
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 20 January 2021
Sec. Animal Nutrition and Metabolism
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.586813
This article is part of the Research Topic The Use of Growth Promoters and their Alternatives in Livestock Production View all 9 articles
Gut inflammatory bowel diseases (IBDs) links to animal medicinal feed and antibiotic-resistance are fueling major economic impacts in the agricultural livestock industry. New animal feeds that promote livestock gut health and control of IBDs without antibiotics are needed. This study investigates the effects of mesobiliverdin IXα (MBV)-enriched microalgae spirulina extracts on the growth performance, blood parameters, intestinal morphology, and gut microbiota of broilers. A total of 288 1-day-old broiler chicks (Arbor Acres) were randomly allotted to six dietary treatments (4 pens/treatment and 12 birds/pen). The dietary treatments comprised a basal diet as control (CON), basal diet plus 0.05 and 0.1% microalgae extract as low and high dose, respectively (SP1 and SP2), basal diet plus 0.05 and 0.1% MBV-enriched microalgae extract as low and high dose, respectively (MBV-SP1 and MBV-SP2), and basal diet plus 0.1% amoxicillin (AMX). All treated animals showed no significant differences in live weight, average daily gain, and feed efficiency compared to control animals. Histological examination showed that AMX treatment decreased the villi lengths of the duodenum and ileum below control villi length (P < 0.05) while MBV-SP1 and particularly MBV-SP2 increased villi lengths in the duodenum, jejunum, and ileum above AMX -treatment lengths (P < 0.05). The Firmicutes/Bacteroidetes ratio increased in the cecum of broilers fed AMX (P < 0.05) while SP2, MBV-SP1, and MBV-SP2-fed animals showed (in order) increasing ratios up to the AMX level. The abundance of bacterial species of the genus Lactobacillus increased in MBV-SP1 and MBV-SP2-fed groups including a striking increase in Lactobacillus salivarius abundance with MBV-SP2 (P < 0.05). Feeding MBV-SP1 and MBV-SP2 decreased the level of pro-inflammatory cytokine IL-6 in plasma of broilers to a greater extent than SP1 and SP2. These results reveal that MBV-enriched microalgae extracts improve the intestinal health and beneficial microflora composition of broilers.
Animal illnesses are globally widespread and challenging clinically, scientifically, and socio-economically (1). Well-known in human diseases, inflammatory bowel diseases (IBDs) are also prevalent and increasingly serious diseases for agricultural livestock. Johne's disease in cattle and other ruminants (2), antibiotic-associated colitis in pigs and horses (3), and necrotic enteritis in chickens (4) are examples of livestock diseases having large impacts on global agro-economies and food security. Complex interactions occur between the gut microbiota and intestinal immune defense systems. Imbalances and disruption of the normal interactions between these systems result in inflammatory responses that characterize IBDs (1, 2, 4, 5). A role for inflammation in IBDs points to anti-inflammatory therapies to combat these diseases (6). However, the majority of anti- inflammatory approaches against IBD either lack effectiveness, are high cost to produce, or have undesirable side effects. For example, a once promising anti-TNFα therapy is expensive and shows loss of response and undesirable side-effects (7). Natural product anti-inflammatories such as curcumin are possibilities though poor bioavailability following ingestion is a limitation (8).
Spirulina microalgae (mainly cyanobacteria Arthrospira platensis and Arthrospira maxima) are grown commercially in large quantities (nearly 3,000 tons per year) for food and feed by many companies worldwide (9). Spirulina not only has potential as a sustainable biofuel, but also as an animal feed (10). Nutrients of spirulina enrichening with protein, carbohydrates, balanced amino acids, carotenoids, fatty acids including γ-linolenic acid, vitamins, and minerals (11, 12). Approximately half of the total spirulina production is currently used for livestock and fish feed. The agricultural use of spirulina as a feedstock is anticipated to gradually grow (9, 13). Furthermore, spirulina has the beneficial effects of anti-oxidation, immunomodulation, and microbial-modulating activities in chickens and pigs, and also good potential for enhancing antibiotic effects (13–15). The cellular protein contents of spirulina and other microalgae are relatively high, and numerous experimental trials with agricultural animals support good productivity, animal health, and product quality with microalgae-supplemented feed (16, 17).
In animals, bioactive tetrapyrrole metabolites are derived by ring cleavage of heme by the enzyme heme oxygenase-1(HO-1). Biliverdin IXα, carbon monoxide, and iron are the initial products from HO-1 action. Biliverdin IXα is subsequently reduced via biliverdin reductase to bilirubin that is excreted in bile. The overall process serves to eliminate heme–which is toxic when accumulated. Biliverdin IXα is also produced by microbes and plants. In microalgae (cyanobacteria and red algae), biliverdin IXα is a precursor to photosensitive tetrapyrroles such as phycocyanobilin and phycoerythrobilin which are chromophores for microalgal light-harvesting systems. Biliverdin IXα, bilirubin and phycocyanobilin are powerful antioxidants (18) and biliverdin IXα and bilirubin are effective cytoprotectants against oxidative stress (19). Biliverdin IXα activates biliverdin reductase to signal downstream pathways for anti-inflammatory cytokine production and activities and suppression of pro-inflammatory gene expression (20). Biliverdin IXα administration has been shown to suppress or protect against several acute and chronic inflammatory diseases such as colitis (21), gastroenteritis (22), sepsis-induced intestinal inflammation and dysmotility (23), and intestinal ischemic/reperfusion injury (24). Thus, biliverdin IXα-based therapies have been suggested for treating human IBD diseases (21, 22).
