
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 12 February 2021
Sec. Veterinary Infectious Diseases
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.604675
 Suresh V. Kuchipudi1,2*
Suresh V. Kuchipudi1,2* Meera Surendran Nair1†
Meera Surendran Nair1† Michele Yon1†
Michele Yon1† Abhinay Gontu1
Abhinay Gontu1 Ruth H. Nissly1
Ruth H. Nissly1 Rhiannon Barry1
Rhiannon Barry1 Denver Greenawalt1
Denver Greenawalt1 Traci Pierre1
Traci Pierre1 Lingling Li1
Lingling Li1 Nagaraja Thirumalapura3
Nagaraja Thirumalapura3 Deepanker Tewari3
Deepanker Tewari3 Bhushan Jayarao1
Bhushan Jayarao1Streptococcus equi subspecies zooepidemicus, a zoonotic bacterial pathogen caused a series of outbreaks with high mortality affecting swine herds in multiple locations of the USA and Canada in 2019. Further genetic analysis revealed that this agent clustered with ATCC 35246, a S. zooepidemicus strain associated with high mortality outbreaks in swine herds of China originally reported in 1977. Rapid and accurate diagnosis is absolutely critical for controlling and limiting further spread of this emerging disease of swine. Currently available diagnostic methods including bacteriological examination and PCR assays do not distinguish between the virulent strains and avirulent commensal strains of S. zooepidemicus, which is critical given that this pathogen is a normal inhabitant of the swine respiratory tract. Based on comparative analyses of whole genome sequences of the virulent isolates and avirulent sequences, we identified a region in the SzM gene that is highly conserved and restricted to virulent S. zooepidemicus strains. We developed and validated a novel probe-based real-time PCR targeting the conserved region of SzM. The assay was highly sensitive and specific to the virulent swine isolates of Streptococcus equi subspecies zooepidemicus. No cross reactivity was observed with avirulent S. zooepidemicus isolates as well as other streptococcal species and a panel of porcine respiratory bacterial and viral pathogens. The PCR efficiency of the assay was 96.64 % and was able to detect as little as 20 fg of the bacterial DNA. We then validated the diagnostic sensitivity and specificity of the new PCR assay using a panel of clinical samples (n = 57) and found that the assay has 100% sensitivity and specificity as compared to bacteriological culture method. In summary, the PCR assay will be an extremely valuable tool for the rapid accurate detection of virulent swine S. zooepidemicus isolates and directly from clinical samples.
Streptococcus equi subspecies zooepidemicus (referred as S. zooepidemicus hereafter), is a zoonotic pathogen of importance to animal and human health and is often associated with sudden epizootics in animals and hence the name zooepidemicus (1). S. zooepidemicus is known to be an opportunistic pathogen affecting a wide range of hosts including horses, pigs, ruminants, guinea pigs, monkeys, cats, dogs, poultry and humans (2–5). S. zooepidemicus infection is manifested in different forms in various hosts, and the disease symptoms include septicemia and arthritis in pigs; mastitis, arthritis, respiratory, and uterine infections in horses; metritis and mastitis in cattle; glomerulonephritis, rheumatic fever, meningitis and purulent arthritis in humans (3, 6, 7). S. zooepidemicus is known to cause pneumonia in equines and canines (5, 8).
Outbreaks of S. zooepidemicus infection in pigs and monkeys with significant morbidity and mortality have been reported previously in Asia (9, 10) and recently in North America (11). S. zooepidemicus infections resulting in sudden death and abortions with high mortality in commercial swine farms were reported in 2019 from the province of Manitoba in Canada (12). Subsequently, several outbreaks of S. zooepidemicus in commercial swine farms with high morbidity and mortality have been reported from Ohio, Tennessee and Pennsylvania in the US (11, 13). The mortality in these outbreaks ranged from 10 to 50% and characterized by sudden onset of death, weakness, lethargy, hyperthermia, and post-mortem lesions included splenomegaly and hemorrhagic lymph nodes. An investigation into the recent outbreaks revealed a striking homology between the recent outbreak isolates and strains of S. zooepidemicus that caused outbreaks with high mortality in swine populations of China in 1977 and with three isolates from human cases with guinea pig exposure (13). Further, the genome analysis of these North American isolates indicated that the virulent swine strains of S. zooepidemicus are epidemiologically related and were significantly different from other swine isolates and most isolates from other animal species (13).
