- 1Department of Veterinary Physiology, Biochemistry and Pharmacology, University of Jos, Jos, Nigeria
- 2vHive, School of Veterinary Medicine, University of Surrey, Guildford, United Kingdom
- 3Zoetis-ALPHA Initiative, Zoetis, Zaventem, Belgium
Peste des petits ruminants (PPR) is a highly contagious viral disease of sheep and goats with high mortality. The disease is of considerable economic importance in countries such as Tanzania, where small ruminant products are important for sustainable livelihoods. This review assesses current knowledge regarding the epidemiology of PPRV in Tanzania, highlighting the challenges with respect to control and suggesting possible interventions. Thirty-three articles were identified after literature searches using Google Scholar and PubMed. Studies revealed that PPRV is endemic in sheep and goats in Tanzania, although seropositivity has also been reported in cattle, camels, buffalo, Grant's gazelle, wildebeest and impala, but with no clinical manifestation. Three lineages (lineage II to IV) of PPRV have been identified in Tanzania, implying at least two separate introductions of the virus. Diagnosis of PPR in Tanzania is mostly by observation of clinical signs and lesions at post mortem. Risk factors in Tanzania include age, sex, species, and close contact of animals from different farms/localities. Although there is an efficacious vaccine available for PPR, poor disease surveillance, low vaccine coverage, and uncontrolled animal movements have been the bane of control efforts for PPR in Tanzania. There is need for collaborative efforts to develop interventions to control and eradicate the disease. The establishment of a national reference laboratory for PPR, conduct of surveillance, the development of high-quality DIVA vaccines, as well as execution of a carefully planned national vaccination campaign may be key to the control and subsequent eradication of PPR in Tanzania and achieving the global goal of eradicating PPR by 2030.
Introduction
Peste des petits ruminants (PPR) is a highly contagious and acute viral disease of sheep and goats, with sub-clinical manifestation in cattle, pigs, and camel. The disease has also been reported in some wildlife species including Dorcas gazelles (Gazella dorcas) (1), Nubian ibex (Capra nubiana), Laristan sheep (Ovis vignei laristanica), and gemsbok (Oryx gazelle) (2). The disease is characterized by fever, anorexia, nasal and ocular discharges, sores in the mouth, pneumonia, profuse diarrhea, and often death (3). Reported morbidity and mortality rates have varied between 90–100% and 50–100%, respectively (2). PPR has also been associated with a high rate of abortion in infected goats (4). Consequently, PPR is a major constraint to small ruminant production in Africa (5, 6) and is thus of high economic importance, especially in areas with a high reliance on small ruminant products (7).
PPR is caused by peste des petits ruminants virus (PPRV), species Small ruminant morbillivirus (SRMV), a member of the genus Morbillivirus, in the family Paramyxoviridae (8, 9). It is closely related to other members of the genus, including rinderpest virus, measles virus, and canine distemper virus (8, 10). The virus is highly contagious, easily transmitted by direct contact of healthy animals with the secretions and/or excretions from infected animals, or by contact with infected fomites (2, 11). PPRV exists as one serotype, but sequence analysis of the nucleoprotein (N) gene and the fusion protein (F) gene has revealed four genetically distinct lineages (10, 12). Lineages I and II are mainly found in West and Central Africa; lineage III is found mainly in East Africa, Yemen and Oman; and lineage IV is found across the Arabian Peninsula, the Middle East, southern Asia and recently, in several African territories (10, 13, 14).
The geographical spread of PPR is wide. The disease was first identified in West Africa in the 1940s (15, 16), and has since been observed in North and Central Africa, the Middle East, and parts of East Africa and Asia (17, 18) and Europe (19). In East Africa, PPRV was first isolated in Ethiopia in 1991 (20), although sick goat herds in the Afar region of Ethiopia were suspected to have PPR much earlier in 1977 (21, 22). In Tanzania, PPR was officially confirmed in 2008 (23, 24). However, Karimuribo et al. (23) suggested that the disease had been in circulation in Tanzania for at least 4 years previously, as farmers had reported “rinderpest-like” syndromes in domestic small ruminants, supported by clinicopathological reports and sero-prevalence data. PPR has since been reported in goats, sheep, and camels in Tanzania (25–28).
Similar to other African countries, the impact of PPR on agriculture in Tanzania has wide implications. Agriculture is a mainstay of Tanzania's economy, with approximately one fifth of the agriculture-derived economy emanating from the livestock subsector (29, 30). About 22% of total household income in Tanzania is from livestock rearing, and ~60% of rural household incomes come from livestock activities (29). Cattle, goats, and sheep constitute a large share of the animals reared by Tanzanian households as sources of protein and livelihood (31), with sheep and goats accounting for about 22 percent of meat consumed in Tanzania (32). Goat and sheep are the species of choice for pastoralists, due to their hardiness and ability to withstand the harsh arid and semi-arid climates. They are mostly kept under extensive management systems with communal grazing and sometimes housing (32).
Currently a global initiative driven by the Food and Agriculture Organization of the United Nations (FAO) and the World Organisation for Animal Health (OIE) exists to eradicate PPR by 2030 (33). For this to be attainable, it is important to understand the specific epidemiological features of the disease and identify the socio-economic factors that must be considered to stop the transmission of the disease (34). This review is aimed at updating knowledge on the epidemiology of PPR in Tanzania, one of the focus countries for the African Livestock Productivity and Health Advancement (A.L.P.H.A.) initiative, which aims to advance livestock health and productivity in sub Saharan Africa. This article investigates the occurrence and distribution of PPR in Tanzania, the circulating strains, risk factors, economic impacts, control and prevention strategies, and challenges to control of PPR. Additionally, this review aims to identify the challenges and research gaps to inform future control efforts, so that small ruminant production may be improved in this region of East Africa.
Methods
Literature searches were conducted in PubMed and Google Scholar. Grey literature was obtained using Google Search and the official websites of FAO and OIE (www.fao.org and www.oie.int). The search terms used were “PPR Tanzania” and “Peste des petits ruminants AND Tanzania.” All searches were carried out between September 2019 and July 2020. First, title and abstract were reviewed to determine their eligibility. For eligible articles, full text was subsequently reviewed while non-eligible articles were excluded.
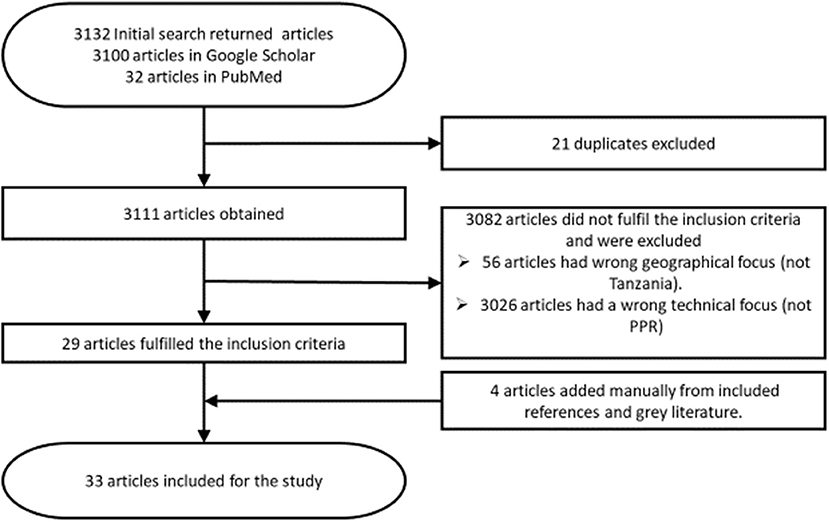
Eligible articles were those published about peste des petits ruminants in Tanzania within the last 16 years (2004–2020), published in or translated to the English language. Only articles concerning case reports, reviews, outbreaks, risk factors, economic losses, control measures, and prevalence of PPR in Tanzania were considered relevant. Additionally, conference papers and theses relating to the topic were included if they were not published in a peer reviewed journal at the time of review. Articles were excluded if they had a geographical focus other than Tanzania or focused on a different disease. Editorials, letters to the editor, opinions or commentaries without original data were also excluded. Data extracted from eligible articles included clinical signs, diagnosis, occurrence, distribution and circulating strains, risk factors, economic losses, control, prevention, and challenges of PPR in Tanzania. The process through which articles were sourced, identified, and selected for this review is shown in Figure 1.
