- 1Universidad de Buenos Aires (UBA), Facultad de Ciencias Veterinarias (FCV), Instituto de Investigación y Tecnología en Reproducción Animal, Cátedra de Teriogenología, Buenos Aires, Argentina
- 2Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina
- 3Universidad de Buenos Aires (UBA), Facultad de Ciencias Veterinarias (FCV), Cátedra de Bioestadística, Buenos Aires, Argentina
The aim of this study was to assess the uterine blood flow (UBF) and corpus luteum blood flow (CLBF) in llamas 8 days post-mating, using color-Doppler ultrasonography (CDU), to determine the possible relationship between vascularization and the presence of an embryo. Adult females (n = 25) were used to monitor ovarian dynamics by palpation and transrectal ultrasonography until detection of a ≥6 mm growing follicle. Females were randomly assigned to one of two groups: Group I (n = 19), were mated and ovulation was induced by a single dose of buserelin (GnRH analog) that same day (Day 0); and Group II (n = 6), only ovulation was induced (control). On Day 8, UBF and CLBF were evaluated transrectally in both groups. The color-flow images obtained were analyzed with Image J1.52a software to determine the vascularization area and the percentage of corpus luteum with blood flow emission (CLBF%) together with the percentage for each uterine horn (UBF%). Statistical analysis was performed using an ANOVA test. In Group I, uterine flushing was performed to obtain the embryos, thus dividing the females into Group I+ (n = 10), when an embryo was recovered and Group I- (n = 9), when no embryo was recovered. Embryo recovery rate was 52.63% (10/19). In Group I+, UBF% was significantly higher compared to Group I- and Group II (P <0.05). UBF appears to be a good predictor for embryo presence, with an area under the curve (AUC) of 0.9 and an optimal cut-off value of 9.37% (with a sensitivity of 90% and specificity of 88.9%). The CLBF% did not differ between groups (P > 0.05). In conclusion, it is possible to detect a local increase of UBF in the presence of an embryo on day 8 post-mating in llamas. This could be useful to achieve an early pregnancy diagnosis or to decide whether to carry out the uterine flushing in a llama embryo transfer program.
Introduction
Llamas are monotocous and have a prolonged gestation period [335–360 days; (1)], hence early pregnancy diagnosis would allow a greater efficiency to obtain one offspring per female per year when conception fails and gives better productive indices. To date, the methods used to diagnose gestation in South American camelids (SAC) are limited. One of the methods that is widespread among SAC producers is to observe the sexual behavior of the females when confronted with a male, from 11 to 13 days after natural mating (2, 3). Although this is an inexpensive technique as it doesn't require sophisticated tools or equipment, behavior interpretation is subjective and prone to error. There are even dominant females that, in absence of high progesterone levels, still reject the male without being pregnant. In addition, this method does not provide information on the number of embryos or fetuses present, their state of development or health. Another diagnostic tool is measuring plasma progesterone levels 11–13 days post-mating (4, 5) as it reflects the presence of a functional corpus luteum (CL), necessary to maintain pregnancy in SAC (6). However, validated progesterone dosage tests for SAC are scarce and difficult to access and the time lag between blood collection and availability of the results is also problematical. Furthermore, reproductive disorders that prolong the luteal phase, such as spontaneous ovulations and luteinized cystic follicles, can produce a 15% of false positive results (5). But, similarly to the first method, this technique doesn't provide information on the number, development, and health of the embryo or fetus either. Pregnancy evaluation by transrectal palpation is possible 35 days after mating in llamas, with greater accuracy 45–50 days after mating (2, 3, 7). This is a simple, low-cost maneuver that although provides fetal viability data, the estimation of gestational age, and therefore prediction of probable date of parturition, are not precise (8). So far, the most effective, precise and early method for diagnosing gestation in SAC is transrectal ultrasonography in Brightness mode (B-mode), which allows visualization of the embryo vesicle 12–14 days after mating, although is more precise as of 16–23 days (9). Unlike the above methods, it not only gives information regarding the number, development and viability of the embryo/s or fetus/es, it also allows one to approximate gestational age (10), even when the date of natural mating is unknown, and hence estimate the probable date of parturition.
In SAC, the embryo reaches the uterine lumen as a hatched blastocyst, between 6 and 6.5 days after ovulation (11, 12), and produces increasing quantities of estradiol-17β during days 7–15 of gestation (13). According to Ford (14), the estradiol produced by embryos of domestic species is responsible for the local increase in uterine blood flow (UBF) registered during early pregnancy. However, it is not known if there is a connection between the presence of the embryo and uterine blood flow in llamas in the early stages of gestation. Color Doppler ultrasonography (CDU) not only allows evaluation of the bidimensional structure of the different organs but also offers the possibility of observing their vascular system (15) as it overlays the color signals of blood flow over the B-mode images (16). To calculate the degree of vascularization of an organ, the percentage of tissue with color signals can be estimated or the images that are captured can be processed and analyzed by a computer using software that allows an objective calculation of the number of colored pixels over the B-mode area (17). The aim of this study was to assess the uterine blood flow and corpus luteum blood flow in llamas, 8 days post-mating using color Doppler ultrasonography to determine the possible relationship between vascularization and the presence of an embryo.
