
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 26 June 2020
Sec. Veterinary Infectious Diseases
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00364
This article is part of the Research Topic An Overview of Current Knowledge on Ticks and Tick-borne Diseases View all 8 articles
The instrumentation of the in vitro culture system has allowed researchers to learn more about the metabolic and growth behavior of Babesia spp. The various applications for in vitro cultivation of Babesia include obtaining attenuated strains for vaccination or pre-munition, the selection of pure lines with different degrees of virulence, studies on biological cloning, ultrastructure, antigen production for diagnostics, drug sensitivity assessments, and different aspects of parasite biology. Although there are different types of vaccines that have been tested against bovine babesiosis, so far, the only procedure that has offered favorable results in terms of protection and safety has been the use of live attenuated vaccines. In countries, such as Australia, Argentina, Brazil, Uruguay and Israel, this type of vaccine has been produced and used. The alternative to live vaccines other than splenectomized calf-derived biological material, has been the in vitro cultivation of Babesia bovis and B. bigemina. The development of in vitro culture of Babesia spp. strains in a defined medium has been the basis for the initiation of a source of parasites and exoantigens for a variety of studies on the biochemistry and immunology of babesiosis. The use of live immunogens from attenuated strains derived from in vitro culture is highlighted, which has been proposed as an alternative to control bovine babesiosis. In several studies performed in Mexico, this type of immunogen applied to susceptible cattle has shown the induction of protection against the experimental heterologous strain challenge with both, Babesia-infected blood and animal exposure to confrontations on tick vector-infested farms. The combination of transfection technologies and the in vitro culture system as integrated methodologies would eventually give rise to the generation of genetically modified live vaccines. However, a greater challenge faced now by researchers is the large-scale cultivation of Babesia parasites for mass production and vaccine distribution.
Bovine babesiosis, also known as Texas fever, “tristeza,” tick fever or red water, is caused by intraerythrocytic protozoa of the genus Babesia that are transmitted by ticks. They can produce an acute disease with clinical findings characterized by fever, hemolytic anemia, hemoglobinuria and death; but abortion can be caused in pregnant females after the first third of pregnancy (1, 2). Clinical signs vary depending on the pathogenicity and virulence of the species and strain of Babesia. There are factors that determine the bovine babesiosis infection, such as age, race and immune status of the animal. Affected animals, recovering from the acute disease, usually remain as asymptomatic carriers for years, can become reservoirs of infection for healthy animals and hardly reach the production levels that were lost by the chronic infection (3).
In Mexico, Babesia parasites are transmitted mainly by Rhipicephalus microplus ticks and the species so far identified are Babesia bovis and B. bigemina (4). Depending on the predominant species, there are variations in the pathogenesis and course of the disease (4, 5). In cattle infected with B. bovis, the presentation is severe and is characterized by high fever, ataxia, anorexia and general circulatory shock. Nervous signs are regularly associated with the sequestration of infected erythrocytes in the cerebral capillaries. Infection with B. bigemina usually manifests more benignly, but infected cattle may present with more severe hemolytic anemia (6). Babesiosis is currently considered as one of the main obstacles to the development of livestock in tropical and subtropical regions of the world. It directly affects the production of meat and milk, affecting the competitiveness of livestock industries (5, 7).
For the control of the disease there are different strategies such as the use of ixodicides for vector control, controlled translocation of cattle, chemotherapy, chemoprophylaxis and selection of tick-resistant cattle. These procedures are effective only if they are included in an integrated control program, which can be costly and impractical (8). Immunization of cattle is currently considered to be the most appropriate procedure for prevention and control of bovine babesiosis; This has been demonstrated with favorable results in terms of protection and safety (9).
There are more than 70 species of protozoa of the genus Babesia, distributed around the world, 18 of which can cause disease in different domestic mammals (10). Four species stand out due to their economic importance in affecting cattle: B. bovis (11), B. bigemina (12), B. divergens (13), and B. major (14). The most important, from an economical point of view, are B. bigemina and B. bovis, widely distributed in areas where their arthropod vectors exist, which are ticks such as R. microplus, R. decoloratus, R. annulatus, and R. geigyi. B. bigemina can also be transmitted by R. decoloratus and R. evertsi (1, 15). B. divergens is transmitted by Ixodes ricinus (16), and B. major, transmitted by Haemaphysalis punctata (16). Babesia is distributed in countries located between 30°S and 40°N of the equator an area that corresponds to the presence of its arthropod vector, R. microplus, R. annulatus, and R. decoloratus (16).
Bovine babesiosis is a serious problem for livestock, especially in developing countries, as they limit the introduction of European type livestock specialized in meat and milk production to tropical regions. In Mexico, bovine babesiosis, described for the first time in early 19th century (17) continues to be a limiting factor for cattle industry production, as the tick vector is distributed in the main tropical livestock production regions. The economic importance of the disease is reflected by the high morbidity and mortality rates in livestock (18).
Due to its wide distribution and effects on livestock (1) bovine babesiosis has been considered the most important among arthropod-borne diseases in cattle (5). Numerous economic losses due to babesiosis, anaplasmosis and ticks have been estimated in different countries of the world. Economical annual loss in amounts of $ 23.3, $ 5.1, $ 5.4, $ 6.8, $ 21.6, $ 19.4, $ 57.2, $ 3.1, and $ 0.6 million USD have been calculated for Australia, Kenya, Zimbabwe, Tanzania, South Africa, China, India, Indonesia and the Philippines, respectively (5). More recently in Mexico, the potential economic losses related to milk and beef production and associated exclusively to the cattle tick R. microplus were estimated at $ 573.61 million USD per year (8). Although well recognized by authorities that there is high mortality as well as drop in milk, meat and calves production caused by babesiosis, a current estimate of the real economic impact of the disease in cattle production is not yet available (8). It has been well established that the Babesia tick complex hinders the importation or mobilization of genetically superior cattle to tropical regions (16, 19). The economic impact of babesiosis with morbidity rates above 50% in endemic areas, reflects the high risk for occurrence of outbreaks in susceptible animals in the Mexican tropics (4).
The first step in invasion is the apparent random interaction between the merozoite and the host cell. For cell invasion to occur, there must be recognition between the surface components of the parasite membrane and the host erythrocyte that leads to the molecular coupling of the surfaces of both cells (20). The erythrocyte invasion by Babesia merozoites is summoned in five steps: (a) Contact between merozoite and erythrocyte; (b) Orientation of the apical pole of the merozoite to the surface of the erythrocyte; (c) Fusion between merozoite and erythrocyte membranes; (d) Release of the rhoptry contents; (e) Erythrocyte membrane invagination. The invasion process occurs when the sporozoites and subsequently the merozoites encounter the erythrocytes. Then, the sporozoites place their apical portion against the surface of the erythrocytes and secrete histidine-rich proteins, contained in the rhoptries and micronemes. Proteins secreted on contact with the membrane facilitate the entry of the parasite into the erythrocyte, without an exoerythrocytic phase. A parasitophorous vacuole is then formed which, by electron microscopic studies on B. microti in hamster erythrocytes (21), and on B. divergens in human erythrocytes (22) was demonstrated to be lost soon upon invasion, leaving the parasites in direct contact with the cytoplasm of the host cell. The parasite is not isolated from the external environment though, suggesting that intraerythrocytic Babesia uses a novel trafficking mechanism for export of proteins into the host. It was shown recently, that vesiculation at the parasite plasma membrane of B. microti produces an interlacement of connected vesicles in the host erythrocyte responsible for the export of antigens from the parasite to the host erythrocyte and subsequently to mouse plasma (23). The parasite differentiates to form the trophozoites (rings), which are then divided by binary fission and give rise to two (or four in the case of B. microti and B. divergens) merozoites. These are united at an angle and once they mature, they separate, leave the erythrocyte and continue to invade new erythrocytes (24).
