistochemical membranous staining of human epidermal growth factor receptor 2 (HER-2). Complete and incomplete membranous staining of neoplastic cells.
The specificity of human anti-HER-2 antibody (Dako A0485) for HER-2 immunolabeling in canine tissues is also controversial. While one study showed no evidence of its specificity in canine tissues by Western blotting and subsequent mass spectrometric analysis (45), another work showed the cross-reactivity of the human anti-HER2 antibody in canine tissue (urothelial) by Western blotting (46).
Triple-negative tumors account for approximately half of CMCs (58.6%) (10), and showed significantly shorter disease-free interval (DFI) and overall survival (OS) in comparison to luminal A tumors. Comparable results were obtained in other studies: a triple-negative phenotype was related to a higher histological grade of malignancy, lymphatic invasion, and poorer prognosis. On the other hand, luminal A tumors were frequently complex tumors associated with better prognosis and longer DFI and OS (10, 38, 42, 43). In a study, HER-2-enriched and triple-negative CMCs presented a downregulation of E-cadherin compared to the luminal A and B subtypes, which are related to invasion and metastasis (43).
Surgery is the primary treatment in the control of CMTs; the goal is to remove the tumor(s) with clean margins and, depending on the case, to prevent the development of new tumors in the remaining glands (4). Clean margins have been found to be predictive of the median survival time (MST) in dogs with stages I–III (19), and very recent publications have elucidated new strategies for the intraoperative assessment of margins using near-infrared light waves to generate real-time, high-resolution images on the microscopic scale, similar to low-power histopathology (47–49).
Despite the elevated frequency of CMTs, there is a lack of prospective clinical trials robust enough to establish the extent of surgical excision: simple lumpectomy, local mastectomy, regional mastectomy, total chain mastectomy, or bilateral total mastectomy (4). Nevertheless, the current literature recommendations are the following: If a single, small (<1 cm) tumor is present, nodulectomy is usually carried out. Simple mastectomy is indicated when the tumor is larger and centrally located within the mammary gland. When multiple tumors are in consecutive glands, or a single tumor is found between two mammary glands, regional mastectomy (excision of adjacent mammary glands, from one to two or from three to five) is performed. Finally, total mastectomy is indicated when multiple tumors are distributed throughout the mammary chain, regardless of the size (4). Those cases in which surgery is not recommended are advanced metastatic (stage V) cancer (17, 50) and inflammatory mammary cancer (IMC) (7, 8, 51).
Additional treatment (adjuvant therapy) can be given after the primary mammary cancer treatment (surgery) to lower the risk of developing further recurrences and metastasis. Adjuvant therapy may include chemotherapy, radiotherapy, and targeted or individualized therapy, this latest based on the specific genetic characteristics of the cancer in a patient (52–55).
Approximately 50% of the dogs with CMTs have at least a malignant neoplasm, and these patients would further profit from adjuvant chemotherapy. However, it has not been demonstrated conclusively if adjuvant chemotherapy offers a significant benefit to dogs with CMTs. Although cases have reported measurable tumor responses to doxorubicin (56–58), carboplatin (59, 60), mitoxantrone, and paclitaxel (61, 62), larger studies have not found a significant improvement of the measurable clinical responses (MST, DFI, or OS) using gemcitabine (17), doxorubicin, docetaxel (16, 19), and mitoxantrone (19). Due to the lack of efficient chemotherapeutics, dogs with malignant CMTs show high rates of recurrence (63) and poor prognosis (64).
Despite this uncertainty, chemotherapy is frequently used in those dogs with tumors considered at high risk of metastasis or recurrence (4).
Another chemotherapeutic approach is oral metronomic chemotherapy, which involves the administration of the lowest biologically effective dose at frequent regular intervals (65). In veterinary medicine, metronomic chemotherapy has been studied since 2007 (66) in tumors that include hemangiosarcoma (67–72), osteosarcoma (73–76), hepatic neuroendocrine carcinoma (77), primary lung carcinoma (78), soft tissue sarcomas (79–82), and transitional cell carcinomas (83), with chemotherapeutics such as cyclophosphamide, lomustine, and chlorambucil. To date, only one study has been published regarding CMC (84), in which longer MSTs were observed in patients treated with surgery and metronomic chemotherapy compared to dogs treated with surgery and conventional chemotherapy.
Although radiotherapy is commonly used in HBCs with locoregional treatment (14), only one study has been published in CMC, specifically in dogs bearing IMC. Radiotherapy, in combination with piroxicam, toceranib, and thalidomide, showed significantly longer time to progression than those patients treated with the same regimen, but without radiotherapy (85).
Targeted therapies involve drugs that block the growth of cancer by interfering with individually expressed specific molecules responsible for tumor cell proliferation, survival, metastasis, or microenvironment (86). Given the poorly efficient available adjuvant therapies in dogs with mammary cancer, several studies have been made in vitro in animal models (mice) and few clinical trials, which are shown below.
Estrogens, progesterone, prolactin (PRL), and growth hormone (GH) are essential for physiological mammary development. The effects of these hormones are mediated through binding to their respective receptors within the mammary gland (6) (Figures 7, 8). Estrogens and progesterone have been historically known to have a main role in tumorigenesis in CMTs, as spaying of female dogs before the first or second heat has significant protective effects (87). On the other hand, exposure to exogenous hormones (both estrogens and progestins) increases the risk of developing CMTs in dogs (88). Furthermore, studies have found that dogs with CMC have higher levels of estrogens in the blood, except in IMC, where the serum estrogens levels are lower than in dogs with other malignant mammary tumors (89, 90). On the other hand, ER, PR, PRL, PRL receptor, and GH receptor have been found to be downregulated in malignant mammary tumors compared to normal mammary gland (91, 92) and benign tumors (92–94). Furthermore, lymphatic invasion, high mitotic index, large tumor size, and tumor grade are significantly associated with low ER/PR expressions. Tumors with low ER/PR expressions have poorer prognosis (89, 93, 94).
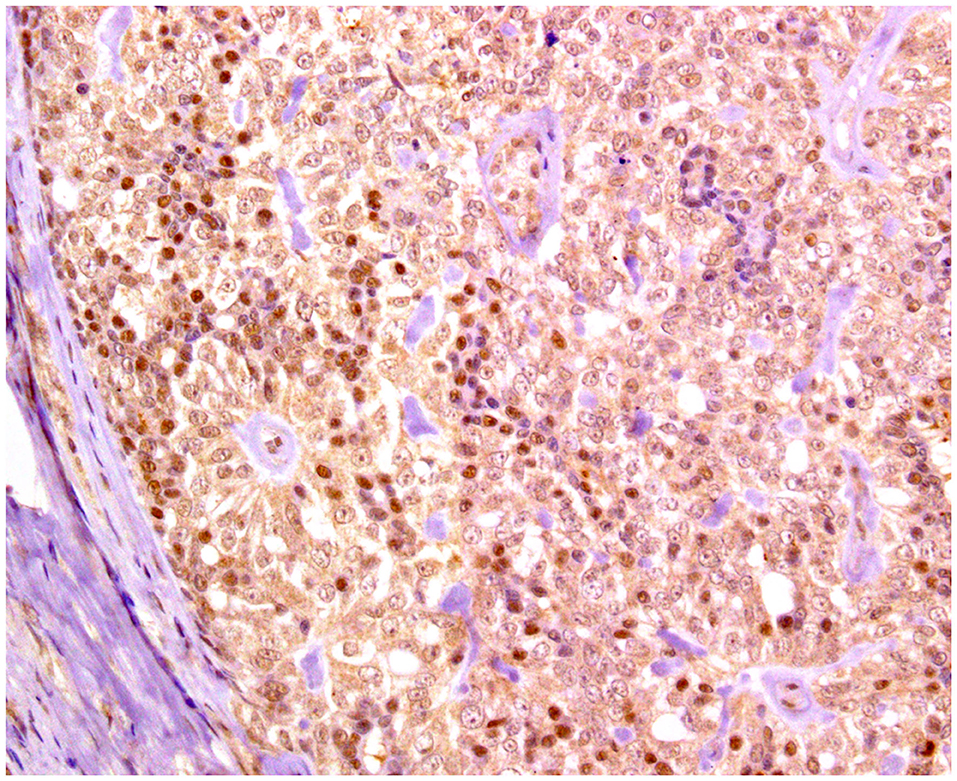
Figure 7. Solid carcinoma, mammary gland, dog. Immunohistochemical nuclear staining of estrogen receptor (ER). Neoplastic cells are positive with different intensities of immunolabeling.
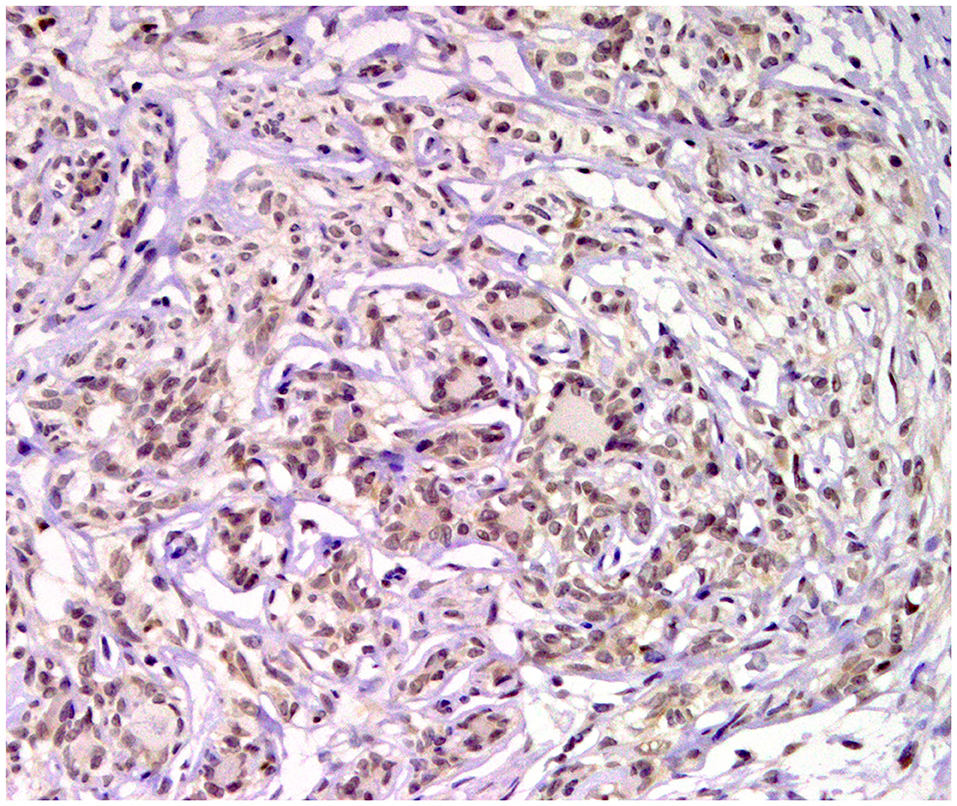
Figure 8. Tubular carcinoma, mammary gland, dog. Immunohistochemical nuclear staining of progesterone receptor (PR). Neoplastic cells are positive.
In human medicine, ER+ breast cancer is the most common subgroup (>70%) (95). The risk of ER+ breast cancer increases with exposure to estrogens during a lifetime, for example due to an earlier menarche or late menopause (14). Moreover, hormone replacement therapy on menopausal women increases the risk of breast cancer (96). In women with HBC, ER+ tumors are susceptible to anti-hormone treatment. This therapy is designed to target mainly ER using antiestrogens, such as tamoxifen or fulvestrant, or by inhibiting the endogenous synthesis of 17β-estradiol using aromatase inhibitors (97).
Tamoxifen is a selective inhibitor of ER that is widely utilized in the treatment of HBC. However, in dogs, severe adverse effects (vulvar edema, vaginal purulent discharge, and pyometra) are repeatedly seen, and that outweighs the possible benefits of this hormone therapy (98, 99).
For this reason, other antiestrogens are being studied for their use in CMC. Indole-3-carbinol is a natural phytochemical found in cruciferous vegetables (i.e., cauliflower, cabbage, and broccoli) that has been proven to suppress cell proliferation and induce apoptosis in breast cancer cell lines by multiple mechanisms such as blocking estrogen receptors (100, 101). In veterinary medicine, a mouse xenograft model of canine IMC was treated with indole-3-carbinol, resulting in decreased tumor proliferation and increased apoptosis, although metastasis and lymphatic embolization were not prevented (102).
When women reach menopause, the ovaries no longer produce estrogen; however, this hormone is produced at other sites (fat, liver, muscle, and mammary tissue) through the aromatase enzyme (103). Aromatase mRNA levels are higher in cancerous tissues than in normal breast tissues in humans (104). Therefore, while ER inhibitors (e.g., tamoxifen) are preferentially given in premenopausal women with ER+ HBC, aromatase inhibitors are used for the treatment of postmenopausal patients with ER+ breast cancer (103). In dogs, IMC has been shown to express higher levels of aromatase than non-IMC. Moreover, in vitro treatment with letrozole, an aromatase inhibitor, significantly reduced cell proliferation in an IMC cell line (105). No clinical trials on the use of aromatase in dogs with CMC have been reported.
Melatonin is a hormone produced by the pineal gland in response to darkness, regulated by photoperiod (106). In breast tissue, melatonin exerts its action through two receptors: melatonin receptors 1 and 2 (MT1 and MT2, respectively) (107). A positive correlation between MT1 and ERα expressions has been demonstrated and recognized as a prognostic marker for OS in HBC (108). Furthermore, melatonin has been shown to suppress the proliferation of HBC cell lines in vitro and in vivo by disrupting estrogen-dependent signaling as well as inhibiting estrogen production in the gonads and in breast tissues through the aromatase pathway (109–111).
In the last decades, the role of androgens on HBC has started to gain the attention of researchers. Not only can androgens be a source of estrogen through the aromatase pathway but they have also been directly implicated as possible carcinogen factors for breast cancer (112). The androgen receptor (AR) is expressed in ~70–90% of invasive human breast cancers, a frequency comparable to or higher than those reported for ER (70–80%) and PR (50–70%) (113, 114). On the other hand, AR has been found in 64% of IMC and 40% of non-inflammatory CMC (89) (Figure 9). To date, in human medicine, AR-targeted drugs have been approved for the treatment of prostate cancer, and different AR inhibitors are being investigated for the treatment of HBC, specifically for the LAR subtype of triple-negative breast cancer (115). Flutamide is an analog of androgen that blocks the AR (116). It is already used in veterinary medicine to treat canine prostate hyperplasia and cancer (117), but has not been tested in patients with CMC. Flutamide has been shown in vitro to decrease the proliferation of an IMC cell line, and reductions in the tumor size and metastasis rates in IMC xenografted mice were found (118).
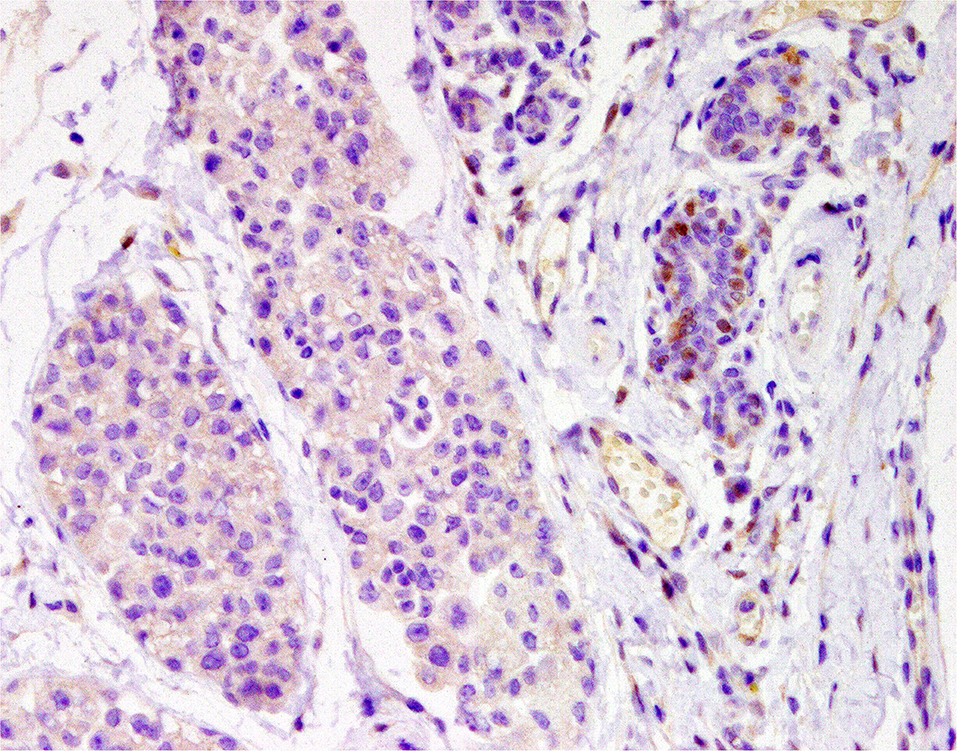
Figure 9. Tubular carcinoma, mammary gland, dog. Immunohistochemical nuclear staining of androgen receptor (AR). Neoplastic cells are negative; adjacent mammary hyperplastic cells are positive.
Progesterone has also a relevant role in HBC (119) and in CMC (105, 120). Progesterone signals via PR, whose expression is stimulated by estrogen (121). Some authors have suggested that progesterone may lead the transition of tumors from luminal to a basal phenotype (122). Upon progesterone exposure, luminal cells secrete growth factors (RANKL and Wnt) that may stimulate the recruitment and differentiation of cancer stem cells (CSCs, characterized by CD40HIGH and CD24LOW) (123).
Therapeutic targeting of PRs have been studied in HBC as a treatment of endocrine refractory breast cancer, the most common drugs being megestrol acetate, medroxyprogesterone acetate, mifepristone, and onaprestone (124). The antiprogestin aglepristone is employed in veterinary medicine for abortion, parturition induction, and pyometra treatment in female dogs (125). The study of antiprogestins in oncology has begun relatively recently. Mifepristone and onapristone have been shown to decrease the number of viable tumor cells in vitro in a canine mammary carcinoma cell line (126). In vivo, aglepristone treatment diminished the expression of PR, reduced the proliferation index in PR+ CMTs (127), and significantly increased DFI and OS in cases with PR+, <3 cm, low and medium grade, low proliferative tumors (128). Although promising for PR+ CMTs, antiprogestins in veterinary oncology need further studies with larger series and longer follow-up periods.
Oxytocin is a peptide hormone mainly synthesized in the hypothalamus that plays a role in uterine contraction and milk ejection, among other functions, and has been linked to the mammary carcinogenic process. Human (129) and canine (130) carcinoma cell lines and HBC xenografted mice (131) have shown reduced proliferation after oxytocin treatment. Recently, it has been shown that the expression of oxytocin receptors in CMTs is associated with ER+, benign tumors, and low-grade malignant tumors compared to high-grade malignant tumors (132).
Desmopressin is a synthetic analogous of vasopressin (antidiuretic hormone) that binds to the V2 membrane receptor (V2R); it has been used for the management of diabetes insipidus in humans (133) and dogs (134). Since V2R is also expressed in endothelial cells, desmopressin has been employed in the treatment of different bleeding disorders due to its effects in the hemostatic system (135). In oncology, a number of studies have shown in mouse models of HBC and in different HBC cell lines that this peptide seems to have anti-metastatic and anti-proliferative effects, probably by targeting V2R-expressing cancer cells and raising intracellular cAMP (136, 137). In a study on canine mammary carcinoma cell lines, desmopressin was shown to decrease cell viability at high concentrations (130). Furthermore, a veterinary clinical trial has demonstrated that the perioperative administration of desmopressin increases the DFI and OS in CMC (138). Although it seemed a very promising therapy, there have been no subsequent clinical trials in veterinary or human medicine performed by this group, with the exception of a phase II trial in HBC patients in 2015 (139), where the safety of perioperative administration was established; however, the effect on DFI, OS, or any other clinical parameter has not been reported. Due to this controversy, Sorenmo et al. conducted a prospective randomized trial in dogs with CMC, in which no metastasis-preventing effect of desmopressin was found (140).
Tyrosine kinase receptors (TKRs) catalyze a series of phosphorylation of target proteins that play a significant role in cell proliferation, metabolism, motility, survival, and apoptosis, as well as endothelial cell activation, leading to neovascularization.
Breakthroughs in biotechnology over the past decades have led to the development of new molecules that act on specific targets, among which small-molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs) stand out. Small-molecule (below 900 Da) tyrosine kinase inhibitors rapidly diffuse across cell membranes and target intracellular or extracellular proteins (kinases). To identify TKIs, the suffix “nib” is placed at the end of the generic name (141). On the other hand, monoclonal antibodies cannot cross cell membranes, therefore acting on the extracellular targets. The stem “mab” at the end of the name corresponds to mAbs. When the monoclonal antibody is completely human, the “umab” substem is used (e.g., nivolumab). If the immunoglobulin is chimeric (human constant domain, plus non-human variable domain), the mAb is named with the “ximab” substem; when the antibody is humanized (human framework with grafted murine complementary determining regions), then the “zumab” substem is utilized (e.g., trastuzumab) (142).
