
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 02 November 2020
Sec. Parasitology
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.573016
This article is part of the Research Topic Neglected and Under-Researched Parasitic Diseases of Veterinary and Zoonotic Interest View all 18 articles
Sarcocystis neurona and Sarcocystis falcatula are protozoan parasites endemic to the Americas. The former is the major cause of equine protozoal myeloencephalitis, and the latter is associated with pulmonary sarcocystosis in birds. The opossum Didelphis virginiana is the definitive host of these parasites in North America. Four Didelphis species are found in Brazil, and in most reports in this country, Sarcocystis species shed by opossums have been classified as S. falcatula–like. It is unknown whether reports on S. neurona–seropositive horses in Brazil are also derived from exposure of horses to S. falcatula–like. The aim of this study was to test the sera reactivity of 409 horses in Brazil using antigens derived from a Brazilian strain of S. falcatula–like (Sarco-BA1) and from a North American strain of S. neurona (SN138). Samples were examined by immunofluorescent antibody tests (IFATs) at start dilutions of 1:20, and a selected number of samples was tested by Western blot (WB). Sera from 43/409 (10.5%) horses were reactive to S. falcatula–like and 70 of 409 (17.1%) were reactive to S. neurona antigen; sera from 25 animals (6.1%) were positive for both parasites by IFAT. A poor agreement was observed between the two employed IFATs (κ = 0.364), indicating that horses were exposed to more than one Sarcocystis species. Horse sera evaluated by WB consisted of four sera reactive to S. falcatula–like by IFAT, six sera positive to S. neurona by IFAT, two sera that tested negative to both parasites by IFAT, and a negative control horse serum from New Zealand. Proteins in the range of 16 and 30 kDa were recognized by part of IFAT-positive sera using both antigen preparations. We concluded that Brazilian horses are exposed to distinct Sarcocystis species that generate different serological responses in exposed animals. Antigens in the range of 16 and 30 kDa are probably homologous in the two parasites. Exposure of the tested horses to other Sarcocystis species, such as Sarcocystis lindsayi, Sarcocystis speeri, and Sarcocystis fayeri, or Sarcocystis bertrami cannot be excluded in the current study.
The coccidian parasite Sarcocystis neurona is the major cause of equine protozoal myeloencephalitis (EPM), a debilitating neurological disease that affects horses in the Americas (1, 2). The opossum Didelphis virginiana serves as definitive host of S. neurona in North America (3, 4), whereas Didelphis albiventris was identified as definitive host of S. neurona in Brazil (5). While only one species of Didelphis is found in North America, four species of this genus are found in Brazil: D. albiventris, Didelphis aurita, Didelphis marsupialis, and Didelphis imperfecta (6). The North American opossum (D. virginiana) is definitive host of three species of Sarcocystis: Sarcocystis falcatula (7), S. neurona (3), and Sarcocystis speeri (8). The South American opossum D. albiventris is definitive host of four species of Sarcocystis: S. neurona, Sarcocystis lindsayi, S. speeri, and S. falcatula (5, 9–11). Under experimental conditions, S. falcatula and S. lindsayi are infective for birds (11, 12), whereas S. neurona and S. speeri are infective for immunodeficient mice (13, 14).
Similarly to the protozoan parasite Toxoplasma gondii, S. neurona also contains several surface antigens (SAGs) which are probably associated with parasite virulence and host cell invasion (15). Three S. neurona SAGs (SnSAGs), named as SnSAG2, SnSAG3, and SnSAG4, were identified in merozoites of all S. neurona isolates and have been employed in an enzyme-linked immunosorbent assay (ELISA) for EPM in horses (16–18). Coding genes for SAGs from Brazilian isolates of S. falcatula–like are very similar to those from North American isolates of S. neurona (19). A high allelic variation is found for SAG2, SAG3, and SAG4 from S. falcatula–like, contrasting to SAGs from S. neurona in North America that possesses low genetic variation (20, 21).
Several serological techniques have been developed to detect S. neurona antibodies, including Western blot (WB), immunofluorescent antibody test (IFAT), S. neurona agglutination test and ELISA (2). WB using serum and cerebrospinal fluid from horses was the first serological test developed for EPM in horses (22). In the last two decades, IFAT and SnSAG ELISA have been validated for S. neurona infections and have been frequently used in veterinary practice and in research investigations (1). WB continues to be a valuable tool on Sarcocystis species investigations; however, its application has been essentially in research studies (2).
S. falcatula is a parasite shed by opossums that causes severe respiratory disease in birds (7, 23, 24). Serologic cross-reactivity between S. falcatula and S. neurona was suspected as some genes coding for immunodominant SAGs for these parasites are very similar (25). In this context, the SnSAG 2-4 ELISA would not be specific for S. neurona and could present cross-reactivity for S. falcatula–infected animals (25). In an experimental study conducted in the late 1990s, four horses in the United States did not seroconvert after experimental inoculation with S. falcatula sporocysts (26); it was assumed that cross-reactivity between S. neurona and S. falcatula would not be a concern when testing horses by SAG ELISA, as S. falcatula would not induce seroconversion in horses (1).