It is frequently highlighted that in order to prevent and/or control bovine babesiosis in endemic countries, the primary goal has been to develop adequate immunogens using live or recombinant vaccines. Conventional procedures so far used and recommended for bovine babesiosis control are: vector control by using ixodicides; controlled mobilization of livestock to prevent asymptomatic and tick-infested livestock from being taken to free zones; both chemotherapy and chemoprophylaxis can be tactically included in a comprehensive program, although they are costly and impractical; the use of resistant livestock has been common in some countries with unsatisfactory results (25). Immunization is the procedure that has been identified for many years as the most appropriate way to prevent and control bovine babesiosis (9).
Historically, premunition has been used for the protection of cattle against babesiosis, which consists of the sub-inoculation of blood from an asymptomatic carrier bovine to susceptible cattle (26). Currently that form of prevention is not recommended because severe disease outbreaks can occur, and other pathogens can be transmitted. This procedure has been empirically applied, and may lead to the spread of other diseases - tuberculosis, brucellosis, IBR, etc. Premunized animals can be clinically affected with babesiosis and may even die if not treated in a timely manner.
Technically supported immunoprophylaxis procedures have been attempted for many years. In Australia, research began with the use of living organisms of reduced virulence, consisting of suspensions of B. bovis or B. bigemina infected erythrocytes with parasite populations previously attenuated by multiple passages in splenectomized or intact calves, respectively, from which blood is obtained with high parasitemias for application as a vaccine (27–31). Some limitations for this type of biological material are the need of an efficient cold chain maintenance and potential risk of contamination with other adventitious pathogens.
Antigens from erythrocytes and plasma from infected animals have also been used. These have provided some protection when applied with Freund's adjuvant or with saponin (32). At about the same time, the in vitro cultivation of Babesia was developed, a system from which attenuated strains that have functional immunogenic proprieties have been derived and tested. These strains have been used both in controlled and field studies, in which protection in vaccinated cattle of at least 80% has been demonstrated when challenged with virulent strains (33–36).
With the use of the in vitro culture system, parasite genes have been identified and cloned in vector expression systems to express recombinant proteins in E. coli, which have subsequently been produced, purified and used as immunogens, highlighting for the case of B. bigemina the GP45 protein which is a membrane surface glycoprotein (37); AMA1 integral membrane protein (38), and RAP-1 protein associated with rhoptries (39); whereas for the case of B. bovis the MSA-1 and MSA-2 proteins which are membrane surface glycoproteins and 12D3, a protein associated with the parasite surface and released into the erythrocyte stroma (15). It has been shown that these recombinant proteins generate high antibody titers in immunized cattle that are capable of inhibiting parasite invasion in vitro. However, in vivo trials demonstrated lack of protection against parasite challenge with field isolates (40, 41). Moreover, cattle immunized with a multi-epitope modified vaccinia Ankara virus and recombinant proteins were not protective after challenging by a virulent B. bovis strain (41).
Recent reviews on the application of molecular type technologies for development of immunogens against babesiosis have reported that effective immunogens against the disease has not been achieved yet. In contrast, it has been described that only live vaccines, whether derived from in vitro culture or from sub-inoculation in splenectomized calves, have demonstrated satisfactory protection in immunized animals against challenge with virulent parasites (15, 42, 43).
The establishment of the Plasmodium culture was fundamental for Babesia in vitro cultivation. In this process, several culture factors were determined to be critical such as; good management sterile practice, use of defibrinated blood, removal of white cells from the cell pack, appropriate incubation temperature, glucose addition, anaerobic conditions and fluid levels (44). Continuous replication cycles were achieved in a velobiosis atmosphere by burning a candle inside a desiccator (45). The need to produce biological material for biochemical, immunological or chemotherapeutic studies of bovine babesiosis was of utmost interest for researchers that developed procedures for establishing the in vitro cultivation of Babesia spp.
For Babesia parasite culture assays, the target cells are mature erythrocytes, which provide the appropriate metabolic requirements for the growth and replication of the intraerythrocytic parasite stages (46). Erythrocytes from a carrier of the same species were shown to be a determining factor, as the proliferation of B. microti was achieved only in hamster erythrocytes by supplementing the culture medium with bovine fetal serum, rat serum or hamster serum (47).
In Mexico, similar procedures were applied to establish the short term culture of B. bovis, for which 50% erythrocyte were resuspended in culture medium 199 with Earle's salts supplemented with 50% bovine serum, in spinner flasks kept under constant agitation and incubation at 37°C in a 5% CO2 atmosphere in air (48). Such studies favored the initiation of B. bovis trials under similar laboratory conditions. The continuous growth of the parasite was established in Mexico for the first time worldwide. Bovine erythrocytes were kept in suspension in culture medium 199 added with HEPES (N-2 hydroxymethylpiperazine N1-2 ethanosulfonic acid) as buffer and supplemented with 50% bovine serum. The pH was adjusted to 7.0 maintaining an atmosphere of 5% of CO2 in air; the complete suspension was kept in agitation at 100 rpm and incubation at 37°C (48). For other Babesia species, such as B. divergens, maintenance in human cells was achieved for 3 days in a supplemented medium with 10% of bovine fetal serum, adding antibiotics, HEPES buffer salt, and incubating at 37°C (49).
Subsequently, by incorporating some modifications to the culture system, the continuous growth was maintained for 32 days, with percentages of parasitized erythrocytes (PPE) reaching 15%, showing that their virulence in cattle remained without changes (50). By studying additional variables, no difference was found when using RPMI 1640, 199, or NCTC-135 as culture media, but it was demonstrated that a pH <6.7 or >7.3 adversely affected the parasite's growth (51). The success so far obtained for in vitro growth of B. bovis, allowed an improvement in the methodology and the development of an in vitro cultivation system named Microaerophilic Stationary Phase (MASP) system, avoiding the cultivation of infected erythrocytes in suspension in spinner flasks. In this system, fluid levels in the culture medium were maintained in an atmosphere of 5% CO2 in air. The depth of the fluid level in the culture vessel was identified as a determining factor. Under these conditions the MASP system allowed an increased invasion of parasites to normal erythrocytes in cumulative dilution levels of up to 1.7 × 1010 over a period of 83 days (52). The development of in vitro cultures for B. bigemina and B. rodhaini was also attempted, with positive results but just for short periods (53).
