
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 30 November 2020
Sec. Animal Nutrition and Metabolism
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.575685
This article is part of the Research Topic Nutritional Intervention for the Intestinal Health of Young Monogastric Animals View all 16 articles
 Cui Ma1,2
Cui Ma1,2 Qiankun Gao1
Qiankun Gao1 Wanghong Zhang1,2
Wanghong Zhang1,2 Qian Zhu1,2
Qian Zhu1,2 Wu Tang1,2
Wu Tang1,2 Francois Blachier3
Francois Blachier3 Hao Ding1
Hao Ding1 Xiangfeng Kong1*
Xiangfeng Kong1*Nutrients in the maternal diet favor the growth and development of suckling piglets and alter their gut microbiota composition and metabolic activity, thus affecting the hosts. The present study analyzed, in suckling piglets from sows receiving antibiotic or synbiotic supplements from pregnancy to lactation, several biochemical parameters, oxidative/anti-oxidative indices, inflammatory cytokines, and ingestion-related factor levels in plasma, as well as colonic microbiota composition and metabolic activity, and mucosal expression of genes related to the intestinal barrier function. Compared with the control group, maternal synbiotic supplementation decreased (P < 0.05) the plasma levels of glucose, AMM, TC, low-density lipoprotein-cholesterol (LDL-C), MDA, H2O2, ghrelin, CCK, PP, IL-1β, IL-2, IL-6, TNF-α, Ala, Cys, Tau, and β-AiBA, the levels of propionate and total short-chain fatty acids (SCFAs) in the colonic luminal content, and colonic abundances of RFN20, Anaerostipes, and Butyricimonas; while increased (P < 0.05) the plasma levels of urea nitrogen (UN), Ile, Leu, α-AAA, α-ABA, and 1-Mehis, as well as colonic abundances of Sphingomonas, Anaerovorax, Sharpea, and Butyricicoccus. Compared with the antibiotic group, maternal synbiotic supplementation decreased (P < 0.05) the plasma levels of glucose, gastrin, and Ala, as well as abundances of Pasteurella and RFN20 and propionate level in the colonic content. Expression of genes coding for E-cadherin, Occludin, ZO-1, ZO-2, IL-10, and interferon-α were down-regulated in the colonic mucosa. The synbiotic supplementation increased (P < 0.05) the plasma levels of UN, Leu, α-ABA, and 1-Mehis, the abundances of Anaerovorax, Sharpea, and Butyricicoccus and expression of genes coding for E-cadherin, Occludin, ZO-1, ZO-2, IL-10, and interferon-α. Spearman correlation analysis showed that there was a positive correlation between colonic Anaerostipes abundance and acetate and SCFAs levels; whereas a negative correlation between Fusobacteria and Fusobacterium abundances and acetate level. These findings suggest that synbiotic supplementation in the maternal diet improved nutrient metabolism and intestinal barrier permeability, reduced oxidative stress, and modified colonic microbiota composition and metabolic activity in suckling piglets.
Economic benefit in swine farm is directly affected by the survival rate, growth and development, and health of suckling piglets (1). The survival and health of suckling piglets are largely dependent on maternal milk quality (2). Maternal nutrition during lactation is an important factor affecting the quality and quantity of the maternal milk. Therefore, improving maternal nutrient level could help to enhance sows lactating performance and promote the growth and development of piglets.
Gut microbiota is involved in the metabolism, growth, and development of the host (3). Short-chain fatty acids (SCFAs) are products of some specific gut bacteria and could serve as luminal energy substrates in colonocytes (4). In addition, SCFAs exert an anti-inflammatory effect in the gut (5). Microbiota colonization in infant gut begins from their mother's wombs (6) and is affected by diets and other environmental factors (7). Exposure to antibiotics via oral administration as a kind medicine (especially the broad-spectrum antibiotics) in newborn animals has a major effect on gut microbiota composition (8). Antibiotics was reported to promote nutrient absorption and increase the piglet growth (9). However, antibiotic overuse leads to drug residues in animals and their products, thus leading to antibiotic resistance and affecting humans health (10). Synbiotics, the mixed additive of prebiotics and probiotics, have shown several beneficial effects in pig production. For instance, several studies showed that dietary synbiotic supplementation improved the intestinal microbiota and growth performance of weaned piglets (11, 12). Therefore, we speculated that synbiotics in the maternal diet could affect the offspring, notably by modifying the gut microbiota and metabolic activity.
Our previous study showed that dietary synbiotic supplementation increased the piglet survival rate by improving the glycolipids absorption and utilization and altering the gut microbiota composition and abundances of sows (13). The present study hypothesizes that maternal synbiotic supplementation may modify beneficially blood indices, gut microbiota composition and metabolic activity, and the mucosal mRNA expression of genes related to the intestinal barrier function. Therefore, the effects of synbiotic supplementation in sows' diets were measured on several parameters in suckling piglets, including plasma biochemical parameters, oxidative/anti-oxidative indices, inflammatory and ingestion-related factors, and free amino acids. In addition, colonic microbiota composition and metabolic activity were measured in piglets, as well as expression of colonic mucosa genes involved in epithelial barrier function and inflammation.
The animal experiment was conducted in Hantang Agriculture Co. Ltd., Shimen, Hunan, China. Forty-eight pregnant Bama mini-pigs were selected and randomly allocated into one of three groups (16 sows per group). The sows in the control group were fed a basal diet, those in the antibiotic group were fed a basal diet supplemented with 50 g/t virginiamycin, and those in the synbiotic group were fed a basal diet supplemented with 200 mL/d fermentation broth per animal and 500 g xylo-oligosaccharides (XOS) per ton diet. The fermentation broth was provided by Hunan Lifeng Biotechnology Co. Ltd. and contained ≥ 1.2 × 108 CFU/g viable Lactobacillus plantarum B90 (BNCC1.12934) ≥ 1.0 × 108 CFU/g and Saccharomyces cerevisiae P11 (BNCC2.3854) ≥ 0.2 × 108 CFU/g. The XOS was provided by Shandong Longlive Biotechnology Co., Ltd., Shandong, China; and contained xylobiose, xylotriose, and xylotetraose at level ≥ 35%. The diet composition and nutrient levels for the sows met the Chinese pig local standard (NY-2004), and the premixes for pregnant and lactating sows met the NRC recommended requirements (NRC, 2012) (Supplementary Table 1). The experimental period was from mating to weaning (postpartum 21 d). During the trial period, there were four sows returned to estrus in the control group, two sows returned to estrus in the antibiotic group, and three sows returned to estrus in the synbiotic group. The diets were fed twice daily (8:00 a.m. and 5:00 p.m.) fluctuating with the physical condition of the sows throughout the trail, and water was available freely.
At 21 day-old (weaned), the piglets from 12 litters were weighed after fasted for about 12 h and one piglet with middle body weight (BW) per litter was selected. Twelve piglets per group were exsanguinated after electrical stunning (120 V, 200 Hz). Each piglet per group was randomly chosen to collect blood samples from precaval vein into 10 mL heparin coated-tubes and plasma was separated by centrifuging at 3,500 g and 4°C for 10 min and stored at −20°C for further analysis. Colonic contents (middle section) were collected in 10 mL sterile centrifuge tubes and stored immediately at −20°C for subsequent analysis of microbiota composition and metabolites. After washing with cold physiological saline, the colonic mucosal tissues were sampled and immediately frozen in liquid nitrogen (~2 g), and then stored at −80°C for mRNA analyses.
The plasma levels of albumin (ALB), alkaline phosphatase (ALP), alanine aminotransferase (ALT), ammonia (AMM), aspartate aminotransferase (AST), glucose (GLU), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), total protein (TP), and urea nitrogen (UN) were determined using commercially available kits (F. Hoffmann-La Roche Ltd, Basel, Switzerland) with the Roche automatic biochemical analyzer (Cobas c311, F. Hoffmann-La Roche Ltd, Basel, Switzerland).
The plasma levels of catalase (CAT), hydrogen peroxide (H2O2), malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC), were determined as per commercially available kit directions (Suzhou keming, Co. Ltd, Jiangsu, China) with Multiscan Spectrum (Tecan, Infinite M200 Pro, Switzerland).
