- 1Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
- 2Department of Medicine and Epidemiology, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
- 3Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, United States
Background: Doxorubicin (DOX) is one of the most effective chemotherapeutics for canine high-grade lymphoma. In addition to dose-dependent chronic cardiotoxicity, DOX can trigger acute cardiac arrhythmias during drug infusion. Diphenhydramine premedication is commonly used, as histamine release is a proposed mechanism for DOX-associated arrhythmogenesis.
Hypothesis/Objectives: The study objectives were to evaluate the incidence and severity of DOX infusion-related cardiac arrhythmias in dogs with high-grade lymphoma and evaluate the effect of diphenhydramine premedication on arrhythmia frequency and severity during and after DOX infusion.
Animals: Twenty-two client-owned dogs with cytologically/histopathologically confirmed high-grade lymphoma were recruited, of which 19 were enrolled and 9 completed the study.
Methods: Dogs were screened by echocardiogram and concurrent electrocardiogram for this randomized prospective crossover study. Group A received no premedication for DOX #1 and was premedicated with diphenhydramine for DOX #2; Group B received diphenhydramine with DOX #1 and no premedication for DOX #2. For both visits, Holter monitor data were collected for 1 h pre-DOX and 3 h post-DOX administration.
Results: Nineteen dogs were enrolled and 9 dogs [Group A (5), Group B (4)] completed the protocol. There was no statistical difference between the DOX alone and DOX + diphenhydramine when evaluating the total number of ventricular premature complexes (VPCs, P = 0.34), change in VPCs/hour (P = 0.25), total number of atrial premature complexes (APCs, P = 0.5), change in APCs/hour (P = 0.06), or ventricular arrhythmia severity score (P > 0.99).
Conclusions and clinical importance: This study demonstrates that in these dogs with rigorous pretreatment cardiovascular screening, DOX infusion did not induce significant arrhythmias. Furthermore, these data suggest that, with this screening approach, diphenhydramine may not alter the arrhythmia number or severity in canine DOX recipients.
Introduction
Doxorubicin (DOX) is one of the most widely used antineoplastic drugs in veterinary and human medicine and demonstrates efficacy against a variety of cancers such as lymphoma and solid tumors like hemangiosarcoma (1–6). Unfortunately, like most chemotherapeutics, DOX can also be associated with adverse effects on tissues and organs. Of these, DOX dose-dependent cardiotoxicity is well-known and can greatly impede its clinical use (7–15).
In humans and dogs, there is significant individual variation in susceptibility regarding DOX cardiotoxicosis, and in general, dogs are considered to be more sensitive than humans (8, 16–19). Many studies have investigated DOX-associated cardiotoxicity, and they are commonly divided into chronic and acute categories. The most important chronic effect is a dose-dependent and irreversible cardiomyopathy (20, 21). This can lead to congestive heart failure and ventricular arrhythmias, and is commonly associated with a poor prognosis, especially in canine patients (22–26). Although cardiotoxicity can occur with any cumulative dose, it is most commonly seen with doses that exceed 180 mg/m2 (or >6 doses in most dogs) or in humans ≥450 mg/m2 (7, 9, 11, 12, 18, 19, 27–29). Acute effects mainly include arrhythmias that are transient and rarely life-threatening, although left ventricular failure, pericarditis, myocarditis, and sudden cardiac death have been reported in people (9, 11, 19, 26, 27, 30–36). In general, acute cardiotoxicities are associated with the peak level of DOX and/or rapid infusion (28, 33, 37). These effects vary from non-specific electrocardiogram ST changes to atrial and ventricular arrhythmias (38–41). In humans, the incidence of acute cardiotoxicity causing arrhythmias ranges from 11 to 40% (2, 19, 33, 38, 42–46).
The underlying cause of acute DOX-induced cardiotoxicity remains unclear. Numerous mechanisms have been proposed in which some reports suggest that histamine and catecholamine release could play a role in the acute cardiotoxic effects (28, 47). Hypotension and allergic reactions related to the drug infusion rate may also play a role in arrhythmogenesis (12, 48, 49).
Considering the previously documented systemic release of histamine during DOX infusion and its potential role in arrhythmogenesis, the use of antihistamine premedication prior to DOX infusion is common practice (28, 41, 50, 51). Currently, there is a paucity of evidence to support or refute the use of antihistamines for infusion-related cardiac arrhythmias in clinical canine patients. The study objectives were to evaluate the incidence and severity of DOX infusion-related cardiac arrhythmias in dogs with high-grade lymphoma, and evaluate the effect of the antihistamine, diphenhydramine, premedication on the arrhythmia number and severity during and after DOX infusion. We hypothesized that diphenhydramine would not impact the number or severity of cardiac arrhythmias observed during DOX infusion in canine lymphoma patients.