Additional modifications for in vitro cultivation of B. bovis included the replacement of HEPES by TES (N-Tris hydroxymethyl methyl 2-aminoethanic acid) as buffer salt (52). This change in buffer also achieved the adaptation and conservation of different parasite strains and allowed that B. bovis could be recovered in vitro by implementing a cryopreservation procedure (54). Biological cloning by limiting dilution techniques was later developed and three B. bovis parasite lines were established in vitro (55). It is noteworthy to mention that in the case of B. bovis, erythrocyte infections exceptionally reach 2% of parasitized erythrocytes (PPE) in cattle, in contrast to the in vitro cultivation system in which PPEs can be much higher (up to 10%), while with Percoll gradient concentration procedures the PPE can reach at least 49% (56) and even higher for B. bigemina (57).
With the experiences generated for B. bovis, the continuous cultivation of B. bigemina was soon established, reaching PPE of 3–6% (58). Similar to what was accomplished with B. bovis, complementary methodologies for in vitro cultivation were developed for cryopreservation (59) and cloning of B. bigemina parasites (57). Thus, with the thoroughly described procedures, the cultivation of B. bovis (60) and B. bigemina was routinely established in Mexico (61).
Overall, the Babesia in vitro culture system is a procedure consisting of a suspension of erythrocytes from a bovine donor in a chemically “defined” culture medium, supplemented with adult bovine serum. Complete medium is the source of nutrients for the growth and multiplication of parasites. In vitro cultivation of Babesia parasites has allowed researchers to identify some nutritional needs of this parasite (62); to study different aspects of parasite biology and its life cycle (43, 63); host-parasite relationships and the identification of proteins involved in the invasion process (64). It has also facilitated to gain knowledge on the metabolic, physiological and reproductive behavior of these protozoa. Biochemical studies of parasite cell lines have been obtained after being biologically cloned (55, 65). In addition, the culture system has been used as a source of soluble antigens for immunoprophylaxis studies (66); to conduct research on parasite ultrastructure (67); for preparation of antigen for serological diagnosis (68, 69); and to conduct drug sensitivity studies (70, 71). Above all, the use of live immunogens derived from in vitro culture has been highlighted as an alternative in the control of bovine babesiosis (15, 31, 33, 34, 72). This topic will be further described in section “Importance and immunoprophylactic applications of in vitro culture-derived parasites.”
The culture medium is the most important component of the culture environment, as it provides the necessary nutrients, growth factors and hormones, and it regulates the pH and the osmotic pressure. In initial cell culture experiments, natural media obtained from tissue extracts and body fluids were used. The need for standardization of media quality and increased demand led to the development of defined media classified as: (a) basal media; (b) media with reduced serum addition; and (c) serum-free media (73). Basal media contain amino acids, vitamins, inorganic salts and glucose as a carbon source, these formulations must be supplemented with serum. Formulations with low serum concentration need to be enriched with nutrients and animal derived factors. Serum-free media requires replacement with nutritional formulations containing growth factors and hormones; the latter are selective means for specific cell lines (74, 75). The propagation of cell cultures in vitro is used as a research model and is currently being used to eliminate the use of animals for experimental purposes (76). Innovations in cell culture have been continuously generated and increased, for application in immunology, pharmacology, physiology, toxicology and oncology among many other applications (77). However, in most studies, the culture depends on the required use of animal derived products, particularly fetal bovine serum as part of the culture medium, as an indispensable factor for cell growth and proliferation (78). Calf serum is a not well-defined supplement, because it varies quantitatively and qualitatively in its composition between batches. In addition, it may contain different amounts of endotoxins, hemoglobin or other adverse factors (79). It can also be a source of viral, bacterial, fungal or parasitic pathogens. Due to all these variables it has been suggested to avoid serum in cell culture in vitro (74, 80), to the extent that there are at least 450 different serum-free commercial media, which function only for a small number of cell types. It has even been indicated that if cell cultures could be available without the use of serum of animal origin, the standardization of protocols and replicas of the experiments between researcher groups could be established (74, 81).
The in vitro cultivation of B. bovis and B. bigemina, routinely requires the basal medium M-199 with Earle's salts and RPMI-1640, invariably supplemented with bovine serum in high proportions, from 20 to 40% (82, 83). Despite its importance, research on in vitro cultivation is carried out in just a few laboratories in the world and there are reports associated with the development of culture systems using chemically defined media. Growth factors to increase the PPEs, such as the replacement of adult bovine serum with fetal bovine serum, equine serum, serum fractions, bovine serum albumin or lipoproteins have been identified (82). For example, in B. divergens cultures, high density lipids (HDL) have been added and the development of cultures with high PPE has been demonstrated (84). In addition, high parasite proliferation with fetal bovine serum reaching a PPE similar to that obtained with adult bovine serum has been described (85). By using a medium enriched with ALBUMAX II, which is a complex mixture of lipids, and hypoxanthine as serum substitute it was possible to grow B. bovis (62). Exclusion of bovine serum for in vitro cultivation of B. bovis was possible by using the GIT medium (70).
Similarly, for the maintenance of B. caballi, the horse serum has been replaced by bovine serum albumin and chemically defined lipids (86). With the addition of hypoxanthine, the concentration of horse serum or fetal bovine serum could be reduced to 10% (87, 88). However, for the in vitro cultivation of B. bigemina, the concentration of bovine serum only was reduced to 20% and for short periods of time (89). The interest in eliminating the animal serum component has generated the commercial availability of different media for cell cultures, of which between 80 and 90% include albumin, transferrin and insulin in its composition (90). Chemically defined media have been developed in recent years, with formulations that partially or totally reduce bovine serum, without altering the morphology and function of cultivated cells. These new media containing vital elements for eukaryotic cell lines, had not been used until very recently for the in vitro cultivation of protozoan parasites where the maintenance and proliferation of Babesia spp. was accomplished (91, 92).
In Mexico, it was shown that B. bovis and B. bigemina can be adequately controlled for in vitro proliferation in a bovine serum-free medium with supplements such as insuline (ins), transferrine (trans) and selenite (sel) at optimal concentrations (91–93). The Advanced DMEM/F12 culture medium (A-DMEM/F12) supplemented with 40% adult bovine serum (v/v), was selected as an alternate medium to the traditional M-199 with Earle's sales (M-199). Improved parasite growth determined as maximal PPE reached, 9.59 and 8.37%, were obtained for B. bovis and B. bigemina, respectively, and the frequency for splitting cultures was reduced to every 24 h; This modification was possible because PPEs >4% were determined. Additionally, with the use of A-DMEM/F12, a period of adaptation of the parasites to the new culture medium was not necessary (91). The A-DMEM/F12 medium proposed in that study included the minimum concentration of the ins-trans-sel mixture as essential components to replace the bovine serum. It has been noted that the concentration of this mixture will depend on the needs of each cell culture (73, 79, 90). When A-DMEM/ F12 medium was supplemented with the ins-trans-sel mixture (at 2000, 1100, 1.34 mg/L, respectively), the proliferation of B. bovis (maximal PPE 9.73%) and B. bigemina (maximal PPE 7.23%) was constantly stimulated, occurring this without an adaptation period, and no changes in parasite morphology were observed by microscopic analysis. Moreover, using this new reformulation of the culture medium, the isolation of B. bovis from an asymptomatic carrier bovine was achieved (91).
