
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 02 December 2020
Sec. Animal Nutrition and Metabolism
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.601336
This article is part of the Research Topic The Use of Growth Promoters and their Alternatives in Livestock Production View all 9 articles
This study aimed to determine the efficacy of dietary algae-derived polysaccharides (ADPs) from Enteromorpha on growth performance, intestinal morphology, intestinal permeability, and antioxidant capacity in serum, liver, and intestinal mucosa of broilers. Three hundred and ninety six day-old male chicks were randomly assigned to six dietary treatments containing 0 (Control), 1,000, 2,500, 4,000, 5,500, and 7,000 mg ADP/kg basal diet in a 35 day feeding trial. During day 1–21, compared with the control group, dietary 1,000–7,000 mg/kg ADP supplementation improved the average daily gain (ADG) and feed conversion ratio (p < 0.05). Overall (day 1–35), dietary inclusion of 1,000 mg/kg ADP increased the final body weight and ADG (p < 0.05). Besides, on day 21, dietary 2,500 mg/kg ADP supplementation increased the serum catalase (CAT) and liver total superoxide dismutase (T-SOD) activities (p < 0.05), whereas dietary 1,000–5,500 mg/kg ADP supplementation decreased malondialdehyde (MDA) contents in serum and liver (p < 0.05). On day 35, supplementation of 1,000 mg/kg ADP increased the serum glutathione peroxidase and CAT activities and liver T-SOD activities (p < 0.05). It decreased the MDA level of serum and liver (p < 0.05). Also, dietary 2,500 mg/kg ADP increased the villus height of jejunum and ileum on day 21 (p < 0.05), and dietary 4,000 mg/kg ADP increased the villus height of duodenum and ileum on day 35 (p < 0.05). On day 21, dietary 4,000 mg/kg ADP increased the CAT activities of the duodenum and T-SOD activities of jejunum and ileum and decreased the MDA contents in the duodenum, jejunum, and ileum (p < 0.05). On day 35, dietary inclusion of 1,000–7,000 mg/kg ADP reduced MDA contents of duodenum and jejunum (p < 0.05). Furthermore, dietary inclusion of ADP at 1,000–7,000 mg/kg decreased serum DAO activities at day 21 and day 35 (p < 0.05), and the serum D-lactic acid concentration was reduced by dietary supplementation of 1,000, 2,500, and 7,000 mg/kg ADP on day 21. In conclusion, dietary ADP exerted beneficial effects on growth performance, antioxidant capacity, and gut health in broilers; based on the studied parameters, the appropriate recommended dose is 1,000–4,000 mg/kg. These findings provided new insights into the potential application of ADP as natural growth promoters in broilers.
Due to restrictions or regulations on the use of antibiotic growth promoters in poultry production, there is an increasing demand for alternatives to antibiotic growth promoters like natural bioactive compounds to maintain the health and productivity of broilers (1–3). Natural polysaccharides, which are widely distributed in the microorganisms and plants, can improve an animal's gut health (4). Previously, numerous studies found that the natural polysaccharides derived from plants and fungus could be used as prebiotics to improve gut microflora balance and barrier function, ultimately promoting the growth of broiler chickens (5–8). Therefore, these natural and functional polysaccharides could replace antibiotics in the poultry industry. The marine ecosystem is an abundant reserve for natural polysaccharides. There are pieces of evidence supporting the biological and pharmacological role of polysaccharides in anti-carcinogenesis, antioxidation, anti-complementation, anti-inflammation, hematopoiesis, immunomodulation, and gastrointestinal tract protection (9, 10). Currently, marine polysaccharides have attracted considerable interest in the feed industry due to their biological activities (8, 11).
A naturally occurring marine green alga, Enteromorpha, is rich in polysaccharides. Many studies have reported multiple health-promoting benefits of Enteromorpha algae-derived polysaccharide (ADP) extract in anti-inflammation, against microbial pathogens, immunological regulation, and free-radical scavenging effects (12, 13). Also, broilers showed improved digestive function and immunity when fed with ADP from Enteromorpha (14, 15). Our recent study found that the ADP of Enteromorpha improves antioxidant performance and intestinal morphology in aged laying hens (16). However, to the best of our knowledge, the effects of ADP extracted from Enteromorpha on the antioxidant property and intestinal barrier function have not been evaluated in broilers yet. Hence, this study was conducted to evaluate the effects of dietary supplementation of ADP on growth performance, antioxidant property, and intestinal barrier function in broiler chickens.
The ADPs were extracted from the Enteromorpha by Qingdao Haida Biotechnology Co., Ltd. (Qingdao, China), with ≥48% purity, with a molecular weight of 4,929 Da. The ADPs are water-soluble sulfated polysaccharides obtained from the natural green alga Enteromorpha by enzymatic extraction, purification, concentration, and spray drying. Briefly, after crushing the algae, the algal powders are soaked in water. Then, the water extracts of the algae are subjected to stepwise enzymatic treatment with pectinase, cellulase, and papain. Then, the enzymes are inactivated, centrifugal concentrated, precipitated with ethanol, and finally spray dried to obtain the ADP used in this study. Based on the analysis by high-performance liquid chromatography, the polysaccharides mainly consisted of rhamnose (Rha), glucuronic acid (GlcA), glucose (Glc), galactose (Gal), and xylose (Xyl) monosaccharides. The molar percentages of monosaccharides in the ADP are as follows: Rha 40.6%, GlcA 9.3%, Glc 38.2%, Gal 5.6%, and Xyl 6.3%.
For this study, 396 day-old male Arbor Acres broiler chicks (initial body weights 44.65 ± 0.56 g) were obtained from a commercial hatchery (Guangxi, China). The chicks were randomly allocated into one of six dietary treatments (6 replicate cages per treatment, with 11 broilers per cage) for a 35 day study period. Dietary treatments were as follows: basal diets supplemented with 0 (control group [CON]), 1,000, 2,500, 4,000, 5,500, and 7,000 mg ADP/kg basal diet. The basal diet was formulated (Table 1) to meet or exceed the nutrient requirements of National Research Council (17) of broilers in two phases—starter (1–21 days) and finisher (22–35 days); all diets were fed as in mash form. To ensure that the ADP was thoroughly mixed into the basal diet, ADP was first mixed with 1 kg of diet by hand, and then, the premix was mixed with the remaining diet by using a blender. The broilers were grown in a temperature-controlled room at 33 ± 1°C for the first 3 days and then gradually reduced by 3°C per week until reaching 24°C and maintaining humidity 65% for the rest of the study period. Stainless steel cages [90 (length) × 70 (width) × 40 (height) cm] with were used for housing. The birds had free access to feed and water.