Materials and Methods
Animals
Non-pregnant, non-lactating female llamas (n = 25) ranging between 4 and 12 years of age and with an average body weight of 120 ± 22 kg were used in this study. All animals were in a good nutritional status (body condition), both healthy and reproductively active at the time of the trial. Females were kept separate from the males and fed with hay and water ad-libitum. The study was conducted between March 2018 and December 2019 at the Faculty of Veterinary Sciences of the University of Buenos Aires, Buenos Aires, Argentina, situated 34° 36' S and 58° 26′ W at sea level. This study was approved by the Committee for the Use and Care of Laboratory Animals (CICUAL, 2017/67) of the Faculty of Veterinary Sciences of the University of Buenos Aires.
Experimental Design
Ovarian dynamics were monitored by transrectal palpation and ultrasonography (Berger LC 2010 plus with a 5 MHz linear-array electronic transducer) until the presence of a growing follicle ≥6 mm was detected. At that moment, all females received a single IV dose of 8 μg of buserelin (GnRH analog; Receptal®, Intervet, Buenos Aires, Argentina) (day 0) to induce endogenous LH release and ovulation. After buserelin injection, llamas were randomly divided in two groups: Group I (n = 19), embryo donor females were mated with a male with proven fertility and uterine flushings were done for embryo recovery on day 8; Group II (n = 6), females without natural mating (control group). Ovulation was confirmed using transrectal ultrasonography based on the disappearance of the dominant follicle on day 2, and control of the ovarian dynamics was carried out every other day (B-mode ultrasonography) until day 8.
Uterine and Corpus Luteum Vascularization
All females were examined by transrectal CDU (MyLabTM 30Gold VET ESAOTE, attached to a 5 MHz linear-array electronic transducer) in order to assess the UBF and corpus luteum blood flow (CLBF) on day 8. The settings (B-mode frequency: 5 MHz with a depth of 8 cm and a gain of 52%; CFM pulse repetition frequency: 1.4 KHz and a gain of 70%) were standardized and remained constant for all examinations. Briefly, the transducer was placed over the middle segment of each uterine horn (UH) as described in llamas (18, 19), to display signals for blood flow in the vessels of all the endometrium, myometrium, and perimetrium, and over the CL. A 3 s video-clip of the vascularization of each structure was registered and downloaded. The video-clips were examined frame by frame (Adobe Premiere Pro CS6®) to select images that showed the maximum vascular signal, three of each UH and three over the maximum cross-sectional area of the CL. A total of 225 images (nine images per female) were saved in tagged image file format (TIFF) and analyzed by an operator without knowledge of the identity of each animal or the result of uterine flushing, using ImageJ 1.52i software (National Institute of Health, USA). The degree of vascularization was estimated by measuring the colored area (cm2) of the vascular flow signals (Doppler mode) over the left UH, right UH and the CL area (cm2) (B-mode). Thus, percent area of vascularization was calculated by the following equation: percent of blood flow area (BF%) = (vascular area/total organ's area) ×100. The average of the three images was considered as the final value for each uterine horn (UBF%) and for the CL (CLBF%) of each animal. In females with two CLs, we calculated the CLBF% of both and considered the average. Uterine flushing was performed in Group I after Doppler ultrasonographic evaluation.
Embryo Recovery and Evaluation
In Group I, uterine flushing was carried out non-surgically for embryo recovery, 8 days after mating (20). The maneuvers were performed with the female either standing or in sternal recumbency. The animal was restrained in stocks, the tail was wrapped and the rectum was emptied of feces. The perineum was then scrubbed using a hypoallergenic detergent, rinsed carefully with clean water and then dried. Restless females received 0.2 mg/kg IV xylazine (Xilazina® 10%, PRO-SER S.A., Buenos Aires, Argentina) before flushing. A Foley catheter (VortexTM; Agtech, Inc.; Manhattan, USA.) 12 or 16 Fr, according to female size, with a stylet inserted into the catheter to keep it from bending during recto-vaginal manipulation, was used. Uterine flushing was done by placing the catheter cuff cranial to the internal cervical os and insufflating it with 5 or 10 ml of air (depending on catheter gauge). The whole uterus was flushed 4 to 5 times using Ringer-Lactate solution (Laboratorios Rivero, Buenos Aires, Argentina), previously warmed (30–35°C), with a total volume of 500 ml. The recovered medium was filtered through a 70 μm EmConTM filter (Agtech, Inc.) for embryos. The fluid in the flushing filter was placed in warmed reticulated Petri dishes and embryos were identified using a stereomicroscope. Embryos were measured and classified according to their morphology following the criteria set by Tibary and Anouassi (21), using a grade scale from 1 to 5. After flushing, donor females received 250 μg IM of PGF2α (cloprostenol; Ciclase DL®, Syntex S.A., Buenos Aires, Argentina) to induce luteolysis. The number of embryos collected, and positive pregnancy diagnoses were recorded. Thus, females were divided into Group I+, when an embryo was recovered (positive uterine flushing) and Group I-, when no embryo was recovered (negative uterine flushing).