The plasma levels of gastrin, ghrelin, cholecystokinin (CCK), interleukin (IL)-1β, IL-2, IL-6, IL-10, interferon (IFN)-α, insulin-like growth factor (IGF)-1, leptin (LEP), pancreatic polypeptide (PP), peptide YY (PPY), and tumor necrosis factor (TNF)-α were measured according to the Meimian ELISA kit directions (Jiangsu Yutong Biological Technology, Co. Ltd., Jiangsu, China) on Multiscan Spectrum (Tecan, Infinite M200 Pro, Switzerland).
Approximately 1.00 mL plasma sample was added into 1.00 mL 8% salicylic acid solution, mixed thoroughly and overnighted at 4°C, and then centrifuged at 8,000 r/min for 10 min to obtain the supernatant. The processed samples were filtered through a 0.45-μm membrane prior to analysis of free amino acids with an automatic AA analyzer (L8900, Hitachi, Tokyo, Japan).
The total genomic DNA of colonic content samples was extracted using the Fast DNA SPIN extraction kits (MP Biomedicals, Santa Ana, CA, USA). The DNA concentration was determined using NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The V3-V4 regions was amplified using the primer 338F (5′-GCACCTAAYTGGGYDTAAAGNG-3′) and 806R (5′-TACNVGGGTATCTAATCC-3′). The protocol of PCR amplification was conducted according to our previous study (13). The PCR products were successfully separated using 1.2% agarose gel electrophoresis, purified using Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN), and further quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Purified amplicons were then subjected to paired-end (2 × 300) sequencing on an Illumina MiSeq platform (Illumina, San Diego, USA) using the MiSeq Reagent Kit v3 (600 cycles) according to the standard protocol, which was performed by Shanghai Personal Biotechnology Co. Ltd., Shanghai, China. The raw Illumina pair-end read data for all samples are available in the NCBI Sequence Read Archive with accession number PRJNA609410.
The SCFAs in colonic contents were measured with gas chromatography (Agilent Technologies 1206, Santa Clara, CA, USA) according to the previous description (14). The levels of bioamines, indole, and skatole in colonic contents were measured using reverse phase-high performance liquid chromatography (Agilent Technologies, Santa Clara, CA, USA) according to a previous study (14).
The primers for target genes and reference gene β-actin (listed in Supplementary Table 2) were designed using Primer-BLAST. RNA extraction and real-time polymerase chain reaction (RT-PCR) analyses were conducted as a previous report (15). The relative expression level of each target gene was determined by RT-PCR with performing on a 480II system (Roche, LightCycler® 480II, Switzerland) and calculated by the 2−ΔΔCt method (16).
The plasma indices, colonic metabolite levels, and colonic microbiota alpha diversity were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's multiple range post hoc test with SPSS 22. The microbial community structural variation among samples was performed by the beta diversity analysis (PERMANOVA) (17) and was showed using the partial least squares-discriminant analysis (PLS-DA). The colonic microbiota abundance and overall composition at phyla and genus levels were analyzed using Metastats (http://metastats.cbcb.umd.edu/) (18). The graph preparation was performed using GraphPad Prism ver7.0 (San Diego, CA, USA). Spearman's correlation between colonic microbiota abundances and metabolite levels was analyzed with the R package. All data were presented as means ± SEM. Differences were considered statistically significant at P < 0.05.
As shown in Figure 1, compared with the control group, maternal synbiotic supplementation increased (P < 0.05) plasma UN level while decreased (P < 0.05) plasma GLU, AMM, TC, and LDL-C levels. Maternal synbiotic supplementation decreased (P < 0.05) plasma ALT and GLU levels, increased (P < 0.05) UN level, and showed an increased trend in TG level (P = 0.074), when compared with the antibiotic group.
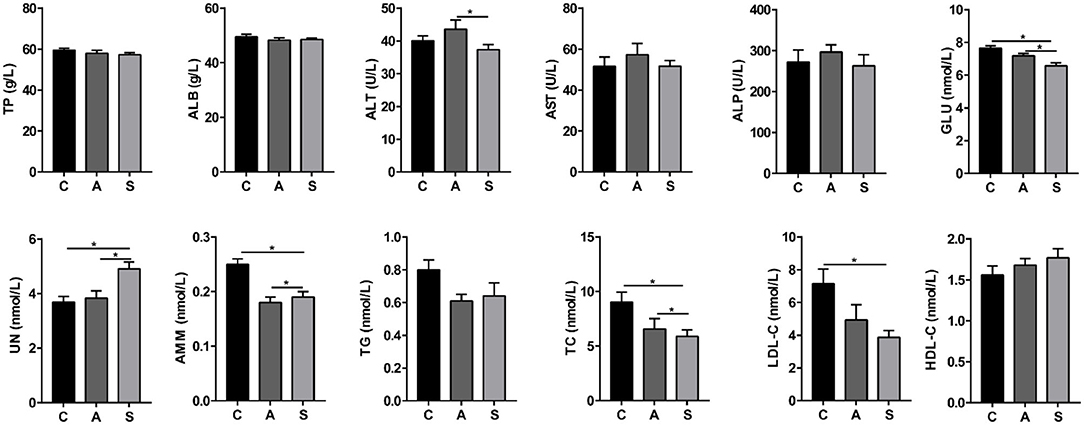
Figure 1. Effect of maternal synbiotic supplementation on plasma biochemical parameters of suckling Bama mini-piglets. C, A, and S present the control group, antibiotic group, and synbiotic group, respectively. The same as below. Data represent the means ± SEM. *indicates statistically significant (P < 0.05). n = 8 per group.
As shown in Figure 2, compared to the control group, maternal synbiotic supplementation decreased (P < 0.05) plasma MDA and H2O2 levels and antibiotic supplementation decreased (P < 0.05) plasma MDA level. However, the plasma T-AOC, SOD, and CAT indices did not reach statistical significance (P > 0.05).
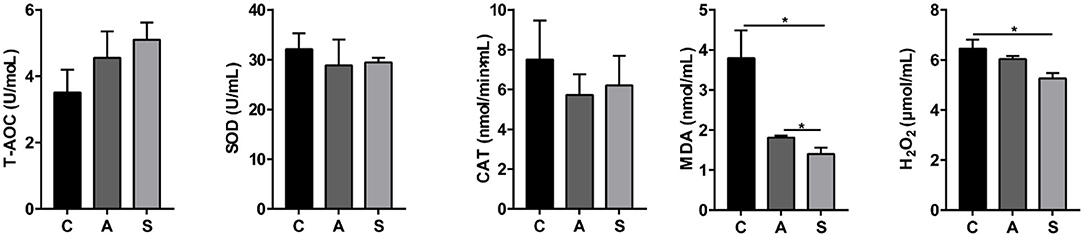
Figure 2. Effect of maternal synbiotic supplementation on plasma oxidative/anti-oxidative levels in suckling Bama mini-piglets. Data represent the means ± SEM. *indicates statistically significant (P < 0.05). n = 8 per group.
As presented in Figure 3, maternal synbiotic supplementation decreased (P < 0.05) plasma levels of IL-1β, IL-2, IL-6, and TNF-α; and antibiotic supplementation decreased (P < 0.05) plasma levels of IGF-1, IL-1β, IL-2, IL-6, and TNF-α, when compared with the control group.