Each component of the ins-tran-sel mixture performs specific functions; Insulin internalizes glucose to cells (94), transferrin collects and transports iron to erythrocytes for hemoglobin synthesis (95, 96), sodium selenite can function as an antioxidant and protector of the erythrocyte membrane (97, 98). In addition, A-DMEM/F12 contains hypoxanthine, which is vital for in vitro proliferation of protozoa (86, 99, 100) as some, including B. bovis, cannot synthesize hypoxanthine de novo (101). Another component of A-DMEM/F12 that facilitated the removal of bovine serum from the culture medium is ALBUMAX II, necessary for the proliferation of Babesia sp. (86, 89, 102). When the combination of medium A-DMEM/F12 with ins-trans-sel (2000, 1100, 1.34 mg/L) and Putrescine (Pu, 0.1012 mg/L) was used, maximal PPE of 17.26 and 14.8% were obtained for B. bovis and B. bigemina, respectively. With this culture medium combination, higher growth rates of parasite proliferation were shown (92, 93).
In these studies, it was demonstrated that there is an optimal concentration for Pu in the culture medium, by which the proliferation of B. bovis and B. bigemina can be best stimulated. It was also shown that Pu is required by both Babesia species to proliferate in vitro in a bovine serum free medium. This suggests that if parasites cannot synthesize Pu de novo, they may obtain it from the culture medium. All this indicates that precise concentrations of Pu should be used to stimulate the proliferation of B. bovis and B. bigemina.
A decisive advance in the improvement of the efficiency for in vitro cultivation of Babesia spp. was the inclusion of a perfusion bioreactor, in addition to the achievements previously obtained. Thus, the A-DMEM / F12 medium, the mixture of ins-trans-sel, Pu, and particularly the lines of B. bovis and B. bigemina adapted to proliferate in a serum-free medium were combined. With these improvements, maximal PPE values of up to 29.7% for B. bovis (Figure 1) and 33.45% for B. bigemina (Figure 2) were reached. This was the first report of the cultivation of Babesia spp. with a perfusion bioreactor, demonstrating the production of Babesia-infected erythrocytes at high density (92, 93).
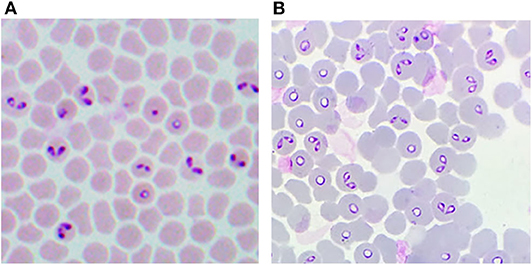
Figure 1. Giemsa-stained blood smear from in vitro cultured-derived Babesia bovis. (A) Conventional culture system. (B) Bioreactor culture system. 100X.
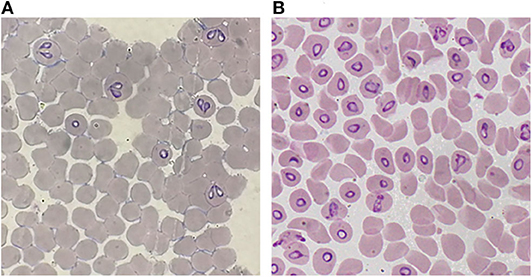
Figure 2. Giemsa-stained blood smear from in vitro cultured-derived Babesia bigemina, (A) Conventional culture system. (B) Bioreactor culture system.100X.
The freeze-thaw process employed in the in vitro culture system has been also modified to try to obtain a larger package of B. bigemina-infected erythrocytes and which could also be recovered in a shorter period once the in vitro culture were to be restarted from infected erythrocytes cryopreserved in liquid nitrogen (93). By modifying the freeze-thaw protocol in which the 20% PVP-40 culture medium was included in A-DMEM/F12 with 40% bovine serum, the in vitro culture system allowed parasite recovery in a short period. At 24 h post-thawing and seeding the cultures, parasites with typical morphology for the species were observed, which allowed establishing the continuity of the in vitro cultivation performing subcultures every 24 h, instead of every 72 h, as it has been reported previously using the standard methodology. Of the reported freezing systems, an efficiency of only 25% has been estimated for the recovery of B. bovis after the application of the freeze-thaw protocols (54). On the other hand, by testing the freezing protocol for B. bigemina it was reported that the recovery of parasites occurred at 48 h post-initiation of the culture, however no indication was made neither for the levels of infection, nor the continuous establishment of the parasites (59). Similarly, by following the standard methodology, PPE levels not exceeding 2% and only for short periods were later reported (61). The results obtained with this modification were probably due to the combination of the PVP-40 with the medium supplemented with serum and the activity of the serum proteins, which facilitated an adequate redox state of the erythrocyte membranes and the reduction of hemolysis (93).
Although there are different types of vaccines that have been tested against bovine babesiosis, so far the only procedure that has offered favorable results in terms of protection and safety has been the use of live attenuated vaccines in Australia (9, 27, 28). In other countries, such as Argentina, Brazil, Uruguay and Israel, this type of vaccine has also been produced and used (103).
The alternative to live vaccines other than splenectomized calf-derived biological material, has been the in vitro cultivation of B. bovis and B. bigemina. The development of the in vitro culture of Babesia spp. strains in a defined medium has been the basis for the initiation of a source of parasites for a variety of studies on the immunology of babesiosis. The various applications of cultivation include the selection of pure lines with different degrees of virulence (104) in order to obtain attenuated strains for vaccination (105–107). The use of live immunogens from attenuated strains derived from in vitro culture has been proposed as an alternative in the control of bovine babesiosis. In several studies, this type of immunogen applied to susceptible cattle has shown the induction of protection against an experimental heterologous challenge under controlled conditions (72, 105–107). Challenge of immunized cattle with the R. microplus tick has also been evaluated in cattle kept in natural conditions, by exposure of vaccinated animals to confrontations on vector-infested farms (34, 68).
In Mexico, as previously described (4) bovine babesiosis is one of the most important diseases transmitted by arthropods to cattle in the tropics. Prevalence rates of up to 96% have been estimated in different livestock regions of the country by serological and molecular assays (35).
Among parasitic diseases, bovine babesiosis is one of the main obstacles to improve production, highlighted by its economic impact on the livestock activity. It is important to mention that out of the total cattle population in Mexico, estimated at about 30 million cattle heads, almost 75% are in areas infested with R. microplus ticks. Therefore, there is always the possibility of infection with Babesia spp.; as up to 89% of Babesia sp. antibodies prevalence rate has been reported in Mexico, thus considering to be a serious obstacle to the introduction of genetically improved cattle, for high bovine beef and milk production (1).