Results
Selected Studies
Thirty-three articles were eligible for this review, 24 were research articles, and one was a review article (Supplementary Material 1). Additionally, there were two conference papers, four theses, and two technical reports.
Clinical signs
Two studies described the clinical signs of PPR in Tanzania and suggest that goats were more susceptible to PPR than sheep, with sheep exhibiting a milder form of the disease (14, 35). The main symptoms of PPR described included anorexia, emaciation, severe depression, fever (40–41°C), diarrhea, muco-purulent nasal, and ocular discharge and erosive and necrotic stomatitis (14, 35). Abortion and nodular lesions were also observed, which were not reported to be common in neighboring Kenya (35). Additionally, when performing post-mortem examination of confirmed cases of PPR, Muse et al. (14) observed lung congestion and consolidation, and increased thickness of inter-alveolar walls, indicating pneumonia.
Diagnosis
In the reviewed studies, diagnosis of PPR in Tanzania was mostly by observation of clinical signs and lesions at post-mortem, followed by monoclonal antibody-based competitive enzyme-linked immunosorbent assay (cELISA) for the detection of PPRV antibodies to determine a previous or current infection (26, 36–40, 50). Additionally, some of the studies also utilized confirmatory molecular methods for the detection of PPRV genome (27, 36, 41–43).
Samples collected for testing included swabs of conjunctival, nasal and oral discharges and ulcers, whole blood, and serum samples for serology (27, 36, 41–43). Portions of intestines, lungs, and lymph nodes were also collected and homogenized for the detection of viral RNA (41, 42). Real-time reverse transcription polymerase chain reaction (rRT-PCR), targeting the PPRV nucleoprotein (N) gene, was used to identify the presence of PPRV genome in buffy coat, homogenized tissue samples, and nasopharyngeal and ocular swabs of suspected cases (12, 27, 36, 41–45). Additionally, phylogenetic analysis based on the N gene has been utilized to determine the PPRV lineage and to establish epidemiological relationships (12, 36, 41, 44). The immunocapture enzyme-linked immunosorbent assay (IC-ELISA) for the rapid identification of PPRV antigen (46), recommended by OIE (47), was not reported to have been used in any of the reviewed articles.
Serological tests performed in the reviewed studies were mostly ELISA techniques such as the competitive PPRV specific anti-H monoclonal based ELISA (c-ELISA) as recommended by the OIE (27, 28, 39, 43, 48–50). The c-ELISA detects antibodies to confirm that the animal has been exposed to PPRV at some point in their lifetime. However, due to the vaccines currently used in Tanzania (live attenuated Nigerian strain 75/1 vaccine) these tests are not able to differentiate between previously infected or vaccinated animals (51).
Occurrence and Distribution
Seven studies reported the occurrence and distribution of PPR in Tanzania (12, 24–26, 43, 49, 52). The studies show PPR to be endemic in goat and sheep populations throughout Tanzania, with several outbreaks reported in different regions of the country (26, 43). Limited evidence of PPRV infection has been observed in wild small ruminants (such as dik-dik, gazelle etc.) and these were reported to be restricted to areas in close proximity with livestock in the Serengeti ecosystem of northern Tanzania, indicating a spill over of infection from livestock populations in Ngorongoro district (24, 26, 52). Seropositivity without clinical manifestation has been observed in cattle, camels, buffalo, Grant's gazelle (Nanger granti), wildebeest, and impala sampled in Ngorongoro district in northern Tanzania (24, 25, 52).
Outbreak History
Eight of the selected studies discussed events that surround the history of PPR outbreaks in Tanzania. Following the serological evidence of PPRV infection in Kenya and Uganda in 1994, the first nationwide serological screening was performed in Tanzania in 2000. Over 3,000 serum samples were screened for PPRV antibodies using the competitive ELISA (cELISA) and all cELISA results were negative (26, 41). A confirmed PPR outbreak in Kenya in August 2006, coupled with reports of clinical signs resembling PPR and high mortality amongst sheep and goats in Ngorongoro, northern Tanzania in December 2007 prompted another investigation (36, 49). Clinical and pathological investigations performed in the Ngorongoro district in March 2008 yielded inconclusive results from 112 sheep and goats, whilst serological investigation was negative for PPR (36). As high mortality persisted amongst the sheep and goat populations in Ngorongoro and the neighboring Mara district, a new investigation confirmed the presence of PPR in Ngorongoro in June 2008, where 129/404 serum samples tested positive for PPR antibody (26, 36). Phylogenetic analysis of isolated PPRV from this investigation identified it as a member of lineage III, the most abundant lineage in eastern Africa (36). Spiegel and Havas (53) suggested that the emergence of PPR in Tanzania in 2008 may have been related to the humanitarian crisis in Kenya in 2007, caused by a highly contested election that led to widespread violence and the displacement of citizens into refugee camps in northern Tanzania. This may have contributed to the introduction of PPRV to Tanzania, due to increased transboundary animal and human movement (2). However, retrospective serological analysis performed by Karimuribo et al. (23) using serum samples collected in 2004 suggested the presence of PPRV in northern Tanzania before 2008, and therefore the time of the true emergence of PPR in Tanzania is unknown.
It was believed that PPR was confined to northern Tanzania until 2009 (42). Negative results were observed in retrospective serological analysis performed using archived sera samples collected from small ruminants for Rift Valley fever surveillance in Mtwara and Lindi regions of southern Tanzania in 2007. Although the sampling strategy of this study was not adequate to confirm absence of infection, these results support the theory that PPR may have been introduced in these regions thereafter (54). PPR was first reported in southern Tanzania in December 2009, in Likuna, a village in the southern Newala district, suspected to be transmitted via goats purchased for Christmas and New Year festivities from Pugu livestock market in the outskirts of Dar es Salaam (14, 36). Since then, outbreaks of PPR have been reported in Tandahimba and Newala districts of Mtwara region of southern Tanzania in 2011 (43), in Ngorongoro and Mvomero districts in northern and eastern Tanzania (respectively) in 2012 (41), and in the Loliondo area in Ngorongoro district of Northern Tanzania in 2016 (27).