Materials and Methods
This was a prospective, randomized, crossover design study that enrolled client-owned dogs diagnosed with high-grade lymphoma presented to the University of California, Davis Veterinary Medical Teaching Hospital (UCD-VMTH) in accordance with the UCD Institutional Animal Care and Use Committee (protocol #19015) from 10/2015 to 10/2018. Dogs were included if they had a cytologic or histopathologic diagnosis of high-grade lymphoma, were receiving a DOX-based multiagent chemotherapy protocol, which at this institution during that timeframe was either a 6-month or 19-week CHOP [cyclophosphamide (cyclophosphamide, Hikma, Columbus, OH), doxorubicin (doxorubicin hydrochloride; Pfizer, New York, NY), vincristine (Hospira, Lake Forest, IL), and prednisone (prednisone, PAR Pharmaceuticals, Chestnut Ridge, NY)] protocol, had not been previously treated with DOX, had a performance status of 0 or 1 (modified Eastern Cooperative Oncology Group), and weighed > 15 kg (4, 52). This body weight restriction was based on this institution's protocol to administer DOX at 30 mg/m2 for dogs > 15 kg and 1 mg/kg for dogs ≤ 15 kg (4, 52–55). Concurrent use of corticosteroids was permissible as per the overseeing clinician's recommendations. Specific supplements (chondroitin sulfate, vitamins, essential fatty acids, and glucosamine) were also permitted if administered for >30 days prior to enrollment, and no new supplements or oral antihistamines were allowed during the study period. At this institution, drugs to manage adverse events such as gastrointestinal toxicity would generally include ondansetron (ondansetron hydrochloride, Rising Health Saddle Brook, NJ), maropitant (Cerenia, Zoetis, Kalamazoo, MI), and/or metronidazole (metronidazole hydrochloride, Blue Point Laboratories, Montgomeryville, PA) or, in the event of clinically important myelosuppression, enrofloxacin (Baytril, Bayer, Shawnee Mission, KS) or amoxicillin clavulanic acid (Clavamox, Zoetis, Kalamazoo, MI) would be prescribed at the discretion of the supervising clinician. Dogs were excluded if they had a clinically significant grade 3 or higher hematologic/biochemical abnormality as defined by the Veterinary Cooperative Oncology Group (VCOG-CTAE 1.1), considered a substage b, received an antihistamine within 72 h of DOX #1, or had significant comorbidities including but not limited to liver or renal insufficiency, heart disease, or any other abnormality deemed incompatible with DOX administration (56). Dogs were also excluded if they were unable to receive DOX at the prescribed 30 mg/m2 dose.
Prior to enrollment, all dogs underwent a complete physical examination and cardiologic examination including a complete echocardiogram with concurrent lead 2 electrocardiogram (ECG) for the duration of the echocardiographic study (Philips EPIQ 7C or ie33; Philips Healthcare; Andover, MA). The screening echocardiogram was used to rule out any concurrent systolic dysfunction as judged by the attending cardiologist. Dogs with observed systolic dysfunction or concomitant cardiac disease beyond mild valvular degeneration/insufficiency as reported by the attending cardiologist were excluded from the study. Additionally, dogs with ventricular or supraventricular arrhythmias observed during the concurrent ECG were excluded from the study. Additional diagnostic tests were performed at the discretion of the owner and overseeing oncology clinician (complete blood count, chemistry, and ± urine analysis, thoracic radiographs, and abdominal ultrasound). Clinical information collected for each dog included age, body weight, sex, breed, and staging information.
Once dogs were enrolled, they were randomized into either Group A or Group B according to a predetermined randomization table. Group A dogs received no premedication for DOX dose #1 and were premedicated with diphenhydramine (diphenhydramine HCl; West-Ward, Eatontown, NJ) for DOX dose #2; Group B dogs received diphenhydramine with DOX dose #1 and no premedication for DOX dose #2. For both Groups A and B, the diphenhydramine premedication was administered intramuscularly 30 min prior to the DOX infusion at a dose of 2 mg/kg. A 5-electrode Holter monitor (Burdick Holter monitor; Ventura, CA) was placed as previously described for a total of 4 h in the clinic, which recorded 1 h prior to DOX infusion to serve as a baseline and for 3 h during/post DOX infusion (57). All dogs received their DOX infusion over 20 min via an intravenous catheter placed in a peripheral vein as per UCD-VMTH's protocol. After DOX administration and Holter monitoring, dogs were discharged with standard recommendations and monitoring. On the second visit, all dogs underwent a physical examination and diagnostic tests as per the clinician's discretion. The Holter monitor data for all patients were evaluated by a cardiology resident in training (CB) and confirmed by a board-certified veterinary cardiologist (JS) using commercially available software (Burdick Holter Analysis Software; Ventura, CA). For each Holter dataset, both observers (CB, JS) were blinded to patient information, randomization assignment, and whether or not DOX was given alone or with diphenhydramine premedication. Ventricular arrhythmias were graded using a standardized scoring system derived for this project where 0 represented no ectopy, 1 for only single ventricular complexes, 2 for ventricular couplets or triplets, 3 for polymorphic ventricular ectopic complexes, and finally 4 if paroxysmal or sustained ventricular tachycardia was observed. If clinically relevant arrhythmias were noted by the cardiologist reading the Holter monitor, then therapeutic recommendations were provided.
