- 1Dipartimento di Medicina Veterinaria, Università degli Studi di Milano, Lodi, Italy
- 2Dipartimento di Scienze Veterinarie, Università degli Studi di Torino, Grugliasco, Italy
T cell lymphoma (TCL) is a heterogenous group of lymphoid malignancies representing about 30–40% of all canine lymphomas and often harboring a very aggressive behavior. WHO classification identifies the majority of TCLs as peripheral TCL, but other subtypes with peculiar presentation and outcome have been recognized. This review aims to explore the use of flow cytometry for refining the diagnosis of canine TCL, putting a particular emphasis on the identification of some peculiar immunotypes, such as T zone lymphoma; on the investigation of putative prognostic markers; and on the evaluation of lymphoma stage and of the minimal residual disease.
Introduction
T cell lymphoma (TCL) is a heterogeneous group of lymphoid neoplasms stemming from T cells. It represents about 30–40% of canine lymphomas. Prevalence of B vs. TCLs varies among different breeds with TCL being highly prevalent in Irish Wolfhound, Shih Tzu, Airedale Terrier, Cavalier King Charles Spaniel, Yorkshire Terrier, Siberian Husky, and some other breeds (1).
Solid presentation with enlarged lymph nodes or the increased volume of any other lymphoid organ are distinctive traits; these features easily allow a distinction between lymphoma and leukemia, such as T chronic lymphocytic leukemia, which, on the contrary, originates from bone marrow or spleen. Despite highly variable cytological aspects, most TCLs fall under the umbrella of peripheral T cell lymphoma (PTCL), not otherwise specified (NOS), according to WHO classification (2). T-zone lymphoma (TZL) and T lymphoblastic lymphoma (LL) are two other commonly found TCL morphotypes, whereas enteropathy-associated TCL, cutaneous epitheliotropic TCL, mediastinal lymphoma, and hepatocytotropic/hepatosplenic lymphoma constitute uncommon findings. The updated Kiel classification identifies several different cytological subtypes of TCL, thus confirming their extremely heterogeneous morphology (3) (Table 1). According to this scheme, TCL could be further divided into low-grade TCLs, characterized by indolent behavior, and high-grade TCLs, characterized by a very aggressive course with median survival time statistically lower than its B cell counterpart (4).
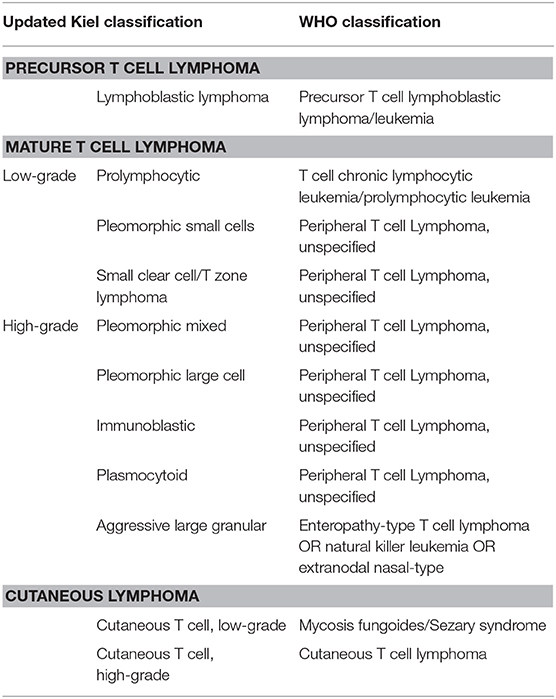
Table 1. Possible correlation between updated Kiel classification and WHO classification for TCLs according to Ponce et al. (3).
Identification of T Cell Lineage
Several antibodies have been generated to recognize specific canine lymphoid markers and to define normal T cell subpopulations via flow cytometry (FC). Antibodies against surface epitopes are highly preferable for FC. Antibodies capable of recognizing intracellular epitopes represent another option, but they do require one or more additional permeabilization steps that may alter cell morphology. This said, in the absence of any definitive reactivity to membrane antigens, intracellular labeling for cytoplasmic CD3 can be performed to confirm a T cell origin. Antibodies prelabeled with different fluorochromes are recommended to identify co-expressions and to reduce the number of cells needed for a complete antibody panel. Common antibodies used to phenotype T cell markers and expected reactivity in non-neoplastic cells are reported in Table 2.
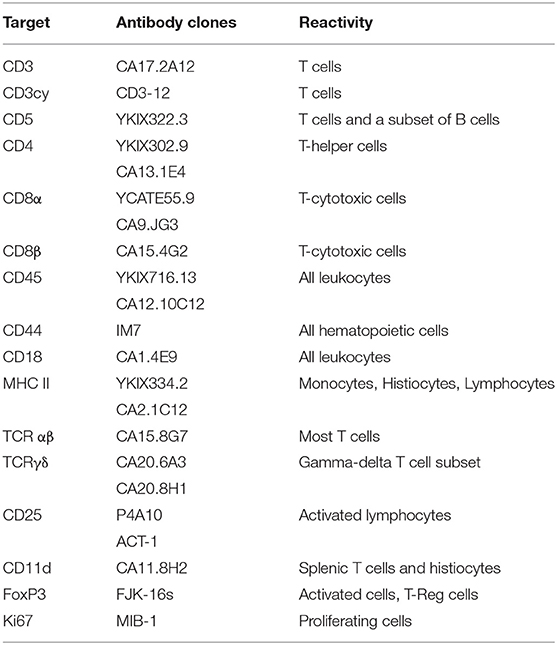
Table 2. Common antibodies used for the characterization of T cells in dog and expected reactivities.