The broilers were weighed, and feed intake was recorded on day 1, 21, and 35. ADGs, average daily feed intake, and feed conversion ratio (FCR) were determined for each study phase. At the end of day 21 and 35, six birds (one bird from each replicate cage) were randomly selected from each treatment, and 3 ml blood samples per bird were collected from the wing vein by using a sterilized syringe with a needle (5 ml, 0.7 mm). The blood samples were then transferred into the vacuum tubes (5 ml, anticoagulant free, Xiangyuan Medical Ltd., Hebei, China). For serum analysis, the blood samples were centrifuged at 2,000 g at 4°C for 10 min to separate the serum and stored at −80°C to determine the antioxidant parameters and intestinal permeability biomarkers. The activities of the total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT), and the malondialdehyde (MDA) contents in serum were measured using commercial analysis kits (Jiancheng Bioengineering Institute, Nanjing, China) according to manufacturer's instructions. The levels of D-lactic acid and diamine oxidase (DAO) activities in serum were determined using enzyme-linked immunosorbent assay kits (96T, Yubo Biotechnology Co., Ltd. Shanghai, China) according to the manufacturer's instructions.
After blood sampling, the same birds were killed (by cutting carotid arteries) to collect the intestinal samples. Subsequently, the duodenum, jejunum, and ileum were separated, and ~2 cm segments of each at the middle location were collected and fixed in 10% buffered formalin until morphology analysis, stained with hematoxylin–eosin using standard histological techniques. The liver and remaining duodenum, jejunum, and ileum were rinsed in ice-cold phosphate-buffered saline (pH 7.0). Approximately 2 g liver tissues and mucosa of the duodenum, jejunum, and ileum samples were collected immediately for antioxidant parameter analysis. The activities of T-SOD, GSH-Px, CAT, and the MDA contents in the liver, mucosa of duodenum, jejunum, and ileum were measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) following manufacturer's instructions. Villus height, crypt depth, and villus width were measured at 40 × magnification using computer software (Olympus, DP72), then villus height-to-crypt depth ratio was calculated. The villus surface area (VSA) was calculated by using the formula (18):
All the data were statistically analyzed using general linear model procedures of SAS (Statistical Analysis System, version 9.2, SAS Institute Inc., Cary, NC, USA) with cage as an experimental unit (19). Data were expressed as means; variability in data was expressed as the standard error of the mean. Orthogonal polynomial contrasts were used to test the linear and quadratic effects of the increasing levels of dietary ADP. The probability value of <0.05 was considered to be statistically significant, and 0.05 ≤ p < 0.10 was considered as a tendency.
The results of growth performance are presented in Table 2. During day 1–21, compared with the CON group, dietary supplementation of 1,000–7,000 mg/kg ADP improved the ADG and FCR (p < 0.05). During day 22–35, no significant differences were observed in ADG, average daily feed intake, and FCR among dietary treatments (p > 0.05). During the overall study period (day 1–35), dietary supplementation of 1,000 mg/kg ADP increased the final BW and ADG (p < 0.05), and supplementation of 1,000, 4,000, and 7,000 mg/kg ADP improved the FCR (p < 0.05).
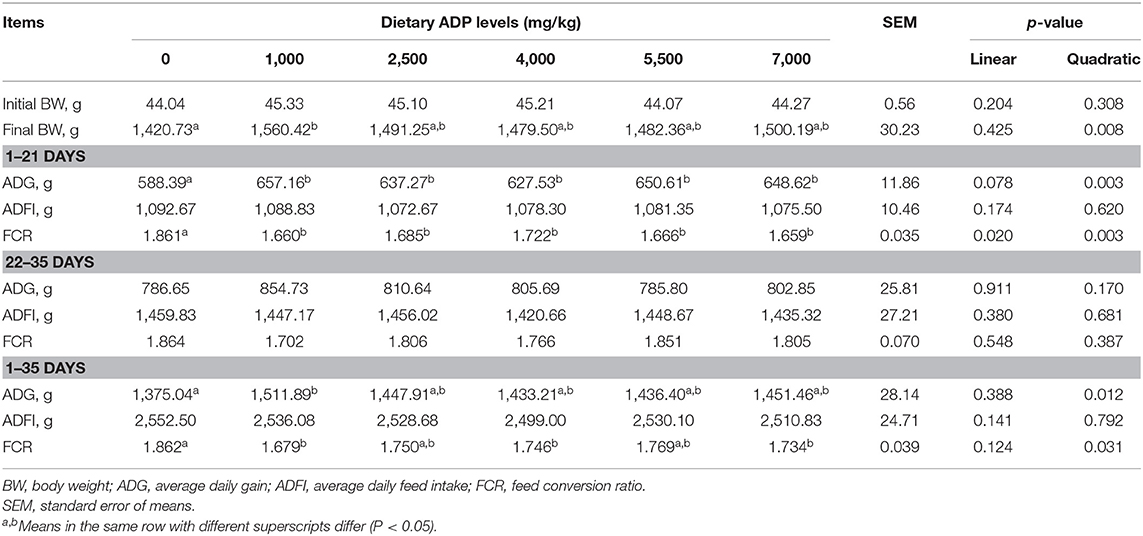
Table 2. Effect of dietary supplementation with graded levels of algae-derived polysaccharides (ADP) on growth performance in broiler chickens.