Statistical Analysis
Continuous data were both visually inspected and tested for normality using a D'Agostino and Pearson normality test. Parametric data are presented as mean ± SD, and non-parametric data are presented as median and minimum to maximum. Paired data (each patient compared to itself when treated with DOX alone or DOX + diphenhydramine premedication) were tested for differences compared using a Wilcoxon matched-pairs signed rank test for non-parametric data and a paired t-test for parametric data. Statistically significant differences were reported at P < 0.05. Comparisons were performed for total atrial premature complexes (APCs) and ventricular premature complexes (VPCs) in 4 h, change in APCs and VPCs comparing the number of arrhythmias in hour 1 to the subsequent 3 h, and lastly the ventricular arrhythmia severity score. All statistics were performed using commercially available software (GraphPad Prism Software, versions 7 and 8; La Jolla, CA).
Results
Clinical Demographics
A total of 22 dogs were screened for the trial, and 19 dogs were randomized (Figure 1). Three dogs failed screening with the identification of a significant ventricular arrhythmia in one dog, a heart base mass along with VPCs in one dog, and VPCs due to arrhythmogenic right ventricular cardiomyopathy in one dog. Of the remaining 19 dogs, they had a median age of 7 years (2–13) with 6 spayed females, 10 castrated males, and 3 intact males. Dogs had a median body weight of 31.7 kg (21.6–52.9 kg). Multiple breeds were represented with Labrador retrievers being the most common (6) followed by mixed breed dogs (4), golden retrievers (2), pitbull terriers (2), and one each of Australian shepherd, German shepherd, border collie, Doberman pinscher, and poodle. Based on the World Health Organization criteria for lymphoma, most dogs were considered at least a stage III (9), with three stage IV, five stage V, and two dogs with mediastinal lymphoma; however, not all dogs were fully staged as this was at the discretion of the pet owner and the clinician (58). All dogs received concurrent corticosteroids as part of their chemotherapy protocol for DOX #1 and two dogs remained on corticosteroids at the time of DOX #2.
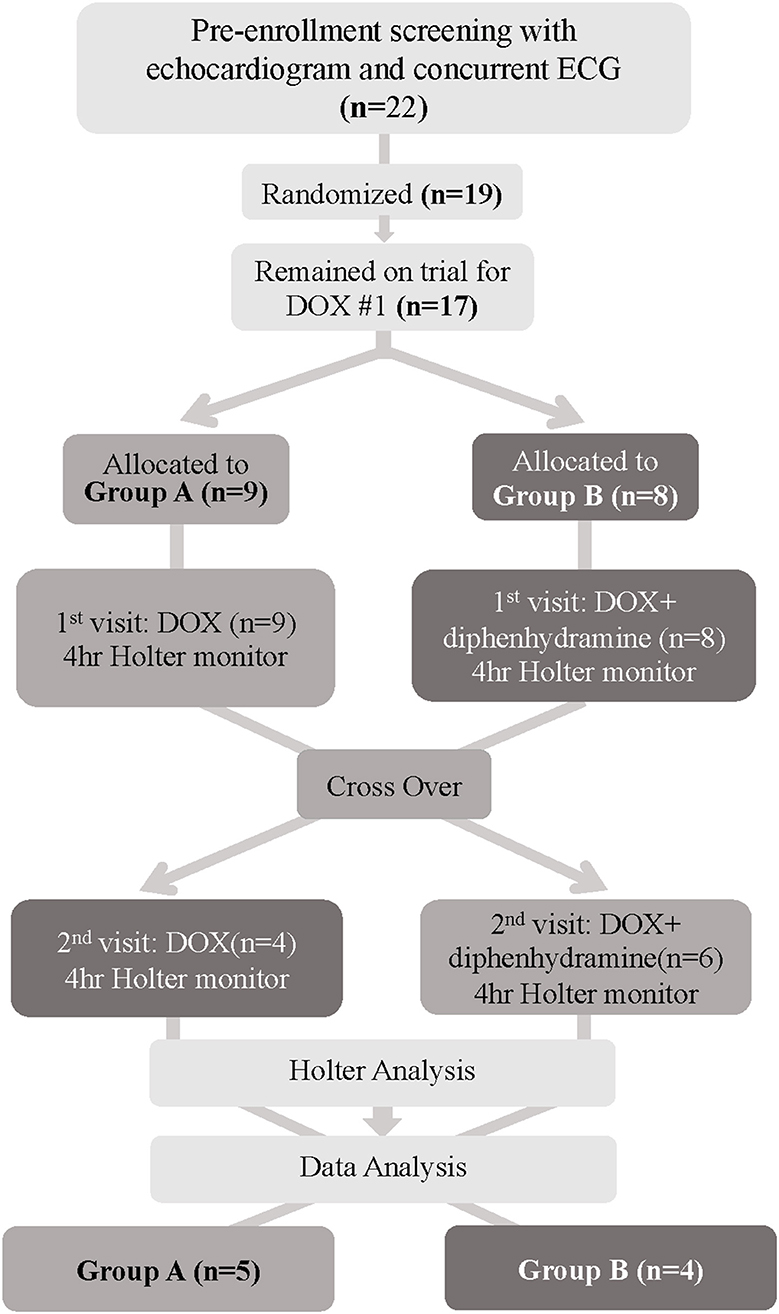
Figure 1. Flowchart depicting patient randomization and progress for dogs enrolled in this trial. DOX, Doxorubicin; ECG, electrocardiogram.
