- 1School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom
- 2Department of Animal Sciences, School of Agriculture, Policy and Development, University of Reading, Reading, United Kingdom
- 3French Agency for Food Environmental and Occupational Health and Safety (ANSES), Regulated Products Assessment Department, Residues and Food Safety Unit, Maisons-Alfort, France
- 4Centre for Organics Research, Southern Cross University, Lismore, NSW, Australia
Low-input (LI) dairy farming, relying heavily on grazing, is increasing in popularity for perceived sustainability, welfare, and milk nutritional quality benefits. However, there is little research into the breed suitability for these systems. The popular Holstein–Friesians are not well-suited to LI production as, to achieve their potential high yields, they require high levels of concentrate intakes and veterinary inputs. Holstein–Friesians were traditionally bred for high milk yields, which often correlate negatively with functional traits, such as fertility and health. This drives the need for alternative breed choices, and UK dairy farmers use several crossbreeding practices. Additionally, classic measures of production efficiency (kilogram feed per liter of milk) are not the sole priority in LI systems, which also aim for improved health, fertility, forage conversion, and milk quality. This study aimed to explore the effect of breeding strategy on LI and organic production in dairy systems, collecting data from 17 farms throughout England and Wales: 7 organic and 10 low-input conventional systems with both purebred and crossbred cows from different breeds. Records from 1,070 cows were collected, including background data, health, fertility, breeding, and parity. Additionally, milk was analyzed on four occasions (autumn 2011 and winter, spring, and summer 2012). Principal components analysis was used to visualize the effect of management, Farm ID, and stage of lactation on LI production. The analysis clustered cows by Farm ID, showing that individual management practice on each farm had the greatest impact on various production traits. Cows were allocated a composite score based on their yield, health records, and milk fatty acid profile, and a linear mixed-effects model indicated (p < 0.01) that crossbred New Zealand Friesian cows scored highest, whereas Dairy Shorthorn cows scored the lowest. This paper highlights weaknesses in current breeding programs for LI and organic farms in the UK, in terms of the alignment of breeds with husbandry practices. Additional research is needed to explore any gene by environment interactions to meet the true potential of individual cows and certain breeds under LI and organic management.
Introduction
Organic farming in the UK is defined by European Union (EU) regulations (1) and certifying bodies such as The Soil Association (2) and Organic Farmers and Growers (3). However, many farms operate low-input (LI) systems, which are not organic and not formally defined or regulated. LI farming refers to the practice of using fewer inputs than conventional agriculture but not necessarily meeting organic or other quality assurance standards. Motivations toward LI farming include economic, environmental, and social parameters (4). The main criticism of organic and LI farming is that, compared with intensive systems, lower yields require more land to produce the same amount of food, leading to poorer biodiversity if seminatural vegetation is converted to agriculture (5). However, rejecting organic production methods by emphasizing yield productivity ignores opportunities for practices that enhance sustainability; therefore, alternative metrics must assess LI systems. Over the past 60 years, dairy farming has typically focused on making better use of inputs, maximizing profit, relying heavily on high yields and improved feed efficiency (kilogram dry matter intake per liter of milk) [e.g., (6) Milkbench + system]. However, in organic and LI dairying, priorities are different, whereas profit is still essential; the production system involves fewer inputs. Feed efficiency is equally important, but the pathway to achieve this is mainly on reducing external inputs rather than maximizing outputs, a practice that may also benefit herd health (7). Reducing the intensity of production lowers veterinary bills and costs associated with inseminations while using optimal grazing strategies (such as mob-grazing) to enhance soil and sward health, meaning cows consume a richer pasture and produce more nutritious milk (8, 9). A robust method to determine sustainability, accounting for animal health/welfare, nutritional quality, and environmental/social impacts, is needed. Although fully exploring the sustainability of LI dairying is beyond the scope of this study, this paper explores aspects of breeding, production output, health and milk quality of LI, and organic dairy farms.
Traditionally, Holstein/Friesians (HF) has been at the forefront of high yielding dairy production globally. Holsteins are primarily selected for their production traits (milk yield and composition), whereas more traditional Friesians can be selected for functional traits (health and fertility). However, HF cows are not well-suited to LI and organic systems, as they require relatively high levels of both concentrates to achieve maximum yield potential and veterinary inputs (10). Instead, breeds able to maintain health and productivity with LIs are preferred. As a cross, HFs are bred for production traits (higher yield), which are often negatively correlated with functional traits, such as a decline in fertility and health (11). To maximize the potential of both alternative and high-yielding breeds, LI and organic dairy systems have increased their interest in crossbreeding dairy cattle, introducing genetics from more robust breeds (12). Additionally, functional traits are heavily influenced by the local environment and have low heritability (11), making it difficult to select genetic lines to improve health and fertility. For this reason, LI and organic systems benefit from crossing with breeds known to have stronger functional traits.
Organic and LI systems often rely on crossbreeding strategies to optimize their herd health and yield potential. A strong reason for crossbreeding is the resulting heterosis or hybrid vigor in the first generation (F1). Crossbred offspring (including HF crosses) outperformance relative to the parental average is one way to improve functional traits (13) without impacting milk production. However, to extend the benefit beyond the first generation, a carefully designed system is required for rotational crossbreeding: crossing two F1 individuals only expresses half the hybrid vigor, whereas introducing a third breed preserves up to 86% of the heterosis (12). Crossbreeding high-production HF with traditional breeds better suited to LI management (with high forage diets) shows potential. For example, recent studies comparing breeds and crossbreeding regimes in Switzerland and the UK showed more traditional breeds, or crossbreeding with traditional breeds can significantly improve the economic performance and milk quality in LI grazing based dairy systems (10, 14). The indicators from these studies are positive, but further research is needed to identify the key mechanisms required to produce predictable, repeatable, efficient, and effective crossbreeds.
There is very little recognized research into breeding for crossbred cattle in smaller LI and organic dairy systems. Yet, these farms have progressed with crossbreeding for many generations within their herds, each using a different strategy to search for breed combinations that perform within their system (15). Therefore, there is not a clear breed (or crossbreed) that typically outperforms others in LI systems, in the way that HF dominates conventional production. In addition, most scientific research has focused on HF because they account for 95% of the EU dairy cow population (16). UK organic milk was valued at £351 million in 2018, with over 25% of UK households purchasing organic milk, representing 5.1% of retail milk sales (17), highlighting the increased need to develop appropriate crossbreeding schemes for such production chains. Studies from a range of countries argue that, due to genetic × environment (GxE) interactions, optimal genetic progress requires either independent breeding programs or an index (to rank sires against requirement) specific for each farming system (18–23). This approach would directly benefit LI and organic systems.
The complexity of breeding support for LI dairying is not well-established in the UK. In LI and organic dairy systems, the diet is predominantly forage; therefore, it is beneficial to have cows that efficiently convert forage, especially grazing, to milk (24). However, current UK breeding objectives available do not include forage conversion as a desirable trait when calculating economic values of genetic gain. Instead, the Agriculture and Horticulture Development Board (AHDB-UK levy board funded by farmers and growers) breeding index for year-round calving focuses on milk production (34.4%), health (21.8%), fertility (15.3%), and temperament, among other traits (25). The AHDB also has a Spring Calving Index, aimed at herds making use of grazed grass by assigning 71.6% of the weighting to fitness traits, but the dominant individual driver is still production (27.4%), and the link between efficiency (with an emphasis on forage conversion) in LI systems has yet to be fully explored. Typically, LI and organic management that supports animal health and mastitis is the main concern (26), whereas health and fertility remain essential in these systems; the risk of illness (for example, acidosis) is much reduced. Although these UK resources for dairy breeding selection exist, other options seem more appropriate for organic and LI production.
