
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 19 July 2021
Sec. Veterinary Infectious Diseases
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.622063
This article is part of the Research Topic Ruminant Mastitis: A 360° View View all 21 articles
 Alessandra Gazzola1
Alessandra Gazzola1 Giulietta Minozzi1
Giulietta Minozzi1 Stefano Biffani2
Stefano Biffani2 Silvana Mattiello3
Silvana Mattiello3 Giovanni Bailo3
Giovanni Bailo3 Renata Piccinini1*
Renata Piccinini1*Mastitis is the most common disease affecting dairy goats and causing economic losses. Although it is accepted that increased somatic cell count (SCC) is mainly a response to infection, its reliability for subclinical mastitis detection in goats is controversial. Indeed, many physiological and extrinsic variables can increase SCC, including breed, parity, age, stage of lactation, seasonal variations, and milking methods. In some animals, milk-secreting tissue is present in the wall of the teat and, in some instances, milk can filter through pores in the skin to the udder surface. This condition is known as “weeping teat” (WT). In these animals, mammary tissue might be prone to develop bacterial infections, although limited information is provided. Weeping teat seems to have a genetic background and is reported to be especially found in goat breeds selected for high milk production. Moreover, it is observed a genetic correlation between WT and decreased milk yield as well as increased somatic cell scores (SCS). Since information on this topic is very limited, this study aimed at investigating any possible relationship between WT, high SCC, and the presence of bacteria in goat milk. Alpine goat farms in Northern Italy were selected based on the presence of WT. Each herd was divided into two age-matched groups, identified as case (WT+) and control (WT–). Half-udder milk samples were collected aseptically at three timepoints; bacteriological analysis was performed, and SCC were determined and transformed in SCS. There was a positive association between SCS and the presence of bacteria in milk (P = 0.037) overall, whereas WT udder defect was associated with positive bacterial culture in just one herd (P = 0.053). Thus, this herd was further investigated, repeating the sampling and the analysis on the following year. The positive association between high SCS and the presence of bacteria in milk was then confirmed (P = 0.007), whereas no association with WT condition was found. These results indicate that WT defect is usually unrelated to both the outcome of milk bacterial culture and SCS. As a side outcome, we could confirm the role of bacterial infection in increasing SCS.
Mastitis is the most common disease affecting dairy goats and represents the main cause of economic losses due to various factors, including the decrease in milk yield and quality as well as the increase of the associated treatment costs.
Somatic cell count (SCC) is the most used indicator of udder health status in cows, but its reliability for subclinical mastitis detection in goats is controversial. Therefore, milk SCC threshold value established for cows is not suitable for goats (1).
Koop et al. (2) showed that high SCC in goat milk is not always associated with a positive bacterial culture. However, various studies reported that increased SCC in goats is mainly a response to infection, thus prevention of IMI can contribute to control SCC in milk (3–5). Rupp et al. (6) provided further evidence that SCC is related to subclinical mastitis in goats, as they observed a positive association between somatic cell score (SCS) and bacterial counts in milk. Moreover, goats with repeated bacteriologically negative udders had the lowest SCS. The degree of the inflammatory reaction may also depend on the microorganisms involved. Rupp et al. (7) detected significant differences between udder halves infected by major pathogens (such as Staphylococcus aureus, Enterobacteriaceae, Streptococcus spp., and Mycoplasma spp.), and those infected by minor pathogens [such as non-aureus staphylococci (NAS), Corynebacterium spp., and Micrococcus spp.], which presented lower SCC. In addition, caprine arthritis-encephalitis virus (CAEV) infection was suggested as a possible cause of increased SCC as well (8, 9).
According to Plummer and Plummer (10), in some animals, milk-secreting tissue may be present in the wall of the teat and, in some instances, milk can filter through tiny pores in the skin to the surface of the udder or the teat, in absence of any invisible orifice. This condition is known as “weeping teat” (WT) and is characterized by the presence of milk cysts in goats, in which the accumulated milk may come out to the outside (11). Weeping teat animals can be easily identified by the presence of milk on the outer surface of the udder, especially right before milking. Seykora and McDaniel (12), hypothesized that this condition may contribute to the developing of bacterial infections, as milk, passing through the pores of the skin onto the external surface of the udder, would facilitate the entry of bacteria into the udder itself; therefore, it can be predicted that this porous tissue might be prone to developing bacterial infections and mastitis. Nevertheless, no data on health effects associated to this condition are available. Differently, other two outcomes can occur: milk may communicate with the teat cistern without visual evidence of the presence of this tissue or it may accumulate forming subcutaneous cysts if the secretory tissue does not have an opening.