As shown in Table 3, on day 21, dietary supplementation of ADP (2,500 mg/kg) increased the serum CAT activities (p < 0.05), whereas dietary supplementation of 1,000, 2,500, 4,000, and 5,500 mg/kg ADP decreased MDA contents in the serum (p < 0.05). Dietary supplementation of 2,500, 5,500, and 7,000 mg/kg ADP enhanced the liver T-SOD activities (p < 0.05), and supplementation of ADP at 1,000–7,000 mg/kg level reduced the liver MDA contents (p < 0.05). On day 35, addition of 1,000 mg/kg ADP increased the GSH-Px and CAT activities (p < 0.05) but decreased the MDA level (p < 0.05) in serum. Dietary supplementation of 1,000–7,000 mg/kg ADP enhanced the T-SOD activities (p < 0.05) but decreased the MDA contents (p < 0.05) in liver.
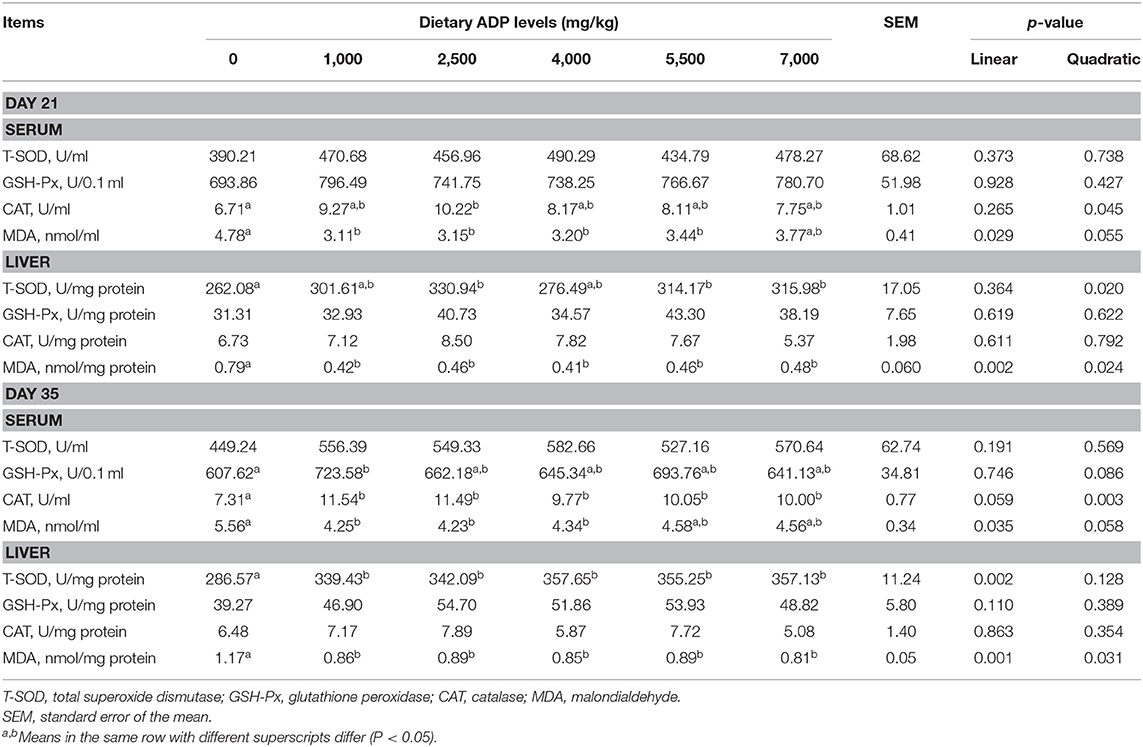
Table 3. Effect of dietary supplementation with graded levels of algae-derived polysaccharides (ADP) on antioxidant status of serum and liver in broiler chickens.
As presented in Table 4, dietary supplementation of ADP at 4,000 and 5,000 mg/kg diet improved the villus height, villus width, and VSA of the duodenum (p < 0.05), dietary inclusion of 1,000, 2,500, 4,000, and 7,000 mg/kg ADP increased the villus height of jejunum (p < 0.05), and dietary inclusion of 2,500, 5,500, and 7,000 mg/kg ADP improved the villus height of ileum (p < 0.05) compared with control diet on day 21. Also, on day 35, dietary ADP increased the villus height and villus height-to-crypt depth ratio of the duodenum at 1,000, 4,000, and 7,000 mg/kg level and improved the VSA of the duodenum at 4,000 and 7,000 mg/kg diet level (p < 0.05). Dietary ADP supplementation improved the villus height of ileum at 2,500, 4,000, 5,500, and 7,000 mg/kg level and the VSA of ileum at 1,000, 2,500, 5,500 mg/kg diet level (p < 0.05).