Statistical Analysis
Statistical analyses were performed using InfoStat software (version 2020; FCA, National University of Córdoba, Argentina). After verifying normal distribution of the data using a Shapiro-Wilks test, an ANOVA test was applied to evaluate uterine and CL vascularization. These data were subjected to Tukey's test to determine significance. In all females, the uterine horn with highest UBF% was chosen. Furthermore, we performed receiver operating characteristics (ROC) analyses focusing on UBF% on day 8, to identify the optimal cutoff value for predicting pregnancy. We evaluated every candidate cutoff value for the optimal cutoff value by geometric distance for 100% sensitivity and specificity. The optimal cutoff value was determined using the data point that minimized the distance (22). Prognostic value was expressed as the area under the curve (AUC) with a 95% confidence interval and a test of significance. A paired T-test was used to evaluate the relationship between the blood flow of each uterine horn and the location of the CL in the females in Group I+. Pearson or Spearman's correlation analysis were used to compare various parameters (Follicle and CL diameter, CLBF). The correlations between parameters were classified according to Taylor (23) as weak (r ≤ 0.35), moderate (r = 0.36–0.67) or strong (r = 0.68–1.00). Values were expressed as mean ± SEM. Differences were considered significant when P < 0.05.
Results
Embryo Recovery Rate and Quality
Embryo recovery rate was 52.63% (10/19), with a total of 11 embryos recovered from 19 flushing procedures (two embryos were obtained from one female). Hence, females in Group I were divided into Group I+: n = 10 and Group I-: n = 9. Of the 11 recovered embryos, nine were grade I, one was grade II and one was an arrested morula (grade V). Embryo size ranged from 350 to 1,500 μm.
Uterine Blood Flow in Pregnant and Non-pregnant Females
In Group I+, UBF% was significantly higher (14.5 ± 1.5%) than in Group I- (6.6 ± 1.2 %) and Group II (5.19 ± 1.94 %; P < 0.05). Moreover, no differences were found between Group I- and Group II (P > 0.05) (Figures 1, 2).
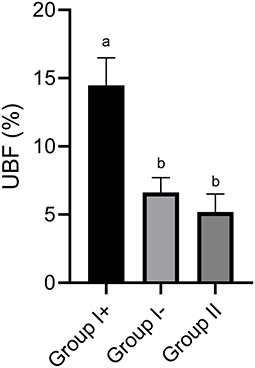
Figure 1. Uterine blood flow (UBF) in the three groups: llamas with an embryo recovered (Group I+), llamas without an embryo recovered (Group I-) and control group (Group II, ovulated, non-mated females). Values are mean ± SEM. a, bGroups with different letters are significantly different (P < 0.05).
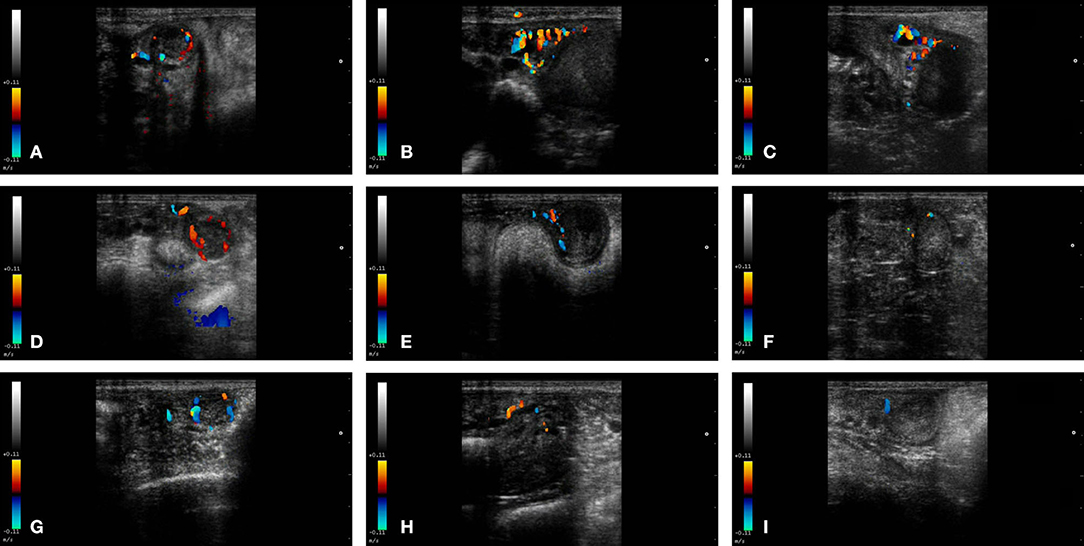
Figure 2. Color-Doppler images from a female llama from each group in the study. From left to right: CL, left uterine horn and right uterine horn. Group I+ (A–C), Group I- (D–F), and Group II (G–I).
Predicting the Presence of an Embryo Using a ROC Curve
To prepare the Receiver Operating Characteristic (ROC) curve, the uterine horn with highest UBF% on day 8 from each female was taken. Differentiation between pregnant and non-pregnant llamas was possible with this technique with an area under the curve (AUC) of 0.900. The optimal cut-off value was 9.37%, with a sensitivity of 90% and specificity of 88.9% (Figure 3).
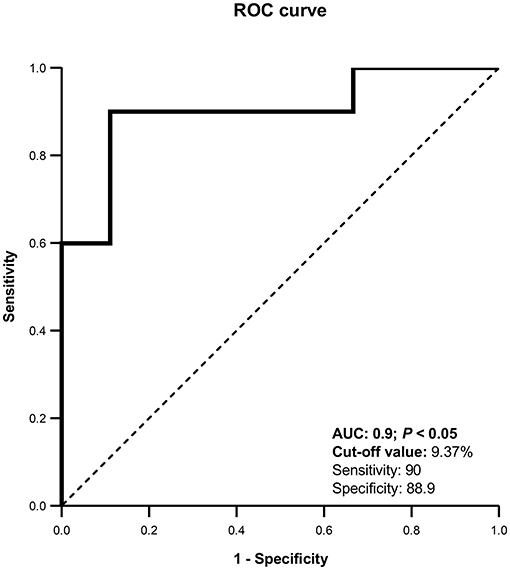
Figure 3. Receiver Operating Characteristic (ROC) curve for uterine blood flow (%). AUC, Area under the curve. The curve was able to detect pregnant llamas when UBF% was >9.37%, with a sensitivity of 90% and a specificity of 88.9%.