Patient Randomization and Attrition (Figure 1)
Of the 19 dogs enrolled and randomized, 2 dogs were removed from the study prior to DOX #1 due to owner withdrawal and lack of pursuit of chemotherapy, leaving 17 dogs. Of the 17 dogs, 9 were randomized to Group A and 8 dogs were allocated to Group B. Group A had 6 dogs remaining at DOX #2, as 3 dogs did not receive the second DOX due to either progressive disease on their chemotherapy protocol (2) or patient non-compliance (1). Group B also experienced attrition of 4 dogs that were removed from the study due to dose reductions in DOX #2 secondary to adverse effects following DOX #1. The adverse events were as follows: the first dog had a grade IV neutropenia and grade 2 vomiting 10 days after DOX #1, the second dog was hospitalized for grade III vomiting and diarrhea 5 days post-DOX #1 and a grade III neutropenia and grade II thrombocytopenia 7 days post-administration, the third dog had grade III diarrhea for which outpatient supportive care was provided 5 days post-DOX #1, and the fourth dog had grade II anorexia, vomiting, and lethargy 2 days post-DOX #1. Finally, once Holter data were evaluated, 1 dog from Group A was identified to have significant ventricular ectopy for their 1-h baseline period and was withdrawn from the study due to recommended antiarrhythmic therapy, leaving a total of 9 evaluable dogs at study completion (Supplementary Table 1). The underlying cause of this dog's preexisting arrhythmia was unknown and could have been secondary to their lymphoma or another cause. Systemic hypersensitivity reactions were not seen in any of the enrolled dogs and thus did not contribute to attrition (Figure 1).
Holter Data
Holter data were assessed for frequency of both VPCs and APCs as well as complexity of VPCs using the provided grading scheme. Overall, arrhythmogenesis was low with no significant difference between the total number of VPCs or APCs over 4 h in the DOX alone or DOX + diphenhydramine groups (Figure 2). Dogs had a median of 2 (0 to 19) VPCs for the DOX alone population and a median of 0 (0 to 66) VPCs for the DOX + diphenhydramine dogs (P = 0.34). A median of 0 (0 to 1) APC was observed in the DOX alone group and a median of 0 (0 to 6) was observed for the DOX + diphenhydramine group (P = 0.5). When the change in the frequency of arrhythmias was assessed comparing the initial baseline hour of Holter monitor data to the subsequent 3 h, there was no significant difference in the change in the number of VPCs [DOX alone median 0, (−5 to 4); DOX + diphenhydramine group median 0, (0 to 10); P = 0.25)] or APCs [DOX alone median 0, (0 to 0.33); DOX + diphenhydramine group median 0, (−2 to 0), (P = 0.06)] (Figure 3). Arrhythmia severity score for ventricular ectopy was not different between groups [(DOX alone median 1, (0 to 2); DOX + diphenhydramine group median 1, (0 to 2); P = 0.99] (Figure 4).
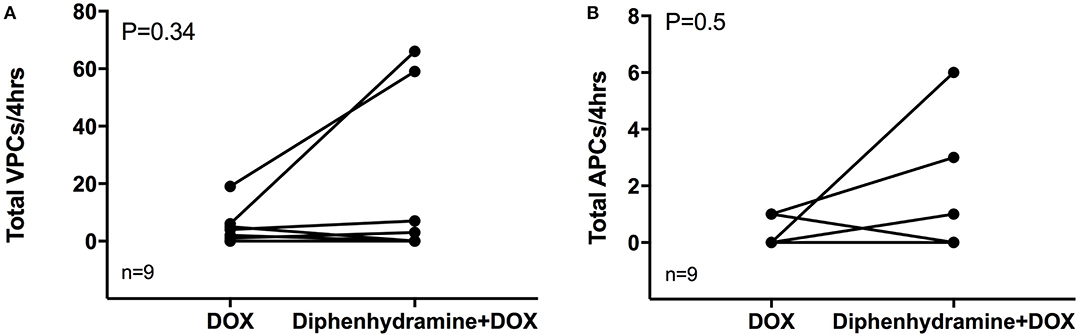
Figure 2. The total number of ventricular premature complexes (VPCs) and atrial premature complexes (APCs) over the observed 4 h is shown in (A) and (B), respectively, for each of the nine evaluable dogs in each treatment group [doxorubicin (DOX) alone and DOX + diphenhydramine]. Each animal is represented by a single point in each treatment group and connected with a single line to represent the paired comparisons employed.