T-helper cells are the most frequent lymphocyte subset in canine lymph nodes (5) and can be easily recognized for their positivity to CD4, CD3, CD5, and MHC II and for their prevalent positivity to TCRαβ and, if activated, to CD25. A peculiar subset of CD4+ T-regulatory cells (T-regs), representing <1% of lymphocytes in circulating blood and <5% in lymph nodes, can be identified for their high expression of CD25 and for the co-expression of nuclear antigen Fox-P3 (6).
T-cytotoxic cells are less frequent in lymph nodes, spleen, and peripheral blood and may be easily identified for positivity to CD8, CD3, CD5, and MHC II.
In the thymus, besides mature T-helper and T-cytotoxic cells, a mixed population of immature T cells co-expressing CD4 and CD8 (double-positive thymocytes) or lacking both antigen expression (double-negative T cells) are found.
Less frequently, T cells may express CD11d and/or TCRγδ. These phenotypes are often suggestive of splenic or, less frequently, intestinal origin (7). These cells, in some instances, can feature a large granular lymphocyte (LGL) morphology (8).
Differentiating Reactive and Neoplastic T Cells
When it comes to differentiating between reactive and neoplastic T cell populations, distinguishing paracortical hyperplasia from TZL represents the most challenging step (9). Paracortical hyperplasia is an infrequent transitory hyperplastic condition due to chronic dermatitis or to some specific antigens stimulating T response (such as in Leishmaniosis or in viral infections). It generally represents the immune response first phase, and it precedes plasma cell proliferation (10). Differentiation via cytology may be inconclusive, and FC should be considered the most rapid and effective way to differentiate. This is due to the very peculiar phenotype of TZL (CD45-CD5+CD21+) that can be easily detected using a multicolor approach (11, 12).
Differentiating between high-grade TCL and reactive lymph node hyperplasia tends to be easier because hyperplastic lymph nodes (with follicular and/or lympho-plasmacellular hyperplasia) are generally composed of a mixed population of T and B cells, sometimes with a slight increase of large B cells. The T cell population is generally composed of a mixed population of both CD4 positive and CD8 positive cells, suggesting polyclonal expansion. On the contrary, most PTCL feature a homogeneous expansion of a single T cell subpopulation. The presence of medium to large T lymphoid cells with forward scatter (FSC) higher than 1.3–1.5 times that of normal lymphocytes also suggests lymphoma. Residual non-neoplastic B and T cells are generally few, and this supports the hypothesis of a clonal expansion, confirming lymphoma.
Neoplasia also may be confirmed through the identification of phenotypic aberrancies. Qualitative and quantitative aberrancies are frequent, occurring in more than 75% of TCLs (13). The most frequent phenotypic aberrancies are loss of CD5 or CD3 expression; loss or co-expression of both CD4 and CD8; marked decrease/loss of MHC class II expression; loss of CD45 or other pan-leukocyte antigen expression; and concurrent expression of a B-specific antigen, such as CD21 or CD79. The use of wide antibody panels increases the odds of detecting aberrant phenotypes, which may be considered a hallmark of neoplasia (13).
When mediastinal masses are composed of a lymphocyte-rich population, FC of the lymphoid population is considered an excellent tool to distinguish mediastinal lymphoma from non-neoplastic normal lymphocytes, thus supporting thymoma. A cutoff value of 10% of double positive CD4+CD8+ thymocytes and small cell size (similar to that of a circulating lymphocyte) is strongly supportive of thymoma (14).
Defining Immunophenotype of Canine Lymphoma
Immunophenotype is a widely accepted prognostic feature in canine high-grade lymphoma (4, 15, 16). Immunohistochemistry is the traditional tool performed to define immunophenotype, but FC has recently gained popularity due to the availability of wide antibody panels, its fast response and the possibility of detecting co-expressions and of accurately quantifying antigen expressions (17).
Consistent approaches and cutoff values to define TCLs, however, are still lacking. Wilkerson et al. (18) considered TCLs if 60% of the population expressed any T cell antigen. A more recent study considered as inclusion criteria to define TCL a lymph node with a cytology/histopathology suggestive of lymphoma together with a positive reactivity to CD3, or CD4, CD8, in more than 10% of large lymphoid cells (19). In another study, more than 65% of large lymphoid cells or more than 80% of total lymphoid cells being positive to CD4 were considered essential to define CD4+ TCLs (20).
In a recent study on CD8+ and CD4–CD8– lymphomas, TCL cases were identified by a discrete majority population of T cells with a homogeneous phenotype or a population of T cells showing at least one feature of aberrancy (21). In another study from the same group, the T cell phenotype was defined upon the expression of CD3 or CD5 with variable expression of T cell subset antigens CD4 and CD8 (22); no cutoff values were reported.