Diagnosis of streptococcal infections is traditionally based on clinical signs and pathological findings in conjunction with laboratory isolation and biochemical identification of the causative agent. However, accurate laboratory identification of S. zooepidemicus poses a challenge as it shares >98% homology in the DNA sequence to the other subspecies, including S. equi subspecies equi (referred as S. equi hereafter), the etiological agent of strangles in horses (6). Molecular tests such as PCR targeting the bacterial genome and mass spectrometric analysis of ribosomal proteins from isolated bacterial colonies have been used in recent years to aid rapid detection and differentiation of S. equi subspecies (14, 15). A dual-target PCR is routinely used in many laboratories to differentiate S. equi subspecies- S. equi and S. zooepidemicus (14). However, all these methods cannot distinguish virulent and avirulent strains of S. zooepidemicus and will not accurately differentiate a mixed culture of S. equi subspecies- S. equi and S. zooepidemicus. It is important to note that mere detection of S. zooepidemicus from animal specimens is of little clinical value as these organisms are found as commensals in many animals. Currently there are no molecular diagnostic assays that can differentiate the virulent S. zooepidemicus isolates responsible for causing lethal disease outbreaks from the rest of the avirulent strains S. zooepidemicus. Therefore, sensitive and specific methods to selectively identify the virulent S. zooepidemicus isolates is urgently needed for early diagnosis and outbreak control.
Several genomic islands and virulence genes in the S. zooepidemicus isolates were attributed to the high mortality in swine populations. SzM gene, which encodes an M-like protein and fibrinogen binding properties, was identified through comparative genome analysis as a key virulence factor of S. zooepidemicus for swine (13). SzM gene is a partial analog of a major virulence factor, SeM of S. equi (16). SzM is highly conserved with 100% homology in the virulent swine isolates including ATCC 35246, and not present in the avirulent S. zooepidemicus strains (13). Similar findings have also been observed with the lethal outbreak isolates from Pennsylvania (PA) and the homology findings are discussed here along with the results to the PCR development and validation experiments described. Therefore, in light of previous genome analysis reports and our findings with the PA isolates, SzM was selected as the target for the development of a probe-based real-time PCR diagnostic assay for the detection of virulent S. zooepidemicus isolates.
Two bacterial cultures were isolated in December 2019 in Pennsylvania from a swine herd, which experienced high mortality. The pure cultures were confirmed as S. zooepidemicus initially using matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) followed by whole genome sequencing. The raw reads have been submitted to the SRA database under the BioProject accession number PRJNA591128. The annotated full genomes of the isolates have been deposited in GenBank under the accession numbers JABDID000000000 and JABMIH000000000.
