- 1Faculty of Veterinary Medicine, University of Teramo, Teramo, Italy
- 2School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 3Department of Sciences of Agriculture, Food and Environment, University of Foggia, Foggia, Italy
Leishmaniosis by Leishmania infantum is a major zoonotic Vector-Borne Disease (VBD) in terms of geographic distribution, pathogenicity and zoonotic potential. While dogs are the main reservoir of L. infantum, the infection in cats is poorly understood although increasingly reported from enzootic and non-enzootic areas. The Mediterranean basin is a key area for leishmaniosis and includes touristic spots that require continuous surveillance for VBDs in consideration of the growing tendency of tourists to travel with their pets. This study evaluated L. infantum seroprevalence in cats living in selected touristic localities of Italy and Greece. A total of 269 cat serum samples from three Sites i.e., 76, 40, and 153 from Adriatic Coast of Abruzzo, Italy (Site A), Giglio Island, Tuscany, Italy (Site B), and Mykonos Island, Greece (Site C), respectively, were included in the survey. Sera samples were subjected to an indirect immunofluorescence antibody assay for the detection of anti-L. infantum specific IgG. Associations between possible risk factors and seropositivity to L. infantum were statistically evaluated. Antibodies against L. infantum were detected in eight out of 269 (3.0%) cats tested i.e., 4/76 (5.3%), 1/40 (2.5%), and 3/153 (2.0%), from sites A, B, and C, respectively. A statistical association between anti-L. infantum antibodies and cohabitation with dogs was shown. This study indicates that feline populations living in the examined Italian and Greek touristic areas are exposed to L. infantum and that they may contribute to the circulation of L. infantum, enhancing the risk of infection for dogs and humans.
Introduction
Leishmaniosis caused by the protozoan parasite Leishmania infantum is a Vector-Borne Disease (VBD) of major veterinary and public health concern in the Mediterranean Basin (1, 2), where phlebotomine sandflies of the genus Phlebotomus transmit L. infantum to a vertebrate host during blood meal (3). Accordingly, geographic spread, epizootiology, and epidemiology of L. infantum are strictly associated with the distribution of these vectors (4, 5).
Current climate changes enhance the reproduction and spread of sandflies and, consequently, promote the rate of L. infantum transmission to a wide range of vertebrates, including humans (6, 7). Recent records have shown a rise of infection rates in companion animals (8–10) and wildlife (11–13). Also, autochthonous cases of infection by L. infantum in people are reported throughout Europe, including Italy and Greece (14). Southern Europe in particular is regarded as a high-burden area for human visceral leishmaniosis due to L. infantum (1).
Dogs are considered the most important domestic reservoir of L. infantum (15) while the role of cats as potential reservoir of this parasite requires further corroboration (16). Although cats are in general considered resistant to L. infantum (17), they may be infected and potentially act as a source of infection for phlebotomine sandflies (18, 19). In particular, infected cats may successfully pass L. infantum to Phlebotomus perniciosus in which the parasite further continues its development (20, 21). Additionally, different studies have documented that L. infantum circulates among feline populations of Mediterranean basin e.g., Greece, Portugal, Turkey, Cyprus, Spain, and Italy, (22–28). Case reports in cats imported from enzootic areas or traveling with their owners in such localities have shown that feline leishmaniosis may be introduced in non-enzootic countries (29, 30). This latter feature is important in regions with intense movements and travels of animals either with their owners (e.g. tourism), or in the frame of adoption programs of stray animals (animal rights associations activity).
Cats are exposed to L. infantum in Italy (28) and Greece (31), which are indeed key epizootiological hubs. Touristic travels are particularly intense in these countries and this is of importance considering the substantial correlation between pet movements and spread of L. infantum from enzootic to free areas (10, 31, 32). In such areas a continuous surveillance of the epizootiology and distribution of feline leishmaniosis is pivotal, toward the protection of animal and human health. Thus, the aim of the present study was to evaluate the exposure to L. infantum in cats from touristic localities of Italy and Greece, and to investigate possible risk factors associated with the seropositivity in feline populations.