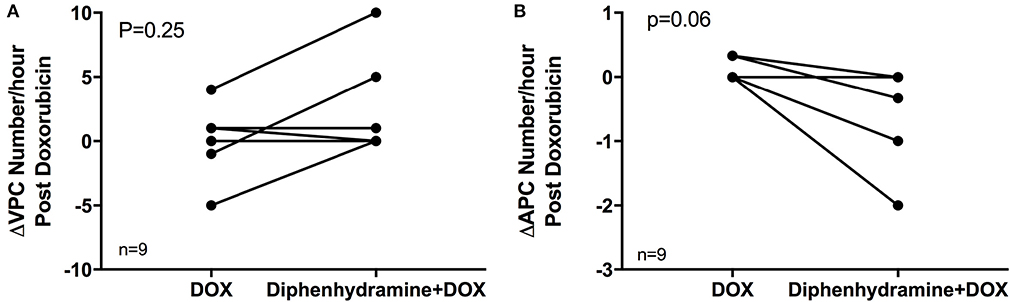
Figure 3. The change in the number of ventricular premature complexes (VPCs) and atrial premature complexes (APCs) comparing the 1 h of pretreatment Holter monitor data to the 3 h during and following doxorubicin (DOX) administration is shown in (A) and (B), respectively, for each of the nine evaluable dogs in each treatment group [doxorubicin (DOX) alone and DOX + diphenhydramine]. Each animal is represented by a single point in each treatment group and connected with a single line to represent the paired comparisons employed.
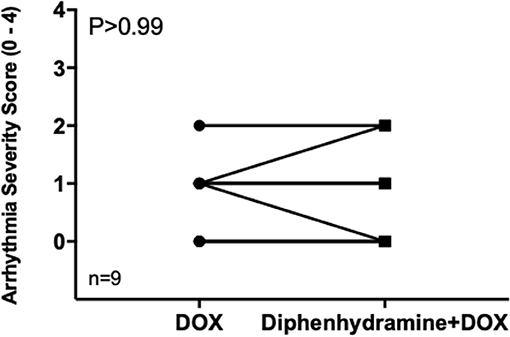
Figure 4. The arrhythmia severity score for ventricular ectopy is shown for each of the nine evaluable dogs in each treatment group [doxorubicin (DOX) alone and DOX + diphenhydramine]. Each animal is represented by a single point in each treatment group and connected with a single line to represent the paired comparisons employed.
Discussion
To the authors' knowledge, this is the first clinical trial investigating the effects of the antihistamine, diphenhydramine, on DOX infusion-related cardiac arrhythmias in client-owned dogs. Our study demonstrates that with the pretreatment cardiovascular screening by echocardiogram and concurrent ECG used in this study, DOX infusion did not commonly induce clinically relevant cardiac arrhythmias in this population of dogs. Overall, arrhythmogenesis was low, and there was no significant difference in the total number of VPCs or APCs, change in VPCs or APCs, or the arrhythmia severity score when dogs received DOX alone or DOX + diphenhydramine premedication. Of the 22 screened dogs, 4 (18%) were excluded from the trial for concomitant cardiac disease, and the study completion rate was low with only 9 of 17 (52%) dogs having evaluable data at study completion.
The lack of diphenhydramine impact could be due in part to the current UCD-VMTH clinical practice for DOX administration as a 20-min infusion. Increased adverse events have been reported when DOX is administered as a bolus rather than as an infusion in dogs and people (28, 59). Bristow et al. (28) demonstrated more significant histamine release when DOX was administered as a bolus rather than a slow infusion, but did not evaluate these dogs for concurrent arrhythmogenesis (28). Additionally, histamine increases have been associated with reduced cardiac systolic function; thus, it may remain reasonable to consider premedication with an antihistamine when DOX is used in patients with known cardiac disease (50). The UCD-VMTH protocol also incorporates the use of prednisone as part of the multiagent chemotherapy protocol for lymphoma. Although corticosteroids are known to reduce blood histamine concentration, it was not eliminated from the study as it remains part of standard therapy for lymphoma at this institution and mirrors clinical practice (4, 52, 60). Comparisons were not made between dogs receiving prednisone vs. those that were not due to small sample size.
This prospective study has several limitations to consider. The first of which is secondary to the small numbers and a low completion rate, which could make results subject to a Type II error. The authors acknowledge the diminished completion rate with only 9 dogs (47%) being evaluable of the 19 dogs randomized. Multiple factors contributed to this attrition including adverse events that triggered dose reductions and thus elimination from the trial. The authors deemed maintenance of dose intensity to be necessary to standardize as reductions may decrease the likelihood of side effects in some patients (61). Other dogs went on to progress on their chemotherapy protocol as DOX was no longer efficacious for their disease, and thus, they were eliminated from the trial. Administration of antihistamines outside of the study protocol was prohibited in this study; however, supportive medications to treat chemotherapy-induced adverse effects were permitted. There is potential for these concomitant medications to impact our data if an idiosyncratic reaction were to occur; but it was deemed unethical to withhold supportive care from these client-owned dogs, and none of the drugs routinely utilized have known arrhythmogenic instigation or suppressive properties. Although no dogs experienced a systemic hypersensitivity reaction, another potential limitation would include that this study was not designed to evaluate non-cardiac implications of diphenhydramine omission such as hypotension, facial swelling, wheals, etc. as the incidence rates are generally low when DOX is administered as a slow infusion rather than a bolus (61)]. Future studies would be necessary to identify potential differences with regard to these reactions between diphenhydramine treated and untreated groups. Finally, it is important to acknowledge that our results may not be applicable to a population with less rigorous screening criteria. Regardless of breed and physical examination findings, all dogs in this study had an echocardiogram with concurrent ECG, which may not mimic clinical practice at all institutions.