In Brazil, Sarcocystis species shed by opossums possess intriguing characteristics. In recent years, more than 50 Sarcocystis species isolates were obtained from Brazilian opossums, and almost 100% of them were infective to birds (budgerigars). However, these isolates were genetically distinct from both S. falcatula and S. neurona and, for this reason, classified as S. falcatula–like (20, 21, 27–29). The Brazilian isolates that were submitted for sequencing of SAG genes possessed a high allelic variation in their coding genes for SAG2, SAG3, and SAG4, which seems to represent genetic recombination between S. neurona, S. falcatula, or other unidentified Sarcocsytis species (19–21, 27–29). Based on this peculiar scenario in Brazil, we hypothesized that Brazilian horses may be exposed and seroconvert to other species of Sarcocystis shed by opossums, besides S. neurona. To address this hypothesis, we tested horse sera using antigen from a North American strain of S. neurona and antigen derived from a recently isolate of S. falcatula–like, which has been propagated in an avian cell line (27).
Serum samples were obtained from 409 adult horses, including males and females, mostly from mixed breeds, and derived from Bahia and Rio Grande do Sul states in Brazil. Samples from Bahia state (n =217) were collected as part of the clinical screening for equine infectious anemia virus and for hematological checking. Samples from Rio Grande do Sul (n =192) were acquired in a commercial slaughterhouse for horse meat exportation. No animals were raised or handled for research purposes. Horse sera were stored for 5 years at −20°C in the Laboratory of Coccidian Protozoa at the School of Veterinary Medicine from Federal University of Bahia.
Antigens for IFATs and for WB consisted of merozoites of a North American strain of S. neurona (SN-138) (30) and merozoites from a South American strain of S. falcatula–like (27). S. neurona merozoites were propagated in Vero cells supplemented with RPMI-1650 + L-glutamine (Invitrogen/Gibco®, Carlsbad, USA), 1% antibiotic–antimycotic (100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B) (Gibco®, Carlsbad, USA), and 5% of inactivate bovine serum (Invitrogen/Gibco®, Auckland, NZ), at 37°C in a humidified incubator containing 5% CO2. S. falcatula–like merozoites were grown in the same conditions as described above, but instead of Vero cells, the parasites were cultured in a permanent chicken cell line (UMNSAH/DF-1) (31), as recently described (27). Cell monolayers containing merozoites of each parasite species were scrapped from the flasks, passed three times through a 26-gauge needle, filtered in Sephadex G-25 (GE Healthcare®) columns, and washed three times in phosphate-buffered saline (PBS) by centrifugation (1,500 g for 5 min).
Volumes of 10 μL, containing of 3 × 103 purified merozoites of S. neurona or S. falcatula–like were added to each 5-mm well of IFAT slides, which were dried at 37°C. Antigen slides were immersed in cold acetone (−20°C) for 10 min for fixation and stored at −20°C until analysis. Antigen-coated slides were stored at a maximum time of 60 days until examination by IFAT.
Serum samples were tested at a starting dilution of 1:20 in PBS. Slides were incubated at 37°C for 30 min in a humid chamber and then washed for 10 min in a FA (fluorescent antibody) buffer (26.9 mM Na2CO3, 100 mM NaHCO3, 70.6 mM NaCl, pH 9.0) and 10 min in PBS and dried at 37°C. A fluorescein isothiocyanate–conjugated anti–horse immunoglobulin G (IgG) (Sigma–Aldrich®, St. Louis, USA) was used as secondary antibody at 1:32 dilution and incubated for 30 min in a dark and humid chamber. Slides were washed in FA and PBS as described above, dried at 37°C, and mounted with buffered glycerin. Reactions were observed under a fluorescent microscope (CiL, Nikon®). Positive controls consisted of sera from naturally exposed horses that reacted at 1:80 solely for each parasite (S. neurona or S. falcatula–like). Negative controls consisted of previously examined horse sera that tested negative for both parasites at dilutions <1:20. Positive reactions were characterized by full peripheral fluorescence of merozoites. Antibody titers were determined by double dilutions for all reactive sera.
The term WB (Western blot) is used here and throughout the manuscript to indicate both WB and immunoblot. Cultured merozoites of S. neurona (4 × 107 per membrane) or S. falcatula–like (2 × 107 per membrane) purified in Sephadex G-25 columns (in Merozoites and Antigen Production) were pelleted by centrifugation and mixed with a reducing sample buffer (1% 2-mercaptoethanol, 2% sodium dodecyl sulfate (SDS), 7% glycerol, 48 mM Tris–HCl, pH 6.8), heated at 97°C for 10 min, and centrifuged at 13,000 g for 10 min at 4°C.
WB was performed similarly as previously reported (32). The solubilized proteins from merozoites were run on a 12.5% polyacrylamide gel electrophoresis with SDS. A prestained molecular weight marker with proteins from 10 to 180 kDa (PageRuler Prestained Protein Ladde, Thermo Scientific™) was used on each gel. Proteins were transferred from the gels to polyvinylidene difluoride (PVDF) membranes, blocked with PBS–Tween–gelatin (0.05% Tween 20 and 2% of gelatin) for 30 min, and stored at −20°C until analysis.