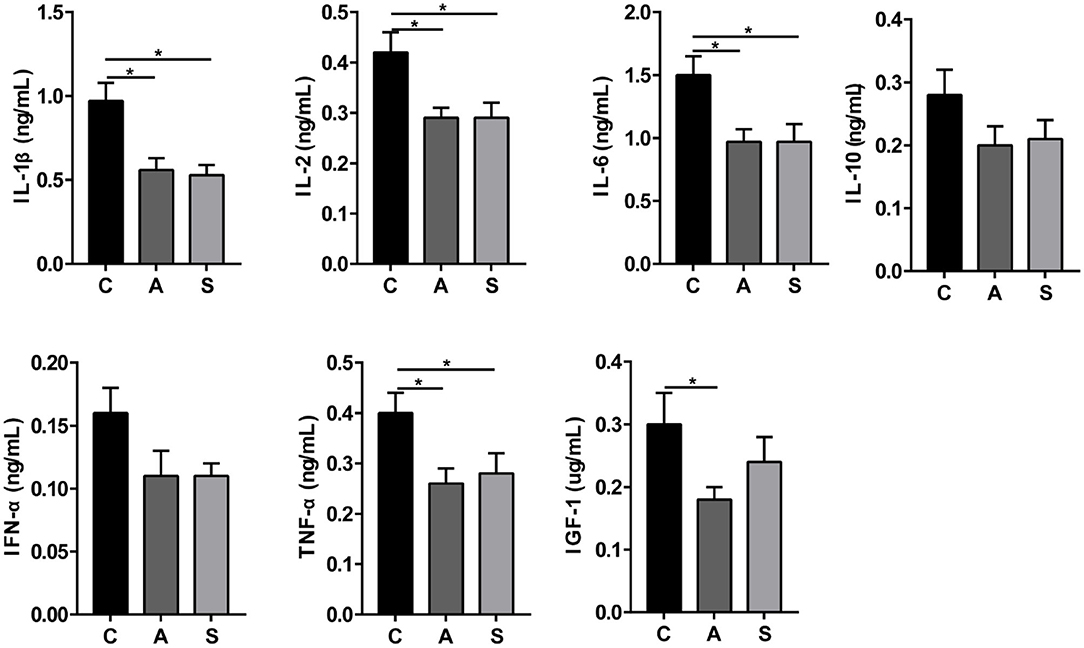
Figure 3. Effect of maternal synbiotic supplementation on plasma inflammatory cytokine levels in suckling Bama mini-piglets. Data represent the means ± SEM. *indicates statistically significant (P < 0.05). n = 8 per group.
As listed in Figure 4, maternal synbiotic supplementation decreased (P < 0.05) plasma ghrelin, CCK, and PP levels and had a decreased trend in LEP level (P = 0.05); and maternal antibiotic supplementation decreased (P < 0.05) plasma gastrin, ghrelin, CCK, PP, LEP, and SS levels, when compared with the control group. Maternal synbiotic supplementation decreased plasma gastrin (P < 0.05) and LEP (P = 0.05) levels relative to the antibiotic group.
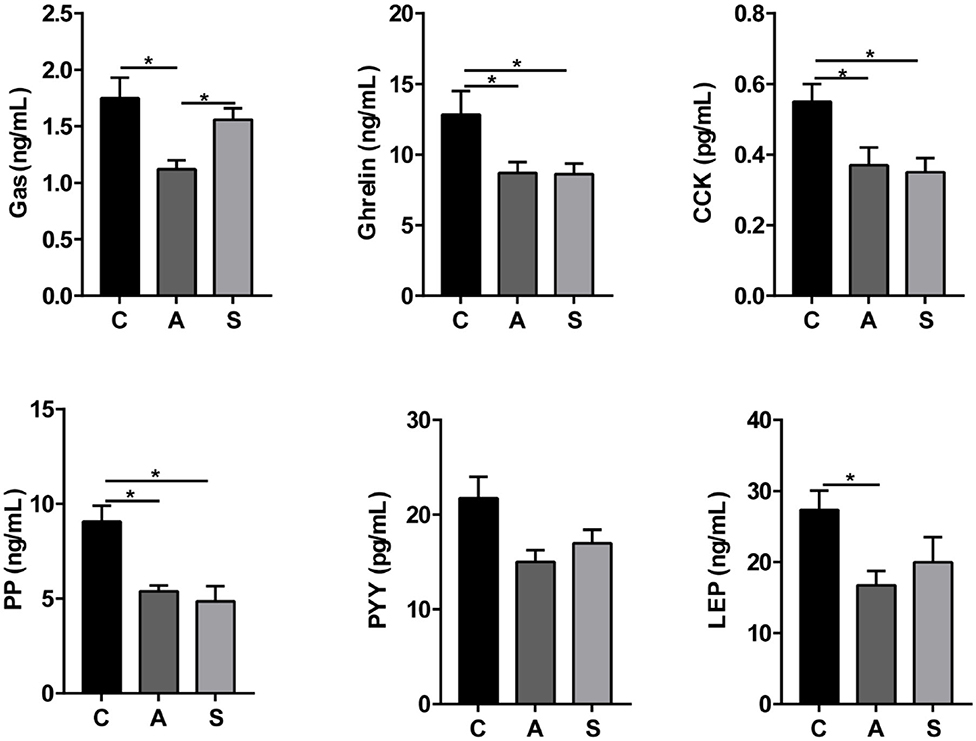
Figure 4. Effect of maternal synbiotic supplementation on plasma ingestion-related factor levels in suckling Bama mini-piglets. Data represent the means ± SEM. *indicates statistically significant (P < 0.05). n = 8 per group.
As shown in Table 1, maternal synbiotic supplementation decreased (P < 0.05) plasma Ile, Leu, α-AAA, α-ABA, and 1-Mehis levels and antibiotic supplementation decreased (P < 0.05) plasma Hypro level, when compared with the control group. The plasma Leu, α-ABA, and 1-Mehis levels in the synbiotic group was higher (P < 0.05) while plasma Ala level was lower (P < 0.05) compared with the antibiotic group.
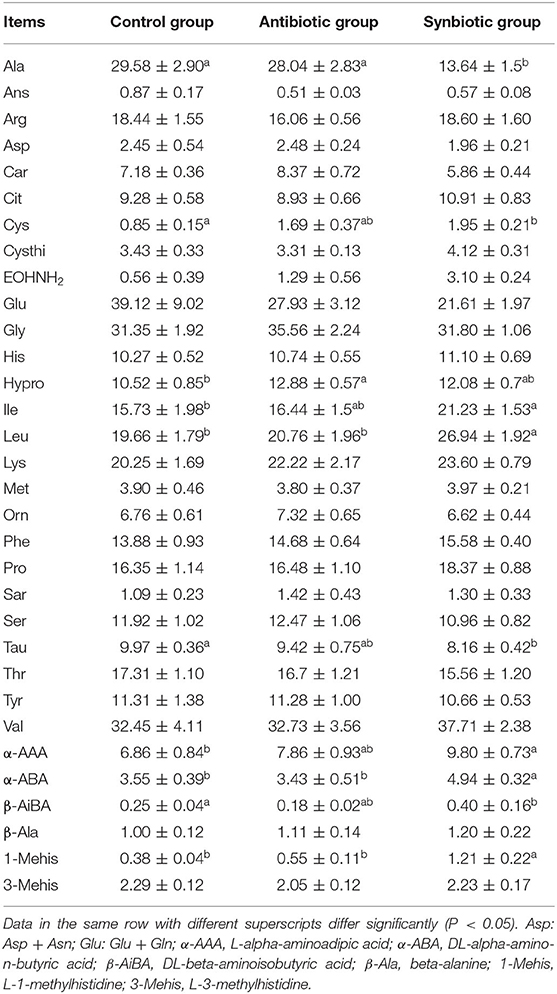
Table 1. Effects of maternal synbiotic supplementation on plasma concentrations of free amino acids in suckling Bama mini-piglets (μg/mL; n = 8).
Total 993,960 high-quality reads were generated from 48 colonic content samples, and each sample contained an average of 41,415 reads (range from 31,377 to 57,987). As shown in Figure 5, the Chao1, ACE, Simpson, and Shannon indices showed no difference among the three groups (P > 0.05). PLS-DA showed that samples from the three groups tended to exhibit a distinct clustering of microbiota composition although there was a partial overlap between the antibiotic group and synbiotic group.