Though biliverdin IXα as a therapeutic has been considered for over 10 years (19), its clinical use is hampered by insufficient quantity, uncertain purity, and derivation from mammalian materials. As an alternative, mesobiliverdin IXα (MBV), a close biliverdin IXα analog, was synthesized from microalgae phycocyanin with high purity and in large amounts by our research group (25, 26). MBV differs from biliverdin IXα with ethyl groups in place of vinyl groups at positions 3 and 18 of the tetrapyrrole structure. MBV and biliverdin IXα are equally good substrates for human BVR, and they impart similar degrees of cytoprotection against oxidative stress in pancreatic islets (27). MBV thus appears to possess therapeutic potential similar to that of biliverdin IXα with the added benefits of scalable production in large amounts from a non-animal source. However, there is no relevant study on the use of MBV in animal feed to promote animal health. The research proposed here will further test these notions in addition to the main focus of exploring MBV's possible beneficial effects in microalgae-based animal feed.
Two hundred and eighty-eight Arbor Acres broilers were reared from day 1 to day 30 and randomly allotted to 6 dietary treatments, 4 replicates for each treatment, and 12 chicks per replicate. Chickens were fed isonitrogenous and isocaloric corn-soybean meal based on calculated values of the ingredients to meet the recommendations for broilers (27). The birds were reared in floor pens (108 × 52 cm) and bedded with rice hulls. The chickens were illuminated at night. Electric incandescent light was used to keep chicks warm. All chicks were kept at 20 lux of light intensity on 23L:1D photoperiod for the week 1. At day 8, the broilers were exposed to the same light intensity and maintain 17L:7D photoperiod thereafter (28). The basal diet composition in the starter and finisher are shown in Table 1. The diets were in a mash form and provided ad libitum, and the birds had free access to tap water. For dietary treatments, basal diet feed was supplemented with amoxicillin (AMX) (antibiotic growth promoter, 0.1% by weight), Spirulina microalgae extracts SP1 and SP2 (0.05 and 0.1%, respectively by weight), or MBV-enriched microalgae extracts MBV-SP1 and MBV-SP2 (0.05 and 0.1%, respectively, by weight). Microalgae extracts for making SP1 and SP2 were prepared as described previously (25) from Spirulina powder (Arthrospira platensis), purchased from Bio-Alternatives, 834 Richmond Street, Klamath Falls, OR 97601 RMac Enterprises Inc. (https://www.bio-alternatives.net/). MBV-enriched microalgae extracts MBV-SP1 and MBV-SP2 were prepared by exposure of microalgae extracts to metal salts as previously described (26). From high-performance liquid chromatography analyses (25), MBV-SP1 and MBV-SP2 extracts were estimated to contain between 0.28 and 0.84 mg of MBV per g of dry microalgae extract, respectively. These levels of MBV-supplementation to animal feed were previously observed to suppress colitis in mouse model feeding studies (YY Lin, unpublished observations).
The feeding strategy was adapted from the Broiler Management Manual that divided growth into different growth stages. The average body weight, average daily gain, average daily feed intake, and feed efficiency (total weight gain/total feed intake) were calculated from days 1 to 30. At the end of experiment, chickens were sacrificed with an anesthetic and stunned with carbon dioxide. All research was approved by the Tunghai University Institutional Animal Care and Use Committee (IACUC Approval No. 107-6) prior to the start of data collection.
On day 30, intestinal sample from 2 broilers per replicate were freshly collected. Four replicates (eight birds/treatment, n = 8) were used for histological examination. Intestinal proximal segment samples (~3 cm in length) of the duodenum, jejunum and ileum were excised and flushed with PBS to remove the residues. The intestinal samples were fixed in a 10% buffered formalin solution for histopathological examination. Tissues were embedded in paraffin wax blocks, sectioned at 5 μm thickness and stained with hematoxylin and eosin. The villus height was examined in eight samples per group and each sample was photographed in 6 different fields randomly.
For microbiome analysis, cecal contents from broilers per replicated were freshly collected at the end of experiment. Five samples from each treatment were randomly selected and used for microbiota analysis. The DNA was extracted using a commercial kit (QIAamp Fast DNA Stool Mini Kit, QIAGEN, Hilden, Germany) following the instructions of the manufacturer. The quality and quantity of DNA was evaluated on a SIMPLINANOTM spectrophotometer (SimpliNano, 29061711). DNA samples were stored at −20°C until further processing.
The 300 bp paired-end raw reads derived from the 16S ribosomal amplicon sequencing were assembled using FLASH v.1.2.11 (29). De-multiplexing was carried out based on barcode identification (30). The raw reads were required to match the correct barcode and primer. Read pairs were then exported into dataset-specific, oriented files. Barcode files were generated at this step, and facilitate incorporation of the datasets into the QIIME. As a quality control, reads with a Q score less than the threshold (Q < 20) were discarded in the QIIME 1.9.1 pipeline (30). If three consecutive bases were Q < 20, the read was truncated and the resulting read retained in the data set only if it was at least 75% of the original length using split_libraries_fastq.py script in QIIME (31). Sequences were chimera-checked using UCHIME to obtain the effective tags (32, 33) and filtered from the data set before operational taxonomic unit (OTU) clustering at 97% sequence identity using the UPARSE function in the USEARCH v.7 pipeline (34). For each representative sequence, the RDP classifier (v.2.2) algorithm (35) was employed to annotate taxonomy classification based on the information retrieved from the Silva Database v.132 (36, 37). Sequences with one-time occurrence (singletons) or present in only one sample were filtered out.
Samples were grouped according to treatment. Analysis was performed at different taxonomical levels separately (phylum, family, and genus). For statistical analysis, significance of all species among groups at various taxonomic level were detected using differential abundance analysis with a zero-inflated Gussian (ZIG) log-normal model as implemented in the “fitFeatureModel” function of the Bioconductor metagenomeSeq package (38). Statistically significant biomarkers were identified by the use of the LEfSe analysis (39). In brief, LEfSe is an approach based on an algorithm that performs the non-parametric Kruskal-Wallis test to identify bacterial taxa whose relative abundance is significantly different between the control and sample of interest. LEfSe applies LDA to those bacterial taxa identified as significantly different and further assesses the effect size of each differentially abundant taxon. In this study, taxa with LDA score (log 10) > 4 was considered significant.