Milk quality has gained a lot of media attention recently, continuing the debate around the role of milk in human diets and the environment (27). Milk fatty acid (FA) profile is strongly influenced by management, and there is a clear difference in the FA profiles of organic and conventional milk (28–30) between the different stages of lactation (31) and seasonally (32, 33). Additionally, FAs can vary as much within- as between-breeds (34, 35), making it harder to isolate breeds that could give an “optimal” FA profile within a specified management system. Some FAs have been studied closely for their effects on human health. The main FAs considered to have a positive effect on human health are alpha-linolenic acid, eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), oleic acid (OA), and cis-9 trans-11 conjugated linoleic acid (CLA9). Alpha-linolenic acid is the most abundant omega-3 (n-3) FA and promotes healthy aging and fetal development (36, 37). The long chain n-3 FAs, EPA, DPA, and DHA are anti-inflammatory and reduce the risk of coronary heart disease (CHD) (38). OA can reduce the risk of CHD and promotes stable cellular membranes (39). CLA9 has been shown to lower the risk of CHD and enhance the immune system (40, 41). In contrast, FAs highlighted as undesirable in human nutrition due to their association with increased CHD risk are lauric (C12:0), myristic (C14:0), and, in particular, palmitic (C16:0) acids (39). Also, the most abundant omega-6 (n-6), linoleic acid, is an essential FA in human diets, but if total n-6 is in excess, as prevalent in Western diets, it becomes pro-inflammatory with negative health effects (42). Of greater relevance is the dietary ratio of n-6/n-3, which, when too high (the exact optimal ratio is unknown), may cause inflammations and increase CHD risk (42, 43). Although there is currently no premium in UK linked to milk fat composition, in the USA, CROPP's organic “Grassmilk™” receives a 15% premium above standard organic milk prices for meeting minimum requirements for the n-6/n-3 ratio, total n-3, and CLA (29). This demonstrates the potential for other sectors and countries to create premium dairy products with an increased concentration of beneficial FAs.
Historic approaches to breeding in dairying have not taken a whole system view, generally resulting in poor health traits and concentrate-dependent cows (16). If robust methods to identify cattle that best suit a particular system are to be developed, there is the potential to improve animal health and welfare, production, nutritional quality, milk FA profile, and efficiency. This paper aims to identify breeds within LI and organic dairy systems that can maintain health and yield while producing milk with a beneficial FA profile. The objectives are to (a) define the variables most relevant to LI and organic farming and observe differences in the management system (individual farms), (b) identify breeds that are similar across the farms and quantify differences, (c) develop a score for LI-production (LI-P) to identify breeds that best suit LI and organic production in terms of production, health, and milk composition with respect to consumer health.
Materials and Methods
Data Collection
Data for this study were collected from 17 dairy farms (7 organic and 10 LI-conventional) throughout England and Wales between November 2011 and October 2012. All herds were a mix of both purebred and different crossbred cows (Table 1). Herd sizes ranged from 150 to 550 cows, and a total of 1,070 cows were recorded to encompass a broad range of breeds and crosses from each farm. A one-off questionnaire was completed to gain information on pre-survey health and parity as well as a breeding pedigree for all individual cows (according to the farmers' records). Milk from each cow was sampled over four dates: autumn 2011 (D1), spring (D2), summer (D3), and autumn 2012 (D4). A corresponding questionnaire for each farm and cow was used to record husbandry practices on all sampling dates, including milk yield, disease incidence, health treatments, cow diet, calving intervals, milking, and grazing management. Organic farming standards require concentrate feed to be sourced organically and have strict land management application practices (2), whereas LI follow similar practices but are not certified organic. Organic and LI farms fed similar levels of concentrate per cow, and organic farms typically fed more conserved forage (Supplementary Table 1). Access to grazing varied across the year and individual management (Supplementary Table 2). All milk samples were analyzed for basic composition, somatic cell count (SCC), and FA profile. All procedures were acceptable to internal ethical review, in accordance with EU Directive 2010/63/EU for animal experiments and approved by the Animal Welfare and Ethical Review Body at Newcastle University.
Milk Analysis
A representative raw milk sample was collected from each cow during milking in the parlor on each sampling date. Milk samples were preserved with Bronopol and kept at ambient temperature during transportation to a commercial National Milk Recording (44) lab. Basic milk composition was analyzed using Milkoscan FT 6000 (Foss Electric, Hillerød, Denmark) (milk fat, protein, urea, and lactose content), and SCC was recorded using a Fossomatic instrument (Foss Electric). The samples were then transported at ambient temperature (10–25°C) to Newcastle University, frozen at −20°C. Bronopol preserves milk for more than 5 days and is effective unless temperatures are consistently high (45); ambient temperature varied by season, but milk was frozen within 4 days of collection. There is some evidence that Bronopol may have a small impact on minor long-chain FAs (46) and protein concentration (47). However, all milk samples in this study were treated the same and are therefore comparable. Milk was defrosted at 4°C, stirred thoroughly to homogenize, and 3–4 ml of milk was transferred in a 7-ml container, frozen at −20°C, and freeze-dried. The lipid was extracted using the method described by Chilliard et al. (48), where 130 μg of lyophilized milk was methylated and esterified. Gas chromatography (Shimadzu, GC-2014, Kyoto, Japan) equipped with a flame ionization detector and by using a Varian CP-SIL 88 fused silica capillary column (100 m × 0.25 mm ID, 0.2 μm film thickness) was used to analyze the FAs. The gas chromatography method has been previously described by Stergiadis et al. (49). Individual FAs were identified against peaks generated by a 52 methyl FA standard, with the area under each peak quantifying the relative proportion of each in the total FAs. An FA methyl ester standard and published chromatograms (50, 51) were used to identify the FAs, and correction factors for short-chain FAs were applied using the method described by Stergiadis et al. (49).
Data Handling
Breed Combinations
The farmers' breeding records categorized all animals. Cows were given a code based on their sire, dam, and predominant breed, for example, a pure-bred Jersey = JE; sire Jersey and dam Ayrshire = JEAYR; sire Jersey × Shorthorn and dam Jersey × Ayrshire = JEX (Table 1). The X indicates a majority genetic contribution and/or a back cross. Including the sire and dam breeds for all cows across the study resulted in around 40 different breed combinations of varying population sizes, depending on the sampling date. This ranged from a single representative on one farm (British Friesian × Montbelliarde) to 119 HF individuals across all farms for D2. To rationalize the number of crossbreed combinations in this study, there is no differentiation between the contribution of genetics by parents' sex. For example, both a cross from a Jersey sire and HF dam and from an HF sire and Jersey dam are labeled HFJE.
Data Analysis
Microsoft Excel was used for data handling, whereas all statistical analysis was completed using “R” (52). The background information on the farms and monitored cows is displayed in Table 1.
Low-Input-Production and Principal Components Analysis
The initial data collection involved 1,070 cows, but for some farms and/or cows on some sampling dates, there are missing and incomplete records. For the observational statistics, the cows selected had records on any given date for production, health, and FA composition results (explained later). This resulted in 299, 757, 772, and 613 cows on D1, D2, D3, and D4, respectively.
Focusing on the available data, using a combination of farm records and results from milk analysis and the priorities of typical LI practices, the variables selected to define LI-P were split into three main criteria:
1. Production:
i. Milk yield (L/day).
ii. Total fat and protein solids (kg/day).
2. Health:
i. Udder health; SCC (×103 cells/ml milk).
ii. Treatments, including antibiotics (e.g., for mastitis or metritis) or other (e.g., for lameness, milk fever, or pain/inflammation).
3. Fatty acid profile:
i. Percentage of total profile with desirable FAs (n-3, OA, CLA9, EPA+ DPA+ DHA).
ii. Percentage of total profile with FAs often consumed in excess and undesirable (C12:0, C14:0, C16:0, n-6, and n-6/n-3 ratio).
The elements of LI-P had different units (FAs were proportional, yield: liters/cow/day, SCC: × 103 cells/ml milk, etc.); thus, the data was standardized (normalization to mean of zero and standard deviation of one) (53) to give each element of LI-P the same weight. Principal components analysis (PCA) in the package “vegan” (54) was used to aid visualization of the effects of Farm ID (2–17) on LI-P. Two sets of graphs were produced from the PCA. First, graphs (Figure 1) in which points represent samples/records from cows (at each farm, one graph for each the four dates), where the closer two points are to each other in PCA ordination space, the more similar their characteristics (in terms of production, health, and milk FA profile). The points in these graphs were color-coded by farm identity to aid interpretation. Second, PCA graphs of these characteristics (Figure 2), in which points close together, indicate co-occurrence on similar farms or farming systems. This second set of PCA graphs were also broken down by date. In other words, the characteristics that are grouped together in Figure 2 can be associated with cows and/or farms that occupy similar ordination space in Figure 1.