For immunoblot, each PVDF membrane coated with S. neurona or S. falcatula–like antigen was cut in 26 strips. Sera from 13 horses were selected for the analysis, including a negative control (horse serum from New Zealand), two horse sera that tested negative for both parasites by IFAT, six samples that tested positive for S. neurona by IFAT, and four sera that tested positive for S. falcatula–like by IFAT. Reactions were conducted in two different ways: 1) using antigen strips that were blocked with bovine serum containing antibodies to Sarcocystis cruzi, as reported by Rossano et al. (33); 2) using antigen strips not treated with anti–S. cruzi serum. Serum containing antibodies to S. cruzi was obtained by testing bovine sera with bradyzoites' antigen by IFAT (34).
Membrane strips from both parasites were blocked for 90 min with anti–S. cruzi bovine serum diluted at 1:65 (33) in PBS–Tween–gelatin. After five washings with 0.05% Tween-20 in PBS (PBS-T), membrane strips were incubated at room temperature with horse sera at 1:10 in PBS-T gelatin for 60 min. Then, they were incubated with anti–horse IgG peroxidase conjugate for 60 min and washed three times with PBS-T and three times with PBS. The reactions were revealed using diaminobenzidine peroxidase tablets and stopped by adding ultrapure water. The same 13 horse sera were tested by WB to both parasite antigens without initial incubation with anti–S. cruzi bovine serum.
To compare the percent agreement in IFATs for S. neurona and S. falcatula–like, Cohen κ statistics was used. Characterization of labeled bands in WB was determined by descriptive statistics, by means of the frequency of the observed bands.
Antibodies to S. neurona (SN138 strain) and to S. falcatula–like (Sarco-BA1 strain) antigens were detected by IFAT in 17.1% (70/409) and in 10.5% (43/409) of the horses, respectively. A 1:20 dilution was used for the study. Simultaneous seropositivities for both antigens were observed in 6.1% (25/409) of the animals. A total of 322 (78.7%) of 409 horses were seronegative to both parasites. The agreement level for the two IFATs, expressed by the κ coefficient, was 0.364, indicating a fair agreement between the two tests. IFAT results are shown in Table 1. The maximum antibody titers observed for the two tested antigens was 1:160; 11 animals were positive at 1:160 for S. neurona, and 2 animals for S. falcatula–like presented titers of 1:160 by IFAT (Supplementary File).

Table 1. Seropositivity of Brazilian horses to Sarcocystis neurona and Sarcocystis falcatula–like tested by immunofluorescent antibody tests.
In WB, reduced antigens from both parasites were tested using sera from 13 horses, including a negative control, two horses that tested negative to both parasites by IFAT, six horses that tested positive to S. neurona, and four horses that tested positive to S. falcatula–like by IFAT (Table 2).
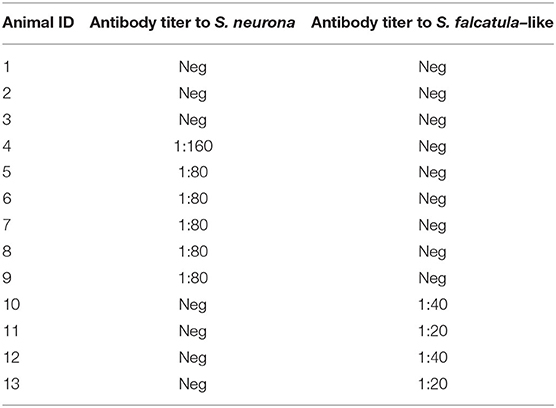
Table 2. Horse sera selected for Western blot and their antibody titers for Sarcocystis neurona and Sarcocystis falcatula determined by IFAT.
Most samples showed varying levels of reactivity to molecules in the regions of 16 and 30 kDa, regardless of S. neurona (Figure 1A) or S. falcatula–like (Figure 1B) were used as antigens in WB. The reactive bands in the antigen strips treated with anti–S. cruzi serum did not show significant differences from those not treated with anti–S. cruzi serum. Labeling of several antigens, besides those in the range of 16 and 30 kDa, were visualized between 10 and 70 kDa, with apparently no diagnostic value.