At the end of experiment, plasma samples from two broilers per replicate (eight birds/treatment, n = 8) were collected from brachial vein and separated through centrifugation at 2,500 × g for 20 min. Plasma from different groups were used to measure the blood biochemistry parameters and inflammation-related cytokines. The samples were diluted with saline, and concentrations of glucose (GLU), triglyceride (TG), cholesterol (CHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL) were measured by biochemistry automatic analyzer (FUJI DRI-CHEM NX 500i, Fujifilm Co., Japan). Total concentration of plasma Interleukin-6 (IL-6) (E12I0006, Shanghai Blue Gene Biotech), and Interleukin (IL-1 β) (E12I0010, Shanghai Blue Gene Biotech) were measured by using an ELISA microplate reader (Epoch2TM, BioTek).
The Kolmogorov-Smirnov test was used to test the normal distribution of the data before statistical analysis was performed. Statistical analyses were performed using GraphPad software (version 5 for Windows). The collected data were tested by means of one-way ANOVA and the mean differences were compared using Tukey's multiple comparison test. Significance was declared at P ≤ 0.05.
During the entire experimental period, the broilers showed good survival rates (Table 2). Live weights, average daily gain, and feed efficiency did not differ for the different dietary treatments (Table 2).
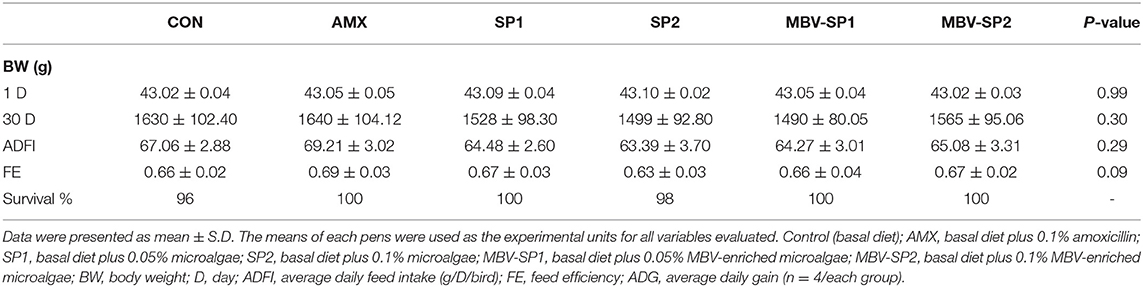
Table 2. Dietary effects of feeding AMX, SP1, SP2, MBV-SP1, and MBV-SP2 on growth performance of broilers.
Histological analysis of intestinal segments showed that AMX treatment decreased villi lengths of the duodenum, jejunum, and ileum below those observed with control (Figure 1). In contrast, MBV-SP1 and MBV-SP2 treatments resulted in increased villi lengths in the duodenum above those observed with control. SP1, SP2, MBV-SP1, and MBV-SP2 treatments showed higher degrees of villi lengthening than observed for AMX treatments with MBV-SP2 showing the highest degree of increased villi lengths (P < 0.05; Figure 1). Representative histological images of chickens of different intestinal segment were presented in Supplementary Figure 1.
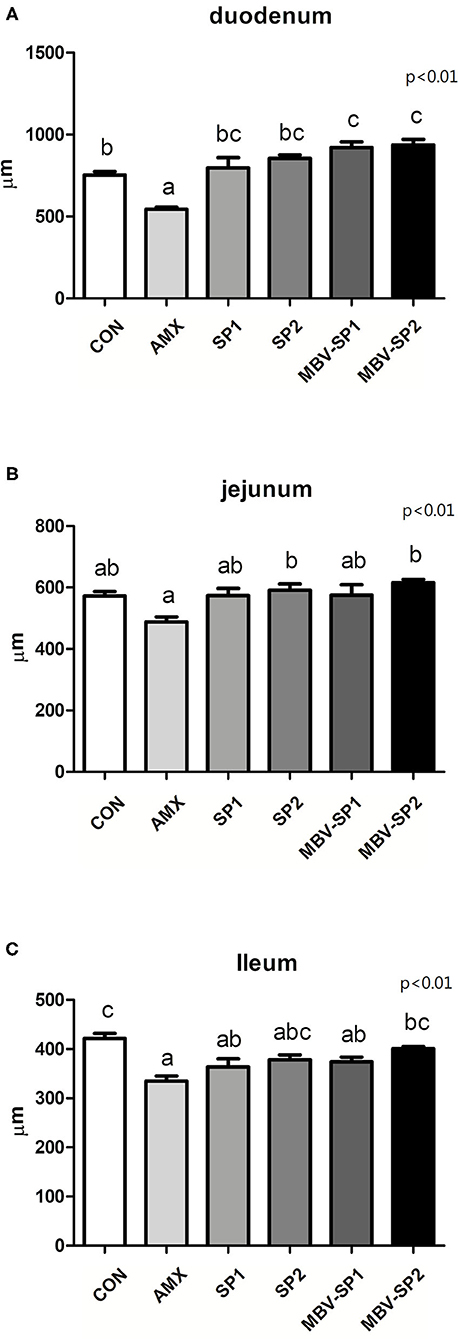
Figure 1. Histological analysis of broiler small intestine after feeding with basal diet (CON), AMX, SP1, SP2, MBV-SP1, and MBV-SP2 of (A) duodenum, (B) jejunum, and (C) ileum. Data were presented as mean ± S.D. and subjected to one-way ANOVA. Different letters indicate significant differences between treatments. Significance was declared when the probability was <5% (P < 0.05; n = 8/each group).
The species accumulation curve (Supplementary Figure 2) indicates an increase in species diversity as the sample size increases. For investigating the microbiota population, the species accumulation curve can be used to assess whether the number of samples provided in the current sequence analysis is sufficient. If the curve rises sharply, a large number of species is found in the community, and when the curve flattens, the species number will not increase with a sample size increase. AMX treatment significantly increased the Firmicutes/Bacteriodetes (F/B) ratio (P < 0.05) while SP1 treatment had no significant impact on the F/B ratio (Supplementary Figure 3). However, SP2, MBV-SP1, and MBV-SP2 treatments increased the F/B ratio.