Blood Flow of Each Uterine Horn and Its Relationship With the Location of the CL in Pregnant Females
Females in Group I+ showed no significant differences in UBF% between both uterine horns irrespective of the location of the CL (P > 0.05). However, those with a CL in the left ovary showed a tendency to have a higher UBF% in the left UH compared to the right UH (P = 0.06), whereas when the CL was in the right ovary, UBF% was similar between uterine horns (P = 0.97).
Corpus Luteum Diameter and Vascularization in Pregnant and Non-pregnant Females
Diameter of the CL on day 8 was similar between all groups (Group I+: 1.06 ± 0.07 cm; Group I-: 1.25 ± 0.06 cm; Group II: 1.25 ± 0.07 cm) (P = 0.76). Neither were significant differences detected in CL blood flow (CLBF%) between Groups I+ (23.29 ± 2.05 %), I- (22.76 ± 2.16 %) and II (22.93 ± 2.64 %) (P = 0.98) (Figure 4).
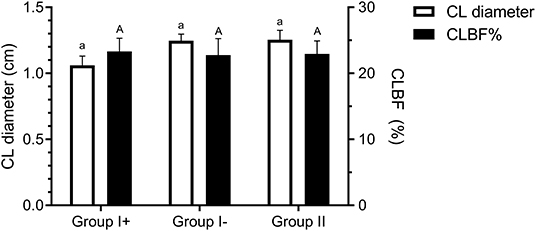
Figure 4. Corpus luteum diameter (cm) and blood flow (CLBF%) on day 8 in the three groups: llamas with an embryo recovered (Group I +), llamas without an embryo recovered (Group I-) and control group (Group II, ovulated, non-mated females). Both variables were expressed as mean ± SEM. a, Aindicate no significant differences between groups for both parameters (P > 0.05).
Correlation Analysis
Follicular diameter on day 0 was moderately correlated to CL diameter on day 8 (Spearman's coefficient of correlation: sρ = 0.61; P = 0.0013) but was not correlated to CLBF% on day 8 (Pearson's coefficient of correlation: ρ = −0.28; P = 0.18); neither was CL diameter on day 8 correlated to CLBF% on the same day (ρ = 0.08; P = 0.72).
Discussion
Embryo transfer is a powerful tool for increasing the number of offspring from a specific donor, but it is also becoming increasingly important for the genetic improvement of livestock herds (20, 24–26). Flushing for embryo recovery from the donor llama's uterus is normally performed on day 8 post-mating, without a previous pregnancy diagnosis. Using CDU, we observed that mated llamas with an embryo recovered (Group I+), had a significantly higher UBF% than mated females without an embryo recovered (Group I-) and llamas that ovulated but not were mated (Group II; control). This increase in UBF in pregnant females vs. non-pregnant females was also observed in embryo donor mares, where Doppler ultrasonography was used to make an early pregnancy diagnosis 8 days after artificial insemination, prior to embryo recovery. They established a cut-off value of 35.55 mm2 to distinguish adult pregnant mares from those that were not gestating, with a 97.2% sensitivity and an 85.7% specificity (27). In inseminated buffalo, increases in UBF were registered after ovulation, with a marked difference observed between pregnant and non-pregnant females from 7 days post-ovulation; hence it was possible to diagnose pregnancy on that date (28). An adequate uterine perfusion is essential in mammals for a correct development of gestation (29), as according to Habara et al. (30), uterine blood supply could regulate endometrial receptivity. It has been suggested that quantifying the increase in uterine blood flow during the luteal phase could be a good predictor for the success of embryo implantation (31). Silva et al. (17) observed that the UBF in mares increased locally at the site of the embryo during the migration phase (days 6 to 16 post-ovulation). They attributed this increase to the possible vasodilator action exerted by vasoactive agents, such as estrogens and prostaglandins, secreted by the equine embryo during that period. This increase in vascularization of the uterine horn ipsilateral to the embryo was also observed by Honnens et al. (32) in pregnant dairy cows from day 11 of gestation. Although the llama with a grade V embryo recovered (arrested morula) was classified in to Group I+ (positive uterine flushing), its UBF was 5.4%, comparable to the mean value registered in mated females without an embryo recovered (Group I-: 6.6%) and llamas that ovulated but not were mated (Group II: 5.19%). This could be due to the metabolic inactivity of the arrested morula and thus, to the lack of secretion and consequent action of its vasoactive agents.
