- 1Department of Clinical Veterinary Medicine, College of Veterinary Medicine, Jilin University, Changchun, China
- 2Nanchang Police Dog Base of the Ministry of Public Security, Nanchang, China
Leptospirosis is a global zoonotic disease caused by pathogenic Leptospira, and those infected animals will show a variety of clinical symptoms and even death. The discovery of endemic strains is crucial to produce effective vaccines. In this study, we report that a strain of Leptospira, isolated from a dog, is pathogenic. Using MLST analysis, the serovar of isolated Leptospira was identified and found it belongs to Leptospira interrogation Serovar Australis. Then, the virulence of this strain was researched by using hamsters. After infection, all the hamsters died within 4–5 days. Typical pathological changes were found in the liver, kidney, and lung of hamsters. These results all indicated that the isolated Leptospira was pathogenic. Thus, this study facilitates to identifying local Leptospira strains and develop a more targeted canine Leptospira vaccine.
Introduction
Leptospirosis, caused by a pathogenic Leptospira, is a zoonotic disease. Human may get leptospirosis directly or indirectly from animals (1). Leptospirosis occurs worldwide, especially in tropical and subtropical regions where rains a lot. On a global scale, it is conservatively estimated that ~500,000 serious cases occur each year, with a case fatality rate ranging from 3 to 70%, depending on severity of clinical symptoms (2, 3). Preliminary results indicate that the currently incidence of Leptospirosis may give rise to a serious underestimation of actual cases. This underestimation is partly due to the no characteristic manifestations in Leptospirosis, which are often confused with other diseases that is prevalent under similar environmental and climatic conditions, such as dengue fever, rickettsia disease, enteritis, and malaria (4).
The first Leptospirosis case report was in 1934 in mainland China. So far, more than 2.5 million people have been infected in China, more than 20,000 people died from Leptospirosis, and at least 80% of cases have occurred from July to December. Because of the climatic factors, September is the peak season during this period:floods are common accompanied by severe weather, such as typhoons (5). Animal Leptospirosis is mainly concentrated in the south of China. In recent years, the domestic pet industry has developed rapidly, which has led to a significant increase in the risk of canine infection with Leptospirosis (6). In the four representative cities in Jiangxi province (Nanchang, Yichun, Shangrao, and Ganzhou), the blood positive rate of stray dogs and pet dogs reached 4.79 and 1.97%, respectively. Because dogs have a closer relationship with humans than economic animals such as pigs and cattle, Leptospira is likely to infect humans through them and pose a serious public health hazard. In addition to human hosts, pathogenic Leptospira also infects a variety of animals, including domestic mammals (livestock) and wild animals, especially rodents, which are considered to be the main source of human Leptospira infection (7). Humans can be infected through direct contact with infected animals, or indirect contact with water and soil contaminated by the urine of infected animals (8). Since it was first confirmed by serology and etiology in Gan County, Jiangxi Province in 1958, it occurred or spread to varying degrees every year.