Among TKRs, HER-2, vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), stem cell factor receptor (c-KitR), and colony-stimulating factor 1 (CSF-1) are overexpressed or constitutively activated in human and canine tumors (143, 144). Tyrosine kinase inhibitors act by competitive inhibition of ATP binding, thus avoiding consecutive phosphorylation reactions and blocking signal transduction to the nucleus, inducing the deregulation of cellular proliferation and differentiation (145). Most of the TKIs are given orally, which means a huge benefit for animal welfare, diminishing stressful situations and providing ease of administration by the owner (146). For these reasons, several attempts to block these receptors have been made in veterinary oncology. Some TKIs designed for human oncology can have multiple actions depending on the receptor blocked (i.e., sunitinib inhibits VEGFR, PDGFR, c-KitR, and CSF-2) (147) and can be served for different types of cancer. Below, we review these targets separately.
The family of EGFRs encompasses four tyrosine kinases receptors also named human epidermal receptors (HERs): HER-1, HER-2, HER-3, and HER-4. When activated, these receptors trigger numerous signaling pathways, which regulate cell proliferation and survival, as well as the metastasis of tumor cells (144). Approximately 15–25% of HBC show overexpression of the HER-2 protein and/or amplification of the HER-2 gene, which is generally associated with a poor prognosis and an aggressive disease course (148).
The targeted therapy with anti-HER-2 agents in HBC is well-established. Several classes of anti-HER-2 agents have been developed, including: (1) monoclonal antibodies that bind to the extracellular domain of HER-2, such as trastuzumab and pertuzumab, which act by direct inhibition of HER-2 and indirect activation of the immune system to evoke antibody-dependent cellular toxicity; (2) small-molecule TKIs, including lapatinib, neratinib, and afatinib, that bind to the intracellular tyrosine kinase domains of HER-2 and other HER family members; and (3) antibody–drug conjugates, e.g., trastuzumab emtansine (T-DM1), composed of a monoclonal antibody directed at the extracellular domain of HER-2 linked to a cytotoxic agent. All of the latter have been approved by the European Medicines Agency (EMA) for clinical use in patients with HER-2+ HBC (149).
The amino acid homology values between canine and human EGFR-1 and HER-2 are reported to be 91 and 92%, respectively (150). In silico studies (research conducted by computer modeling or simulation) have shown that cetuximab (monoclonal antibody against EGFR-1) epitopes only differ by four amino acids in canines, while the trastuzumab (monoclonal antibody against HER-2) binding site is identical in humans and canines. In vitro studies with canine mammary carcinoma cells have reported a significant growth inhibition and G0/G1 phase arrest when treated with either cetuximab or trastuzumab (150). A “caninized” version of cetuximab developed by the same group, fusing the canine constant heavy-chain genes with the variable region murine genes of cetuximab, was able to inhibit the proliferation of canine mammary carcinoma cell lines, enhancing tumor cell killing via (151) phagocytosis.
With regard to anti-HER-2 TKIs, only gefitinib has been attested in CMTs. In vitro studies showed anti-proliferative effects in a canine mammary carcinoma cell line comparable to those with small interfering RNA (siRNA) targeting EGFR and HER-2 (152).
There are no clinical trials published on the use of HER-2 inhibitors in CMC, and their potential use in veterinary medicine is still far from daily routine.
Since 1971, it is well-known that the growth of solid tumors, beyond the size of 1–2 mm3, is conditioned to a sufficient supply of nutrients and oxygen, for which the new development of blood vessels was hypothesized and called angiogenesis (153). In early tumor development, neoplastic cells are oxygenated through simple diffusion in a phase defined as “avascular state.” With time and growth, tumor cells are deprived of oxygen and undergo a phenotypical change into a pro-angiogenic state (angiogenic switch) by inducing specific gene expression to overcome hypoxia through sprouting angiogenesis, vasculogenic mimicry (VM), and/or vascular co-option (VCO) (154). The sprouting angiogenic process occurs in both normal and neoplastic tissues. It is a complex process regulated by pro- and anti-angiogenic factors, among which stands out the VEGF family receptors, especially VEGF-A and its receptor (VEGFR-2), the fibroblast growth factor receptor 2 (FGFR-2), and PDGFR (154). VM and VCO are only found in highly aggressive cancers, as is explained below.
In both, humans (155) and dogs (156), VEGFR-2 and PDGFR are increased in malignant, triple-negative mammary tumors, being higher in those cases with metastatic disease (distant > regional) and positively correlated with tumor grade. Further, in HBC, microvascular density (MVD) is significantly correlated with metastasis, OS, and DFI (157); likewise, MVD is increased in canine primary mammary tumors with distant metastasis (156, 158).
In human medicine, about one third of the molecular therapeutics in clinical development are directed against angiogenesis. Angiogenesis is mediated by two major molecular routes: the VEGF axis-dependent route and the non-VEGF-mediated mechanisms (159). The anti-angiogenic therapies against the VEGF family block either the ligands or the receptors (160). The most widely studied anti-angiogenic therapeutic is bevacizumab (monoclonal antibody against the anti-VEGF-A ligand). In 2008, it became the first Food and Drug Administration (FDA)-approved anti-angiogenic drug for HBC; however, due to the lack of significant clinical improvements in subsequent studies, the approval was revoked in 2011 (161).
Small-molecule TKIs that block the VEGF family receptors have also been developed (pazopanib, sunitinib, and sorafenib, among others), many of them not only acting against angiogenesis but also diminishing other tumor metabolic pathways (162). For instance, pazopanib acts as an anti-angiogenic through the inhibition of VEGFR, PDGFR, and c-Kit in human renal carcinoma, soft tissue sarcoma, and breast cancer in combination with the anti-HER-2 lapatinib (163). In addition, sunitinib, which inhibits VEGFR, PDGFR, c-KitR, and CSF-1R, has shown promising activity as a single agent for advanced HBC (164). On the other hand, sorafenib, a VEGFR, PDGFR, and rapid accelerated fibrosarcoma-1 (Raf-1) kinase inhibitor, has antitumor effects in vitro and inhibits neovascularization in xenograft models of HBC (165). Sorafenib has been assessed in veterinary medicine, and it has shown a promising ability to inhibit VM in CMC cell lines in vitro (166). Other TKIs that target VEGFR-2 and that have been proven to significantly diminish the level of active (phosphorylated) VEGFR2, reduce cell proliferation and migration, and increase apoptosis in in vitro studies against CMT cell lines are rivoceranib (apatinib) (167) and vandetanib (168).
Despite the logical targeting of angiogenic pathways in cancer treatment and significant efforts in new drug development and HBC clinical trials, no significant clinical benefit has been achieved that outweighs the potential side effects. Some authors have hypothesized that a multi-target approach to angiogenesis is needed to overcome the apparent resistance of tumors to anti-angiogenesis treatment (159).
As stated earlier, angiogenesis is not the exclusive method to nourish tumor cells; two other mechanisms have been discovered in highly aggressive neoplasms—VM (169, 170) and VCO (171)—which have been hypothesized as responsible for the resistance to anti-angiogenic therapy (172–174). VM describes the formation of de novo vascular channels lined by genetically deregulated highly malignant cancer cells (175). These cancer cells, also called endothelial-like cells, exhibit cancer stem cell markers and characteristic endothelial morphology under electron microscopy in cell lines of human and canine inflammatory mammary cancer (176). VM has been associated with the spread and metastasis of human (177) and canine mammary tumors (178). VM was found in 33% of canine mammary tumors, and its presence was correlated with histological grade of malignancy and shorter survival times (166).
In VCO, neoplastic cells closely adhere to preexisting blood vessels to obtain nutrients and oxygen and further develop sprouting angiogenesis after the hypoxia switch is turned on (174, 179). In veterinary medicine, VCO has not been recognized yet.
The stem cell factor receptor c-Kit is an active participant in many vital functions in humans and animals, such as homeostasis, cell maintenance, differentiation, and melanogenesis, in a wide variety of cells (180). However, overexpression or mutation where the receptor is constitutively activated has been detected in a number of tumors in humans (181) and dogs, notably in canine mast cell tumors (143). In canine mast cell tumors, the c-Kit receptor can be labeled by immunohistochemistry in three different staining patterns: pattern I (perimembranous staining), pattern II (focal or stippled cytoplasmic), and pattern III (diffuse cytoplasmic staining), pattern III being the more aggressive (182). In women, c-Kit is expressed in normal breast tissue and is gradually lost during the malignant progression of breast tumors due to a downregulation at the mRNA level (183). On the contrary, c-Kit has been found to be present in 38.5% of CMTs (184) (Figure 10) and seems to be overexpressed in malignant mammary tumors (185, 186), in addition to being correlated with the proliferation index (187) and angiogenesis (188).
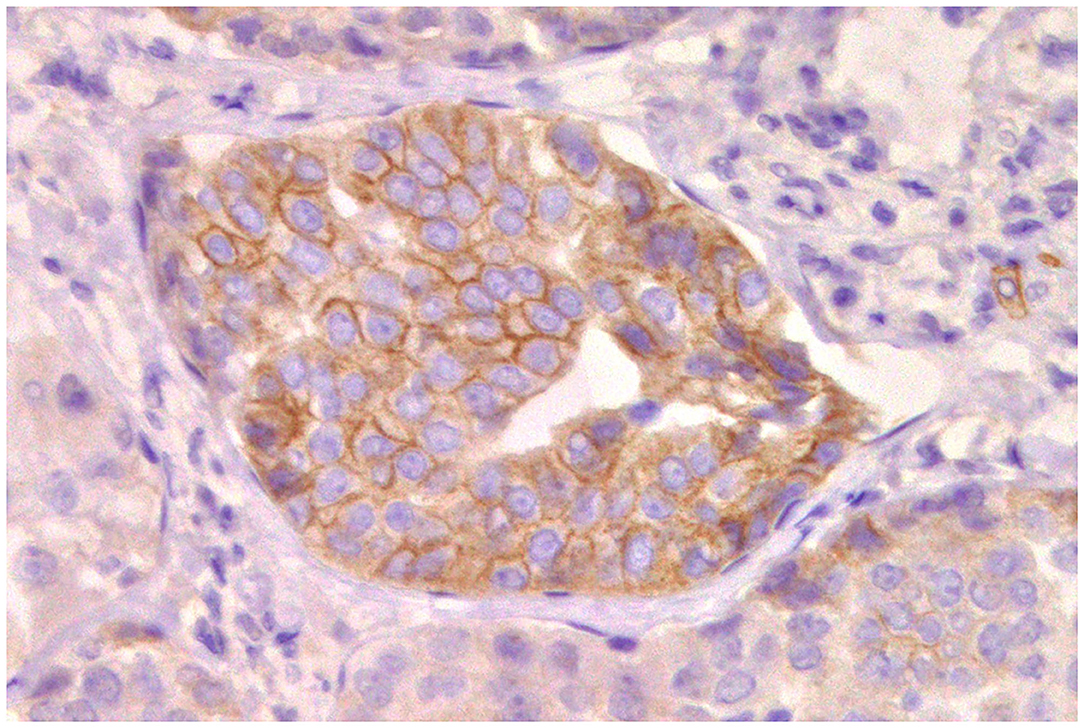
Figure 10. Tubular carcinoma, mammary gland, dog. Immunohistochemical membranous staining of stem cell factor receptor (c-KitR). Neoplastic cells are positive.
In veterinary medicine, there are only two approved targeted anti-c-Kit TKIs: toceranib (Palladia) and masitinib. Toceranib, designed and approved for mast cell tumors in dogs, acts mostly through the inhibition of the c-KitR, and it is a sister compound to sunitinib, which was later approved for human therapies (189). Toceranib appears to exert antitumor activity against a variety of dog cancers, including CMC, particularly in cases with pulmonary metastases (143). Toceranib also has anti-angiogenic effects, inhibiting VEGFR and PDGFR, and produces a significant decrease in regulatory T cells, which may increase immune surveillance (189). Toceranib has shown a discrete in vitro reduction in cell proliferation on canine mammary carcinoma cell lines (184).
The evaluation of toceranib in clinical trials with dogs with CMC is very limited. In a study with dogs presenting IMC, toceranib was given in combination with piroxicam (anti-COX-2) and thalidomide (an immunomodulatory and anti-angiogenic agent) (190), with or without hypofractionated radiation therapy. The authors found a significant improvement in the clinical benefit rate and overall survival time compared with historical palliative treatment. The response was better when radiotherapy was employed (85).
Masitinib is also a potent and selective inhibitor of the c-Kit receptor that has been recommended for its use in canine mast cell tumors (191). It also inhibits the PDGF receptor and the fibroblast growth factor receptor (FGFR-3). Masitinib has been studied in vitro as a “chemosensitizer” by enhancing the anti-proliferative effects of a cytotoxic drug, gemcitabine (192). There are no other studies on the treatment of CMC.
Although TKIs (in particular toceranib) are commonly used for CMC treatment, in the veterinary clinic, the evidence is merely anecdotal, and only the aforementioned studies, one in vitro (184) and two in vivo (85, 189), have been conducted. There is an urgent need for prospective randomized studies to adequately evaluate the effectiveness of toceranib in patients with CMC in c-Kit-positive or c-Kit-negative tumors.
Other tyrosine kinase inhibitors are under study. Palbociclib is an inhibitor of cyclin-dependent kinase 4 (CDK4) and CDK6, which are key regulators of the cell cycle machinery and, thus, cell proliferation (193). In women, palbociclib improves progression-free survival in ER+, HER-2– breast cancer when combined with an aromatase inhibitor (letrozole) or an ER downregulator (fulvestrant), so it received approval from the FDA and EMA (194). CDK6 has been consistently detected in CMT cells with no association to histotype or grade (64). In vitro studies with canine mammary cell lines have shown that palbociclib induces cell cycle arrest, prevents colony formation, and impairs cell migration activity (64). There are no clinical trials on dogs with CMC.
The tumor suppressor gene p53 plays a central role in tumorigenesis. The p53 protein, after tetramerization, inhibits cell proliferation, functioning as an initiator of cell cycle arrest and apoptosis. Mutations of p53 often cause a disruption of its tumor-suppressor function and induce genomic instabilities (195). Mutations in the p53 gene are associated with more than half of all human cancers and have been described in multiple cancers in dogs (196), including mammary tumors (197, 198). However, its expression and mutation status as prognostic factors in veterinary medicine are controversial: while some studies have found no correlation (199, 200), other authors have associated higher levels of p53 with poor overall survival (201, 202).
Mutation of the p53 gene can lead to a stable protein that is identifiable by immunohistochemistry (203), although a truncating mutation of the p53 gene can lead to an immunohistochemically undetectable protein (204). Therefore, four different patterns of p53 immunolabeling have been published: overexpression, complete absence, cytoplasmic staining, and wild-type staining. The first three patterns are related to an underlying p53 mutation, while the fourth pattern is primarily associated with no mutation (204, 205). Altered expressions of p53 may result from a direct mutation of p53 within tumor cells or from an altered localization of p53 by increased export of the protein from the nucleus, thereby decreasing its downstream targets and inhibiting apoptosis and cell cycle arrest (195).
Approximately 30% of HBCs have a p53 mutation, but this frequency is dependent on the molecular subtype, as the luminal subgroup has the lowest mutation rate and TNBC has the highest (up to 88%) (206). Additionally, p53 overexpression has been correlated with more aggressive HBCs and worst outcomes (207–209).
In CMC, malignant tumors have shown higher levels of p53 than benign tumors (210), and the increase of p53 is greater in higher-grade tumors with higher proliferation rates (211). Likewise, a significant correlation between increases in p53 expression and mutations with shorter OS has been found (212).
Since mutations in the p53 gene have an enormous impact on cancer development, great efforts are being made into the mechanisms that can counteract this effect and are brought together in several extensive reviews (213, 214) that compile different experimental approaches to target p53 in human cancer: inhibition of mutant p53 by promoting its protein degradation, restoration of the wildlife activity of mutant p53, and immune stimulation against p53 activity.
In contrast, there is only one study on p53 therapy in canine mammary cancer cells. Neoplastic cells can increase the cytoplasmic translocation of nuclear p53 by overexpressing exportin-1, thus preventing its binding to DNA and its anti-proliferative activities (215). The addition of KPT-185 and KPT-355, engineered molecules that inhibit exportin-1 on canine mammary carcinoma cells, in vitro induced cell cycle arrest, apoptosis, and reduced growth (216).
Cyclooxygenases (COX) are a group of enzymes that catalyzes the conversion of arachidonic acid to prostanoids (prostaglandins, prostacyclins, and thromboxanes). In humans and animals, COX exist in three isoforms: COX-1, constitutively found in most cells, in charge of maintaining homeostasis, protection of the gastric mucosa, and regulation of platelet aggregation and renal blood flow; cyclooxygenase-2 (COX-2), an inducible isoform that is detected in neoplastic and normal cells induced through several stimuli (e.g., mitogens, growth factors, hormones, and pro-inflammatory cytokines); and COX-3, which is expressed mainly in the central nervous system and the aortic wall (217, 218).
Since 1897, with the development of aspirin, non-steroidal anti-inflammatory drugs (NSAIDs) have been developed as analgesic, antipyretic, anti-inflammatory, and anti-rheumatic treatment (219). However, a meta-analysis showed a remarkable preventive effect against colon cancer (220). Other studies found the same effects in different types of cancer, such as breast cancer (221). Nevertheless, the adverse effects of long-term use of NSAIDs were significant, mainly gastrointestinal bleeding, increased uric acid, and coagulation inhibition, among others (222). In an attempt to avoid these adverse effects and target specifically COX-2 for its participation in the neoplastic process, selective COX-2 inhibitors were designed and named as “coxibs.” Experimentally, both NSAIDs and coxibs have been shown to inhibit tumorigenesis by inhibiting cancer cell growth and proliferation, modulating apoptotic activity, reducing the metastatic and invasive potential of cells, and by inhibiting angiogenesis (223, 224).
Coxibs had been related in human medicine to thrombotic cardiovascular events, myocardial infarction, and stroke, especially in long-term use (225). Although subsequent meta-analysis revealed that the risk of cardiovascular events was not related to the usage of coxibs (226), the use of coxibs as adjuvants in the treatment of human cancer is an ongoing intense field of research, especially in combination with chemotherapeutic agents (227).
COX-2 is overexpressed in various canine epithelial malignant tumors, including ovarian carcinomas, prostate carcinomas, urothelial and transitional cell carcinomas, colorectal and small intestine tumors, squamous cell carcinomas, osteosarcoma, and melanoma (228).
Since 2003, COX-2 expression has been reported in both benign and malignant canine mammary tumors (229) (Figure 11). Cyclooxygenase-2 is overexpressed in 83–95% of the CMC in association with characteristics of aggressiveness, such as high histological and nuclear grades, mitotic index, and lymph node metastasis (230–232). COX-2 has an important role in the angiogenesis of CMC: its presence is correlated with MVD and EGFR and VEGF expressions (233). Interestingly, in canine IMC, which is characterized by exacerbated angiogenesis, lymphangiogenesis, and lymphangiotropism, COX-2 is associated with higher lymphatic proliferation index, VEGF-D (a lymphangiogenic factor), and its receptor VEGFR-3; in contrast, COX-2 is associated with VEGF-A in non-inflammatory mammary cancer (234), indicating a different role of COX-2 in the angiogenesis of IMC.
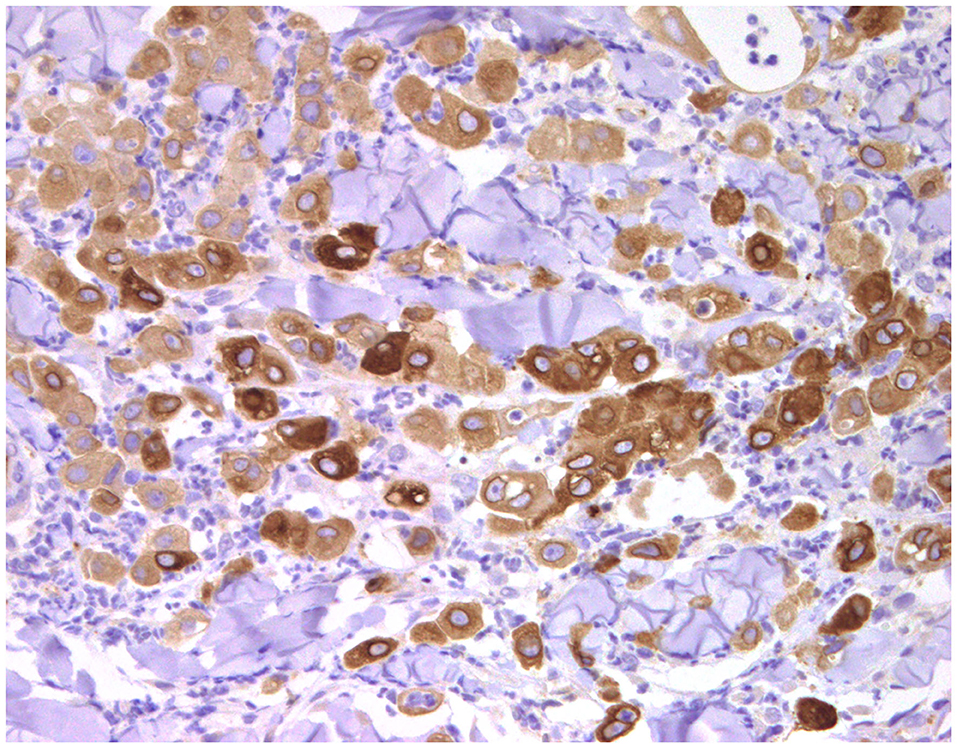
Figure 11. Anaplastic carcinoma, mammary gland, dog, inflammatory carcinoma. Immunohistochemical cytoplasmic staining of cyclooxygenase-2 (COX-2). Neoplastic cells are positive with different intensities of immunolabeling.
Moreover, COX-2 also participates in the immunomodulation of the tumor microenvironment by promoting the M2 phenotype of macrophages, inhibiting antigen-presenting cells and reducing CD8+ T cells, all of which impair the antitumoral immune response (235).