In SAC, ovulations occur with equal frequency from both ovaries, however, most pregnancies are located in the left uterine horn [alpaca: 97.5 and 99.3% with a CL in the right and left ovaries, respectively, (33, 34); llama: 100%, (6)]. For this reason, an embryo resulting from an ovulation in the right ovary needs to migrate or emit some type of signal from the right uterine horn to the left to somehow indicate its presence to both uterine horns and thus prevent luteolysis (11, 20, 35, 36). Although the antiluteolytic signal has not yet been identified, it is believed that the estradiol-17β secreted by the blastocyst could be involved (37). The possible migration and resulting contact with both uterine horns of the embryo proceeding from an ovulation in the right ovary could explain the increase in blood flow registered in both uterine horns in the females of Group I+ with a CL in the right ovary. Powell et al. (13), suggested that llama embryo mobility through the uterus could be mediated by the estradiol-17β it secretes during the early stages of pregnancy, generating a localized increase in myometrial contractility and so allowing its propulsion toward the left uterine horn. Embryo estrogens would exercise this action by binding to the estrogen receptor β, whose expression in the myometrium and perimetrium is increased in the presence of a CL and is greater in pregnant vs. non-pregnant females (35). Whereas, if ovulation occurs in the left ovary, the embryo would not need to migrate because the right uterine horn is incapable of lysing a CL in the left ovary (11, 20, 36). Estradiol has a potent vasodilator effect (18, 38, 39) and it is possible that for this reason, the females in Group I+ with a CL in the left ovary register higher levels of blood flow in the ipsilateral uterine horn. However, increasing the number of animals evaluated could take this difference in UBF% between uterine horns to become statistically significant.
The positive correlation between the follicle diameter on day 0 and the CL diameter on day 8 that we detected was also observed in cows (40, 41), ewes (42), and mares (43–45) and could be because the size of the pre-ovulatory follicle would regulate the size of the CL in its early stages, mainly due to a spatial constraint (46). Nevertheless, the vertical diameter of the CL on day 8 was similar between pregnant females (Group I+) and non-pregnant females (Groups I- and II). According to previous studies in llamas (47, 48), after mating or inducing ovulation, the CL that is formed reaches its maximum diameter 10 days after the stimulus, both in pregnant and non-pregnant females. Only after this date does the CL commence to decrease in size in non-pregnant females while maintaining a plateau in pregnant animals and the difference between both groups becomes evident only after 14 days. Similarly, CLBF% on day 8 showed no significant differences between pregnant and non-pregnant females. Considering that the CL is the most highly vascularized temporary tissue of the body and receives the greatest rate of blood flow per unit of tissue (49), no apparent differences between pregnant and non-pregnant females in luteal blood flow would be expected during early angiogenesis in CL development (40). Our findings coincide with a recent report by Gallelli et al. (48), who evaluated CL blood flow using CDU in llamas after natural mating and observed that the CLBF was similar in all females up to 8 days post-mating. After that, CLBF remained high in pregnant females, while in non-pregnant animals it decreased dramatically until it disappeared between 14 and 16 days after mating. The difference in CLBF between pregnant and non-pregnant llamas was only evident after day 12 (48). Similar CLBF profiles between non-pregnant females were also observed using Doppler ultrasound in llamas (50, 51), alpacas (52), and dromedaries (53, 54), after inducing ovulation. In these cases, CLBF reached maximum values between 7 and 9 days after induction of ovulation and after that, they began to descend and reached basal levels between days 12 and 14. In all cases, CLBF reduction in non-pregnant females coincided with the process of luteolysis, which occurs over this period in these species. According to different authors, CL function (progesterone production) would be determined by its vascularity more than by its size [llamas: (48); dromedaries: (53); bovines: (55–57)]. In cows, de Tarso et al. (40, 41) evaluated the diameter and blood flow of the CL and found a moderate correlation (ρ = 0.43 a 0.6) between both parameters. However, in our study no correlation between the diameter of the CL and its blood flow was observed on day 8, similar to what had been previously reported by Gallelli et al. (48).
According to our study, CDU would predict the outcome of transcervical uterine flushings based on the UBF% 8 days after natural mating in llamas. By constructing a ROC curvewith an AUC of 0.900 we established that, if 9.37% is used as the cut-off value, it is possible to estimate the result of a uterine flushing with a 90% sensitivity and an 88.9% specificity. This would provide valuable information when deciding whether or not to perform a uterine flushing in an embryo transfer program. In addition, females diagnosed as pregnant could be separated early to adopt management and nutrition maneuvers appropriate to this new reproductive status, first adapting their nutritional plan and minimizing stress situations in order to prevent embryo mortality (58) which is high in these species (3). On the other hand, non-pregnant females detected early could be separated to be mated again. However, due to the high percentage of early embryo losses registered in these species, it would be best to combine this early detection method with other methods implemented later on, such as B-mode ultrasonography or transrectal palpation, to confirm pregnancy diagnosis.
Conclusion
In conclusion, the results of the present study indicate that evaluation of uterine blood flow by color Doppler ultrasound combined with computer assisted analysis of images are reliable techniques for detection of early pregnancy prior to embryo recovery on day 8 post-mating in llamas.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Committee for the Use and Care of Laboratory Animals (CICUAL).
Author Contributions
VT and EZ contributed conception, design of the study, and wrote the manuscript. EZ, VT, and MFG provided help with field work. MG performed the statistical analysis. MC, DN, and MM made critical revisions to the paper. DN contributed to language editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by grants from the University of Buenos Aires (UBACyT 2014-2017 and UBACyT 2018) and the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015-2220, PICT 2016-0145, and PICT 2018-2527).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank M. F. Veiga for her help in the experiments.
References
1. Aba MA. Hormonal Interrelationships in Reproduction of Female Llamas and Alpacas. (Doctoral Thesis). Sweden: Swedish University of Agriculture. (1998).