Considering the abovementioned papers, despite the lack of a consensus, TCL is likely to be correctly diagnosed if a lymph node presents with cytology/histology suggestive of lymphoma and if (1) a prevalent population of medium-to-large lymphoid cells (≥1.3 times the size of normal T-lymphocytes) expresses uniformly CD3 and/or CD5; (2) more than 80% of total lymphoid cells have a homogeneous expression of CD4 or CD8; or (3) any aberrant phenotype in CD3 or CD5 positive cells is present, including the loss of membrane expression of CD3 in cells retaining cytoplasmic expression of CD3 only.
In rare instances, co-expression of T and B lineage-specific markers may occur. More specifically, CD21 may be expressed in TZL, and CD79 may be aberrantly expressed in some PTCLs. A comprehensive evaluation of a complete panel of anti-T antibodies is normally enough to differentiate B cells from TCLs with an aberrant expression of B cell markers, but in some cases, some other ancillary tests (PARR and histopathology) might be necessary although caution should be made using PARR as an immunotyping method (23).
Refining the Diagnosis of TCL Subtypes
Flow Cytometry is a valid and rapid tool not only to define T cell lineage, but also to refine the classification of TCL subtypes in addition to cytomorphology and histopathology.
Peripheral T Cell Lymphoma
Peripheral T cell lymphoma is the most widely distributed TCL subtype and comprises a heterogeneous group of different immunotypes with different cytological presentations and variable outcomes. According to the updated Kiel classification scheme adapted to the dog, PTCLs are characterized by six morphological subtypes, namely pleomorphic small-cell, small clear-cell, pleomorphic mixed, pleomorphic large-cell, immunoblastic, and plasmacytoid lymphomas (3). Among PTCLs, the most frequent phenotype is CD45+CD3+ CD4+MHC II–/low (Figure 1) (19, 20). In all PTCL subtypes, CD5 expression is usually positive, but a loss of CD5 and CD3 may aberrantly occur and have a possible prognostic meaning. CD25 is generally negative, suggesting that they are inactive cells. TCRαβ is highly prevalent. This immunophenotype occurs in 43–45% of multicentric TCLs according to two different studies (19, 22). Forward scatter properties show a medium-to-large size with median FSC 1.4 times greater than in non-neoplastic lymphocytes (22). This subtype exhibits a consistent gene expression profile with the positive regulation of phosphatidylinositol 3-kinase (PI3K) activity. Phosphatidylinositol 3-kinase works together with AKT and mTOR to regulate the cell cycle, leading to increased cell proliferation and survival. The AKT/PI3K/mTOR signaling axis is usually antagonized by the product of phosphatase and tensin homolog gene (PTEN), a tumor suppressor gene that is downregulated in CD4+ TCL as well (22).
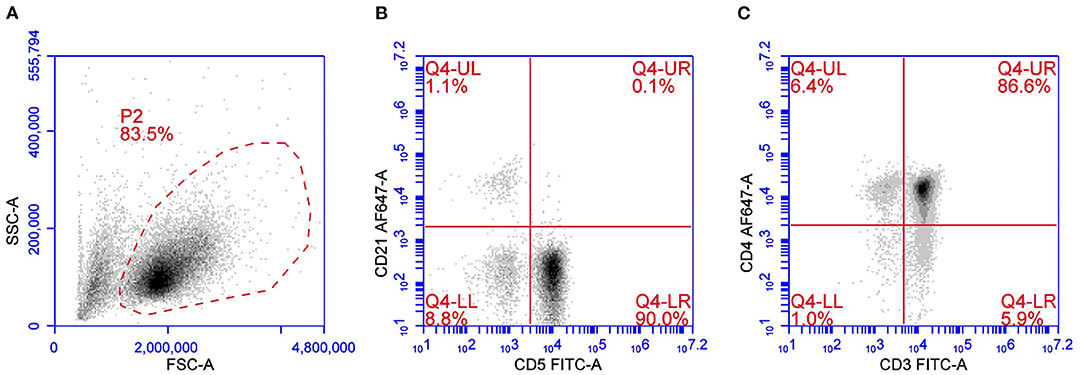
Figure 1. Flow cytometric presentation of a PTCL. (A) Forward scatter (FSC) vs. side scatter (SSC) plot after doublet exclusion showing a unique population of medium-to-large sized cells. (B,C) CD5 vs. CD21 plot (B) and CD3 vs. CD4 plot (C) of P2-gated cells showing a predominant population of CD5+CD3+CD4+ cells.
The less frequent immunophenotypes of PTCL are CD45+CD3+CD8+MHC II– and CD45+CD3+CD8–CD4–MHCII– comprising about 11.9 and 13.5% of TCLs, respectively (19).
A recent study based on 119 cases demonstrated that the former immunophenotype is often associated with a cutaneous localization, whereas the latter more frequently affects mediastinal lymph nodes (21). Forward scatter properties are similar between the two subtypes, with median values of FSC >1.2 times in neoplastic cells than in normal lymphocytes.
Other possible immunotypes are rare, but may include CD3+CD4+CD8+, CD3+CD4+MHC II+, and CD3+CD8+MHCII+ (19). However, specific studies on these immunotypes including histopathology and the evaluation of CD45 expression to exclude TZL are still lacking.