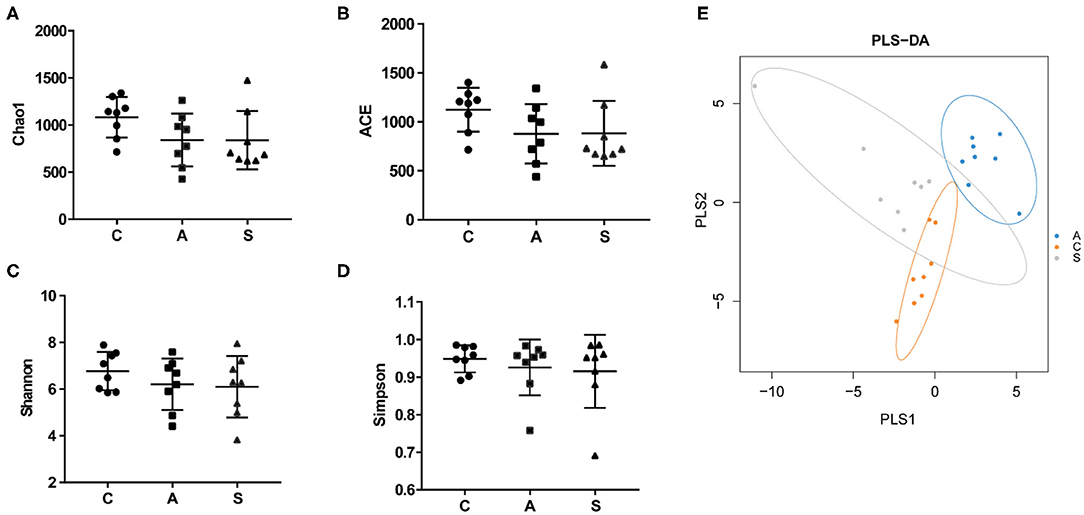
Figure 5. Effect of maternal synbiotic supplementation on alpha diversity of colonic microbiota in suckling Bama mini-piglets. (A–D) The microbial diversity is estimated by Chao, ACE, Shannon, and Simpson indices. (E) Partial least squares discrimination analysis (PLS-DA) of the colonic microbial community. Data represent the means ± SEM. The data were analyzed by One-way analysis of variance and Duncan's multiple range test. n = 8 per group.
As shown in Figure 6, the top five dominant phyla were Firmicutes (80.7%), Proteobacteria (7.3%), Bacteroidetes (6.3%), Spirochaetes (2.8%), and Fusobacteria (1.4%), which account for > 98% of total colonic bacteria. At phylum level, only Fusobacteria relative abundance in the antibiotic group was higher (P < 0.01) than that in the control group.
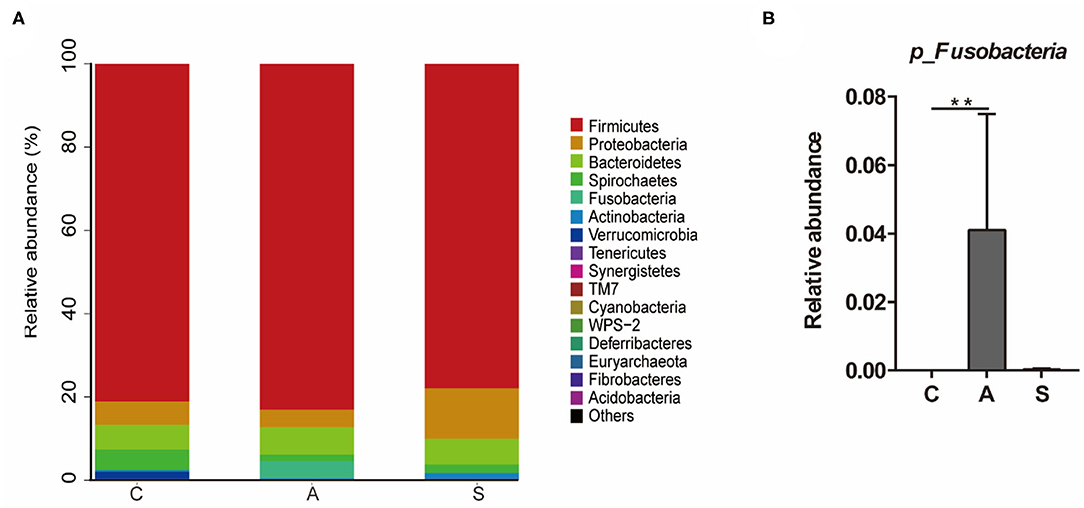
Figure 6. Effect of maternal synbiotic supplementation on the colonic microbial community structure in suckling Bama mini-piglets. Colonic microbiota distributed at the phylum level (A) and all phyla were listed. A comparison of relative abundances at the phylum level (B) was analyzed by Metastats analysis, and the discrepancy of the top 10 colonic microbiota was listed. Phyla with proportion < 0.001 were grouped in others. **P < 0.01. n = 8 per group.
At genus level, Lactobacillus (23.2%), p-75-a5 (3.4%), Herbaspirillum (3.3%), Treponema (2.5%), and Oscillospira (2.5%) were the top dominant genera of colonic microbiota with a clear classification status (Figure 7). Further, the abundances of colonic microbiota with a clear classification status of 20 most abundant bacterial genera were analyzed. Relative to the control group, maternal synbiotic supplementation increased (P < 0.05) the abundances of p_Proteobacteria;g_Sphingomonas, p_Firmicutes;g_Anaerovorax, p_Firmicutes;g_Holdemania, p_Firmicutes;g_Sharpea, p_Firmicutes;g_Butyricicoccus, and p_Firmicutes;g_Anaerostipes; while decreased (P < 0.05) the abundances of p_Firmicutes;g_Facklamia, p_Firmicutes;g_RFN20, p_Actinobacteria;g_Arcanobacterium, and p_Proteobacteria;g_Brevundimonas. Maternal antibiotic supplementation decreased (P < 0.05) the abundances of p_Proteobacteria;g_Acinetobacter, p_Firmicutes;g_Facklamia, p_Firmicutes;g_Streptococcus, and p_Proteobacteria;g_Brevundimonas while increased (P < 0.05) p_Fusobacteria;g_Fusobacterium abundance. Compared with the antibiotic group, maternal synbiotic supplementation decreased (P < 0.05) the abundances of p_Proteobacteria;g_Pasteurella and p_Firmicutes;g_RFN20, while increased (P < 0.01) the abundances of p_Firmicutes;g_Anaerovorax, p_Firmicutes;g_Holdemania, p_Firmicutes;g_Sharpea, and p_Firmicutes;g_Butyricicoccus.
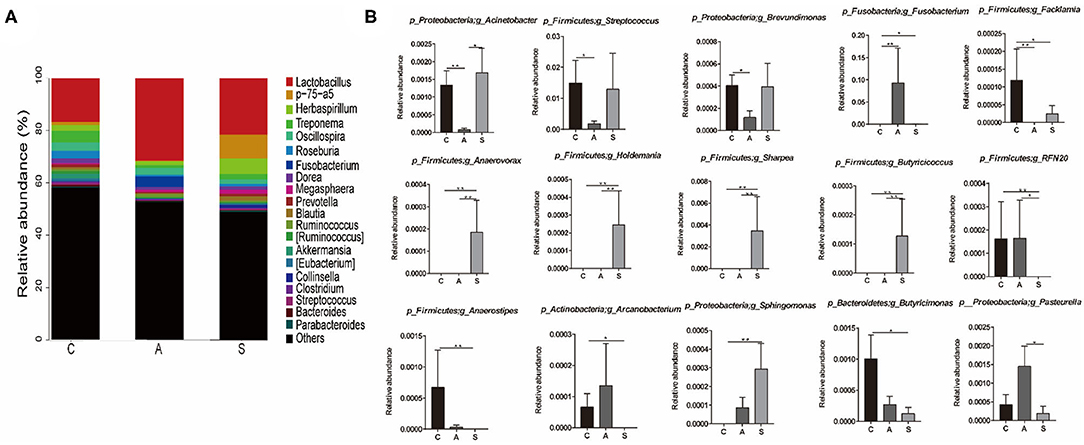
Figure 7. Effect of maternal synbiotic supplementation on the colonic microbial community structure in suckling Bama mini-piglets. Colonic microbiota distributed at the genus level (A) and only the top 20 genera were listed. A comparison of relative abundances at the genus level (B) was analyzed by Metastats analysis. The 20 most abundant bacterial genera with a clear classification status were presented and compared. *P < 0.05; **P < 0.01. n = 8 per group.
As shown in Figure 8, compared with the control group, the levels of propionate, straight-chain fatty acids, and SCFAs were decreased (P < 0.05) and spermidine level showed a decreased trend (P = 0.055) in the synbiotic group. Moreover, maternal synbiotic supplementation decreased (P < 0.05) the propionate level and increased (P = 0.055) spermidine level compared with the antibiotic group. The differences in other determined metabolites among the three groups did not present statistically significant (P > 0.05) (Supplementary Figure 1).