Currently, very limited data are available on either the frequency of WT or its economic impact in goat farms. In Italy, genetic evaluations for type traits of dairy goats started in 2000, providing information on possible defects with potential functional impact, including the presence/absence of WT. The WT condition is reported to be especially associated with goat breeds selected for high milk production (10, 13). In Italy, mammary gland abnormality has been reported in around 4 and 13% of primiparous Saanen and Alpine females kidding from 2009 to 2014, respectively (14), with an observed incidence, respectively, of 3.6 and 7.5% for primiparous Saanen and Alpine goats. However, this proportion could be underestimated because of voluntary culling or inaccurate evaluation of WT. Biffani et al. (14) observed genetic correlation between WT and milk production or SCS, but the standard error of the estimates was very large. In particular, primiparous Alpine goats showed a loss of 0.046 kg/day milk in comparison with normal does, while SCS increased 0.26–0.21 in pluriparous or primiparous WT animals, respectively (13).
Since information on the role played by WT on the occurrence of intramammary infections is almost unknown, the present case-control study aimed at investigating the possible association between WT, the increase of SCC, and the presence of bacteria in milk of Alpine goats reared in Italy.
Four Alpine goat farms located in Lombardy region (Northern Italy) and registered in genealogical herd books of Associazione Nazionale della Pastorizia (ASSONAPA, Rome, Italy) were selected based on the phenotypic presence of WT. Alpine goats were chosen as they have a higher frequency of WT compared to the Saanen breed. Herd size ranged from 39 to 116 lactating goats (mean ± S.E.: 65.3 ± 17.3). The prevalence of WT in the four herds was 13.6, 14.1, 7.2, and 9.9%. Goats were housed indoor on permanent straw litter, with occasional access to outdoor pasture, and were milked twice/day. All the goats in their second, third or fourth lactation presenting WTs were included as case groups (WT+); the same number of goats, matched with WT+ for age and parity, was recruited as control group (WT–). We decided to exclude parities higher than the fourth one, as older goats show usually more intramammary infections than younger ones. Three WT-goats were culled during the trial period, and therefore were excluded from final analysis. Our follow-up study was performed in 2018, and then repeated in a single herd (herd A) during the following year, to further investigate it.
Half udder milk samples were collected from goats in their second, third, or fourth lactation at three timepoints, at the beginning, in the middle, and at the end of lactation. Samples were taken before milking with an aseptic procedure, by disinfecting the teat with wipes containing chlorhexidine, discarding a few streams of milk from the teat (foremilk), and collecting 10 ml of milk into sterile tubes. After collection, samples were immediately placed on ice and then transported chilled at +4°C to the laboratory.
Bacteriological analysis was performed with standard techniques on the day of sampling. In detail, for each sample, 10 μl of milk was plated onto blood agar supplemented with 5% defibrinated bovine blood using a sterile inoculating loop. Plates were then incubated at 37°C and analyzed after 24 and 48 h. Colonies grown on agar plates were isolated and identified following National Mastitis Council guidelines (15), then confirmed by API system. A sample was defined as polymicrobic when more than two distinct colony types were present. The presence of Mycoplasma spp. was not investigated, because contagious agalactia of goats is a notifiable disease and no case was officially reported since years. Somatic cell count was determined as well, using a SomacountTM 150 (Bentley Instruments, Minnesota, USA). Cell counts were expressed as cells/μl.
A general linear mixed model was used to investigate the effect of WT phenotypes and SCC on the observed microbiology outcome (MO).
The general model (Model 1) was:
where MO was the dependent variable considered as a binomial trait (0 = no infection, 1 = at least one infected teat); wt (two classes) is the presence/absence of a WT phenotype; sampling (three classes) is the milk sampling at the beginning, the middle, and the end of lactation; parity (three classes) is the parity class; SCS is the mean of the SCC of the two teats transformed to SCS as
according to Shook (16); animal is the random permanent environmental effect; herd (4 classes) is the herd where data were collected.
Model 1 was fitted to complete data. Successively, a data subset (A) was created including only records from herd A. The same model, hereinafter called model 2, was fitted after excluding the herd effect. Lastly, dataset A was additionally subset in two datasets, namely B and C. Dataset B included records collected in year 2018, while dataset C included records collected in 2019. Model 2 was fitted to both dataset B and C.
The general linear mixed model was fitted using the function glmer of the package “lme4” implemented in the R environment1 for statistical programming. Odds ratio have been calculated as exponential of the results of the respective linear general mix model in R statistical environment. All graphical representations were produced using R1.
To further corroborate our results, we performed bootstrap resampling to calculate the 95% Confidence Intervals (C.I.) of the estimates of the effects included in the models (17). All bootstrap analyses were performed with the R libraries boot (18, 19), based on 5,000 bootstrap replicates. Further libraries were used, tidyverse, knitr, tidyr, and broom.