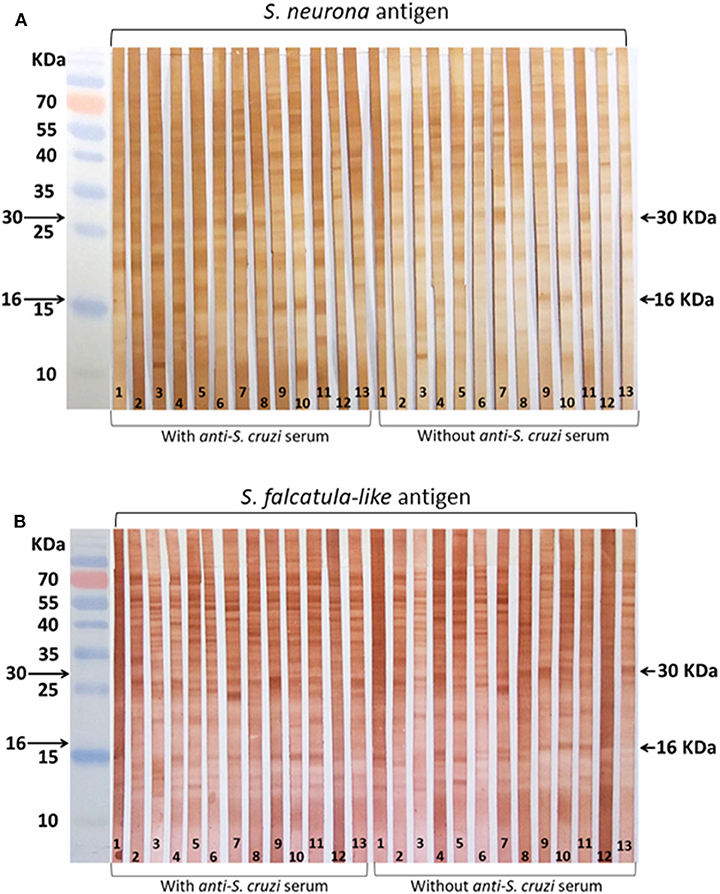
Figure 1. Horse sera tested by Western blot (WB). Serum samples were selected for WB by immunofluorescence antibody tests (IFATs). (A) Membrane strips coated with Sarcocystis neurona antigen; (B) membrane strips coated with Sarcocystis falcatula–like antigen. Reactions were conducted with and without blocking treatment with bovine anti–Sarcocystis cruzi serum. Strip 1: negative control (horse serum from New Zealand); 2–3: sera that tested negative for both parasites by IFAT; 4–9: sera that tested positive for S. neurona by IFAT; 10–13: sera that tested positive for S. falcatula–like by IFAT.
To test the hypothesis that Brazilian horses may be exposed and seroconvert to more than one Sarcocystis species shed by opossums, we examined horse sera from Bahia and Rio Grande do Sul states using two Sarcocystis species as antigens. Merozoites from a North American strain of S. neurona (SN-138) and from a South American strain of S. falcatula–like (Sarco-BA1) were employed as antigens in IFAT and WB. All samples were examined by IFAT, and 13 selected sera were tested by WB using antigens from both parasites. The sera tested by WB were processed with and without a blocking step with bovine serum anti–S. cruzi.
Results obtained by IFAT indicated that the examined horses reacted to more than one species of Sarcocystis; the κ coefficient was 0.364, supporting that the frequency of seropositivity in the IFAT for S. neurona had a fair agreement with the IFAT for S. falcatula–like. A total of 45 horses reacted solely to S. neurona antigen by IFAT, and 18 horses showed positive reactions solely to S. falcatula–like. A starting serum dilution of 1:20 was selected for the current study because in a previous work, a gold standard panel of horses infected with S. neurona had seropositivities between 1:20 and 1:80 by IFAT (35); these authors recommended a 1:80 cutoff in IFAT for horses suspected to have EPM. In the current work, equine sera were derived from horses with no neurological disease, and for this reason, we decided to use a less conservative cutoff.
The labeling patterns in WB using antigens of S. neurona and S. falcatula–like were very similar, indicating serological cross-reactivity for several shared antigens. The WB reactions using a blocking step with bovine anti–S. cruzi serum to minimize nonspecific reactions did not lead to any significant change in the results. Slight differences were observed in the intensity of the labeled antigens; however, it may be related to the time that enzymatic reactions were stopped during immunoblot. In literature, antigens regarded as immunodominant for S. neurona infection in horses possess approximate molecular weights of 16 and 30 kDa (33). Some authors also include proteins in the range of 7 to 10 kDa, besides the 16- and 30-kDa antigens as specific for S. neurona exposure (36). Positive reactions to one of the two proteins (16 or 30 kDa) are considered suspect for S. neurona infection (35). In subsequent studies with larger sample sets, the main concern of WB for S. neurona in horses corresponded to its low specificity (35, 37, 38).
Several reports on S. neurona infections or exposure in Brazilian animals have been conducted using North American strains of the parasite (39–43). It is worth to note that S. neurona antigen derived from Brazilian isolates of the parasite is not available for serological tests. The opossum species identified as definitive host for S. neurona in the United States (D. virginiana) does not exist in Brazil. The only description of S. neurona in a Brazilian opossum (D. albiventris) was reported in 2001 (5); parasite identification was mainly based on its infectivity to immunodeficient mice and by polymerase chain reaction–restriction fragment length polymorphism according to primers designed by Tanhauser et al. (44). At the time S. neurona was described in D. albiventris (5), the employed molecular tools were believed to precisely identify the Sarcocystis species infecting the opossum. In recent years, it became clear that additional molecular techniques are needed to differentiate S. neurona from closely related Sarcocystis species shed by opossums, including S. falcatula–like organisms (27).
In a recent publication, a Brazilian cat was reported to have S. neurona infection (45); based on the internal transcribed spacer 1 (ITS1) of the rDNA, this Sarcocystis species differed from organisms classified as S. neurona or S. falcatula. These authors used additional molecular markers, including SAG loci, 18S, and COX1; the combined molecular data, mostly based on ITS1 and SAG loci, allowed the classification of this cat isolate as S. neurona, although it is clearly distinct from the North American isolates of S. neurona (45).