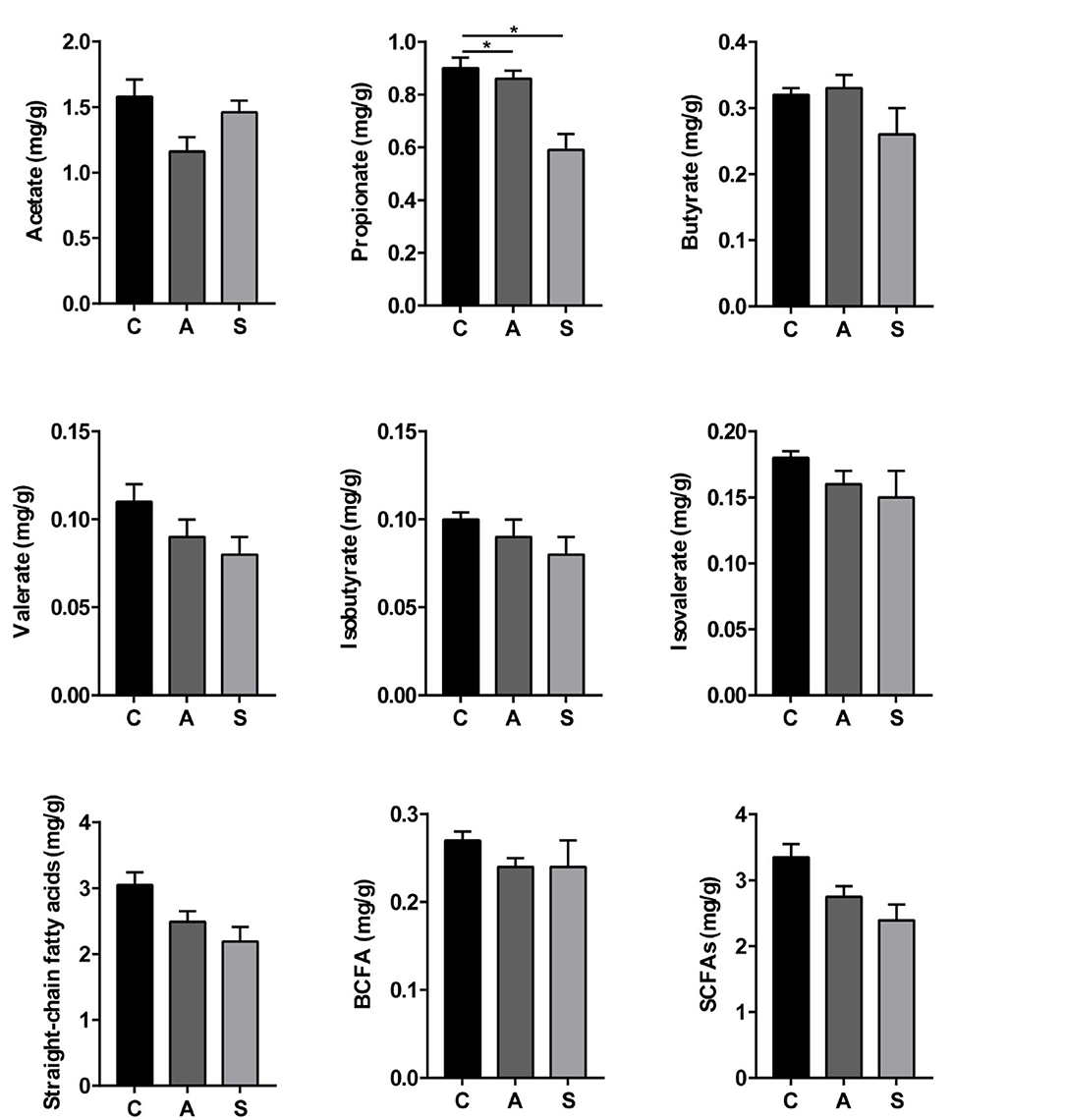
Figure 8. Effect of maternal synbiotic supplementation on colonic short-chain fatty acids levels of suckling Bama mini-piglets. The data were analyzed by Duncan's multiple range test using One-way analysis of variance. Data represent the means ± SEM. *P < 0.05. n = 8 per group.
As shown in Figure 9, p_Firmicutes;g_Butyricicoccus abundance was positively correlated (P < 0.05) with isovalerate and branched-chain fatty acid (BCFA) levels, as well as p_Firmicutes;g_Anaerostipes abundance with acetate and SCFAs levels. However, a significant negative correlation (P < 0.05) was observed between p_Fusobacteria and p_Fusobacteria;g_Fusobacterium abundances and acetate level. In addition, there was a negative correlation (P < 0.05) between p_Firmicutes;g_Facklamia abundance and tryptamine level, as well as p_Actinobacteria;g_Arcanobacterium abundance and tryptamine and skatole levels.
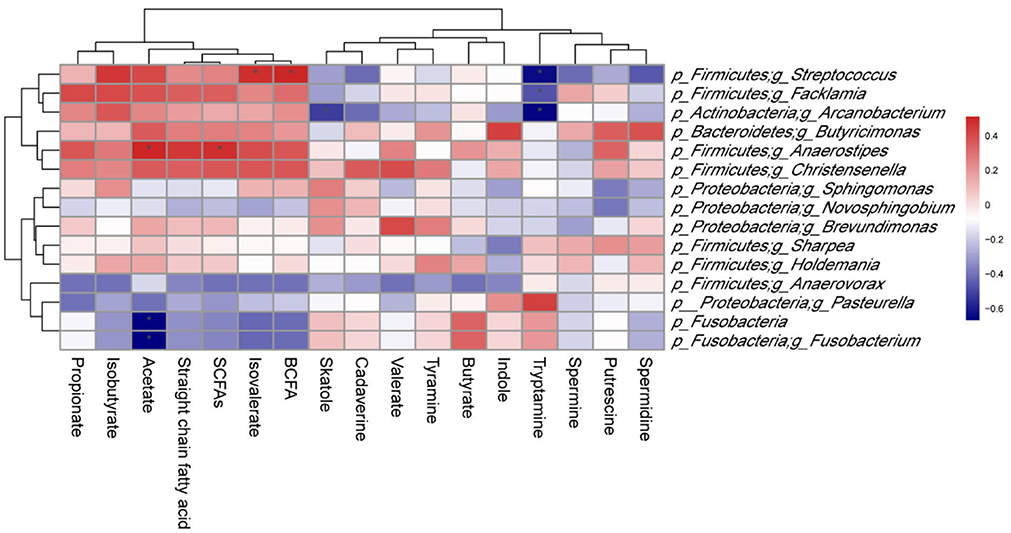
Figure 9. Correlation between colonic microbiota and their metabolites in suckling Bama mini-piglets. Spearman (r) correlations were used, and * means that the correlation is significant. SCFAs, short-chain fatty acids; BCFA, branched-chain fatty acid.
As shown in Figure 10, maternal synbiotic supplementation up-regulated (P < 0.05) the mRNA expression of colonic E-cadherin, Occludin, ZO-1, ZO-2, IL-10, and IFN-α compared with the antibiotic group. Compared with the control group, maternal synbiotic and antibiotic supplementation failed to affect the expression of determined genes.