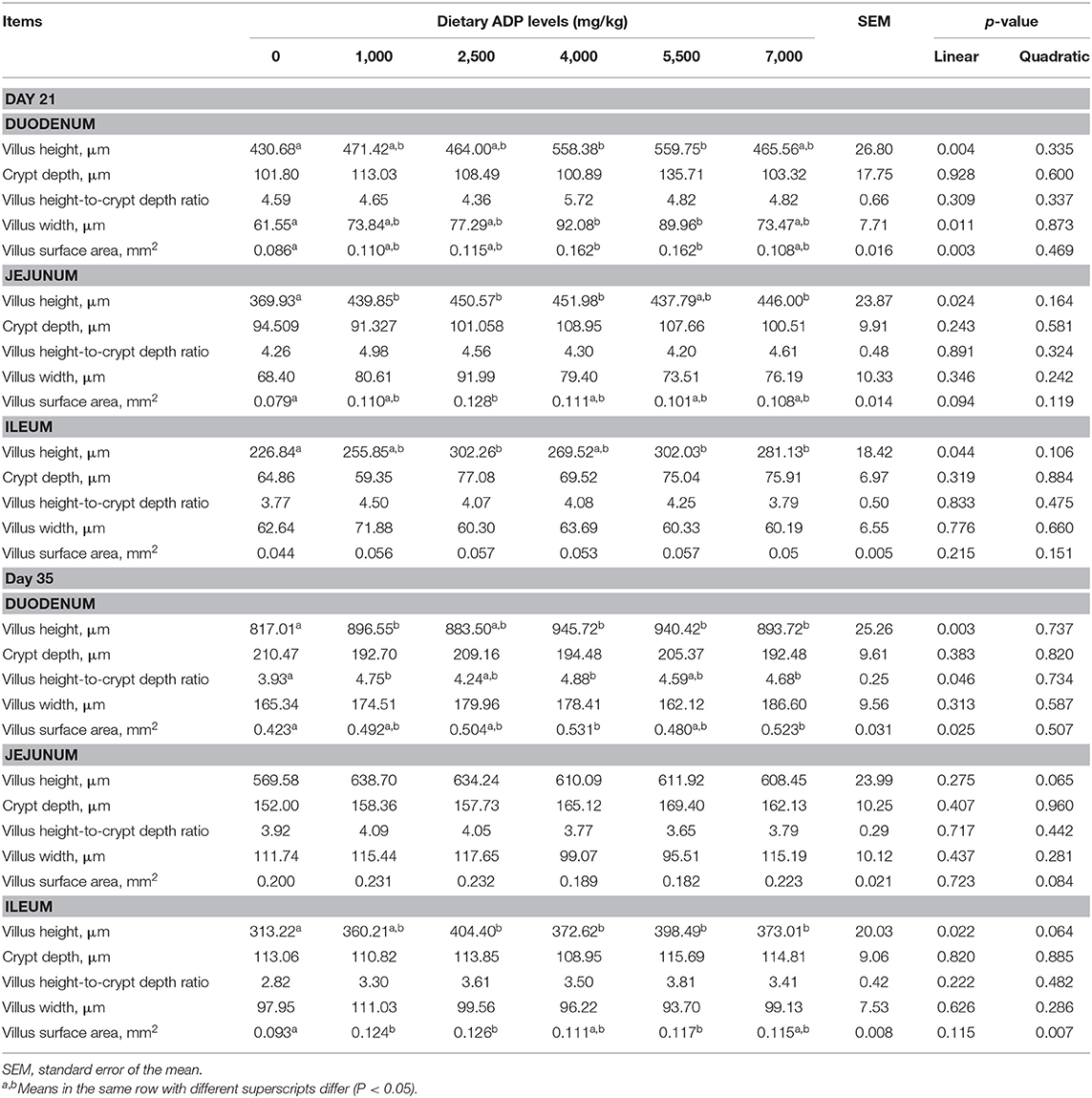
Table 4. Effect of dietary supplementation with graded levels of algae-derived polysaccharides (ADP) on intestinal morphology in broiler chickens.
On day 21 of the study, compared with the CON group, dietary 4,000 mg/kg increased the CAT activities, and 1,000–7,000 mg/kg ADP supplementation decreased the MDA contents in the duodenum (p < 0.05, Table 5). The inclusion of 4,000 mg/kg ADP in diet enhanced the T-SOD activities and reduced the MDA level of the jejunum (p < 0.05). Besides, dietary supplementation of ADP at 2,500, 4,000, 5,500, and 7,000 levels improved the T-SOD activities, 4,000, 5,500, and 7,000 mg/kg ADP improved the CAT activities, and 1,000–7,000 mg/kg ADP reduced the MDA contents in the ileum (p < 0.05). On day 35, dietary inclusion of 1,000–7,000 mg/kg ADP led to a reduction in MDA contents of the duodenum and jejunum in comparison with the CON group (p < 0.05). Dietary ADP at 1,000, 2,500, 4,000, and 7,000 mg/kg improved the T-SOD activities of jejunum (p < 0.05). Meanwhile, dietary addition of 5,500–7,000 mg/kg ADP decreased the MDA level of ileum (p < 0.05).
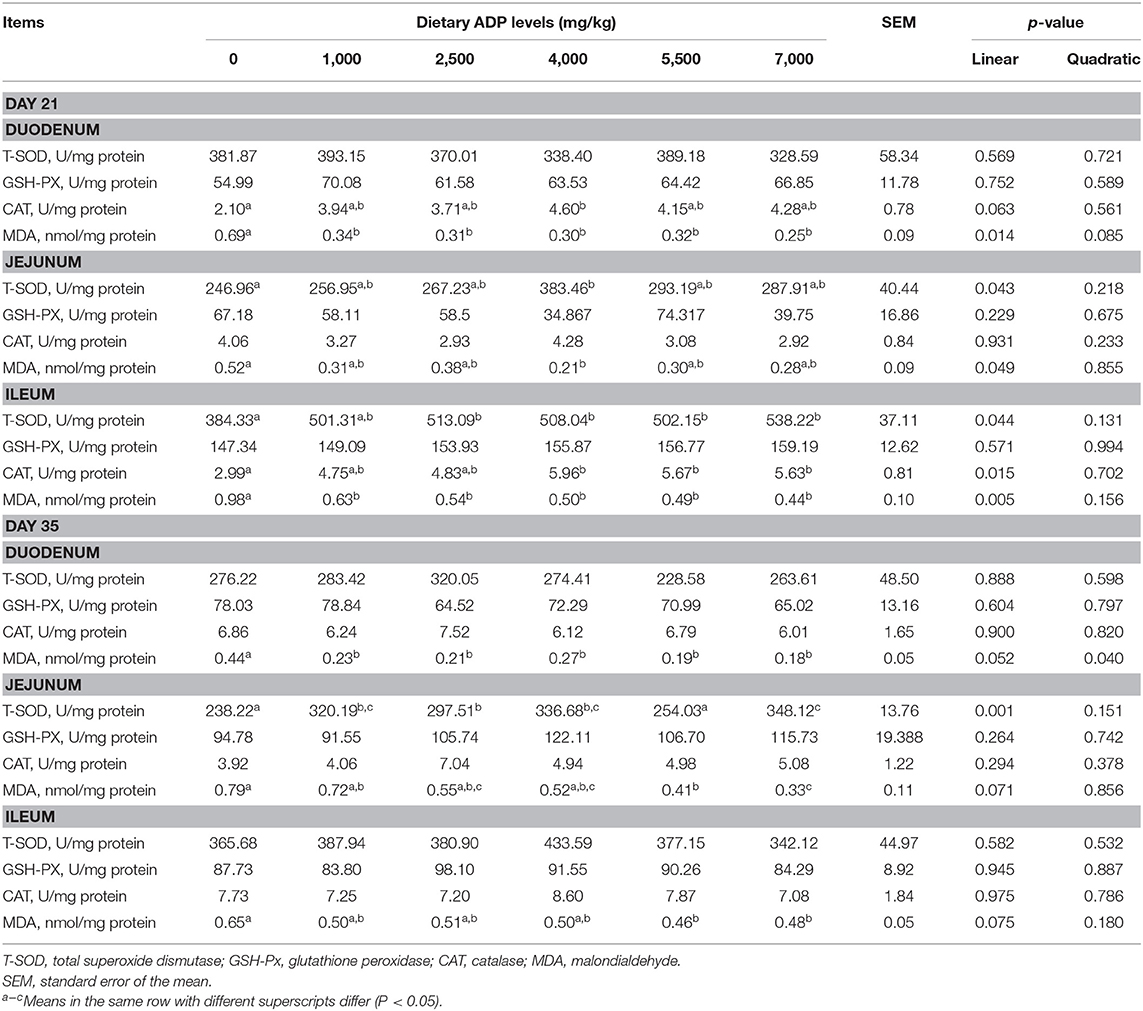
Table 5. Effect of dietary supplementation with graded levels of algae-derived polysaccharides (ADP) on antioxidant capacity of intestinal mucosa in broiler chickens.