Studies on Brazilian Sarcocystis species shed by opossums, based on bioassay and different molecular markers, revealed that all isolates differed from S. neurona, in both biological and molecular aspects (19–21, 27, 29); the reported isolates were infective to budgerigars and possessed a high level of genetic recombination. In light of the peculiar scenario observed in Brazil, some questions have been raised by our research group: 1) May horses be infected with or present seroconversion to S. falcatula–like? 2) Are there other Sarcocystis species shed by opossums that are capable of infecting horses in Brazil? Although EPM associated to S. neurona was reported in Brazilian horses, identification of the parasite was mainly based on clinical, morphological, and immunological tests, including serology and immunohistochemistry (46, 47). So far, there is no isolation or molecular identification of S. neurona from any Brazilian horse. Serological studies in the country have been conducted with North American S. neurona antigens, in both IFAT and ELISA. In a serological survey performed with Brazilian horses from several states, the overall frequency of S. neurona antibodies was 69.6% (669/961) using an ELISA with SAG4 as antigen (40). Interestingly, in two recent seroepidemiological studies in Brazil using IFAT, frequencies of antibodies to the parasite were 26% (n = 506) in Minas Gerais (48), and 2.8% (n = 427) in the state of Alagoas (49); in these two studies, North American isolates of S. neurona were used with a 1:80 cutoff. In the present study, we detected 17.1% (n = 409) of seropositive animals by IFAT using S. neurona as antigen and 1:20 as cutoff. The differences in seropositivities between SAG4 ELISA (69.6%) and IFAT (2.8–26%) for Brazilian horses raise suspicions that results were overestimated by using SAG4 ELISA or underestimated by using IFAT. Inclusion of SnSAG ELISA (17, 18) in the current study, as well as a sample set of sera from horses with confirmed S. neurona infections, would highly aid on evaluation of cross reactions between anti–S. neurona antibodies and S. falcatula–like antigens. Performing immunoblotting with Neospora spp. antigens would also contribute to test potential cross-reactivity between this genus and Sarcocystis spp.
In the current work, we detected horses that reacted solely to S. falcatula–like antigen by IFAT and horses with positive reactions solely to S. neurona antigen by IFAT. For this reason, we suspected that more than one species of Sarcocystis species shed by Brazilian opossums induce seroconversion in horses. In a recent study, we performed experimental infection in Mongolian gerbils using S. neurona and S. falcatula–like (50). Serological cross-reactivity between the two parasites was clearly demonstrated by WB, whereas IFAT was able to distinguish infections caused by each of these parasites (50).
In conclusion, we demonstrated that using IFAT as serological test, Brazilian horses reacted differently to S. neurona and S. falcatula–like antigens. A 1:20 cutoff was employed in each IFAT; however, the optimal cutoff was not determined in this study, as no gold standard sera are available for Brazilian horses. Seroconversion of the tested animals to other Sarcocystis species that infect horses, including Sarcocystis bertrami or Sarcocystis fayeri (51), as well as to other Sarcocystis species shed by opossums, such as S. lindsayi or S. speeri, cannot be excluded in the current work. It is crucial to conduct further studies on molecular identification of Sarcocystis species in Brazilian horses with EPM, as well as to determine whether Brazilian strains of S. falcatula–like induce seroconversion in horses by oral ingestion of sporocysts of the parasite. To our knowledge, this is the first study to test horse sera with S. falcatula–like antigens and to provide evidence of serologic cross-reactivity in horses involving S. neurona and S. falcatula–like. Further studies are needed to determine an appropriate serological test to aid on diagnosis of EPM in Brazilian horses.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the animal study because no animals were raised or handled for research purposes, therefore, no license was required for the experiments. Samples from Bahia state (n = 267) derived from regular clinical screening for equine infectious anemia virus and for hematological checking. Samples from Rio Grande do Sul state (n = 142) were acquired in a commercial slaughterhouse for horse meat exportation.
WB-S: analysis and drafting of the manuscript. RJ: Western blot analysis and revision of the manuscript. RF: assistance in cell culture and IFAT. LG: designed the experiment and revised the manuscript drafts. All authors approved of the final version of the submitted manuscript.