Comparative gene-based analysis of the two isolates was performed along with virulent type strain ATCC 35246 using Molecular Evolutionary Genetics Analysis software (MEGA-X®) (17). The gene SzM was conserved among the two isolates and was earlier reported to be present only in the virulent strains of S. zooepidemicus from swine (13). Primers and probe targeting 85 bp region of the SzM gene were designed using Primer Express v. 3.0.1® (Applied Biosystems). NCBI Primer-Blast® analysis was used to confirm the specificity of primers by confirming the absence of targets other than virulent strains of S. zooepidemicus in the nucleotide sequence database (18). The sequence of SzM in S. zooepidemicus isolates from other animals for example equines is different and hence this assay will not cross react and is specific for virulent swine isolates. The forward primer (5′—AAGTCGTTGCTCAACTTCATCTATTAAC–3′), reverse primer (5′—TAGGTAATGACCGTCCTAATGATGTT–3′) and the probe (5′—6FAM-AGTTTAACCCTCTTGATCTAT-MGBNFQ–3′) were manufactured by Applied Biosystems, USA. The qPCR assay mixture consisted of 0.4 mM of each primer, 0.3 mM probe and VetMax-Plus qPCR Master Mix® (Applied Biosystems, USA) for a volume of 20 μl. A template DNA volume of 5 μl was added and the assay was performed on an ABI 7500 FAST system® (Applied Biosystems, USA). The optimal cycling conditions were standardized as: 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 45 s, with data collection at the end of each 60°C step.
Assay specificity was tested with a panel of reference bacterial isolates and viral pathogens commonly associated with porcine respiratory disease syndrome (Table 1). Additionally, commensal strains of S. zooepidemicus isolated from horses (n = 10) obtained from Pennsylvania were tested by the developed assay. Avirulent S. zooepidemicus isolates obtained from diagnostic cases submitted to the Pennsylvania Animal Diagnostic Lab system (PADLS), were also tested with the developed assay. All the isolates were confirmed by MALDI-TOF MS and conventional bacterial identification (data not shown).
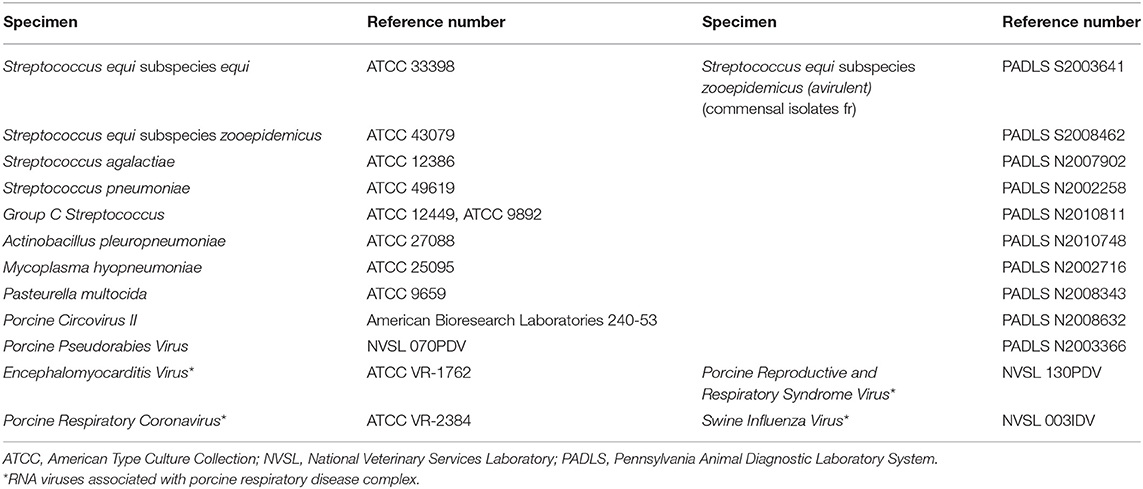
Table 1. Panel of microbial pathogens used for validating the specificity of the developed PCR assay.
The analytical sensitivity and limit of detection was determined using serial dilutions of the S. zooepidemicus DNA. The diagnostic sensitivity and specificity of the PCR assay was established comparing with bacterial culture method using samples (n = 57) from pigs with and without respiratory disease that were submitted to the Animal Diagnostic Lab. The PCR assay was also tested for its range of detection, linearity, efficiency, precision, and repeatability. The validation of the assay was performed based on the guidelines laid out by American Association of Veterinary Laboratory Diagnosticians (AAVLD).