The effects of dietary ADP on intestinal permeability biomarkers are presented in Table 6. Dietary inclusion of ADP at 1,000–7,000 mg/kg decreased serum DAO activities on day 21 and 35 (p < 0.05). Also, the serum D-lactic acid concentration was reduced by dietary supplementation of 1,000, 2,500, and 7,000 mg/kg ADP on day 21.
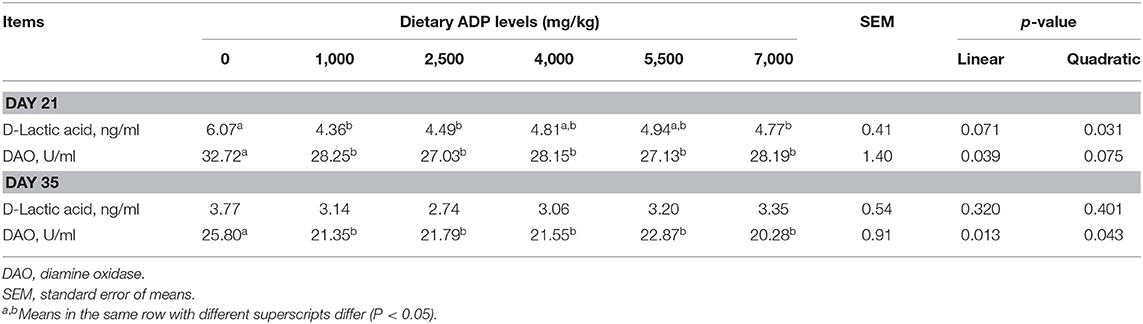
Table 6. Effect of dietary supplementation with graded levels of algae-derived polysaccharides (ADP) on serum markers of intestinal permeability in broiler chickens.
In recent years, utilizing polysaccharide compounds in the feed as prebiotics and growth promoters are at the center of attention in the poultry industry. The functional polysaccharides are non-digestible ingredients due to the presence of β-1,4 linkages (11, 16). Consumption of functional polysaccharides from medicinal plants and fungus could modulate the gut microbiota and improve health (3), thereby improving the growth performance in broilers (5–7, 20). The findings of the present study indicated that the inclusion of dietary ADP could promote the growth performance of broiler chickens. Similar to our results, Li et al. (21) observed that the dietary ADP of Enteromorpha at the inclusion rate of 0.5% improved the ADG and FCR of broilers through enhancing immune function. Similarly, other sources of marine polysaccharides, such as chitooligosaccharide (COS), have also been reported to improve the growth rate in broilers. According to Li et al. (22), broilers fed diet that contained 30 mg/kg COS showed higher FCR when compared with control. Likewise, Huang et al. (23) and Li et al. (24) found that the dietary inclusion of 150 or 100 mg/kg COS improved ADG and FCR in broilers. A wide range of mechanisms can underlay to decipher the beneficial effects of these natural marine polysaccharides on enhancing the growth performances, antioxidant capacity, and intestinal health status (22), promoting the growth of beneficial gut microbiota (8), immunity (14, 21), and nutrient digestibility (15) in broilers. However, Biggs et al. (25) and Shang et al. (6) observed no significant difference in the growth performance of broiler chickens fed a diet containing natural polysaccharides. The variation in response to natural dietary polysaccharides in different trials could be attributed to the polymerization levels, purity, supplemental dosage, experimental period and conditions, and the source of polysaccharides. Besides, it was remarkable that the positive effects of ADP on growth performance were mainly manifested during the starter phase (day 1–21) in this study. It was probably due to external stressors leading to altered physiology and weak immunity in broilers (22). The functional polysaccharides could improve the physiological function and immune function against a variety of stressors, subsequently stimulating the growth rate of broilers.