Materials and Methods
Animals and Study Areas
Overall, 269 cats (116 in Italy and 153 in Greece) living in three touristic areas were included in the study: 76 cats from Adriatic Coast of Abruzzo, Italy (Site A), 40 cats from Giglio Island, Tuscany, Italy (Site B), and 153 cats from Mykonos Island, Greece (Site C). Detailed information about sex, age, and lifestyle was recorded for each cat. Consent for sample collection and screening for leishmaniosis was obtained from the animal owners or the local municipality authorities and animal rights association authorities, as applicable.
Sampling and Serology
Blood samples were collected individually in tubes without anticoagulant and centrifuged after clot formation for serum separation and collection. Sera were subjected to an indirect immunofluorescence antibody test (IFAT) for the detection of specific IgG against L. infantum. Commercially available slides coated with promastigotes (MegaFLUO Leish—Megacor Diagnostik GmbH) and anti-cat IgG conjugate (FLUO FITC anti-cat IgG conjugate) were used, following the instructions provided by the manufacturer. The cut-off dilution of 1:80 was applied, as indicated in the LeishVet guidelines1.
Statistical Analysis
A statistical analysis was carried out using GraphPad Prism 8 (GraphPad Software, LLC). The Fisher's exact test was used to evaluate the eventual significant associations (p ≤ 0.05) between exposure to L. infantum and possible risk factors (age, sex, lifestyle, cohabitation with other animals).
Cats were divided in two age classes i.e., aging <36 months old (n. 128), and ≥36 months (n. 141). One hundred and forty cats were male and 129 were female. Overall, 45 cats were housed indoor and were not allowed to go outside, while 224 had constant outdoor access or lived permanently outdoor.
Results
Overall, antibodies against L. infantum were detected in 8/269 cats (3.0%; 95% CI ±2.0) i.e., 4/76 (5.3%; 95% CI ±5.0) from site A, 1/40 (2.5%; 95% CI ±4.5) from site B, and 3/153 (2.0%; 95% CI ±2.2) from site C.
The Fisher's exact test revealed a statistically significant (p < 0.05) association between cat seropositivity and cohabitation with dogs (p = 0.0360). According to the statistical analysis no other relevant associations were found. The number of seropositive and seronegative cats for each possible risk factor is detailed in Table 1. The median age of both seropositive and seronegative cats was 36 months.
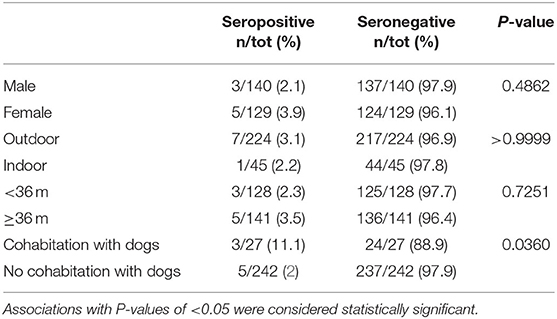
Table 1. Number and percentage of cats seropositive and seronegative at IFAT (cut-off dilution 1:80) for Leishmania infantum antibodies for each possible risk factor considered in the present study.
Discussion
The data generated in the present study add new information on the exposure to L. infantum of cat populations in the examined areas of Italy and Greece.
The overall seroprevalence (3.0%) recorded is in line with values registered in central Spain (1.3–3.2%), Portugal (2.8%) and, very recently, throughout Italy (3.3%) (28, 33, 34). A recent systematic review with meta-analysis of the literature of the last two decades has indicated higher seroprevalence rates in cats from these countries i.e., 11.0% in Greece and 24.0% in Italy (2). Indeed, studies are difficult to compare when different molecular/serological techniques are used, while differences in the prevalence rates could also be attributed to the selection of the sample population (e.g., age, lifestyle, and number of cats), to the selected antibody titer cut-off and to the sampling areas. In fact, IFAT is the most widely used method and, although different values have been applied in different studies (34–39), a cut-off of 1:80 is recommended in the LeishVet group guidelines (see text footnote 1).