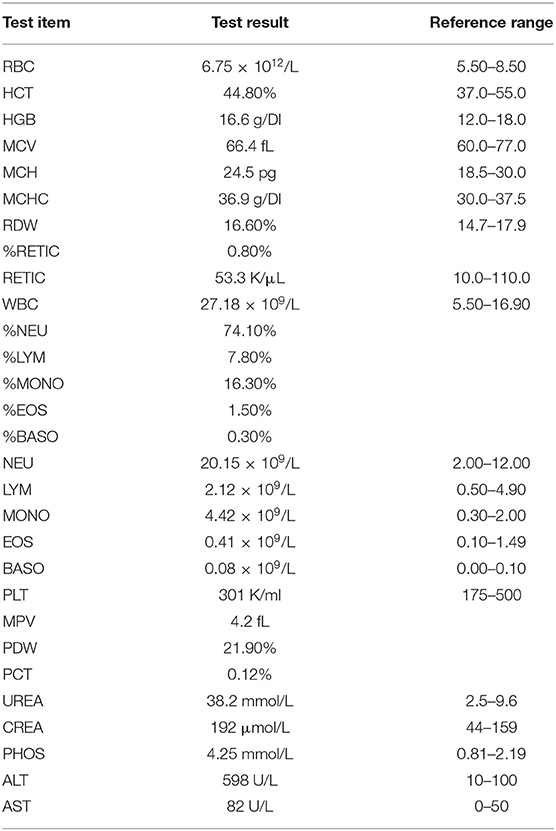
In recent years, the typing method based on multi-site sequence analysis has gradually been applied to bacteriological genetyping and molecular epidemiological research. The dog, we studied in this study, has clinical symptoms of vomiting, loss of appetite, and jaundice, which is like the symptoms of Leptospirosis. Therefore, the dog was subjected to blood routine, biochemical examination (Table 1), and serological examination (MAT). However, the sick dog failed to recover and died. In this study, experimental techniques such as MAT, PCR, and MLST were mainly used to identify Leptospira isolated from the sick dog and determine its molecular type and serovar. We also preliminary studied the virulence of this strain, which provided a basis for the prevention and control of Leptospira. This indicates that the Leptospira that is prevalent in dogs in Nanchang maybe this strain. It also helps to develop a canine vaccine against the endemic Leptospira, effective prevention of Leptospirosis from dogs to humans.
Materials and Methods
Case Description
A 3 year-old female mixed breed dog was sent to the Nanchang Police Dog Base Animal Hospital for treatment. According to the owner of the sick dog, the dog lacked energy with decreased appetite, vomiting, and yellow urine. The course of the disease is about 5 days. Temperature, heart rate, and respiratory rate were recorded at 39.8°C, 130, and 40 bpm, respectively. Icteric mucous membranes were found in the dog by the clinical examination, and the color of the serum was dark yellow. The complete blood count and serum biochemistry showed an increase in WBC, NEU, and MONO, CREA, UREA, PHOS, ALT, and AST, indicating serious problems with liver and kidney function (Table 1). Fluid therapy was initiated immediately through an intravenous catheter. However, the sick dog failed to recover and died. With the consent of the owner, we performed an autopsy and collected its serum, liver, and kidneys.
Ethics Statement
During the experiment, in a 12 h light and a 12 h dark cycle, hamsters drank freely with standard rodent food. Hamsters experiments were performed according to regulations of the Administration of Affairs Concerning Experimental Animals in China. Due to the serious condition of the dog, it eventually fell ill and died. With the consent of the owner, we performed an autopsy and collected its serum, liver, and kidneys. All the protocol include hamsters and dog (Autopsy of the cadaver and sample collection), was approved by the Institutional Animal Care and Use Committee of Jilin University (20170318).
Bacterial Strain Isolation, Cultivation, and Animals
All the strains of serotype pathogenic Leptospira interrogans and L. biflexa serovar Patoc were cultivated in Ellinghausen–McCullough–Johnson–Harris (EMJH) medium at 29°C for 5–7 days to observe bacterial growth (9). Before use, bacterial concentration was determined using a Petroff–Hausser counting chamber. The count was performed at 400x magnification under a darkfield, and DNA was extracted when the number of Leptospira reached 2 × 108 cells/ml.
The liver and kidney of dog were disinfected with 75% alcohol. Then the kidney cortex and liver parenchymal tissue pieces (about the size of a rice grain) were cut out in a clean bench and cultured in EMJH medium for 5–7 days to observe bacterial growth. When Leptospira was observed in darkfield, EMJH solid medium was prepared, and the bacterial diluent was smeared on the solid medium for cultivation. Later, single colonies that had grown were picked and inoculated in the EMJH medium for culture. Syrian golden hamsters (Mesocricetus auratus) were provided by the Animal Center of Jilin University.
Preparation of DNA
When the number of Leptospira reached 2 × 108 cells/ml, 1.2 ml of 15 pathogenic Leptospira, the newly isolated Leptospira, L. biflexa serovar Patoc and were taken and centrifuged at 12,000 rpm for 10 min. Then the supernatant was discarded to obtain a precipitate. The DNA was then extracted using the TIAN-amp DNA Minikit.