With the ongoing intensification of the poultry industry, broiler chickens are continuously exposed to various external stressors resulting in excess production of reactive oxygen species (ROS) and disturbing the redox balance in the chicken's body, contributing to oxidative stress (26, 27). Oxidative stress is one of the major factors adversely influencing the performance of chickens and leads to many metabolic disorders (16, 28, 29). Antioxidant enzymes, such as SOD and CAT, are known for their scavenging oxygen free radical property, thereby providing the first line of cellular defense against oxidative damage (30, 31). As an end product from lipid peroxidation, the MDA is used as a biomarker to measure the level of oxidative stress (32). The results of this study showed that the dietary ADP supplementation elevates the SOD activities of liver and CAT activities of serum but reduced the MDA levels in serum and liver. Duan et al. (13) provided direct evidence about the antioxidant properties of Enteromorpha ADP using in vitro studies. However, scientific information from the in vivo studies on the antioxidant capacity of ADP from Enteromorpha in broilers is relatively scarce. Due to the antioxidant bioactivities, the natural polysaccharides have received broad interest in animal nutrition, and several researchers have evaluated the antioxidant effects of marine polysaccharides in poultry and suggested that the antioxidant activity of marine polysaccharides may be due to the hemiacetal hydroxyl in the structure, which can scavenge the free radicals (9). Park et al. (33) reported that the inclusion of COS in drinking water could enhance the antioxidant capacity of broilers, and the present study found that the ADP supplementation improves serum SOD and liver CAT activities with a reduction in serum and liver MDA contents, which is similar to the finding in laying hens by Guo et al. (16). Similarly, Long et al. (34) reported that the dietary addition of 2,000 mg/kg Lycium barbarum polysaccharides improved SOD and GSH-Px activities with decreased MDA concentration in the serum and liver of broilers on day 42. It can be presumed that the improved antioxidant capacity observed in the present study is primarily attributed to the antioxidant characteristics of ADP (35). Thus, the marine polysaccharide ADP might be used as an ideal natural antioxidant agent in broilers due to synthetic antioxidants' toxicity. Also, improved antioxidant capacity might be closely linked with the enhanced growth performance in this study.
The gut is the center for digestion and absorption in animals. Additionally, it is known to function as an intestinal barrier, intestinal structure, antioxidant status, and permeability and play a critical role in nutrient processing and enhancing animal performance (4, 16). In this study, dietary supplementation of graded dosages of ADP exerted beneficial effects on intestinal morphology in villus height in both the duodenum and ileum. Similar to our results, Li et al. (22) found that dietary 30 mg/kg COS exerted a positive effect on duodenal morphology in broilers, as evidenced by the increased villus height and the ratio of villus height to crypt depth. Shang et al. (6) also reported that the natural dietary 0.5% polysaccharides of fructo-oligosaccharides could improve the villus height and total mucosa thickness of the ileum in broiler chickens on day 21. Similarly, Berrocoso et al. (36) reported increased villus height and villus height:crypt depth ratio when broilers were fed in ovo with raffinose as a prebiotic. Also, Abolfathi et al. (27) reported that dietary 1,000 mg/kg extract of elecampane (Inula helenium L.) rhizome that contains polysaccharide compounds increase villus height and villus height:crypt depth ratio of jejunum and ileum in broilers. These favorable alterations in intestinal morphology might be due to the prebiotic function of polysaccharides that could work as a substrate for the intestinal microflora and aids in stimulating the fermentation rate and increasing the production of short-chain fatty acids. The short-chain fatty acid benefits the differentiation and proliferation of intestinal epithelial cells (37). Similarly, other researchers suggest a positive association between intestinal morphological and antioxidant capacity in broilers (38–40), which was seen in our study as well.
The intestinal mucosa acts as a barrier to the external environment. Also, it is vulnerable to oxidative damage due to the massive workload and high rate of oxidative metabolism of the intestine, which results in the abundant release of ROS (28). Therefore, the intestine is a key source of ROS and particularly sensitive to oxidative stress compared with other tissues (41). Our current study's findings indicate that the basal diet supplemented with ADP enhances antioxidant enzyme (SOD, CAT) activities and successfully inhibits lipid peroxidation as observed in the decrease of MDA content in the intestinal mucosa. The results strongly agree with the available literature on natural polysaccharides. For instance, the inclusion of 30 or 350 mg/kg COS in broilers' diet was reported to improve the intestinal mucosa oxidative damage by enhancing SOD and GSH-Px enzyme activities and reducing the content of MDA (22, 42). The Artemisia argyi aqueous extract that contains an abundant amount of natural polysaccharides, feeding to broilers at 500–2,000 mg/kg level, increased the total antioxidant capacity, SOD, and GSH-Px activities by decreasing the MDA levels in small intestinal tissues (43). Thus, the natural polysaccharides can act as a defensive mechanism for antioxidant status in broilers' intestinal mucosa and can prevent the uncontrolled formation of ROS and/or directly scavenging the free radicals.
Serum D-lactic acid levels and DAO activity levels are considered sensitive biomarkers for intestinal permeability and generally determined to examine the extent of the damage or repair in the intestinal tract (44). An increase in serum D-lactic acid levels reflects an increased intestinal permeability (45). The DAO is secreted from the epithelial cells of the intestine and known for its hyper-endocellular enzymatic activity. The presence of higher DAO levels in serum indicates elevated functioning of intestinal barrier and permeability (44). In the present study, both D-lactic acid levels and DAO activities were significantly decreased by the inclusion of ADP in the broiler's diets, which indicated that the ADP improved the intestinal permeability and ultimately protected the intestinal barrier function. Similarly, previous literature showed that dietary supplementation of marine polysaccharides of 30 mg/kg COS resulted in a lower serum DAO activity and tended to reduce the concentration of D-lactic acid in broilers (22). The beneficial effects of ADPs on intestinal permeability might be related to improvements in tight junction protein expression of the intestine; however, the specific mechanism warrants further investigations. Coupled with the findings of higher antioxidant capacity of the intestinal epithelium along with improved morphological characteristics and enhanced intestinal barrier function observed in our study, it could provide the right consistency in improving the growth performance of broilers. The assessment of D-lactic acid and DAO can be performed in blood, which is a less invasive method. However, additional studies are required to recommend it for practical applications.
Our study shows that the dietary inclusion of ADP could promote growth performance, enhance the antioxidant property, and improve intestinal barrier function in terms of morphology, antioxidant status, and permeability of the intestinal mucosa (Figure 1). According to the study results, supplementation levels could be recommended at 1,000–4,000 mg/kg ADP in the broilers' diet. These findings provided a reference and new insights on dietary inclusion of ADP to promote growth and natural antioxidants, thus reducing the usage of antibiotics and synthetic antioxidants in broiler nutrition.