Conclusion
These data support that diphenhydramine premedication provided no appreciable benefit in the arrhythmia number or severity in this population of dogs with cardiovascular prescreening.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by University of California, Davis Institutional Animal Care and Use Committee (IACUC, protocol #19015) from 10/2015 to 10/2018. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
JW, JS, CB, and JB were involved in study idea, design, implementation, data acquisition, data analysis, and manuscript preparation. LY, YU, LV, and KS were involved in study implementation, data acquisition, data analysis, and manuscript preparation. All authors provided approval and accountability for this work.
Funding
Funding for this work by a grant (2015-45-R) from the Center for Companion Animal Health at the University of California, Davis. Jenna Burton was supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA138464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The National Institutes of Health had no role in the design of the study, collection, analysis, and interpretation of data, nor writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We graciously acknowledge the expert technical assistance of Denise Berger and Judy Schettler in the execution of this project. Presented in part at the European College of Veterinary Internal Medicine Congress 2019, Milan, Italy.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.00368/full#supplementary-material
Abbreviations
APC, Atrial Premature Complex; DOX, Doxorubicin; UCD-VMTH, University of California, Davis, Veterinary Medical Teaching Hospital; VPC, Ventricular Premature Complex; VCOG-CTAE 1. 1, Veterinary Comparative Oncology Group, Common Terminology of Adverse Events version 1.1.
References
1. Ogilvie GK, Reynolds HA, Richardson RC, Withrow SJ, Norris AM, Henderson RA, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. (1989) 195:1580–3.
2. O'Bryan RM, Luce JK, Talley RW, Gottlieb JA, Baker LH, Bonadonna G. Phase II evaluation of adriamycin in human neoplasia. Cancer. (1973) 32:1–8. doi: 10.1002/1097-0142(197307)32:1<1::AID-CNCR2820320101>3.0.CO;2-X
3. Blum RH, Carter SK. Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Med. (1974) 80:249–59. doi: 10.7326/0003-4819-80-2-249
4. Hosoya K, Kisseberth WC, Lord LK, Alvarez FJ, Lara-Garcia A, Kosarek CE, et al. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J Vet Intern Med. (2007) 21:1355–63. doi: 10.1111/j.1939-1676.2007.tb01959.x
5. Batschinski K, Nobre A, Vargas-Mendez E, Tedardi MV, Cirillo J, Cestari G, et al. Canine visceral hemangiosarcoma treated with surgery alone or surgery and doxorubicin: 37 cases (2005-2014). Can Vet J. (2018)59:967–72.
6. Morabito A, Gattuso D, Stani SC, Fanelli M, Ferrau F, De Sio L, et al. Safety and activity of the combination of pegylated liposomal doxorubicin and weekly docetaxel in advanced breast cancer. Breast Cancer Res Treat. (2004) 86:249–57. doi: 10.1023/B:BREA.0000036898.45123.e9
7. Fowler MM, Jesty SA, Gompf RE, Johns SM. ECG of the month. Doxorubicin cardiotoxicosis. J Am Vet Med Assoc. (2013) 243:52–4. doi: 10.2460/javma.243.1.52
8. Banco B, Grieco V, Servida F, Giudice C. Sudden death in a dog after doxorubicin chemotherapy. Vet Pathol. (2011) 48:1035–7. doi: 10.1177/0300985810377185
9. Friess GG, Boyd JF, Geer MR, Garcia JC. Effects of first-dose doxorubicin on cardiac rhythm as evaluated by continuous 24-hour monitoring. Cancer. (1985) 56:2762–4. doi: 10.1002/1097-0142(19851215)56:12<2762::AID-CNCR2820561207>3.0.CO;2-E
10. Xin YF, Zhang S, Gu LQ, Liu SP, Gao HY, You ZQ, et al. Electrocardiographic and biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin-induced cardiotoxicity in the beagle dogs. Biol Pharm Bull. (2011) 34:1523–6. doi: 10.1248/bpb.34.1523
11. Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. (2007) 49:330–52. doi: 10.1016/j.pcad.2006.10.002
12. Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf . (2000) 22:263–302. doi: 10.2165/00002018-200022040-00002
13. Ratterree W, Gieger T, Pariaut R, Saelinger C, Strickland K. Value of echocardiography and electrocardiography as screening tools prior to Doxorubicin administration. J Am Anim Hosp Assoc. (2012) 48:89–96. doi: 10.5326/JAAHA-MS-5680
14. O'Bryan RM, Baker LH, Gottlieb JE, Rivkin SE, Balcerzak SP, Grumet GN, et al. Dose response evaluation of adriamycin in human neoplasia. Cancer. (1977) 39:1940–8. doi: 10.1002/1097-0142(197705)39:5<1940::AID-CNCR2820390505>3.0.CO;2-0
15. Al-Malky HS, Al Harthi SE, Osman AM. Major obstacles to doxorubicin therapy: cardiotoxicity and drug resistance. J Oncol Pharm Pract. (2019) 26:434–44. doi: 10.1177/1078155219877931
17. Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol. (2000) 7:53–62. doi: 10.1067/mnc.2000.103324
18. Alves de Souza RC, Camacho AA. Neurohormonal, hemodynamic, and electrocardiographic evaluations of healthy dogs receiving long-term administration of doxorubicin. Am J Vet Res. (2006) 67:1319–25. doi: 10.2460/ajvr.67.8.1319
19. Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. (1973) 32:302–14. doi: 10.1002/1097-0142(197308)32:2<302::AID-CNCR2820320205>3.0.CO;2-2
20. Van Vleet JF, Ferrans VJ. Clinical observations, cutaneous lesions, and hematologic alterations in chronic adriamycin intoxication in dogs with and without vitamin E and selenium supplementation. Am J Vet Res. (1980) 41:691–9.