In 2018, a total of 286 half-udder milk samples were collected from 49 Alpine goats (23 cases and 26 controls). The results of bacteriological analysis and SCC determination are shown in Table 1. Overall, most of isolates were NAS (91.7%), the most prevalent being Staphylococcus chromogenes (23.6%), Staphylococcus caprae (21.8%), unidentified NAS (Staphylococcus sp., 20%), and Staphylococcus epidermidis (14.5%). Non-aureus staphylococci species varied from herd to herd. In particular, S. caprae was isolated mainly in herd A, whereas S. epidermidis just in herd D. S. chromogenes was equally distributed in three herds, being absent in herd C. S. aureus was isolated only in herd C, in two animals. One of them was infected at all the sampling points (goat n. 26), while the other one (n. 31) was positive for S. aureus at two supplemental samplings carried out by the farmer and was then culled.
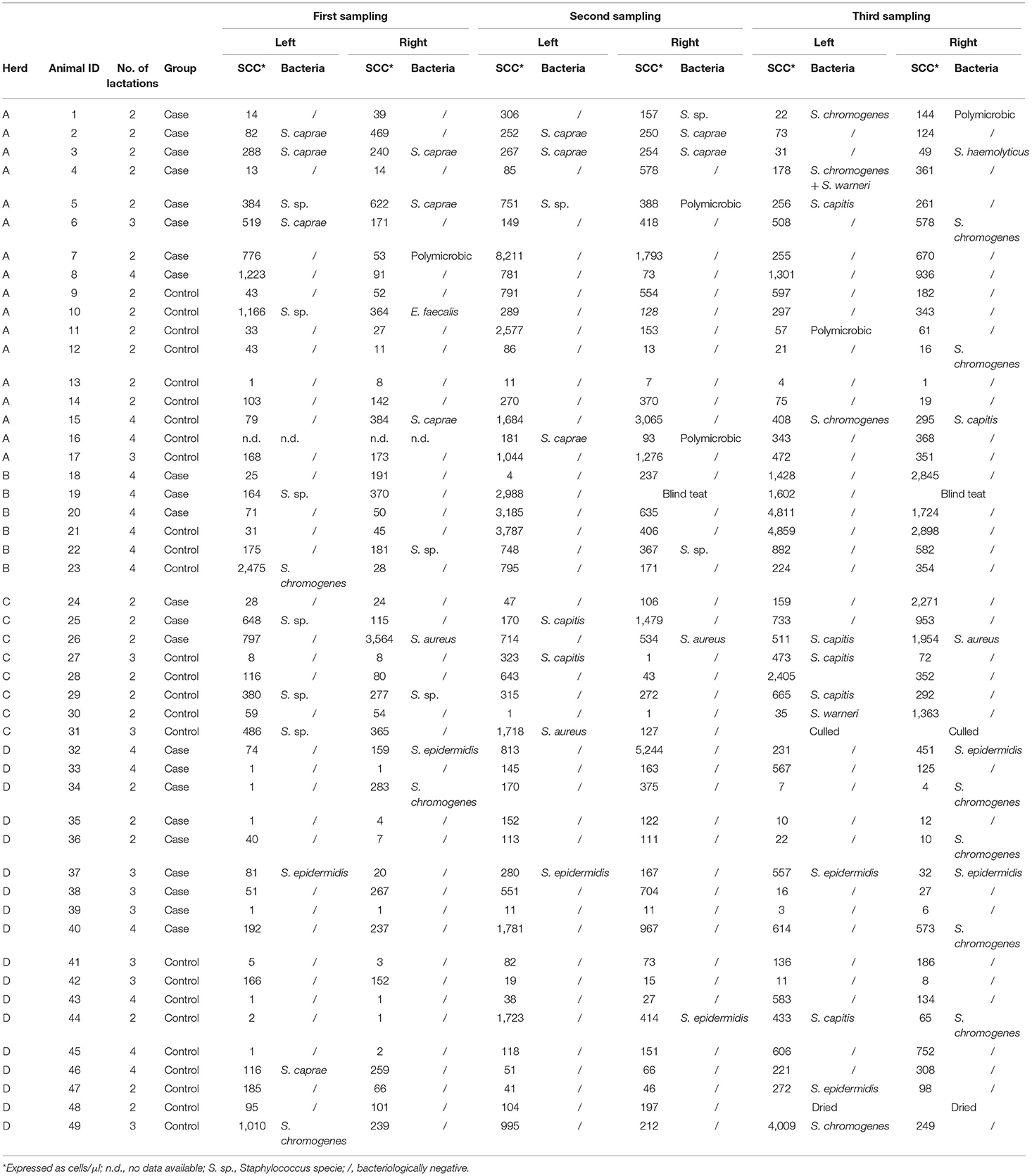
Table 1. Results of bacteriological analysis and SCC determination on half-udder milk samples from 49 Alpine goats (23 cases and 26 controls) collected in 2018 at three timepoints (at the beginning, in the middle and at the end of lactation).