The non-selective (anti-COX 1 and 2) NSAIDs meloxicam and piroxicam have been tested in vitro against CMC cells, which resulted in apoptosis induction, migration inhibition, and cell cycle arrest (236, 237). Additionally, piroxicam was utilized in a xenograft model of CMC, showing a tumor size reduction (238). Further, a small group of dogs with IMC were treated with piroxicam. The mean survival time was significantly longer in dogs treated with piroxicam compared to those treated with doxorubicin-based chemotherapy (239).
Due to the availability of COX-2 selective drugs in veterinary medicine (firocoxib, deracoxib, cimicoxib, robenacoxib, and mavacoxib), their effectiveness in CMC has been studied in cell lines, mice xenografts, and, in lesser extent, in clinical trials. Celecoxib and mavacoxib have been used in CMC cell lines, showing their cytotoxic activity, proliferation inhibition, apoptosis induction, and migration reduction, even in those cell lines with low COX-2 expression, suggesting their extraordinary therapeutic applicability in COX-2-positive or COX-2-negative CMC (217, 240, 241). Deracoxib has been proven alone or in combination with piroxicam, displaying a significant decrease in cell viability, achieving an arrest in cell cycle, and induction of apoptosis in CMC cell lines (237). Additionally, when combined with doxorubicin, a strong synergistic activity was seen, allowing to reduce the dose of doxorubicin in vitro (242). In CMT xenografted mice, deracoxib was given in comparison to piroxicam, although its effect was lower than piroxicam. Nevertheless, the authors did not evaluate the dose of deracoxib, thus concluding that mice could have been underdosed (238).
In a clinical trial, piroxicam showed an increased in OS compared to doxorubicin in 12 IMC-bearing dogs with COX-2-positive tumor cells (239). Likewise, in a case–control prospective study, firocoxib showed higher disease-free survival and overall survival compared to mitoxantrone in dogs with highly malignant CMT-expressing COX-2 (18).
As mentioned above, in the available clinical trials on the use of NSAIDs/coxibs in CMC, patients had COX-2-positive tumors. However, in vitro evidence has shown that anti-COX-2 therapy may be therapeutically useful regardless of the COX-2 expression status (217).
As a conclusion, the anti-COX-2 drugs approved for their use in veterinary medicine are being used in veterinary clinics as anti-inflammatory drugs, but also as an adjuvant in neoplastic patients, no matter the expression of the enzyme in the patients' tumors and, in many cases, the lack of proper clinical trials.
Epithelial–mesenchymal transition (EMT) is a complex process in which epithelial cells lose their characteristics and acquire mesenchymal properties. It is essential in different embryonic stages and organ development, wound healing, and neoplastic infiltration and metastasis, cell motility, and invasiveness (243). By this process, epithelial cells undergo detachment and acquire the capacities of motility and invasiveness through the extracellular matrix to finally enter blood and lymphatic vessels and colonize different organs.
EMT is driven by the dysregulation of the adhesion molecules (mainly cadherins). Cadherins are calcium-dependent adhesion molecules responsible for cell-to-cell attachment and for maintenance of the normal structure and polarization of tissues. The most relevant are P-cadherin (placental cadherin), N-cadherin (neural cadherin), and E-cadherin (epithelial cadherin) (244). In normal human mammary tissue, E-cadherin is expressed in luminal epithelial cells, while P-cadherin is found in myoepithelial cells (245). In HBC, E-cadherin is known to be an inhibitor of metastasis, and its downregulation or inactivation leads to aggressive forms of breast cancer, EMT, lymphovascular invasion and metastasis (246, 247), as well as higher histological grade (248). Likewise, in CMC, reduction of E-cadherin expression has been related to large size and ulceration of mammary tumors (249), infiltrative growth, high histological grade, and lymph node metastasis (250–253), as well as shorter overall and disease-free survivals (254). EMT involves a range of transcription factors, including zinc finger E-box-binding homeobox 1 and 2 (ZEB1 and ZEB2), Snail, signal transducer and activator of transcription 3 (STAT3), and Twist, as well as transcriptional targets such as transforming growth factor-β (TGF-β), all of which suppress the expression of epithelial-associated molecules (E-cadherin and membranous β-catenin) and promote molecules associated with mesenchymal cell phenotypes (N-cadherin, cytoplasmic β-catenin, fibronectin, and vimentin) (255, 256). In CMC, Snail and ZEB2 have been proven to be related to E-cadherin downregulation in invasive micropapillary carcinomas (257, 258).
Not only EMT is required for successful metastatic colonization, but also cells that are capable of initiating tumorigenesis may undergo self-renewal and differentiate into various subsets of cells found in the primary tumor; these cells are called cancer stem cells (CSCs) (259). Molecular pathways that lead to EMT are markedly overlapping with those of CSC generation, allowing neoplastic epithelial cells to pass through the EMT and generate a population of CSCs (260). Additionally, CSCs are associated with therapeutic resistance (261).
Several reviews are centered on EMT as a source of metastasis, CSC generation, and therapy resistance in HBC (261–263). Likewise, studies on CMC have revealed a positive correlation between cells undergoing EMT and higher tumor grade and metastasis (264) and have identified ZEB1 and ZEB2 as potential therapeutic targets in CMT cells in vitro, intended to restore E-cadherin and inhibit EMT, although to date, there are no drugs targeting these molecules (265).
A number of attempts have been made for the therapeutic targeting of EMT and CSCs in HBC and CMC, which are presented below.
As aforementioned, melatonin is capable of disrupting estrogen-dependent cell signaling. In addition, in vitro studies on HBC and CMC cell lines have shown that melatonin is also able to reduce EMT through the degradation of β-catenin, an E-cadherin repressor (266), reducing cell migration, invasion, and CSC generation (267, 268). Significantly, the effect of melatonin is higher on ER+ CMC cells overexpressing melatonin receptors (269). In a more recent in vitro study, CMC cell lines treated with melatonin plus IL-25 significantly reduced cell viability, increased caspase-3-mediated apoptosis, and reduced pro-angiogenic VEGF-A (268).
Metformin, a commonly used drug in human medicine as oral treatment for type II diabetes, is being experimentally studied due to its anti-carcinogenic properties associated with the inhibition of the EMT process. Metformin has been shown to inhibit EMT in HBC cells by repressing the drivers of TGF-β (270). In vitro studies on CMC cell lines proved that metformin is able to induce cell cycle arrest and reduce cell migration and N-cadherin expression while increasing E-cadherin expression. The foreseen effect was ever more prominent when combined with silencing of TGF-β in the CMC cell line (271). In CMC xenografted mice treated with metformin, there was a significant decline in CSCs and reduction of lung metastasis and tumor growth (272–274). When combined with LY294002 (an inhibitor of the PI3K/AKT/mTOR pathway with anti-angiogenic properties), metformin showed a marked reduction in viability and tumor cell growth in vitro, while in CMC xenografted mice, both drugs decreased the tumor size and showed an important anti-angiogenic effect (reduction of VEGF-A expression and MVD) (275).
Cancer stem cells are also characterized by the presence of multidrug-resistant (MDR) adenosine triphosphate-binding cassette (ABC) transporter efflux pumps, influencing chemotherapy resistance in cancer due to their capacity to export a wide variety of cell substances (276). It has been seen that the Wnt/β-catenin pathway can upregulate the multidrug resistance protein 1 (MDR-1). Simvastatin (a lipid-lowering drug) has been studied in mammary oncology due to retrospective evidence of an improved OS and recurrence-free survival in HBC-bearing patients (277), and further in vitro analyses revealed an induction of apoptosis on HBC cell lines (278). In vitro studies using simvastatin on CMC cell lines showed that the expressions of MDR-1 and β-catenin were reduced, contributing to a chemosensitizing effect on CMC cells (279). Furthermore, when combined with doxorubicin, simvastatin exhibited a synergic cytotoxic effect on CMC cells (280). However, despite good in vitro results, discouraging large meta-analyses have found no significant benefit from the use of simvastatin in patients suffering from HBC (281–283).
Artemisinin, a derivate from the plant Artemisia annua, employed in Chinese traditional medicine, was discovered by Tu Youyou in 1972 for the treatment of malaria. By this discovery, Tu Youyou was co-recipient of the 2015 Nobel Prize in Medicine. Studies on HBC cells showed the in vitro suppression of N-cadherin, involved in EMT (284). Additionally, synthetic derivates of artemisinin (e.g., artesunate) are capable of inducing caspase-dependent apoptosis in HBC cells (285). The in vitro treatment of CMC cells with an artemisinin derivative (dihydroartemisinin, DHA) showed an inhibition of cell migration and invasiveness by downregulating the expression of EMT-related genes (Slug, ZEB1, ZEB2, and Twist) (286). In human medicine, a few phase I clinical trials have been conducted without significant adverse effects (287, 288), although a patient with HBC on artemisinin treatment showed toxic encephalopathy (289). Knowing the neurotoxicity of artemisinin in experimental animal models (290), a phase I clinical trial should be performed in domestic animals before giving any general recommendation on its use in veterinary patients.
In human oncology, after decades of depleting the immune system of cancer patients with chemotherapy, the tendency is now to protect and increase the action of the immune system against cancer cells (291).
Cancer immunoediting is the process by which the immune system tries to destroy neoplastic cells and is composed of three steps: elimination, equilibrium, and escape (292). In the first step, the host's immune system responds to the newly formed tumor and removes it prior to any clinical evidence. If some resistant clones of the tumor are present, they survive and remain inactive in the second stage, the equilibrium. In the escape or evasion stage, tumor cells improve their ability to evade the immune system, eventually leading to clinical manifestation (293). Some of the evasion mechanisms are as follows: decreased or absent expression of major histocompatibility complex (MHC) molecules, activation of immunoregulatory pathways (immune checkpoints) such as the inhibitory molecule CTLA-4 (cytotoxic T lymphocyte antigen 4), upregulation of PD-L1 (programmed death ligand 1) that binds to the PD-1 receptor on T lymphocytes and represses their function, secretion of immunosuppressive factors such as TGF-β, interleukin-10, and VEGF, as well as induction of regulatory T cells (294). Although many of these mechanisms are already known, the true interplay between the pathways, and the interaction between tumor cells and the immune system and microenvironment, is still largely unknown.
Some mAbs that target specific molecules that intervene in a signaling pathway (e.g., anti-HER-2) have been mentioned in this review. In this section, we will discuss an intriguing class of antibodies designed to modulate the immune response and the use of vaccines, including cellular immunotherapy and DNA vaccines. Cellular immunotherapy, in particular hybrid cell vaccines, are based on cells generated by fusing antigen-presenting cells (i.e., dendritic cells) with the tumor cells of the recipient, intending to present to the immune system the whole tumor-associated antigens and activate an immune response (295). DNA vaccines are based on the introduction of one or more genes (e.g., tumor antigen, cytokines, etc.) into plasmids that are delivered into the patient with the subsequent expression of the introduced gene (296). Finally, the role of oncolytic viruses, which show selective cytotoxicity toward cancer cells and may favor the restoration of the anticancer immune function, will be reviewed (297).
The most successful human immunotherapies to date include mAbs against lymphoma antigens (i.e., CD20—rituximab) as well as mAbs against immune checkpoint molecules such as PD-1 (i.e., pembrolizumab, atezolizumab, and nivolumab) and CTLA-4 (i.e., tremelimumab and ipilimumab), which are able to release the cytotoxic activity of T lymphocytes and activate other immune responses such as antigen presentation and cytokine production (298). Besides targeting immune checkpoint molecules, other immunotherapies are being developed, such as tumor-specific cytotoxic immune cells and cytokines. However, although some cancers, such as melanoma and lymphoma, respond well to immunotherapy, other solid tumors still have weak responses (299).
Cancer cell lines and mouse models, including transgenic mice and patient-derived xenografts, have been extremely useful in the study of human cancer, yielding valuable insights into cancer biology, genetics, and biochemistry (300). However, they have limitations and lack essential features inherent only to spontaneous cancers, like an intact complex immune system (301, 302). Because normal immunocompetent mice reject human tumor grafts, there are no preclinical experimental models to investigate immunotherapy for cancer patient tumors. Canine patients with CMC have been used as an intermediate model in several clinical trials with novel immunotherapy (303).
Several clinical trials have revealed relevant information regarding canine cancer immunotherapy in osteosarcoma, lymphoma, melanoma, meningioma, bladder cancer, soft tissue sarcoma, and hemangiosarcoma using multiple immunotherapeutic approaches (DNA vaccines, cellular immunotherapy, mAbs, bacteria, etc.) (303–310), which are out of the scope of this review.
Even though HBC is not considered a highly immunogenic cancer, immunotherapeutic strategies are being successfully tested especially against TNBC (311). Proof of this is that the FDA approved an immunotherapy for advanced PD-L1+ TNBC, atezolizumab (anti-PD-1 mAB), in combination with paclitaxel (312). Another anti-PD-L1 mAb, pembrolizumab, has also been shown to improve the progression-free survival in PD-1+ TNBC when combined with paclitaxel, gemcitabine, carboplatin, or eribulin (313, 314). Although PD-1 has been detected in CMTs (315, 316), no attempt has been made to use anti-PD-1 or anti-PD-L1 mAbs against CMC, perhaps due to cost restrictions. The only published clinical trial with mAbs against CMC (including IMC) used the mouse monoclonal antibody BR96, which recognizes a specific antigen (Lewisy-related carbohydrate, Ley) expressed in several solid tumors and gastrointestinal epithelium, conjugated with a truncated, non-binding derivate of Pseudomonas exotoxin A. Stable disease or partial response was achieved in IMC cases, as well as neutralizing antibodies (317). In addition to this, in 2014, a canine anti-EGFR-1 mAb was developed, but it never reached the clinical or preclinical level (151).
Cellular immunotherapy is an emerging field of research in which immune cells are extracted, modified, and reinfused into the patient (318). In HBC, two main types of cellular immunotherapy have been studied: adoptive cell therapy (ACT, based on T lymphocytes) and dendritic cell therapy (319). ACT is based on the isolation of T lymphocytes in the resected tumor, in vitro expansion or modification, and subsequent reinfusion (318). Few information on HBC are available to date. Direct T lymphocyte reinfusion in combination with pembrolizumab led to a complete durable regression in a patient with chemotherapy-refractory HBC (320). Chimeric antigen receptor (CAR) T cell therapy is a type of modification where the receptor is engineered to target a specific antigen and combine antigen-binding and T cell-activating functions, hence recognizing antigens in the absence of the presentation by the MHC (321). To date, only preclinical studies have been published using HER-2, mucin 1 cell surface associated (MUC-1), mesothelin (MSLN), epithelial cell adhesion molecule (EPCAM), and carcinoembryonic antigen (CEA) as targets (322). On the other hand, in dendritic cell therapy, the cells are isolated and combined with tumor antigens before reinfusion into the patient (319). Despite clinical settings being currently limited to phase I/II human trials, preclinical studies on HER-2-loaded and cyclin D1-loaded dendritic cell vaccines have been shown to significantly inhibit the HBC xenografted tumor growth in mice (323). Recently, autologous hybrid-cell vaccines were produced for a clinical trial in CMC as an intermediate model for HBC. The therapy was combined with immunostimulatory oligonucleotides and gemcitabine and achieved 3.3 times longer median survival times than the control group, except for the case of IMC, which only resulted in a median of 42 days (324).
DNA, or gene-based, vaccines are intended to deliver functional genes to the target cells for the expression of functional proteins (325). Since naked DNA is readily accessible for endonucleases, the DNA needs an effective and safe delivery method, which can be biological (e.g., viruses and bacteria) or non-biological (e.g., physical methods such as electroporation or chemical methods such as nanoparticles) (326). In HBC, different delivery methods have been tested to transport tumoral antigens, such as HER-2, p53, MUC1, Twist, and mammaglobin-1, as well as immunostimulatory molecules such as IL-6 and IL-12 (326, 327).
Since the intravenous administration of IL-12 has been associated with grave toxicity in humans (328), a clinical trial using nine dogs, one of them with a mammary tumor, used intratumoral IL-12 (plasmid DNA by electroporation) as an immunostimulatory cytokine. Despite transient increases in serum and tumor IL-12 and IFN-⋎, no clinically relevant outcome benefits were seen (329). Similarly to this, recombinant viral vaccines, based on replication-defective recombinant adenoviruses, have been proven to be safe and to induce strong antibody and cellular antigen-specific immune responses in non-human primates (330). A study evaluated the ability of DNA electroporation and a recombinant adenovirus serotype 6, both expressing telomerase reverse transcriptase (overexpressed in tumor cells, while low to absent in normal cells) and HER-2, to induce immune responses in healthy dogs against these proteins. A detectable and long-standing cellular and humoral immune response was detected in the absence of side effects or autoimmunity (331).
An anticancer DNA vaccine based on p62 (a protein involved in selective macroautophagy that is dispensable for most tissues, but essential for the development and survival of tumors) was utilized in CMC xenografted mice and dogs bearing mammary carcinomas. The intramuscular administration of the p62 DNA vaccine achieved a partial response or stable disease in the absence of noteworthy secondary effects. Antitumoral activity was related to lymphocyte infiltration, particularly T lymphocytes, and tumor encapsulation via fibrosis (332, 333).
Another clinical trial with dogs presenting CMC utilized nanoparticles carrying DNA plasmids coding canine interferon-β and herpes simplex virus (HSV) thymidine kinase (a suicide gene), which were injected into the tumor bed during mastectomy; afterwards, subcutaneous injections of the nanoparticles associated with a human granulocyte–macrophage colony-stimulating factor and interleukin-2, mixed with allogeneic mammary carcinoma extracts, were periodically administered. The therapy was well-tolerated; only one out of 26 patients had recurrence and none displayed distant metastasis, and overall survival was also improved (334).
Oncolytic viruses show selective cytotoxicity toward cancer cells and may favor the restoration of immune anticancer function (297). Clinical trials with oncolytic viruses are currently ongoing in humans, and two viruses have been approved for commercial use. The first, H101 or Oncorine®, is an adenoviral construct with an E1B deletion (in order to avoid replication in normal cells), approved in China in 2005 for the treatment of head-and-neck squamous cell carcinoma (335). The second, T-VEC or ImlygicTM, is an engineered herpes simplex virus type I (HSV-1) that expresses the human granulocyte–monocyte colony-stimulating factor as an immune stimulant, approved in 2015 by the United States FDA for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery (336). In veterinary medicine, a number of viruses with natural oncolytic capacity, as well as engineered viruses, are being studied for several neoplasms (337, 337–349). Among them are morbillivirus, poxvirus, and reovirus.
In human medicine, the measles virus has demonstrated an oncolytic potential since anecdotical reports describing the regression of hematopoietic neoplasms after natural infection with measles virus (350). In HBC, the measles virus has shown a strong cytolytic effect in cancer cell lines in vitro (351–354). Additionally, attenuated measles virus has been proven to overcome chemoresistance in HBC cells. Several studies in mice, however, showed that viral replication also existed in the organs and cells of infected mice and not only in the targeted tumor cells (355–357). Therefore, some modifications have been done to the viral strains. For instance, a recombinant measles virus strain was created by eliminating its ability to bind to the signaling lymphocyte activation molecule (SLAM), a major receptor used by the wild-type virus to infect immune cells in naturally occurring infections. Cancer cells do not express SLAM molecules, but the measles virus is able to use the Nectin-4 receptor to bind and infect the cell (351). Interestingly, Nectin-4 expression has been found in HBC cells (358) and in 45% of CMT tissue samples and in CMC cell lines. Recombinant measles virus strain exerts cytotoxic effects in Nectin-4-expressing CMC cell lines. In Nectin-4-expressing CMC xenografted mice, this oncolytic therapy showed significant suppression of tumor growth without any noticeable adverse effect (340).
Canine distemper virus (CDV) has been shown to induce apoptosis in the cerebellum and lymphoid tissue of naturally infected dogs through the extrinsic pathway, activating caspase-8 and caspase-3 (359, 360). Therefore, this morbillivirus is considered to be a candidate for potential treatment in canine malignancies. An attenuated strain of CDV showed a substantial oncolytic effect in CMC cells both in vitro and in xenografted mice, without significant adverse events, by inducing apoptosis through the same pathways as natural infection (extrinsic pathway) with participation of nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) (346, 361).
Several studies on the use of multiple strains of vaccinia virus (362–366) have demonstrated the in vitro and preclinical effects against HBC, which have led to a randomized phase III clinical trial in patients with metastatic HBC in which the use of a poxviral vaccine in combination with docetaxel resulted in an increase in progression-free survival (367).
Two oncolytic strains of vaccinia virus (strain GLV-1h68 and strain GLV-5b451 expressing GLAF-2, an antibody against VEGF) have been tested against CMC cells in vitro or in xenografted mice, resulting in efficient infection and lysis of cells in vitro while achieving significant tumor growth inhibition in vivo with strong inflammatory and oncolytic-associated effects (368, 369) and a reduction of MVD in the tumors treated with strain GLV-5b451 (337).
An attenuated form of Myxoma virus lacking the serp2 gene (an anti-apoptotic virulence factor) was used to evaluate its oncolytic activity in canine mammary cancer cells and showed severe cytopathic effects and adequate viral replication (370).
The oncolytic virus pelareorep (REOLYSIN®) is a non-modified serotype 3 reovirus strain that has shown antitumor activity in clinical and preclinical models, especially in pancreatic cancer, and currently is being tested in clinical trials to assess its efficacy as an oncolytic agent against several cancers. In HBC cells, REOLYSIN® infection in the presence of DNA-damaging agents enhances infection and triple-negative breast cancer cell killing by the reovirus (371).