In order to explore the relative abundance of microbiota (phylum, class, order, family, and genus) we select the top 10 populations of species for relative abundance in each feed treatment group identified by the gene sequence analyses for species annotation. The relative proportions of different taxonomic classes were obtained for each feed treatment (Figure 2). The analyses show that Firmicutes and Bacteroidetes are the most common phyla in chicken ceca, and Proteobacteria and Cyanobacteria account for the remainder (Figure 2A). Changing the feed treatments significantly altered the F/B ratios (Supplementary Figure 3 and Figure 2A). AMX, MBV-SP1, and MBV-SP2 treatments increased the Firmicutes and reduced the Bacteroidetes populations. At the class level, the majority were Clostridia, Bacteroidia, Bacilli, and Negativicutes (Figure 2B), and at the order level, Clostridiales, Bacteroidales, Lactobacillales, and Selenomonadales comprised the majority (Figure 2C). At the family and genus levels, AMX, SP1, SP2, MBV-SP1, and MBV-SP2 each increased the proportions of Lactobacillaceae and Lactobacillus, respectively, with MBV-SP1 and MBV-SP2 having similar and the most dramatic effects (Figures 2D,E). Partial Least Squares Discriminant Analysis (PLS-DA) conducted to examine the functional distinction of microbiota revealed statistically significant discrimination among the groups (Supplementary Figure 4).
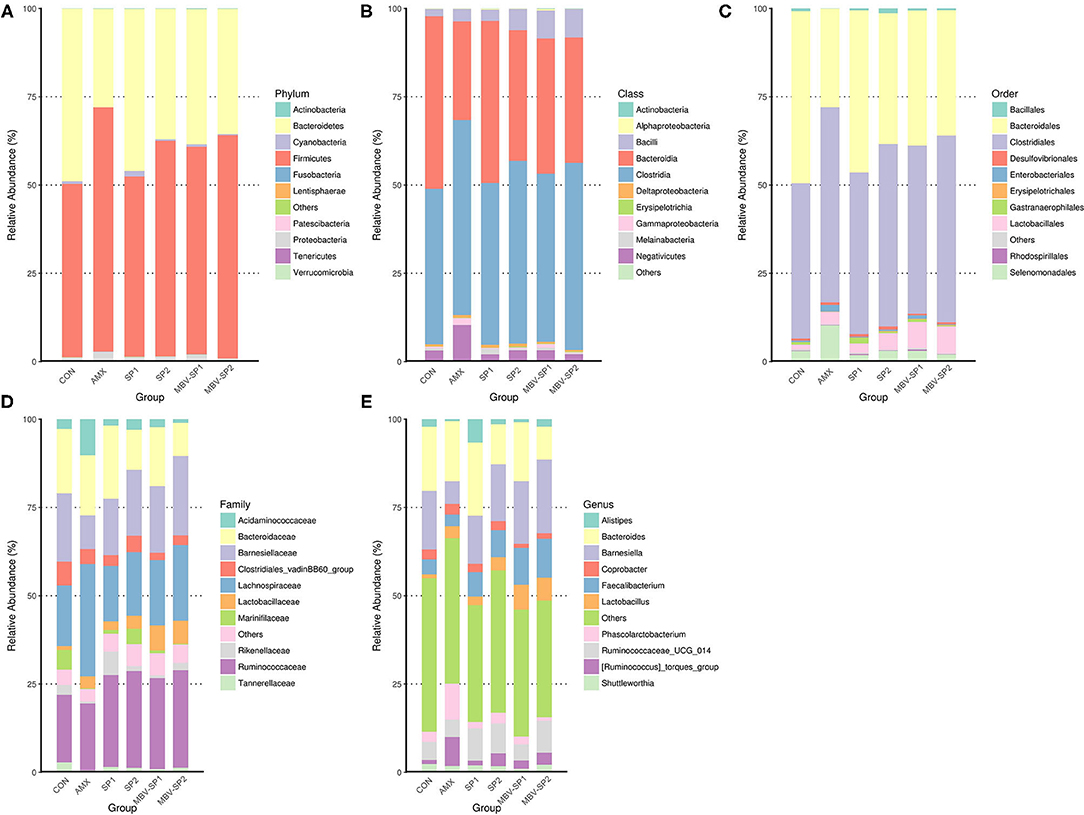
Figure 2. The relatively abundant microbiota in different taxonomic levels. (A) Phylum, (B) Class, (C) Order, (D) Family, and (E) Genus level. Each color represents a different taxonomic unit (n = 5/each group).
LEfSe applies LDA to those bacterial taxa identified as significantly different (P < 0.05) are presented as Figure 3. Statistical analyses of metagenomics profiles were used to perform Welch's t-test between pairs of feed treatment groups to determine which gut bacterial species displayed the largest difference in abundance when comparing two different feeding treatments (P < 0.05; Figures 4A,B). The analyses revealed that the proportion of Lactobacillus salivarius increased to the largest extent when comparing CON vs. MBV-SP2 (P < 0.05) and AMX vs. MBV-SP2 feeding treatments (P < 0.05). The calculated OTU relative abundance of L. salivarius, confirmed that MBV-SP2 is best among all treatments for increasing its abundance (Figure 4C).