Overall SCS mean value in 2018 was 3.8 ± 2.12. Regarding the presence/absence of WT, the SCS-value was 4.1 or 3.5, respectively. When the udder half and the microbiological outcome was considered, the mean value of SCS was 4.6 or 4.3 in the bacteriologically positive left or right half udders, respectively, decreasing to 3.6 or 3.2 in the negative halves. The presence of bacteria in milk was significantly associated with SCS (P = 0.037), but neither with WT udder defect, nor to parity or sampling time (Table 2). Considering herd A only, an almost significant effect of WT udder defect on the response to bacterial culture was observed, with a mean SCS-value of 4.4 ± 1.7 in WT udders (median value 4.5) and 3.5 ± 2.3 in normal glands (median value 3.9; P = 0.053; Figure 1, Table 3). The box plot of SCS in cases and controls in herd A is shown in Figure 1.
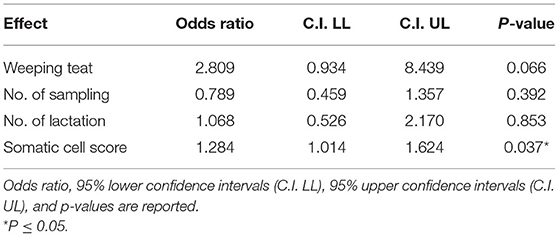
Table 2. Results of the linear general mix model on four herds sampled in 2018 on fixed effects in Model 1.
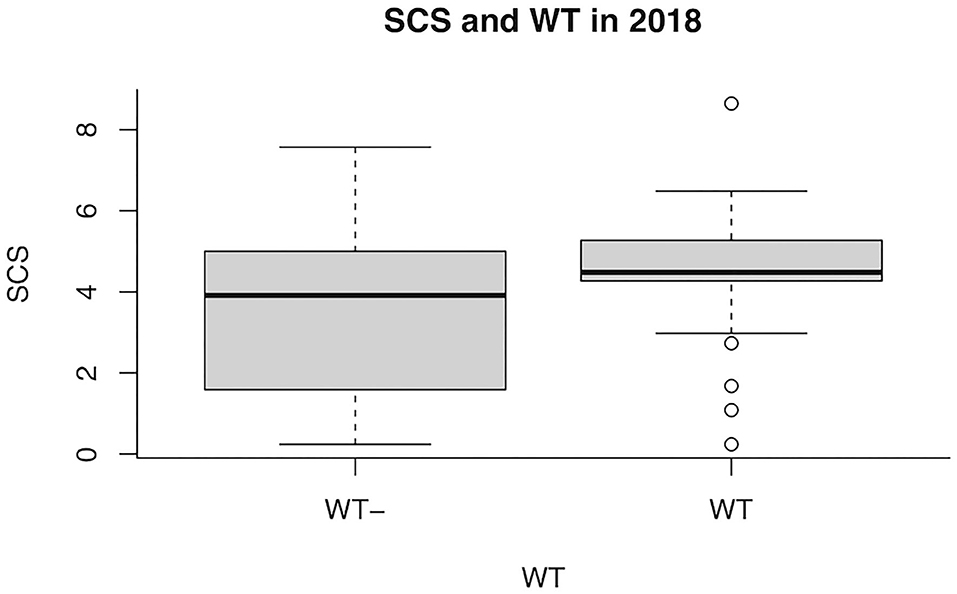
Figure 1. Box plot of somatic cell score (SCS) in goats with (WT+) and without (WT–) weeping teats in herd A in 2018.
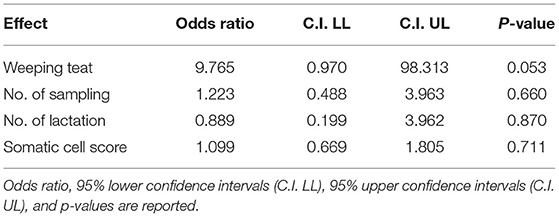
Table 3. Results of the linear general mix model in herd A sampled in 2018 on fixed effects in Model 2.
Therefore, herd A was further investigated on the following year (2019). A total of 109 half-udder milk samples were collected from 19 goats (9 cases and 10 controls), including the surviving goats sampled in 2018, plus three new animals. The results are shown in Table 4.