Lymphoblastic Lymphoma
Lymphoblastic lymphoma is a rare subtype identified in about 10% of TCL cases and about 3% of all canine lymphoma (3). According to the WHO classification, LL is considered a solid variant of T cell acute LL and is generally classified as a neoplasm arising from precursor cells. Despite the homogeneous population of small cells without an evident nucleolus, LL is an aggressive high-grade lymphoma, and the mitotic index is often very high. The outcome is generally poor without any statistical difference compared with CD4+ PTCL. One study (24) described a high prevalence of LL in the boxer breed, harboring a less aggressive course and a longer survival. A recent study on CD4+ TCL reported an overlapping of gene expression profiles between PTCL and LL in dogs (22). In terms of FC, the prevalent immunophenotype of LL is similar to that of PTCL (CD45+CD3+CD4+MHC II–), and despite the supposed origin from precursor cells, CD34 is generally negative. Terminal deoxynucleotidyl transferase (TdT), which is often used to confirm lymphoblastic nature in human LL (25), has not yet been optimized for dogs.
In cases characterized by small-sized cells and no expression of precursor markers, the FC determination of proliferative activity can be useful to grade the tumor. A significant difference between low- and high-grade lymphomas, according to the updated Kiel classification, has been shown both for Ki67 expression (26) and S-phase fraction (27), and a cutoff of 12.2 and 3.15% was proposed, respectively, independent of the lineage. Nevertheless, in the study by Poggi et al. (26), T cell high-grade lymphomas presented higher Ki67 values compared with B cell high-grade forms (45.0 vs. 29.3%).
T-Zone Lymphoma
T-zone lymphoma is a low-grade indolent lymphoma occurring in about 10% of TCL. Cytologically TZL can be described as a lymphocytic, small clear-cell lymphoma. Neoplastic cells are easily recognized using a multicolor approach for their characteristic loss of CD45 and for the frequent aberrant expression of CD21, a B cell antigen, in the context of a CD3+CD5+MHC II+ lymphoma (11, 12) (Figure 2). Although rare, loss of CD45 expression may also occur in other lymphoma subtypes besides TZL (28). CD25 is frequently expressed in TZL, thus confirming the neoplastic cell activation status. Expression of CD4 and CD8 may be variable (CD4+CD8–, CD4–CD8+, CD4–CD8–, or CD4+CD8+) without any evident correlation with outcome. Due to the very peculiar phenotype, TZL also may be easy to recognize in its earlier phases, when a mixed population of residual non-neoplastic lymphocytes is still present, in cases of concurrent lymphomas in the same node (29–31) or, again, when they are detected in non-lymphoid tissues, such as peripheral blood and bone marrow, which are infiltrated in more than 90% of cases (32). The low proliferative activity reported by TZLs is described in two studies focusing on B and TCLs and supports the low aggressiveness of the tumor (26, 27). Several studies confirm the indolent behavior of this subtype, making aggressive chemotherapy unnecessary in most dogs with TZL (4, 11, 32, 33).
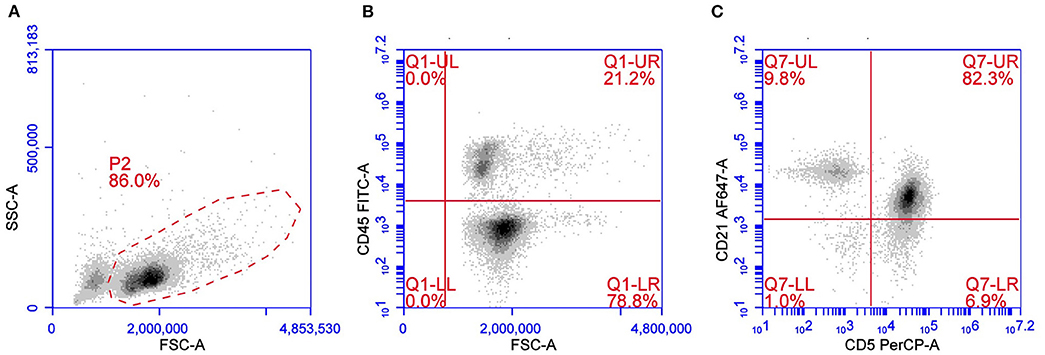
Figure 2. Flow cytometric presentation of a TZL. (A) Forward scatter (FSC) vs. side scatter (SSC) plot after doublet exclusion showing a prevalent population of medium sized cells. (B,C) FSC vs. CD45 plot (B) and CD5 vs. CD21 plot (C) of P2-gated cells showing that medium-sized cells are CD45–CD5+CD21+dim.
Studies on FC aspects in dogs with other TCL subtypes are fragmentary and based on very limited caseloads. Limited data are due to the frequent extranodal localizations that impair the overall quality of FC samples. According to a study, in fact, <60% of extranodal lymphoma were suitable for FC processing, compared with more than 90% of nodal ones (34). Immunohistochemistry and other techniques are more suitable to investigate this kind of lesions.
Hepatosplenic/Hepatocytotropic Lymphomas
Keller et al. (35) described a series of nine cases of hepatosplenic/hepatocytotropic lymphomas. Immunophenotype was assessed via FC, and immunohistochemistry and neoplastic cells expressed CD3+ (5/7), TCRαβ- (5/5), TCRγδ+ (3/5), CD11d+ (6/7). CD8α was inconsistently expressed in 4/6 cases, and CD4 and CD8β were not expressed in any of the cases. The positivity to CD11d suggests an origin from the splenic red pulp and may help to differentiate hepatosplenic from hepatocytotropic lymphomas; these findings are consistent with what has been described in human medicine (36).