21. Van Vleet JF, Ferrans VJ, Weirich WE. Cardiac disease induced by chronic adriamycin administration in dogs and an evaluation of vitamin E and selenium as cardioprotectants. Am J Pathol. (1980) 99:13–42.
22. Gralla EJ, Fleischman RW, Luthra YK, Stadnicki SW. The dosing schedule dependent toxicities of adriamycin in beagle dogs and rhesus monkeys. Toxicology. (1979) 13:263–73. doi: 10.1016/S0300-483X(79)80030-X
23. Hallman BE, Hauck ML, Williams LE, Hess PR, Suter SE. Incidence and risk factors associated with development of clinical cardiotoxicity in dogs receiving doxorubicin. J Vet Intern Med. (2019) 33:783–91. doi: 10.1111/jvim.15414
24. Tallaj JA, Franco V, Rayburn BK, Pinderski L, Benza RL, Pamboukian S, et al. Response of doxorubicin-induced cardiomyopathy to the current management strategy of heart failure. J Heart Lung Transpl. (2005) 24:2196–201. doi: 10.1016/j.healun.2004.12.108
25. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. (2000) 342:1077–84. doi: 10.1056/NEJM200004133421502
26. Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med. (1978) 65:823–32. doi: 10.1016/0002-9343(78)90802-1
27. Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. (1979) 91:710–7. doi: 10.7326/0003-4819-91-5-710
28. Bristow MR, Sageman WS, Scott RH, Billingham ME, Bowden RE, Kernoff RS, et al. Acute and chronic cardiovascular effects of doxorubicin in the dog: the cardiovascular pharmacology of drug-induced histamine release. J Cardiovasc Pharmacol. (1980) 2:487–515. doi: 10.1097/00005344-198009000-00002
29. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. (2003) 97:2869–79. doi: 10.1002/cncr.11407
30. Couch RD, Loh KK, Sugino J. Sudden cardiac death following adriamycin therapy. Cancer. (1981) 48:38–9. doi: 10.1002/1097-0142(19810701)48:1<38::AID-CNCR2820480109>3.0.CO;2-H
31. Gilladoga AC, Manuel C, Tan CT, Wollner N, Sternberg SS, Murphy ML. The cardiotoxicity of adriamycin and daunomycin in children. Cancer. (1976) 37(Suppl. 2):1070–8. doi: 10.1002/1097-0142(197602)37:2+<1070::AID-CNCR2820370814>3.0.CO;2-6
32. Wortman JE, Lucas VS Jr, Schuster E, Thiele D, Logue GL. Sudden death during doxorubicin administration. Cancer. (1979) 44:1588–91. doi: 10.1002/1097-0142(197911)44:5<1588::AID-CNCR2820440508>3.0.CO;2-X
33. Praga C, Beretta G, Vigo PL, Lenaz GR, Pollini C, Bonadonna G, et al. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep. (1979) 63:827–34.
34. Kehoe R, Singer DH, Trapani A, Billingham M, Levandowski R, Elson J. Adriamycin-induced cardiac dysrhythmias in an experimental dog model. Cancer Treat Rep. (1978) 62:963–78.
35. Loar AS, Susaneck SJ. Doxorubicin-induced cardiotoxicity in five dogs. Semin Vet Med Surg. (1986) 1:68–71.
36. Mauldin GE, Fox PR, Patnaik AK, Bond BR, Mooney SC, Matus RE. Doxorubicin-induced cardiotoxicosis. Clinical features in 32 dogs. J Vet Intern Med. (1992) 6:82–8. doi: 10.1111/j.1939-1676.1992.tb03156.x
37. van Dalen EC, van der Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. (2009) Cd005008. doi: 10.1002/14651858.CD005008.pub3
38. Wang SQ. [Electrocardiogram analysis of adriamycin cardiotoxicity in 160 cases]. Zhonghua Zhong Liu Za Zhi. (1991) 13:71–3.
39. Okuma K, Furuta I, Ota K. [Acute cardiotoxicity of anthracyclines–analysis by using Holter ECG]. Gan To Kagaku Ryoho. (1984) 11:902–11.