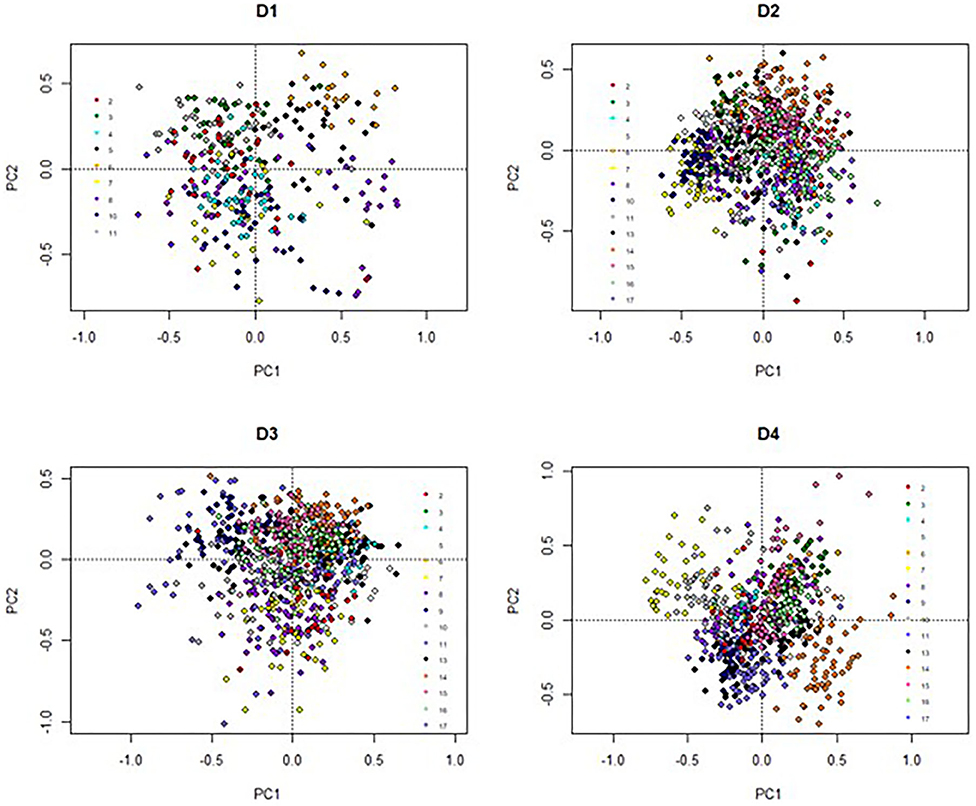
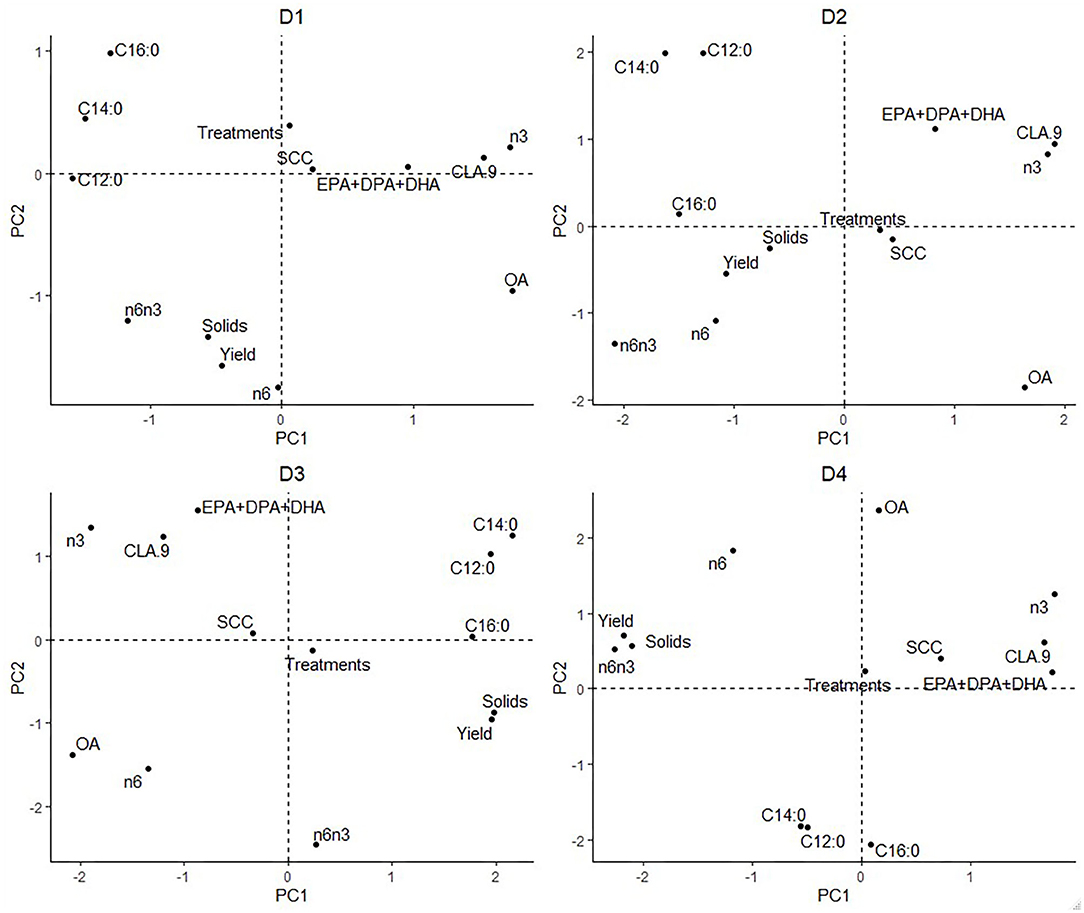
Figure 1. Principal components analysis based on low-input-production, highlighted by Farm ID. C12:0 = Lauric Acid, C14:0 = Myristic Acid, C16:0 = Palmitic Acid, CLA.9 = Conjugated linoleic acid (C18:2, c9t11 isomer), n3 = omega-3, n6 = omega-6, n6n3 = omega-6/ omega-3 ratio, EPA + DPA + DHA = EPA = Eicosapentaenoic Acid + DPA = Docosapentaenoic Acid + DHA = Docosahexaenoic Acid, SCC = Somatic Cell Count, Treatments = Health Treatments.
Descriptive Statistical Analysis
For the descriptive statistical analysis, additional inclusion criteria were considered: on any sampling date, records existing for at least six cows of the same breed (combination) from at least three different farms. These criteria resulted in the most breed combinations and ensured comparison between breeds rather than individual farm management style. After these additional inclusion criteria had been applied, there were eight breeds for comparison: Ayrshire cross (AYRX, n = 100), HF (HF, n = 325), HF × Jersey (HFJE, n = 184), HF × Scandinavian Red (HFSR, n = 274), Jersey cross (JEX, n = 121), New Zealand Friesian cross (NZFX, n = 90), Dairy Shorthorn (SH, n = 80), and Scandinavian Red cross (SRX, n = 140). The number of cows represented by each breed from each farm is available in Supplementary Table 3.
The “R” package “nlme” (55) was used to model “Breed” against the variables described for the LI-P, with Season and Farm ID as random factors. The linear mixed-effects model accounts for variation explained by the fixed effects (Breed) and random effects (Season and Farm ID). As farms were observed across the four sampling dates, these related measures would violate the independence assumptions made by a linear model, hence, the use of “Farm ID” and “Season” as random factors. Days in milk did not differ between the breeds (F-statistic = 1.50, p = 0.165), allowing the breed to be compared without differentiating or adjusting for the stage of lactation. On each date all cows from the same farm were fed the same ration, not as individuals (Supplementary Table 1). The feed data did not meet the assumptions of the model; therefore, mean and standard deviations are given, but a p-value is not provided. Observationally, there was no big difference in the amount of concentrate fed between the breeds, but there was a notable difference in the amount of conserved forage-fed between breeds (Table 2). Concentrate feeding is thought to have the biggest impact on the FA profile (56); therefore, no corrections were made to the data before analysis.