The seroprevalence herein detected in Site A (5.6%) is higher than the rates registered recently in the same region (27). This may be attributed to the fact that 3/4 (75.0%) of the positive cats from Site A cohabited with dogs, as this was identified as a statistically significant risk factor by the statistical analysis in the present study. Accordingly, a recent study has demonstrated that a close contact with infected dogs can lead to high seroprevalence rates in cats (40). Nevertheless, this association should be interpreted with caution, because (i) it is generally accepted that the risk of infection does not increase in animals or humans cohabiting with infected dogs, but rather if a high seroprevalence occurs in local dog population and (ii) the parasitological status of dogs cohabiting with positive cats of this study was unknown. Another explanation could be that only cats living in coastal areas of Site A have been examined, while in the previous survey cats were sampled in both mountainous and coastal areas (27). Indeed, altitude and cold temperatures have a negative influence on the biology of L. infantum vectors (41, 42). Moreover, some efficient vectors of L. infantum e.g., Phlebotomus neglectus, find optimal habitat conditions at <1,000 m from the seashore (42, 43). The higher prevalence rates obtained in cats from Abruzzo 15 years ago by IFAT (16.3%) (44), can be explained by the low cut-off value (1:40) which has most likely overestimated the results.
The present data are the first generated on the exposure to L. infantum of feline populations living in Site B, a popular touristic island belonging to Tuscany regional territory. The prevalence rate recorded is in line with that detected in a previous study carried out in continental Tuscany in early 2000's (0.9%), (45). Importantly, the single cat that seroreacted was locally born and never moved to other areas. This confirms the presence of the pathogen on the island, which is not surprising, given that Tuscany is a known enzootic area of canine leishmaniosis (46). It should be also considered that Giglio island is a dog-friendly island for tourists2 and dogs traveling with their owners from enzootic areas of continental Italy can constantly contribute to the circulation of L. infantum among the local fauna. This result thus represents a potential alarm bell ringing for pet populations for the near future.
In a recent study on feline VBDs performed in continental and insular Greece, no L. infantum seropositive cats were detected in Mykonos Island (31), a fact that could be attributed to the small size of sampled cats. Therefore, the present results provide new information for this geographic region. In fact, in previous studies in Greece, specific antibodies to Leishmania spp. in cats have been detected in Crete Island (14.7%) and in continental Greece i.e., Thessaloniki (3.8%), Athens (8.3%), Thessaly and Macedonia regions (10%) (22, 31, 47). As suggested for Italy, the circulation of L. infantum in Site C could have been favored by sociological factors. Indeed, in Greek Islands (including Site C) numerous colonies of stray cats managed by charities and animal welfare organizations arrange adoptions and re-homing of cats3 (31). Nonetheless, these commendable initiatives may further concur to the spread of feline leishmaniosis, because cats are not tested before their re-location. The lack of studies on seropositivity in dog populations living in Mykonos Island does not allow a comparison of the prevalence between species. However, L. infantum is widely distributed in canine populations of Greece, and seroprevalence rates in regions of continental Greece range from 2% up to 50.2% (48, 49). An overall prevalence of 6.5% emerged in a recent serological and molecular study carried out on the Islands of Ios, Santorini, Tinos, and Skiathos (50). The prevalence of L. infantum in Greece and its presence in surrounding islands suggest that this protozoan most likely circulates also among canine populations of Mykonos.
Animals living outdoors are considered at high risk of infection with arthropod-transmitted pathogens, including L. infantum (51–53). Nevertheless, a significant correlation between outdoor lifestyle and the presence of L. infantum antibodies in the here examined cats was not observed. This is consistent with the results of a recent study, where 4/5 seropositive cats had, unexpectedly, an indoor lifestyle (27). Therefore, the present data suggest that indoor housing is not a sufficient preventative measure against leishmaniosis.
On the whole, this study confirms that cats living in the examined touristic areas of the Mediterranean basin are exposed to L. infantum. Infection rates in cats are generally lower than in dogs and the role of cats in the epidemiology of zoonotic leishmaniosis is still controversial and should be further investigated (16, 54). Recent findings have ultimately confirmed that dogs are better reservoir and spreaders of L. infantum than cats because under the same exposure conditions they display higher parasitic load, a determining factor for infectivity to sandflies (40, 55). Nonetheless, the role of cats as persistent source of infection for vectors (54) should not be overlooked, as they may amplify infection chances for dogs and, subsequently, for people.