Sero-Prevalence
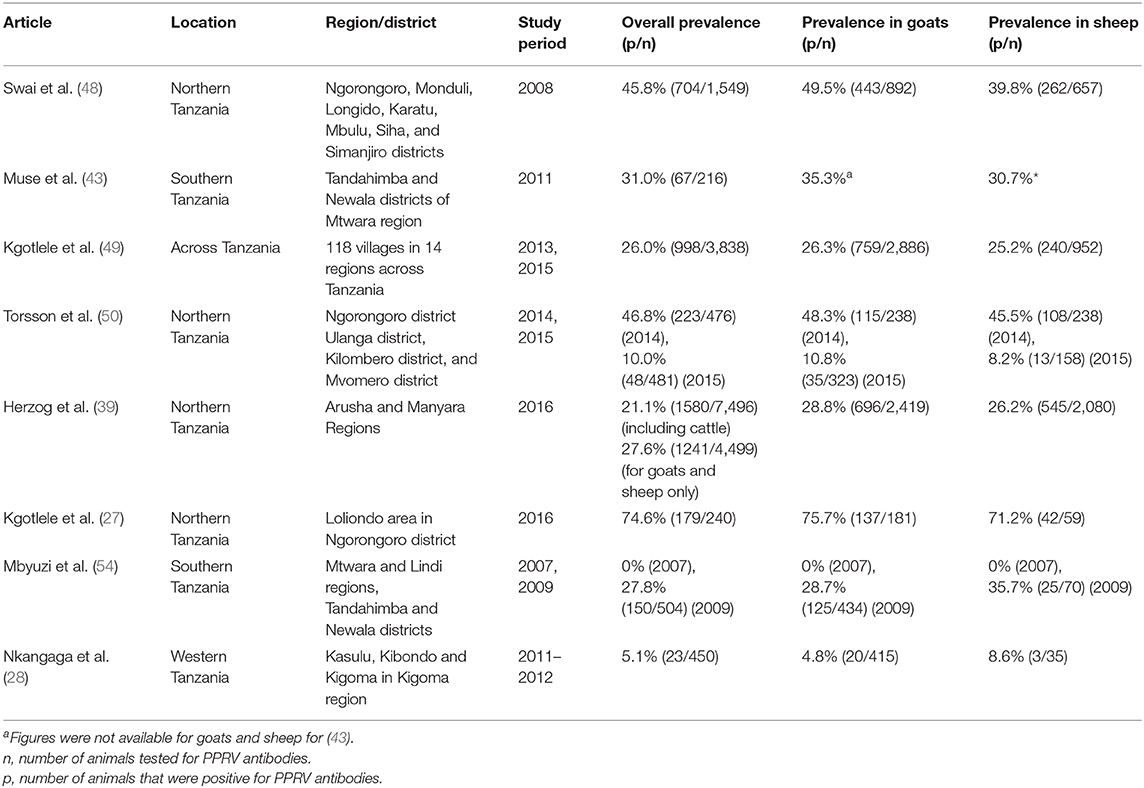
The sero-prevalence of PPR in Tanzania was reported in six of the studies performed between 2008 and 2016 and results are summarized in Table 1. The national prevalence of PPR was estimated in a study performed using samples collected in 2013 and 2015 as 26.0% with a true prevalence estimated as 27.1% (95% confidence interval: 25.6–28.5%), although prevalence differed widely by region, varying from 2.6% in Katavi region to 67.3% in Arusha and 70.0% in Morogoro (49). Indeed, the authors suggested that the high sero-prevalence observed may have been due to previous PPR vaccination in these regions. A study performed by the same authors in 2016 (27) also observed a high sero-prevalance (74.6%) in Arusha region, however, they reported no history of PPR vaccination, according to records from the District Veterinary Office.
Torsson et al. (50) observed a decrease in the sero-prevalence of PPR from 49.3% in 2014 to 10.0% in 2015, in a study performed at the wildlife–livestock interface in Ngorongoro district in the northern Arusha region, and Ulanga, Kilombero, and Mvomero districts in the south-eastern Morogoro region. The authors attributed the difference in sero-prevalence to vaccination that was performed in the Morogoro and Mtwara regions prior to sample collection in 2014, and therefore it is likely that the high seropositivity was influenced by vaccine-induced antibodies, compared with a population containing more naïve susceptible animals (3–12 months of age) during the 2015 sample collection.
Kgotlele et al. (49) reported that the sero-prevalence of PPR did not differ significantly between goat (26.3%) and sheep (25.2%) populations. However, Swai et al. (48) and Nkangaga et al. (28) observed a significantly higher sero-prevalence in goats when compared to sheep (Table 1).
Circulating Strains of PPRV
Only 4/33 of the eligible studies characterized the strains of PPRV present in Tanzania. Kgotlele et al. (41) carried out phylogenetic analysis based on the N gene of PPRV, on nasal and ocular swabs and whole blood samples obtained from PPR cases in northern and eastern Tanzania. They identified lineage III, with a high genetic identity to PPRVs from Sudan and Ethiopia. Jones et al. (45) also identified PPRV lineage III in samples collected in Ngorongoro District in 2015, which clustered with isolates from Uganda, Kenya and Democratic Republic of Congo. Additionally, Misinzo et al. (12) identified lineage II and IV from goats in the 2011 PPR outbreak in southern Tanzania (52). Therefore, this suggests at least three separate introductions of PPR into Tanzania.
Risk Factors
The risk factors for PPRV infection were investigated by eight of the eligible studies, using questionnaires and sero-prevalence data. The risk factors identified as major contributors to PPR occurrence in Tanzania included communal grazing and housing (14, 42, 55, 56); the practice of selling sick animals at cheap prices and bought by livestock keepers for slaughtering in other villages (14); the mixing of infected with healthy animals in markets; and poor access to veterinary services (14).
Torsson et al. (50) reported that female sheep and goats may be at higher risk of PPR than males because they are kept longer on the farms and therefore have a longer risk period for PPRV exposure. Additionally, a higher prevalence of PPR was reported in pastoral (primarily livestock) management systems, compared to agropastoral systems (a mix of crop and livestock) in Northern Tanzania potentially indicating pastoral management as a risk factor (36, 39, 40, 48). Mbyuzi et al. (57) observed a significantly higher incidence of PPR as reported by farmers in the rainy than the dry season. Additionally, Mdetele et al. (58) reported a significantly higher seroprevalence of PPR in semi-arid and coastal agro-ecological zones in Tanzania, when compared to the plateau ecological zones, suggesting coastal, and semi-arid regions are high risk ecological zones. The practice of grazing sheep and goats in close proximity to or on wildlife grazing areas was also shown to increase the risk of PPR occurrence in wild ruminants (24, 52).
Control and Prevention
PPR control programs initiated by the Tanzanian government were discussed by five of the reviewed studies. Between 2006 and 2008, an estimated 64,661 animals were culled in Tanzania, in attempts to control PPR (59). In response to the incursion of PPR in Tanzania in 2008, the United Republic of Tanzania Ministry of Livestock and Fisheries carried out mass (blanket) vaccination of sheep and goats in the Northern and Lake Zones bordering Kenya through the Vaccination for Control of Neglected Animal Diseases in Africa (VACNADA) project, funded by the European Union Food Facility (37). The VACNADA project achieved 71.1% seroconversion following vaccination, which according to Baron et al. (60), may have been enough to successfully prevent PPR transmission. Despite this, PPR was observed a few months later in southern Tanzania in 2009 and proceeded to spread across the country, including to northern Tanzania (14, 36, 42, 43). Since then several vaccination campaigns have been executed, including in northern Tanzania in 2010 (23), in small ruminants along livestock marketing routes in 2011, and in herds in the area around Mikumi National Park in 2013 (61). The Nigerian strain 75/1 PPR vaccine is often used for PPR control in Tanzania, and other Southern African Development Community (SADC) member countries (26, 41). Karimuribo et al. (23) reported that farmers in Tanzania used antibiotics to treat clinical cases of PPR in their flock.