REOLYSIN® was tested in CMC cells in vitro and in xenografted mice, demonstrating significant cell death via caspase-3-mediated apoptosis (338). When combining this oncolytic therapy with low doses of paclitaxel, carboplatin, gemcitabine, or toceranib, its activity was enhanced with all the therapeutic agents, except toceranib (341). A series of cases of dogs with various malignancies, which included two cases of CMC (one IMC and one non-IMC), were treated with intratumoral or intravenous REOLYSIN®. Less than 50% of the dogs presented grade I or II adverse effects, which included vomiting, diarrhea, and inflammation of the injected tumor. Dogs did not shed virus and had elevated neutralizing antibodies. No specific information about the antitumoral response in CMC patients was provided (341, 345).
Nanotechnology has been evolving rapidly in recent years, providing new therapeutic tools for several diseases. Nanoparticles are defined as particles below 100 nm of dimension, although their surface is generally large enough to bind and carry therapeutic compounds (372). Nanoparticles have been proposed as drug carriers in cancer treatment since they can increase drug accumulation in target tissues, optimizing the therapeutic effect (373).
Several nanoparticle-based delivery platforms have been approved by the US FDA, and two nano-based drugs are already in the market for HBC—Doxil® (doxorubicin-loaded nanoparticles) and Abraxane® (albumin-bound paclitaxel-loaded nanoparticles)—whose coating evades the immune system, allowing a precise targeting delivery (374). Additionally, several clinical trials with HBC patients are currently under study using these delivery platforms to carry doxorubicin, paclitaxel, cisplatin, irinotecan, annamycin (synthetic derivate of doxorubicin), and docetaxel (375–379).
In dogs, doxorubicin is a commonly used chemotherapeutic for CMC. However, its toxicity is dose-limiting, reducing treatment efficacy (58). Aldoxorubicin (a prodoxorubicin bound to albumin, which is cleaved from the drug in the acidic tumor microenvironment) (380) was constructed into nanofiber peptides and administered parenterally to xenografted mice bearing HBC, reducing primary tumor burden and lung metastasis as well as improving survival (381). This was also tested in canine mammary tumor cells in vitro, showing an excellent anti-proliferative effect at lower doses than free aldoxorubicin or doxorubicin (382).
Gold nanoparticles, associated with other minerals, have been used in human oncology as an aid in diagnostic methods (due to the molecular weight of gold, it captures many x-rays) and as support in thermo- and phototherapy (383). However, direct in vitro toxicity has been seen against human colorectal, hepatocellular, and mammary carcinoma cell lines (384). Two metal compounds, Co(III) and Zn(III), that have previously shown remarkable anti-proliferative activity against human colorectal carcinoma cells, hepatocellular carcinoma cells, and breast carcinoma cells were loaded onto 14-nm gold nanoparticles. These metal compounds demonstrated to efficiently lyse CMC cells in vitro; moreover, when loaded onto nanoparticles, the effect was even stronger at lower doses (385).
Herbal medicine has long been administered to treat malignancies in Asian countries (386). While 86.4% of HBC patients with Asian background tend to use herbal medicine, the figures in the Western world, although significantly lower, are continually increasing (387). Over the years, numerous herbal compounds with anticancer activities, such as proliferation inhibition, apoptosis induction, anti-angiogenic, and anti-metastatic, have been identified. The most common natural products are curcumin, berberine, artemisinins, ginsenoides, ursolic acid, silibinin, emodin, triptolide, cucurbitacins, tanshinones, ordonin, shikonin, gambogic acid (GA), artesunate, wogonin, β-elemene, and cepharanthine (388).
Few phytochemicals have been studied in veterinary oncology. The following are those related to CMC.
Curcumin is a phytochemical isolated from the rhizome of turmeric (Curcuma longa), used in traditional medicine in India and China for a long time (389). In vitro studies have demonstrated its anti-proliferative, pro-apoptotic, anti-angiogenic, and chemosensitizing activities against several tumor types, including breast cancer cells (390–396). In veterinary medicine, curcumin and carnosic acid, derived from rosemary leaf extract, were used alone or in combination on mastocytoma, osteosarcoma, and CMC cell lines and resulted in caspase 3 and 7 activation and apoptosis, with a potent synergistic effect when used in combination (397). A major limitation of curcumin is its low absorption (< 1% of orally administered curcumin will be absorbed) (398). Therefore, a liposome-encapsulated curcumin formulation that enables intravenous delivery was developed and tested on canine cancer cells such as CMC, melanoma, and osteosarcoma cell lines, as well as endothelial cells. Cell proliferation was effectively inhibited with the treatment; likewise, the viability, migration, and tube formation of endothelial cells were suppressed. In the same study, a pilot clinical trial was conducted with cancer-bearing dogs (CMC, pulmonary carcinoma, thyroid carcinoma, chest wall sarcoma, osteosarcoma, and malignant melanoma), achieving stable disease in ~60% of dogs (399). Finally, a combination of curcumin and paclitaxel was loaded into silica nanoparticles and delivered into CMC cell lines, manifesting a clear and persistent cytotoxic effect (400, 401).
The edible wild ginger Zingiber zerumbet contains several phytochemicals with healing properties; zerumbone is one of the most important due to its antitumor, anti-inflammatory, antioxidant, antimicrobial, antinociceptive, hepatoprotective, and immunomodulatory activities (402). However, its poor absorption and bioavailability are the main issues for its therapeutic application (403). Therefore, nanostructured lipid carriers loaded with zerumbone have been used as an apoptogenic agent in several neoplastic human and canine cell lines, including CMC. The effect was attributable to increases in caspase-8, caspase-9, caspase-3, and caspase-7 (404).
The last one of the herbal compounds that have been used for canine mammary tumors is berberine, an isoquinolone alkaloid of the plant Berberis vulgaris L. that inhibited the proliferation of CMC cells in vitro (405).
Ivermectin is a well-known anti-parasitic agent used to treat a variety of canine parasitic infestations. The mechanism of action of ivermectin in parasites is due to blockade of the parasite chloride channel (406). Currently, ivermectin has been linked to a potential anticancer effect in different tumor types, including breast cancer (407). In vitro studies using ivermectin in CMC cell lines, and further in xenografted mice, effectively inhibited cell growth in a dose- and time-dependent manner. The effects were associated with cell cycle arrest via the downregulation of CDK4 and cyclin D1 expressions and reduced WNT/β-catenin signaling (408).
Selenium possesses different anti-neoplastic mechanisms: promotion of cell apoptosis, anti-angiogenesis, and immune system regulation. Also, its antioxidant effect may reduce the toxicity of conventional chemotherapeutics if used in combination (409). Different selenium compounds (sodium selenite, methylseleninic acid, and methylselenocysteine) showed in vitro anti-proliferative effects on CMC that were even greater when combined with cyclophosphamide. An increase of apoptosis, downregulation of pro-angiogenic VEGFA, angiopoietin-2, and hypoxia-inducible factor-1 alpha, and upregulation of the anti-angiogenic and anti-proliferative phosphatase and tensin homolog (PTEN) were the major features (409). In further CMC xenografted mouse models, the different selenium compounds significantly inhibited tumor growth, generated large necrotic areas, and reduced the MVD compared to the untreated control. This in vivo study also found a reduction in pro-angiogenic factors (VEGFA, PDGF, and angiopoietin-2) (410).
Salinomycin is an ionophore antibiotic isolated from Streptomyces albus, which is widely used in farm animals as an anticoccidial drug (411). Several studies with HBC cell lines demonstrated that salinomycin inhibits in vitro growth by inducing apoptosis and selectively targeting CSC (412–419). Likewise, salinomycin was found to have a profound effect in CMC cell lines by selectively depleting canine mammary CSC and inhibiting the Wnt/β-catenin signaling pathway (preventing cell invasion and migration) (420, 421).
A total of 71 studies focused on adjuvant therapies in CMTs, not including those related to surgery or conventional chemotherapy, were analyzed in this review. The majority of those studies were performed in vitro (49 studies); 15 used xenografted mice to study CMC (in total, 15 papers of mouse models of CMTs). Only six of those investigations done with CMC cells in vitro or in mouse models, have reached the clinical setting (138, 143, 239, 332, 333, 341, 345, 399).
Setting clinical studies of conventional chemotherapy aside, to date, 18 clinical trials have been conducted in dogs with CMC, and a third of them are new immunotherapies, which demonstrate the usefulness of spontaneous CMC as a natural model for the study of HBC in a natural model with a complete immune system. They are also a reflection of the current state of cancer research and the important trend to stimulate the immune system against cancer cells (291). In spite of the relevance of such investigations in dogs, the number of patients recruited in them is very low to obtain conclusive and extrapolative results: five of the 18 clinical studies were done with less than eight dogs with mammary cancer; four of them with less than three dogs. In addition, two more studies were executed in non-tumor-bearing dogs. As a main conclusion, larger prospective randomized studies are needed to provide a strong level of evidence that allows a widespread use of some of these new approaches. Considering that CMTs are the most common malignancy in dogs and the low rate of success of routine adjuvant therapies (i.e., conventional chemotherapy), these clinical trials seem to not be enough. Significantly greater effort must be made to generate knowledge and develop canine-specific targeted therapies. There is a great need for well-planned large prospective randomized clinical trials in dogs with CMC to obtain valid results for both species, humans and dogs, on the use of new therapies.
Following the One Health concept, human and veterinary oncology will have to join forces to take advantage of both the economic and technological resources that are invested in HBC research, together with the innumerable advantages of dogs with CMC as a spontaneous animal model.
LP and GV devised, structured, and wrote the manuscript. GV reviewed the literature. ÁA-D and GV performed the immunohistochemical slides and the photographs. All the authors reviewed and corrected the manuscript.
GV has a PhD grant funded through the Mexican Council for Science and Technology (CONACYT), 515916. ÁA-D has specialization in Veterinary Pathology (ECVP Residency) grant funded by the Complutense University of Madrid, 69/2018. The research of the Oncology Mammary Unit of the Complutense Veterinary Teaching Hospital was funded by the Spanish Ministry of Science, Innovation and Technology, project PGC2018-094516-B-I00.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Moe L. Population-based incidence of mammary tumours in some dog breeds. J Reprod Fertil Suppl. (2001) 57:439–43.
2. Salas Y, Márquez A, Diaz D, Romero L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002-2012: a growing animal health problem. PLoS ONE. (2015) 10:e0127381. doi: 10.1371/journal.pone.0127381
3. Goldschmidt MH, Peña L, Zappulli V. Tumors of the mammary gland. In: Meuten DJ, editor. Tumors in Domestic Animals. Fifth ed. Danvers, MA: John Wiley & Sons, Inc (2017). p. 723–65. doi: 10.1002/9781119181200.ch17
4. Sorenmo K, Worley D, Zappulli V. Tumors of the mammary gland. In: Vail D, Thamm D, Liptack J, editors. Withrow and MacEwen's Small Animal Clinical Oncology. 6 ed. St. Louis, MO: Elsevier (2020). p. 604–25. doi: 10.1016/B978-0-323-59496-7.00028-1
5. Goldschmidt M, Pena L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Pathol. (2011) 48:117–31. doi: 10.1177/0300985810393258
6. Sorenmo KU, Rasotto R, Zappulli V, Goldschmidt MH. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet Pathol. (2011) 48:85–97. doi: 10.1177/0300985810389480
7. Peña L, Perez-Alenza MD, Rodriguez-Bertos A, Nieto A. Canine inflammatory mammary carcinoma: histopathology, immunohistochemistry and clinical implications of 21 cases. Breast Cancer Res Treat. (2003) 78:141–8. doi: 10.1023/A:1022991802116
8. Clemente M, De Andres PJ, Pena L, Perez-Alenza MD. Survival time of dogs with inflammatory mammary cancer treated with palliative therapy alone or palliative therapy plus chemotherapy. Vet Rec. (2009) 165:78–81. doi: 10.1136/vetrec.165.3.78
9. Peña L, De Andres PJ, Clemente M, Cuesta P, Perez-Alenza MD. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up: relationship with clinical and histological characteristics. Vet Pathol. (2013) 50:94–105. doi: 10.1177/0300985812447830
10. Abadie J, Nguyen F, Loussouarn D, Peña L, Gama A, Rieder N, et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: immunophenotypes and prognostic significance. Breast Cancer Res Treatment. (2018) 167:459–68. doi: 10.1007/s10549-017-4542-8
11. Lee K-H, Park H-M, Son K-H, Shin T-J, Cho J-Y. Transcriptome signatures of canine mammary gland tumors and its comparison to human breast cancers. Cancers. (2018) 10:317. doi: 10.3390/cancers10090317
12. Jeong SJ, Lee KH, Nam AR, Cho JY. Genome-wide methylation profiling in canine mammary tumor reveals miRNA candidates associated with human breast cancer. Cancers. (2019) 11:1466. doi: 10.3390/cancers11101466
13. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. (2016) 17:43–46. doi: 10.7314/APJCP.2016.17.S3.43
14. Harbeck N, Penault-llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers. (2019) 5:66. doi: 10.1038/s41572-019-0111-2
15. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. (2000) 406:747–52. doi: 10.1038/35021093
16. Simon D, Schoenrock D, Baumgärtner W, Nolte I. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J Vet Intern Med. (2006) 20:1184–90. doi: 10.1111/j.1939-1676.2006.tb00720.x
17. Marconato L, Lorenzo RM, Abramo F, Ratto A, Zini E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet Comp Oncol. (2008) 6:90–101. doi: 10.1111/j.1476-5829.2007.00143.x
18. Arenas C, Peña L, Granados-Soler JL, Pérez-Alenza MD. Adjuvant therapy for highly malignant canine mammary tumours: cox-2 inhibitor versus chemotherapy: a case–control prospective study. Vet Rec. (2016) 179:125. doi: 10.1136/vr.103398
19. Tran CM, Moore AS, Frimberger AE. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet Comp Oncol. (2016) 14:252–62. doi: 10.1111/vco.12092
20. Nguyen F, Pena L, Ibisch C, Loussouarn D, Gama A, Rieder N, et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: natural history and prognostic factors. Breast Cancer Res Treat. (2018) 167:635–48. doi: 10.1007/s10549-017-4548-2
21. Rutteman G, Withrow SEGM. Tumors of the mammary gland. In: Withrow SJ, MacEwen E, editors. Small Animal Clinical Oncology. 3rd ed. Philadelphia, PA: WB Saunders (2001). p. 455–77.
22. Chocteau F, Abadie J, Loussouarn D, Nguyen F. Proposal for a histological staging system of mammary carcinomas in dogs and cats. Part 1: canine mammary carcinomas. Front Vet Sci. (2019) 6:388. doi: 10.3389/fvets.2019.00388
23. Sorenmo KU, Durham AC, Kristiansen V, Pena L, Goldschmidt MH, Stefanovski D. Developing and testing prognostic bio-scoring systems for canine mammary gland carcinomas. Vet Comp Oncol. (2019) 17:479–88. doi: 10.1111/vco.12509
24. Zappulli V, Peña L, Rasotto R, Goldschmidt M, Gama A, Scruggs J, et al. Surgical Pathology of Tumors of Domestic Animals. United States of America: Davis-Thompson DVM Foundation (2019).
25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. (1991) 19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x
26. Rasotto R, Berlato D, Goldschmidt MH, Zappulli V. Prognostic significance of canine mammary tumor histologic subtypes: an observational cohort study of 229 cases. Vet Pathol. (2017) 54:571–8. doi: 10.1177/0300985817698208
27. Canadas A, França M, Pereira C, Vilaça R, Vilhena H, Tinoco F, et al. Canine mammary tumors: comparison of classification and grading methods in a survival study. Vet Pathol. (2019) 56:208–19. doi: 10.1177/0300985818806968
28. Destexhe E, Lespagnard L, Degeyter M, Heymann R, Coignoul F. Immunohistochemical identification of myoepithelial, epithelial, and connective tissue cells in canine mammary tumors. Vet Pathol. (1993) 30:146–54. doi: 10.1177/030098589303000207
29. Espinosa Los de Monteros A, Millán MY, Ordás J, Carrasco L, Reymundo C, Martín Las de Mulas J. Immunolocalization of the smooth muscle-specific protein calponin in complex and mixed tumors of the mammary gland of the dog: assessment of the morphogenetic role of the myoepithelium. Vet Pathol. (2002) 39:247–56. doi: 10.1354/vp.39-2-247
30. Gama A, Alves A, Gartner F, Schmitt F. p63: a novel myoepithelial cell marker in canine mammary tissues. Vet Pathol. (2003) 40:412–20. doi: 10.1354/vp.40-4-412
31. Peña L, Gama A, Goldschmidt MH, Abadie J, Benazzi C, Castagnaro M, et al. Canine mammary tumors: a review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet Pathol. (2014) 51:127–45. doi: 10.1177/0300985813509388
32. Bocker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological concept. Lab Invest. (2002) 82:737–46. doi: 10.1097/01.LAB.0000017371.72714.C5
33. Rasotto R, Goldschmidt MH, Castagnaro M, Carnier P, Caliari D, Zappulli V. The dog as a natural animal model for study of the mammary myoepithelial basal cell lineage and its role in mammary carcinogenesis. J Comp Pathol. (2014) 151:166–80. doi: 10.1016/j.jcpa.2014.04.013
34. Boecker W, Buerger H. Evidence of progenitor cells of glandular and myoepithelial cell lineages in the human adult female breast epithelium: a new progenitor (adult stem) cell concept. Cell Prolif. (2003) 36:73–84. doi: 10.1046/j.1365-2184.36.s.1.7.x
35. Payne SJ, Bowen RL, Jones JL, Wells CA. Predictive markers in breast cancer–the present. Histopathology. (2008) 52:82–90. doi: 10.1111/j.1365-2559.2007.02897.x
36. Lokuhetty D, White V, Watanabe R, Cree I. WHO Classification of Tumours. Breast Tumors. 5 ed. Lyon: International Agency for Research on Cancer (2019).
37. Rakha EA, Green AR. Molecular classification of breast cancer: what the pathologist needs to know. Pathology. (2017) 49:111–9. doi: 10.1016/j.pathol.2016.10.012
38. Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Archiv. (2008) 453:123–32. doi: 10.1007/s00428-008-0644-3
39. Sassi F, Benazzi C, Castellani G, Sarli G. Molecular-based tumour subtypes of canine mammary carcinomas assessed by immunohistochemistry. BMC Vet Res. (2010) 6:5. doi: 10.1186/1746-6148-6-5
40. Ressel L, Puleio R, Loria GR, Vannozzi I, Millanta F, Caracappa S, et al. HER-2 expression in canine morphologically normal, hyperplastic and neoplastic mammary tissues and its correlation with the clinical outcome. Res Vet Sci. (2013) 94:299–305. doi: 10.1016/j.rvsc.2012.09.016
41. Shinoda H, Legare ME, Mason GL, Berkbigler JL, Afzali MF, Flint AF, et al. Significance of ERα, HER2, and CAV1 expression and molecular subtype classification to canine mammary gland tumor. J Vet Diagn Invest. (2014) 26:390–403. doi: 10.1177/1040638714527289
42. Im KS, Kim NH, Lim HY, Kim HW, Shin JI, Sur JH. Analysis of a new histological and molecular-based classification of canine mammary neoplasia. Vet Pathol. (2014) 51:549–59. doi: 10.1177/0300985813498780
43. Varallo GR, Gelaleti GB, Maschio-Signorini LB, Moschetta MG, Lopes JR, De Nardi AB, et al. Prognostic phenotyping classification for canine mammary tumors. Oncol Lett. (2019) 18:6545–53. doi: 10.3892/ol.2019.11052
44. Seung BJ, Cho SH, Kim SH, Lim HY, Sur JH. Quantitative analysis of HER2 mRNA expression by RNA in situ hybridization in canine mammary gland tumors: comparison with immunohistochemistry analysis. PLoS ONE. (2020) 15:e0229031. doi: 10.1371/journal.pone.0229031
45. Burrai GP, Tanca A, De Miglio MR, Abbondio M, Pisanu S, Polinas M, et al. Investigation of HER2 expression in canine mammary tumors by antibody-based, transcriptomic and mass spectrometry analysis: is the dog a suitable animal model for human breast cancer? Tumor Biol. (2015) 36:9083–91. doi: 10.1007/s13277-015-3661-2
46. Tsuboi M, Sakai K, Maeda S, Chambers JK, Yonezawa T, Matsuki N, et al. Assessment of HER2 expression in canine urothelial carcinoma of the urinary bladder. Vet Pathol. (2019) 56:369–76. doi: 10.1177/0300985818817024
47. Fabelo C, Selmic LE, Huang PC, Samuelson JP, Reagan JK, Kalamaras A, et al. Evaluating optical coherence tomography for surgical margin assessment of canine mammary tumors. Vet Comp Oncol. (2020). doi: 10.1111/vco.12632. [Epub ahead of print].