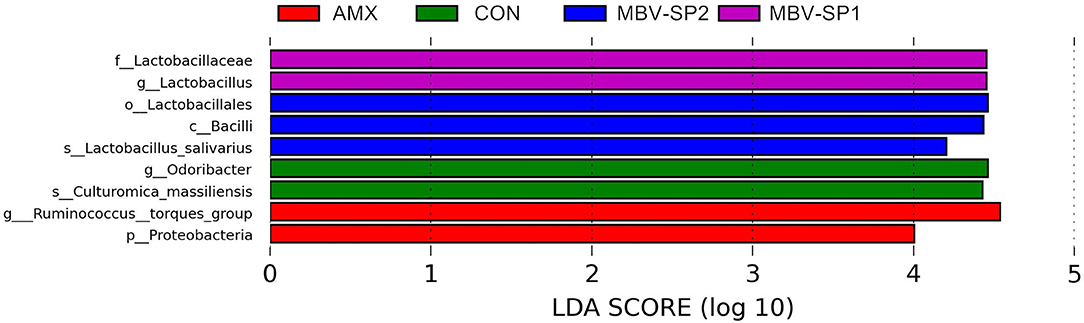
Figure 3. LEfSe analysis showing the most differentially abundant taxa between control and treatment groups. Only taxa with LDA > 4 are shown. The letter in front of the strains indicates the taxon level; p, phylum; c, class; o, order; f, family; g, genus; s, species (n = 5/each group).
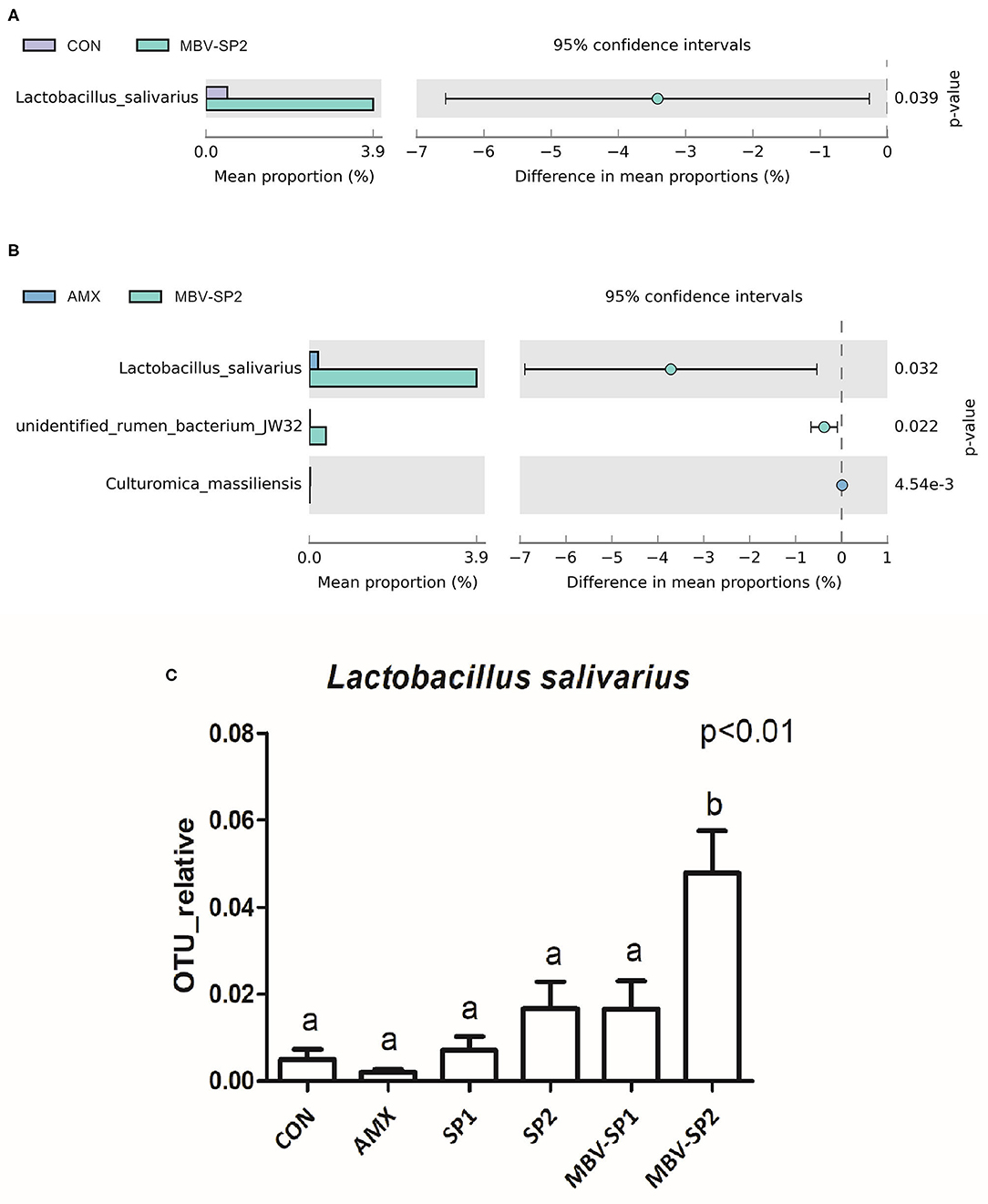
Figure 4. (A) The Welch's test of group CON and MBV-SP2, (B) The Welch's test of group AMX and MBV-SP2, (C) The OTU relative abundance of Lactobacillus salivarius after feeding with basal diet (CON), AMX, SP1, SP2, MBV-SP1, and MBV-SP2. Data were presented as mean ± S.D. and subjected to one-way ANOVA. The mean differences were compared using Tukey's multiple comparison test. Different letters indicate significant differences between treatments. Significance was declared when the probability was <5% (P < 0.05; n = 5/each group).
No significant differences between the blood biochemical values were observed for all of the feeding treatment and control groups (Table 3). It should be noted that a trend of decreased values for LDL (P = 0.1) levels was evident with MBV-SP2 treatment. Cytokine analysis revealed that MBV-SP1 and MBV-SP2 feeding treatment significantly decreased (P < 0.05) blood IL-6–an inflammatory biomarker in chickens–below the control and AMX treatments (Figure 5A). SP1 and SP2 feeding lowered IL-6 as well but to a lesser extent. Another prominent cytokine in chicken, IL-1β did not change levels with any of the feed treatments (Figure 5B). However, the downward trend can be observed in MBV-SP1 and MBV-SP2 feeding treatment.