Challenges for the Control of PPR
Despite numerous vaccination campaigns, PPR has spread throughout most of Tanzania. Two articles outlined the challenges hindering the control of PPR in Tanzania. Torsson et al. (26) highlighted low awareness among small ruminant farmers, traders, and transporters; uncontrolled livestock movements; poor availability of diagnostic tools, poor surveillance and reporting; and a lack of capacity to enforce regulations as major constraints in the control of PPR. In addition to uncontrolled livestock movement, Kivaria et al. (36) reported that poor zoo-sanitary habits by farmers and a lack of proper local and national control strategies are the main factors responsible for the persistence of PPRV in Tanzania.
Economic Impact
The economic losses attributed to PPR in Tanzania were reported by one grey literature report, two theses and a review article. Economic losses may be due to depletion of the small ruminant population, by mortalities associated with the disease, or by culling as a control measure (59). Other economic losses may result from the cost of medication, vaccination, veterinary and labor services, a reduced market value due to poor body condition, and the embargo on livestock markets imposed by authorities (44, 51, 59). A study in 2012 in Tandahimba and Ulanga districts in southern Tanzania found that the outbreaks of PPR reduced the average value of small ruminants by 10%, caused a decrease in flock size, and increased the inputs and risks of small ruminant production (26). This resulted in a loss of potential income and a reduced ability of the flock to support household livelihood (by ~30%). Consequently, the estimated total loss of income to PPR was estimated to be TZS 335,420 (155 Euro) per household per year, amounting to a cumulative national loss in excess of TZS 200 billion (92 million Euro) per year (26).
Discussion
There is a dearth of literature on the status of PPR in Tanzania, indicated by the low number of eligible articles obtained for this review. Reviewed studies have shown that the incursion of PPR into Tanzania in 2008 may be directly linked with the emergence and spread of PPR in neighboring Kenya in 2006 (53). A pointer to this is the fact that the first report of PPR in Tanzania was an outbreak in Ngorongoro district, bordering Kenya (36, 53), and the strain of PPRV isolated belonged to lineage III, the same lineage predominant in Kenya, and other countries in East Africa at that time (36, 62). Subsequent isolation of PPRV belonging to lineage II and IV (12, 52) suggest that PPRV may have been imported into the country on more than one occasion (12, 36). Lineage II PPRV in Tanzania may have come from Uganda (12, 36), however, the origin of Lineage IV may be difficult to discern as it is widely spread across the world and in East Africa (63). Of the eight countries bordering Tanzania, PPR has been reported in four: Kenya, Uganda, Democratic Republic of Congo and Burundi (64, 65). Indeed, the existence of an informal cross border livestock trade in the eastern and southern African regions (66, 67) presents a continuous risk of PPR incursion, persistence and spread among these countries and beyond (51, 65, 68, 69).
Studies reviewed show that PPR is endemic throughout Tanzania, and it has had devastating effects on the small ruminant population and the livelihoods of pastoralists across the country over the last several years (36). This is attributable to the high transmissibility and morbidity of PPR (2), which has resulted in its rapid spread in small ruminant populations through large areas of Africa and Asia within the past 20 years (70). Evidence of interspecies transmission of PPR has been observed in several studies (1, 71). Munir (72) reported that most epidemics in wild small ruminants appear to originate from nearby infected domestic sheep and goats and although there is no plausible evidence of self-sustaining PPRV infection in wild ruminant populations, the potential importance of wildlife in the epidemiology of the disease cannot be ignored. The endemicity of PPR therefore poses a threat, not only to the pastoralists and their livelihoods, but potentially also to the conservation of wildlife and endangered wild small ruminant species (24, 52, 73).
Events/activities that bring together flocks/herds from different farms/localities or introduce sick animals to healthy ones have been identified as major risk factors for PPR in Tanzania and Kenya (35). These activities include communal grazing and housing, the mixing of infected animals with healthy animals in livestock markets, and the introduction of recently purchased or rustled animals to a herd. Similar risk factors for PPR have been identified by other studies in Djibouti (74), Chad (75), India (76), and Pakistan (77, 78). Poor access to veterinary services was identified as a risk factor for PPR in Tanzania (14), and is the bane of livestock production in most of Africa (79). There is a lack of veterinarians or community animal health workers in rural Tanzania, the hub of small ruminant production (29, 80). Consequently, PPR control in rural Tanzania is not highly prioritized (68).
The yearly economic losses attributable to PPR worldwide are enormous (33). Losses due to PPR identified in this review include: mortalities associated with the disease, reduced market value caused by poor body condition, culling, the cost of medication, vaccination, veterinary and labor services, and the cost of embargo on livestock markets imposed by authorities. These agree with those identified in studies from other PPR endemic countries for example in Ethiopia (81, 82), Kenya (83), India (84), and globally (33). The estimated total national loss of income to PPR (92 Million Euros per year) is a huge burden to the Tanzanian economy and underscores the need to eliminate the disease in the country (26).
Control of PPR may be achieved by culling, confinement of infected animals, biosecurity measures to reduce infectious fomites, refusal of imports of sheep and goats from regions suffering outbreaks, and mass vaccination (85). In addition to mass/blanket vaccination, it is also important to target vaccination and sero-surveillance activities at the borders with other PPR endemic areas/countries, to establish immune belts and prevent importation of outbreaks (86). Since the suspected incursion of PPR into Tanzania in 2008, the disease has continued to spread throughout the country, and is now endemic in most regions, despite vaccination campaigns. Mdetele et al. (37) reported a significant increase in antibody detected between pre- and post-vaccination goat and sheep in Northern Tanzania, which suggests that the vaccine may be effective in an outbreak. It is likely therefore, that the inability of vaccination programs to effectively contain the disease may be attributed to other factors such as poor coverage of vaccination programs, lack of control of livestock movement, and the high fecundity due to the dynamic nature of small ruminant populations (26, 87). Herd immunity levels required for successful prevention of PPR transmission is in the range of 70–90% (60), and previous vaccination campaigns in Tanzania may have fallen short of this estimate. Continuous effort is required to maintain high levels of immunity to prevent transmission, especially in small ruminants with a short generation time and high turnover of new/naïve animals (87). Additionally, interference of maternal immunity in young animals, poor vaccine quality, and deficiency in the maintenance of cold chain may also cause vaccination failure (82). Consequently, the reasons for vaccine failure and the persistence of disease transmission in Tanzania should be elucidated. Investigations should be encouraged to further evaluate the barriers to vaccine use, and factors that may affect vaccine efficacy and uptake, including the maintenance of cold-chain storage, and the correct administration. Control by vaccination requires that farmers are aware of the benefits, and that they and their veterinary extension advisors appreciate that frequency of vaccination is related to herd dynamics. Additionally, proper animal identification is necessary for traceability, adequate vaccination coverage, and accurate sero-monitoring (88, 89). Establishing herd status through clinical history and serological testing would be advantageous provided that laboratory access and costs can be managed.
Adequate surveillance of PPR is vital for control and to inform vaccination programs, as demonstrated in countries with successful PPR control policies such as Morocco (90). Indeed, the epidemiological studies accessed for this work covered only few districts/areas of Tanzania, leaving huge areas without data on the status of PPR. For this review, searches were done online only, thus theses, articles and reports not available online were not used for this study. Consequently, the methods used to collect data for this review may have resulted in bias in the study locations, and data from certain locations may have been exempted from this study.