This work was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (process no. 308795/2015-6). WB-S was granted with a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Code 001) and LG was recipient of a productivity fellowship by CNPq, Brazil.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.573016/full#supplementary-material
1. Reed SM, Furr M, Howe DK, Johnson AL, MacKay RJ, Morrow JK, et al. Equine protozoal myeloencephalitis: an updated consensus statement with a focus on parasite biology, diagnosis, treatment, and prevention. J Vet Intern Med. (2016) 30:491–502. doi: 10.1111/jvim.13834
2. Dubey JP, Howe DK, Furr M, Saville WJ, Marsh AE, Reed SM, et al. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM). Vet Parasitol. (2015) 209:1–42. doi: 10.1016/j.vetpar.2015.01.026
3. Fenger CK, Granstrom DE, Langemeier JL, Stamper S, Donahue JM, Patterson JS, et al. Identification of opossums (Didelphis virginiana) as the putative definitive host of Sarcocystis neurona. J Parasitol. (1995) 81:916–9. doi: 10.2307/3284040
4. Fenger CK, Granstrom DE, Gajadhar AA, Williams NM, McCrillis SA, Stamper S, et al. Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis sp. sporocysts from the opossum (Didelphis virginiana). Vet Parasitol. (1997) 68:199–213. doi: 10.1016/S0304-4017(96)01112-0
5. Dubey JP, Lindsay DS, Kerber CE, Kasai N, Pena HF, Gennari SM, et al. First isolation of Sarcocystis neurona from the South American opossum, Didelphis albiventris, from Brazil. Vet Parasitol. (2001) 95:295–304. doi: 10.1016/S0304-4017(00)00395-2
6. Cerqueira R. The distribution of Didelphis in South America (Polyprotodontia, Didelphidae). J Biogeogr. (1985) 12:135–45. doi: 10.2307/2844837
7. Box ED, Meier JL, Smith JH. Description of Sarcocystis falcatula stiles, 1893, a parasite of birds and opossums. J Protozool. (1984) 31:521–4. doi: 10.1111/j.1550-7408.1984.tb05495.x
8. Dubey JP, Lindsay DS. Sarcocystis speeri N. sp. (Protozoa. Sarcocystidae) from the opossum (Didelphis virginiana). J Parasitol. (1999) 85:903–9. doi: 10.2307/3285830
9. Dubey JP, Speer CA, Bowman DD, Horton KM, Venturini C, Venturini L. Experimental transmission of Sarcocystis speeri dubey and lindsay, 1999 from the south American opossum (Didelphis albiventris) to the North American opossum (Didelphis virginiana). J Parasitol. (2000) 86:624–7. doi: 10.1645/0022-3395(2000)086[0624:ETOSSD]2.0.CO;2
10. Dubey JP, Lindsay DS, Venturini L, Venturini C. Characterization of Sarcocystis falcatula isolates from the Argentinian opossum, Didelphis albiventris. J Eukaryot Microbiol. (2000) 47:260–3. doi: 10.1111/j.1550-7408.2000.tb00045.x
11. Dubey JP, Rosenthal BM, Speer CA. Sarcocystis lindsayi n. sp. (Protozoa. Sarcocystidae) from the South American opossum, Didelphis albiventris from Brazil. J Eukaryot Microbiol. (2001) 48:595–603. doi: 10.1111/j.1550-7408.2001.tb00196.x
12. Marsh AE, Barr BC, Tell L, Koski M, Greiner E, Dame J, et al. In vitro cultivation and experimental inoculation of Sarcocystis falcatula and Sarcocystis neurona merozoites into budgerigars (Melopsittacus undulatus). J Parasitol. (1997) 83:1189–92. doi: 10.2307/3284386
13. Dubey JP, Lindsay DS. Isolation in immunodeficient mice of Sarcocystis neurona from opossum (Didelphis virginiana) faeces, and its differentiation from Sarcocystis falcatula. Int J Parasitol. (1998) 28:1823–8. doi: 10.1016/S0020-7519(98)00166-0
14. Dubey JP, Speer CA, Lindsay DS. Isolation of a third species of Sarcocystis in immunodeficient mice fed feces from opossums (Didelphis virginiana) and its differentiation from Sarcocystis falcatula and Sarcocystis neurona. J Parasitol. (1998) 84:1158–64. doi: 10.2307/3284665
15. Lekutis C, Ferguson DJ, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii: variations on a theme. Int J Parasitol. (2001) 31:1285–92. doi: 10.1016/S0020-7519(01)00261-2
16. Howe DK, Gaji RY, Marsh AE, Patil BA, Saville WJ, Lindsay DS, et al. Strains of Sarcocystis neurona exhibit differences in their surface antigens, including the absence of the major surface antigen SnSAG1. Int J Parasitol. (2008) 38:623–31. doi: 10.1016/j.ijpara.2007.09.007
17. Yeargan MR, Howe DK. Improved detection of equine antibodies against Sarcocystis neurona using polyvalent ELISAs based on the parasite SnSAG surface antigens. Vet Parasitol. (2011) 176:16–22. doi: 10.1016/j.vetpar.2010.10.034
18. Reed SM, Howe DK, Morrow JK, Graves A, Yeargan MR, Johnson AL, et al. Accurate antemortem diagnosis of equine protozoal myeloencephalitis (EPM) based on detecting intrathecal antibodies against Sarcocystis neurona using the SnSAG2 and SnSAG4/3 ELISAs. J Vet Intern Med. (2013) 27:1193–200. doi: 10.1111/jvim.12158
19. Monteiro RM, Keid LB, Richtzenhain LJ, Valadas SY, Muller G, Soares RM. Extensively variable surface antigens of Sarcocystis spp. infecting Brazilian marsupials in the genus Didelphis occur in myriad allelic combinations, suggesting sexual recombination has aided their diversification. Vet Parasitol. (2013) 196:64–70. doi: 10.1016/j.vetpar.2013.01.019
20. Cesar MO, Matushima ER, Zwarg T, de Oliveira AS, Sanches TC, Joppert AM, et al. Multilocus characterization of Sarcocystis falcatula-related organisms isolated in Brazil supports genetic admixture of high diverse SAG alleles among the isolates. Exp Parasitol. (2018) 188:42–9. doi: 10.1016/j.exppara.2018.03.004
21. Gondim LSQ, Jesus RF, Ribeiro-Andrade M, Silva JCR, Siqueira DB, Marvulo MFV, et al. Sarcocystis neurona and Neospora caninum in Brazilian opossums (Didelphis spp.): molecular investigation and in vitro isolation of Sarcocystis spp. Vet Parasitol. (2017) 243:192–8. doi: 10.1016/j.vetpar.2017.07.002
22. Granstrom DE, Dubey JP, Davis SW, Fayer R, Fox JC, Poonacha KB, et al. Equine protozoal myeloencephalitis: antigen analysis of cultured Sarcocystis neurona merozoites. J Vet Diagn Invest. (1993) 5:88–90. doi: 10.1177/104063879300500118
23. Smith JH, Meier JL, Neill PJ, Box ED. Pathogenesis of Sarcocystis falcatula in the budgerigar. II. Pulmonary pathology. Lab Invest. (1987) 56:72–84.