In conclusion, the awareness toward feline infections should be kept at higher levels. Further studies evaluating the occurrence of L. infantum both in cats and dogs living in touristic destinations like Mykonos and Giglio Islands are warranted. Also, it would be interesting to conduct molecular studies to evaluate if species other than L. infantum occur in animal populations living in Mediterranean touristic spots. The importance of a constant surveillance is underlined by the fact that leishmaniosis has been regarded as an emerging threat in travelers (56) and cases of human leishmaniosis in tourists that visited Italy and Greece have been documented (57, 58). As routine examinations for Leishmania infection and application of chemoprophylactic measures in dogs and cats traveling with their owners are not regulated by laws, the establishment of standard preventative protocols for traveling animals is of importance for limiting the spread of zoonotic leishmaniosis and to minimize Public Health risks.
Data Availability Statement
The datasets presented in this article are not readily available because they are represented only by the number of seropositive/seronegative cats in each study areas. Requests to access the datasets should be directed to c21vcmVsbGlAdW5pdGUuaXQ=.
Ethics Statement
Ethical review and approval was not required for the animal study because Blood samples were collected from cats upon a written consent obtained from the animal owners or the local municipality authorities and animal rights association authorities, in the framework of animal health screening programmes. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
DT has supervised the whole study and drafted the manuscript. SM, MC, and AD participated in all project activities and in drafting the manuscript, after critically revising it. DD, AB, ADC, IR, RS, and BP have participated in the field, laboratory work, and in drafting the manuscript in various ways. All authors contributed to the article and approved the submitted version.
Funding
The present study has been founded by the University of Teramo, Italy (Funds for Basic Research Activities—FARDIB).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Athina Tsokana, Elisa Di Gennaro, Giulia Sarrocco, Ramona Latino, and Lorenzo Salvati for their valuable support in the field activities. The authors thank all cat owners and representatives who were willing to participate in the study.
Abbreviations
VBD, Vector-borne Diseases; IFAT, Immunofluorescence Antibody Test; CI, Confidence Interval.
Footnotes
1. ^http://www.leishvet.org/fact-sheet-feline-leishmaniosis/feline-leishmaniosis-clinical-diagnosis/
2. ^https://www.traghetti-giglio.it/en/tp-magazine/dog-friendly-holiday-giglio-island/
References
1. Burza S, Croft S, Boelaert M. Leishmaniasis. Lancet. (2018) 392:951–70. doi: 10.1016/S0140-6736(18)31204-2
2. Asfaram S, Fakhar M, Teshnizi SH. Is the cat an important reservoir host for visceral leishmaniasis? A systematic review with meta-analysis. J Venom Anim Toxins Incl Trop Dis. (2019) 25:e20190012. doi: 10.1590/1678-9199-JVATITD-2019-0012
3. Torres-Guerrero E, Quintanilla-Cedillo M, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. (2017) 6:750. doi: 10.12688/f1000research.11120.1
4. Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. (2016) 10:e0004349. doi: 10.1371/journal.pntd.0004349
5. Steverding D. The history of leishmaniasis. Parasit Vectors. (2017) 10:82. doi: 10.1186/s13071-017-2028-5
6. Semenza J, Menne B. Climate change and infectious diseases in Europe. Lancet Infect Dis. (2009) 9:365–75. doi: 10.1016/S1473-3099(09)70104-5
7. Trájer A, Sebestyén V. The changing distribution of Leishmania infantum Nicolle, 1908 and its Mediterranean sandfly vectors in the last 140 kys. Sci Rep. (2019) 9:11820. doi: 10.1038/s41598-019-48350-7