Cutaneous Epitheliotropic T Cell Lymphoma
Cutaneous Epitheliotropic T Cell Lymphoma (CETL) is commonly investigated via immunohistochemistry because cutaneous lesions are difficult to sample for FC and the presence of non-neoplastic reactive lymphocytes may affect FC interpretation. Flow cytometry may be useful to evaluate immunophenotype in infiltrated lymph nodes from primary CETL or in peripheral blood if Sezary syndrome is present (37). In these cases, neoplastic cells are CD3+CD8+TCRγδ+ and frequently lack CD5 expression (38).
Mediastinal Lymphoma
Flow cytometric features from seven cases of mediastinal lymphoma were reported in a small case series (14). Four out of seven cases expressed CD4; one expressed only CD45, CD34, and CD14 and exhibited T cell clonality by PARR analysis, and one was of B cell origin. The last case was double positive for CD4+CD8+ but was mainly composed of large cells. The authors concluded that FC is a useful tool for discriminating mediastinal masses and that thymoma could be ruled out in all cases in which <10% of the small lymphocytes were CD4+CD8+. However, a more recent paper (39) describes expansion of CD4–CD8– cells in a case of canine thymoma, partially contradicting the previously published paper.
Enteropathy-Associated T Cell Lymphoma
Enteropathy-associated T Cell Lymphoma (EATL) is generally investigated via immunohistochemistry and shows positivity to CD3 and occasionally to CD30, a marker of anaplastic lymphoma in humans (40). In rare instances, aberrant co-expression of B cell antigen CD20 is possible (41). To the best of our knowledge, no FC studies in EATL are available in dogs.
Refining the Prognosis of T Cell Lymphoma
Outcomes of TCLs vary from poorly aggressive TZL with an indolent course (the majority of cases) to highly aggressive PTCL and hepatosplenic lymphoma. Being that TCLs comprise a wide spectrum of different entities, each of them featuring a different biological behavior and outcome, an accurate definition of the immunophenotype is crucial for predicting outcome and setting up a tailored therapy. Even though specific studies on prognosis in TCLs are fragmentary, different subtypes could likely have different prognostic factors.
Flow Cytometry is likely to be the best available tool to accurately detect TZL given the very peculiar immunophenotype and the loss of CD45 expression. The presence of CD45–CD5+ cells in lymph nodes and, in most cases, in peripheral blood allows the suspicion of TZL also in its earlier stages, despite the lack of a marked lymphoadenomegaly in palpable lymph nodes. To date, no specific immunophenotypic prognostic markers for TZL have been described, but median survival time is reported to range between 637 and 930 days according to different studies (11, 12, 42). However, some aggressive cases featuring a similar immunophenotype but leading to short survival time are sometimes described (28, 43).
Peripheral T cell lymphomas are generally associated with short survival times [162 days according to (15)]. A recent study defined shorter survival times and progression-free intervals (PFI) if CD4+MHC II– phenotype is found (160 and 108 days, respectively) (19). Unfortunately, CD45 expression was not included in this study, and the inclusion of some TZLs (expressing high MHC II) in the caseload may have contributed to bias in the comparison. In another study (22), no differences were found in survival times among CD4+MHCII–, CD8+MHCII–, and CD4–CD8–MHCII–, but large cell size was associated with a significantly shorter PFI and overall survival (20, 22). CD5 expression (20) or loss of CD3 expression or co-expression of CD79a (44) were also moderately associated with shorter survival time in CD4+MHC II– PTCLs.
Considering all these findings, FC immunophenotyping seems to be useful to better refine the subtype of TCL and to differentiate PTCL from TZL. Furthermore, large size and aberrant loss of CD3, or aberrant expression of CD79 may be linked to a more aggressive course.
In addition to defining the degree of malignancy, Ki67 values have proven useful for stratifying high-grade B cell lymphomas with different prognosis (45). Different levels of Ki67 expression were also described within high-grade TCLs (26). However, whether it can represent a prognostic index for TCLs has yet to be determined.
Staging T Cell Lymphoma
Flow cytometry is an effective tool for defining infiltration of neoplastic cells in different tissues, mainly in liquid ones such as peripheral blood (PB) and bone marrow (BM). The impact of the stage on the prognosis of canine lymphoma is not consistent among different studies, and the current findings are often influenced by the inclusion of different lymphoma subtypes. Also, the strategy to detect infiltration (microscopic evaluation, immunohistochemistry, FC, PARR) and the cutoff values used vary among studies, thus strongly affecting results. Some studies, however, suggest that stages, in particular, stage V (i.e., infiltration of PB and/or BM or any other extra lymphoid tissue) may affect outcomes differently in different subtypes. Most studies on this issue have been focused on B cell lymphomas (46, 47), and no specific studies on the prognostic significance of PB and BM infiltration are available when it comes to TCLs.
As described, PTCLs are a very heterogeneous group in terms of immunophenotype and morphology, suggesting that the strategies used to detect neoplastic infiltration in peripheral and medullary blood may differ. Multicolor FC may help to easily detect the percentage of CD3+CD4+MHC II– neoplastic cells in PB and BM and to distinguish this circulating neoplastic cells from residual non-neoplastic T-helper cells (MHC II+). Similarly, CD3+CD8+MHC II–, CD3+CD4–CD8–, or CD3+CD4+CD8+ lymphomas may be accurately quantified using a multicolor approach. Other strategies may be used if aberrant patterns are expressed, such as loss of CD3 or CD5 expression. In these cases, double labeling for CD3 and CD5 may easily identify neoplastic cells. In contrast, differentiation between neoplastic and residual circulating lymphocytes can be challenging if the immunophenotype of neoplastic cells is similar to that of normal T-helper (CD4+MHC II+) or T-cytotoxic (CD8+MHC II+) lymphocytes and no aberrancies are present. In these instances, a larger cell size compared with that of circulating lymphocytes can help to suspect neoplastic infiltration.