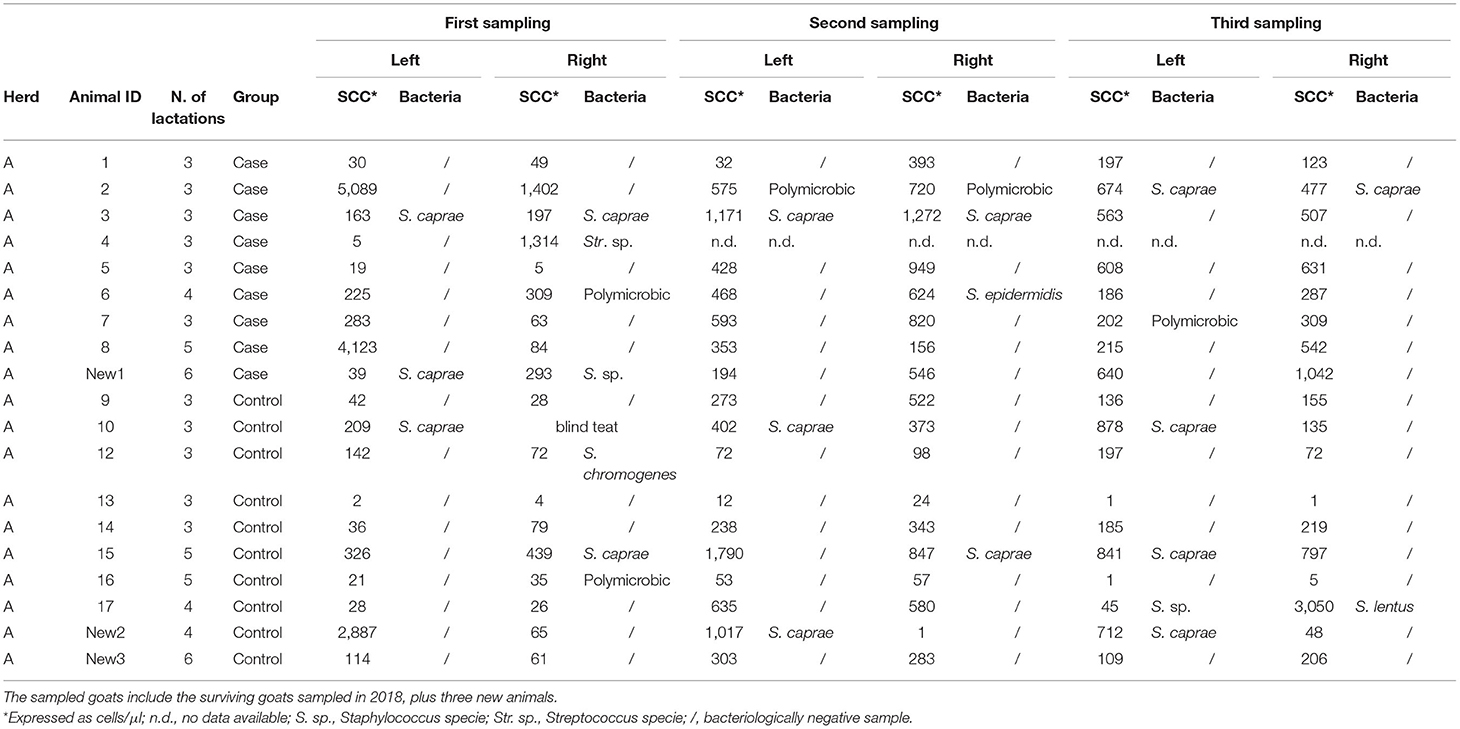
Table 4. Results of bacteriological analysis and SCC determination on half-udder milk samples from 19 Alpine goats (9 cases and 10 controls) collected in herd A in 2019 at three timepoints (at the beginning, in the middle, and at the end of lactation).
Most of the isolates were NAS (90.5%), identified almost exclusively as S. caprae (78.9%). No contagious microorganism was detected.
In 2019, the presence of bacteria in milk was no more associated with the WT udder defect, whereas the effect on SCS became statistically significant (P = 0.008; Table 5). The box plot of SCS and bacterial infections in herd A in 2019 is shown in Figure 2.
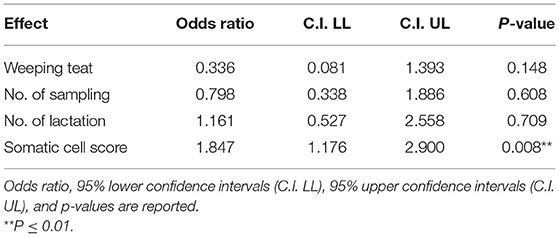
Table 5. Results of the linear general mix model on herd A sampled in 2019 on fixed effects in Model 2.