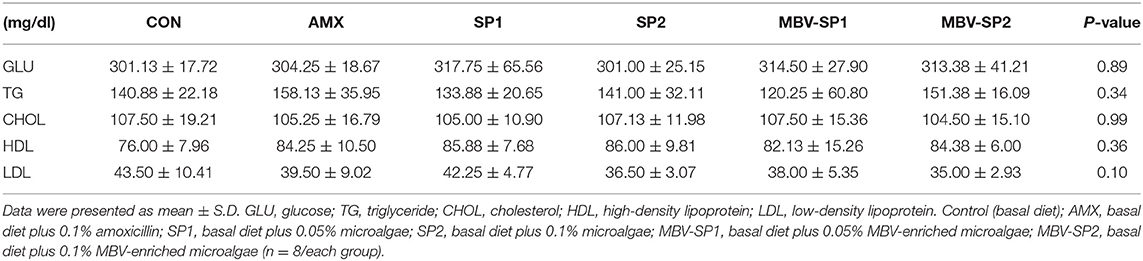
Table 3. Effects of feeding AMX, SP1, SP2, MBV-SP1, and MBV-SP2 on blood biochemistry parameters in broilers.
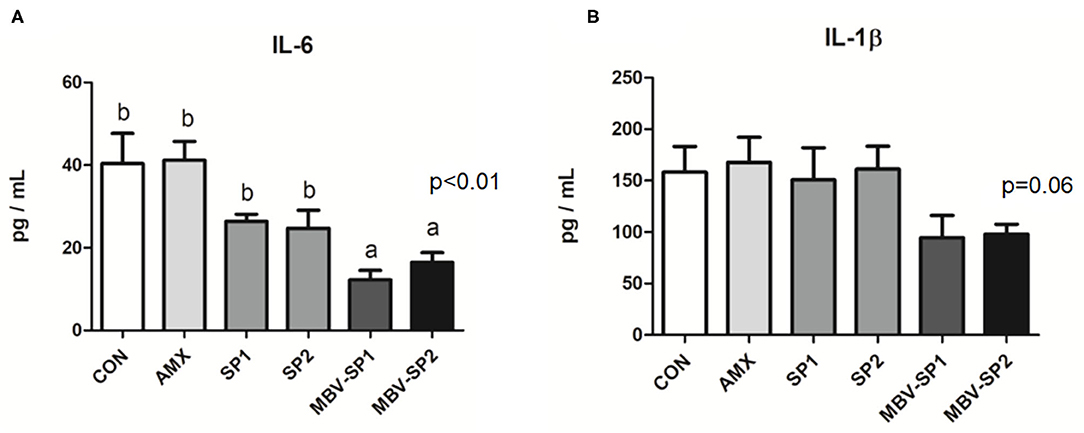
Figure 5. Enzyme-linked immunosorbent assay of IL-6 (A) and IL-1 beta (B) in chicken plasma after feeding with basal diet (CON), AMX, SP1, SP2, MBV-SP1, and MBV-SP2. Data were presented as mean ± S.D. and subjected to one-way ANOVA. The mean differences were compared using Tukey's multiple comparison test. Different letters indicate significant differences between treatments. Significance was declared when the probability was <5% (P < 0.05; n = 8/each group).
Spirulina microalgae is not only used as a raw material for animal and aquatic feed, but it also has microbial-modulating activities, both for Gram-negative and Gram-positive bacteria (40–42). Moreover, spirulina also has beneficial effects on metabolic disease in clinical trial and alleviates adverse impacts due to high ambient temperature that reduces immunity function and increases production of free radicals in broilers (15, 43) which implies spirulina's potential as a nutraceutical in animals and humans. In the current study, we observe that spirulina extract enriched with MBV appears to possess even greater protective and beneficial effects for gut health than spirulina extract alone. This study suggests the possible use of MBV-enriched microalgae as a beneficial or medicinal animal feed or supplement.
Our study found that providing spirulina as feed supplement does not cause adverse growth effects (Table 2), and it alters the gut microbiota (Figures 2, 4). Our results are the first to show that microalgal spirulina extract containing MBV increases the abundance of Lactobacillus, especially L. salivarius in the gut of broilers (Figure 4C). Various studies have demonstrated that L. salivarius is a promising probiotic that produces bacteriocins inhibitory to the growth of other bacteria and occurs in human, porcine and poultry gastrointestinal tracts (44–47). Furthermore, L. salivarius has been shown to modulate inflammatory cytokines against critical gut pathogens Salmonella and Campylobacter jejuni (45, 46, 48). Our results are consistent with these previous findings and further show the improvement of intestinal structure and immune-modulating function by MBV-containing spirulina extract (Figures 1, 5). As for the mechanism underlying the effects of the adopted treatment, no previous published study on the use of MBV in animal feed exists. Biliverdin has been considered as a therapeutic agent for many years (19). However, considering its clinical application, there are insufficient quantities, uncertain purity, and obstacles derived from mammalian materials. As an alternative, our group synthesized MBV from microalgae phycocyanin with high purity and in large amounts. In the current study, we do not clarify the specific mechanisms underlying the effects of MBV-SP treatment. However, two important gut health parameters are correlated with the observed gut microbiota index changes: normal intestinal histopathology (Figure 1) and lowered plasma IL-6 and IL-1β levels (Figure 5) both known to be associated with suppression of intestinal bowel disease (49–52). Therefore, it is speculated that MBV improves gut health via modulation of gut microbiota and lowered circulatory inflammatory cytokines. In recent experiments using pig intestinal cells as a platform and purified MBV, we observed a cytoprotective effect (unpublished data) that is conceivably relevant to our findings reported here.
The current research examines the possibility of a new feed additive of livestock feed devoid of antibiotics and that could promote animal gut health. It exploits recent discoveries about MBV, an analog of the animal metabolite biliverdin IXα known to protect against inflammatory conditions such as IBDs. Future research is aimed at scaled production of MBV-enriched microalgae extract and determination of efficacy toward development of a next- generation animal feed.