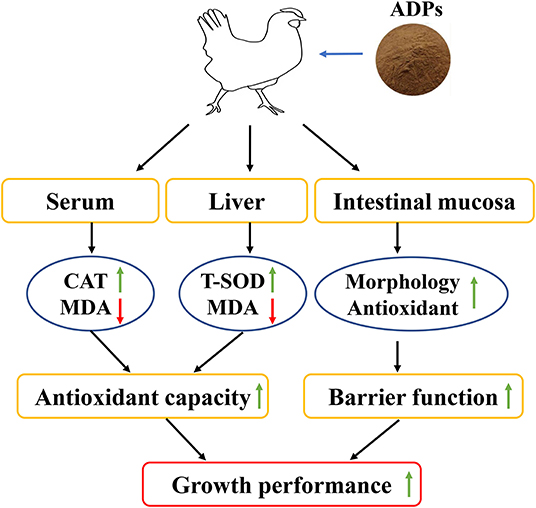
Figure 1. Schematic diagram showing how Algae-derived polysaccharides (ADP) promote broiler chicken's growth performance by improving antioxidant capacity and intestinal barrier function.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Animal Care Committee, Guangdong Ocean University.
W-CL, RJ, and BB: conceptualization. W-CL: methodology. W-CL and YG: analysis and data curation. W-CL and BB: writing-original draft preparation. RJ and BB: writing-review and editing. RJ: supervision. W-CL and Z-HZ: project administration. W-CL: funding acquisition. All authors contributed to the article and approved the submitted version.
This research was funded by the Natural Science Foundation of Guangdong Province (2018A030307023), National Nature Science Foundation of China (32002196), Talent Research Start-up Project of Guangdong Ocean University (R18007), South China Sea Scholar by Guangdong Ocean University (573118025), Innovative Strong School Engineering Youth Talent Project (2017KQNCX090) by Department of Education in Guangdong Province, and National Research Foundation of South Korea (2018R1C1B5086232) funded by Korean Government (MEST).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Chueh CC, Lin LJ, Lin WC, Huang SH, Jan MS, Chang SC, et al. Antioxidant capacity of banana peel and its modulation of Nrf2-ARE associated gene expression in broiler chickens. Ital J Anim Sci. (2019) 18:1394–103. doi: 10.1080/1828051X.2019.1667884
2. Liu W, Yuan Y, Sun C, Balasubramanian B, Zhao Z, An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals. (2019) 9:506. doi: 10.3390/ani9080506
3. Yadav S, Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J Anim Sci Biotechnol. (2019) 10:2. doi: 10.1186/s40104-018-0310-9
4. Jha R, Fouhse JM, Tiwari UP, Li L, Willing BP. Dietary fiber and intestinal health of monogastric animals. Front Vet Sci. (2019) 6:48. doi: 10.3389/fvets.2019.00048
5. Kim GB, Seo YM, Kim CH, Paik IK. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult Sci. (2011) 90:75–82. doi: 10.3382/ps.2010-00732
6. Shang Y, Regassa A, Kim JH, Kim WK. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poult Sci. (2015) 94:2887–97. doi: 10.3382/ps/pev275
7. Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult Sci. (2018) 97:3489–93. doi: 10.3382/ps/pey220
8. Zhang J, Cai K, Mishra R, Jha R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affect cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult Sci. (2020) 99:4776–85. doi: 10.1016/j.psj.2020.06.061
9. Laurienzo P. Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs. (2010) 8:2435–65. doi: 10.3390/md8092435
10. Jiao Y, Jha R, Zhang WL, Kim IH. Effects of chitooligosaccharide supplementation on egg production, egg quality and blood profiles in laying hens. Indian J Anim Res. (2019) 53:1199–204. doi: 10.18805/ijar.B-881
11. Liu WC, Zhou SH, Balamuralikrishnan B, Zeng FY, Sun CB, Pang HY. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immun. (2020) 104:202–12. doi: 10.1016/j.fsi.2020.05.079
12. Guo Y, Balasubramanian B, Zhao ZH, Liu WC. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult Sci. (2020). doi: 10.1016/j.psj.2020.10.050. [Epub ahead of print].
13. Duan K, Shan H, Lin YT, Lv HT. Free radical degradation of Enteromorpha polysaccharides and its antioxidant activity. Feed Res. (2015) 24:51–5.
14. Sun JF, Zhao J, Qi R, Xiao Y, Lin YT. Effects of adding levels of Enteromorpha prolifera into a diet on immunity and serum biochemical indices of broiler chickens. Chin J Anim Nutr. (2010) 22:682–8.
15. Du HY, Liu HM, Yang GY, Yu C, Wang SB. Effects of Enteromorpha prolifera polysaccharides on intestinal digestive enzyme activity, microbial number and nutrient apparent utilization of broilers. Chin J Anim Nutr. (2019) 31:956–61. doi: 10.3969/j.issn.1006-267x.2019.02.053
16. Guo Y, Zhao ZH, Pan ZY, An LL, Balasubramanian B, Liu WC. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult Sci. (2020) 99:2100–7. doi: 10.1016/j.psj.2019.12.032