Primer Design
The primer was designed by the laboratory with an annealing temperature of 60°C. The GeneBank database was used to query and download the published sequence of the common pathogenic Leptospira LipL32 gene. All the sequences obtained were analyzed and compared by DNAMAN software to select a highly conserved sequence among them. Primers were designed using the biological software Primer Premier 6.0 according to the principle of primer design. To ensure the high specificity of the primers involved, all involved primer sequences were validated in the NCBI database. The sequence was sent to Comate Bioscience Company Limited for synthesis, and after centrifugation according to the instructions, the corresponding volume of TE was added for dissolution. The size of the target fragment was 468 bp. The forward primer was 5′-CRGGCGACGGAGAYTTAG-3′; The downstream primer was 5′-AGAYTCTTCRGCDGCKATRG-3′.
PCR Amplification
The PCR program for the primers was as follows: predenaturation at 94 °C for 3 min; denaturation at 94°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 30 s for 35 cycles, and an extension at 72°C for 5 min. The reaction volume was 25 μl and consisted of 1 μl of each primer, 1 μl of DNA template,12.5 μl of Taq, and 5.5 μl of sterile water. After PCR run, the newly isolated Leptospira sample's PCR production was sequenced. Then the amplification sequences were comparative with outer membrane protein (lipL32) gene of Leptospira.
MLST Analysis
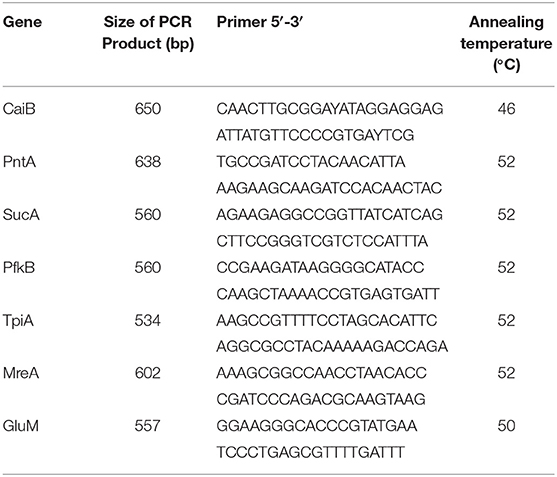
MLST website was established to provide public access to these data and to provide the resource to other investigators who can use this to assign the ST of further strains. This was accessed at http://leptospira.mlst.net. DNA was extracted from the cultures of the Leptospira by using the TIAN-amp DNA Minikit. Based on other literature, seven locis (pntA, sucA, caiB, tpiA, mreA, pfkB, and glmU) were used for MLST (10). Primer sequences were showed in Table 3. Amplifications were performed in a 25 μl reaction system containing 1 μL (1 ng/μL) of genomic DNA, 1 μl of each primer (0.2 μmol). PCR was performed with an initial denaturing step at 94°C for 5 min, followed by 30 cycles of 94°C for 10 s, 52°C (mreA, pfkB, pntA, sucA, and tpiA), or 50°C (caiB and glmU) for 15 s, 72°C for 50 s, then 72°C for 7 min. PCR product size ranged from 555 to 638 bp. MLST was performed for the isolates by using these 7 loci. Following the standard MLST protocol, each allele was assigned a different allele number and the allelic profile (a string of seven integers) was used to define the sequence type (ST).
Infection in the Hamster Model and Histopathological Examination
To determine whether the isolated strain was pathogenic, four 21 day-old hamsters were inoculated intraperitoneally with ~108 cells/ml of this strain (11), four were inoculated 107 cells/ml, four were inoculated 106 cells/ml, the other four were injected with an equal volume of saline as control group. All of the hamsters were monitored daily for the appearance of clinical signs. Necropsy was performed when the hamster dead. Kidneys, lung and liver were aseptically removed, macerated and suspended in a liquid medium for re-isolation. Organs for histopathological examination were fixed in 10% formalin for 48–72 h, dehydrated in gradient alcohol series, embedded in paraffin, and stained with hematoxylin and eosin (H&E) (12).