Our data reveal MBV-SP1 and particularly MBV-SP2 increased villi lengths in the duodenum, jejunum, and ileum above AMX-treatment lengths. In addition, feeding MBV-SP1 and MBV-SP2 decreased the level of pro-inflammatory cytokine IL-6 in plasma of broilers to a greater extent than SP1 and SP2. The microbiota analysis also showed that MBV-enriched microalgae spirulina have beneficial effects for gut health. In sum, MBV-enriched microalgae extracts may replace the antibiotics used in livestock industry.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: European Bioinformatics Institute, accession no: PRJEB41302.
The animal study was reviewed and approved by Tunghai University.
Y-YL and P-EC designed the research. C-WC, JT, and MA provide materials (microalgae extract and MBV-enriched microalgae extract). Y-YL and P-EC performed the research and analyzed the data. Y-YL wrote the manuscript. C-WC, JT, and Y-YL participated in the revision of the manuscript. All authors contributed to data interpretation and approved the final version of the manuscript.
Funds supporting this study were provided by the Ministry of Science and Technology, Taiwan (project no. 108-2321-B-003-001, 108-2313-B-002-062, and 109-2321-B-003-001), National Taiwan University (project no. NTU-JP-109L7244, NTU-JP-109L7338, and NTU-JP-110L7225), USDA-NIFA Utah Agricultural Experiment Station (Project 1271), and the Utah Science, Technology and Research (USTAR) initiative.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Dr. Yu-Lun Kuo at BIOTOOLS Co. Ltd., in Taiwan for kindly supporting analysis of NGS data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.586813/full#supplementary-material
Supplemental Figure 1. Representative histological images of different intestinal segment. Sections were stained with hematoxylin and eosin and viewed at X40 magnification with scale bars indicating 500 μm.
Supplemental Figure 2. Species accumulation curve. Data were presented as mean ± S.D.
Supplemental Figure 3. Effect of SP1, SP2, MBV-SP1, and MBV-SP2 feed on broiler gut Firmicutes/Bacteroidetes (F/B) ratios. Data were presented as mean ± S.D.
Supplemental Figure 4. Partial Least Squares Discriminant Analysis of the cecal contents. Control (basal diet); AMX, basal diet plus 0.1% amoxicillin; SP1, basal diet plus 0.05% microalgae; SP2, basal diet plus 0.1% microalgae; MBV-SP1, basal diet plus 0.05% MBV-enriched microalgae; MBV-SP2, basal diet plus 0.1% MBV-enriched microalgae.
1. Kirsner JB. Inflammatory bowel diseases at the University of Chicago-early experiences: a personal historical account. Inflamm Bowel Dis. (2005) 11:407–16. doi: 10.1097/01.mib.0000164101.96028.ac
2. Rathnaiah G, Zinniel DK, Bannantine JP, Stabel JR, Gröhn YT, Collins MT, et al. Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne's disease. Front Vet Sci. (2017) 4:187. doi: 10.3389/fvets.2017.00187
3. Sekyere OJ. Antibiotic types and handling practices in disease management among pig farms in Ashanti region, Ghana. J Vet Med. (2014) 2014:531952. doi: 10.1155/2014/531952
4. Arsenault RJ, Li Y, Bell K, Doig K, Potter A, Griebel PJ, et al. Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's Disease. Infect Immun. (2012) 80:3039–48. doi: 10.1128/IAI.00406-12
5. Arsenault RJ, Li Y, Maattanen P, Scruten E, Doig K, Poter A, et al. Altered toll-like receptor 9 signaling in Mycobacterium avium subsp. Paratuberculosis-infected bovine monocytes reveals potential therapeutic targets. Infect Immun. (2013) 81:226–37. doi: 10.1128/IAI.00785-12
6. Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. (2010) 7:37. doi: 10.1186/1742-2094-7-37
7. Reddy JG, Loftus EV Jr. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am. (2006) 35:837–55. doi: 10.1016/j.gtc.2006.09.008
8. Toden S, Theiss AL, Wang X, Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci Rep. (2017) 7:814. doi: 10.1038/s41598-017-00812-6
9. Holman BWB, Malau-Aduli AEO. Spirulina as a livestock supplement and animal feed. J Anim Physiol Anim Nutr. (2013) 97:61523. doi: 10.1111/j.1439-0396.2012.01328.x
10. Lum KK, Kim J, Lei XG. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J Anim Sci Biotechnol. (2013) 4:53. doi: 10.1186/2049-1891-4-53
12. El-Moataaz S, Ismael H, Aborhyem S. Assessment of chemical composition of Spirulina platensis and its effect on fasting blood glucose and lipid profile in diabetic Rats. J High Instit Public Health. (2019) 49:199–211. doi: 10.21608/JHIPH.2019.64463
13. Finamore A, Palmery M, Bensehaila S, Peluso I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxid Med Cell Longev. (2017) 2017:3247528. doi: 10.1155/2017/3247528
14. Neumann C, Velten S, Liebert F. N balance studies emphasize the superior protein quality of pig diets at high inclusion level of algae meal (Spirulina platensis) or insect meal (Hermetia illucens) when adequate amino acid supplementation is ensured. Animals. (2018) 8:172. doi: 10.3390/ani8100172
15. Mirzaie S, Zirak-Khattab F, Hosseini SA, Donyaei-Darian H. Effects of dietary spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas J Anim Sci. (2018) 31:556–63. doi: 10.5713/ajas.17.0483
16. Zheng J, Inoguchi T, Sasaki S, Maeda Y, McCarty MF, Fujii M, et al. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol. (2012) 304:R110–20. doi: 10.1152/ajpregu.00648.2011
17. Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. (2016) 90:1817–40. doi: 10.1007/s00204-016-1744-5
18. Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. (1990) 186:301–9. doi: 10.1016/0076-6879(90)86123-d
19. Soares MP, Bach FH. Heme oxygenase-1: from biology to therapeutic potential. Trends Mol Med. (2009) 15:50–8. doi: 10.1016/j.molmed.2008.12.004
20. Wegiel B, Otterbein LE. Go green: the anti-inflammatory effects of biliverdin reductase. Front Pharmacol. (2012) 3:47. doi: 10.3389/fphar.2012.00047
21. Berberat PO, A-Rahim YI, Yamashita K, Warny MM, Csizmadia E, Robson SC, et al. Heme oxygenase-1-generated biliverdin ameliorates experimental murine colitis. Inflamm. Bowel Dis. (2005) 11:350–9. doi: 10.1097/01.mib.0000164017.06538.8a
22. Gomes AS, Gadelha GG, Lima SJ, Garcia JA, Medeiros JVR, Havt A, et al. Gastroprotective effect of heme-oxygenase 1/biliverdin/CO pathway in ethanol-induced gastric damage in mice. Eur J Pharmacol. (2010) 642:140–5. doi: 10.1016/j.ejphar.2010.05.023
23. Overhaus M, Moore BA, Barbato JE, Behrendt FF, Doering JG, Bauer AJ. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. (2006) 290:G695–703. doi: 10.1152/ajpgi.00152.2005
24. Nakao A, Kaczorowski DJ, Sugimoto R, Billiar TR, McCurry KR. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J Clin Biochem Nutr. (2008) 42:78–88. doi: 10.3164/jcbn.2008013
25. Ito T, Chen D, Chang CWT, Kenmochi T, Saito T, Suzuki S, et al. Mesobiliverdin-IXα enhances rat pancreatic islet yield and function. Front Pharmacol. (2013) 4:50. doi: 10.3389/fphar.2013.00050
26. Takemoto JY, Chen D, Chang CWT, Wood J. Therapeutic meso-biliverdin IXα compositions and associated methods. US Patent. Patent No. US 9119842 B2, U.S. Patent and Trademark Office.