The developed qPCR failed to amplify any region in the tested related and unrelated pathogens which can cause porcine respiratory diseases. The panel of pathogens also included avirulent S. zooepidemicus, which was not amplified by the assay.
Triplicates of ten-fold dilutions of the DNA from one of the virulent S. zooepidemicus were tested by the developed assay. The linearity (Figure 1), range of detection and efficiency of the assay was determined (Table 2). The analytical sensitivity corresponding to the lowest limit of detection was determined as 20 fg of the target DNA (Ct = 34.10 ± 0.61).
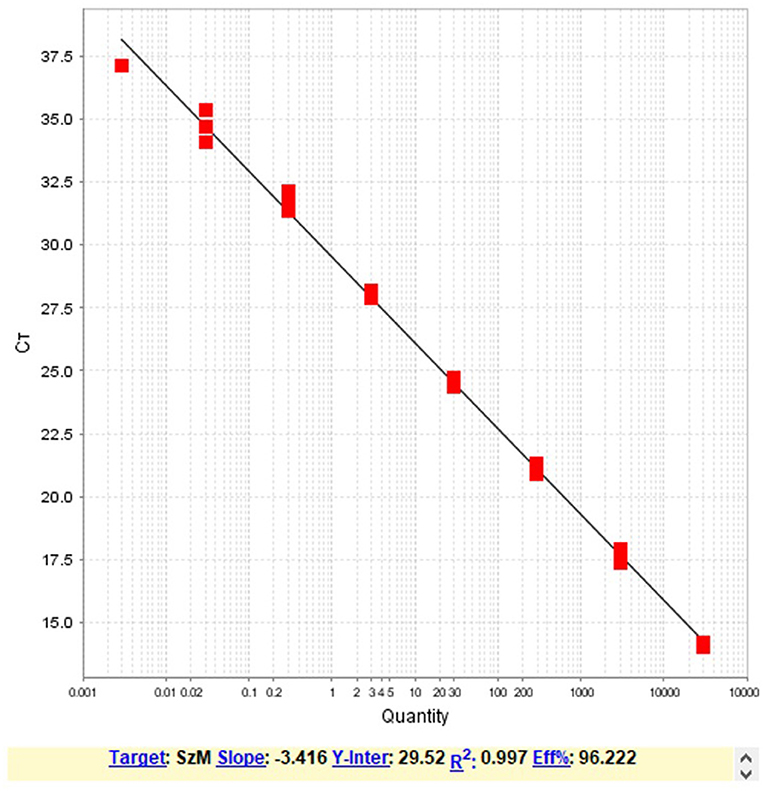
Figure 1. The regression analysis of the resultant average Ct value with the Log10 dilutions of the DNA template showing the linearity and the efficiency of the PCR assay.

Table 2. The Ct values for the log dilutions of the DNA template, giving the range of maximal and minimal dilution of CFU of the pathogen which could be detected by the developed PCR assay.
The resulting Ct values from five replicates of three dilutions of the template DNA tested within a day was analyzed to calculate the Coefficient of variance (CV) % ranging from 0.19 to 1.16 % (Table 3). The inter-day variation of the assay ranged between a CV % of 0.51 to 1.49 % (for dilutions from 10∧−1 to 10∧−3) (Table 3). The intermediate precision both within day and between days resulting in <2 % CV, confirmed high repeatability of the assay.
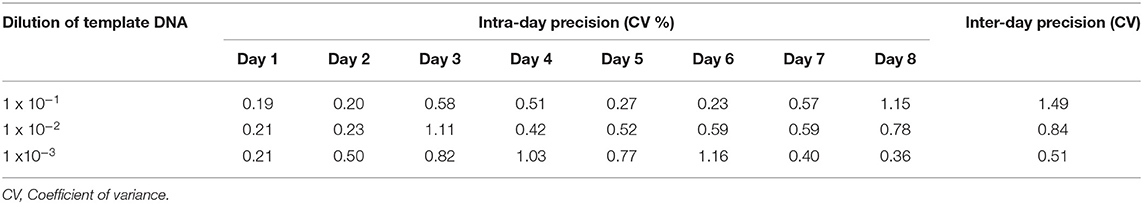
Table 3. Intra-day and inter-day precision of the resulting Ct values from five replicates tested each day for eight days were <2 % co-efficient of variance.