A major hinderance to adequate surveillance is the inability of most antibody tests to distinguish between infected and vaccinated animals (91). This may be overcome with the use of vaccines with DIVA (Differentiating Infected from Vaccinated Animals) capability with their accompanying diagnostic tests, allowing for the discrimination of infected and vaccinated animals (10, 91). This is important for proper planning, execution, and evaluation of control programs (86, 91, 92). Additionally, the use of low-cost, easy to use, point of care diagnostic techniques, and alternative non-invasive sample types may improve surveillance (93–95). At present, there is no official national reference laboratory for PPR in Tanzania, however, the Center for Infectious Diseases and Biologicals (CIDB) of the Tanzania Veterinary Laboratories Agency (TVLA) performs routine testing for PPR and has recently joined a twinning project with OIE Reference Laboratories to improve capacity for PPR diagnosis and expertise (96). International collaborations with organizations such as OIE and FAO should be sought, with local efforts to solve this problem if the target of eradicating PPR globally by 2030 is to be achieved.
This review demonstrates the endemicity of PPR in Tanzania that has major socio-economic impacts on pastoralists and agro-pastoralists in the country, and consequently to the local economy. Uncontrolled animal movement, poor vaccination coverage, mixing of herds/flocks from different farms/localities and sick with healthy animals have aided the transmission and persistence of the disease. Interventions are required to control and eradicate PPR in Tanzania which may be achieved by the collaboration of stakeholders, including: farmers, the Tanzanian government, international organizations (such as FAO and OIE), researchers, and multinational veterinary pharmaceutical companies. An effective widespread/national vaccination campaign must be planned and executed; along with policies aimed at improving awareness of the disease, improving diagnostics, surveillance, disease reporting, and controlling livestock movement; to arrest the spread of the virus and stop the disease incursion into neighboring countries, and achieve the global goal of eradicating PPR by 2030.
Author Contributions
AE, EM, and RA: conceptualization. AE, RA, EI, and BA: methodology. EI and AO: analysis. EI, BA, RA, AE, AC, AO, EM, and GV: writing and review. AC and GV: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Zoetis—Africa Livestock Productivity and Health Advancement (ALPHA) Initiative, co-funded by Zoetis and the Bill & Melinda Gates Foundation (Grant No. OPP1165393).
Conflict of Interest
The authors declare that this study received funding from Zoetis and the Bill and Melinda Gates Foundation. The funder (Zoetis) had the following involvement with the study: three co-authors (AO, GV, and EM) were Zoetis employees and were involved in study design and the writing of this article.
Acknowledgments
The authors would like to thank the following for their support. We grateful to Tetiana Miroshnychenko of the Zoetis-ALPHA Initiative Zaventem team and Dr. Isaac Odeyemi from Zoetis Outcomes Research team for guidance. We thank Adam Trish of the vHive team at the University of Surrey and Dr. Abubakar Bala Muhammad of Life Stock Management Services Limited, for administrative assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.592662/full#supplementary-material
References
1. Asil RM, Ludlow M, Ballal A, Alsarraj S, Ali WH, Mohamed BA, et al. First detection and genetic characterization of peste des petits ruminants virus from dorcas gazelles “Gazella dorcas” in the Sudan, 2016-2017. Arch Virol. (2019) 164:2537–43. doi: 10.1007/s00705-019-04330-w
3. Kulkarni DD, Bhikane AU, Shaila MS, Varalakshmi P, Apte MP, Narladkar BW. Peste des petits ruminants in goats in India. Veterinary Rec. (1996) 138:187. doi: 10.1136/vr.138.8.187
4. Abubakar M, Ali Q, Khan HA. Prevalence and mortality rate of peste des petits ruminant (PPR): possible association with abortion in goat. Trop Animal Health Produc. (2008) 40:317–21. doi: 10.1007/s11250-007-9105-2
5. Mariner JC, Jones BA, Rich KM, Thevasagayam S, Anderson J, Jeggo M, et al. The opportunity to eradicate Peste des Petits Ruminants. J Immunol. (2016) 196:3499–506. doi: 10.4049/jimmunol.1502625
6. Ugochukwu IC, Ezeasor CK, Agina OA, Anyogu DC, Chukwudi IC, Idoko SI, et al. Peste des petits ruminants: aetiology, pathology, immunology, disease status in Africa, diagnosis, control, prevention and treatment: a review. Notulae Scientia Biol. (2019) 11:12–20. doi: 10.15835/nsb11110355
7. Elsawalhy A, Mariner JC, Chibeu D, Wamway H, Wakhusama S, Olaho-Mukani W, et al. Pan African strategy for the progressive control of peste des petits ruminants (Pan African PPR Strategy). Bullet Anim Health Produc Africa. (2011) 58:185–93. doi: 10.4314/bahpa.v58i3.64206
8. Gibbs PJ, Taylor WP, Lawman MJ, Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus Morbillivirus. Intervirology. (1979) 11:268–74. doi: 10.1159/000149044
9. Amarasinghe GK, Ayllón MA, Bào Y, Basler CF, Bavari S, Blasdell KR, et al. Taxonomy of the order Mononegavirales: update 2019. Arch Virol. (2019) 164:1967–80. doi: 10.1007/s00705-019-04247-4
10. Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gener Virol. (2010) 91:2885–97. doi: 10.1099/vir.0.025841-0
11. Parida S, Selvaraj M, Gubbins S, Pope R, Banyard A, Mahapatra M. Quantifying levels of peste des petits ruminants (PPR) virus in excretions from experimentally infected goats and its importance for nascent PPR eradication programme. Viruses. (2019) 11:249. doi: 10.3390/v11030249
12. Misinzo G, Kgotlele T, Muse EA, Van Doorsselaere J, Berg M, Munir M. Peste des petits ruminants virus lineage II and IV from goats in southern tanzania during an outbreak in 2011. Br J Virol. (2015) 2:1–4.
13. Banyard AC, Wang Z, Parida S. Peste des petits ruminants virus, eastern Asia. Emerg Infect Dis. (2014) 20:2176–8. doi: 10.3201/eid2012.140907
14. Muse EA, Karimuribo ED, Gitao C, Misinzo G, Mellau LS, Msoffe PL, et al. Epidemiological investigation into the introduction and factors for spread of Peste des Petits Ruminants, southern Tanzania. Onderstepoort J. Vet Res. (2012) 79:49–54. doi: 10.4102/ojvr.v79i2.457
15. Shaila MS, Shamaki D, Forsyth MA, Diallo A, Goatley L, Kitching RP, et al. (1996). Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 43:149–53. doi: 10.1016/0168-1702(96)01312-3
16. Gargadennec L, Lalanne A. La peste des petits ruminants. Bull des Serv Zootechniques et des Epizooties de l'Afrique Occidentale Francaise. (1942) 5:16–21.