17. National Research Council. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC. (1994).
18. Yamauchi K, Buwjoom T, Koge K, Ebashi T. Histological intestinal recovery in chickens refed dietary sugar cane extract. Poult Sci. (2006) 85:645–51. doi: 10.1093/ps/85.4.645
20. Liu L, Shen J, Zhao C, Wang X, Yao J, Gong Y, et al. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int J Biol Macromol. (2015) 72:624–32. doi: 10.1016/j.ijbiomac.2014.08.057
21. Li Q, Wang C, Luo J, Lu X, Wang L, Luo F, et al. Effects of dietary Enteromorpha prolifera polysaccharide on growth performance and immune function of broilers. Chin Poult. (2017) 39:24–8. doi: 10.16372/j.issn.1004-6364.2017.09.006
22. Li J, Cheng Y, Chen Y, Qu H, Zhao Y, Wen C, Zhou Y. Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals. (2019) 9:493. doi: 10.3390/ani9080493
23. Huang RL, Yin YL, Wu GY, Zhang YG, Li TJ, Li LL, et al. Effect of dietary oligochitosan supplementation on ileal digestibility of nutrients and performance in broilers. Poult Sci. (2005) 84:1383–8. doi: 10.1093/ps/84.9.1383
24. Li XJ, Piao XS, Kim SW, Liu P, Wang L, Shen YB, et al. Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poult Sci. (2007) 86:1107–14. doi: 10.1093/ps/86.6.1107
25. Biggs P, Parsons CM, Fahey GC. The effects of several oligosaccharides on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult Sci. (2007) 86:2327–36. doi: 10.3382/ps.2007-00427
26. Quintiliani M. The oxygen effect in radiation inactivation of DNA and enzymes. Int J Radiat Biol. (1986) 50:573–94. doi: 10.1080/09553008614550981
27. Lee MT, Lin WC, Lee TT. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals–a review. Asian Australas J Anim Sci. (2019) 32:309–19. doi: 10.5713/ajas.18.0538
28. Mishra B, Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front Vet Sci. (2019) 6:60. doi: 10.3389/fvets.2019.00060
29. Wang J, Yang Z, Celi P, Yan L, Ding X, Bai S, et al. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants. (2019) 8:503. doi: 10.3390/antiox8100503
30. Yin J, Ren W, Liu G, Duan J, Yang G, Wu L, et al. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res. (2013) 47:1027–35. doi: 10.3109/10715762.2013.848277
31. Farag MR, Elhady WM, Ahmed SYA, Taha HSA, Alagawany M. Astragalus polysaccharides alleviate tilmicosin-induced toxicity in rats by inhibiting oxidative damage and modulating the expressions of HSP70, NF-kB and Nrf2/HO-1 pathway. Res Vet Sci. (2019) 124:137–48. doi: 10.1016/j.rvsc.2019.03.010
32. Yang RL, Li W, Shi YH, Le GW. Lipoic acid prevents high-fat diet-induced dyslipidemia and oxidative stress: a microarray analysis. Nutrition. (2008) 24:582–8. doi: 10.1016/j.nut.2008.02.002
33. Park SB, Kim SW, Kim YS, Na CS, Shim KS. Effect of inclusion of chitosan-oligosaccahariede in drinking water on the blood component profile, immunity and antioxidative enzyme in broiler chickens. Korean J Org Agric. (2014) 22:483–90. doi: 10.11625/KJOA.2014.22.3.483
34. Long LN, Kang BJ, Jiang Q, Chen JS. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult Sci. (2020) 99:744–51. doi: 10.1016/j.psj.2019.10.043
35. Alagawany M, Abd El-Hack ME, Farag MR, Elnesr SS, El-Kholy MS, Saadeldin IM, et al. Dietary supplementation of Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult Sci. (2018) 97:3126–37. doi: 10.3382/ps/pey186
36. Berrocoso JD, Kida R, Singh AK, Kim YS, Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult Sci. (2017) 96:1573–80. doi: 10.3382/ps/pew430
37. Rémésy C, Levrat MA, Gamet L, Demigné C. Cecal fermentations in rats fed oligosaccharides (inulin) are modulated by dietary calcium level. Am J Physiol. (1993) 264:855–62. doi: 10.1152/ajpgi.1993.264.5.G855
38. Abolfathi ME, Tabeidian SA, Foroozandeh Shahraki AD, Tabatabaei SN, Habibian M. Effects of ethanol extract of elecampane (Inula helenium L.) rhizome on growth performance, diet digestibility, gut health, and antioxidant status in broiler chickens. Livest Sci. (2019) 223:68–75. doi: 10.1016/j.livsci.2019.03.006
39. Gu XH, Hao Y, Wang XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult Sci. (2012) 91:790–9. doi: 10.3382/ps.2011-01628
40. Wu Y, Zhou Y, Lu C, Ahmad H, Zhang H, He J, et al. Influence of butyrate loaded clinoptilolite dietary supplementation on growth performance, development of intestine and antioxidant capacity in broiler chickens. PLoS ONE. (2016) 11:e0154410. doi: 10.1371/journal.pone.0154410
41. Habibian M, Sadeghi G, Karimi A. Comparative effects of powder, aqueous and methanolic extracts of purslane (Portulaca oleracea L.) on growth performance, antioxidant status, abdominal fat deposition and plasma lipids in broiler chickens. Anim Prod Sci. (2019) 59:89–100. doi: 10.1071/AN17352
42. Li X, Ding X, Peng X, Chi X, Cui H, Zuo Z, et al. Effect of chitosan oligosaccharides on antioxidant function, lymphocyte cycle and apoptosis in ileum mucosa of broiler. Kafkas Univ Vet Fak Derg. (2017) 23:571–7. doi: 10.9775/kvfd.2016.17329
43. Zhao F, Shi B, Sun D, Chen H, Tong M, Zhang P, et al. Effects of dietary supplementation of Artemisia argyi aqueous extract on antioxidant indexes of small intestine in broilers. Anim Nutr. (2016) 2:198–203. doi: 10.1016/j.aninu.2016.06.006
44. Gilani S, Gordon SH, Soressa MK, Rebecca EAF, Cuong DT, Robert JH. New biomarkers for intestinal permeability induced by lipopolysaccharide in chickens. Anim Prod Sci. (2016) 56:1984–97. doi: 10.1071/AN15725
Keywords: algae-derived polysaccharides, antioxidant capacity, broilers, growth performance, gut health
Citation: Liu W-C, Guo Y, Zhao Z-H, Jha R and Balasubramanian B (2020) Algae-Derived Polysaccharides Promote Growth Performance by Improving Antioxidant Capacity and Intestinal Barrier Function in Broiler Chickens. Front. Vet. Sci. 7:601336. doi: 10.3389/fvets.2020.601336
Received: 31 August 2020; Accepted: 02 November 2020;
Published: 02 December 2020.
Edited by:
Minoru Tanaka, Nippon Veterinary and Life Science University, JapanReviewed by:
Siaka Seriba Diarra, University of the South Pacific, FijiCopyright © 2020 Liu, Guo, Zhao, Jha and Balasubramanian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajesh Jha, cmpoYUBoYXdhaWkuZWR1; Balamuralikrishnan Balasubramanian, YmFsYS5tLmtAc2Vqb25nLmFjLmty; Z2VuZXRpY3NtdXJhbGlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.