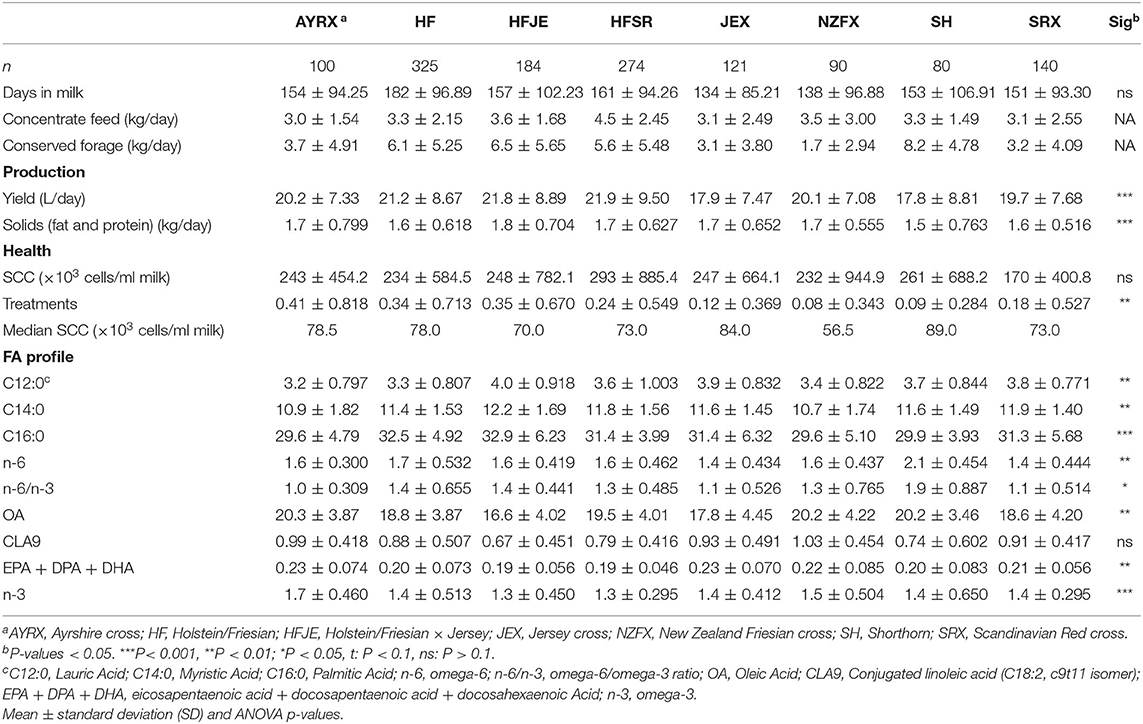
Table 2. Effect of breed on components of low-input-production: production (milk yield and total fat and protein solids), health (health treatments and SCC), and nutritionally relevant FA in milk (expressed as a percentage of the entire FA profile).
Traditionally, post-hoc Tukey honest significant difference tests are used for multiple comparisons of levels within a factor. However, due to the complexity of this data set with multiple levels of comparison (8), some with few replicates, controlling the familywise error rate even by this approach would risk numerous type 1 errors (false-positives) and would be misleading (57–59).
Low-Input Production Score
To create a universal score for each record, common units are required. Using the variables selected for LI-P, scores were created for each cow record to assess the best performing breed. Milk yield, total fat and protein solids, SCC, and proportions of desirable and undesirable FAs were (higher rankings indicate more beneficial qualities) scored as described next.
1. Production records [milk yield (L/day) and total fat and protein solids (kg)] were allocated into five groups of equal observations, rated 1–5 with 5 the highest and 1 the lowest. Scores were combined to make a total production score, out of 10.
2. SCC (×103 cells/ml milk) was allocated into five groups of equal observations rated 1–5 with 5 the lowest and 1 the highest. For veterinary treatments, cows were given a 1 if they received no treatments and 0 if they had been given antibiotics or an alternative (e.g., for mastitis or metritis or other, e.g., for lameness, milk fever or pain/inflammation) at least once since the previous collection date, which was added to the SCC category resulting in a total health score, out of 6.
3. For desirable FAs (OA, CLA9, n-3, and EPA + DPA + DHA), concentrations were ranked and allocated to five equal groups with a score of 5 was given to the highest and 1 to the lowest group, whereas undesirable FA (often consumed in excess) (C12:0, C14:0, C16:0, n-6, and n-6/n-3 ratio) scores were reversed, 5 to the lowest group. FA categories were combined to create an FA score, out of 45.
These individual assessments were then used to calculate a single score (out of one) for each cow record using two alternative approaches. The score weightings are based organic and LI values, the AHDB Spring and Autumn calving indices (60) and the premium offered for FA quality by Organic Valley's Grassmilk® (29).
• Weighted health score: the scores were weighted at 30% production, 50% health, and 20% FA.
• Weighted production score: 60% production, 30% health, and 10% FA.
For example:
Weighted health score = 30% * (production score/10) + 50% * (health score/ 6) + 20% * (FA score/45).
Results
Low-Input-Production and Principal Components Analysis
The PCA result is displayed in Figures 1, 2. On D1, 46% of the total variance was explained by PC1 (29%) and PC2 (17%). On D2, 43% of the variance was explained by PC1 (25%) and PC2 (18%). On D3, 51% of the variance was explained by PC1 (31%) and PC2 (20%). On D4, 55% of the variance was explained by PC1 (29%) and PC2 (26%).
The individual farm had major influences on LI-P, especially on D4 (autumn 2012) (Figure 1), where cows from the same farm are clearly clustered together. Farm 7 cows are tightly clustered in the negative PC1 axis and positive PC2 axis, whereas Farm 14 cows are clustered in the negative PC1 and PC2 axis. The beneficial FAs n-3 and CLA9 and EPA + DPA + DHA generally occurred close together in PCA ordination space, whereas the detrimental saturated FAs C12:0, C14:0, and C16:0 are together in the opposite axes quadrants on all four sampling dates, D1–D4 (Figure 2).
Interpretation of Figure 1 is aided by cross-referencing with Figure 2 to superimpose the latter onto Figure 1. For example, on date D1, many cows from Farm 8 are associated with high levels of CLA9 and OA in milk. In contrast, Farms 2, 3, and 11 have higher saturated FA: C12:0, C14:0, and C16:0 concentrations. However, D2 cows from Farms 6, 15, and 16 are associated with the beneficial FA EPA + DPA + DHA and CLA9 and Farm 17 with n-6 and a high ratio of n-6/n-3. Across all four sampling dates, Farm 7 (yellow) stands out for producing milk with elevated n-6 content and n-6/n-3, although no farm is consistently associated with beneficial FA in milk.
Effect of Breed on Low-Input-Production
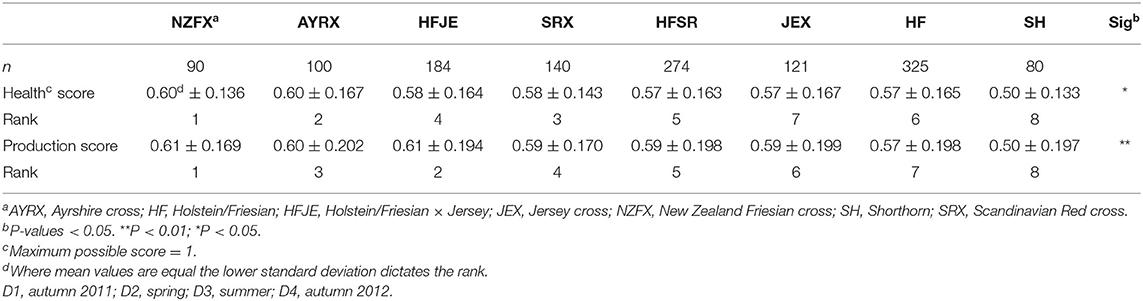
The mean values for the components of LI-P for the eight most common breeds and crosses are shown in Table 2. Averaging data (over four dates) from multiple farms with similar breed combinations indicated that the individual parameters used to define LI-P did significantly differ between breeds, although, again, there was no difference in the stage of lactation between the breeds in this data set. The highest yielding breed was the HF (21.2 L) and the HF crosses (HFJE: 21.8 L and HFSR: 21.9 L), and HFJE had the highest fat and protein solids (1.8 kg). However, HF and the crosses had the lowest concentrations of long-chain n-3 FAs [EPA + DPA + DHA [HF: 0.20%, HFJE: 0.19%, and HFSR: 0.19%]], and HFJE and HFSR had the lowest total n-3 (both 1.3%). Additionally, HFJE had the highest concentrations of C12:0 (4%), C14:0 (11.2%), and C16:0 (32.9%). AYRX had the lowest concentration of C12:0 (3.2%), C16:0 (29.6%), and n-6/n-3 (1.0) and also had the highest concentration of OA (20.3%), CLA9 (0.99%—not significant), EPA + DPA + DHA (0.23%), and n-3 (1.7%). SH had the lowest average daily yield (17.8 L) and solids (1.5 kg), a high average cell count (261 × 103 cells/ml milk), the highest concentration of n-6 (2.1%) and n-6/n-3 (1.9) and had a low concentration of EPA + DPA + DHA (0.20%), n-3 (1.40%), and CLA9 (0.74%).