17. Dhar P, Sreenivasa BP, Barrett T, Corteyn M, Singh RP, Bandyopadhyay SK. Recent epidemiology of peste des petits ruminants virus (PPRV). Veterinary Microbiol. (2002) 88:153–9. doi: 10.1016/S0378-1135(02)00102-5
18. Liu F, Wu X, Liu W, Li L, Wang Z. Current perspectives on conventional and novel vaccines against peste des petits ruminants. Veterinary Res. Commun. 38:307–22. doi: 10.1007/s11259-014-9618-x
19. Parida S, Muniraju M, Altan E, Baazizi R, Raj GD, Mahapatra M. Emergence of PPR and its threat to Europe. Small Ruminant Res. (2016) 142:16–21. doi: 10.1016/j.smallrumres.2016.02.018
20. Roeder PL, Abraham G, Kenfe G, Barrett T. Peste des petits ruminants in Ethiopian goats. Trop Anim Health Product. (1994) 26:69–73. doi: 10.1007/BF02239901
21. Pegram RG, Tereke F. Observation on the health of Afar livestock. Ethiop Veterinary J. (1981) 5:11–4.
22. Agga GE, Raboisson D, Walch L, Alemayu F, Tesfaye D, Bahiru G, et al. Epidemiological survey of peste des petits ruminants in Ethiopia: cattle as potential sentinel for surveillance. Front Veterinary Sci. (2019) 6:302. doi: 10.3389/fvets.2019.00302
23. Karimuribo ED, Loomu PM, Mellau LSB, Swai E. Retrospective study on sero-epidemiology of peste des petits ruminants before its official confirmation in northern Tanzania in 2008. Res Opini Anim Veterinary Sci. (2011) 1:184–7.
24. Lembo T, Oura C, Parida S, Hoare R, Frost L, Fyumagwa R, et al. Peste des petits ruminants infection among cattle and Wildlife in Northern Tanzania. Emerg Infect Dis. 19:2037–40. doi: 10.3201/eid1912.130973
25. Swai ES, Moshy W, Mbise E, Kaaya J, Bwanga S. (2011). Serological evidence of camel exposure to Peste des Petits Ruminants virus in Tanzania. Res Opin Anim Veterinary Sci. 1:325–9. doi: 10.1007/s11250-014-0747-6
26. Torsson E, Kgotlele T, Berg M, Mtui-Malamsha N, Swai ES, Wensman JJ, et al. History and current status of peste des petits ruminants virus in Tanzania. Infect Ecol Epidemiol. (2016) 6:1–7. doi: 10.3402/iee.v6.32701
27. Kgotlele T, Chota A, Chubwa CC, Nyasebwa O, Lyimo B, Torsson E, et al. Detection of peste des petits ruminants and concurrent secondary diseases in sheep and goats in Ngorongoro district, Tanzania. Compar Clin Pathol. (2019) 28:755–9. doi: 10.1007/s00580-018-2848-5
28. Nkangaga JJ, Matondo AB, Karimuribo E. Peste des petits ruminants (PPR) in western Tanzania. Tanzania Veterinary J. (2019) 34:44–53.
29. Covarrubias K, Nsiima L, Zezza A. Livestock and livelihoods in Rural Tanzania. A Descriptive Analysis of the 2009 National Panel Survey. (2012).
30. Matthew M, Mruttu H, Gebru G. Animal Health Strategy and Vision for Tanzania. Nairobi: Tanzania Ministry of Agriculture, Livestock and Fisheries and International Livestock Research Institute (ILRI) (2016).
31. Michael S, Mbwambo N, Mruttu H, Dotto MM, Ndomba C, da Silva M, et al. Tanzania Livestock Master Plan Developed by the Tanzania Ministry of Livestock and Fisheries (MLF) and the International Livestock Research Institute (ILRI) Livestock Master Plan Team. (2018). Available online at: https://cgspace.cgiar.org/bitstream/handle/10568/92405/livestockMasterPlan.Tanzania.pdf?sequence=1 (accessed December, 2019).
32. Wilson RT. The Red Meat Value Chain in Tanzania A Report From the Southern Highlands Food Systems Programme. Food and Agriculture Organization of the United Nations (FAO) (2013). p. 14–36.
33. Jones BA, Rich KM, Mariner JC, Anderson J, Jeggo M, Thevasagayam S, et al. The economic impact of eradicating peste des petits ruminants: a benefit-cost analysis. PLoS ONE. (2016) 11:e0149982. doi: 10.1371/journal.pone.0149982
34. Albina E, Kwiatek O, Minet C, Lancelot R, de Almeida RS, Libeau G. Peste des petits ruminants, the next eradicated animal disease?. Veterinary Microbiol. (2013) 165:38–44. doi: 10.1016/j.vetmic.2012.12.013
35. Gitao CG, Kihu SM, Bebora LC, Njenga JM, Wairire GG, Maingi N, et al. Comparison of Peste des petits ruminants (PPR) disease between Tanzania and Kenya. In: The 3rd RUFORUM Biennial Conference. (2012). Entebe.
36. Kivaria FM, Kwiatek O, Kapaga AM, Swai ES, Libeau G, Moshy W, et al. The incursion, persistence and spread of peste des petits ruminants in Tanzania: epidemiological patterns and predictions. Onderstepoort J. Veterinary Res. (2014) 81:593. doi: 10.4102/ojvr.v80i1.593
37. Mdetele D, Mwakabumbe S, Seth M, Madege M. Evaluation of effectiveness of pest des petits ruminants vaccine in Northern Tanzania. Res Opin Anim Veterinary Sci. (2015) 5:401–5.
38. Kamwendo GC. Assessment of the epidemiological status, seroprevalence and molecular detection of Peste des Petits Ruminants in goats and sheep along Tanzania-Malawi Border (Doctoral dissertation) Sokoine University of Agriculture (2016) p. 45.
39. Herzog CM, de Glanville WA, Willett BJ, Kibona TJ, Cattadori IM, Kapur V, et al. Pastoral production is associated with increased peste des petits ruminants seroprevalence in northern Tanzania across sheep, goats and cattle. Epidemiol Infect. (2019) 147:e242. doi: 10.1017/S0950268819001262
40. Herzog CM, de Glanville WA, Willett BJ, Cattadori IM, Kapur V, Hudson PJ, et al. Identifying age cohorts responsible for Peste des petits ruminants virus transmission among sheep, goats, and cattle in northern Tanzania. Viruses. (2020) 12:186. doi: 10.3390/v12020186
41. Kgotlele T, Macha ES, Kasanga CJ, Kusiluka LJM, Karimuribo ED, Van Doorsselaere J, et al. Partial genetic characterization of peste des petits ruminants virus from goats in Northern and Eastern Tanzania. Transbound Emerg Dis. (2014) 61:56–62. doi: 10.1111/tbed.12229
42. Kgotlele T, Kasanga CJ, Kusiluka LJ, Misinzo G. Preliminary investigation on presence of peste des petits ruminants in Dakawa, Mvomero district, Morogoro region, Tanzania. Onderstepoort J Veterinary Res. (2014) 81:2–4. doi: 10.4102/ojvr.v81i2.732
43. Muse EA, Matondo RB, Karimuribo ED, Misinzo G, Mellau LSB, Msoffe PLMAMO GGC. Peste des Petits Ruminants (PPR) outbreak in southern, Tanzania. In: The 3rd RUFORUM Biennial Conference, 24th – 28th September 2012. Entebbe (2012). p. 1–3.
44. Namtimba AM. Seroprevalence and genetic characterisation Of Peste Des Petits Ruminants Virus in selected areas of Tanzania. Morogoro: Sokoine University of Agriculture (2015).
45. Jones BA, Mahapatra M, Chubwa C, Clarke B, Batten C, Hicks H, et al. Characterisation of Peste Des Petits Ruminants Disease in pastoralist flocks in Ngorongoro District of Northern Tanzania and Bluetongue Virus Co-Infection. Viruses. (2020) 12:389. doi: 10.3390/v12040389
46. Abraham G, Berhan A. The use of antigen-capture enzyme-linked immunosorbent assay (ELISA) for the diagnosis of rinderpest and peste des petits ruminants in Ethiopia. Trop Anim Health Product. (2001) 33:423–30. doi: 10.1023/A:1010547907730
47. OIE. Peste des Petits Ruminants (Infection With Peste des Petits Ruminants) in OIE Terrestrial Mannual 2019. (2019). p. 1–16.