To the best of our knowledge, no specific studies (featuring a consistent caseload of PTCLs) on the prognostic significance of PB or BM infiltrations are available, but some findings suggest that the presence of leukemic cells is related to a bad outcome in TCL (19), thus suggesting a possible prognostic role. This said, cutoff values to define leukemic infiltration were not specified.
T-zone lymphoma is associated with the presence of neoplastic circulating cells in more than 90% of cases (32) with or without lymphocytosis. BM is often mildly or not infiltrated, and cytopenia is generally scarce. Neoplastic circulating cells are easy to detect using multicolor FC and double labeling for CD45/CD5. Sometimes their detection in PB may precede the development of an evident solid mass. Despite the frequent peripheral infiltration, the prognosis of TZL is generally quite good, and no data support a prognostic role of PB or BM infiltration to date.
Evaluation of Minimal Residual Disease
From a technical standpoint, the same strategies used to stage TCLs can be used to evaluate the effects of chemotherapy and to monitor the minimal residual disease (MRD). Minimal residual disease via FC has been suggested as a possible feasible method to monitor the effect of chemotherapy and to predict relapse in canine diffuse large B cell lymphoma (48). At present, however, no specific studies on the evaluation of MRD via FC and its possible prognostic role in canine TCL are available.
Future Perspectives
Although the availability of specific antibodies for most T cell subpopulations improved the diagnosis of TCL, there is still a lack of specific antibodies for some peculiar subtypes, such as natural killer cells, precursor T cells, and/or antibodies that may potentially be useful to better understand the prognosis and peculiar risk factors. Again, the commercial availability of antibodies labeled with more different fluorochromes would improve the multicolor approach, allowing detection also of a rare population of cells.
Also, the clinical significance of staging PB and BM infiltration of TCLs is far from being completely understood, and multi-institutional prospective studies with a standardized approach and therapy are needed to obtain adequate statistic power.
Author Contributions
All authors have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Modiano JF, Breen M, Burnett RC, Parker HG, Inusah S, Thomas R, et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. (2005) 65:5654–61. doi: 10.1158/0008-5472.CAN-04-4613
2. Valli VE, San Myint M, Barthel A, Bienzle D, Caswell J, Colbatzky F, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. (2011) 48:198–211. doi: 10.1177/0300985810379428
3. Ponce F, Marchal T, Magnol JP, Turinelli V, Ledieu D, Bonnefont C, et al. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet Pathol. (2010) 47:414–33. doi: 10.1177/0300985810363902
4. Ponce F, Magnol JP, Ledieu D, Marchal T, Turinelli V, Chalvet-Monfray K, et al. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet J. (2004) 167:158–66. doi: 10.1016/j.tvjl.2003.10.009
5. Rütgen BC, König R, Hammer SE, Groiss S, Saalmüller A, Schwendenwein I. Composition of lymphocyte subpopulations in normal canine lymph nodes. Vet Clin Pathol. (2015) 44:58–69. doi: 10.1111/vcp.12221
6. Pinheiro D, Singh Y, Grant CR, Appleton RC, Sacchini F, Walker KR, et al. Phenotypic and functional characterization of a CD4(+) CD25(high) FOXP3(high) regulatory T-cell population in the dog. Immunology. (2011) 132:111–22. doi: 10.1111/j.1365-2567.2010.03346.x
7. Faldyna M, Sinkora J, Knotigova P, Leva L, Toman M. Lymphatic organ development in dogs: major lymphocyte subsets and activity. Vet Immunol Immunopathol. (2005) 104:239–47. doi: 10.1016/j.vetimm.2004.12.002
8. McDonough SP, Moore PF. Clinical, hematologic, and immunophenotypic characterization of canine large granular lymphocytosis. Vet Pathol. (2000) 37:637–46. doi: 10.1354/vp.37-6-637
9. Fournel-Fleury C, Magnol JP, Guelfi JF. editors. Chapter IV: The lymph node. In: Color Atlas of Cancer of the Dog and Cat. Paris: CNVSPA Edition (1994). p. 241–67.
10. Comazzi S, MacNeill A. Cytology of lymphoid tissues. In AM Barger and AL MacNeill, Small Animal Cytological Diagnosis, Boca Raton, FL: CRC Press (2017). p. 241–80.