There was no difference in the SCC between breeds, but 12% of SCC recordings from individual cows were above the EU standard, ranging from 400,000 to 9,000,000 cells/ml milk. This resulted in SCC having a very wide standard deviation; therefore, the median values were included in Table 2 (as well as mean values) for a more representative SCC status. The median cell counts for each breed are below 90,000 cells/ml milk. Most health treatments were given to the AYRX (0.41), whereas the NZFX (0.08) and SH (0.09) received the least.
Low-Input-Production Score
The two LI-P scores for each breed combination are presented in Table 3. The NZFX was the highest-scoring breed, ranking first under both the weighted health and production scenarios, whereas SH was the lowest-scoring breed ranking last in both scenarios. The largest change in the LI-P score with the different weightings was HFJE, which scored fourth in the health score, but second, emphasizing production.
Discussion
The data collected for this paper provides valuable information from commercial farms of direct practical application for farmers, in an area lacking in the scientific literature. As a study monitoring on-farm activities, many variables are not controlled, but the statistical model mitigates some of these effects. The data collected is of sufficient quality and range to provide invaluable insights into LI-P systems in the UK. This includes the effects of breed combinations on LI-P and determining how and why breeds are suited to different farms. Although this paper does not draw definitive conclusions, it explores the current status of dairy breeding strategies and highlights how farmer's decision-making should direct future LI (cross) breeding research.
Low-Input Production
The influence of farm management (e.g., breed, diet, calving date, and nutrition) on milk composition, yield, and animal health has been well documented (28, 29, 61, 62). These effects are seen in the PCA analyses (Figure 1), where each farm system clusters (apart from D1, with fewer records). Most organic cows were autumn calving, and many LI were spring calving (Table 1). Due to this collinearity, it would be statistically difficult to identify if management (organic vs. LI) or stage in lactation affected LI-P. Additionally, the collinearity violates the assumptions of most statistical models on the independent influence of factors; it would therefore be incorrect to separate these in an attempt to identify whether the management or lactation stage has the strongest influence on LI-P. It is clear, nevertheless, from Figure 1 that LI-P is very closely associated with individual farms. The specific aims and preferences of individual farmers result in decisions about suitable breeds for that particular system, and as these management decisions are unique to each farm, the effect of breed on LI-P is multifaceted.
Feeding
Although the scoring system aimed to identify breeds well-suited to LI farming, there were differences in supplementary feeding between breeds, which could influence findings. The amount of concentrate feed offered was fairly consistent across breeds (from 3.0 to 4.5 kg per head per day), although conserved forage offered was more variable, ranging from 1.7 to 8.6 kg per head per day. Increasing fresh forage in the diet influences milk fat composition, raising CLA9 and omega-3 (29, 63), and if we assume fresh forage consumption is indirectly proportional to the amounts of other feeds offered (32), we could expect the ranking of the breeds to follow a similar pattern—driven by the positive influence of milk fat composition to these composite scores. However, although this holds for the best and worst ranked breeds under both scores [NZFX ranked first on both scores, had the lowest supplementary feeding, the highest concentration of CLA9 [1.03%], and the second highest concentration of n-3 [1.5%] and SH, eighth on both scores, had the highest level of supplementation offered], the ranking of all other breeds does not follow combined supplementary feeding rates. The AYRX outranked both SRX and JEX in health and production scores, whereas HFJE outranked JEX in both scores and SRX in production score; yet, both AYRX and HFJE received more supplementary feed than JEX and SRX. Despite receiving higher levels of supplementary feed than JEX and SRX, milk from AYRX cows had the highest concentration of n-3 (1.7%) and second-highest CLA9 (0.99%) among all the breeds. At the other end of the health and production ranking, JEX cows were judged seventh and sixth yet were offered the second-lowest level of supplementary feeding, hence expected to have a relatively high grazing intake. Despite the evidence that feeds management has the greatest impact on the FA profile (29, 61), this study sampled milk from a wide variety of farms and breeds where the effect of diet was possibly minimized, potentially displaying differences between the breed.
Animal Health
SCC is an indication of udder health, cow welfare, and milk quality. Generally, if SCC is below 100,000 cells/ml milk, the cow is considered healthy, whereas above 200,000 cells/ml milk, the cow is likely to have at least one mastitic quarter, and, although some cows naturally have higher SCC, above 400,000 cells/ml milk is deemed unfit for human consumption by the EU (64). During the study, only 19% of high SCC (>400,000 cells/ml milk) cows received a health treatment (veterinary or other). Under EU organic guidelines, cows are expected to resist infection through effective management (65), suggesting that the farmers in this study were more likely to allow cows to build immunity to fight infection rather than treat with antibiotics. Interestingly, HF and HF crosses were responsible for 41% of the high cell counts, whereas only 4% of cows with SCC over 400,000 cells/ml milk were the best performing breed (NZFX), providing evidence that NZ genetics have effective health traits. Additionally, this portion of animals with high cell counts highlights the need and potential benefit of breeding for improved health traits, especially in organic production systems, when prophylactic treatment is not an option. A recent report found antibiotic use in livestock decreased 40% from 2013 to 2017 (66), but there is still pressure on dairy industries to reduce antibiotic use due to antimicrobial resistance, which already impacts human and animal health (67).
Breeding Objectives
The effect of forage diets on milk FA profile has been well-researched (29, 46, 61), but forage conversion by diverse breeds in LI systems has not. Most of the research into forage conversion has predominantly focused on HF (68, 69). Other studies have suggested that the JE × HF cross is better suited to a pasture-based system (70–72) but only compared with HF. As a generalization, HFs were bred for their production traits rather than milk composition or health traits (73). This was reflected in this study, as cows with HF genetics had the highest yield (21.2–21.9 L/day), and HFJE had the most protein and fat solids (1.8 kg/day), but SCC was highest for HFSR (294,000 cells/ml milk) and HFJE (third highest: 248,000 cells/ml milk). Although HFs are important in the UK, and their crosses have worked well in some grazing based systems, further research into forage conversion in more diverse breeds is needed to improve LI and organic dairy systems. Although cattle diets might be the dominant factor controlling milk FA profiles, there is also evidence that heritability affects milk fat composition both within and between breeds (20, 34). This suggests a combination of feeding forage and selective breeding may optimize FA composition for consumer health. Despite breeding bodies and milk purchasers prioritizing milk fat and protein content, there is currently no premium to reward fat composition in the UK. Organic Valley's “Grassmilk™” (USA) receives a 15% premium above organic prices for n-3, CLA9 content, and n-6/n-3 ratio (74). This demonstrates a market for optimizing milk fat composition and thus creates a marketing opportunity for UK milk.
An alternative benchmark for LI dairy is the New Zealand National Breeding Objectives, in which grazing is emphasized and priority placed on forage conversion, the yield of milk components (protein and fat %), health, and fertility (75). Based on the importance of forage in NZ dairying, it is unsurprising that the NZ Friesian cross outperformed all other breeds in this study, ranking first in both performance scores (Table 3). Although the breed is an important component of management, diet is the strongest factor that influences FA composition in milk (46), whereas high intakes of forage in the diet increase milk n-3 concentrations and reduce n-6/n-3 ratio (28, 29). The contribution of milk FA profile to the LI-P score identifies a breed's ability to graze and use grass efficiently; therefore, the concentrations of n-3, CLA9, or n-6/n-3 ratio in milk could be used to predict how well forage is converted to milk.