48. Swai ES, Kapaga A, Kivaria F, Tinuga D, Joshua G, Sanka P. Prevalence and distribution of Peste des petits ruminants virus antibodies in various districts of Tanzania. Veterinary Res Commun. (2009) 33:927–36. doi: 10.1007/s11259-009-9311-7
49. Kgotlele T, Torsson E, Kasanga CJ. Seroprevalence of Peste Des Petits Ruminants virus from samples collected in different regions of Tanzania in 2013 and 2015. J Veterinary Sci Technol. (2016) 7:1–5. doi: 10.4172/2157-7579.1000394
50. Torsson E, Berg M, Misinzo G, Herbe I, Kgotlele T, Päärni M, et al. Seroprevalence and risk factors for peste des petits ruminants and selected differential diagnosis in sheep and goats in Tanzania. Infect Ecol Epidemiol. (2017) 7. doi: 10.1080/20008686.2017.1368336
51. Nkangaga JJ. Disease Status And Risk Factors for Peste Des Petits Ruminants Along Tanzania-Burundi and Democratic Republic of Congo Border. Morogoro: Sokoine University of Agriculture (2014).
52. Mahapatra M, Sayalel K, Muniraju M, Eblate E, Fyumagwa R, Shilinde S, et al. Spillover of peste des petits ruminants virus from domestic to wild ruminants in the Serengeti ecosystem, Tanzania. Emerg Infect Dis. (2015) 21:2230–4. doi: 10.3201/eid2112.150223
53. Spiegel KA, Havas KA. The socioeconomic factors surrounding the initial emergence of peste des petits ruminants in Kenya, Uganda, and Tanzania from 2006 through 2008. Transbound Emerg Dis. (2019) 66:627–33. doi: 10.1111/tbed.13116
54. Mbyuzi AO, Komba EV, Kimera SI, Kambarage DM. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuro-pneumonia in goats and sheep in the Southern Zone of Tanzania. Prevent Veterinary Med. (2014) 116:138–44. doi: 10.1016/j.prevetmed.2014.06.013
55. Chota A, Shirima G, Kusiluka L. Risk factors associated with Mycoplasma capricolum subspecies capripneumoniae and morbillivirus infection in small ruminants in Tanzania. Trop Anim Health Product. (2019) 51:1807–15. doi: 10.1007/s11250-019-01981-4
56. Herzog CM, de Glanville WA, Willett BJ, Cattadori IM, Kapur V, Hudson PJ, et al. Peste des petits ruminants virus transmission scaling and husbandry practices that contribute to increased transmission risk: an investigation among sheep, goats, and cattle in Northern Tanzania. Viruses. (2020) 12:930. doi: 10.3390/v12090930
57. Mbyuzi AO, Komba EVG, Cordery-Cotter R, Magwisha HB, Kimera SI, Kambarage DM. Descriptive survey of Peste des Petits Ruminants and Contagious Caprine Pleuropneumonia outbreaks in traditional goat flocks in Southern Tanzania: producers concerns, knowledge and attitudes. Livestock Res Rural Dev. (2015) 27:1–9.
58. Mdetele D, Seth M, Kabululu M, Misinzo G. (2020). A comparative study of the sero-prevalence of Peste Des Petits Ruminants Virus among districts of different agro-ecological zones in Tanzania. East Af J Sci Technol Innovat. 1:1–12. doi: 10.37425/eajsti.v1i3.167
59. FAO and OIE. Peste des Petits Ruminants Global Eradication Programme. Rome: The Food and Agriculture Organization of the United Nations and the World Organisation for Animal Health (2016).
60. Baron MD, Diop B, Njeumi F, Willett BJ, Bailey D. Future research to underpin successful peste des petits ruminants virus (PPRV) eradication. J General Virol. (2017) 98:2635–44. doi: 10.1099/jgv.0.000944
61. Roos N. Seroepidemiology of Peste des petits Ruminants in central Tanzania Alongside an Evaluation of Filter Paper as Transport Medium. Uppsala: Swedish University of Agricultural Sciences Faculty (2016).
62. Dundon WG, Kihu SM, Gitao GC, Bebora LC, John NM, Oyugi JO, et al. Detection and genome analysis of a lineage III peste des petits ruminants virus in Kenya in 2011. Transbound Emerg Dis. (2017) 64: 644–50. doi: 10.1111/tbed.12374
63. Kumar KS, Babu A, Sundarapandian G, Roy P, Thangavelu A, Kumar KS, et al. Molecular characterisation of lineage IV peste des petits ruminants virus using multi gene sequence data. Veterinary Microbiol. (2014) 174:39–49. doi: 10.1016/j.vetmic.2014.08.031
64. Tshilenge GM, Walandila JS, Kikukama DB, Masumu J, Balowa LK, Cattoli G, et al. Peste des petits ruminants viruses of lineages II and III identified in the Democratic Republic of the Congo. Veterinary Microbiol. (2019) 239:108493. doi: 10.1016/j.vetmic.2019.108493
65. Dundon WG, Diallo A, Cattoli G. Peste des petits ruminants in Africa: a review of currently available molecular epidemiological data, 2020. Arch Virol. (2020) 165:2147–63. doi: 10.1007/s00705-020-04732-1
66. Little PD. Hidden Value on the Hoof: Cross-Border Livestock Trade in Eastern Africa. Common Market for Eastern and Southern Africa Comprehensive African Agriculture Development Program, Policy Brief number 2, February 2009. Midrand: CAADP (2009).
67. Afrika JG, Ajumbo G. Informal cross border trade in Africa: Implications and policy recommendations. Afr Econ Brief . (2012) 3:1–13.