8. Le Rutte EA, van Straten R, Overgaauw PAM. Awareness and control of canine leishmaniosis: a survey among Spanish and French veterinarians. Vet Parasitol. (2018) 253:87–93. doi: 10.1016/j.vetpar.2018.01.013
9. Escobar TA, Dowich G, Dos Santos TP, Zuravski L, Duarte CA, Lübeck I, et al. Assessment of Leishmania infantum infection in equine populations in a canine visceral leishmaniosis transmission area. BMC Vet Res. (2019) 15:381. doi: 10.1186/s12917-019-2108-1
10. Spada E, Perego R, Vitale F, Bruno F, Castelli G, Tarantola G, et al. Feline Leishmania spp. infection in a non-endemic area of northern Italy. Animals. (2020) 10:817. doi: 10.3390/ani10050817
11. Millán J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. (2014) 113:2005–14. doi: 10.1007/s00436-014-3929-2
12. Tsakmakidis I, Pavlou C, Tamvakis A, Papadopoulos T, Christodoulou V, Angelopoulou K, et al. Leishmania infection in lagomorphs and minks in Greece. Vet Parasitol Reg Stud Rep. (2019) 16:100279. doi: 10.1016/j.vprsr.2019.100279
13. Cantos-Barreda A, Navarro R, Pardo-Marín L, Martínez-Subiela S, Ortega E, Cerón JJ, et al. Clinical leishmaniosis in a captive Eurasian otter (Lutra lutra) in Spain: a case report. BMC Vet Res. (2020) 16:312. doi: 10.1186/s12917-020-02509-x
15. Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. (2009) 136:1915–34. doi: 10.1017/S0031182009991156
16. Dalvi APR, Carvalho TDG, Werneck GL. Is There an association between exposure to cats and occurrence of visceral leishmaniasis in humans and dogs? Vector Borne Zoonotic Dis. (2018) 18:335–42. doi: 10.1089/vbz.2017.2162
17. Day M. Cats are not small dogs: is there an immunological explanation for why cats are less affected by arthropod-borne disease than dogs? Parasit Vectors. (2016) 9:507. doi: 10.1186/s13071-016-1798-5
18. da Silva S, Rabelo P, Gontijo N, Ribeiro R, Melo M, Ribeiro V, et al. First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet Parasitol. (2010) 174:150–4. doi: 10.1016/j.vetpar.2010.08.005
19. Afonso M, Duarte R, Miranda J, Caranha L, Rangel E. Studies on the feeding habits of Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) populations from endemic areas of american visceral leishmaniasis in Northeastern Brazil. J Trop Med. (2012) 2012:858657. doi: 10.1155/2012/858657
20. Maroli M, Pennisi MG, Di Muccio T, Khoury C, Gradoni L, Gramiccia M. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet Parasitol. (2007) 145:357–60. doi: 10.1016/j.vetpar.2006.11.009
21. González E, Jiménez M, Hernández S, Martín-Martín I, Molina R. Phlebotomine sand fly survey in the focus of leishmaniasis in Madrid, Spain (2012–2014): seasonal dynamics, Leishmania infantum infection rates and blood meal preferences. Parasit Vectors. (2017) 10:368. doi: 10.1186/s13071-017-2309-z
22. Diakou A, Papadopoulos E, Lazarides K. Specific anti-Leishmania spp. antibodies in stray cats in Greece. J Feline Med Surg. (2009) 11:728–30. doi: 10.1016/j.jfms.2008.01.009
23. Maia C, Gomes J, Cristòvão J, Nunes M, Martins A, Rebêlo E, et al. Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet Parasitol. (2010) 174:336–40. doi: 10.1016/j.vetpar.2010.08.030
24. Can H, Döşkaya M, Özdemir H, Sahar E, Karakavuk M, Pektaş B, et al. Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of Izmir, Turkey. Exp Parasitol. (2016) 167:109–14. doi: 10.1016/j.exppara.2016.05.011
25. Attipa C, Papasouliotis K, Solano-Gallego L, Baneth G, Nachum-Biala Y, Sarvani E, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vector. (2017) 10:130. doi: 10.1186/s13071-017-2063-2
26. Montoya A, García M, Gálvez R, Checa R, Marino V, Sarquis J, et al. Implications of zoonotic and vector-borne parasites to free-roaming cats in central Spain. Vet Parasitol. (2018) 251:125–30. doi: 10.1016/j.vetpar.2018.01.009