Since instrumentation of the in vitro cultivation system, which allowed for selection of various strains with different growth patterns in vitro, culture-derived parasites were then tested as live immunogens, for which the pathogenicity and immunogenicity of different Babesia spp. cell lines were evaluated. These early studies attempted to assess the reduced virulence of both, parasite lines and clones after being inoculated in susceptible animals (104, 108). Subsequent studies were carried out to determine the immunoprotective dose for the immunogen containing B. bovis parasites, in which different groups of cattle were inoculated at increasing doses, from 1 × 105to1 × 109 infected erythrocytes (IE). In these vaccine evaluation experiments, the variables registered have usually included: Determination of the packed cell volume (PCV) by the microhematocrit method; parasitemia expressed as percent of parasitized erythrocytes (PPE) and presence or absence of characteristic clinical signs of bovine babesiosis, basically fever, hemoglobinuria or mortality. Also, to establish the challenge dose with pathogenic parasites a virulent strain was obtained from a clinical babesiosis case, cryopreserved in liquid nitrogen and reactivated in a splenectomized calf. Then, increasing doses from 1 × 104to1 × 108 infected erythrocytes were assessed in terms of virulence. All these experiments included cattle inoculated with uninfected erythrocytes (UE) that were considered as a non-vaccinated control group. In this way, an inoculum containing 1 × 108 B. bovis, or B. bigemina-infected erythrocytes was chosen as the challenge doses (33, 69). At challenge, none of the vaccinated animals showed clinical disease, whereas the control animals inoculated with UE showed severe clinical disease involving declines in PCV, fever (>40°C) and presence of parasites in peripheral blood smears. The most suitable vaccine dose selected was 1 × 107 infected erythrocytes for both Babesia species (33, 69, 109).
Afterwards, and in order to prove cross-immunity between B. bovis and B. bigemina, cattle were inoculated with the monovalent B. bigemina or B. bovis vaccine and a third group with a combined immunogen containing both species; a control group was inoculated with UE. At challenge, protection assessed was 25, 50, and 100% for cattle immunized with B. bigemina, B. bovis, and B. bigemina/B. bovis, respectively. As for animals inoculated with UE, all were severely affected, one died, and the rest were treated to avoid unnecessary death. It was concluded that cross-protection is not good enough to induce solid immunity and, therefore it is necessary the application of the combined B. bigemina/B. bovis immunogen is necessary (110).
The bivalent immunogen containing fresh B. bigemina and B. bovis was evaluated under a controlled challenge. At 3 months post-vaccination (PV) animals showed slight decrease in the PCV with no changes in rectal temperature values and parasitemias were as low as 0.01 up to 0.06 PPE due to B. bovis and B. bigemina, respectively. In contrast, control group animals showed fever, decrease in PCV values of up to 29% and parasitemias as high as 0.5 for B. bigemina and 0.03 for B. bovis, respectively. Thus, adequate protection was induced by the combined live attenuated vaccine (109).
To make a continuous use of the attenuated live vaccine, another study in a two-phase experiment was conducted. First, cattle were injected with the combined vaccine and a control group with UE. Animals were lodged in a tick-free area for 2 months; then cattle were moved into a farm with 80% of seroprevalence to Babesia spp. As described for other evaluations, vaccinated cattle were moderately affected, but treatment was not required. In contrast, unvaccinated animals showed severe clinical signs, such as fever (41°C), decreasing PCV higher than 50%, and parasites were detected on the peripheral bloodstream by Giemsa stained smears (72). Another experiment in which the only difference was that the animals were vaccinated and kept in a ranch under hyperendemic conditions for Babesia spp., cattle were maintained in tick-restricted stables for 21 days after which animals were released into tick-infested pastures. Under these circumstances, protection was reduced to 70% in the vaccinated animals, although the entire control group showed very severe signs of the disease (34).
The attenuated vaccine evaluations were successful, both under controlled and field conditions. However, the handling and storage of fresh material showed serious limitations (7 days of half shelf life). Therefore, B. bovis and B. bigemina at different doses, from 1 × 107 to 5 × 108 IE were cryopreserved in 20% PVP in a liquid nitrogen tank. The inocula were injected in cattle, including as a positive control a group of cattle vaccinated with 1 × 107 IE of fresh material. Vaccinated animals were then challenged in the field by tick exposure, finding a protection conferred of 90% in vaccinated cattle (35). Similar results were observed by applying the bivalent attenuated vaccine added with Lactobacillus casei in naive cattle (111).
An interesting evaluation of the attenuated vaccine in native cattle was carried out in an enzootically unstable farm for bovine babesiosis. Due to the excessive control of ticks, clinical and fatal cases had been described in this cattle farm. In that scenario, vaccination of cattle with frozen stabilates of culture-derived parasites helped restoring the enzootic stability. Therefore, the vaccine would be useful not only for prevention but also to control clinical outbreaks (112).
More recently, a live vaccine containing B. bovis and B. bigemina-infected erythrocytes cultured in vitro with a serum-free medium was assessed for protection conferred to naïve cattle, under natural tick-challenge in a high endemicity zone for Babesia spp. Vaccinated animals with Babesia-infected erythrocytes derived from both the serum-free culture system or the traditional bovine serum-containing culture system showed an excellent protection level, whereas the control unvaccinated animals were not protected and showed severe clinical signs, closely related to bovine babesiosis (113).
Studies on the improvement of the in vitro culture system of B. bovis and B. bigemina have demonstrated that it is possible to expand from a flask of 72 cm2 containing a PPE of 4% to a perfusion bioreactor, steadily increasing the maximal PPE up to 30% while harvesting material every 24 h. This has been achieved for both B. bovis and B. bigemina culture systems which implies obtaining a larger number of vaccine doses in a shorter period (92, 113).
Despite the development and continuous improvement for the in vitro culture methodology, currently there is not a commercially available vaccine in Mexico. However, farmers in enzootically unstable areas of bovine babesiosis have been utilizing the vaccine in validation programs to prevent babesiosis outbreaks, particularly on pure breed cattle that is translocated to tropical regions. In this sense, establishment of adequate farm production practices such as the vaccination strategy for disease prevention is essential.
Ever since 1964, live attenuated vaccine strains of B. bovis and B. bigemina derived from splenectomized calves have been described in Australia (9). However, by using the vaccine produced by multiple passages in calves some failures have been described. Several studies have concluded that even though a high immunogenic response can be attained, the vaccine has a limited lifespan. Apparently, this is due in part to the presence of variant antigenic parasite populations in the field; thus, presenting the need for changing the vaccine strain every 3–5 years to prevent vaccine failures (114). It has also been described that around 27 million doses of vaccine had been utilized in Australia up until 1996 (115).
An attenuated Australian B. bigemina G strain was tested in South Africa, and although slightly affected upon vaccination, animals were protected after challenge with a virulent strain (116). In field trials, the immunity provided by the calf-derived attenuated vaccine is long lasting, observing only failures associated to immunogenicity of the strain, or lack of responsiveness for some animals. On the other hand, some disadvantages have been described for the use of calf-derived live vaccines including the development of acute disease, dissemination of other pathogens that are contaminants, as well as the sensitization of vaccinated animals leading to hemolytic anemias of newborn calves (28).
In Paraguay, pregnant heifers were vaccinated with the Australian vaccine strains. After a syringe challenge with local B. bovis parasites, vaccinated animals showed an effective immune response. However, the response to B. bigemina was not conclusive due to the apparent avirulent nature of the local strain (117).
Regarding Babesia vaccines using in vitro culture-derived parasites, Yunker et al. (105) described a virulent strain adapted to grow in vitro by using equine serum in the culture medium. It was demonstrated that after an undetermined number of serial passages in vitro, an attenuated B. bovis strain was attained which when inoculated in experimental animals, was able to induce solid immunity by challenging experimental cattle with a virulent strain 2 months after vaccination. Unvaccinated animals were severely affected, and nervous signs were observed.