68. Chazya R, Muma JB, Mwacalimba KK, Karimuribo E, Mkandawire E, Simuunza M. A qualitative assessment of the risk of introducing peste des petits ruminants into northern Zambia from Tanzania. Veterinary Med Int. (2014) 2014:202618. doi: 10.1155/2014/202618
69. Niyokwishimira A, de D, Baziki J, Dundon WG, Nwankpa N, Njoroge C, Boussini H, et al. Detection and molecular characterization of Peste des Petits Ruminants virus from outbreaks in Burundi, December 2017–January 2018. Transbound Emerg Dis. (2019) 66:2067–73. doi: 10.1111/tbed.13255
70. Sghaier S, Cosseddu GM, Hassen SB, Hammami S, Ammar HH, Petrini A, et al. Peste des petits ruminants virus, Tunisia, 2012–2013. Emerg Infect Dis. (2014) 20:2184. doi: 10.3201/eid2012.141116
71. Pruvot M, Fine AE, Hollinger C, Strindberg S, Damdinjav B, Buuveibaatar B, et al. Outbreak of Peste des Petits Ruminants among critically endangered Mongolian Saiga and Other Wild Ungulates, Mongolia, 2016–2017. Emerg Infect Dis. (2020) 26:51. doi: 10.3201/eid2601.181998
72. Munir M. Role of wild small ruminants in the epidemiology of peste des petits ruminants. Transbound Emerg Dis. (2014) 61:411–24. doi: 10.1111/tbed.12052
73. Njeumi F, Bailey D, Soula J, Diop B, Tekola BG. Eradicating the Scourge of Peste Des Petits Ruminants from the World. Viruses. (2020) 12:313. doi: 10.3390/v12030313
74. Moumin G, Moussa C, Teshale S, Gezahegne M. Seroprevalence and risk factors for peste des petits ruminants in sheep and goats in Djibouti. Rev Sci Tech. (2018) 37:961–9. doi: 10.20506/37.3.2899
75. Mahamat O, Doungous T, Kebkiba B, Oumar HA, Oussiguéré A, Yacoub AH, et al. Seroprevalence, geographical distribution, and risk factors of peste des petits ruminants in the Republic of Chad. J Adv Veterinary Animal Res. (2018) 5:420. doi: 10.5455/javar.2018.e293
76. Singh RP. Control strategies for peste des petits ruminants in small ruminants of India. Rev Sci Techni-OIE. (2011) 30:879. doi: 10.20506/rst.30.3.2079
77. Zahur AB, Ullah A, Irshad H, Latif A, Ullah RW, Jahangir M, et al. Epidemiological analysis of Peste des Petits Ruminants (PPR) outbreaks in Pakistan. J Biosci Med. (2014) 2:18. doi: 10.4236/jbm.2014.26004
78. Abubakar M, Rasool MH, Manzoor S, Saqalein M, Rizwan M, Munir M, et al. Evaluation of risk factors for peste des petits ruminants virus in sheep and goats at the Wildlife-Livestock Interface in Punjab Province, Pakistan. BioMed Res Int. (2016) 2016:7826245. doi: 10.1155/2016/7826245
79. Ilukor J. Improving the delivery of veterinary services in Africa: insights from the empirical application of transaction costs theory in Uganda and Kenya. Rev Sci Techn. (2017) 36:279–89. doi: 10.20506/rst.36.1.2628
80. Gustafson CR, VanWormer E, Kazwala R, Makweta A, Paul G, Smith W, et al. Educating pastoralists and extension officers on diverse livestock diseases in a changing environment in Tanzania. Pastoralism. (2015) 5:1–12. doi: 10.1186/s13570-014-0022-5
81. Bedore B, Mustefa M, Tamire M, Geinoro T. Current status of occurrence and socio-economic impacts of peste des petits ruminants virus (PPRV) on small ruminant population in Ethiopia. Am J Epidemiol Public Health. (2019) 3: 12–6.
82. Lyons NA, Jemberu WT, Chaka H, Salt JS, Rushton J. Field-derived estimates of costs for Peste des Petits Ruminants vaccination in Ethiopia. Prevent Veterinary Med. (2019) 163:37–43. doi: 10.1016/j.prevetmed.2018.12.007
83. Kihu SM, Gitao GC, Bebora LC, John NM, Wairire GG, Maingi N, et al. Economic losses associated with Peste des petits ruminants in Turkana County Kenya. Pastoralism. (2015) 5:9. doi: 10.1186/s13570-015-0029-6
84. Thombare NN, Sinha MK. Economic implications of peste des petits ruminants (PPR) disease in sheep and goats: a sample analysis of district Pune, Maharastra. Agric Econ Res Rev. (2009) 22:319–22. doi: 10.22004/ag.econ.57469
85. Kabir A, Abro DHKSH, Kalhoro MS, Yousafzai HA, Shams S, Khan IU, et al. Peste des petits ruminants: a review. Pure Appl Biol. (2019) 8:1214–22. doi: 10.19045/bspab.2019.80063
86. Singh RP, Bandyopadhyay SK. Peste des petits ruminants vaccine and vaccination in India: sharing experience with disease endemic countries. Virus Dis. (2015) 26:215–24. doi: 10.1007/s13337-015-0281-9
87. Bora M, Yousuf RW, Dhar P, Singh RP. An overview of process intensification and thermo stabilization for upscaling of Peste des petits ruminants vaccines in view of global control and eradication. Virus Dis. (2018) 29:285–96. doi: 10.1007/s13337-018-0455-3
88. ElArbi AS, Metras R, Hammami P, Ciss M, Beye A, Diallo A, et al. PPR control in a Sahelian Setting: what vaccination strategy for Mauritania. Front Veterinary Sci. (2019) 6:242. doi: 10.3389/fvets.2019.00242
89. Salih HAM, Elfadil AAM. Preliminary qualitative risk assessment for Peste des petits ruminants (PPR) in sheep exported from Sudan during 2012. J Veterinary Sci Med. (2016) 4:9. doi: 10.13188/2325-4645.1000021
90. Kardjadj M. Epidemiological situation of transboundary animal diseases in North African countries—proposition of a regional control strategy. Trop Anim Health Product. (2018) 50:459–67. doi: 10.1007/s11250-017-1453-y
91. Pasick J. Application of DIVA vaccines and their companion diagnostic tests to foreign animal disease eradication. Anim Health Res Rev. (2004) 5:257–62. doi: 10.1079/AHR200479
92. Sen A, Saravanan P, Balamurugan V, Rajak KK, Sudhakar SB, Bhanuprakash V, et al. Vaccines against peste des petits ruminants virus. Exp Rev Vac. (2010) 9:785–96. doi: 10.1586/erv.10.74
93. Clarke BD, Islam MR, Yusuf MA, Mahapatra M, Parida S. Molecular detection, isolation and characterization of Peste-des-petits ruminants virus from goat milk from outbreaks in Bangladesh and its implication for eradication strategy. Transbound Emerg Dis. (2018) 65:1597–604. doi: 10.1111/tbed.12911
94. Bataille A, Kwiatek O, Belfkhi S, Mounier L, Parida S, Mahapatra M, et al. Optimization and evaluation of a non-invasive tool for peste des petits ruminants surveillance and control. Sci Rep. (2019) 9:4742. doi: 10.1038/s41598-019-41232-y
95. Rajko-Nenow P, Flannery J, Arnold H, Howson EL, Darpel K, Stedman A, et al. A rapid RT-LAMP assay for the detection of all four lineages of Peste des Petits Ruminants virus. J Virol Methods. (2019) 274:113730. doi: 10.1016/j.jviromet.2019.113730
Keywords: peste des petit ruminants, PPRV, small ruminant morbillivirus, sheep, goats, small ruminant
Citation: Idoga ES, Armson B, Alafiatayo R, Ogwuche A, Mijten E, Ekiri AB, Varga G and Cook AJC (2020) A Review of the Current Status of Peste des Petits Ruminants Epidemiology in Small Ruminants in Tanzania. Front. Vet. Sci. 7:592662. doi: 10.3389/fvets.2020.592662
Received: 07 August 2020; Accepted: 29 October 2020;
Published: 25 November 2020.
Edited by:
Adama Diallo, Centre de Coopération Internationale en Recherche Agronomique pour le Développement, FranceReviewed by:
William Dundon, International Atomic Energy Agency, AustriaRabindra Prasad Singh, Indian Veterinary Research Institute (IVRI), India
Copyright © 2020 Idoga, Armson, Alafiatayo, Ogwuche, Mijten, Ekiri, Varga and Cook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abel B. Ekiri, YWIuZWtpcmlAc3VycmV5LmFjLnVr
 Enokela S. Idoga
Enokela S. Idoga Bryony Armson
Bryony Armson Ruth Alafiatayo
Ruth Alafiatayo Adah Ogwuche3
Adah Ogwuche3 Abel B. Ekiri
Abel B. Ekiri Alasdair J. C. Cook
Alasdair J. C. Cook