Serology
Currently, MAT is one of the most important diagnostic tests and it is commonly used as a serological gold standard (13). Firstly, we performed MAT qualitative experiments on the serum of the sick dog, and evaluated the titer after confirming its serology. Secondly, we injected this strain into the abdominal cavity of four 3 week-old golden hamsters at 2 × 108 cells/ml. Before the experimental group of hamsters died, blood was collected from the ocular nerve plexus, and the serum was collected by centrifugation for MAT.
Results
Clinical Symptoms of a Sick Dog and Bacterial Isolation
At autopsy, the dog was found to have jaundice (Figure 1A). Hemorrhage in lung was severe (Figure 1B). No obvious abnormalities were found in the liver and kidneys. At the 10th day of culture, the Leptospira in liver and kidney were observed under a dark-field microscope (Figure 1C). At the 15th day of culture, a single colony of Leptospira was observed on the EMJH solid medium (Figure 1D).
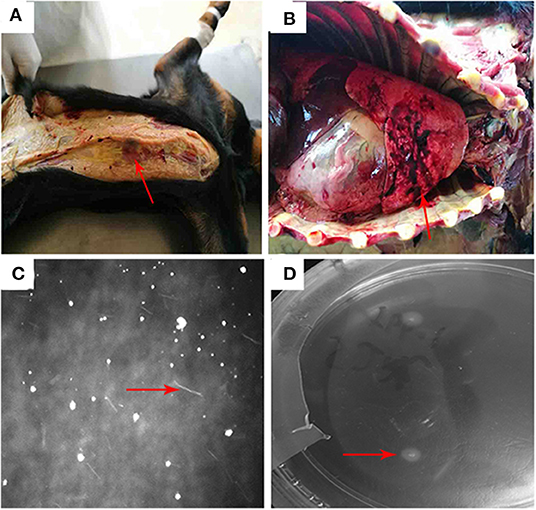
Figure 1. (A) The dog was found to have jaundice. (B) hemorrhage from the lungs was relatively serious: (C) Leptospira in liver and kidney cultures were observed under a dark field microscope. (D) a single colony of Leptospira was observed on the EMJH solid medium.
The Reliability of the Primer
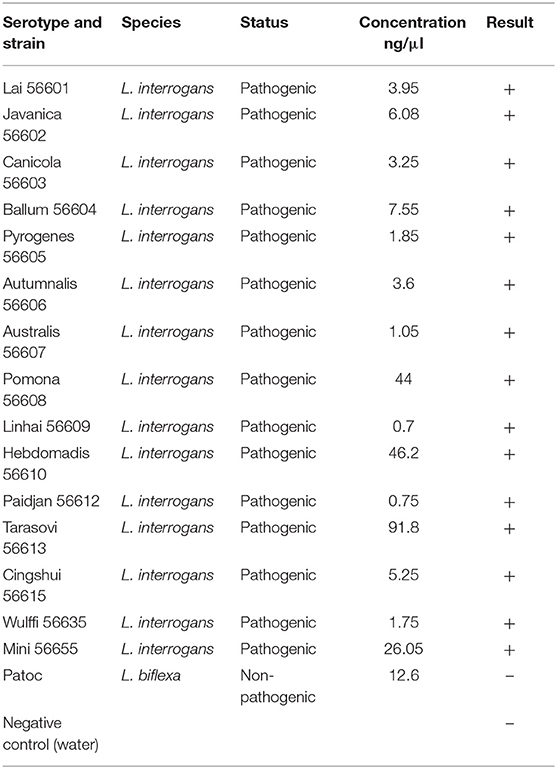
To analyze the reliability of the primer, the DNA of 15 pathogenic Leptospira serovar and Patoc was extracted and their concentrations were determined (Table 2). Then, PCR was performed to observe the band's condition (Figure 2A). All the 15 pathogenic Leptospira Serovar were detected. All experiments were conducted more than three times.