24. Smith JH, Meier JL, Neill PJ, Box ED. Pathogenesis of Sarcocystis falcatula in the budgerigar. I. Early pulmonary schizogony. Lab Invest. (1987) 56:60–71.
25. Wendte JM, Miller MA, Nandra AK, Peat SM, Crosbie PR, Conrad PA, et al. Limited genetic diversity among Sarcocystis neurona strains infecting southern sea otters precludes distinction between marine and terrestrial isolates. Vet Parasitol. (2010) 169:37–44. doi: 10.1016/j.vetpar.2009.12.020
26. Cutler TJ, MacKay RJ, Ginn PE, Greiner EC, Porter R, Yowell CA, et al. Are Sarcocystis neurona and Sarcocystis falcatula synonymous? A horse infection challenge. J Parasitol. (1999) 85:301–5. doi: 10.2307/3285638
27. Gondim LFP, Soares RM, Tavares AS, Borges-Silva W, de Jesus RF, Llano HAB, et al. Sarcocystis falcatula-like derived from opossum in Northeastern Brazil: In vitro propagation in avian cells, molecular characterization and bioassay in birds. Int J Parasitol Parasites Wildl. (2019) 10:132–37. doi: 10.1016/j.ijppaw.2019.08.008
28. Acosta ICL, Soares RM, Mayorga L, Alves BF, Soares HS, Gennari SM. Occurrence of tissue cyst forming coccidia in magellanic penguins (Spheniscus magellanicus) rescued on the coast of Brazil. PLoS ONE. (2018) 13:e0209007. doi: 10.1371/journal.pone.0209007
29. Valadas SY, da Silva JI, Lopes EG, Keid LB, Zwarg T, de Oliveira AS, et al. Diversity of Sarcocystis spp. shed by opossums in Brazil inferred with phylogenetic analysis of DNA coding ITS1, cytochrome B, surface antigens. Exp Parasitol. (2016) 164:71–8. doi: 10.1016/j.exppara.2016.02.008
30. Lindsay DS, Mitchell SM, Vianna MC, Dubey JP. Sarcocystis neurona (Protozoa: Apicomplexa): description of oocysts, sporocysts, sporozoites, excystation, early development. J Parasitol. (2004) 90:461–5. doi: 10.1645/GE-230R
31. Foster DN, Foster LK. Immortalized Cell Lines for Virus Growth. Minneapolis: University of Minnesota (1997).
32. Gondim LF, Wolf A, Vrhovec MG, Pantchev N, Bauer C, Langenmayer MC, et al. Characterization of an IgG monoclonal antibody targeted to both tissue cyst and sporocyst walls of Toxoplasma gondii. Exp Parasitol. (2016) 163:46–56. doi: 10.1016/j.exppara.2016.01.014
33. Rossano MG, Mansfield LS, Kaneene JB, Murphy AJ, Brown CM, Schott HC, et al. Fox. Improvement of western blot test specificity for detecting equine serum antibodies to Sarcocystis neurona. J Vet Diagn Invest. (2000) 12:28–32. doi: 10.1177/104063870001200105
34. More G, Basso W, Bacigalupe D, Venturini MC, Venturini L. Diagnosis of Sarcocystis cruzi, Neospora caninum, and Toxoplasma gondii infections in cattle. Parasitol Res. (2008) 102:671–5. doi: 10.1007/s00436-007-0810-6
35. Duarte PC, Daft BM, Conrad PA, Packham AE, Gardner IA. Comparison of a serum indirect fluorescent antibody test with two Western blot tests for the diagnosis of equine protozoal myeloencephalitis. J Vet Diagn Invest. (2003) 15:8–13. doi: 10.1177/104063870301500103
36. Moré G, Vissani A, Pardini L, Monina M, Muriel M, Howe D, et al. Seroprevalence of Sarcocystis neurona and its association with neurologic disorders in Argentinean horses. J Equine Vet Sci. (2014) 34:1051–54. doi: 10.1016/j.jevs.2014.06.002
37. Arias M, Yeargan M, Francisco I, Dangoudoubiyam S, Becerra P, Francisco R, et al. Exposure to Sarcocystis spp. in horses from Spain determined by Western blot analysis using Sarcocystis neurona merozoites as heterologous antigen. Vet Parasitol. (2011) 185:301–4. doi: 10.1016/j.vetpar.2011.09.042
38. Daft BM, Barr BC, Gardner IA, Read D, Bell W, Peyser KG, et al. Sensitivity and specificity of western blot testing of cerebrospinal fluid and serum for diagnosis of equine protozoal myeloencephalitis in horses with and without neurologic abnormalities. J Am Vet Med Assoc. (2002) 221:1007–13. doi: 10.2460/javma.2002.221.1007