2. Calderón W, Novoa C, Franco E. Exámen de la Preñez en la alpaca. Lima: Cuarto Boletín Extraordinario, IVITA. (1970). p. 43–48.
3. Fernandez-Baca S, Hansel W, Novoa C. Embryonic mortality in the Alpaca. Biol Reprod. (1970) 3:243–51. doi: 10.1093/biolreprod/3.2.243
4. Aba MA, Forsberg M, Kindahl H, Sumar J, Edqvist LE. Endocrine changes after mating in pregnant and non-pregnant llamas and alpacas. Acta Vet Scand. (1995) 36:489–98.
5. Adam CL, Moir CE, Shiach P. Plasma progesterone concentrations in pregnant and non-pregnant llamas (Lama glama). Vet Record. (1989) 125:618–20.
6. Sumar JB. Removal of the ovaries or ablation of the corpus luteum and its effect on the maintenance of gestation in the alpaca and llama. Acta Vet Scand Supl. (1988) 83:133–41.
7. Alarcon V, Sumar JB, Riera Foote WC. Comparison of three methods of pregnancy diagnosis in alpacas and llamas. Theriogenology. (1990) 34:1119–27. doi: 10.1016/S0093-691X(05)80011-1
8. Aller J, Alberio R, Rebuffi G. Pregnancy and age of gestation diagnosis by means rectal palpation and ultrasonography in llamas (Lama glama). Archiv Zootecnia. (1998) 47:43–50.
9. Bravo WP, Mayta MM, Ordoñez CA. Growth of the conceptus in alpacas. Am J Vet Res. (2000) 61:1508–11. doi: 10.2460/ajvr.2000.61.1508
10. Parraguez VH, Cortéz S, Gazitúa FJ, Ferrando G, MacNiven V, Raggi LA. Early pregnancy diagnosis in alpaca (Lama pacos) and llama (Lama glama) by ultrasound. Anim Reprod Sci. (1997) 47:113–21. doi: 10.1016/S0378-4320(96)01630-2
11. Picha Y, Tibary A, Memon M, Kasimanickam R, Sumar JB. Chronology of early embryonic development and embryo uterine migration in alpacas. Theriogenology. (2013) 79:702–8. doi: 10.1016/j.theriogenology.2012.11.027
12. Tibary A, Campbell A, Pearson L. Evolution of embryo transfer in domestic animals. Spermova. (2013) 3:1–9.
13. Powell SA, Smith BB, Timm KI, Menino AR. Estradiol production by preimplantation blastocysts and increased serum progesterone following estradiol treatment in llamas. Anim Reprod Sci. (2007) 102:66–75. doi: 10.1016/j.anireprosci.2006.10.002
14. Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci. (1982) 55:32–42.
15. Ginther OJ. How ultrasound technologies have expanded and revolutionized research in reproduction in large animals. Theriogenology. (2014) 81:112–25. doi: 10.1016/j.theriogenology.2013.09.007
16. Ginther OJ, Gastal EL, Gastal MO, Utt MD, Beg MA. Luteal blood flow and progesterone production in mares. Anim Reprod Sci. (2007) 99:213–20. doi: 10.1016/j.anireprosci.2006.05.018
17. Silva LA, Gastal EL, Beg MA, Ginther OJ. Changes in vascular perfusion of the endometrium in association with changes in location of the embryonic vesicle in mares. Biol Reprod. (2005) 72:755–61. doi: 10.1095/biolreprod.104.036384
18. Silva M, Urra F, Ratto M. Uterine endometrial vascularization during ovarian follicular growth in llamas: the effect of estradiol plasma concentration. Theriogenology. (2018) 106:164–9. doi: 10.1016/j.theriogenology.2017.10.027
19. Urra F, Ratto MH, Silva M. Evaluation of the effect of mating, intrauterine deposition of raw seminal plasma or seminal plasma purified β-NGF on endometrial vascularization in llamas. Theriogenology. (2019) 125:18–23. doi: 10.1016/j.theriogenology.2018.10.007
20. Trasorras VL, Chaves MG, Neild DM, Gambarotta M, Aba M, Agüero A. Embryo transfer technique: factors affecting the viability of the corpus luteum in llamas. Anim Reprod Sci. (2010) 121:279–85. doi: 10.1016/j.anireprosci.2010.06.004
21. Tibary A, Anouassi A. Theriogenology in Camelidae. Anatomy, physiology, pathology and artificial breeding. In: Reproductive Physiology in female camelidae. Actes Éditions, Rabat: Institut Agronomique et Vetérinaire Hassan II (Maroc), printed by Abu Dhabi Printing and Publishing Company. (1997).
22. Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. (2006) 163:670–5. doi: 10.1093/aje/kwj063
23. Taylor R. Interpretation of the correlation coefficient: a basic review. J Diagnostic Med Sonogr. (1990) 6:35–9. doi: 10.1177/875647939000600106
24. Sumar JB. Embryo transfer in domestic South American camelids. Anim Reprod Sci. (2013) 136:170–7. doi: 10.1016/j.anireprosci.2012.10.029
25. Vaughan J, Mihm M, Wittek T. Factors influencing embryo transfer success in alpacas-A retrospective study. Anim Reprod Sci. (2013) 136:194–204. doi: 10.1016/j.anireprosci.2012.10.010
26. Trasorras VL, Carretero MI, Neild DM, Chaves MG, Giuliano SM, Miragaya MH. Production, preservation, and transfer of South American Camelid embryos. Front Vet Sci. (2017) 4:190. doi: 10.3389/fvets.2017.00190
27. Nieto-Olmedo P, Martín-Cano FE, Gaitskell-Phillips G, Ortiz-Rodríguez JM, Peña FJ, Ortega-Ferrusola C. Power Doppler can detect the presence of 7–8 day conceptuses prior to flushing in an equine embryo transfer program. Theriogenology. (2020) 145:1–9. doi: 10.1016/j.theriogenology.2020.01.015
28. Lasheen ME, Badr HM, Kandiel MMM, Abo El-Maaty AM, Samir H, Farouk M, et al. Predicting early pregnancy in Egyptian buffalo cows via measuring uterine and luteal blood flows, and serum and saliva progesterone. Trop Anim Health Prod. (2018) 50:137–42. doi: 10.1007/s11250-017-1413-6
29. Mansour GM, Hussein SH, Abd El Hady RM, Mohammed HF, Abd El Gawad MM, AbouGabal AI, et al. Uterine artery flow velocity waveform (FVW) type and subendometrial vascularity in recurrent pregnancy loss. J Maternal Fetal Neonatal Med. (2020) 33:527–32. doi: 10.1080/14767058.2018.1495190
30. Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. Elevated blood flow resistance in uterine arteries of women with unexplained recurrent pregnancy loss. Hum Reprod. (2002) 17:190–4. doi: 10.1093/humrep/17.1.190
31. Rossal LP, Bellver Pradas J, Escudero E, Gaytán J, Pellicer A. Ecografía-Doopler en el estudio de la Implantación. Rev Iberoamericana Fertilidad Reprod Humana. (2002) 19:309–17.
32. Honnens A, Voss C, Herzog K, Niemann H, Rath D, Bollwein H. Uterine blood flow during the first 3 weeks of pregnancy in dairy cows. Theriogenology. (2008) 70:1048–56. doi: 10.1016/j.theriogenology.2008.06.022
33. Fernandez-Baca S, Hansel W, Saatman R, Sumar JB, Novoa C. Differential luteolytic effects of right and left uterine horns in the Alpaca. Biol Reprod. (1979) 20:586–95. doi: 10.1095/biolreprod20.3.586
34. Fernández-Baca S, Sumar JB, Novoa C, Leyva V. Relación entre la ubicación del cuerpo lúteo y la localización del embrión en la alpaca. Revista Investigaciones Pecuarias. (1973) 2:131–5.
35. Powell SA, Smith BB, Timm KI, Menino AR. Expression of estrogen receptors α and β in the corpus luteum and uterus from non-pregnant and pregnant llamas. Mol Reprod Dev. (2007) 74:1043–52. doi: 10.1002/mrd.20684
36. Sumar JB, Leyva V. Relationship between location of the corpus luteum and location of the embryo in the llama (Lama glama). In Res. Proyectos Investigación, Univ. Nac. Mayor de San Marcos. Lima, Perú. (1979).
37. Bianchi CP, Meikle A, Benavente MA, Álvarez MA, Trasorras VL, Miragaya MH, et al. Oestrogen and progesterone receptors and COX-2 Expression In Endometrial Biopsy Samples During Maternal Recognition Of Pregnancy In Llamas (Lama glama). Reprod Domestic Anim. (2015) 50:980–8. doi: 10.1111/rda.12618
38. Mattioli M, Barboni B, Turriani M, Galeati G, Zannoni A, Castellani G, et al. Follicle activation involves vascular endothelial growth factor production and increased blood vessel extension. Biol Reprod. (2001) 65:1014–9. doi: 10.1095/biolreprod65.4.1014
39. Moor RM, Hay MF, Seamark RF. The sheep ovary: regulation of steroidogenic, haemodynamic and structural changes in the largest follicle and adjacent tissue before ovulation. J Reprod Fertil. (1975) 45:595–604. doi: 10.1530/jrf.0.0450595
40. de Tarso SGS, Apgar GA, Gastal MO, Gastal EL. Relationships between follicle and corpus luteum diameter, blood flow, and progesterone production in beef cows and heifers: preliminary results. Anim Reprod. (2016) 13:81–92. doi: 10.21451/1984-3143-AR797
41. de Tarso SGS, Gastal GDA, Bashir ST, Gastal MO, Apgar GA, Gastal EL. Follicle vascularity coordinates corpus luteum blood flow and progesterone production. Reprod Fertil Dev. (2017) 29:448–57. doi: 10.1071/RD15223
42. Murdoch WJ, Kirk EA van. Luteal dysfunction in ewes induced to ovulate early in the follicular phase. Endocrinology. (1998) 139:3480–4. doi: 10.1210/endo.139.8.6137
43. Ginther OJ. Reproductive biology of the mare: basic and applied aspects, 2nd edn. (1992). Available online at: https://agris.fao.org/agris-search/search.do?recordID=US19930099223 (accessed August 20, 2020).
44. Ishak GM, Bashir ST, Gastal MO, Gastal EL. Pre-ovulatory follicle affects corpus luteum diameter, blood flow, and progesterone production in mares. Anim Reprod Sci. (2017) 187:1–12. doi: 10.1016/j.anireprosci.2017.09.003
45. Silva LA, Gastal EL, Gastal MO, Beg MA, Ginther OJ. Relationship between vascularity of the preovulatory follicle and establishment of pregnancy in mares. Anim Reprod. (2006) 3:339−46. Available online at: https://www.animal-reproduction.org/journal/animreprod/article/5b5a6080f7783717068b47cd (accessed August 20, 2020).