48. Favril S, Brioschi C, Vanderperren K, Abma E, Stock E, Devriendt N, et al. Preliminary safety and imaging efficacy of the near-infrared fluorescent contrast agent DA364 during fluorescence-guided surgery in dogs with spontaneous superficial tumors. Oncotarget. (2020) 11:2310–26. doi: 10.18632/oncotarget.27633
49. Newton A, Predina J, Mison M, Runge J, Bradley C, Stefanovski D, et al. Intraoperative near-infrared imaging can identify canine mammary tumors, a spontaneously occurring, large animal model of human breast cancer. PLoS ONE. (2020) 15:e0234791. doi: 10.1371/journal.pone.0234791
50. Chang SC, Chang CC, Chang TJ, Wong ML. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998-2002). J Am Vet Med Assoc. (2005) 227:1625–9. doi: 10.2460/javma.2005.227.1625
51. Marconato L, Romanelli G, Stefanello D, Giacoboni C, Bonfanti U, Bettini G, et al. Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003-2008). J Am Vet Med Assoc. (2009) 235:967–72. doi: 10.2460/javma.235.8.967
52. Haga S, Nakayama M, Tatsumi K, Maeda M, Imai S, Umesako S, et al. Overexpression of the p53 gene product in canine mammary tumors. Oncol Rep. (2001) 8:1215–19. doi: 10.3892/or.8.6.1215
53. Enginler SO, Akiş I, Toydemir TS, Oztabak K, Haktanir D, Gündüz MC, et al. Genetic variations of BRCA1 and BRCA2 genes in dogs with mammary tumours. Vet Res Commun. (2014) 38:21–7. doi: 10.1007/s11259-013-9577-7
54. Kaszak I, Ruszczak A, Kanafa S, Kacprzak K, Król M, Jurka P. Current biomarkers of canine mammary tumors. Acta Vet Scand. (2018) 60:66. doi: 10.1186/s13028-018-0417-1
55. Thumser-Henner P, Nytko KJ, Rohrer Bley C. Mutations of BRCA2 in canine mammary tumors and their targeting potential in clinical therapy. BMC Vet Res. (2020) 16:30. doi: 10.1186/s12917-020-2247-4
56. Hahn K, Richardson R, Acvim D, Knapp D. Canine malignant mammary neoplasia: biological behavior, diagnosis, and treatment alternatives. J Am Anim Hosp Assoc. (1992) 28:251.
57. Ogilvie GK, Reynolds HA, Richardson RC, Withrow SJ, Norris AM, Henderson RA, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. (1989) 195:1580–3.
58. Zambrano-Estrada X, Landaverde-Quiroz B, Dueñas-Bocanegra AA, De Paz-Campos MA, Hernández-Alberto G, Solorio-Perusquia B, et al. Molecular iodine/doxorubicin neoadjuvant treatment impair invasive capacity and attenuate side effect in canine mammary cancer. BMC Vet Res. (2018) 14:87. doi: 10.1186/s12917-018-1411-6
59. Sorenmo K. Canine mammary gland tumors. Vet Clin North Am Small Anim Pract. (2003) 33:573–96. doi: 10.1016/S0195-5616(03)00020-2
60. Lavalle GE, De Campos CB, Bertagnolli AC, Cassali GD. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. In Vivo. (2012) 26:375–9.
61. Poirier VJ, Hershey AE, Burgess KE, Phillips B, Turek MM, Forrest LJ, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. (2004) 18:219–22. doi: 10.1111/j.1939-1676.2004.tb00164.x
62. von Euler H, Rivera P, Nyman H, Häggström J, Borgå O. A dose-finding study with a novel water-soluble formulation of paclitaxel for the treatment of malignant high-grade solid tumours in dogs. Vet Comp Oncol. (2013) 11:243–55. doi: 10.1111/j.1476-5829.2011.00314.x
63. Stratmann N, Failing K, Richter A, Wehrend A. Mammary tumor recurrence in bitches after regional mastectomy. Vet Surg. (2008) 37:82–6. doi: 10.1111/j.1532-950X.2007.00351.x
64. Schoos A, Knab VM, Gabriel C, Tripolt S, Wagner DA, Bauder B, et al. In vitro study to assess the efficacy of CDK4/6 inhibitor Palbociclib (PD-0332991) for treating canine mammary tumours. Vet Comp Oncol. (2019) 17:507–21. doi: 10.1111/vco.12514
65. Kareva I, Waxman DJ, Lakka Klement G. Metronomic chemotherapy: an attractive alternative to maximum tolerated dose therapy that can activate anti-tumor immunity and minimize therapeutic resistance. Cancer Lett. (2015) 358:100–6. doi: 10.1016/j.canlet.2014.12.039
66. Lana S, U'Ren L, Plaza S, Elmslie R, Gustafson D, Morley P, et al. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. (2007) 21:764–9. doi: 10.1111/j.1939-1676.2007.tb03019.x
67. Wendelburg KM, Price LL, Burgess KE, Lyons JA, Lew FH, Berg J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001-2012). J Am Vet Med Assoc. (2015) 247:393–403. doi: 10.2460/javma.247.4.393
68. Finotello R, Henriques J, Sabattini S, Stefanello D, Felisberto R, Pizzoni S, et al. A retrospective analysis of chemotherapy switch suggests improved outcome in surgically removed, biologically aggressive canine haemangiosarcoma. Vet Comp Oncol. (2017) 15:493–503. doi: 10.1111/vco.12193
69. Matsuyama A, Poirier VJ, Mantovani F, Foster RA, Mutsaers AJ. Adjuvant doxorubicin with or without metronomic cyclophosphamide for canine splenic hemangiosarcoma. J Am Anim Hosp Assoc. (2017) 53:304–12. doi: 10.5326/JAAHA-MS-6540
70. Alexander CK, Cronin KL, Silver M, Gardner HL, London C. The addition of metronomic chemotherapy does not improve outcome for canine splenic haemangiosarcoma. J Small Anim Pract. (2019) 60:32–7. doi: 10.1111/jsap.12926
71. Marconato L, Chalfon C, Finotello R, Polton G, Vasconi ME, Annoni M, et al. Adjuvant anthracycline-based vs metronomic chemotherapy vs no medical treatment for dogs with metastatic splenic hemangiosarcoma: a multi-institutional retrospective study of the Italian society of veterinary oncology. Vet Comp Oncol. (2019) 17:537–44. doi: 10.1111/vco.12519
72. Treggiari E, Borrego JF, Gramer I, Valenti P, Harper A, Finotello R, et al. Retrospective comparison of first-line adjuvant anthracycline vs metronomic-based chemotherapy protocols in the treatment of stage I and II canine splenic haemangiosarcoma. Vet Comp Oncol. (2020) 18:43–51. doi: 10.1111/vco.12548
73. Bracha S, Walshaw R, Danton T, Holland S, Ruaux C, Obradovich J. Evaluation of toxicities from combined metronomic and maximal-tolerated dose chemotherapy in dogs with osteosarcoma. J Small Anim Pract. (2014) 55:369–74. doi: 10.1111/jsap.12228
74. London CA, Gardner HL, Mathie T, Stingle N, Portela R, Pennell ML, et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: a multi-institutional study. PLoS ONE. (2015) 10:e0124889. doi: 10.1371/journal.pone.0124889
75. Duffy ME, Anderson CL, Choy K, Fidel JL. Metronomic administration of lomustine following palliative radiation therapy for appendicular osteosarcoma in dogs. Can Vet J. (2018) 59:136–42.
76. Matsuyama A, Schott CR, Wood GA, Richardson D, Woods JP, Mutsaers AJ. Evaluation of metronomic cyclophosphamide chemotherapy as maintenance treatment for dogs with appendicular osteosarcoma following limb amputation and carboplatin chemotherapy. J Am Vet Med Assoc. (2018) 252:1377–83. doi: 10.2460/javma.252.11.1377
77. Morgan E, O'Connell K, Thomson M, Boyd S, Sandy J. Primary hepatic neuroendocrine carcinoma treated with doxorubicin and cyclophosphamide in a dog. J Am Anim Hosp Assoc. (2019) 55:e55305. doi: 10.5326/JAAHA-MS-6887
78. Polton G, Finotello R, Sabattini S, Rossi F, Laganga P, Vasconi ME, et al. Survival analysis of dogs with advanced primary lung carcinoma treated by metronomic cyclophosphamide, piroxicam and thalidomide. Vet Comp Oncol. (2018) 16:399–408. doi: 10.1111/vco.12393
79. Elmslie RE, Glawe P, Dow SW. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J Vet Intern Med. (2008) 22:1373–9. doi: 10.1111/j.1939-1676.2008.0179.x
80. Burton JH, Mitchell L, Thamm DH, Dow SW, Biller BJ. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J Vet Intern Med. (2011) 25:920–6. doi: 10.1111/j.1939-1676.2011.0753.x
81. Leach TN, Childress MO, Greene SN, Mohamed AS, Moore GE, Schrempp DR, et al. Prospective trial of metronomic chlorambucil chemotherapy in dogs with naturally occurring cancer. Vet Comp Oncol. (2012) 10:102–12. doi: 10.1111/j.1476-5829.2011.00280.x
82. Cancedda S, Marconato L, Meier V, Laganga P, Roos M, Leone VF, et al. Hypofractionated radiotherapy for macroscopic canine soft tissue sarcoma: a retrospective study of 50 cases treated with A 5 × 6 gy protocol with or without metronomic chemotherapy. Vet Radiol Ultrasound. (2016) 57:75–83. doi: 10.1111/vru.12308
83. Schrempp DR, Childress MO, Stewart JC, Leach TN, Tan KM, Abbo AH, et al. Metronomic administration of chlorambucil for treatment of dogs with urinary bladder transitional cell carcinoma. J Am Vet Med Assoc. (2013) 242:1534–8. doi: 10.2460/javma.242.11.1534
84. Campos CBDE, Lavalle GE, Monteiro LN, Pêgas GRA, Fialho SL, Balabram D. Adjuvant thalidomide and metronomic chemotherapy for the treatment of canine malignant mammary gland neoplasms. In Vivo. (2018) 32:1659–66. doi: 10.21873/invivo.11429
85. Rossi F, Sabattini S, Vascellari M, Marconato L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet Comp Oncol. (2018) 16:497–504. doi: 10.1111/vco.12407
86. Masoud V, Pagès G. Targeted therapies in breast cancer: new challenges to fight against resistance. World J Clin Oncol. (2017) 8:120. doi: 10.5306/wjco.v8.i2.120
87. Schneider R, Dorn CR, Taylor DO. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst. (1969) 43:1249–61.
88. Misdorp W. Canine mammary tumours: protective effect of late ovariectomy and stimulating effect of progestins. Vet Q. (1988) 10:26–33. doi: 10.1080/01652176.1988.9694142
89. Illera JC, Pérez-Alenza MD, Nieto A, Jiménez MA, Silvan G, Dunner S, et al. Steroids and receptors in canine mammary cancer. Steroids. (2006) 71:541–8. doi: 10.1016/j.steroids.2005.11.007
90. Queiroga FL, Pérez-Alenza MD, Silvan G, Peña L, Lopes C, Illera JC. Role of steroid hormones and prolactin in canine mammary cancer. J Steroid Biochem Mol Biol. (2005) 94:181–7. doi: 10.1016/j.jsbmb.2004.12.014
91. Spoerri M, Guscetti F, Hartnack S, Boos A, Oei C, Balogh O, et al. Endocrine control of canine mammary neoplasms: serum reproductive hormone levels and tissue expression of steroid hormone, prolactin and growth hormone receptors. BMC Vet Res. (2015) 11:235. doi: 10.1186/s12917-015-0546-y
92. Mohr A, Lüder Ripoli F, Hammer SC, Willenbrock S, Hewicker-Trautwein M, Kiełbowicz Z, et al. Hormone receptor expression analyses in neoplastic and non-neoplastic canine mammary tissue by a bead based multiplex branched DNA assay: a gene expression study in fresh frozen and formalin-fixed, paraffin-embedded samples. PLoS ONE. (2016) 11:e0163311. doi: 10.1371/journal.pone.0163311
93. Nieto A, Peña L, Pérez-Alenza MD, Sánchez MA, Flores JM, Castaño M. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: clinical and pathologic associations and prognostic significance. Vet Pathol. (2000) 37:239–47. doi: 10.1354/vp.37-3-239
94. Mainenti M, Rasotto R, Carnier P, Zappulli V. Oestrogen and progesterone receptor expression in subtypes of canine mammary tumours in intact and ovariectomised dogs. Vet J. (2014) 202:62–8. doi: 10.1016/j.tvjl.2014.06.003
95. Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. (2014) 10:2293–301. doi: 10.2217/fon.14.110
96. Padilla-Rodriguez M, Parker SS, Adams DG, Westerling T, Puleo JI, Watson AW, et al. The actin cytoskeletal architecture of estrogen receptor positive breast cancer cells suppresses invasion. Nat Commun. (2018) 9:2980. doi: 10.1038/s41467-018-05367-2
97. Lanari C, Wargon V, Rojas P, Molinolo AA. Antiprogestins in breast cancer treatment: are we ready? Endocr Relat Cancer. (2012) 19:R35–50. doi: 10.1530/ERC-11-0378
98. Morris JS, Dobson JM, Bostock DE. Use of tamoxifen in the control of canine mammary neoplasia. Vet Rec. (1993) 133:539–42. doi: 10.1136/vr.133.22.539
99. Tavares WL, Lavalle GE, Figueiredo MS, Souza AG, Bertagnolli AC, Viana FA, et al. Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Vet Scand. (2010) 52:67. doi: 10.1186/1751-0147-52-67
100. Meng Q, Yuan F, Goldberg ID, Rosen EM, Auborn K, Fan S. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. (2000) 130:2927–31. doi: 10.1093/jn/130.12.2927
101. Weng J-R, Tsai C-H, Kulp SK, Chen C-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. (2008) 262:153–63. doi: 10.1016/j.canlet.2008.01.033
102. Martín-Ruiz A, Peña L, González-Gil A, Díez-Córdova LT, Cáceres S, Illera JC. Effects of indole-3-carbinol on steroid hormone profile and tumor progression in a mice model of canine inflammatory mammarycancer. BMC Cancer. (2018) 18:626. doi: 10.1186/s12885-018-4518-z
103. Tremont A, Lu J, Cole JT. Endocrine therapy for early breast cancer: updated review. Ochsner J. (2017) 17:405–11.
104. Suzuki T, Moriya T, Ishida T, Ohuchi N, Sasano H. Intracrine mechanism of estrogen synthesis in breast cancer. Biomed Pharmacother. (2003) 57:460–2. doi: 10.1016/j.biopha.2003.09.007
105. De Andres PJ, Caceres S, Clemente M, Perez-Alenza MD, Illera JC, Pena L. Profile of steroid receptors and increased aromatase immunoexpression in canine inflammatory mammary cancer as a potential therapeutic target. Reprod Domest Anim. (2016) 51:269–75. doi: 10.1111/rda.12676
106. Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. (2001) 21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001
107. Brydon L, Roka F, Petit L, de Coppet P, Tissot M, Barrett P, et al. Dual signaling of human Mel1a melatonin receptors via G(i2), G(i3), and G(q/11) proteins. Mol Endocrinol. (1999) 13:2025–38. doi: 10.1210/mend.13.12.0390
108. Jablonska K, Pula B, Zemla A, Owczarek T, Wojnar A, Rys J, et al. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J Pineal Res. (2013) 54:334–45. doi: 10.1111/jpi.12032
109. Kiefer T, Ram PT, Yuan L, Hill SM. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res Treat. (2002) 71:37–45. doi: 10.1023/A:1013301408464
110. Martínez-Campa C, González A, Mediavilla MD, Alonso-González C, Alvarez-García V, Sánchez-Barceló EJ, et al. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br J Cancer. (2009) 101:1613–19. doi: 10.1038/sj.bjc.6605336
111. Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, et al. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res. (2011) 51:259–69. doi: 10.1111/j.1600-079X.2011.00888.x
112. Dorgan JF, Longcope C, Stephenson HE Jr, Falk RT, Miller R, Franz C, et al. Serum sex hormone levels are related to breast cancer risk in postmenopausal women. Environ Health Perspect. (1997) 105(Suppl. 3):583–5. doi: 10.1289/ehp.97105s3583
113. Gonzalez LO, Corte MD, Vazquez J, Junquera S, Sanchez R, Alvarez AC, et al. Androgen receptor expresion in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer. (2008) 8:149. doi: 10.1186/1471-2407-8-149
114. Esfahlan RJ, Zarghami N, Esfahlan AJ, Mollazadeh M, Nejati K, Nasiri M. The possible impact of obesity on androgen, progesterone and estrogen receptors (ERα and ERβ) gene expression in breast cancer patients. Breast Cancer. (2011) 5:227–37. doi: 10.4137/BCBCR.S7707
115. Kono M, Fujii T, Lim B, Karuturi MS, Tripathy D, Ueno NT. Androgen receptor function and androgen receptor-targeted therapies in breast cancer: a review. JAMA Oncol. (2017) 3:1266–73. doi: 10.1001/jamaoncol.2016.4975
116. Ji YK, Lee GS, Choi KC, Jeung EB. Anti-progestogenic effect of flutamide on uterine expression of calbindin-D9k mRNA and protein in immature mice. Reprod Toxicol. (2006) 22:694–701. doi: 10.1016/j.reprotox.2006.04.015
117. Frank D, Sharpe N, Scott MC, Mirro E, Hartman B, Halliwell WH. Chronic effects of flutamide in male beagle dogs. Toxicol Pathol. (2004) 32:243–9. doi: 10.1080/01926230490274416
118. Caceres S, Monsalve B, Peña L, de Andres PJ, Alonso-Diez A, Illera MJ, et al. In vitro and in vivo effect of flutamide on steroid hormone secretion in canine and human inflammatory breast cancer cell lines. Vet Comp Oncol. (2018) 16:148–8. doi: 10.1111/vco.12324
119. McGuire WL, Horwitz KB. A role for progesterone in breast cancer. Ann N Y Acad Sci. (1977) 286:90–100. doi: 10.1111/j.1749-6632.1977.tb29408.x
120. Timmermans-Sprang EPM, Gracanin A, Mol JA. Molecular signaling of progesterone, growth hormone, Wnt, and HER in mammary glands of dogs, rodents, and humans: new treatment target identification. Front Vet Sci. (2017) 4:53. doi: 10.3389/fvets.2017.00053
121. Huber JC, Ott J. The dialectic role of progesterone. Maturitas. (2009) 62:326–9. doi: 10.1016/j.maturitas.2008.12.009
122. Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit Rev Eukaryotic Gene Exp. (2008) 18:11–33. doi: 10.1615/CritRevEukarGeneExpr.v18.i1.20
123. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. (2010) 465:803–7. doi: 10.1038/nature09091
124. Horwitz KB, Sartorius CA. 90 YEARS OF PROGESTERONE: Progesterone and progesterone receptors in breast cancer: past, present, future. J Mol Endocrinol. (2020) 65:T49–63. doi: 10.1530/JME-20-0104
125. Ros L, Holst BS, Hagman R. A retrospective study of bitches with pyometra, medically treated with aglepristone. Theriogenology. (2014) 82:1281–6. doi: 10.1016/j.theriogenology.2014.08.011
126. Guil-Luna S, Hellmén E, Sánchez-Céspedes R, Millán Y, Martín de las Mulas J. The antiprogestins mifepristone and onapristone reduce cell proliferation in the canine mammary carcinoma cell line CMT-U27. Histol Histopathol. (2014) 29:949–55. doi: 10.14670/HH-29.949
127. Guil-Luna S, Sánchez-Céspedes R, Millán Y, De Andrés FJ, Rollón E, Domingo V, et al. Aglepristone decreases proliferation in progesterone receptor-positive canine mammary carcinomas. J Vet Intern Med. (2011) 25:518–23. doi: 10.1111/j.1939-1676.2011.0723.x
128. Guil-Luna S, Millán Y, De Andres J, Rollón E, Domingo V, García-Macías J, et al. Prognostic impact of neoadjuvant aglepristone treatment in clinicopathological parameters of progesterone receptor-positive canine mammary carcinomas. Vet Comp Oncol. (2017) 15:391–9. doi: 10.1111/vco.12175
129. Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer. (1997) 72:340–4. doi: 10.1002/(SICI)1097-0215(19970717)72:2<340::AID-IJC23>3.0.CO;2-I
130. Benavente MA, Bianchi CP, Imperiale F, Aba MA. Antiproliferative effects of oxytocin and desmopressin on canine mammary cancer cells. Front Vet Sci. (2016) 3:119. doi: 10.3389/fvets.2016.00119
131. Cassoni P, Sapino A, Papotti M, Bussolati G. Oxytocin and oxytocin-analogue F314 inhibit cell proliferation and tumor growth of rat and mouse mammary carcinomas. Int J Cancer. (1996) 66:817–20. doi: 10.1002/(SICI)1097-0215(19960611)66:6<817::AID-IJC18>3.0.CO;2-#
132. Benavente MA, Bianchi CP, Aba MA. Expression of Oxytocin receptors in canine mammary tumours. J Comp Pathol. (2019) 170:26–33. doi: 10.1016/j.jcpa.2019.05.005
133. Chanson P, Salenave S. Diabetes insipidus and pregnancy. Ann Endocrinol. (2016) 77:135–8. doi: 10.1016/j.ando.2016.04.005
134. Vande Walle J, Stockner M, Raes A, Nørgaard JP. Desmopressin 30 years in clinical use: a safety review. Curr Drug Saf. (2007) 2:232–8. doi: 10.2174/157488607781668891
135. Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J Thromb Haemost. (2003) 1:682–9. doi: 10.1046/j.1538-7836.2003.00190.x
136. Giron S, Tejera AM, Ripoll GV, Gomez DE, Alonso DF. Desmopressin inhibits lung and lymph node metastasis in a mouse mammary carcinoma model of surgical manipulation. J Surg Oncol. (2002) 81:38–44. doi: 10.1002/jso.10141
137. Iannucci NB, Ripoll GV, Garona J, Cascone O, Ciccia GN, Gomez DE, et al. Antiproliferative effect of 1-deamino-8-D-arginine vasopressin analogs on human breast cancer cells. Future Med Chem. (2011) 3:1987–93. doi: 10.4155/fmc.11.152
138. Hermo GA, Turic E, Angelico D, Scursoni AM, Gomez DE, Gobello C, et al. Effect of adjuvant perioperative desmopressin in locally advanced canine mammary carcinoma and its relation to histologic grade. J Am Anim Hosp Assoc. (2011) 47:21–7. doi: 10.5326/JAAHA-MS-5509
139. Weinberg RS, Grecco MO, Ferro GS, Seigelshifer DJ, Perroni NV, Terrier FJ, et al. A phase II dose-escalation trial of perioperative desmopressin (1-desamino-8-d-arginine vasopressin) in breast cancer patients. Springerplus. (2015) 4:428. doi: 10.1186/s40064-015-1217-y
140. Sorenmo K, Durham AC, Evans B, Scavello H, Stefanovski D. A prospective randomized trial of desmopressin in canine mammary carcinoma. Vet Comp Oncol. (2020) 18:796–803. doi: 10.1111/vco.12619
141. Link W. Principles of Cancer Treatment and Anticancer Drug Development. Cham: Springer International Publishing (2019). doi: 10.1007/978-3-030-18722-4
142. Mayrhofer P, Kunert R. Nomenclature of humanized mAbs: early concepts, current challenges and future perspectives. Hum Antibodies. (2019) 27:37–51. doi: 10.3233/HAB-180347
143. London C, Mathie T, Stingle N, Clifford C, Haney S, Klein MK, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia(®)) in solid tumours. Vet Comp Oncol. (2012) 10:194–205. doi: 10.1111/j.1476-5829.2011.00275.x
144. Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. (2015) 66:111–28. doi: 10.1146/annurev-med-042513-015127
145. London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. (2009) 15:3856–65. doi: 10.1158/1078-0432.CCR-08-1860
146. Ranieri G, Pantaleo M, Piccinno M, Roncetti M, Mutinati M, Marech I, et al. Tyrosine kinase inhibitors (TKIs) in human and pet tumours with special reference to breast cancer: a comparative review. Crit Rev Oncol Hematol. (2013) 88:293–308. doi: 10.1016/j.critrevonc.2013.05.009
147. Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. (2009) 23:377–89. doi: 10.2165/11318860-000000000-00000
148. Mazzotta M, Krasniqi E, Barchiesi G, Pizzuti L, Tomao F, Barba M, et al. Long-term safety and real-world effectiveness of trastuzumab in breast cancer. J Clin Med. (2019) 8:254. doi: 10.3390/jcm8020254
149. Goutsouliak K, Veeraraghavan J, Sethunath V, De Angelis C, Osborne CK, Rimawi MF, et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat Rev Clin Oncol. (2020) 17:233–50. doi: 10.1038/s41571-019-0299-9
150. Singer J, Weichselbaumer M, Stockner T, Mechtcheriakova D, Sobanov Y, Bajna E, et al. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol Immunol. (2012) 50:200–9. doi: 10.1016/j.molimm.2012.01.002
151. Singer J, Fazekas J, Wang W, Weichselbaumer M, Matz M, Mader A, et al. Generation of a canine anti-EGFR (ErbB-1) antibody for passive immunotherapy in dog cancer patients. Mol Cancer Ther. (2014) 13:1777–90. doi: 10.1158/1535-7163.MCT-13-0288
152. Gray ME, Lee S, McDowell AL, Erskine M, Loh QTM, Grice O, et al. Dual targeting of EGFR and ERBB2 pathways produces a synergistic effect on cancer cell proliferation and migration in vitro. Vet Comp Oncol. (2016) 15:890–909. doi: 10.1111/vco.12230
153. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. (1971) 285:1182–6. doi: 10.1056/NEJM197111182852108
154. Petrovic N. Targeting angiogenesis in cancer treatments: where do we stand? J Pharm Pharm Sci. (2016) 19:226–38. doi: 10.18433/J30033
155. Ghosh S, Sullivan CA, Zerkowski MP, Molinaro AM, Rimm DL, Camp RL, et al. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. (2008) 39:1835–43. doi: 10.1016/j.humpath.2008.06.004
156. Dos Anjos DS, Vital AF, Lainetti PF, Leis-Filho AF, Dalmolin F, Elias F, et al. Deregulation of VEGFR-2 and PDGFR expression and microvascular density in a triple-negative model of canine malignant mammary tumors with lymph node or lung metastasis. Vet Sci. (2019) 6:3. doi: 10.3390/vetsci6010003
157. Ribatti D, Nico B, Ruggieri S, Tamma R, Simone G, Mangia A. Angiogenesis and antiangiogenesis in triple-negative breast cancer. Transl Oncol. (2016) 9:453–7. doi: 10.1016/j.tranon.2016.07.002
158. Graham JC, Myers RK. The prognostic significance of angiogenesis in canine mammary tumors. J Vet Intern Med. (1999) 13:416–18. doi: 10.1111/j.1939-1676.1999.tb01456.x
159. Clarke JM, Hurwitz HI. Understanding and targeting resistance to anti-angiogenic therapies. J Gastrointest Oncol. (2013) 4:253–63.
160. Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. (2006) 13:1845–57. doi: 10.2174/092986706777585059
161. Li Q, Yan H, Zhao P, Yang Y, Cao B. Efficacy and safety of bevacizumab combined with chemotherapy for managing metastatic breast cancer: a meta-analysis of randomized controlled trials. Sci Rep. (2015) 5:15746. doi: 10.1038/srep15746
162. Zhou Z, Yao H, Hu H. Disrupting tumor angiogenesis and “the hunger games” for breast cancer. Adv Exp Med Biol. (2017) 1026:171–95. doi: 10.1007/978-981-10-6020-5_8
163. Slamon D, Gomez HL, Kabbinavar FF, Amit O, Richie M, Pandite L, et al. Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2-positive advanced or metastatic breast cancer. J Clin Oncol. (2008) 26:1016. doi: 10.1200/jco.2008.26.15_suppl.1016
164. Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. (2005) 4:677–85. doi: 10.1158/1535-7163.MCT-04-0297
165. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. (2004) 64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443
166. Mangini Prado MC, Losant Macedo SDA, Gumiero Guiraldelli G, Lainetti Patricia DF, Fernando Leis-Filho A, Emiko Kobayashi P, et al. Investigation of the prognostic significance of vasculogenic mimicry and its inhibition by sorafenib in canine mammary gland tumors. Front Oncol. (2019) 9:1445. doi: 10.3389/fonc.2019.01445
167. Lee JH, Li Q, An JH, Chae HK, Choi JW, Kim BJ, et al. Antitumor activity of rivoceranib against canine mammary gland tumor cell lines. Anticancer Res. (2019) 39:5483–94. doi: 10.21873/anticanres.13741
168. Kennedy KC, Qurollo BA, Rose BJ, Thamm DH. Epidermal growth factor enhances the malignant phenotype in canine mammary carcinoma cell lines. Vet Comp Oncol. (2011) 9:196–206. doi: 10.1111/j.1476-5829.2010.00248.x
169. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. (1999) 155:739–52. doi: 10.1016/S0002-9440(10)65173-5
170. Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. (2000) 156:361–81. doi: 10.1016/S0002-9440(10)64739-6
171. Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. (1999) 284:1994–8. doi: 10.1126/science.284.5422.1994
172. Kuczynski EA, Reynolds AR. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis. (2020) 23:55–74. doi: 10.1007/s10456-019-09698-6
173. Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. (2012) 31:16. doi: 10.1186/1756-9966-31-16
174. Pinto MP, Sotomayor P, Carrasco-Avino G, Corvalan AH, Owen GI. Escaping antiangiogenic therapy: strategies employed by cancer cells. Int J Mol Sci. (2016) 17:1489. doi: 10.3390/ijms17091489
175. Folberg R, Maniotis AJ. Vasculogenic mimicry. Apmis. (2004) 112:508–25. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x
176. Barreno L, Cáceres S, Alonso-Diez Á, Vicente-Montaña A, García ML, Clemente M, et al. Vasculogenic mimicry-associated ultrastructural findings in human and canine inflammatory breast cancer cell lines. BMC Cancer. (2019) 19:750. doi: 10.1186/s12885-019-5955-z
177. Zhang J, Qiao L, Liang N, Xie J, Luo H, Deng G. Vasculogenic mimicry and tumor metastasis. J Buon. (2016) 21:533–41.
178. Clemente M, Pérez-Alenza MD, Illera JC, Peña L. Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet Pathol. (2010) 47:265–74. doi: 10.1177/0300985809353167
179. Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. (2019) 16:469–93. doi: 10.1038/s41571-019-0181-9
180. Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. (1999) 31:1037–51. doi: 10.1016/S1357-2725(99)00076-X
181. Abbaspour Babaei M, Kamalidehghan B, Saleem M, Huri HZ, Ahmadipour F. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Dev Ther. (2016) 10:2443–59. doi: 10.2147/DDDT.S89114
182. Kiupel M, Webster JD, Kaneene JB, Miller R, Yuzbasiyan-Gurkan V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. (2004) 41:371–7. doi: 10.1354/vp.41-4-371
183. Janostiak R, Vyas M, Cicek AF, Wajapeyee N, Harigopal M. Loss of c-KIT expression in breast cancer correlates with malignant transformation of breast epithelium and is mediated by KIT gene promoter DNA hypermethylation. Exp Mol Pathol. (2018) 105:41–9. doi: 10.1016/j.yexmp.2018.05.011
184. Gattino F, Maniscalco L, Iussich S, Biasato I, Martano M, Morello E, et al. PDGFR-α, PDGFR-β, VEGFR-2 and CD117 expression in canine mammary tumours and evaluation of the in vitro effects of toceranib phosphate in neoplastic mammary cell lines. Vet Rec. (2018) 183:221. doi: 10.1136/vr.104414
185. Kubo K, Matsuyama S, Katayama K, Tsutsumi C, Yonezawa K, Shimada T, et al. Frequent expression of the c-kit proto-oncogene in canine malignant mammary tumor. J Vet Med Sci. (1998) 60:1335–40. doi: 10.1292/jvms.60.1335
186. Koltai Z, Szabó B, Jakus J, Vajdovich P. Tyrosine kinase expression analyses in canine mammary gland tumours - A pilot study. Acta Vet Hung. (2018) 66:294–308. doi: 10.1556/004.2018.027
187. Brunetti B, Beha G, Benazzi C, Bondin V, De Tolla L, Sarli G. CD117 expression influences proliferation but not survival in canine mammary tumours. J Comp Pathol. (2014) 151:202–6. doi: 10.1016/j.jcpa.2014.04.018
188. Carvalho MI, Pires I, Dias M, Prada J, Gregório H, Lobo L, et al. Intratumoral CD3+ T-lymphocytes immunoexpression and its association with c-Kit, angiogenesis, and overall survival in malignant canine mammary tumors. Anal Cell Pathol. (2015) 2015:920409. doi: 10.1155/2015/920409
189. London CA, Hannah AL, Zadovoskaya R, Chien MB, Kollias-Baker C, Rosenberg M, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. (2003) 9:2755–68.
190. de Campos CB, Lavalle GE, Fialho Ligório S, Camargo Nunes F, Carneiro RA, Amorim RL, et al. Absence of significant adverse events following thalidomide administration in bitches diagnosed with mammary gland carcinomas. Vet Rec. (2016) 179:514. doi: 10.1136/vr.103764
191. Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, Legendre A, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. (2008) 22:1301–9. doi: 10.1111/j.1939-1676.2008.0190.x
192. Thamm DH, Rose B, Kow K, Humbert M, Mansfield CD, Moussy A, et al. Masitinib as a chemosensitizer of canine tumor cell lines: a proof of concept study. Vet J. (2012) 191:131–4. doi: 10.1016/j.tvjl.2011.01.001
193. Clark AS, Karasic TB, DeMichele A, Vaughn DJ, O'Hara M, Perini R, et al. Palbociclib (PD0332991)-a selective and potent cyclin-dependent kinase inhibitor: a review of pharmacodynamics and clinical development. JAMA Oncol. (2016) 2:253–60. doi: 10.1001/jamaoncol.2015.4701
194. Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. (2015) 373:209–19. doi: 10.1056/NEJMoa1505270
195. Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. (2010) 2:a001008. doi: 10.1101/cshperspect.a001008
196. Setoguchi A, Sakai T, Okuda M, Minehata K, Yazawa M, Ishizaka T, et al. Aberrations of the p53 tumor suppressor gene in various tumors in dogs. Am J Vet Res. (2001) 62:433–9. doi: 10.2460/ajvr.2001.62.433
197. Ochiai K, Azakami D, Morimatsu M, Hirama H, Kawakami S, Nakagawa T, et al. Endogenous Leu332Gln mutation in p53 disrupts the tetramerization ability in a canine mammary gland tumor cell line. Oncol Rep. (2018) 40:488–94. doi: 10.3892/or.2018.6409
198. Abdelmegeed SM, Mohammed S. Canine mammary tumors as a model for human disease. Oncol Lett. (2018) 15:8195–205. doi: 10.3892/ol.2018.8411
199. Oliveira TF, Maués T, Ramundo MS, Figueiredo AMS, de Mello MFV, El-Jaick KB, et al. TP53 gene expression levels and tumor aggressiveness in canine mammary carcinomas. J Vet Diagn Invest. (2017) 29:865–8. doi: 10.1177/1040638717721730
200. Munday JS, Ariyarathna H, Aberdein D, Thomson NA. Immunostaining for p53 and p16(CDKN2A) protein is not predictive of prognosis for dogs with malignant mammary gland neoplasms. Vet Sci. (2019) 6:34. doi: 10.3390/vetsci6010034
201. Dolka I, Czopowicz M, Gruk-Jurka A, Wojtkowska A, Sapierzynski R, Jurka P. Diagnostic efficacy of smear cytology and robinson's cytological grading of canine mammary tumors with respect to histopathology, cytomorphometry, metastases and overall survival. PLoS ONE. (2018) 13:e0191595. doi: 10.1371/journal.pone.0191595
202. Klopfleisch R, Gruber AD. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Res Vet Sci. (2009) 87:91–6. doi: 10.1016/j.rvsc.2008.12.010
203. Bartley AN, Ross DW. Validation of p53 immunohistochemistry as a prognostic factor in breast cancer in clinical practice. Arch Pathol Lab Med. (2002) 126:456–8. doi: 10.1043/0003-9985(2002)126<0456:VOPIAA>2.0.CO;2
204. Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Modern Pathol. (2011) 24:1248–53. doi: 10.1038/modpathol.2011.85
205. Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. (2019) 38(Suppl. 1):S123–31. doi: 10.1097/PGP.0000000000000488
206. Dumay A, Feugeas JP, Wittmer E, Lehmann-Che J, Bertheau P, Espié M, et al. Distinct tumor protein p53 mutants in breast cancer subgroups. Int J Cancer. (2013) 132:1227–31. doi: 10.1002/ijc.27767
207. Pan Y, Yuan Y, Liu G, Wei Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients. PLoS ONE. (2017) 12:e0172324. doi: 10.1371/journal.pone.0172324
208. Abubakar M, Guo C, Koka H, Sung H, Shao N, Guida J, et al. Clinicopathological and epidemiological significance of breast cancer subtype reclassification based on p53 immunohistochemical expression. NPJ Breast Cancer. (2019) 5:20. doi: 10.1038/s41523-019-0117-7
209. Fabi A, Mottolese M, Di Benedett A, Sperati F, Ercolani C, Buglioni S, et al. p53 and BLC2 immunohistochemical expression across molecular subtypes in 1099 early breast cancer patients with long-term follow-up: an observational study. Clin Breast Cancer. (2020) 20:e760–70. doi: 10.1016/j.clbc.2020.05.005
210. Morris JS, Nixon C, King OJ, Morgan IM, Philbey AW. Expression of TopBP1 in canine mammary neoplasia in relation to histological type, Ki67, ERalpha and p53. Vet J. (2009) 179:422–9. doi: 10.1016/j.tvjl.2007.10.025
211. Rodo A, Malicka E. Immunohistochemical expression of protein p53 in neoplasms of the mammary gland in bitches. Pol J Vet Sci. (2008) 11:89–95.
212. Lee CH, Kim WH, Lim JH, Kang MS, Kim DY, Kweon OK. Mutation and overexpression of p53 as a prognostic factor in canine mammary tumors. J Vet Sci. (2004) 5:63–9. doi: 10.4142/jvs.2004.5.1.63
213. Levine AJ. Targeting therapies for the p53 protein in cancer treatments. Ann Rev Cancer Biol. (2019) 3:21–34. doi: 10.1146/annurev-cancerbio-030518-055455
214. Zhou X, Hao Q, Lu H. Mutant p53 in cancer therapy—the barrier or the path. J Mol Cell Biol. (2019) 11:293–305. doi: 10.1093/jmcb/mjy072
215. Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. (2012) 83:1021–32. doi: 10.1016/j.bcp.2011.12.016
216. Grayton JE, Miller T, Wilson-Robles H. In vitro evaluation of selective inhibitors of nuclear export (SINE) drugs KPT-185 and KPT-335 against canine mammary carcinoma and transitional cell carcinoma tumor initiating cells. Vet Comp Oncol. (2017) 15:1455–67. doi: 10.1111/vco.12289
217. Tamura D, Saito T, Murata K, Kawashima M, Asano R. Celecoxib exerts antitumor effects in canine mammary tumor cells via COX-2-independent mechanisms. Int J Oncol. (2015) 46:1393–404. doi: 10.3892/ijo.2015.2820
218. Szweda M, Rychlik A, Babińska I, Pomianowski A. Significance of cyclooxygenase-2 in oncogenesis. J Vet Res. (2019) 63:215–24. doi: 10.2478/jvetres-2019-0030
219. Zhang Z, Chen F, Shang L. Advances in antitumor effects of NSAIDs. Cancer Manage Res. (2018) 10:4631–40. doi: 10.2147/CMAR.S175212
220. Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. (2009) 10:501–7. doi: 10.1016/S1470-2045(09)70035-X
221. Retsky M, Rogers R, Demicheli R, Hrushesky WJ, Gukas I, Vaidya JS, et al. NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat. (2012) 134:881–8. doi: 10.1007/s10549-012-2094-5
222. Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. (2000) 321:1183–7. doi: 10.1136/bmj.321.7270.1183
223. Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. (2004) 31:22–9. doi: 10.1053/j.seminoncol.2004.03.042
224. Gurpinar E, Grizzle WE, Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res. (2014) 20:1104–13. doi: 10.1158/1078-0432.CCR-13-1573
225. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. (2001) 286:954–9. doi: 10.1001/jama.286.8.954
226. White WB, West CR, Borer JS, Gorelick PB, Lavange L, Pan SX, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol. (2007) 99:91–8. doi: 10.1016/j.amjcard.2006.07.069
227. Li S, Jiang M, Wang L, Yu S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: recent advancement. Biomed Pharmacother. (2020) 129:110389. doi: 10.1016/j.biopha.2020.110389
228. Doré M. Cyclooxygenase-2 expression in animal cancers. Vet Pathol. (2011) 48:254–65. doi: 10.1177/0300985810379434
229. Doré M, Lanthier I, Sirois J. Cyclooxygenase-2 expression in canine mammary tumors. Vet Pathol. (2003) 40:207–12. doi: 10.1354/vp.40-2-207
230. Badowska-Kozakiewicz AM, Malicka E. Expression of cyclooxygenase-2 in neoplasms of the mammary gland in bitches. Pol J Vet Sci. (2010) 13:337–42.
231. Guimarães MJ, Carvalho MI, Pires I, Prada J, Gil AG, Lopes C, et al. Concurrent expression of cyclo-oxygenase-2 and epidermal growth factor receptor in canine malignant mammary tumours. J Comp Pathol. (2014) 150:27–34. doi: 10.1016/j.jcpa.2013.07.005
232. Millanta F, Asproni P, Canale A, Citi S, Poli A. COX-2, mPGES-1 and EP2 receptor immunohistochemical expression in canine and feline malignant mammary tumours. Vet Comp Oncol. (2016) 14:270–80. doi: 10.1111/vco.12096
233. Queiroga FL, Pires I, Parente M, Gregório H, Lopes CS. COX-2 over-expression correlates with VEGF and tumour angiogenesis in canine mammary cancer. Vet J. (2011) 189:77–82. doi: 10.1016/j.tvjl.2010.06.022
234. Clemente M, Sanchez-Archidona AR, Sardon D, Diez L, Martin-Ruiz A, Caceres S, et al. Different role of COX-2 and angiogenesis in canine inflammatory and non-inflammatory mammary cancer. Vet J. (2013) 197:427–32. doi: 10.1016/j.tvjl.2013.02.009
235. Carvalho MI, Pires I, Prada J, Raposo TP, Gregório H, Lobo L, et al. High COX-2 expression is associated with increased angiogenesis, proliferation and tumoural inflammatory infiltrate in canine malignant mammary tumours: a multivariate survival study. Vet Comp Oncol. (2017) 15:619–31. doi: 10.1111/vco.12206
236. Iturriaga MP, Paredes R, Arias JI, Torres CG. Meloxicam decreases the migration and invasion of CF41.Mg canine mammary carcinoma cells. Oncol Lett. (2017) 14:2198–206. doi: 10.3892/ol.2017.6400
237. Ustün Alkan F, Ustüner O, Bakirel T, Cinar S, Erten G, Deniz G. The effects of piroxicam and deracoxib on canine mammary tumour cell line. ScientificWorldJournal. (2012) 2012:976740. doi: 10.1100/2012/976740
238. Sonzogni-Desautels K, Knapp DW, Sartin E, Doré M. Effect of cyclooxygenase inhibitors in a xenograft model of canine mammary tumours. Vet Comp Oncol. (2011) 9:161–71. doi: 10.1111/j.1476-5829.2010.00242.x
239. de M, Souza CH, Toledo-Piza E, Amorin R, Barboza A, Tobias KM. Inflammatory mammary carcinoma in 12 dogs: clinical features, cyclooxygenase-2 expression, and response to piroxicam treatment. Can Vet J. (2009) 50:506–10.