In Argentina, an in vitro culture-derived B. bigemina vaccine, showed good protection in naïve cattle. Vaccinated animals were able to stand the virulent challenge without specific treatment. In fact, a vaccine which includes B. bovis and B. bigemina is currently commercially available (118).
Some drawbacks for the use of attenuated vaccines have been described that include the spread of adventitious pathogens, difficulties in dose standardization, parasite virulence reversion and quality control stringencies during the production and handling of the vaccines. However, vaccines containing in vitro culture-derived parasites are processed under more controlled conditions, and have a lower risk to acquire adventitious pathogens due to gamma radiation of the serum, and more recently by using bovine serum-free culture medium (31, 36, 91, 93). As for the handling of the vaccine inoculum, the usefulness of the vaccine as fresh or frozen material has been demonstrated (35, 119).
Despite many shortcomings the attenuated vaccines at the present time are the best strategy for bovine babesiosis prevention (31). B. bovis attenuated vaccines produced both in vivo or in vitro, are infective and can induce similar solid protection against virulent challenge (106, 107, 120, 121). Timms and Stewart (122) combined the stationary culture and suspension culture systems to scale B. bovis proliferation as a live vaccine. This procedure was recognized as a system providing for enough quantity and quality of parasites to be feasible for massive vaccination in the field.
Since the B. bovis genome was sequenced (101), transfection technologies have facilitated carrying out genetic studies in this parasite (123, 124). Furthermore, an attenuated vaccine with a genetically transfected strain would allow to easily discriminate among vaccinated and naturally infected animals in the field (125). By using transfection technologies combined with the in vitro culture system, there would probably be improvements in the production of genetically modified live vaccines (15, 126).
Currently, the research community is discussing on what type of methodology is needed for an effective vaccine against bovine babesiosis, beyond the methodology of in vitro cultivation of Babesia spp. One approach suggested is the use of a blood-stage vaccine to prevent clinical disease or to avoid transmission from tick to host. Another approach is a tick-stage vaccine to prevent parasite transmission between host and ticks (127).
In vitro cultivation of B. bovis and B. bigemina provides for a wide number of applications: as a source of biological material for immunological studies, for the production of antigens for serological diagnostic techniques; moreover, it is an indispensable methodology for the development of subunit and recombinant vaccines, although currently one of the main benefits is the production of live attenuated vaccines. In spite of having been demonstrated its usefulness in terms of safety and protection, particularly in susceptible cattle introduced to hyperendemic regions of babesiosis in Mexico, the culture-derived live vaccine faces now a greater challenge, the parasite cultivation at large scale for mass production and distribution, due to the specific requirements for this type of vaccines.
JA and JF: conceptualization and writing, review and editing. JA, JF, and CR: formal analysis. JA and CR: writing original draft preparation. JF: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
This work was partially funded by CONACYT, Mexico, Problemas Nacionales 2015, Project No. 1336, and SEP-CONACYT CB2017-2018, Project No. A1-S-43508.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors thank INIFAP and CONACYT in Mexico for providing financial support for research projects in the Babesia laboratory unit at (formerly) CENID-Parasitologia Veterinaria, now part of CENID-Salud Animal e Inocuidad (National Research Center on Animal Health and Safety).
1. McCosker PG. The global importance of babesiosis. In: Ristic M, Kreier JP, editors. Babesiosis. New York, NY: Academic Press (1981). p. 1–24.
2. Purnell RE. Babesiosis in varios hosts. In: Ristic M, Kreir JP, editors. Babesiosis. New York, NY: Academic Press (1981). p. 25–32.
3. Mahoney DF, Ross DR. Epizootiological factors in the control of bovine babesiosis. Aust Vet J. (1972) 48:292–8. doi: 10.1111/j.1751-0813.1972.tb05160.x
4. Alvarez JA, Rojas C, Figueroa JV. Diagnostic tools for the identification of Babesia sp. in persistently infected cattle. Pathogens. (2019) 8:143. doi: 10.3390/pathogens8030143
5. Bock RE, Jackson L, de Vos A, Jorgensen W. Bovine babesiosis. Parasitol. (2004) 129:247–69. doi: 10.1017/S0031182004005190
6. Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. (2008) 38:1219–37. doi: 10.1016/j.ijpara.2008.03.001
7. Grisi L, Leite RC, Martins JR, Barros AT, Andreotti R, Cançado PH, et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet. (2014) 23:150–6. doi: 10.1590/S1984-29612014042
8. Rodriguez-Vivas RI, Grisi L, Perez AA, Silva VH, Torres JF, Fragoso H, et al. Potential economic impact assessment for cattle parasites in Mexico. Review Rev Mex Cienc Pec. (2017) 8:61–74. doi: 10.22319/rmcp.v8i1.4305
9. Bock RE, de Vos AJ. lmmunity following use of Australian tick fever vaccine: a review of the evidence. Aust Vet J. (2001) 79:832–9. doi: 10.1111/j.1751-0813.2001.tb10931.x
10. Levine ND, Corliss JO, Cox FEG, Deroux G, Grain J, Honigberg BM, et al. A newly revised classification of the protozoa. J Protozool. (1980) 27:37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x
11. Babes V. Sur 1'hemoglobinurie bacterienne du boeuf. C R Hebd Seances Acad Sci. (1888) 107:692–4.
12. Smith T, Kilborne FL. lnvestigations into the nature, causation, and prevention of Texas or southern cattle fever, U.S.D.A. Bureau of Anim lnd Bull. (1893) 1:1–372. doi: 10.5962/bhl.title.124068
13. M'Fadyean J, Stockman S. A new species of piroplasm found in the blood of British cattle. J Comp Path. (1911) 24:340–54. doi: 10.1016/S0368-17421180062-7
14. Sergent E, Donatien A, Parrot L, Lestoquard F, Plantureux E. Les piroplasmoses bovines dues aux Babesella. Etude d'ensemble, avec Description d'une espece nouvelle B major originaire de France. Arch Inst Past Algerie. (1926) 4:318–39.
15. Florin-Christensen M, Suarez CE, Rodriguez AE, Flores DA, Schnittger L. Vaccines against bovine babesiosis: where we are now and possible roads ahead. Parasitol. (2014) 141:1563–92. doi: 10.1017/S0031182014000961
16. Uilenberg G. Epizootiology of tick-borne diseases. In: FAO, editor. Animal Production and Health Paper 36; Tick-Borne Livestock Diseases and Their Vectors. (1983). Available online at: http://www.fao.org/3/x6538e/X6538E02.htm#ch2.2 (accessed January 15, 2020).
17. Tussaint M. Piroplasmosis bigeminum en Mexico. Boletin del Instituto patologico, Descrito por el M. V. Z. Eutimio Lopez Vallejo en 1910. Algunas enfermedades microbianas y parasitarias. Mexico DF: Estacion Agricola Central (1905).
18. Alvarez-Martinez JA, Rojas-Martinez C, Lira-Amaya JJ, Figueroa-Millan JV. Development of a live attenuated vaccine for the control of bovine babesiosis in Mexico. Arch Palliat Care. (2017) 2:1015.
19. Solorio-Rivera JL, Rodriguez-Vivas RI. Epidemiologia de la babesiosis bovina I. Componentes epidemiologicos. Rev Biomed. (1997) 8:37–47.