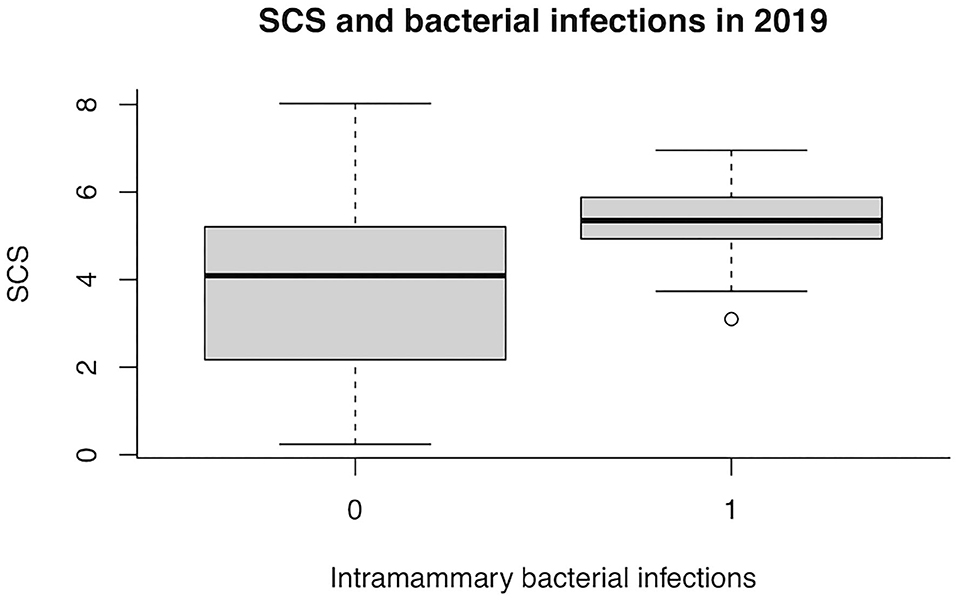
Figure 2. Box plot of somatic cell score (SCS) and bacterial infections in herd A in 2019. 0, no infection; 1, at least one infected teat.
Pooling together data collected in 2018 and 2019 in Herd A, no significant effect of SCS nor WT udder on intramammary bacterial infections was observed (Table 6).
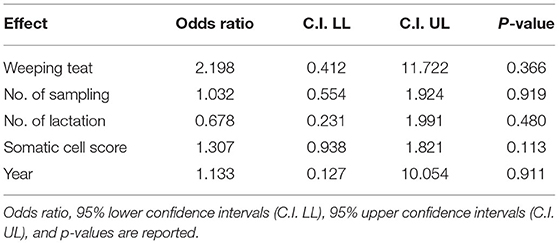
Table 6. Results of the linear general mix model on herd A sampled in 2018 and 2019 on fixed effects in Model 2.
The results of the bootstrap C.I. estimates confirmed all previous significant findings. In detail, significant association for SCS levels and MO was confirmed in the analysis of the four Herds sampled in 2018. Furthermore, association between MO and WT was confirmed in herd A in 2018 and between MO and SCS in 2019. All non-significant effects in all models were further confirmed (Supplementary Tables 1–4).
The importance of dairy goats has significantly increased during last decades, providing an alternative to dairy cow products for human consumption (20). A healthy mammary gland is essential for dairy farms and directly correlated with milk yield and quality.
Various pathological conditions of the udder in goats have been described, including supernumerary and abnormal teats, gynecomastia, precocious udders, and fibrocystic disease (11, 21). Among udder defects, information about the WT is extremely limited, but raises concern about its possible role in the development of mastitis. Yet, both its etiology and consequences are not fully understood. In this respect, our study provides new evidence suggesting that WT may be usually unrelated to both the outcome of milk bacterial culture and SCS. However, in one herd out of four we found a positive association of WT defect with positive bacterial culture, although this was not further confirmed in the following year.
In order to define the udder health status, SCC is the most used indicator in cows, but its ability to predict subclinical mastitis in goats has been questioned. Indeed, average SCC values in goats are higher than those in cattle and sheep, since they are influenced by several physiological and environmental factors, such as parity, stage of lactation, season, and milk yield (22–24). Our results highlighted significantly higher SCS in goat udders presenting bacterial infections, independently of parity, season, or managerial factors. This result was in accordance with different studies reporting increased SCC in goats in response to infection (2–5).