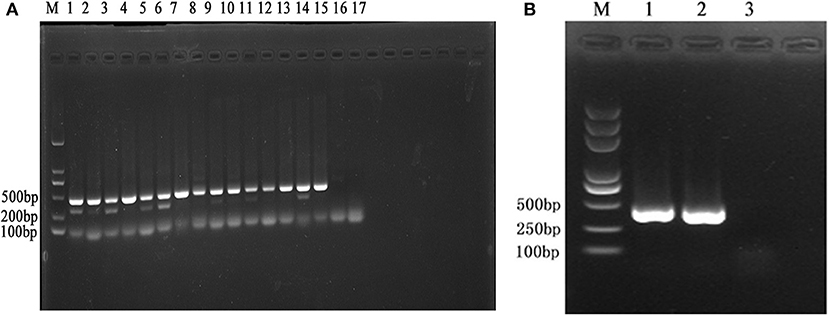
Figure 2. (A) M is expressed as DNA maker, 1–17 is expressed as Leptospira standard strain Lai 56601, Javanica 56602, Canicola 56603, Ballum 56604, Pyrogenes 56605, Autumnalis 56606, Australis 56607, Pomona 56608, Linhai 56609, Hebdomadis 56610. Paidjan 56612, Tarasovi 56613, Cingshui 56615, Wulffi 56635, Mini 56655, L. biflexa serovar Patoc, negative control. (B) M is expressed as DNA maker, 1–3 is expressed as: Leptospira standard strain Lai 56601, sample, negative control.
The Newly Isolated Leptospira Sample Detection
The results showed that a band appears at 468 bp,it was Leptospira interrogans (Figure 2B). The sequence comparative analysis result shows it was Leptospira interrogans. The amplification sequences were showed in Table 4. All experiments were conducted more than three times.
MLST Result
All the seven locis were successfully amplified from the isolate Leptospira. MLST was performed for the isolate by using the 7 loci. Following the standard MLST protocol which was accessed at http://leptospira.mlst.net, an allele number was assigned to all the allele of different Leptospiral strains and the allelic profile was defined as sequence type (ST) 93(a string of seven allele number for the single colony of Leptospira was 1-2-3-5-6-6-7). According to the ST profile, the Leptospiral strains were defined as Leptospira interrogans Serovar Australis.
Infection and Histopathological Examination Result
After infection, the isolated strain caused clinical symptoms, including dehydration, fluffy hair, poor mental state, and curled into the corner alone. All the hamsters in the group of inoculated 108 cells/ml of this strain dead (Figure 3A) at the fourth day after infecton. Two hamsters in the group of inoculated 107 cells/ml died quickly on the fourth and fifth days (Figure 3B). However, all hamsters in the group of inoculated 106 cells/ml were alive (Figure 3C). Pathological changes in the livers of infected animals were distinct with increased permeability of hepatic tight junctions and areas of necrosis and inflammatory infiltration. Meanwhile, the renal tissues of the infected group showed a dramatic lesion with hemorrhage. We also found that generalized interstitial pneumonia was noted in the lungs of the infected group, with hemorrhagic areas, alveolar congestion, and infiltrate of mononuclear cells (Figures 3D–F).