20. Yokohama N, Okamura M, Igarashi I. Erythrocyte invasion by Babesia parasites: current advances in the elucidation of the molecular interactions between the protozoan ligands and host receptors in the invasion stage. Vet Parasitol. (2006) 138:22–32. doi: 10.1016/j.vetpar.2006.01.037
21. Rudzinska MA, Trager W, Lewengrub SJ, Gubert E. An electronmicroscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. (1976) 169:323–34. doi: 10.1007/BF00219605
22. Repnik U, Gangopadhyay P, Bietz S, Przyborski JM, Griffiths G, Lingelbach K. The apicomplexan parasite Babesia divergens internalizes band 3, glycophorin A and spectrin during invasion of human red blood cells. Cell Microbiol. (2015) 17:1052–8. doi: 10.1111/cmi.12422
23. Thekkiniath J, Kilian N, Lawres L, Gewirtz MA, Graham MM, Liu X, et al. Evidence for vesicle-mediated antigen export by the human pathogen Babesia microti. Life Sci Alliance. (2019) 2:e201900382. doi: 10.26508/lsa.201900382
24. Sevilla E, Gonzalez LM, Luque D, Gray J, Montero E. Kinetics of the invasion and egress processes of Babesia divergens, observed by time-lapse video microscopy. Scientific Rep. (2018) 8:14116. doi: 10.1038/s41598-018-32349-7
25. FAO. Revision of Strategies for the Control of Ticks and Tick-Borne Diseases and Their Vectors. Rome ltaly: FAO expert consultation (1989).
26. de Waal DT, Combrink MP. Live vaccines against bovine babesiosis. Vet Parasitol. (2006) 138:88–96. doi: 10.1016/j.vetpar.2006.01.042
27. Callow LL, Mellors LT. A new vaccine against Babesia argentina infection prepared in splenectomized calves. Austr Vet J. (1967) 42:464–5. doi: 10.1111/j.1751-0813.1966.tb14476.x
28. Callow LL. Vaccination against bovine babesiosis. In: Miller H, Pino JA, McKelvey JJ, editors. lmmunity to Blood Parasites of Animals and Man Advances in Experimental Medicine and Biology. New York, NY: Plenum Press (1977). p. 121–49.
29. Callow LL, Mellors LT, McGregor W. Reduction in virulence of Babesia bovis due to rapid passage in splenectomized cattle. Int J Parasitol. (1979) 9:333–8. doi: 10.1016/0020-7519(79)90083-3
30. Dalgliesh T, Jorgensen WK, de Vos AJJ. New Australian vaccines for the control of babesiosis and Anaplasmosis in the world cattle trade. Trop Anim Health Prod. (1990) 22:44–52. doi: 10.1007/BF02243499
31. Shkap V, de Vos AJ, Zweygarth E, Jongejan F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: the continuing necessity. Trends Parasitol. (2007) 23:420–6. doi: 10.1016/j.pt.2007.07.003
32. Kuttler KL, Johnson LW. Immunization of cattle with a Babesia bigemina antigen in Freund's complete adjuvant. Am J Vet Res. (1980) 41:536–8.
33. Canto AG, Figueroa MJV, Alvarez MJA, Ramos AJA, Vega MCA. Capacidad inmunoprotectora de una clona irradiada de Babesia bovis derivada del cultivo in vitro. Tec Pecu Mex. (1996) 34:127–35.
34. Canto AGJ, Rojas EE, Alvarez JA, Ramos JA, Mosqueda JJ, Vega CA, et al. Protection against bovine babesiosis with a mixed in vitro culture-derived Babesia bovis and Babesia bigemina vaccine under field challenge. Immunization in an endemic area. Tec Pecu Mex. (2003)41:307–15.
35. Alvarez MJA, Ramos JA, Rojas E, Mosqueda JJ, Vega CA, Olvera A, et al. Field challenge of cattle vaccinated with a combined Babesia bovis and Babesia bigemina frozen immunogen. Ann N Y Acad Sci. (2004) 1026:277–83. doi: 10.1196/annals.1307.043
36. Rojas C, Figueroa JV, Alvarado A, Mejia P, Mosqueda JJ, Falcon A, et al. Bovine babesiosis live vaccine production: use of gamma irradiation on the substrate. Ann NY Acad Sci. (2006) 1081:405–16. doi: 10.1196/annals.1373.059
37. McElwain TF, Perryman LE, Musoke AJ, McGuire TC. Molecular characterization and immunogenicity of neutralization sensitive Babesia bigemina merozoite surface proteins. Mol Biochem Parasitol. (1991) 47:213–22. doi: 10.1016/0166-6851(91)90181-5
38. Torina A, Agnone A, Sireci G, Mosqueda JJ, Blanda V, Albanese I, et al. Characterization of the apical membrane antigen-1 in Italian strains of Babesia bigemina. Transboud Emerg Dis. (2010) 57:52–6. doi: 10.1111/j.1865-1682.2010.01118.x
39. Norimine J, Mosqueda J, Suarez C, Palmer GH, McElwain TF, Mbassa G, et al. Stimulation of T-Helper cell gamma interferon and immunoglobulin G responses specific for Babesia bovis Rhoptry-Associated Protein 1 (RAP-1) or a RAP-1 Protein lacking the carboxy-terminal repeat region is insufficient to provide protective immunity against virulent B. bovis challenge. Infect Immun. (2003) 71:5021–32. doi: 10.1128/IAI.71.9.5021-5032.2003
40. Alvarez JA, Lopez U, Rojas MC, Borgonio VM, Sanchez V, Castaneda R, et al. Immunization of Bos taurus steers with Babesia bovis recombinant antigens MSA-1, MSA-2c and 12D3. Transbound Emerg Dis. (2010) 57:87–90. doi: 10.1111/j.1865-1682.2010.01117.x
41. Jaramillo OJA, de la Fournière SAM, Valenzano NM, Nicole M, Guillemia CE, Valentini B, et al. Immunisation of cattle against Babesia bovis combining a multi-epitope modified vaccinia Ankara virus and a recombinant protein induce strong Th1 cell responses but fails to trigger neutralising antibodies required for protection. Ticks Tick-borne Dis. (2019) 10:101270. doi: 10.1016/j.ttbdis.2019.101270
42. Brown WC, Palmer GH. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol Today. (1999) 15:275–81. doi: 10.1016/S0169-4758(99)01471-4
43. Mosqueda J, Olvera-Ramirez A, Aguilar-Tipacamu G, Canto GJ. Current advances in detection and treatment of babesiosis. Curr Med Chem. (2012) 19:1504–18. doi: 10.2174/092986712799828355
44. Bass CC, Johns FM. The cultivation of malarial Plasmodia (Plasmodium vivax and Plasmodium falciparum) in vitro. J Exp Med. (1912) 16:567–79. doi: 10.1084/jem.16.4.567
45. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. (1976) 193:673–5. doi: 10.1126/science.781840
46. Nutall GHF, Graham-Smith GS. The development of Piroplasma canis culture. Parasitol. (1908) 1:243–60. doi: 10.1017/S0031182000003498
47. Bautista CR, Kreier JP. Effect of immune serum on the growth of Babesia microti in hamster erythrocytes in short-term culture. Infect Immun. (1979) 25:470–47. doi: 10.1128/IAI.25.1.470-472.1979
48. Erp EE, Gravely SM, Smith RD, Ristic M, Osorno BM, Carson CA. Growth of Babesia bovis in bovine erythrocyte cultures. Am J Trop Med Hyg. (1978) 27:1061. doi: 10.4269/ajtmh.1978.27.1061
49. Irvin AD, Young ER. Further studies on the up take of tritiated nucleic acid precursors by Babesia spp. of cattle and mice. lnt J Parasitol. (1979) 9:109–14. doi: 10.1016/0020-75197990099-7
50. Erp EE, Smith RD, Ristic M, Osorno BM. Continuous cultivation in vitro of Babesia bovis. Am J Vet Res. (1980) 41:1141–2.