Various bacteria can be implicated in goat subclinical mastitis. The most frequently isolated bacteria in the four herds were NAS, mostly S. chromogenes and S. caprae, followed by unidentified Staphylococci and S. epidermidis. Accordingly, in the literature NAS are the most prevalent bacteria isolated from udder halves in goats and appear to behave as minor and opportunistic pathogens (2, 4, 25). Also Koop et al. (2) reported that NAS species have a high prevalence in goat mastitis, and cause persistent infections. Among NAS, S. caprae was one of the most frequently isolated Staphylococci, followed by S. epidermidis, Staphylococcus xylosus, S. chromogenes, and Staphylococcus simulans. Analogously, Rupp et al. (6), reported NAS as the prevalent agents of goat mastitis with a decreasing frequency of isolation from S. xylosus, to S. caprae and S. epidermidis.
In conclusion, our results cannot confirm the hypothesis that WT udder condition facilitates the entry of bacteria into the udder and that WT goats are more likely to develop localized bacterial infections. However, we cannot exclude that the WT defect could represent a risk for the health of the udder of dairy goats, when associated with particular conditions. Indeed, in our follow-up study only a single herd showed a significant effect of WT on intramammary infection just in the first year, that could not be confirmed in the following year. Additionally, our results showed that the presence of bacteria in milk is positively related with the increase in SCS, despite the physiological increase during lactation. It is necessary to extend the research to a larger number of farms in order to investigate the reasons for this variability and understand if and when the presence of WT could represent a risk for the health of goat's udder.
Mastitis is the most common disease affecting dairy goats and causing economic losses. Although it is accepted that increased SCSs is mainly a response to infection, its reliability for subclinical mastitis detection in goats is controversial, since it is influenced by many physiological and extrinsic variables, including breed, parity, age, stage of lactation, seasonal variations, and milking methods.
In some animals, milk-secreting tissue is present in the wall of the teat and, in some instances, milk can filter through pores in the skin to the surface of the udder. This condition is known as “weeping teat,” and it is hypothesized that the mammary gland might be prone to develop bacterial infections, although very few information is provided. Our results cannot exclude that the WT defect could represent a risk for udder health of dairy goats, when associated with particular conditions. Indeed, in our follow-up study only a single herd showed a significant effect of WT on intramammary infection, and this was not confirmed by further investigations. As a side outcome, our results showed that the presence of bacteria in milk is positively related with the increase in SCS, despite the physiological increase during lactation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because Our study could not be classified as a clinical one and we did not apply interventions outside of routine care, since we just collected milk during routine milking.
GM, SB, and RP: design of study and experiments. AG, GB, and SM: laboratory and field activities. AG, GM, SM, and RP: analysis of results, data interpretation, and manuscript drafting. All authors have read and approved the final manuscript.
This study was funded by Università degli Studi di Milano, PSR2017_DIP_026.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.622063/full#supplementary-material
1. Persson Y, Olofsson I. Direct and indirect measurement of somatic cell count as indicator of intramammary infection in dairy goats. Acta Vet Scand. (2011). 53:15. doi: 10.1186/1751-0147-53-15
2. Koop G, De Vliegher S, De Visscher A, Supré K, Haesebrouck F, Nielen M, et al. Differences between coagulase-negative Staphylococcus species in persistence and in effect on somatic cell count and milk yield in dairy goats. J Dairy Sci. (2012) 95:5075–84. doi: 10.3168/jds.2012-5615
3. Poutrel B, de Crémoux R, Ducelliez M, Verneau D. Control of intramammary infections in goats: impact on somatic cell counts. J Anim Sci. (1997) 75:566–70. doi: 10.2527/1997.752566x
4. Bergonier D, de Crémoux R, Rupp R, Lagriffoul G, Berthelot X. Mastitis of dairy small ruminants. Vet Res. (2003) 34:689–716. doi: 10.1051/vetres:2003030
5. Moroni P, Pisoni G, Ruffo G, Boettcher PJ. Risk factors for intramammary infections and relationship with somatic-cell counts in Italian dairy goats. Prev Vet Med. (2005) 69:163–73. doi: 10.1016/j.prevetmed.2004.10.013
6. Rupp R, Huau C, Caillat H, Fassier T, Bouvier F, Pampouille E, et al. Divergent selection on milk somatic cell count in goats improves udder health and milk quality with no effect on nematode resistance. J Dairy Sci. (2019) 102:5242–53. doi: 10.3168/jds.2018-15664
7. Rupp R, Clément V, Piacere A, Robert-Granié C, Manfredi E. Genetic parameters for milk somatic cell score and relationship with production and udder type traits in dairy Alpine and Saanen primiparous goats. J Dairy Sci. (2011) 94:3629–34. doi: 10.3168/jds.2010-3694
8. Nord K, Adnøy T. Effects of infection by caprine arthritis-encephalitis virus on milk production of goats. J Dairy Sci. (1997) 80:2391–7. doi: 10.3168/jds.S0022-0302(97)76190-3
9. Sánchez A, Contreras A, Corrales JJ, Marco JC. Relationships between infection with caprine arthritis encephalitis virus, intramammary bacterial infection and somatic cell counts in dairy goats. Vet Rec. (2001) 148:711–4. doi: 10.1136/vr.148.23.711
10. Plummer PJ, Plummer C. Diseases of the mammary gland. In: Pugh DG, Baird N, editors. Sheep and Goat Medicine. Saunders: Elsevier (2012). pp. 442–465.