27. National Research Council. Nutrient Requirements of Poultry, 9th ed. Washington, DC: National Academic Press (1994).
28. Rault JL, Clark K, Groves PJ, Cronin GM. Light intensity of 5 or 20 lux on broiler behavior, welfare and productivity. Poult Sci. (2017) 96:779–87. doi: 10.3382/ps/pew423
29. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
30. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6. doi: 10.1038/nmeth.f.303
31. Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. (2013) 10:57–9. doi: 10.1038/nmeth.2276
32. Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. (2011) 21:494–504. doi: 10.1101/gr.112730.110
33. Edgar RC, Hass BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. (2011) 27:2194–200. doi: 10.1093/bioinformatics/btr381
34. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
35. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. (2007) 73:5261–7. doi: 10.1128/AEM.00062-07
36. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
37. Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. (2014) 42:D643–8. doi: 10.1093/nar/gkt1209
38. Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. (2013) 10:1200–2. doi: 10.1038/nmeth.2658
39. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. (2011) 12:R60. doi: 10.1186/gb-2011-12-6-r60
40. Bhowmik D, Dubey J, Mehra S. Probiotic efficiency of Spirulina platensis—stimulating growth of lactic acid bacteria. World J Dairy Food Sci. (2009) 4:160–3.
41. El-Sheekh MM, Daboor SM, Swelim MA, Mohamed S. Production and characterization of antimicrobial active substance from Spirulina platensis. Iran J Microbiol. (2014) 6:112–9.
42. Suganya KSU, Govindaraju K, Kumar VG, Dhas TS, Karthick V, Singaravelu G, et al. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater Sci Eng C Mater Biol Appl. (2015) 47:351–6. doi: 10.1016/j.msec.2014.11.043
43. Serban MC, Sahebkar A, Dragan S, Stoichescu-Hogea G, Ursoniu S, Andrica F, et al. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin Nutr. (2016) 35:842–51. doi: 10.1016/j.clnu.2015.09.007
44. O'Shea EF, O'Connor PM, Raftis EJ, O'Toole PW, Stanton C, Cotter PD, et al. Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. J Bacteriol. (2011) 193:6973–82. doi: 10.1128/JB.06221-11
45. Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, et al. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. (2006) 50:3111–6. doi: 10.1128/AAC.00259-06
46. Zhang J, Deng J, Wang Z, Che C, Li YF, Yang Q. Modulatory effects of Lactobacillus salivarius on intestinal mucosal immunity of piglets. Curr Microbiol. (2011) 62:1623–31. doi: 10.1007/s00284-011-9906-4
47. Shokryazdan P, Faseleh Jahromi M, Liang JB, Ramasamy K, Sieo CC, Ho YW. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE. (2017) 12:e0175959. doi: 10.1371/journal.pone.0175959
48. Ryan KA, O'Hara AM, van Pijkeren JP, Douillard FP, O'Toole PW. Lactobacillus salivarius modulates cytokine induction and virulence factor gene expression in Helicobacter pylori. J Med Microbiol. (2009) 58:996–1005. doi: 10.1099/jmm.0.009407-0
49. Hyams JS, Fitzgerald JE, Treem WR, Wyzga N, Kreutzer DL. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology. (1993) 104:1285–92. doi: 10.1016/0016-5085(93)90336-b
50. Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. (2007) 13:1016–23. doi: 10.1002/ibd.20148
51. Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. (2008) 180:5653–61. doi: 10.4049/jimmunol.180.8.5653
Keywords: gut health, microbiota, mesobiliverdin, spirulina, poultry
Citation: Chang C-WT, Takemoto JY, Chang P-E, AlFindee MN and Lin Y-Y (2021) Effects of Mesobiliverdin IXα-Enriched Microalgae Feed on Gut Health and Microbiota of Broilers. Front. Vet. Sci. 7:586813. doi: 10.3389/fvets.2020.586813
Received: 24 July 2020; Accepted: 22 December 2020;
Published: 20 January 2021.
Edited by:
Einar Vargas-Bello-Pérez, University of Copenhagen, DenmarkReviewed by:
Mabrouk Elsabagh, Kafrelsheikh University, EgyptCopyright © 2021 Chang, Takemoto, Chang, AlFindee and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Yu Lin, eXlsaW5AbnR1LmVkdS50dw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.