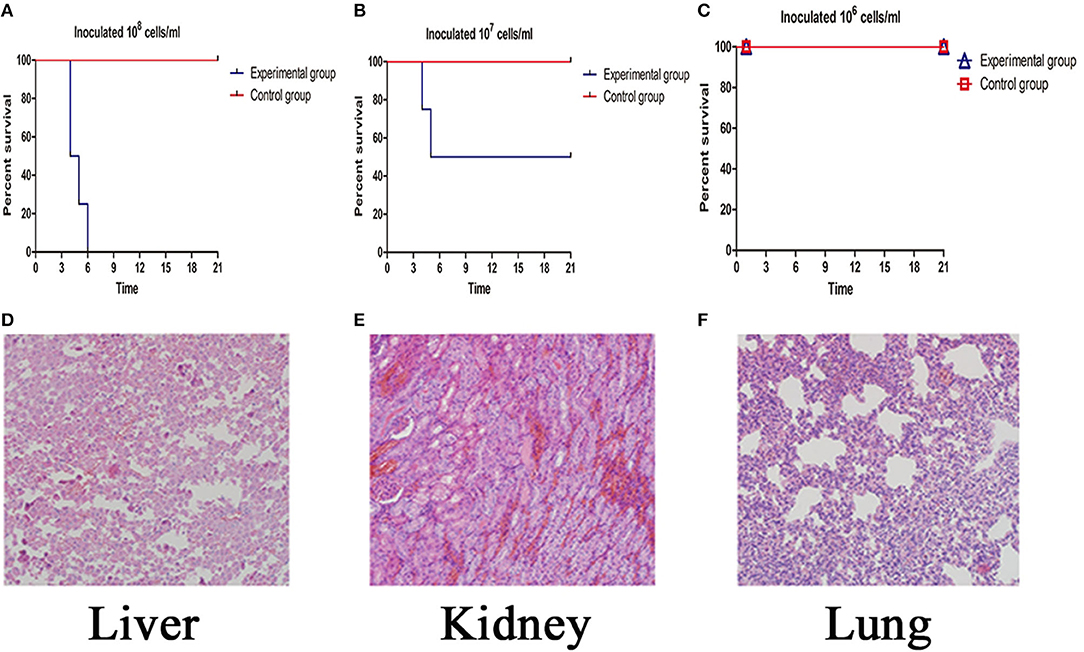
Figure 3. (A–C) The survival rate of the inoculated 108-106 cells/ml Leptospira experimental group. (D–F) Histopathology of the liver (200×), kidney (200×), lung (200×).
Serology
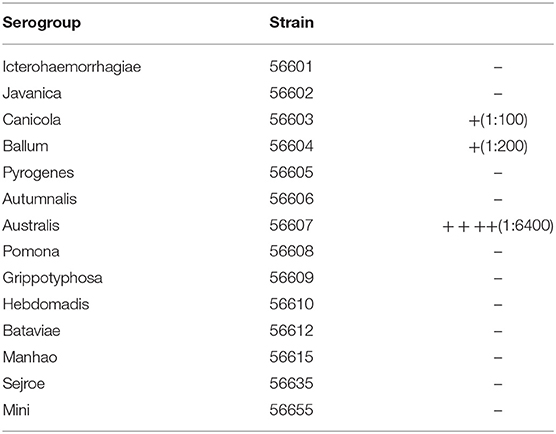
After MAT, serum from the sick dog had the most obvious anti-serum agglutination with Leptospira interrogans Serovar Australis, whose Strain number is 56607 (Table 5). Serum from the infected hamster showed the same result as the dog, further proving that the isolated strain was Leptospira interrogans Serovar Australis (Table 6).
Discussion
Leptospira has a long and thin spiral shape and a more active state of motion can be observed under the microscope. Its diameter is approximately about 0.2 μm, and the length ranges from 5 to 25 μm (14). The outer membrane contains lipopolysaccharide, like the genus Pseudomonas but not to Borrelia and Treponema pallidum. The main pathogenic Leptospira is mainly Leptospira interrogans. In this study, we showed that Leptospira isolated from the kidney of a dog in Nanchang, Jiangxi Province belonged to Leptospira interrogans Serovar Australis by PCR, MLST, and MAT analysis. The isolate strain was virulent by using the hamster model. Our results facilitated the active surveillance of Leptospirosis, local investigations, and source tracking.
Jiangxi province is an ancient source of Leptospirosis. Every year several cases of Leptospirosis are reported in Jiangxi provinces. From 2016 to 2018, 354, 201, and 157 cases of Leptospirosis were reported nationwide (excluding Hong Kong, Macao and Taiwan regions), with reported incidences of 0.0258/100,000, 0.0146/100,000, and 0.0113/100,000, respectively. From 2016 to 2018, Jiangxi Province reported a total of 19, 21 and 9 cases, with reported incidences of 0.0416/100,000, 0.0261/100,000, and 0.0195/100,000, respectively. The Leptospira icterohaemorrhagiae is the dominating epidemic serovar in Jiangxi Province, followed by the Javanica. L. introrogans is the main pathogenic gene species. ST1 is the main dominant ST type, followed by ST143. It is reported that L. introrogans gene species are more suitable for living in humid environments, while the L. bergerpersenii gene species can survive in both wet and dry conditions (15).