Effect of Breed on Low-Input-Production
The results of this study confirm that although management on individual farms affects LI-P, the breed also plays an important role. Despite ranking last under both scenarios (Table 3), shorthorns are well known for their positive temperament, high fertility, and efficiency in converting forage to milk (76), which are all metrics important for LI dairying although not formally analyzed in this study. In terms of desirable milk–fat composition, AYRX had the most desirable FA profile. However, AYRX yielded less milk (20.2 L/day) than the more productive HF crosses (21.2–21.9 L/day) and came fourth for SCC (243,000 cells/ml milk). Despite this, the AYRX ranked second in health and third in the production score. Ayrshires are commonly used in organic systems because of their ease of management, forage to milk conversion, and overall health and longevity (77). The Jersey crosses did not rank well (rank = seventh weighted health and sixth in production score), but the Jersey has many desirable traits for organic and LI systems (78). The Ayrshire, Shorthorn, and Jersey have merits beyond the scope of this study to measure; additionally, the low UK population of these breeds offers less scope for selection than the more popular HF.
Scandinavian Reds have a reputation for good udder health (79), and the SRX had the lowest average SCC (170,000 cells/ml milk); however, the HFSR had the highest average SCC (294,000 cells/ml milk), but interestingly, the median of both SR crosses was the same (73,000 cells/ml milk). This suggests that farms with a high mastitis challenge might cross HF with SR due to their reputation and breeding history, potentially instead of changing management to reduce infection risk.
The breeds in this study are generally popular and well-suited to organic and LI farming. Despite this, many of the desirable traits for organic and LI dairying were not measured in this study (forage conversion, fertility, temperament, ease of calving, etc.). It is easy to pick and choose the characteristics that could make a breed look “better” or “worse;” it can be subjective, but farmers make their decisions based on their priorities and what works best for their specific system, and despite the low score for LI-P, many of these breeds are all essential for LI and organic dairying.
Heterosis
Another important factor to consider in a crossbreeding program is heterosis and the effects of back-crossing, as demonstrated from the breeding approach used on these organic and LI farms. All farms had at least three core breeds (Table 1), most of which get crossed and back-crossed. In this study, of the 1,070 cows selected from 17 farms, 40% were F1 (first generation crosses), 40% were F2 or subsequent generations, and only 20% were purebred. This confirms that in these LI and organic systems, cross-breeding is essential to develop robust, productive cows. As discussed, much of the published research is centered on HF crosses, which, as demonstrated by this study, are not representative of LI and organic management practices on the UK farms studied. Additionally, maximizing the benefits of hybrid vigor can be complicated and unpredictable, but challenging organic conditions often make heterosis worthwhile (80). Partially due to the emphasis on specific breeds, such as HF, there is little readily available, independent advice for farmers with alternative breeds, regarding heterosis. Further studies are needed using a diverse range of breeds to fully understand this effect and the benefits it offers (81), but as demonstrated by the predominance of crossbreeding in this study, the industry is ahead of the science—farmers are investigating the effects for themselves.
Genotype by Environment Interaction
The genotype by environment interaction (GxE) is key to distinguishing between intensive and LI or organic breeding programs. Nauta et al. (18) first explored the GxE differences between organic and conventional dairying and reported heritabilities of SCC and production traits that warrant a re-ranking of dairy bulls for organic systems. The abundance of cross-breeding in this study indicates that farmers are learning about how (cross) breeds interact with their environments, potentially observing heterosis and GxE independently, suggesting that for LI and organic breeding objectives to be successful, the science will have to align with farming practices. Rodriguez-Bermudez et al. (23) conclude that by breeding for intensive systems, organic cows will not meet their potential due to the impact of GxE interactions on performance. To improve efficiency in LI/organic dairying, genotypes must be well-adapted to their systems, which has less emphasis on production but a greater focus on fertility and resilience (81). Keeping the GxE interaction in mind when developing and evaluating breeding programs is essential to allow livestock to meet their potential—regardless of the system that they are kept in.
To conclude, this paper highlights weaknesses in current UK breeding programs for LI and organic dairying due to limited past research on forage conversion to healthy milk and a bias toward HFs. The lack of robust scientific evidence necessary to advance breeding systems has resulted in the science-base often being behind best farming practices. Evidence from this study indicates that New Zealand Friesian and Ayrshire genetics could suit some LI/organic farms. Thorough further research is needed to explore the GxE and forage intake and conversion to meet the true potential of cows under these management systems. The ideal scenario would be for farmers to access an interactive flow chart to guide them through breed selection based on inputs, constraints, and priorities within their system, resulting in an indexing system unique to each farm.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by all procedures were acceptable to internal ethical review, in accordance with EU Directive 2010/63/EU for animal experiments and approved by the Animal Welfare and Ethical Review Body at Newcastle University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
HD conducted the data analysis, writing, and formatting of the manuscript. EC and SS collected the data and managed all the laboratory work. RS provided statistical advice and editing. CL, GB, and SS developed the experimental design, editing, style, and formatting. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was funded by the Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities as part of the Low Input Breeds Project (http://www.lowinputbreeds.org/home.html; grant no: 222623) and Newcastle University SAgE faculty PhD program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thank you to the farmers who put their time and effort into this project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.544149/full#supplementary-material
References
1. European Comission. Organic Farming. Policy, Rules, Organic Certifications, Support and Criteria for Organic Farming. (2008). Available online at: https://ec.europa.eu/info/food-farming-fisheries/farming/organic-farming
2. Soil Association. Soil Association Organic Standards, Farming and Growing. (2018). Available online at: https://www.soilassociation.org/media/15931/farming-and-growing-standards.pdf (accessed January 10, 2019).
3. Organic Farmers and Growers. OF&G Organic Standards and Certification Manual. (2013). Available online at: https://ofgorganic.org/useful-info/organic-standards (accessed February 13, 2020).
4. Bijttebier J, Hamerlinck J, Moakes S, Scollan N, Van Meensel J, Lauwers L. Low-input dairy farming in Europe: exploring a context-specific notion. Agricult Syst. (2017) 156:43–51. doi: 10.1016/j.agsy.2017.05.016
5. Seufert V, Ramankutty N, Foley JA. Comparing the yields of organic and conventional agriculture. Nature. (2012) 485:229–32. doi: 10.1038/nature11069
6. DairyCo. Profiting From Efficient Milk Production. Available online at: file://campus/home/home05/b3039679/Downloads/dairyco_milkbench__report.pdf (accessed November 8, 17).
7. Wagenaar JP, Klocke P, Butler G, Smolders G, Nielsen JH, Canever A, et al. Effect of production system, alternative treatments and calf rearing system on udder health in organic dairy cows. NJAS Wageningen J Life Sci. (2011) 58:157–62. doi: 10.1016/j.njas.2011.06.001
8. Stergiadis S, Leifert C, Seal CJ, Eyre MD, Larsen MK, Slots T, et al. A 2-year study on milk quality from three pasture-based dairy systems of contrasting production intensities in wales. J Agricult Sci. (2015) 153:708–31. doi: 10.1017/S0021859614000963
9. Scollan N, Padel S, Halberg N, Hermansen J, Nicholas P, Rinne M, et al. Organic and low-input dairy farming: avenues to enhance sustainability and competitiveness in the EU. EuroChoices. (2017) 16:40–5. doi: 10.1111/1746-692X.12162
10. Butler G. LowInputBreeds Final Report: Developing Integrated Livestock Breeding and Management Strategies to Improve Animal Health, Product Quality and Performance in European Organic and ‘Low Input' Milk, Meat and Egg Production. (2014). Available online at: http://www.lowinputbreeds.org/fileadmin/documents_organicresearch/lowinputbreeds/newsletter-no-10.pdf
11. Simianer H, Bieber A. Genomic Breeding Programs–A Large Step Forward For Low-Input Dairy Cattle Breeding? (2012). Available online at: http://www.lowinputbreeds.org/fileadmin/documents_organicresearch/lowinputbreeds/tn-1-1-simianer-etal-2014-dairy-cow-breeding.pdf
12. Sørensen MK, Norberg E, Pedersen J, Christensen LG. Invited review: crossbreeding in dairy cattle: a danish perspective. J Dairy Sci. (2008) 91:4116–28. doi: 10.3168/jds.2008-1273
13. Kargo M, Ettema JF, Sørensen LH, Fjordside M, Hjortø L. Combi-Cross–the use of New Technologies for Improving Dairy Crossbreeding Programs. Vancouver, BC: 10th World Congress on Genetics Applied to Livestock Production (WCGALP) (2014).