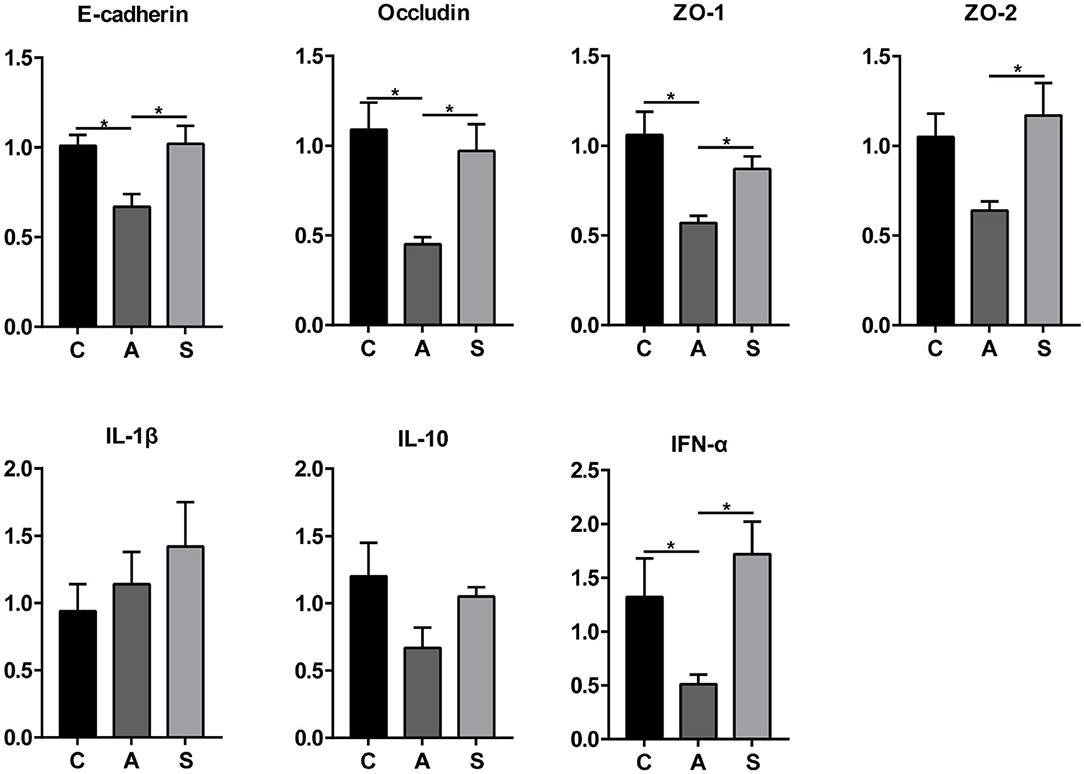
Figure 10. Effect of maternal synbiotic supplementation on mRNA expression of colonic mucosal genes related to the intestinal barrier function in suckling Bama mini-piglets. The data were analyzed by Duncan's multiple range test using One-way analysis of variance. Data showed the means ± SEM. *P < 0.05. n = 8 per group.
The present study explored the effects of synbiotic supplementation in the maternal diets from pregnancy to lactation on the intestine health of suckling piglets by determining colonic microbiota composition, metabolite levels, and mucosal gene expression, as well as plasma parameters. We found that maternal antibiotic supplementation is counter-productive for the intestinal health based on the measurement of parameters related to the intestinal barrier permeability, whereas synbiotic supplementation improved parameters related to nutrient metabolism and intestinal health.
The piglets utilize efficiently dietary fat when blood TC level decreases. LDL-C transports TC synthesized by the liver to extrahepatic tissue, thus preventing excessive lipid deposition in the liver (19). In the present study, maternal synbiotic supplementation decreased plasma TC and LDL-C levels, suggesting that dietary fat was highly utilized by piglets to favor their growth. Shakeri et al. (20) reported that supplementing synbiotics reduced the blood TC level by altering gut microbiota metabolism. UN is a metabolite of amino acid and/or protein (21), plasma level of which reflects the profiles of protein absorption and utilization in the animal body (22). AMM reflects the liver function and the decrease of plasma AMM level indicates the increase of liver ability for synthesizing urea (23). The present study showed that plasma UN level increased while AMM level decreased in the synbiotic group, suggesting that maternal synbiotic supplementation promoted protein utilization of suckling piglets. These findings suggest that maternal synbiotic supplementation, but not antibiotic supplementation, would enhance the nitrogen metabolism of suckling piglets.
Amino acids (AAs), apart for being an important component of tissue protein, play several important roles in protein metabolism in animals (24). Weanling piglets use branched-chain amino acids (including Ile, Leu, and Val) to maintain their growth and development, especially Leu which contributes to regulate protein synthesis and tissue growth of animals (25). In the present study, maternal synbiotic supplementation increased the plasma Ile and Leu levels in suckling piglets. In addition, previous studies showed that Tau and Cys, main products of Met metabolism, play a vital role in the growth and health of piglets (26). Ala is the main substrate for glucose synthesis in the liver, which can play a role in the body's immune function (27). Tau, mostly found at a high level in animal tissues, has been shown to improve animal lipid metabolism (28). The present study showed that maternal synbiotic supplementation decreased the plasma levels of Tau, Cys, and Ala in piglets, suggesting that dietary synbiotics may modify amino acid metabolism in the offspring. These above-mentioned findings suggested that maternal synbiotic supplementation affects the protein synthesis by altering plasma amino acids levels.
Plasma MDA level reflects lipid peroxidation in the body tissues (29). H2O2 is a reactive oxygen species (ROS) that can increase the oxidative stress in tissues (30). A previous study showed that piglets may produce excessive reactive oxygen species thus leading to oxidative stress, which may lead to intestinal barrier dysfunction in weaned piglets (31). Interestingly, we found that maternal synbiotic supplementation decreased plasma MDA and H2O2 levels, suggesting that the synbiotics could relieve the oxidative stress exposure to suckling piglets. Among prebiotics, XOS produces SCFAs which may reduce ROS production (32), Lactobacillus reduces MDA production (33), and synbiotic addition reduces the MDA level and relieves oxidative stress in tissues (29).
Gut microbiota is involved in nutrient utilization and affects the growth and development of the host (34). Maternal nutrition during pregnancy and lactation modified the gut microbiota composition and health of offspring (35). Gut microbiota diversity was closely related with the host's health (36). The α-diversity of microbiota is decreased, which may be associated with a higher occurrence of low-grade inflammation and some metabolic diseases (37). In the present study, after maternal antibiotic or synbiotic supplementation, the α-diversity of colonic microbiota in piglets did not change, whereas the microbiota composition and abundances changed markedly, suggesting that maternal synbiotic might not exert a negative effect on suckling piglets.
In the animal gut, the dominant phyla usually includes Firmicutes, Bacteroides, Proteobacteria, and Fusobacterium (38). In the present study, the abundances of Firmicutes, Bacteroides, and Proteobacteria accounted for 94.3% of the total sequences. Firmicutes plays a vital role in the degradation of polysaccharides and oligosaccharides (39), which involves some key metabolic conversions by the gut microbial community (40). In addition, maternal synbiotic supplementation increased the abundances of Butyricicoccus and Sharpea belonged to Firmicutes. Butyricicoccus can reduce the production of pro-inflammatory cytokines to inhibit the host's inflammation (41). We found that maternal synbiotic supplementation increased Butyricicoccus abundance, which might reduce the inflammation occurrence of suckling piglets via altering gut microbiota composition and abundance. Sharpea promotes SCFAs (especially butyrate) and lactate production (42). Our study showed that maternal synbiotic supplementation increased Sharpea abundance in the offspring, which may favor inhibition of the proliferation of potential pathogenic bacteria by reducing the gut pH value. Additively, Fusobacterium can use glucose as a carbon source, the abundance of which is increased by polysaccharide degradation (43). Several studies reported that Fusobacterium might be a contributing factor for inflammation (44), the abundance of which increased in neonatal piglets with diarrhea (45). In the present study, the Fusobacterium abundance showed a decreased trend in the synbiotic group, implying that maternal synbiotic supplementation reduced this potential pathogenic bacteria.
Colonic SCFAs can exert crucial effects on intestinal function and health of the host before and after absorption in the blood (46). In addition of providing 60–70% of total energy to colonic cells (47), the SCFAs are associated with the reduction of the host's inflammation (48) and the relieving symptoms of other metabolic diseases (49). Among them, propionate reduces the serum cholesterol level and liver lipogenesis of rats (50). Our study showed that maternal synbiotic supplementation decreased propionate level in the colonic content. These findings suggested that maternal synbiotic supplementation increased certain gut microbiota species and promoted the production of specific metabolites. In addition, colonic p_Firmicutes;g_Anaerostipes abundance was positively correlated with acetate and SCFAs levels; and Fusobacteria and p_Fusobacteria;g_Fusobacterium abundances were negatively correlated with acetate level, suggesting that Anaerostipes might promote the SCFAs production while Fusobacteria and Fusobacterium would diminish them by a underlying mechanism that needs to be determined.