11. Matthews J, . Diseases of the mammary gland. In: Diseases of the goat. 4th Ed. Chichester: John Wiley and Sons, Ltd (2016). pp. 198–201.
12. Seykora AJ, McDaniel BT. Udder and teat morphology related to mastitis resistance: a review. J Dairy Sci. (1985) 68:2087–93. doi: 10.3168/jds.S0022-0302(85)81072-9
13. Biffani S, Minozzi G, Piccinini R, Castiglioni B, Grande S, Fresi P, et al. Effect of weeping udder on the level of somatic cell counts and production traits in Italian Alpine and Saanen dairy goats. In: International Bovine Mastitis Conference In Proc. National Mastitis Council. Milan (2018). doi: 10.13140/RG.2.2.19023.38569
14. Biffani S, Tiezzi F, Fresi P, Stella A, Minozzi G. Genetic parameters of weeping teats in Italian Saanen and Alpine dairy goats and their relationship with milk production and somatic cell score. J Dairy Sci. (2020) 103:9167–76. doi: 10.3168/jds.2020-18175
15. Hogan JS, Gonzales RN, Harmon RJ, Nickerson SC, Oliver SP, Pankey JW, et al. Laboratory Handjournal on Bovine Mastitis, revised ed. Madison WI: National Mastitis Council Inc. (1999). P. 222.
16. Shook GE. Genetic improvement of mastitis through selection on somatic cell count. Vet Clin North Am Food Anim Pract. (1993) 9:563–81. doi: 10.1016/s0749-0720(15)30622-8
17. Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman and Hall, Inc. (1993).
18. Canty A, Ripley BD. Boot: Bootstrap R (S-Plus) Functions. R package version 1.3–25. (2020). Available online at: http://CRAN.R-project.org/package=boot (accessed December 27, 2020).
19. Davison AC, Hinkley DV. Bootstrap Methods and Their Applications. Cambridge: Cambridge University Press (1997). Available online at: http://statwww.epfl.ch/davison/BMA/ (accessed January 11, 2021).
20. Lérias JR, Hernández-Castellano LE, Suárez-Trujillo A, Castro N, Pourlis A, Almeida AM. The mammary gland in small ruminants: major morphological and functional events underlying milk production–a review. J Dairy Res. (2014) 81:304–18. doi: 10.1017/S0022029914000235
21. Yeruham I, Sharir B, Friedman S, Perl S. Cystic dilation of the teat sinuses in doe goats. Vet Rec. (2005) 156:844. doi: 10.1136/vr.156.26.844
22. Paape MJ, Poutrel B, Contreras A, Marco JC, Capuco AV. Milk somatic cells and lactation in small ruminants. J Dairy Sci. (2001). 84(Suppl.):E237–44. doi: 10.3168/jds.S0022-0302(01)70223-8
23. Jimenez-Granado R, Sanchez-Rodriguez M, Arce C, Rodriguez-Estevez V. Factors affecting somatic cell count in dairy goats: a review. Span J Agric Res. (2014) 12:133–50. doi: 10.5424/sjar/2014121-3803
24. Tedde V, Bronzo V, Puggioni GMG, Pollera C, Casula A, Curone G, et al. Milk cathelicidin and somatic cell counts in dairy goats along the course of lactation. J Dairy Res. (2019) 86:217–21. doi: 10.1017/S0022029919000335
Keywords: goat, weeping teat, udder defect, milk, microbiology, somatic cell count
Citation: Gazzola A, Minozzi G, Biffani S, Mattiello S, Bailo G and Piccinini R (2021) Effect of Weeping Teats on Intramammary Infection and Somatic Cell Score in Dairy Goats. Front. Vet. Sci. 8:622063. doi: 10.3389/fvets.2021.622063
Received: 27 October 2020; Accepted: 22 June 2021;
Published: 19 July 2021.
Edited by:
Alejandra Andrea Latorre, University of Concepción, ChileReviewed by:
Anastasio Arguello, University of Las Palmas de Gran Canaria, SpainCopyright © 2021 Gazzola, Minozzi, Biffani, Mattiello, Bailo and Piccinini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renata Piccinini, cmVuYXRhLnBpY2NpbmluaUB1bmltaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.