Multi-locus sequence typing (MLST) is an analysis method based on housekeeping gene sequence determination, which is used for strains genomic correlation and genetic diversity. MLST is a typing method established in recent years (16). It is based on the MLEE principle and a typing method of the nucleotide sequence of pathogenic bacteria. MLST is the product of a combination of high-throughput sequencing technology and mature population genetics. By analyzing the nucleotide sequences of core fragments of multiple housekeeping genes, 7–8 housekeeping genes were sequenced (17, 18). MLST is based on DNA sequencing data, which can make it specific, repeatable, and comparable, and it is also suitable for detecting strain evolution and genetic analysis of microbial populations (18). This method has high-resolution and good-reproducibility between different laboratories (19). The combined use of MLST and MAT will identify Leptospira more accurately (20).
Up to now, the epidemic situation of Leptospira in Nanchang has rarely been reported in China. In this study, we have characterized a pathogenic Leptospira, which belongs to the Leptospira interrogans serovar Australis. Further characterization of this isolate is underway and may help to understand the virulence and pathogenic mechanisms of Leptospira spp. We will also conduct a local epidemiological investigation to determine the prevalence of Leptospirosis and the strains. These efforts will have a positive effect on the prevention and control of Leptospirosis in the local area, and contribute to the preparation of a canine vaccine against the local epidemic of Leptospira.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Jilin University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
YC: conceptualization. NS and WZ: data curation. WZ: formal analysis and project administration. YC and LX: funding acquisition and writing—review and editing. NS and YD: investigation. NS and DW: methodology. DW and YD: software. YD: visualization. ZD: supervision. NS: writing—original draft.
Funding
We thank Dr. Xiaokui Guo (Shanghai Jiao Tong University, Shanghai, China) for providing Leptospira interrogans serovar Lai strain Lai (56601), the Key Project of Chinese National Programs for Research and Development (No. 2016YFD0501005). This work was supported by General project of Jiangxi province (20171BBG70075), the National Natural Science Foundation of China (No. 31802261), China Postdoctoral Science Foundation-funded project and Interdisciplinary Research Funding Program for Doctoral Students of Jilin University (Nos. 101832020DJX096 and 101832020CX327).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Xiaokui Guo (Shanghai Jiao Tong University, Shanghai, China) for providing Leptospira interrogans serovar Lai strain Lai (56601).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.607115/full#supplementary-material
References
1. Adler B, de la Peña Moctezuma A. Leptospira and Leptospirosis. Vet Microbiol. (2010) 140:287–96. doi: 10.1016/j.vetmic.2009.03.012
2. Romero EC, Yasuda PH. Human Leptospirosis: guidance for diagnosis, surveillance and control. Rev Inst Med Trop São Paulo. (2003) 45:292. doi: 10.1590/S0036-46652003000500015
3. Søndergaard MM, Tursunovic A, Thye-Rønn P, Bang JC, Hansen IM. Leptospirosis-associated severe pulmonary hemorrhagic syndrome with lower back pain as an initial symptom. Am J Case Rep. (2016) 17:883–6. doi: 10.12659/AJCR.900477
4. Ahmed A, Engelberts MFM, Boer KR, Ahmed N, Hartskeerl RA. Development and validation of a real-time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS ONE. (2009) 4:e7093. doi: 10.1371/journal.pone.0007093
5. Weilin H, Xu'Ai L, Jie Y. Leptospira and leptospirosis in China. Curr Opin Infect Dis. (2014) 27:432–6. doi: 10.1097/QCO.0000000000000097