240. Saito T, Tamura D, Asano R. Usefulness of selective COX-2 inhibitors as therapeutic agents against canine mammary tumors. Oncol Rep. (2014) 31:1637–44. doi: 10.3892/or.2014.3010
241. Hurst EA, Pang LY, Argyle DJ. The selective cyclooxygenase-2 inhibitor mavacoxib (Trocoxil) exerts anti-tumour effects in vitro independent of cyclooxygenase-2 expression levels. Vet Comp Oncol. (2019) 17:194–207. doi: 10.1111/vco.12470
242. Bakirel T, Alkan FÜ, ÜStÜNer O, ÇInar S, Yildirim F, Erten G. Synergistic growth inhibitory effect of deracoxib with doxorubicin against a canine mammary tumor cell line, CMT-U27. J Vet Med Sci. (2016) 78:657–68. doi: 10.1292/jvms.15-0387
243. Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. (2009) 174:1588–93. doi: 10.2353/ajpath.2009.080545
244. Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. (1993) 5:806–11. doi: 10.1016/0955-0674(93)90029-P
245. Knudsen KA, Wheelock MJ. Cadherins and the mammary gland. J Cell Biochem. (2005) 95:488–96. doi: 10.1002/jcb.20419
246. Hunt NC, Douglas-Jones AG, Jasani B, Morgan JM, Pignatelli M. Loss of E-cadherin expression associated with lymph node metastases in small breast carcinomas. Virchows Arch. (1997) 430:285–9. doi: 10.1007/BF01092751
247. Corso G, Figueiredo J, De Angelis SP, Corso F, Girardi A, Pereira J, et al. E-cadherin deregulation in breast cancer. J Cell Mol Med. (2020) 24:5930–6. doi: 10.1111/jcmm.15140
248. Li Z, Yin S, Zhang L, Liu W, Chen B. Prognostic value of reduced E-cadherin expression in breast cancer: a meta-analysis. Oncotarget. (2017) 8:16445–55. doi: 10.18632/oncotarget.14860
249. Matos AJ, Lopes C, Carvalheira J, Santos M, Rutteman GR, Gärtner F. E-cadherin expression in canine malignant mammary tumours: relationship to other clinico-pathological variables. J Comp Pathol. (2006) 134:182–9. doi: 10.1016/j.jcpa.2005.10.004
250. Brunetti B, Sarli G, Preziosi R, Leprotti S, Benazzi C. E-cadherin expression in canine mammary carcinomas with regional lymph node metastases. J Vet Med A Physiol Pathol Clin Med. (2003) 50:496–500. doi: 10.1111/j.1439-0442.2003.00577.x
251. Brunetti B, Sarli G, Preziosi R, Monari I, Benazzi C. E-cadherin and beta-catenin reduction influence invasion but not proliferation and survival in canine malignant mammary tumors. Vet Pathol. (2005) 42:781–7. doi: 10.1354/vp.42-6-781
252. De Matos AJ, Lopes CC, Faustino AM, Carvalheira JG, Rutteman GR, Gärtner Mde F. E-cadherin, beta-catenin, invasion and lymph node metastases in canine malignant mammary tumours. Apmis. (2007) 115:327–34. doi: 10.1111/j.1600-0463.2007.apm_544.x
253. Asproni P, Ressel L, Millanta F, Vannozzi I, Poli A. Co-localization of PTEN and E-cadherin in canine mammary hyperplasias and benign and malignant mammary tumors. Res Vet Sci. (2015) 103:113–18. doi: 10.1016/j.rvsc.2015.09.022
254. Gama A, Paredes J, Gärtner F, Alves A, Schmitt F. Expression of E-cadherin, P-cadherin and beta-catenin in canine malignant mammary tumours in relation to clinicopathological parameters, proliferation and survival. Vet J. (2008) 177:45–53. doi: 10.1016/j.tvjl.2007.05.024
255. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. (2009) 119:1420–8. doi: 10.1172/JCI39104
256. Wu Y, Zhou BP. Snail: more than EMT. Cell Adhesion Migr. (2010) 4:199–203. doi: 10.4161/cam.4.2.10943
257. Gamba CO, Rodrigues MA, Gomes DA, Estrela-Lima A, Ferreira E, Cassali GD. The relationship between E-cadherin and its transcriptional repressors in spontaneously arising canine invasive micropapillary mammary carcinoma. J Comp Pathol. (2015) 153:256–65. doi: 10.1016/j.jcpa.2015.08.006
258. Gamba CO, Damasceno KA, Ferreira IC, Rodrigues MA, Gomes DA, Alves MR, et al. The investigation of transcriptional repression mediated by ZEB2 in canine invasive micropapillary carcinoma in mammary gland. PLoS ONE. (2019) 14:e0209497. doi: 10.1371/journal.pone.0209497
259. Schatton T, Frank NY, Frank MH. Identification and targeting of cancer stem cells. Bioessays. (2009) 31:1038–49. doi: 10.1002/bies.200900058
260. Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. (2008) 3:e2888. doi: 10.1371/journal.pone.0002888
261. Gooding AJ, Schiemann WP. Epithelial-mesenchymal transition programs and cancer stem cell phenotypes: mediators of breast cancer therapy resistance. Mol Cancer Res. (2020) 18:1257–70. doi: 10.1158/1541-7786.MCR-20-0067
262. Sun X, Wang M, Yao L, Li X, Dong H, Li M, et al. Exploring the metabolic vulnerabilities of epithelial-mesenchymal transition in breast cancer. Front Cell Dev Biol. (2020) 8:655. doi: 10.3389/fcell.2020.00655
263. Kong D, Hughes CJ, Ford HL. Cellular plasticity in breast cancer progression and therapy. Front Mol Biosci. (2020) 7:72. doi: 10.3389/fmolb.2020.00072
264. Raposo-Ferreira TMM, Brisson BK, Durham AC, Laufer-Amorim R, Kristiansen V, Puré E, et al. Characteristics of the epithelial-mesenchymal transition in primary and paired metastatic canine mammary carcinomas. Vet Pathol. (2018) 55:622–33. doi: 10.1177/0300985818776054
265. Xavier PLP, Cordeiro YG, Rochetti AL, Sangalli JR, Zuccari DAPC, Silveira JC, et al. ZEB1 and ZEB2 transcription factors are potential therapeutic targets of canine mammary cancer cells. Vet Comp Oncol. (2018) 16:596–605. doi: 10.1111/vco.12427
266. Mao L, Dauchy RT, Blask DE, Slakey LM, Xiang S, Yuan L, et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3β. Mol Endocrinol. (2012) 26:1808–20. doi: 10.1210/me.2012-1071
267. Gonçalves Ndo N, Colombo J, Lopes JR, Gelaleti GB, Moschetta MG, Sonehara NM, et al. Effect of melatonin in epithelial mesenchymal transition markers and invasive properties of breast cancer stem cells of canine and human cell lines. PLoS ONE. (2016) 11:e0150407. doi: 10.1371/journal.pone.0150407
268. Gelaleti GB, Borin TF, Maschio-Signorini LB, Moschetta MG, Hellmén E, Viloria-Petit AM, et al. Melatonin and IL-25 modulate apoptosis and angiogenesis mediators in metastatic (CF-41) and non-metastatic (CMT-U229) canine mammary tumour cells. Vet Comp Oncol. (2017) 15:1572–84. doi: 10.1111/vco.12303
269. Ramos Lopes J, Bazela Maschio L, Victorasso Jardim-Perassi B, Gobbe Moschetta M, Carvalho Ferreira L, Rodrigues Martins G, et al. Evaluation of melatonin treatment in primary culture of canine mammary tumors. Oncol Rep. (2015) 33:311–19. doi: 10.3892/or.2014.3596
270. Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFβ-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis. Cell Cycle. (2010) 9:4461–8. doi: 10.4161/cc.9.22.14048
271. Custódio PR, Colombo J, Ventura FV, Castro TB, Zuccari D. Melatonin treatment combined with TGF-β silencing inhibits epithelial-mesenchymal transition in CF41 canine mammary cancer cell line. Anticancer Agents Med Chem. (2020) 20:989–97. doi: 10.2174/1871520620666200407122635
272. Saeki K, Watanabe M, Tsuboi M, Sugano S, Yoshitake R, Tanaka Y, et al. Anti-tumour effect of metformin in canine mammary gland tumour cells. Vet J. (2015) 205:297–304. doi: 10.1016/j.tvjl.2015.04.026
273. Barbieri F, Thellung S, Ratto A, Carra E, Marini V, Fucile C, et al. In vitro and in vivo antiproliferative activity of metformin on stem-like cells isolated from spontaneous canine mammary carcinomas: translational implications for human tumors. BMC Cancer. (2015) 15:228. doi: 10.1186/s12885-015-1235-8
274. Leonel C, Borin TF, de Carvalho Ferreira L, Moschetta MG, Bajgelman MC, Viloria-Petit AM, et al. Inhibition of epithelial-mesenchymal transition and metastasis by combined TGFbeta knockdown and metformin treatment in a canine mammary cancer xenograft model. J Mammary Gland Biol Neoplasia. (2017) 22:27–41. doi: 10.1007/s10911-016-9370-7
275. Moschetta MG, Leonel C, Maschio-Signorini LB, Borin TF, Gelaleti GB, Jardim-Perassi BV, et al. Evaluation of angiogenesis process after metformin and LY294002 treatment in mammary tumor. Anticancer Agents Med Chem. (2019) 19:655–66. doi: 10.2174/1871520619666181218164050
276. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. (2002) 2:48–58. doi: 10.1038/nrc706
277. Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int J Cancer. (2016) 139:1281–8. doi: 10.1002/ijc.30185
278. Bai F, Yu Z, Gao X, Gong J, Fan L, Liu F. Simvastatin induces breast cancer cell death through oxidative stress up-regulating miR-140-5p. Aging. (2019) 11:3198–19. doi: 10.18632/aging.101974
279. Cruz P, Reyes F, Torres CG. Simvastatin modulates β-catenin/MDR1 expression on spheres derived from CF41.Mg canine mammary carcinoma cells. Pol J Vet Sci. (2018) 21:95–9.
280. Torres CG, Olivares A, Stoore C. Simvastatin exhibits antiproliferative effects on spheres derived from canine mammary carcinoma cells. Oncol Rep. (2015) 33:2235–44. doi: 10.3892/or.2015.3850
281. Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. (2005) 23:8606–12. doi: 10.1200/JCO.2005.02.7045
282. Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival. A population-based cohort study. Epidemiology. (2015) 26:68–78. doi: 10.1097/EDE.0000000000000189
283. Ahern TP, Damkier P, Feddersen S, Kjærsgaard A, Lash TL, Hamilton-Dutoit S, et al. Predictive pharmacogenetic biomarkers for breast cancer recurrence prevention by simvastatin. Acta Oncol. (2020) 59:1009–15. doi: 10.1080/0284186X.2020.1759820
284. Ko YS, Lee WS, Panchanathan R, Joo YN, Choi YH, Kim GS, et al. Polyphenols from artemisia annua L inhibit adhesion and EMT of highly metastatic breast cancer cells MDA-MB-231. Phytother Res. (2016) 30:1180–8. doi: 10.1002/ptr.5626
285. Pirali M, Taheri M, Zarei S, Majidi M, Ghafouri H. Artesunate, as a HSP70 ATPase activity inhibitor, induces apoptosis in breast cancer cells. Int J Biol Macromol. (2020) 164:3369–75. doi: 10.1016/j.ijbiomac.2020.08.198
286. Dong J, Yang W, Han J, Cheng R, Guo X, Li L. Effect of dihydroartemisinin on epithelial-to-mesenchymal transition in canine mammary tumour cells. Res Vet Sci. (2019) 124:240–7. doi: 10.1016/j.rvsc.2019.03.020
287. von Hagens C, Walter-Sack I, Goeckenjan M, Storch-Hagenlocher B, Sertel S, Elsässer M, et al. Long-term add-on therapy (compassionate use) with oral artesunate in patients with metastatic breast cancer after participating in a phase I study (ARTIC M33/2). Phytomedicine. (2019) 54:140–8. doi: 10.1016/j.phymed.2018.09.178
288. von Hagens C, Walter-Sack I, Goeckenjan M, Osburg J, Storch-Hagenlocher B, Sertel S, et al. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res Treat. (2017) 164:359–69. doi: 10.1007/s10549-017-4261-1
289. Panossian LA, Garga NI, Pelletier D. Toxic brainstem encephalopathy after artemisinin treatment for breast cancer. Ann Neurol. (2005) 58:812–13. doi: 10.1002/ana.20620
290. Gordi T, Lepist EI. Artemisinin derivatives: toxic for laboratory animals, safe for humans? Toxicol Lett. (2004) 147:99–107. doi: 10.1016/j.toxlet.2003.12.009
291. Lohmueller J, Finn OJ. Current modalities in cancer immunotherapy: immunomodulatory antibodies, CARs and vaccines. Pharmacol Ther. (2017) 178:31–47. doi: 10.1016/j.pharmthera.2017.03.008
292. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. (2007) 121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x
293. Bates JP, Derakhshandeh R, Jones L, Webb TJ. Mechanisms of immune evasion in breast cancer. BMC Cancer. (2018) 18:556. doi: 10.1186/s12885-018-4441-3
294. Spranger S, Gajewski TF. Mechanisms of tumor cell–intrinsic immune evasion. Ann Rev Cancer Biol. (2018) 2:213–28. doi: 10.1146/annurev-cancerbio-030617-050606
295. Trefzer U, Walden P. Hybrid-cell vaccines for cancer immune therapy. Mol Biotechnol. (2003) 25:63–9. doi: 10.1385/MB:25:1:63
296. Lopes A, Vandermeulen G, Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res. (2019) 38:146. doi: 10.1186/s13046-019-1154-7
297. White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. (2008) 15:911–20. doi: 10.1038/gt.2008.21
298. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
299. Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. (2017) 46:210–19. doi: 10.1016/j.intimp.2017.03.015
300. Bock BC, Stein U, Schmitt CA, Augustin HG. Mouse models of human cancer. Cancer Res. (2014) 74:4671–5. doi: 10.1158/0008-5472.CAN-14-1424
301. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. (2004) 172:2731–8. doi: 10.4049/jimmunol.172.5.2731
302. Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. (2012) 18:5160–2. doi: 10.1158/1078-0432.CCR-12-2408
303. Dow S. A role for dogs in advancing cancer immunotherapy research. Front Immunol. (2020). 10:2935. doi: 10.3389/fimmu.2019.02935
304. Regan D, Dow S. Manipulation of innate immunity for cancer therapy in dogs. Vet Sci. (2015) 2:423–39. doi: 10.3390/vetsci2040423
305. Park JS, Withers SS, Modiano JF, Kent MS, Chen M, Luna JI, et al. Canine cancer immunotherapy studies: linking mouse and human. J Immunother Cancer. (2016) 4:97. doi: 10.1186/s40425-016-0200-7
306. Me Finocchiaro L, Glikin G. Recent clinical trials of cancer immunogene therapy in companion animals. World J Exp Med. (2017) 7:42–8. doi: 10.5493/wjem.v7.i2.42
307. Addissie S, Klingemann H. Cellular immunotherapy of canine cancer. Vet Sci. (2018) 5:100. doi: 10.3390/vetsci5040100
308. Klingemann H. Immunotherapy for dogs: running behind humans. Front. Immunol. (2018) 9:133. doi: 10.3389/fimmu.2018.00133
309. Bergman PJ. Cancer immunotherapies. Vet Clin North Am Small Anim Pract. (2019) 49:881–902. doi: 10.1016/j.cvsm.2019.04.010
310. Tarone L, Barutello G, Iussich S, Giacobino D, Quaglino E, Buracco P, et al. Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy. Cancer Immunol Immunother. (2019) 68:1839–53. doi: 10.1007/s00262-019-02360-6
311. de Melo Gagliato D, Buzaid AC, Perez-Garcia J, Cortes J. Immunotherapy in breast cancer. Curr Pract Clin Challenges. BioDrugs. (2020) 34:611–23. doi: 10.1007/s40259-020-00436-9
312. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
313. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. (2020) 38:1000. doi: 10.1200/JCO.2020.38.15_suppl.1000
314. Tolaney SM, Kalinsky K, Kaklamani VG, D'Adamo DR, Aktan G, Tsai ML, et al. A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). J Clin Oncol. (2020) 38:1015. doi: 10.1200/JCO.2020.38.15_suppl.1015
315. Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE. (2016) 11:e0157176. doi: 10.1371/journal.pone.0157176
316. Shosu K, Sakurai M, Inoue K, Nakagawa T, Sakai H, Morimoto M, et al. Programmed cell death ligand 1 expression in canine cancer. In Vivo. (2016) 30:195–204.
317. Henry CJ, Buss MS, Hellström I, Hellström KE, Brewer WG, Bryan JN, et al. Clinical evaluation of BR96 sFv-PE40 immunotoxin therapy in canine models of spontaneously occurring invasive carcinoma. Clin Cancer Res. (2005) 11:751–5.
318. Cirillo M, Tan P, Sturm M, Cole C. Cellular immunotherapy for hematologic malignancies: beyond bone marrow transplantation. Biol Blood Marrow Transpl. (2018) 24:433–42. doi: 10.1016/j.bbmt.2017.10.035
319. Huber A, Dammeijer F, Aerts JGJV, Vroman H. Current state of dendritic cell-based immunotherapy: opportunities for in vitro antigen loading of different DC subsets? Front Immunol. (2018) 9:2804. doi: 10.3389/fimmu.2018.02804
320. Zacharakis N, Chinnasamy H, Black M, Xu H, Lu Y-C, Zheng Z, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. (2018) 24:724–30. doi: 10.1038/s41591-018-0040-8
321. Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. (2016) 37:220–30. doi: 10.1016/j.tips.2015.11.004
322. Venetis K, Invernizzi M, Sajjadi E, Curigliano G, Fusco N. Cellular immunotherapy in breast cancer: the quest for consistent biomarkers. Cancer Treat Rev. (2020) 90:102089. doi: 10.1016/j.ctrv.2020.102089
323. Gibb MJ, Kroll M, Su Q, Zurawski S, Zurawski G, Igyarto B. Novel cyclin D1-based DC vaccine inhibits TNBC tumor growth. J Immunol. (2018) 200:181. Available online at: https://www.jimmunol.org/content/200/1_Supplement/181.13
324. Bird RC, DeInnocentes P, Church Bird AE, Lutful Kabir FM, Martinez-Romero EG, Smith AN, et al. Autologous hybrid cell fusion vaccine in a spontaneous intermediate model of breast carcinoma. J Vet Sci. (2019) 20:e48. doi: 10.4142/jvs.2019.20.e48
325. Santos-Carballal B, Fernández Fernández E, Goycoolea FM. Chitosan in non-viral gene delivery: role of structure, characterization methods, and insights in cancer and rare diseases therapies. Polymers. (2018) 10:444. doi: 10.3390/polym10040444
326. Shanmugaraj B, Priya LB, Mahalakshmi B, Subbiah S, Hu RM, Velmurugan BK, et al. Bacterial and viral vectors as vaccine delivery vehicles for breast cancer therapy. Life Sci. (2020) 250:117550. doi: 10.1016/j.lfs.2020.117550
327. Allahverdiyev A, Tari G, Bagirova M, Abamor ES. Current approaches in development of immunotherapeutic vaccines for breast cancer. J Breast Cancer. (2018) 21:343–53. doi: 10.4048/jbc.2018.21.e47
328. Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. (1999) 27:58–63. doi: 10.1177/019262339902700112
329. Cicchelero L, Denies S, Haers H, Vanderperren K, Stock E, Van Brantegem L, et al. Intratumoural interleukin 12 gene therapy stimulates the immune system and decreases angiogenesis in dogs with spontaneous cancer. Vet Comp Oncol. (2017) 15:1187–205. doi: 10.1111/vco.12255
330. Aurisicchio L, Mennuni C, Giannetti P, Calvaruso F, Nuzzo M, Cipriani B, et al. Immunogenicity and safety of a DNA prime/adenovirus boost vaccine against rhesus CEA in nonhuman primates. Int J. Cancer. (2007) 120:2290–300. doi: 10.1002/ijc.22555
331. Peruzzi D, Mesiti G, Ciliberto G, La Monica N, Aurisicchio L. Telomerase and HER-2/neu as targets of genetic cancer vaccines in dogs. Vaccine. (2010). 28:1201–8. doi: 10.1016/j.vaccine.2009.11.031
332. Gabai V, Venanzi FM, Bagashova E, Rud O, Mariotti F, Vullo C, et al. Pilot study of p62 DNA vaccine in dogs with mammary tumors. Oncotarget. (2014) 5:12803–10. doi: 10.18632/oncotarget.2516
333. Venanzi FM, Gabai V, Mariotti F, Magi GE, Vullo C, Sufianov AA, et al. p62-DNA-encoding plasmid reverts tumor grade, changes tumor stroma, and enhances anticancer immunity. Aging. (2019) 11:10711–22. doi: 10.18632/aging.102486
334. Finocchiaro LME, Spector AIM, Agnetti L, Arbe MF, Glikin GC. Combination of suicide and cytokine gene therapies as surgery adjuvant for canine mammary carcinoma. Vet Sci. (2018) 5:70. doi: 10.3390/vetsci5030070
335. Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. (2006) 98:298–300. doi: 10.1093/jnci/djj111
336. Dolgin E. Oncolytic viruses get a boost with first FDA-approval recommendation. Nat Rev Drug Discov. (2015) 14:369–71. doi: 10.1038/nrd4643
337. Adelfinger M, Bessler S, Frentzen A, Cecil A, Langbein-Laugwitz J, Gentschev I, et al. Preclinical testing oncolytic vaccinia virus strain GLV-5b451 expressing an anti-VEGF single-chain antibody for canine cancer therapy. Viruses. (2015) 7:4075–92. doi: 10.3390/v7072811
338. Igase M, Hwang CC, Coffey M, Okuda M, Noguchi S, Mizuno T. The oncolytic effects of reovirus in canine solid tumor cell lines. J Vet Med Sci. (2015) 77:541–8. doi: 10.1292/jvms.14-0570
339. Gupta SK, Yadav PK, Gandham RK, Sahoo AP, Harish DR, Singh AK, et al. Canine parvovirus NS1 protein exhibits anti-tumor activity in a mouse mammary tumor model. Virus Res. (2016) 213:289–98. doi: 10.1016/j.virusres.2015.12.017
340. Shoji K, Yoneda M, Fujiyuki T, Amagai Y, Tanaka A, Matsuda A, et al. Development of new therapy for canine mammary cancer with recombinant measles virus. Mol Ther Oncolytics. (2016) 3:15022. doi: 10.1038/mto.2015.22
341. Igase M, Hwang CC, Kambayashi S, Kubo M, Coffey M, Miyama TS, et al. Oncolytic reovirus synergizes with chemotherapeutic agents to promote cell death in canine mammary gland tumor. Can J Vet Res. (2016) 80:21–31.