11. Seelig DM, Avery P, Webb T, Yoshimoto J, Bromberek J, Ehrhart EJ, et al. Canine T-zone lymphoma: unique immunophenotypic features, outcome, and population characteristics. J Vet Intern Med. (2014) 28:878–86. doi: 10.1111/jvim.12343
12. Martini V, Poggi A, Riondato F, Gelain ME, Aresu L, Comazzi S. Flow-cytometric detection of phenotypic aberrancies in canine small clear cell lymphoma. Vet Comp Oncol. (2015) 13:281–7. doi: 10.1111/vco.12043
13. Gelain ME, Mazzilli M, Riondato F, Marconato L, Comazzi S. Aberrant phenotypes and quantitative antigen expression in different subtypes of canine lymphoma by flow cytometry. Vet Immunol Immunopathol. (2008) 121:179–88. doi: 10.1016/j.vetimm.2007.09.018
14. Lana S, Plaza S, Hampe K, Burnett R, Avery AC. Diagnosis of mediastinal masses in dogs by flow cytometry. J Vet Intern Med. (2006) 20:1161–5. doi: 10.1892/0891-6640(2006)20[1161:dommid]2.0.co;2
15. Valli VE, Kass PH, San Myint M, Scott F. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol. (2013) 50:738–48. doi: 10.1177/0300985813478210
16. Frantz AM, Sarver AL, Ito D, Phang TL, Karimpour-Fard A, Scott MC, et al. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Vet Pathol. (2013) 50:693–703. doi: 10.1177/0300985812465325
17. Comazzi S, Gelain ME. Use of flow cytometric immunophenotyping to refine the cytological diagnosis of canine lymphoma. Vet J. (2011) 188:149–55. doi: 10.1016/j.tvjl.2010.03.011
18. Wilkerson MJ, Dolce K, Koopman T, Shuman W, Chun R, Garrett L, et al. Lineage differentiation of canine lymphoma/leukemias and aberrant expression of CD molecules. Vet Immunol Immunopathol. (2005) 106:179–96. doi: 10.1016/j.vetimm.2005.02.020
19. Deravi N, Berke O, Woods JP, Bienzle D. Specific immunotypes of canine T cell lymphoma are associated with different outcomes. Vet Immunol Immunopathol. (2017) 191:5–13. doi: 10.1016/j.vetimm.2017.07.008
20. Avery PR, Burton J, Bromberek JL, Seelig DM, Elmslie R, Correa S, et al. Flow cytometric characterization and clinical outcome of CD4+ T-cell lymphoma in dogs: 67 cases. J Vet Intern Med. (2014) 28:538–46. doi: 10.1111/jvim.12304
21. Harris LJ, Rout ED, Labadie JD, Avery PR, Fernandez M, Yoshimoto J, et al. Clinical features of canine nodal T-cell lymphomas classified as CD8+ or CD4-CD8- by flow cytometry. Vet Comp Oncol. (2020) 18:416–27. doi: 10.1111/vco.12568
22. Harris LJ, Hughes KL, Ehrhart EJ, Labadie JD, Yoshimoto J, Avery AC. Canine CD4+ T-cell lymphoma identified by flow cytometry exhibits a consistent histomorphology and gene expression profile. Vet Comp Oncol. (2019) 17:253–64. doi: 10.1111/vco.12460
23. Thalheim L, Williams LE, Borst LB, Fogle JE, Suter SE. Lymphoma immunophenotype of dogs determined by immunohistochemistry, flow cytometry, and polymerase chain reaction for antigen receptor rearrangements. J Vet Intern Med. 27:1509–16. doi: 10.1111/jvim.12185
24. Lurie DM, Milner RJ, Suter SE, Vernau W. Immunophenotypic and cytomorphologic subclassification of T-cell lymphoma in the boxer breed. Vet Immunol Immunopathol. (2008) 125:102–10. doi: 10.1016/j.vetimm.2008.05.009
25. Cortelazzo S, Ponzoni M, Ferreri AJM, Hoelzer D. Lymphoblastic lymphoma. Review Crit Rev Oncol Hematol. (2011) 79:330–43. doi: 10.1016/j.critrevonc.2010.12.003
26. Poggi A, Miniscalco B, Morello E, Comazzi S, Gelain ME, Aresu L, et al. Flow cytometric evaluation of ki67 for the determination of malignancy grade in canine lymphoma. Vet Comp Oncol. (2015) 13:475–80. doi: 10.1111/vco.12078
27. Miniscalco B, Poggi A, Martini V, Morello E, Sulce M, Melega M. Flow cytometric characterization of S-phase fraction and ploidy in lymph node aspirates from dogs with lymphoma. J Comp Pathol. (2018) 161:34–2. doi: 10.1016/j.jcpa.2018.04.005
28. Parachini-Winter C, Curran KM, Russell DS, Gorman E. A case of canine high-grade T-cell lymphoma immunophenotypically consistent with T-zone lymphoma. Vet Clin Pathol. (2018) 47:643–8. doi: 10.1111/vcp.12657
29. Suwa A, Shimoda T. Concurrent with T-zone lymphoma and high-grade gastrointestinal cytotoxic T-cell lymphoma in a dog. J Vet Med Sci. (2017) 79:736–9. doi: 10.1292/jvms.16-0542
30. Matsuyama A, Bienzle D, Richardson D, Deravi N, Hwang MH, Darzentas N, et al. Composite lymphoma of concurrent T zone lymphoma and large cell B cell lymphoma in a dog. BMC Vet Res. (2019) 15:413. doi: 10.1186/s12917-019-2154-8