14. Stergiadis S, Bieber A, Franceschin E, Isensee A, Eyre MD, Maurer V, et al. Impact of US brown Swiss genetics on milk quality from low-input herds in Switzerland: interactions with grazing intake and pasture type. Food Chem. (2015) 175:609–18. doi: 10.1016/j.foodchem.2014.11.079
15. Nauta WJ, Baars T, Saatkamp H, Weenink D, Roep D. Farming strategies in organic dairy farming: effects on breeding goal and choice of breed. Explor Study Livestock Sci. (2009) 121:187–99. doi: 10.1016/j.livsci.2008.06.011
16. Oltenacu PA, Broom DM. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim Welfare. (2010) 19:39–49.
17. OMSCo. Global Organic Dairy Market Report 2019. (2019). Available online at: https://www.omsco.co.uk/issuu/ (accessed April 12, 2019).
18. Nauta WJ, Veerkamp RF, Brascamp EW, Bovenhuis H. Genotype by environment interaction for milk production traits between organic and conventional dairy cattle production in the Netherlands. J Dairy Sci. (2006) 89:2729–37. doi: 10.3168/jds.S0022-0302(06)72349-9
19. Rozzi P, Miglior F, Hand KJ. A total merit selection index for ontario organic dairy farmers. J Dairy Sci. (2007) 90:1584–93. doi: 10.3168/jds.S0022-0302(07)71644-2
20. Garnsworthy PC, Feng S, Lock AL, Royal MD. Short communication: heritability of milk fatty acid composition and stearoyl-CoA desaturase indices in dairy cows1. J Dairy Sci. (2010) 93:1743–8. doi: 10.3168/jds.2009-2695
21. Yin T, Bapst B, Borstel UU, Simianer H, König S. Genetic parameters for gaussian and categorical traits in organic and low input dairy cattle herds based on random regression methodology. Livest Sci. (2012) 147:159–69. doi: 10.1016/j.livsci.2012.04.017
22. Martin-Collado D, Byrne TJ, Amer PR, Santos BFS, Axford M, Pryce JE. Analyzing the heterogeneity of farmers' preferences for improvements in dairy cow traits using farmer typologies. J Dairy Sci. (2015) 98:4148–61. doi: 10.3168/jds.2014-9194
23. Rodriguez-Bermudez R, Miranda M, Lopez-Alonso M, Garcia-Vaquero M. Chapter 2: New breeding strategies in organic dairy farming. In: Jenkins O, editor. Advances in Animal Science and Zoology, Vol. 11. Nova Science (2018).
24. Buckley F, Holmes C, Keane M. Utilisation of Grazed Grass in Temperate Animal Systems: Utilisation of Grazed Grass in Temperate Animal Systems 61Genetic Characteristics Required in Dairy and Beef Cattle for Temperate Grazing System. (2005) p. 61–79. doi: 10.3920/978-90-8686-554-3
25. AHDBDairy. UK Breeding Objectives. (2018). Available online at: https://dairy.ahdb.org.uk/technical-information/breeding-genetics/uk-breeding-objectives/#.XKR1yflKhpg (accessed April 03, 2019).
26. Richert RM, Cicconi KM, Gamroth MJ, Schukken YH, Stiglbauer KE, Ruegg PL. Management factors associated with veterinary usage by organic and conventional dairy farms. J Am Vet Med Assoc. (2013) 242:1732–43. doi: 10.2460/javma.242.12.1732
27. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. (2016) 60:32527. doi: 10.3402/fnr.v60.32527
28. Srednicka-Tober D, Barański M, Seal CJ, Sanderson R, Benbrook C, Steinshamn H, et al. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: a systematic literature review and meta-and redundancy analyses. Br J Nutr. (2016) 115:1043–60. doi: 10.1017/S0007114516000349
29. Benbrook CM, Davis DR, Heins BJ, Latif MA, Leifert C, Peterman L, et al. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci Nutr. (2018) 6:681–700. doi: 10.1002/fsn3.610
30. Stergiadis S, Berlitz CB, Hunt B, Garg S, Ian Givens D, Kliem KE. An update to the fatty acid profiles of bovine retail milk in the United Kingdom: implications for nutrition in different age and gender groups. Food Chem. (2019) 276:218–30. doi: 10.1016/j.foodchem.2018.09.165
31. Nantapo CTW, Muchenje V, Hugo A. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system. Food Chem. (2014) 146:127–33. doi: 10.1016/j.foodchem.2013.09.009
32. Butler G, Nielsen JH, Slots T, Seal C, Eyre MD, Sanderson R, et al. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: seasonal variation. J Sci Food Agric. (2008) 88:1431–41. doi: 10.1002/jsfa.3235
33. Kliem KE, Shingfield KJ, Livingstone KM, Givens DI. Seasonal variation in the fatty acid composition of milk available at retail in the United Kingdom and implications for dietary intake. Food Chem. (2013) 141:274–81. doi: 10.1016/j.foodchem.2013.02.116
34. Soyeurt H, Dardenne P, Gillon A, Croquet C, Vanderick S, Mayeres P, et al. Variation in fatty acid contents of milk and milk fat within and across breeds. J Dairy Sci. (2006) 89:4858–65. doi: 10.3168/jds.S0022-0302(06)72534-6
35. Stergiadis S, Seal C, Leifert C, Eyre M, Larsen MK, Butler G. Variation in nutritionally relevant components in retail Jersey and Guernsey whole milk. Food Chem. (2013) 139:540–8. doi: 10.1016/j.foodchem.2013.01.078
36. Su K-P, Huang S-Y, Chiu T-H, Huang K-C, Huang C-L, Chang H-C, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. (2008) 69:644–51. doi: 10.4088/JCP.v69n0418
37. Swanson D, Block R, Mousa SA. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. (2012) 3:1–7. doi: 10.3945/an.111.000893
38. Givens DI, Gibbs RA. Very long chain n-3 polyunsaturated fatty acids in the food chain in the UK and the potential of animal-derived foods to increase intake. Nutr. Bull. (2006) 31:104–10. doi: 10.1111/j.1467-3010.2006.00554.x
39. Haug A, Hostmark AT, Harstad OM. Bovine milk in human nutrition - a review. Lipids Health Dis. (2007) 6:25. doi: 10.1186/1476-511X-6-25
40. Wahle KWJ, Heys SD, Rotondo D. Conjugated linoleic acids: are they beneficial or detrimental to health? Progr Lipid Res. (2004) 43:553–87. doi: 10.1016/j.plipres.2004.08.002
41. Viladomiu M, Hontecillas R, Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur J Pharmacol. (2016) 785:87–95. doi: 10.1016/j.ejphar.2015.03.095
42. Simopoulos A. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. (2016) 8:128. doi: 10.3390/nu8030128
43. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. (2008) 233:674–88. doi: 10.3181/0711-MR-311
44. National Milk Recording (2018). Available online at: https://www.nmr.co.uk/my-nmr (accessed April 30, 2018).
45. Ruttan G. United States Patent, Milk Sample Preservative. (1993). Available online at: https://patentimages.storage.googleapis.com/b8/a1/ef/2c66c7833de8d1/US5196344.pdf (accessed June 20, 2020).