27. Morelli S, Crisi P, Di Cesare A, De Santis F, Barlaam A, Santoprete G, et al. Exposure of client-owned cats to zoonotic vector-borne pathogens: clinic-pathological alterations and infection risk analysis. Comp Immunol Microbiol Infect Dis. (2019) 66:101344. doi: 10.1016/j.cimid.2019.101344
28. Iatta R, Furlanello T, Colella V, Tarallo V, Latrofa M, Brianti E, et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl Trop Dis. (2019) 13: e0007594. doi: 10.1371/journal.pntd.0007594
29. Rüfenacht S, Sager H, Muller N, Schaerer V, Heier A, Welle M, et al. Two cases of feline leishmaniosis in Switzerland. Vet Rec. (2005) 156:542–5. doi: 10.1136/vr.156.17.542
30. Richter M, Schaarschmidt-Kiener D, Krudewig C. Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweiz Arch Tierheilkd. (2014) 156:289–94 doi: 10.1024/0036-7281/a000593
31. Diakou A, Di Cesare A, Accettura P, Barros L, Iorio R, Paoletti B, et al. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl Trop Dis. (2017) 11: e0005335. doi: 10.1371/journal.pntd.0005335
32. Maia C, Cardoso L. Spread of Leishmania infantum in Europe with dog travelling. Vet Parasitol. (2015) 213:2–11. doi: 10.1016/j.vetpar.2015.05.003
33. Cardoso L, Lopes A, Sherry K, Schallig H, Solano-Gallego L. Low seroprevalence of Leishmania infantum infection in cats from northern Portugal based on DAT and ELISA. Vet Parasitol. (2010) 174:37–42. doi: 10.1016/j.vetpar.2010.08.022
34. Miró G, Rupérez C, Checa R, Álvez R, Hernández L, García M, et al. Current status of L. infantum infection in stray cats in the Madrid region (Spain): implications for the recent outbreak of human leishmaniosis? Parasit Vectors. (2014) 7:112. doi: 10.1186/1756-3305-7-112
35. Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: pathogen and vector circulation in a confined environment. Vet Parasitol. (2017) 236:144–51. doi: 10.1016/j.vetpar.2017.01.019
36. Morganti G, Veronesi F, Stefanetti V, Di Muccio T, Fiorentino E, Diaferia M, et al. Emerging feline vector-borne pathogens in Italy. Parasit Vectors. (2019) 12:193. doi: 10.1186/s13071-019-3409-8
37. Silaghi C, Knaus M, Rapti D, Kusi I, Shukullari E, Hamel D, et al. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasit Vectors. (2014) 7:62. doi: 10.1186/1756-3305-7-62
38. Pennisi M, Lupo T, Malara D, Masucci M, Migliazzo A, Lombardo G. Serological and molecular prevalence of Leishmania infantum infection in cats from Southern Italy. J Feline Med Surg. (2012) 14:656–57. doi: 10.5402/2013/916376
39. Persichetti M, Solano-Gallego L, Serrano L, Altet L, Reale S, Masucci M, et al. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors. (2016) 9:247. doi: 10.1186/s13071-016-1534-1
40. Baneth G, Nachum-Biala Y, Zuberi A, Zipori-Barki N, Orshan L, Kleinerman G, et al. Leishmania infection in cats and dogs housed together in an animal shelter reveals a higher parasite load in infected dogs despite a greater seroprevalence among cats. Parasite Vectors. (2020) 13:115. doi: 10.1186/s13071-020-3989-3
41. Koch LK, Kochmann J, Klimpel S, Cunze S. Modeling the climatic suitability of leishmaniasis vector species in Europe. Sci Rep. (2017) 7:13325.
42. Tsirigotakis N, Pavlou C, Christodoulou V, Dokianakis E, Kourouniotis C, Alten B, et al. Phlebotomine sand flies (Diptera: Psychodidae) in the Greek Aegean Islands: ecological approaches. Parasit Vectors. (2018) 11:97. doi: 10.1186/s13071-018-2680-4
43. Latrofa MS, Iatta R, Dantas-Torres F, Annoscia G, Gabrielli S, Pombi M, et al. Detection of Leishmania infantum DNA in phlebotomine sand flies from an area where canine leishmaniosis is endemic in southern Italy. Vet Parasitol. (2018) 253:39–42. doi: 10.1016/j.vetpar.2018.02.006
44. Vita S, Santori D, Aguzzi I, Petrotta E, Luciani A. Feline leishmaniasis and ehrlichiosis: serological investigation in Abruzzo region. Vet Res Commun. (2005) 2:319–21. doi: 10.1007/s11259-005-0071-8