We used a panel of porcine clinical samples that comprised tissues, contact swabs or isolated cultures that were examined by culture followed by MALDI-TOF MS identification. Both the PCR and culture method identified 27 samples as positive and 30 samples as negative, confirming the 100% sensitivity and specificity of the PCR assay. In addition, we extracted DNA from avirulent S. zooepidemicus isolates which gave a negative test as expected.
Evolution of microbial pathogens is an ongoing process, and new pathogens are continually emerging from nature. In addition to the newly emerging pathogens, reemergence of pathogens into new regions continues to be a major global threat to animal and human health. Emerging and remerging animal infectious diseases have the potential to negatively impact animal health, food safety and trade. In addition, several animal infectious diseases have zoonotic potential and hence can have a significant impact on public health.
The recent outbreaks of a virulent S. zooepidemicus flag the re-emergence of the infection after more than four decades of its appearance in China in the 1970s. Conventionally, differential diagnosis of S. zooepidemicus from the closely related S. equi subspecies often involve biochemical characterization, mass spectrometric analysis, polymerase chain reactions or genome sequence analyses (14, 19, 20). The molecular diagnostics described to date are largely focused on presence of genes of S. equi and their corresponding absence in S. zooepidemicus. Båverud et al. (14) described a PCR method which amplifies regions in SodA and SeeI genes (14). The superoxide dismutase A (SodA) gene is amplified in both the subspecies- S. equi and S. zooepidemicus, which were later differentiated by amplification of SeeI in S. equi isolates alone. A few diagnostics were based on detection of SeM, an M-like protein of S. equi as a unique gene absent in S. zooepidemicus (6, 21). SeeH and SeeI genes were also targeted with the purpose of identifying S. equi from S. zooepidemicus (22). All the above-mentioned molecular diagnostics have limitations in that they cannot distinguish a mixed cultures of S. equi and S. zooepidemicus. Moreover, all the described two-step PCR assays are based on the absence of SeeI genes for confirming the presence of S. zooepidemicus. As such these assays are useful to establish that S. zooepidemicus is not present (rule out) but are not very specific to confirm (rule in) the diagnosis. Furthermore, the analyses of 16s rRNA reveal that the sequences are identical among the S. equi strains but vary widely among the S. zooepidemicus strains (14). There is a wide genetic variation reported among isolates of S. zooepidemicus as compared to S. equi subspecies (23).
With the recent outbreak of S. zooepidemicus, the swine farms have been kept on high alert owing to the high mortality produced by the pathogen. Accurate and rapid diagnosis of the virulent S. zooepidemicus is of utmost importance to not only treat the affected animals but also to swiftly implement mitigation measures to further prevent the spread of this deadly infection. The genome sequencing analyses of the isolates from North America revealed the various virulence factors and their conserved nature among the virulent strains of S. zooepidemicus- SzM, lmb, fbpz, skc, has operon & mag regulon (12). Our genomic analyses of the isolates from the recent US outbreak with other virulent strains of S. zooepidemicus revealed that SzM, the gene for a M-like protein of the bacterium is highly conserved and an important virulence contributing factor as was previously reported (13).
The probe based real-time PCR assay developed and validated in this study provides a highly specific means to make a rule in diagnosis of the virulent S. zooepidemicus infection. As this assay can differentiate the virulent S. zooepidemicus isolates from both avirulent S. zooepidemicus and S. equi isolates, it could help in better understanding the ecology and epidemiology of these bacterial agents. A key question yet to be answered is whether susceptible animals of other species serve as reservoirs for S. zooepidemicus. This PCR assay can be used to answer this question and further investigate the host range S. zooepidemicus. In summary, this novel assay which can give the result in <4 h provided a practical solution to the hitherto unsolved problem of diagnosing virulent S. zooepidemicus.