Cytokines can regulate the systemic inflammatory response of the body. The SCFAs promote the migration of leukocytes to the inflammatory site and production of several anti- and pro-inflammatory cytokines, including TNF-α, IL-1β, IL-2, IL-6, and IL-10 (51). Acetate, propionate, and butyrate reduce the production of TNF-α (52), IL-1β, and IL-6 (53). Interestingly, we found that maternal synbiotic supplementation decreased the plasma levels of TNF-α, IL-1β, IL-2, and IL-6 in offspring piglets, suggesting that dietary synbiotics might reduce inflammation in piglets via modifying several bacterial metabolite productions. Additionally, cytokines have the function of regulating immune and inflammatory responses and maintaining barrier integrity (54). In the present study, maternal synbiotics up-regulated the mRNA expression of colonic mucosal IFN-α, suggesting that the synbiotic addition in the maternal diets enhances the immune response of suckling piglets via regulating gut microbiota composition and metabolic activity as previously proposed (55).
The SCFAs can modulate hormone secretion (e.g., Leptin) (56) and are involved in modulating the production of Ghrelin (57). CCK can suppress the appetite by acting on the central nervous system (58). Ghrelin can act on appetite (59) and satiety by regulating the gut microbial community of the host. The PP secretion can be stimulated by dietary fat (60). Our study showed that maternal synbiotic supplementation decreased the plasma levels of Ghrelin, CCK, and PP of piglets, suggesting that maternal synbiotic addition might affect plasma hormone secretion of suckling piglet by mediating gut microbiota and their metabolites.
When the intestinal mucosal barrier is damaged, the permeability of which would increase, thus causing intestinal inflammation or other diseases due to harmful substances invading the body tissues (55). Compared with the antibiotic group, dietary synbiotic supplementation up-regulated the mRNA expression of colonic mucosal E-Cadherin, Occludin, ZO-1, and ZO-2, suggesting that the maternal synbiotic administration might improve tight-junction integrity of colonic intestinal epithelial cells via colonic microbiota. Shi et al. (61) found that the mixture of Lactobacillus species increased the colonic mucosal tight-junction proteins and relieved inflammation in antibiotic-supplemented mice by modulating their microbiota structure. Yin et al. (62) also showed that dietary XOS supplementation improved the intestinal barrier by up-regulating ZO-1 expression. Further work is required to explore the dose of synbiotic supplementation in maternal diets presenting an impact on the intestinal permeability in piglets.
In conclusion, maternal synbiotic supplementation from pregnancy to lactation may improve glycolipid and protein metabolism, reduce oxidative stress level, and improve the intestinal health of suckling piglets. Notably, these findings provide a new perspective for manipulating gut microbiota with synbiotic addition to improve the nutrient metabolism and intestine health of offspring. The changes in maternal milk composition after maternal synbiotic supplementation need further analysis in the future to full interpret the findings of the present study.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA609410.
The animal study was reviewed and approved by Animal Care and Use Committee of the Institute of Subtropical Agriculture. Written informed consent was obtained from the owners for the participation of their animals in this study.
XK designed the experiment. CM, QG, WZ, QZ, HD, and WT carried out the animal trail, and sample collection and analysis. CM and WZ performed the statistical analyses. CM wrote the manuscript. FB and XK revised the manuscript. All authors reviewed this manuscript.
This present study was jointly supported by the National Key Research and Development Project (2018YFD0500404-4), National Natural Science Foundation of China (31772613), STS regional key project of the Chinese Academy of Sciences (KFJ-STS-QYZD-052), Talent Projects of Guangxi Science and Technology Department (AD17195043), and Special Funds for Construction of Innovative Provinces in Hunan Province (2019RS3022).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the staff and postgraduate students of Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process for collecting samples, and technicians from CAS Key Laboratory of Agro-ecological Processes in Subtropical Region for providing technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.575685/full#supplementary-material
1. Liang H, Yan L, Chuan Y, Xie P, Qin X, Yue X, et al. Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction. Br J Nutr. (2015) 114:53–62. doi: 10.1017/S0007114515001579
2. Noble MS, Rodriguez-Zas S, Cook JB, Bleck GT, Hurley WL, Wheeler MB. Lactational performance of first-parity transgenic gilts expressing bovine alpha-lactalbumin in their milk. J Anim Sci. (2002) 80:1090–6. doi: 10.2527/2002.8041090x
3. Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol Spectr. (2015) 3:3. doi: 10.1128/microbiolspec.MBP-0008-2014
4. Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. (2010) 16:684–695. doi: 10.1002/ibd.21108
5. Ziar H, Gérard P, Riazi A. Effect of prebiotic carbohydrates on growth, bile survival and cholesterol uptake abilities of dairy-related bacteria. J Sci Food Agric. (2014) 94:1184–90. doi: 10.1002/jsfa.6395
6. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. (2014) 6:237. doi: 10.1126/scitranslmed.3008599
7. Doré J, Blottière H. The influence of diet on the gut microbiota and its consequences for health. Curr Opin Biotechnol. (2015) 32:195–9. doi: 10.1016/j.copbio.2015.01.002
8. Jin Y, Wu S, Zeng Z, Fu Z. Effects of environmental pollutants on gut microbiota. Environ Pollut. (2017) 222:1–9. doi: 10.1016/j.envpol.2016.11.045
9. Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. (2015) 6:7486. doi: 10.1038/ncomms8486
10. Maxwell A, Dowson CG, Spencer J. The molecular basis of antibiotic action and resistance. J Mol Biol. (2019) 431:3367–9. doi: 10.1016/j.jmb.2019.06.018
11. Mair C, Plitzner C, Domig KJ, Schedle K, Windisch W. Impact of inulin and a multispecies probiotic formulation on performance, microbial ecology and concomitant fermentation patterns in newly weaned piglets. J Anim Physiol Anim Nutr (Berl). (2010) 94:e164–e77. doi: 10.1111/j.1439-0396.2010.01000.x
12. Umu ÖC, Frank JA, Fangel JU, Oostindjer M, da Silva CS, Bolhuis EJ, et al. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome. (2015) 3:16. doi: 10.1186/s40168-015-0078-5
13. Ma C, Zhang WH, Gao QK, Zhu Q, Song MT, Ding H, et al. Dietary synbiotic supplementation alters reproductive performance, plasma biochemical parameters, fecal microbiota composition, and metabolite levels in pregnant and lactating sows. J Funct Food. (2020) 75:104221. doi: 10.1016/j.jff.2020.104221
14. Hu CJ, Li FN, Duan YH, Yin YL, Kong XF. Glutamic acid supplementation reduces body fat weight in finishing pigs when provided solely or in combination with arginine and it is associated with colonic propionate and butyrate concentrations. Food Funct. (2019) 10:4693–704. doi: 10.1039/c9fo00520j
15. Duan Y, Zeng L, Li F, Wang W, Li Y, Guo Q, et al. Effect of branched-chain amino acid ratio on the proliferation, differentiation, and expression levels of key regulators involved in protein metabolism of myocytes. Nutrition. (2017) 36:8–16. doi: 10.1016/j.nut.2016.10.016
16. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. (2008) 3:1101. doi: 10.1038/nprot.2008.73
17. McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. (2001) 82:290–297. doi: 10.1890/0012-96582001082[0290:Fmmtcd]2.0.Co;2
18. White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. (2009) 5:e1000352. doi: 10.1371/journal.pcbi.1000352
19. Chen Y, Gong X, Li G, Lin M, Huo Y, Li S, et al. Effects of dietary alfalfa flavonoids extraction on growth performance, organ development and blood biochemical indexes of Yangzhou geese aged from 28 to 70 days. Anim Nutr. (2016) 2:318–322. doi: 10.1016/j.aninu.2016.09.004
20. Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, et al. Consumption of synbiotic bread decreases triacylglycerol and vldl levels while increasing hdl levels in serum from patients with type-2 diabetes. Lipids. (2014) 49:695–701. doi: 10.1007/s11745-014-3901-z
21. Blachier F, Mariotti F, Huneau JF, Tome D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids. (2007) 33:547–62. doi: 10.1007/s00726-006-0477-9
22. Cole NA, Clark RN, Todd RW, Richardson CR, Gueye A, Greene LW, et al. Influence of dietary crude protein concentration and source on potential ammonia emissions from beef cattle manure. J Anim Sci. (2005) 83:722–31. doi: 10.2527/2005.833722x
23. Tian QY, Zeng ZK, Zhang YX, Long SF, Piao XS. Effect of L- or DL-methionine supplementation on nitrogen retention, serum amino acid concentrations and blood metabolites profile in starter pigs. Asian-Aust J Anim Sci. (2016) 29:689–94. doi: 10.5713/ajas.15.0730
24. Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci. (2014) 2:387–417. doi: 10.1146/annurev-animal-022513-114113
25. Mao X, Zeng X, Huang Z, Wang J, Qiao S. Leptin and leucine synergistically regulate protein metabolism in C2C12 myotubes and mouse skeletal muscles. Br J Nutr. (2013) 110:256–64. doi: 10.1017/s0007114512004849
26. Wang WW, Qiao SY, Li DF. Amino acids and gut function. Amino Acids. (2009) 37:105–10. doi: 10.1007/s00726-008-0152-4
27. Li P, Yin YL, Li FN, Kim SW, Wu GY. Amino acids and immune function. Br J Nutr. (2007) 98:237–52. doi: 10.1017/S000711450769936X29
28. Ripps H, Shen, W. Review: taurine: a “very essential” amino acid. Mol Vision. (2012) 18:2673–86. Available online at: http://hdl.handle.net/1912/5720
29. Salehi-Abargouei A, Ghiasvand R, Hariri M. Prebiotics, prosynbiotics and synbiotics: can they reduce plasma oxidative stress parameters? A systematic review. Probiotics Antimicrob Proteins. (2017) 9:1–11. doi: 10.1007/s12602-016-9248-4
30. Hussain T, Tan B, Yin YL, Blachier F, Tossou MCB, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. (2016) 2016:7432797. doi: 10.1155/2016/7432797
31. Zhu LH, Zhao KL, Chen XL, Xu JX. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. (2012) 90:2581–9. doi: 10.2527/jas.2012-4444
32. Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. (2011) 3:858–76. doi: 10.3390/nu3100858
33. Ito M, Sawada H, Ohishi K, Yoshida Y, Yokoi W, Watanabe T, et al. Suppressive effects of bifidobacteria on lipid peroxidation in the colonic mucosa of iron-overloaded mice. J Dairy Sci. (2001) 84:1583–9. doi: 10.3168/jds.S0022-0302(01)74591-2
34. Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science. (2016) 352:1533. doi: 10.1126/science.aad9359
35. De Waard M, Brands B, Kouwenhoven SMP, Lerma JC, Crespo-Escobar P, Koletzko B, et al. Optimal nutrition in lactating women and its effect on later health of offspring: a systematic review of current evidence and recommendations (Early Nutrition project). Crit Rev Food Sci Nutr. (2017) 57:4003–16. doi: 10.1080/10408398.2016.1158149
36. Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. (2014) 63:1913–20. doi: 10.1136/gutjnl-2013-306541
37. Wilmanski T, Rappaport N, Earls JC, Magis AT, Manor O, Lovejoy J, et al. Blood metabolome predicts gut microbiome α-diversity in humans. Nat Biotechnol. (2019) 37:1217–28. doi: 10.1038/s41587-019-0233-9
38. Guevarra RB, Hong SH, Cho JH, Kim BR, Shin J, Lee JH, et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J Anim Sci Biotechnol. (2018) 9:54. doi: 10.1186/s40104-018-0269-6
39. Tyakht AV, Kostryukova ES, Popenko AS, Belenikin MS, Pavlenko AV, Larin AK, et al. Human gut microbiota community structures in urban and rural populations in Russia. Nat Commun. (2013) 4:2469. doi: 10.1038/ncomms3469
40. Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. (2007) 9:1101–11. doi: 10.1111/j.1462-2920.2007.01281.x
41. Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. (2013) 62:1745–52. doi: 10.1136/gutjnl-2012-303611
42. Kamke J, Kittelmann S, Soni P, Li Y, Tavendale M, Ganesh S, et al. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome. (2016) 4:56. doi: 10.1186/s40168-016-0201-2
43. Honma K, Ruscitto A, Sharma A. β-glucanase activity of the oral bacterium tannerella forsythia contributes to the growth of a partner species, fusobacterium nucleatum, in cobiofilms. Appl Environ Microbiol. (2017) 84: e01759-17. doi: 10.1128/aem.01759-17
44. Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. (2016) 22:557–6. doi: 10.3748/wjg.v22.i2.557
45. Hermann-Bank ML, Skovgaard K, Stockmarr A, Strube ML, Larsen N, Kongsted H, et al. Characterization of the bacterial gut microbiota of piglets suffering from new neonatal porcine diarrhoea. BMC Vet Res. (2015) 11:139. doi: 10.1186/s12917-015-0419-4
46. Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. (2015) 64:1744–54. doi: 10.1136/gutjnl-2014-307913
47. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. (2001) 81:1031–64. doi: 10.1152/physrev.2001.81.3.1031
48. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TM. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes (Lond). (2014) 38:1525–31. doi: 10.1038/ijo.2014.46
49. Pluznick JL. Microbial short-chain fatty acids and blood pressure regulation. Curr Hypertens Rep. (2017) 19:25. doi: 10.1007/s11906-017-0722-5
50. Delzenne NM, Williams CM, Prebiotics and lipid metabolism. Curr Opin Lipidol. (2002) 13:61. doi: 10.1097/00041433-200202000-00009
51. Vinolo MA, Rodrigues HG, Hatanaka E, Hebeda CB, Farsky SH, Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci. (2009) 117:331–8. doi: 10.1042/cs20080642
52. Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. (2011) 22:849–55. doi: 10.1016/j.jnutbio.2010.07.009
53. Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E-2 and cytokines. World J Gastroenterol. (2009) 15:5549–57. doi: 10.3748/wjg.15.5549
54. Andrews C, McLean MH, Durum SK. Cytokine tuning of intestinal epithelial function. Front Immunol. (2018) 9:1270. doi: 10.3389/fimmu.2018.01270
55. Frank DN, Amand ALS, Feldman RA, Boedeker EC, Harpaz N, Pace, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. P Natl Acad Sci USA. (2007) 104:13780–5. doi: 10.1073/pnas.0706625104
56. Zaibi MS, Stocker CJ, O'Dowd J, Davies A, Bellahcene M, Cawthorne M A, et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. (2010) 584:2381–6. doi: 10.1016/j.febslet.2010.04.027
57. Delzenne NM., Cani PD, Daubioul C, Neyrinck AM. Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr. (2005) 93:S157–S61. doi: 10.1079/bjn20041342
58. Fakhry J, Wang J, Martins P, Fothergill LJ, Hunne B, Prieur P, et al. Distribution and characterisation of CCK containing entero endocrine cells of the mouse small and large intestine. Cell Tissue Res. (2017) 369:245–53. doi: 10.1007/s00441-017-2612-1
59. Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. (2004) 50:1511–25. doi: 10.1373/clinchem.2004.032482
60. Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. (2007) 10:729–34. doi: 10.1097/MCO.0b013e3282efdebb
61. Shi Y, Zhao X, Zhao J, Zhang H, Zhai Q, Narbad A, et al. A mixture of Lactobacillus species isolated from traditional fermented foods promote recovery from antibiotic-induced intestinal disruption in mice. J Appl Microbiol. (2018) 124:842–54. doi: 10.1111/jam.13687
Keywords: biochemical parameters, gut microbiota, metabolites, sows, suckling piglets, synbiotic
Citation: Ma C, Gao Q, Zhang W, Zhu Q, Tang W, Blachier F, Ding H and Kong X (2020) Supplementing Synbiotic in Sows' Diets Modifies Beneficially Blood Parameters and Colonic Microbiota Composition and Metabolic Activity in Suckling Piglets. Front. Vet. Sci. 7:575685. doi: 10.3389/fvets.2020.575685
Received: 01 July 2020; Accepted: 04 November 2020;
Published: 30 November 2020.
Edited by:
Amlan Kumar Patra, West Bengal University of Animal and Fishery Sciences, IndiaCopyright © 2020 Ma, Gao, Zhang, Zhu, Tang, Blachier, Ding and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangfeng Kong, bm5reGZAaXNhLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.