46. Butler G, Nielsen JH, Larsen MK, Rehberger B, Stergiadis S, Canever A, et al. The effects of dairy management and processing on quality characteristics of milk and dairy products. NJAS Wageningen J Life Sci. (2011) 58:97–102. doi: 10.1016/j.njas.2011.04.002
47. Barbano DM, Wojciechowski KL, Lynch JM. Effect of preservatives on the accuracy of mid-infrared milk component testing. J Dairy Sci. (2010) 93:6000–11. doi: 10.3168/jds.2010-3601
48. Chilliard Y, Martin C, Rouel J, Doreau M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output1. J Dairy Sci. (2009) 92:5199–211. doi: 10.3168/jds.2009-2375
49. Stergiadis S, Leifert C, Seal CJ, Eyre MD, Steinshamn H, Butler G. Improving the fatty acid profile of winter milk from housed cows with contrasting feeding regimes by oilseed supplementation. Food Chem. (2014) 164:293–300. doi: 10.1016/j.foodchem.2014.05.021
50. Loor JJ, Ueda K, Ferlay A, Chilliard Y, Doreau M. Biohydrogenation, duodenal flow, and intestinal digestibility of trans fatty acids and conjugated linoleic acids in response to dietary forage:concentrate ratio and linseed oil in dairy cows. J Dairy Sci. (2004) 87:2472–85. doi: 10.3168/jds.S0022-0302(04)73372-X
51. Shingfield KJ, Reynolds CK, Hervás G, Griinari JM, Grandison AS, Beever DE. Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. J Dairy Sci. (2006) 89:714–32. doi: 10.3168/jds.S0022-0302(06)72134-8
52. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (2017). Available online at: https://www.R-project.org/
53. Milligan GW, Cooper MC. A study of standardization of variables in cluster analysis. J Classificat. (1988) 5:181–204. doi: 10.1007/BF01897163
54. Oksanen JB, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Vegan: Community Ecology Package. (2017). Available online at: https://CRAN.R-project.org/package=vegan
55. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. {nlme}: Linear and Nonlinear Mixed Effects Models. (2017). Available online at: https://CRAN.R-project.org/package=nlme
56. Butler G, Stergiadis S, Seal C, Eyre M, Leifert C. Fat composition of organic and conventional retail milk in northeast England. J Dairy Sci. (2011) 94:24–36. doi: 10.3168/jds.2010-3331
57. Fletcher HJ, Daw H, Young J. Controlling multiple F test errors with an overall F test. J Appl Behav Sci. (1989) 25:101–8. doi: 10.1177/0021886389251008
58. Smith RA, Levine TR, Lachlan KA, Fediuk TA. The high cost of complexity in experimental design and data analysis: type I and type II error rates in multiway ANOVA. Hum Commun Res. (2002) 28:515–30. doi: 10.1111/j.1468-2958.2002.tb00821.x
59. Cramer AOJ, van Ravenzwaaij D, Matzke D, Steingroever H, Wetzels R, Grasman RPPP, et al. Hidden multiplicity in exploratory multiway ANOVA: prevalence and remedies. Psychon Bull Rev. (2016) 23:640–7. doi: 10.3758/s13423-015-0913-5
60. AHDB Dairy. Block Calving Breeding Indexes- £SCI and £ACI. (2018). Available online at: https://dairy.ahdb.org.uk/technical-information/breeding-genetics/block-calving-breeding-indexes-%C2%A3sci-and-%C2%A3aci/#.XvCrA2hKjIU (accessed June 20, 2020).
61. Kalač P, Samková E. The effects of feeding various forages on fatty acid composition of bovine milk fat: a review. Czech J Anim Sci. (2010) 55:521–37. doi: 10.17221/2485-CJAS
62. Schwendel BH, Wester TJ, Morel PCH, Tavendale MH, Deadman C, Shadbolt NM, et al. Invited review: organic and conventionally produced milk—an evaluation of factors influencing milk composition. J Dairy Sci. (2015) 98:721–46. doi: 10.3168/jds.2014-8389
63. Davis H, Chatzidimitriou E, Leifert C, Butler G. Evidence that forage-fed cows can enhance milk quality. Sustainability. (2020) 12:3688. doi: 10.3390/su12093688
64. AHDBDairy. Somatic Cell Count- Milk Quality Indicator. (2018). Available online at: https://dairy.ahdb.org.uk/technical-information/animal-health-welfare/mastitis/symptoms-of-mastitis/somatic-cell-count-milk-quality-indicator/#.Ww_KevmnFpg (accessed May 1, 2018).
65. Council Regulation. Council Regulation (EC) No 1804/1999 of 19 July 1999 supplementing Regulation (EEC) No 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs to include livestock production. Off J Eur Commun. (1999) 222:08.
66. Government H. UK One Health Report Joint Report on Antibiotic use and Antibiotic Resistance 2013–2017. (2019). Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/775075/one_health_report_2019_v45.pdf
67. Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res. (2017) 13:211. doi: 10.1186/s12917-017-1131-3
68. Tozer PR, Bargo F, Muller LD. The effect of pasture allowance and supplementation on feed efficiency and profitability of dairy systems†. J Dairy Sci. (2004) 87:2902–11. doi: 10.3168/jds.S0022-0302(04)73421-9
69. McCarthy B, Pierce KM, Delaby L, Brennan A, Fleming C, Horan B. The effect of stocking rate and calving date on grass production, utilization and nutritive value of the sward during the grazing season. Grass Forage Sci. (2013) 68:364–77. doi: 10.1111/j.1365-2494.2012.00904.x
70. Prendiville R, Pierce KM, Delaby L, Buckley F. Animal performance and production efficiencies of holstein-friesian, Jersey and Jersey × Holstein-Friesian cows throughout lactation. Livest Sci. (2011) 138:25–33. doi: 10.1016/j.livsci.2010.11.023
71. Beecher M, Buckley F, Waters SM, Boland TM, Enriquez-Hidalgo D, Deighton MH, et al. Gastrointestinal tract size, total-tract digestibility, and rumen microflora in different dairy cow genotypes. J Dairy Sci. (2014) 97:3906–17. doi: 10.3168/jds.2013-7708
72. Coffey EL, Delaby L, Fitzgerald S, Galvin N, Pierce KM, Horan B. Effect of stocking rate and animal genotype on dry matter intake, milk production, body weight, and body condition score in spring-calving, grass-fed dairy cows. J Dairy Sci. (2017) 100:7556–68. doi: 10.3168/jds.2017-12672
73. Miglior F, Muir BL, Van Doormaal BJ. Selection indices in holstein cattle of various countries. J Dairy Sci. (2005) 88:1255–63. doi: 10.3168/jds.S0022-0302(05)72792-2
74. Organic Valley. Why is Organic Valley Adding Omega-3 to Some of its Organic Milk? (2019). Available online at: http://organicvalley.custhelp.com/app/answers/detail/a_id/335/~/why-is-organic-valley-adding-omega-3-to-some-of-its-organic-milk%3F (accessed March 21, 2019).
75. NZAEL. Economic Values. (2019). Available online at: https://www.dairynz.co.nz/animal/animal-evaluation/interpreting-the-info/economic-values/ (accessed April 02, 2019).
76. The Shorthorn Society. Shorthorn: History of the Breed. (2019). Available online at: http://www.shorthorn.co.uk/dairyshorthorn/index.php/breed/history-of-the-breed (accessed April 05, 2019).
77. Ayreshire Cattle Society. The Ayreshire Breed. (2018). Available online at: https://www.ayrshirescs.org/ayrshires-cattle-society/society/the-breed/ (accessed April 10, 2019).
78. Jersey Cattle Society. Jersey Breed Facts. (2019). Available online at: http://www.ukjerseys.com/breed/facts.html (accessed April 10, 2019).
79. Clasen JB, Fogh A, Kargo M. Differences between performance of F1 crossbreds and holsteins at different production levels. J Dairy Sci. (2019) 102:436–41. doi: 10.3168/jds.2018-14975
80. de Haas Y, Smolders EAA, Hoorneman JN, Nauta WJ, Veerkamp RF. Suitability of cross-bred cows for organic farms based on cross-breeding effects on production and functional traits. Animal. (2013) 7:655–65. doi: 10.1017/S1751731112002042
Keywords: low-input, organic, dairy farming, milk quality, grazing
Citation: Davis H, Stergiadis S, Chatzidimitriou E, Sanderson R, Leifert C and Butler G (2020) Meeting Breeding Potential in Organic and Low-Input Dairy Farming. Front. Vet. Sci. 7:544149. doi: 10.3389/fvets.2020.544149
Received: 19 March 2020; Accepted: 16 September 2020;
Published: 28 October 2020.
Edited by:
Mauro Coppa, Institut National de la Recherche Agronomique (INRA), FranceReviewed by:
Bruno Martin, INRAE Clermont-Auvergne-Rhône-Alpes, FranceLuiz Carlos Pinheiro Machado F°, Federal University of Santa Catarina, Brazil
Copyright © 2020 Davis, Stergiadis, Chatzidimitriou, Sanderson, Leifert and Butler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah Davis, aGFubmFoLmRhdmlzQG5ld2Nhc3RsZS5hYy51aw==
 Hannah Davis
Hannah Davis Sokratis Stergiadis
Sokratis Stergiadis Eleni Chatzidimitriou3
Eleni Chatzidimitriou3