The raw reads have been submitted to the SRA database under the BioProject accession number PRJNA591128. The annotated full genomes of the isolates have been deposited in GenBank under the accession numbers JABDID000000000 and JABMIH000000000.
SK conceived the study. MY and MS assisted in study design. RN, RB, DG, TP, LL, NT, DT, and BJ helped in data collection, analysis, and interpretation. AG, MS, MY, and SK wrote the manuscript. All authors reviewed and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was funded by grant support from the Pennsylvania Soybean Board (OSP#213542), Pennsylvania Department of Agriculture (OSP#189021) and the United States Department of Agriculture (OSP#197702). We wish to thank Dr. Susan Brockmeier of USDA-ARS-National Animal Disease Center; Nubia Resende-de-Macedo and Karen Harmon of Iowa State University, Katherine Dolan of the C.E. Kord Animal Health Diagnostic Lab, Joany van Balen and Dubraska Diaz-Campos of The Ohio State University, Denise Barnhart of Penn Vet New Bolton Center and their support staff for generously providing clinical samples for assay validation. We would also like to thank the staff of all PADLS laboratories for their technical assistance.
1. Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. (2002) 15:613–30. doi: 10.1128/CMR.15.4.613-630.2002
2. Byun JW, Yoon SS, Woo GH, Jung BY, Joo YS. An outbreak of fatal hemorrhagic pneumonia caused by Streptococcus equi subsp. zooepidemicus in shelter dogs. J Vet Sci. (2009) 10:269–71. doi: 10.4142/jvs.2009.10.3.269
3. Bisgaard M, Bojesen AM, Petersen MR, Christensen H. A major outbreak of Streptococcus equi subsp. zooepidemicus infections in free-range chickens is linked to horses. Avian Dis. (2012) 56:561–6. doi: 10.1637/10123-030712-Reg.1
4. Fulde M, Valentin-Weigand P. Epidemiology and pathogenicity of zoonotic streptococci. Curr. Top. Microbiol. Immunol. (2013) 368:49–81. doi: 10.1007/82_2012_277
5. Velineni S, Timoney JF, Russell K, Hamlen HJ, Pesavento P, Fortney WD, et al. Clones of Streptococcus zooepidemicus from outbreaks of hemorrhagic canine pneumonia and associated immune responses. Clin Vaccine Immunol. (2014) 21:1246. doi: 10.1128/CVI.00222-14
6. Timoney JF. The pathogenic equine streptococci. Vet Res. (2004) 35:397–409. doi: 10.1051/vetres:2004025
7. Gruszynski K, Young A, Levine SJ, Garvin JP, Brown S, Turner L, et al. Streptococcus equi subsp. zooepidemicus infections associated with guinea pigs. Emerg Infect Dis. (2015) 21:156–8. doi: 10.3201/eid2101.140640
8. Barr BS. Pneumonia in weanlings. Vet Clin N Am Equine Prac. (2003) 19:35–49. doi: 10.1016/S0749-0739(02)00065-2
9. Soedarmanto I, Pasaribu FH, Wibawan IW, Lammler C. Identification and molecular characterization of serological group C streptococci isolated from diseased pigs and monkeys in Indonesia. J Clin Microbiol. (1996) 34:2201–4. doi: 10.1128/JCM.34.9.2201-2204.1996
10. Ma Z, Geng J, Zhang H, Yu H, Yi L, Lei M, et al. Complete genome sequence of Streptococcus equi subsp. zooepidemicus strain ATCC 35246. J Bacteriol. (2011) 193:5583–4. doi: 10.1128/JB.05700-11
11. Usda. Emerging Risk Notice: Streptococcus equi subspecies zooepidemicus [Online]. USA. (2019). Available online at: https://www.aphis.usda.gov/animal_health/downloads/streptococcus-zooepidemicus-notice.pdf [Accessed].