45. Poli A, Abramo F, Barsotti P, Leva S, Gramiccia M, Ludovisi A, et al. Feline leishmaniosis due to Leishmania infantum in Italy. Vet Parasitol. (2002) 106:181–91. doi: 10.1016/s0304-4017(02)00081-x
46. Sauda F, Malandrucco L, Macrì G, Scarpulla M, De Liberato C, Terracciano G, et al. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in central Italy. Parasite. (2018) 25:2. doi: 10.1051/parasite/2018001
47. Chatzis M, Andreadou M, Leontides L, Kasabalis D, Mylonakis M, Koutinas A, et al. Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Vet Parasitol. (2014) 202:217–25. doi: 10.1016/j.vetpar.2014.02.044
48. Athanasiou LV, Kontos VI, Saridomichelakis MN, Rallis TS, Diakou A. A cross-sectional sero-epidemiological study of canine leishmaniasis in Greek mainland. Acta Trop. (2012) 122:291–5. doi: 10.1016/j.actatropica.2012.02.003
49. Ntais P, Sifaki-Pistola D, Christodoulou V, Messaritakis I, Pratlong F, Poupalos G, et al. Leishmaniases in Greece. Am J Trop Med Hyg. (2013) 89:906–15. doi: 10.4269/ajtmh.13-0070
50. Diakou A, Di Cesare A, Morelli S, Colombo M, Halos L, Simonato G, et al. Endoparasites and vector-borne pathogens in dogs from Greek islands: pathogen distribution and zoonotic implications. PLoS Negl Trop Dis. (2019) 13:e0007003 doi: 10.1371/journal.pntd.0007003
51. Beugnet F, Halos L. Parasitoses and Vector Borne Diseases of Cats, 1st Edn. Merial, Lyon: Press (2015).
52. Spada E, Canzi I, Baggiani L, Perego R, Vitale F, Migliazzo A, et al. Prevalence of Leishmania infantum and co-infections in stray cats in northern Italy. Comp Immunol Microbiol Infect Dis. (2016) 45:53–8. doi: 10.1016/j.cimid.2016.03.001
53. Persichetti M, Pennisi M, Vullo A, Masucci M, Migliazzo A, Solano-Gallego L. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasit Vectors. (2018) 11:136. doi: 10.1186/s13071-018-2725-8
54. Pennisi M, Cardoso L, Baneth G, Bourdeau P, Koutinas A, Miró G, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors. (2015) 8:302. doi: 10.1186/s13071-015-0909-z
55. Borja LS, Sousa OMF, Solcà MDS, Bastos LA, Bordoni M, Magalhães JT, et al. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet Parasitol. (2016) 229:110–7. doi: 10.1016/j.vetpar.2016.10.004
56. Mansueto P, Seidita A, Vitale G, Cascio A. Leishmaniasis in travelers: a literature review. Travel Med Infect Dis. (2014) 12:563–81. doi: 10.1016/j.tmaid.2014.09.007
57. Berens-Riha N, Fleischmann E, Pratlong F, Bretzel G, von Sonnenburg F, Löscher T. Cutaneous leishmaniasis (Leishmania tropica) in a German tourist after travel to Greece. J Travel Med. (2009) 16:220–2. doi: 10.1111/j.1708-8305.2008.00291.x
Keywords: Leishmania infantum, cats, seroprevalence, Italy, Greece, zoonosis
Citation: Morelli S, Colombo M, Dimzas D, Barlaam A, Traversa D, Di Cesare A, Russi I, Spoletini R, Paoletti B and Diakou A (2020) Leishmania infantum Seroprevalence in Cats From Touristic Areas of Italy and Greece. Front. Vet. Sci. 7:616566. doi: 10.3389/fvets.2020.616566
Received: 12 October 2020; Accepted: 23 November 2020;
Published: 11 December 2020.
Edited by:
Simona Gabrielli, Sapienza University of Rome, ItalyReviewed by:
Fabrizia Veronesi, University of Perugia, ItalyRudi Cassini, University of Padua, Italy
Copyright © 2020 Morelli, Colombo, Dimzas, Barlaam, Traversa, Di Cesare, Russi, Spoletini, Paoletti and Diakou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donato Traversa, ZHRyYXZlcnNhQHVuaXRlLml0
 Simone Morelli
Simone Morelli Mariasole Colombo1
Mariasole Colombo1 Alessandra Barlaam
Alessandra Barlaam Donato Traversa
Donato Traversa Angela Di Cesare
Angela Di Cesare Roberta Spoletini
Roberta Spoletini Barbara Paoletti
Barbara Paoletti Anastasia Diakou
Anastasia Diakou