31. Long ME, Evans B, Avery AC, Wellman ML. Lymphocytosis and lymphadenopathy in a dog arising from two distinct lymphoid neoplasms. Vet Clin Pathol. (2020) 49:307–11. doi: 10.1111/vcp.12855
32. Martini V, Marconato L, Poggi A, Riondato F, Aresu L, Cozzi M. Canine small clear cell/T-zone lymphoma: clinical presentation and outcome in a retrospective case series. Vet Comp Oncol. (2016) 14(Suppl 1):117–26. doi: 10.1111/vco.12155
33. Flood-Knapik KE, Durham AC, Gregor TP, Sánchez MD, Durney ME, Sorenmo KU. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet Comp Oncol. (2013) 11:272–86. doi: 10.1111/j.1476-5829.2011.00317.x
34. Martini V, Melega M, Riondato F, Marconato L, Cozzi M, Bernardi S. A retrospective study of flow cytometric characterization of suspected extranodal lymphomas in dogs. J Vet Diagn Invest. (2018) 30:830–6. doi: 10.1177/1040638718804301
35. Keller SM, Vernau W, Hodges J, Kass PH, Vilches-Moure JG, McElliot V, et al. Hepatosplenic and hepatocytotropic T-cell lymphoma: two distinct types of T-cell lymphoma in dogs. Vet Pathol. (2013) 50:281–90. doi: 10.1177/0300985812451625
36. Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. (2000) 18:975–1026. doi: 10.1146/annurev.immunol.18.1.975
37. Rütgen BC, Flickinger I, Wolfesberger B, Litschauer B, Fuchs-Baumgartinger A, Hammer SE, et al. Cutaneous T-cell lymphoma - Sézary syndrome in a Boxer. Vet Clin Pathol. (2016) 45:172–8. doi: 10.1111/vcp.12311
38. Moore PF, Affolter VK, Graham PS, Hirt B. Canine epitheliotropic cutaneous T-cell lymphoma: an investigation of T-cell receptor immunophenotype, lesion topography and molecular clonality. Vet Dermatol. (2009) 20:569–76. doi: 10.1111/j.1365-3164.2009.00814.x
39. Wikander YM, Knights K, Coffee C, Vernau W, Biller DS, Higginbotham ML, et al. CD4 and CD8 double-negative immunophenotype of thymoma-associated lymphocytes in a dog. J Vet Diagn Invest. (2020) 32:918–22. doi: 10.1177/1040638720948628
40. Stranahan LW, Whitley D, Thaiwong T, Kiupel M, Oliveira F. Anaplastic large T-cell lymphoma in the intestine of dogs. Vet Pathol. (2019) 56:878–84. doi: 10.1177/0300985819852132
41. Noland EL, Kiupel M. Coexpression of CD3 and CD20 in canine enteropathy-associated T-cell lymphoma. Vet Pathol. (2018) 55:241–4. doi: 10.1177/0300985817747326
42. Mizutani N, Goto-Koshino Y, Takahashi M, Uchida K, Tsujimoto H. Clinical and histopathological evaluation of 16 dogs with T-zone lymphoma. J Vet Med Sci. (2016) 78:1237–44. doi: 10.1292/jvms.15-0688
43. Comazzi S, Martini V, Aresu L, Marconato L, Parachini-Winter, et al. “A case of canine high-grade T-cell lymphoma immunophenotypically consistent with T-zone lymphoma. Vet Clin Pathol. (2019) 48:5–6. doi: 10.1111/vcp.12718
44. Purzycka K, Peters LM, Desmas I, Davies O, Chang YM, Lara-Garcia A. Clinicopathological characteristics and prognostic factors for canine multicentric non-indolent T-cell lymphoma: 107 cases. Vet Comp Oncol. (2020) 18:656–63. doi: 10.1111/vco.12589
45. Poggi A, Miniscalco B, Morello E, Gattino F, Delaude A, Ferrero Poschetto L, et al. Prognostic significance of Ki67 evaluated by flow cytometry in dogs with high-grade B-cell lymphoma. Vet Comp Oncol. (2017) 15:431–40. doi: 10.1111/vco.12184
46. Marconato L, Martini V, Aresu L, Sampaolo M, Valentini F, Rinaldi V. Assessment of bone marrow infiltration diagnosed by flow cytometry in canine large B cell lymphoma: prognostic significance and proposal of a cut-off value. Vet J. (2013) 197:776–81. doi: 10.1016/j.tvjl.2013.05.003
47. Marconato L, Comazzi S, Aresu L, Riondato F, Stefanello D, Ferrari R. Prognostic significance of peripheral blood and bone marrow infiltration in newly-diagnosed canine nodal marginal zone lymphoma. J. Vet. (2019) 246:78–84. doi: 10.1016/j.tvjl.2019.02.002
Keywords: dog, lymphoma, T-cell, flow cytometry, diagnosis, prognosis
Citation: Comazzi S and Riondato F (2021) Flow Cytometry in the Diagnosis of Canine T-Cell Lymphoma. Front. Vet. Sci. 8:600963. doi: 10.3389/fvets.2021.600963
Received: 31 August 2020; Accepted: 26 February 2021;
Published: 21 April 2021.
Edited by:
Steven E. Suter, North Carolina State University, United StatesReviewed by:
Davis Seelig, University of Minnesota Twin Cities, United StatesArianna Miglio, University of Perugia, Italy
Copyright © 2021 Comazzi and Riondato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Comazzi, c3RlZmFuby5jb21henppQHVuaW1pLml0
 Stefano Comazzi
Stefano Comazzi Fulvio Riondato
Fulvio Riondato