46. Mann GE. Corpus luteum size and plasma progesterone concentration in cows. Anim Reprod Sci. (2009) 115:296–9. doi: 10.1016/j.anireprosci.2008.11.006
47. Adams GP, Sumar JB, Ginther OJ. Form and function of the corpus luteum in llamas. Anim Reprod Sci. (1991) 24:127–38. doi: 10.1016/0378-4320(91)90088-H
48. Gallelli MF, Bianchi C, Zampini E, Trasorras V, Gambarotta M, Miragaya M. Corpus luteum vascularization during the maternal recognition of pregnancy in llamas (Lama glama). Reprod Domestic Anim. (2020) 55:13588. doi: 10.1111/rda.13588
49. Wiltbank MC, Dysko RC, Gallagher KP, Keyes PL. Relationship between blood flow and steroidogenesis in the rabbit corpus luteum. J Reprod Fertil. (1988) 84:513–20. doi: 10.1530/jrf.0.0840513
50. Fernández A, Ulloa-Leal C, Silva M, Norambuena C, Adams GP, Guerra M, et al. The effect of repeated administrations of llama ovulation-inducing factor (OIF/NGF) during the peri-ovulatory period on corpus luteum development and function in llamas. Anim Reprod Sci. (2014) 149:345–52. doi: 10.1016/j.anireprosci.2014.08.001
51. Ulloa-Leal C, Bogle OA, Adams GP, Ratto MH. Luteotrophic effect of ovulation-inducing factor/nerve growth factor present in the seminal plasma of llamas. Theriogenology. (2014) 81:38. doi: 10.1016/j.theriogenology.2014.01.038
52. Norambuena MC, Hernández F, Maureira J, Rubilar C, Alfaro J, Silva G, et al. Effects of leptin administration on development, vascularization and function of Corpus luteum in alpacas submitted to pre-ovulatory fasting. Anim Reprod Sci. (2017) 182:28–34. doi: 10.1016/j.anireprosci.2017.04.006
53. Rawy MS, Derar DR, El-Sherry TM, Megahed GA. Characterisation of follicular and luteal blood flow in female dromedary camel induced to ovulate using GnRH analogue. J Camel Pract Res.(2012) 19:269–75.
54. Rawy MS, Derar RI, El-Sherry TM, Megahed GA. Plasma steroid hormone concentrations and blood flow of the ovarian structures of the female dromedary (Camelus dromedarius) during growth, dominance, spontaneous ovulation, luteinization and regression of the follicular wave. Anim Reprod Sci. (2014) 148:137–44. doi: 10.1016/j.anireprosci.2014.05.004
55. Baumgartner U. Farbdopplersonographische untersuchung der Arteria uterina und des Corpus luteum beim Rind. Munchen: Tierarztliche Fakultat Der Ludwig-Maximilians-Universitat Munchen (1998) 67–89.
56. Herzog K, Brockhan-Lüdemann M, Kaske M, Beindorff N, Paul V, Niemann H, et al. Luteal blood flow is a more appropriate indicator for luteal function during the bovine estrous cycle than luteal size. Theriogenology. (2010) 73:691–7. doi: 10.1016/j.theriogenology.2009.11.016
57. Herzog K, Voss C, Kastelic JP, Beindorff N, Paul V, Niemann H, et al. Luteal blood flow increases during the first 3 weeks of pregnancy in lactating dairy cows. Theriogenology. (2011) 75:549–54. doi: 10.1016/j.theriogenology.2010.09.024
58. Ferrer MS. Diagnosis of pregnancy and evaluation of high-risk pregnancy. In: Cebra C, Anderson DE, Tibary A, Van Saun RJ, Johnson V, and LaRue W, editors. Llama and Alpaca Care. Medicine, Surgery, Reproduction, Nutrition, and Herd Health. Linda Duncan (2014). p. 250–6. doi: 10.1016/B978-1-4377-2352-6.00022-5
Keywords: camelids, embryo transfer, Doppler ultrasonography, pregnancy, biotechnologies
Citation: Zampini EG, Gallelli MF, Chaves MG, Neild DM, Gambarotta M, Miragaya MH and Trasorras VL (2020) Uterine and Corpus Luteum Blood Flow Evaluation Prior to Uterine Flushing in Llama Embryo Donors. Front. Vet. Sci. 7:597960. doi: 10.3389/fvets.2020.597960
Received: 23 August 2020; Accepted: 12 October 2020;
Published: 17 November 2020.
Edited by:
Khalid El Allali, Agronomic and Veterinary Institute Hassan II, MoroccoReviewed by:
Dale Kelley, Oklahoma State University, United StatesTom Stout, Utrecht University, Netherlands
Copyright © 2020 Zampini, Gallelli, Chaves, Neild, Gambarotta, Miragaya and Trasorras. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enzo G. Zampini, ZWd6YW1waW5pQGZ2ZXQudWJhLmFy
 Enzo G. Zampini
Enzo G. Zampini María F. Gallelli
María F. Gallelli María G. Chaves
María G. Chaves Deborah M. Neild
Deborah M. Neild Mariana Gambarotta3
Mariana Gambarotta3 Marcelo H. Miragaya
Marcelo H. Miragaya Virginia L. Trasorras
Virginia L. Trasorras