342. Bhat AH, Ganguly B, Tiwari AK, Das AK. Canine parvovirus ns1 gene and chicken anemia vp3 gene induce partial oncolysis of canine transmissible venereal tumor. Sci Rep. (2017) 7:15419. doi: 10.1038/s41598-017-15734-6
343. MacNeill AL, Weishaar KM, Séguin B, Powers BE. Safety of an oncolytic myxoma virus in dogs with soft tissue sarcoma. Viruses. (2018) 10:398. doi: 10.3390/v10080398
344. Sánchez D, Cesarman-Maus G, Amador-Molina A, Lizano M. Oncolytic viruses for canine cancer treatment. Cancers. (2018) 10:404. doi: 10.3390/cancers10110404
345. Hwang CC, Igase M, Sakurai M, Haraguchi T, Tani K, Itamoto K, et al. Oncolytic reovirus therapy: pilot study in dogs with spontaneously occurring tumours. Vet Comp Oncol. (2018) 16:229–38. doi: 10.1111/vco.12361
346. Li P, Wang J, Chen G, Zhang X, Lin D, Zhou Y, et al. Oncolytic activity of canine distemper virus in canine mammary tubular adenocarcinoma cells. Vet Comp Oncol. (2019) 17:174–83. doi: 10.1111/vco.12466
347. Cecil A, Gentschev I, Adelfinger M, Dandekar T, Szalay AA. Vaccinia virus injected human tumors: oncolytic virus efficiency predicted by antigen profiling analysis fitted boolean models. Bioengineered. (2019) 10:190–6. doi: 10.1080/21655979.2019.1622220
348. Igase M, Shousu K, Fujiki N, Sakurai M, Bonkobara M, Hwang CC, et al. Anti-tumour activity of oncolytic reovirus against canine histiocytic sarcoma cells. Vet Comp Oncol. (2019) 17:184–93. doi: 10.1111/vco.12468
349. Hamada K, Takagi S, Kuboshima H, Shimada H, Takagi K, Yasuoka T, et al. Cloning of carrier cells infected with oncolytic adenovirus driven by midkine promoter and biosafety studies. J Gene Med. (2019) 21:e3064. doi: 10.1002/jgm.3064
350. Zygiert Z. Hodgkin's disease: remissions after measles. Lancet. (1971) 1:593. doi: 10.1016/S0140-6736(71)91186-X
351. Sugiyama T, Yoneda M, Kuraishi T, Hattori S, Inoue Y, Sato H, et al. Measles virus selectively blind to signaling lymphocyte activation molecule as a novel oncolytic virus for breast cancer treatment. Gene Ther. (2013) 20:338–47. doi: 10.1038/gt.2012.44
352. Delpeut S, Sisson G, Black KM, Richardson CD. Measles virus enters breast and colon cancer cell lines through a PVRL4-mediated macropinocytosis pathway. J Virol. (2017) 91:e02191–16. doi: 10.1128/JVI.02191-16
353. Tai CJ, Liu CH, Pan YC, Wong SH, Richardson CD, Lin LT. Chemovirotherapeutic treatment using camptothecin enhances oncolytic measles virus-mediated killing of breast cancer cells. Sci Rep. (2019) 9:6767. doi: 10.1038/s41598-019-43047-3
354. Abdullah SA, Al-Shammari AM, Lateef SA. Attenuated measles vaccine strain have potent oncolytic activity against Iraqi patient derived breast cancer cell line. Saudi J Biol Sci. (2020) 27:865–72. doi: 10.1016/j.sjbs.2019.12.015
355. Gentschev I, Adelfinger M, Josupeit R, Rudolph S, Ehrig K, Donat U, et al. Preclinical evaluation of oncolytic vaccinia virus for therapy of canine soft tissue sarcoma. PLoS ONE. (2012) 7:e37239. doi: 10.1371/journal.pone.0037239
356. Hwang CC, Umeki S, Kubo M, Hayashi T, Shimoda H, Mochizuki M, et al. Oncolytic reovirus in canine mast cell tumor. PLoS ONE. (2013) 8:e73555. doi: 10.1371/journal.pone.0073555
357. Laborda E, Puig-Saus C, Rodriguez-García A, Moreno R, Cascalló M, Pastor J, et al. A pRb-responsive, RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus for application in veterinary oncology. Mol Ther. (2014) 22:986–98. doi: 10.1038/mt.2014.7
358. Siddharth S, Goutam K, Das S, Nayak A, Nayak D, Sethy C, et al. Nectin-4 is a breast cancer stem cell marker that induces WNT/β-catenin signaling via Pi3k/Akt axis. Int J Biochem Cell Biol. (2017) 89:85–94. doi: 10.1016/j.biocel.2017.06.007
359. Moro L, Martins AS, Alves CM, Santos FG, Del Puerto HL, Vasconcelos AC. Apoptosis in the cerebellum of dogs with distemper. J Vet Med B Infect Dis Vet Public Health. (2003) 50:221–5. doi: 10.1046/j.1439-0450.2003.00657.x
360. Del Puerto HL, Martins AS, Moro L, Milsted A, Alves F, Braz GF, et al. Caspase-3/-8/-9, Bax and Bcl-2 expression in the cerebellum, lymph nodes and leukocytes of dogs naturally infected with canine distemper virus. Genet Mol Res. (2010) 9:151–61. doi: 10.4238/vol9-1gmr717
361. Garcia JA, Ferreira HL, Vieira FV, Gameiro R, Andrade AL, Eugênio FR, et al. Tumour necrosis factor-alpha-induced protein 8 (TNFAIP8) expression associated with cell survival and death in cancer cell lines infected with canine distemper virus. Vet Comp Oncol. (2017) 15:336–44. doi: 10.1111/vco.12168
362. Chon HJ, Lee WS, Yang H, Kong SJ, Lee NK, Moon ES, et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res. (2019) 25:1612–23. doi: 10.1158/1078-0432.CCR-18-1932
363. Choi AH, O'Leary MP, Chaurasiya S, Lu J, Kim SI, Fong Y, et al. Novel chimeric parapoxvirus CF189 as an oncolytic immunotherapy in triple-negative breast cancer. Surgery. (2018) 163:336–42. doi: 10.1016/j.surg.2017.09.030
364. Kwilas AR, Ardiani A, Dirmeier U, Wottawah C, Schlom J, Hodge JW. A poxviral-based cancer vaccine the transcription factor twist inhibits primary tumor growth and metastases in a model of metastatic breast cancer and improves survival in a spontaneous prostate cancer model. Oncotarget. (2015) 6:28194–210. doi: 10.18632/oncotarget.4442
365. Gholami S, Marano A, Chen NG, Aguilar RJ, Frentzen A, Chen CH, et al. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. (2014) 148:489–99. doi: 10.1007/s10549-014-3180-7
366. Chaurasiya S, Yang A, Kang S, Lu J, Kim SI, Park AK, et al. Oncolytic poxvirus CF33-hNIS-ΔF14.5 favorably modulates tumor immune microenvironment and works synergistically with anti-PD-L1 antibody in a triple-negative breast cancer model. Oncoimmunology. (2020) 9:1729300. doi: 10.1080/2162402X.2020.1729300
367. Heery CR, Ibrahim NK, Arlen PM, Mohebtash M, Murray JL, Koenig K, et al. Docetaxel alone or in combination with a therapeutic cancer vaccine (PANVAC) in patients with metastatic breast cancer: a randomized clinical trial. JAMA Oncol. (2015) 1:1087–95. doi: 10.1001/jamaoncol.2015.2736
368. Gentschev I, Stritzker J, Hofmann E, Weibel S, Yu YA, Chen N, et al. Use of an oncolytic vaccinia virus for the treatment of canine breast cancer in nude mice: preclinical development of a therapeutic agent. Cancer Gene Ther. (2009) 16:320–8. doi: 10.1038/cgt.2008.87
369. Gentschev I, Ehrig K, Donat U, Hess M, Rudolph S, Chen N, et al. Significant growth inhibition of canine mammary carcinoma xenografts following treatment with oncolytic vaccinia virus GLV-1h68. J Oncol. (2010) 2010:736907. doi: 10.1155/2010/736907
370. Urbasic AS, Hynes S, Somrak A, Contakos S, Rahman MM, Liu J, et al. Oncolysis of canine tumor cells by myxoma virus lacking the serp2 gene. Am J Vet Res. (2012) 73:1252–61. doi: 10.2460/ajvr.73.8.1252
371. Rodríguez Stewart RM, Berry JTL, Berger AK, Yoon SB, Hirsch AL, Guberman JA, et al. Enhanced killing of triple-negative breast cancer cells by reassortant reovirus and topoisomerase inhibitors. J Virol. (2019) 93:e01411–19. doi: 10.1128/JVI.01411-19
372. De Jong WH, Borm PJA. Drug delivery and nanoparticles:applications and hazards. Int J Nanomed. (2008) 3:133–49. doi: 10.2147/IJN.S596
373. Zocchi MR, Tosetti F, Benelli R, Poggi A. Cancer nanomedicine special issue review anticancer drug delivery with nanoparticles: extracellular vesicles or synthetic nanobeads as therapeutic tools for conventional treatment or immunotherapy. Cancers. (2020) 12:1886. doi: 10.3390/cancers12071886
374. Mirza Z, Karim S. Nanoparticles-based drug delivery and gene therapy for breast cancer: Recent advancements and future challenges. Semin Cancer Biol. (2019). doi: 10.1016/j.semcancer.2019.10.020. [Epub ahead of print].
375. Baselga J, Manikhas A, Cortés J, Llombart A, Roman L, Semiglazov VF, et al. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Ann Oncol. (2014) 25:592–8. doi: 10.1093/annonc/mdt543
376. Park IH, Sohn JH, Kim SB, Lee KS, Chung JS, Lee SH, et al. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res Treat. (2017) 49:569–77. doi: 10.4143/crt.2016.289
377. Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. (2017) 9:12. doi: 10.3390/pharmaceutics9020012
378. Li Y, Humphries B, Yang C, Wang Z. Nanoparticle-mediated therapeutic agent delivery for treating metastatic breast cancer-challenges and opportunities. Nanomaterials. (2018) 8:361. doi: 10.3390/nano8060361
379. Fujiwara Y, Mukai H, Saeki T, Ro J, Lin YC, Nagai SE, et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br J Cancer. (2019) 120:475–80. doi: 10.1038/s41416-019-0391-z
380. Kratz F. DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Invest Drugs. (2007) 16:855–66. doi: 10.1517/13543784.16.6.855
381. Bellat V, Ting R, Southard TL, Vahdat L, Molina H, Fernandez J, et al. Functional peptide nanofibers with unique tumor targeting and enzyme-induced local retention properties. Adv Func Mater. (2018) 28:1803969. doi: 10.1002/adfm.201803969
382. Stokol T, Wan C, Blakely R, Bellat V, Law B. Aldoxorubicin-loaded nanofibers are cytotoxic for canine mammary carcinoma and osteosarcoma cell lines in vitro: a short communication. Res Vet Sci. (2020) 128:86–9. doi: 10.1016/j.rvsc.2019.11.003
383. Núñez C, Estévez SV, Del Pilar Chantada M. Inorganic nanoparticles in diagnosis and treatment of breast cancer. J Biol Inorg Chem. (2018) 23:331–45. doi: 10.1007/s00775-018-1542-z
384. Silva A, Luís D, Santos S, Silva J, Mendo AS, Coito L, et al. Biological characterization of the antiproliferative potential of Co(II) and Sn(IV) coordination compounds in human cancer cell lines: a comparative proteomic approach. Drug Metabol Drug Interact. (2013) 28:167–76. doi: 10.1515/dmdi-2013-0015
385. Raposo LR, Roma-Rodrigues C, Jesus J, Martins LMDRS, Pombeiro AJ, Baptista PV, et al. Targeting canine mammary tumours via gold nanoparticles functionalized with promising Co(II) and Zn(II) compounds. Vet Comp Oncol. (2017) 15:1537–42. doi: 10.1111/vco.12298
386. Efferth T, Miyachi H, Bartsch H. Pharmacogenomics of a traditional Japanese herbal medicine (Kampo) for cancer therapy. Cancer Genomics Proteomics. (2007) 4:81–91.
387. Zhu L, Li L, Li Y, Wang J, Wang Q. Chinese herbal medicine as an adjunctive therapy for breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2016) 2016:9469276. doi: 10.1155/2016/1819794
388. Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu L, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med. (2019) 14:48. doi: 10.1186/s13020-019-0270-9
389. Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: barriers to obtaining a health claim. Nutr Rev. (2015) 73:155–65. doi: 10.1093/nutrit/nuu064
390. Holy J. Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell Motil Cytoskeleton. (2004) 58:253–68. doi: 10.1002/cm.20012
391. Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. (2008) 26:289–97. doi: 10.1007/s10637-007-9099-7
392. Tian B, Wang Z, Zhao Y, Wang D, Li Y, Ma L, et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. (2008) 264:299–308. doi: 10.1016/j.canlet.2008.01.041
393. Liu Q, Loo WT, Sze SC, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. (2009) 16:916–22. doi: 10.1016/j.phymed.2009.04.008
394. Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. (2010) 297:1–8. doi: 10.1016/j.canlet.2010.04.018
395. Yang CL, Ma YG, Xue YX, Liu YY, Xie H, Qiu GR. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. (2012) 31:139–50. doi: 10.1089/dna.2011.1300
396. Hassan ZK, Daghestani MH. Curcumin effect on MMPs and TIMPs genes in a breast cancer cell line. Asian Pac J Cancer Prev. (2012) 13:3259–64. doi: 10.7314/APJCP.2012.13.7.3259
397. Levine CB, Bayle J, Biourge V, Wakshlag JJ. Cellular effects of a turmeric root and rosemary leaf extract on canine neoplastic cell lines. BMC Vet Res. (2017) 13:388. doi: 10.1186/s12917-017-1302-2
398. Helson L. Curcumin (diferuloylmethane) delivery methods: a review. Biofactors. (2013) 39:21–6. doi: 10.1002/biof.1080
399. Withers SS, York D, Johnson E, Al-Nadaf S, Skorupski KA, Rodriguez CO Jr, et al. In vitro and in vivo activity of liposome-encapsulated curcumin for naturally occurring canine cancers. Vet Comp Oncol. (2018) 16:571–9. doi: 10.1111/vco.12424
400. Jiahao Lin, Qiang Cai, Yinian Tang, Yanjun Xu, Qian Wang, Tingting Li, et al. PEGylated Lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: design, characterization and its cytotoxic effect. Int J Pharm. (2018) 536:272–82. doi: 10.1016/j.ijpharm.2017.10.043
401. Gao J, Fan K, Jin Y, Zhao L, Wang Q, Tang Y, et al. PEGylated lipid bilayer coated mesoporous silica nanoparticles co-delivery of paclitaxel and curcumin leads to increased tumor site drug accumulation and reduced tumor burden. Eur J Pharm Sci. (2019) 140:105070. doi: 10.1016/j.ejps.2019.105070
402. Rahman HS, Rasedee A, Yeap SK, Othman HH, Chartrand MS, Namvar F, et al. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. BioMed Res Int. (2014) 2014:920742. doi: 10.1155/2014/920742
403. Rahman HS, Rasedee A, How CW, Abdul AB, Zeenathul NA, Othman HH, et al. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomed. (2013) 8:2769–81. doi: 10.2147/IJN.S45313
404. Foong JN, Selvarajah GT, Rasedee A, Rahman HS, How CW, Beh CY, et al. Zerumbone-loaded nanostructured lipid carrier induces apoptosis of canine mammary adenocarcinoma cells. BioMed Res Int. (2018) 2018:8691569. doi: 10.1155/2018/8691569
405. Sefidabi R, Mortazavi P, Hosseini S. Antiproliferative effect of berberine on canine mammary gland cancer cell culture. Biomed Rep. (2017) 6:95–8. doi: 10.3892/br.2016.809
406. Lynagh T, Lynch JW. Molecular mechanisms of Cys-loop ion channel receptor modulation by ivermectin. Front Mol Neurosci. (2012) 5:60. doi: 10.3389/fnmol.2012.00060
407. Dou Q, Chen HN, Wang K, Yuan K, Lei Y, Li K, et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. (2016) 76:4457–69. doi: 10.1158/0008-5472.CAN-15-2887
408. Diao H, Cheng N, Zhao Y, Xu H, Dong H, Thamm DH, et al. Ivermectin inhibits canine mammary tumor growth by regulating cell cycle progression and WNT signaling. BMC Vet Res. (2019) 15:276. doi: 10.1186/s12917-019-2026-2
409. Liu Y, Li W, Guo M, Li C, Qiu C. Protective role of selenium compounds on the proliferation, apoptosis, and angiogenesis of a canine breast cancer cell line. Biol Trace Element Res. (2016) 169:86–93. doi: 10.1007/s12011-015-0387-3
410. Li W, Guo M, Liu Y, Mu W, Deng G, Li C, et al. Selenium induces an anti-tumor effect via inhibiting intratumoral angiogenesis in a mouse model of transplanted canine mammary tumor cells. Biol Trace Element Res. (2016) 171:371–9. doi: 10.1007/s12011-015-0554-6
411. Zhou S, Wang F, Wong ET, Fonkem E, Hsieh TC, Wu JM, et al. Salinomycin: a novel anti-cancer agent with known anti-coccidial activities. Curr Med Chem. (2013) 20:4095–101. doi: 10.2174/15672050113109990199
412. Mai TT, Hamaï A, Hienzsch A, Cañeque T, Müller S, Wicinski J, et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. (2017) 9:1025–33. doi: 10.1038/nchem.2778
413. Kim KY, Park KI, Kim SH, Yu SN, Lee D, Kim YW, et al. Salinomycin induces reactive oxygen species and apoptosis in aggressive breast cancer cells as mediated with regulation of autophagy. Anticancer Res. (2017) 37:1747–58. doi: 10.21873/anticanres.11507
414. Li W, Yang H, Li X, Han L, Xu N, Shi A. Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer. Oncol Rep. (2019) 41:437–46. doi: 10.3892/or.2018.6805
415. Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomed. (2019) 14:9199–216. doi: 10.2147/IJN.S230376
416. Dewangan J, Srivastava S, Mishra S, Divakar A, Kumar S, Rath SK. Salinomycin inhibits breast cancer progression via targeting HIF-1α/VEGF mediated tumor angiogenesis in vitro and in vivo. Biochem Pharmacol. (2019) 164:326–35. doi: 10.1016/j.bcp.2019.04.026
417. Tyagi M, Patro BS. Salinomycin reduces growth, proliferation and metastasis of cisplatin resistant breast cancer cells via NF-kB deregulation. Toxicol In Vitro. (2019) 60:125–33. doi: 10.1016/j.tiv.2019.05.004
418. Hero T, Bühler H, Kouam PN, Priesch-Grzeszowiak B, Lateit T, Adamietz IA. The triple-negative breast cancer cell line MDA-MB 231 is specifically inhibited by the ionophore salinomycin. Anticancer Res. (2019) 39:2821–7. doi: 10.21873/anticanres.13410
419. Versini A, Colombeau L, Hienzsch A, Gaillet C, Retailleau P, Debieu S, et al. Salinomycin derivatives kill breast cancer stem cells by lysosomal iron targeting. Chemistry. (2020) 26:7416–24. doi: 10.1002/chem.202000335
420. Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. (2012) 2012:950658. doi: 10.1155/2012/950658
Keywords: canine mammary cancer, targeted therapy, markers, immunotherapy, immunophenotyping, hormonal therapy, tyrosine kinase receptors inhibitors
Citation: Valdivia G, Alonso-Diez Á, Pérez-Alenza D and Peña L (2021) From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 8:623800. doi: 10.3389/fvets.2021.623800
Received: 30 October 2020; Accepted: 11 January 2021;
Published: 17 February 2021.
Edited by:
Renee Laufer Amorim, São Paulo State University, BrazilReviewed by:
Maria Gärtner, University of Porto, PortugalCopyright © 2021 Valdivia, Alonso-Diez, Pérez-Alenza and Peña. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Peña, bGF1cmFwZUB1Y20uZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.