39. Dubey JP, Lindsay DS, Hill D, Romand S, Thulliez P, Kwok OC, et al. Prevalence of antibodies to Neospora caninum and Sarcocystis neurona in sera of domestic cats from Brazil. J Parasitol. (2002) 88:1251–2. doi: 10.1645/0022-3395(2002)088[1251:POATNC]2.0.CO;2
40. Hoane JS, Gennari SM, Dubey JP, Ribeiro MG, Borges AS, Yai LE, et al. Prevalence of Sarcocystis neurona and Neospora spp. infection in horses from Brazil based on presence of serum antibodies to parasite surface antigen. Vet Parasitol. (2006) 136:155–9. doi: 10.1016/j.vetpar.2005.10.023
41. Meneses ID, Andrade MR, Uzeda RS, Bittencourt MV, Lindsay DS, Gondim LF. Frequency of antibodies against Sarcocystis neurona and Neospora caninum in domestic cats in the state of Bahia, Brazil. Rev Bras Parasitol Vet. (2014) 23:526–9. doi: 10.1590/s1984-29612014080
42. Onuma SS, Melo AL, Kantek DL, Crawshaw-Junior PG, Morato RG, May-Junior JA, et al. Exposure of free-living jaguars to Toxoplasma gondii, Neospora caninum and Sarcocystis neurona in the Brazilian Pantanal. Rev Bras Parasitol Vet. (2014) 23:547–53. doi: 10.1590/s1984-29612014077
43. Gennari SM, Pena HF, Lindsay DS, Lopes MG, Soares HS, Cabral AD, et al. Prevalence of antibodies against Neospora spp, and Sarcocystis neurona in donkeys from northeastern Brazil. Rev Bras Parasitol Vet. (2016) 25:109–11. doi: 10.1590/S1984-29612016003
44. Tanhauser SM, Yowell CA, Cutler TJ, Greiner EC, MacKay RJ, Dame JB. Multiple DNA markers differentiate Sarcocystis neurona and Sarcocystis falcatula. J Parasitol. (1999) 85:221–8. doi: 10.2307/3285623
45. Hammerschmitt ME, Henker LC, Lichtler J, da Costa FVA, Soares RM, Llano HAB, et al. First molecular characterization of Sarcocystis neurona causing meningoencephalitis in a domestic cat in Brazil. Parasitol Res. (2020) 119:675–82. doi: 10.1007/s00436-019-06570-w
46. Masri MD, Alda JL, Dubey JP. Sarcocystis neurona-associated ataxia in horses in Brazil. Vet Parasitol. (1992) 44:311–4. doi: 10.1016/0304-4017(92)90128-V
47. da PaixãoTA, de Paula Rêgo IO, de Lima Santos R. Anti-Sarcocystis neurona immunostaining associated with equine protozoal myeloencephalitis in Brazil. Ciência Rural. (2007) 37:1820–3. doi: 10.1590/S0103-84782007000600052
48. Ribeiro MJM, Rosa MHF, Bruhn FRP, de Mello Garcia A, da Rocha CMBM, Guimarães AM. Seroepidemiology of Sarcocystis neurona, Toxoplasma gondii and Neospora spp. among horses in the south of the state of minas gerais, Brazil. Rev Bras Parasitol Vet. (2016) 25:142–50. doi: 10.1590/S1984-29612016029
49. Valenca S, Ribeiro-Andrade M, More G, Albuquerque PPF, Pinheiro Junior JW, Mota RA. Low prevalence of infection by Sarcocystis neurona in horses from the State of Alagoas, Brazil. Rev Bras Parasitol Vet. (2019) 28:298–302. doi: 10.1590/s1984-29612019027
50. de Jesus RF, Borges-Silva W, Bezerra TL, Gondim LQ, Uzeda RS, Gondim LFP. Serologic cross-reactivity between Sarcocystis neurona and Sarcocystis falcatula-like in experimentally infected Mongolian gerbils. Vet Parasitol. (2019) 276:108962. doi: 10.1016/j.vetpar.2019.108962
Keywords: Sarcocystis sp., equine, antibody, Western blot, immunoblot
Citation: Borges-Silva W, de Jesus RF, Ferreira R and Gondim LFP (2020) Reactivity of Horse Sera to Antigens Derived From Sarcocystis falcatula–Like and Sarcocystis neurona. Front. Vet. Sci. 7:573016. doi: 10.3389/fvets.2020.573016
Received: 15 June 2020; Accepted: 17 September 2020;
Published: 02 November 2020.
Edited by:
Annunziata Giangaspero, University of Foggia, ItalyReviewed by:
Daniel K. Howe, University of Kentucky, United StatesCopyright © 2020 Borges-Silva, de Jesus, Ferreira and Gondim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luís F. P. Gondim, cGl0YUB1ZmJhLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.