40. Jacobs GJ. Secondary canine cardiomyopathies: their causes and characteristics. Vet Med. (1996) 91:534–42.
42. Steinberg JS, Cohen AJ, Wasserman AG, Cohen P, Ross AM. Acute arrhythmogenicity of doxorubicin administration. Cancer. (1987) 60:1213–8. doi: 10.1002/1097-0142(19870915)60:6<1213::AID-CNCR2820600609>3.0.CO;2-V
43. Tan C, Etcubanas E, Wollner N, Rosen G, Gilladoga A, Showel J, et al. Adriamycin–an antitumor antibiotic in the treatment of neoplastic diseases. Cancer. (1973) 32:9–17. doi: 10.1002/1097-0142(197307)32:1<9::AID-CNCR2820320102>3.0.CO;2-6
44. Middleman E, Luce J, Frei E III. Clinical trials with adriamycin. Cancer. (1971) 28:844–50.3. doi: 10.1002/1097-0142(1971)28:4<844::AID-CNCR2820280407>3.0.CO;2-9
45. Cortes EP, Lutman G, Wanka J, Wang JJ, Pickren J, Wallace J, et al. Adriamycin (Nsc-123127) Cardiotoxicity - Clinicopathologic Correlation. Cancer Chemoth Rep 3. (1975) 6:215–25.
46. Dindogru A, Barcos M, Henderson ES, Wallace HJ Jr. Electrocardiographic changes following adriamycin treatment. Med Pediatr Oncol. (1978) 5:65–71. doi: 10.1002/mpo.2950050110
47. Wolff AA, Levi R. Histamine and cardiac arrhythmias. Circ Res. (1986) 58:1–16. doi: 10.1161/01.RES.58.1.1
48. Kilickap S, Akgul E, Aksoy S, Aytemir K, Barista I. Doxorubicin-induced second degree and complete atrioventricular block. Europace. (2005) 7:227–30. doi: 10.1016/j.eupc.2004.12.012
49. Eschalier A, Lavarenne J, Burtin C, Renoux M, Chapuy E, Rodriguez M. Study of histamine release induced by acute administration of antitumor agents in dogs. Cancer Chemother Pharmacol. (1988) 21:246–50. doi: 10.1007/BF00262779
50. Gebbia N, Flandina C, Leto G, Tumminello FM, Sanguedolce R, Candiloro V, et al. The role of histamine in doxorubicin and teniposide-induced cardiotoxicity in dog and mouse. Tumori. (1987) 73:279–87. doi: 10.1177/030089168707300312
51. Craig JA, Richardson RC, Rudd RG. A practical guide to clinical oncology-4: chemotherapy and immunotherapy. Vet Med. (1986) 81:226–41.
52. Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. (2002) 16:704–9. doi: 10.1111/j.1939-1676.2002.tb02411.x
53. Curran K, Thamm DH. Retrospective analysis for treatment of naive canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet Comp Oncol. (2016) 14 Suppl 1:147–55. doi: 10.1111/vco.12163
54. Ogilvie GK, Richardson RC, Curtis CR, Withrow SJ, Reynolds HA, Norris AM, et al. Acute and short-term toxicoses associated with the administration of doxorubicin to dogs with malignant tumors. J Am Vet Med Assoc. (1989) 195:1584–7.
55. Finotello R, Stefanello D, Zini E, Marconato L. Comparison of doxorubicin-cyclophosphamide with doxorubicin-dacarbazine for the adjuvant treatment of canine hemangiosarcoma. Vet Comp Oncol. (2017) 15:25–35. doi: 10.1111/vco.12139
56. Veterinary cooperative oncology group - common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. (2011) 14:417–46. doi: 10.1111/vco.283
57. Stern JA, Meurs KM, Spier AW, Koplitz SL, Baumwart RD. Ambulatory electrocardiographic evaluation of clinically normal adult Boxers. J Am Vet Med Assoc. (2010) 236:430–3. doi: 10.2460/javma.236.4.430
58. TNM Classification of Tumours in Domestic Animals. World Health Organization. (1980). Available online at: https://apps.who.int/iris/handle/10665/68618 (accessed February 1, 2020).
59. Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. (2010) 10:337. doi: 10.1186/1471-2407-10-337
60. Porter JF, Young JA, Mitchell RG. Effects of corticosteroids on distribution of histamine in the blood of asthmatic children. Arch Dis Child. (1970) 45:54–8. doi: 10.1136/adc.45.239.54
Keywords: histamine, cardiac, chemotherapy, Adriamycin, Benadryl
Citation: Willcox JL, Belanger C, Burton JH, Yu L, Ueda Y, Visser LC, Skorupski K and Stern JA (2020) Intramuscular Diphenhydramine Does Not Affect Acute Doxorubicin Infusion-Related Arrhythmia Number or Severity in a Prospective Crossover Study in Canine Lymphoma: A Pilot Study. Front. Vet. Sci. 7:368. doi: 10.3389/fvets.2020.00368
Received: 12 February 2020; Accepted: 27 May 2020;
Published: 17 July 2020.
Edited by:
David Bruyette, Anivive Lifesciences, United StatesReviewed by:
Domenico Caivano, University of Perugia, ItalyKirstie Barrett, VCA Inc., United States
Copyright © 2020 Willcox, Belanger, Burton, Yu, Ueda, Visser, Skorupski and Stern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Lindley Willcox, amx3aWxsY294QHVjZGF2aXMuZWR1; Joshua A. Stern, anN0ZXJuQHVjZGF2aXMuZWR1
 Jennifer Lindley Willcox
Jennifer Lindley Willcox Catherine Belanger2
Catherine Belanger2 Jenna Hart Burton
Jenna Hart Burton Lance C. Visser
Lance C. Visser Joshua A. Stern
Joshua A. Stern