12. De Costa MO, Lage B. Streptococcus equi subsp. zooepidemicus associated with sudden death of swine in North America. bioRxiv. (2019) 81:2636. doi: 10.1101/812636
13. Chen X, Resende-De-Macedo N, Sitthicharoenchai P, Sahin O, Burrough E, Clavijo M, et al. Genetic characterization of Streptococcus equi subspecies zooepidemicus associated with high swine mortality in United States. Transb Emerg Dis. (2020) 00:1–12. doi: 10.1111/tbed.13645
14. Baverud V, Johansson SK, Aspan A. Real-time PCR for detection and differentiation of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus. Vet Microbiol. (2007) 124:219–29. doi: 10.1016/j.vetmic.2007.04.020
15. Webb K, Barker C, Harrison T, Heather Z, Steward KF, Robinson C, et al. Detection of Streptococcus equi subspecies equi using a triplex qPCR assay. Vet J. (2013) 195:300–4. doi: 10.1016/j.tvjl.2012.07.007
16. Velineni S, Timoney JF. Characterization and protective immunogenicity of the SzM protein of Streptococcus zooepidemicus NC78 from a clonal outbreak of equine respiratory disease. Clin Vacc Immunol. (2013) 20:1181. doi: 10.1128/CVI.00069-13
17. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. (2018) 35:1547–9. doi: 10.1093/molbev/msy096
18. Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. (2012) 13:134. doi: 10.1186/1471-2105-13-134
19. Mir IA, Kumar B, Taku A, Faridi F, Bhat MA, Baba NA, et al. Bacteriological and Molecular Detection of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus in Equines of Northern India. J Equine Sci. (2013) 24:53–55. doi: 10.1294/jes.24.53
20. Mani RJ, Thachil AJ, Ramachandran A. Discrimination of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Vet Diagn Invest. (2017) 29:622–7. doi: 10.1177/1040638717702687
21. Jannatabadi AA, Mohammadi GR, Rad M, Maleki M. Molecular identification of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus in nasal swabs samples from horses suffering respiratory infections in Iran. Pak J Biol Sci. (2008) 11:468–71. doi: 10.3923/pjbs.2008.468.471
22. Alber J, El-Sayed A, Lammler C, Hassan AA, Weiss R, Zschock M. Multiplex polymerase chain reaction for identification and differentiation of Streptococcus equi subsp. zooepidemicus and Streptococcus equi subsp. equi. J Vet Med B Infect Dis Vet Public Health. (2004) 51:455–8. doi: 10.1111/j.1439-0450.2004.00799.x
Keywords: SzM gene, real time PCR, pig mortality, virulent strains, Streptococcus equi subspecies zooepidemicus
Citation: Kuchipudi SV, Surendran Nair M, Yon M, Gontu A, Nissly RH, Barry R, Greenawalt D, Pierre T, Li L, Thirumalapura N, Tewari D and Jayarao B (2021) A Novel Real-Time PCR Assay for the Rapid Detection of Virulent Streptococcus equi Subspecies zooepidemicus—An Emerging Pathogen of Swine. Front. Vet. Sci. 8:604675. doi: 10.3389/fvets.2021.604675
Received: 10 September 2020; Accepted: 20 January 2021;
Published: 12 February 2021.
Edited by:
Camilla Luzzago, University of Milan, ItalyReviewed by:
Filip Boyen, Ghent University, BelgiumCopyright © 2021 Kuchipudi, Surendran Nair, Yon, Gontu, Nissly, Barry, Greenawalt, Pierre, Li, Thirumalapura, Tewari and Jayarao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suresh V. Kuchipudi, c2t1Y2hpcHVkaUBwc3UuZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.