6. Harkin KR, Roshto YM, Sullivan JT. Clinical application of a polymerase chain reaction assay for diagnosis of Leptospirosis in dogs. J Am Vet Med Assoc. (2003) 222:1224–9. doi: 10.2460/javma.2003.222.1224
7. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. (2015) 387:65–97. doi: 10.1007/978-3-662-45059-8_5
8. Ellis WA. Animal leptospirosis. Curr Top Microbiol Immunol. (2015) 387:99–137. doi: 10.1007/978-3-662-45059-8_6
9. Jin X, Zhang W, Ding Z, Wang H, Wu D, Xie X, et al. Efficacy of the rabbit polyclonal anti-Leptospira antibody against homotype or heterotype Leptospira infection in hamster. PLoS Negl Trop Dis. (2016) 10:e0005191. doi: 10.1371/journal.pntd.0005191
10. Boonsilp S, Thaipadungpanit J, Amornchai P, Wuthiekanun V, Bailey MS, Holden MTG, et al. A Single Multilocus Sequence Typing (MLST) scheme for seven pathogenic Leptospira species. PLoS Negl. Trop Dis. (2013) 7:e1954. doi: 10.1371/journal.pntd.0001954
11. Silva EF, Santos CS, Athanazio DA, Seyffert N, Seixas FK, Cerqueira GM, et al. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine. (2008) 26:3892–6. doi: 10.1016/j.vaccine.2008.04.085
12. Zhang W, Zhang N, Wang W, Wang F, Gong Y, Jiang H, et al. Efficacy of cefepime, ertapenem and norfloxacin against Leptospirosis and for the clearance of pathogens in a hamster model. Microbial Pathog. (2014) 77:78–83. doi: 10.1016/j.micpath.2014.11.006
13. Weiss S, Menezes A, Woods K, Chanthongthip A, Dittrich S, Opoku-Boateng A, et al. An extended Multilocus Sequence Typing (MLST) scheme for rapid direct typing of Leptospira from clinical samples. PLoS Negl Trop Dis. (2016) 10:e0004996. doi: 10.1371/journal.pntd.0004996
14. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. (2003) 3:757–71. doi: 10.1016/S1473-3099(03)00830-2
15. Cosson JF, Picardeau M, Mielcarek M, Tatard C, Chaval Y, Suputtamongkol Y, et al. Epidemiology of Leptospira transmitted by rodents in Southeast Asia. PLoS Negl Trop Dis. (2014) 8:e2902. doi: 10.1371/journal.pntd.0002902
16. Maiden MCJ. Multilocus sequence typing of bacteria. Annu Rev Microbiol. (2006) 60:561–88. doi: 10.1146/annurev.micro.59.030804.121325
17. Feil EJ, Enright MC. Analyses of clonality and the evolution of bacterial pathogens. Curr Opin Microbiol. (2004) 7:308–13. doi: 10.1016/j.mib.2004.04.002
18. Qi WH, Lacher DW, Bumbaugh AC, Hyma KE, Ouellette LM, Large TM, et al. EcMLST: an online database for multi locus sequence typing of pathogenic Escherichia coli. In: 2004 IEEE Computational Systems Bioinformatics Conference, Proceedings. Stanford, CA, (2004) p. 520–21.
19. Kaas RS, Friis C, Ussery DW, Aarestrup FM. Estimating variation within the genes and inferring the phylogeny of 186 sequenced diverse Escherichia coli genomes. BMC Genomics. (2012) 13:577. doi: 10.1186/1471-2164-13-577
Keywords: Leptospira interrogans Serovar Australis, PCR, MAT, MLST (multilocus sequence typing), dog, hamster
Citation: Song N, Zhang W, Ding Y, Wu D, Dai Z, Xu L and Cao Y (2021) Preliminary Characterization of Dog Derived Pathogenic Strains of Leptospira interrogans Serovar Australis in Nanchang of Jiangxi Province, China. Front. Vet. Sci. 7:607115. doi: 10.3389/fvets.2020.607115
Received: 16 September 2020; Accepted: 07 December 2020;
Published: 14 January 2021.
Edited by:
Joy Scaria, South Dakota State University, United StatesReviewed by:
Sérgio Jorge, Federal University of Pelotas, BrazilSharon Yvette Angelina Manalo Villanueva, University of the Philippines Manila, Philippines
Copyright © 2021 Song, Zhang, Ding, Wu, Dai, Xu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongguo Cao, eWdjYW84MkBqbHUuZWR1LmNu; Li Xu, eHVsaTc0MDMxMUAxNjMuY29t
†These authors have contributed equally to this work
 Ning Song1†
Ning Song1† Yongguo Cao
Yongguo Cao