51. Erp EE, Smith RD, Ristic M, Osorno BM. Optimization of the suspension culture method for in vitro cultivation of Babesia bovis. Am J Vet Res. (1980) 41:2059–62.
52. Levy M, Ristic M. Babesia bovis: Continuous cultivation in a microaerophilus stationary phase culture. Science. (1980) 207:1218–20. doi: 10.1126/science.7355284
53. Timms P. Short term cultivation of Babesia species. Res Vet Sci. (1980) 29:102–4. doi: 10.1016/S0034-5288(18)32694-8
54. Palmer DA, Buening GM, Carson CA. Cryopreservation of Babesia bovis for in vitro cultivation. Parasitol. (1982) 62:221–31.
55. Rodriguez SD, Buening GM, Green TJ, Carson CA. Cloning of Babesia bovis by in vitro cultivation. Infect Immun. (1983) 42:15–8. doi: 10.1128/IAI.42.1.15-18.1983
56. Rodriguez SD, Buening GM, Vega CA, Carson CA. Babesis bovis: Purification and concentration of merozoites and infected bovine erythrocytes. Exp Parasitol. (1986) 61:236–43. doi: 10.1016/0014-4894(86)90157-8
57. Vega CA, Buening GM, Rodriguez SD, Carson CA. Cloning of in vitro propagated Babesia bigemina. Vet Parasitol. (1986) 22:223–33. doi: 10.1016/0304-4017(86)90109-3
58. Vega CA, Buening GM, Green TJ, Carson CA. In vitro cultivation of Babesia bigemina. Am J Vet Res. (1985) 46:416–20.
59. Vega CA, Buening GM, Rodriguez SD, Carson CA, Mclaughlin K. Cryopreservation of Babesia bigemina for in vitro cultivation. Am J Vet Res. (1985) 46:421–3.
60. Figueroa MJV, Canto AG, Juarez FJ, Ruiz LF. Babesia bovis: Establecimiento y condiciones optimas de multiplicacion. Tec Pecu Mex. (1984) 46:46.
61. Monroy BM, Romero OG, Torres AR, Alvarez MJA, Canto AGJ, Vega MCA. Establecimiento en Mexico del cultivo in vitro de Babesia bigemina. Tec Pecu Mex. (1987) 25:141–50.
62. Jackson LA, Waldron SJ, Weier HM, Nicoll CL, Cooke BM. Babesia bovis: Culture of laboratory-adapted parasite lines and clinical isolates in a chemically defined medium. Exp Parasitol. (2001) 99:168–74. doi: 10.1006/expr.2001.4655
63. Mosqueda JJ, Falcon NA, Alvarez JA, Ramos AJA, Oropeza HL, Figueroa MJ. Babesia bigemina sexual stages are induced in vitro and are specially recognized by antibodies in the midgut of infected Boophilus microplus ticks. Int J Parasitol. (2004) 34:1229–36. doi: 10.1016/j.ijpara.2004.07.003
64. Lobo CA. Babesia divergens and Plasmodium falciparum use common receptors, glycophorins A and, B, to invade the human red blood cell. Infect Immun. (2005) 73:649–51. doi: 10.1128/IAI.73.1.649-651.2005
65. Rodriguez SD, Buening GM, Carson CA. Caracterizacion bioquimica preliminar de clonas de Babesia bovis irradiadas con Co 60. Tec Pecu Mex. (1993) 31:16–21.
66. Smith RD, James MA, Ristic M, Aikawa M, Vega CA. Bovine babesiosis: protection of cattle with culture-derived soluble Babesia bovis antigen. Science. (1981) 212:335–8. doi: 10.1126/science.7209532
67. Gorenflot A, Brasseur P, Precigout E, L'Hostis M, Marchand A, Scherevel J. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol Res. (1991) 77:3–12. doi: 10.1007/BF00934377
68. Canto GJ, Vega CA, Smith R. Ensayos de vacunacion contra Babesia bovis utilizando antigenos procedentes del cultivo in vitro. Tec Pecu Mex. (1982) 43:43–54.
69. Figueroa VJ, Canto AG, Alvarez MJA, Lona R, Ramos JA, Vega MCA. Capacidad protectora en bovinos de una cepa de Babesia bigemina derivada del cultivo in vitro. Tec Pecu Mex. (1998) 36:95–107.
70. Bork S, Yokoyama N, Matsuo T, Claveria FG, Fukisaki K, lgarashi I. Clotrimazole, ketoconazole, and clodinafop-propargyl inhibit the in vitro growth of Babesia bigemina and Babesia bovis (Phylum apicomplexa). Parasitol. (2003) 127:311–5. doi: 10.1017/S0031182003003895
71. Bork S, Yokoyama N, Matsuo T, Claveria FG, Fukisaki K, lgarashi I. Clotrimazole, ketoconazole, and clodinafop-propargyl as potent growth inhibitors of equine Babesia parasites during in vitro culture. J Parasitol. (2003) 89:604–6. doi: 10.1645/0022-339520030890604:CKACAP2.0.CO;2
72. Canto GJ, Alvarez JA, Rojas EE, Ramos JA, Mosqueda JJ, Vega CA, et al. Protection against bovine babesiosis with a mixed in vitro culture-derived Babesia bovis and Babesia bigemina vaccine under field challenge. Immunization in a disease-free area. Vet Mex. (2003) 34:323–32. doi: 10.21753/vmoa.34.004.98
73. van der Valk J, Brunner D, de Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, et al. Optimization of chemically defined cell culture media replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. (2010) 24:1053–63. doi: 10.1016/j.tiv.2010.03.016
74. Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. Altex. (2003) 20:275–81.
75. Even MS, Sandusky CB, Barnard ND. Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends Biotechnol. (2006) 24:105–8. doi: 10.1016/j.tibtech.2006.01.001
76. Falkner E, Appl H, Eder C, Losert UM, Schöffl H, Pfaller W. Serum free cell culture: the free access online database. Toxicol In Vitro. (2006) 20:395–400. doi: 10.1016/j.tiv,.2005.09.006
77. Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. Cytokines, inducers and inhibitors modulate MMP-2 and MMP-9 secretion by human Fanconi anemia immortalized fibroblasts. Oncol Rep. (2017) 37:3. doi: 10.3892/or.2017.5368
78. Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. Altex. (2010) 27:53–62. doi: 10.14573/altex.2010.1.53
79. Barnes D, Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. (1980) 102:255–70. doi: 10.1016/0003-2697(80)90151-7
80. Dessels C, Potgieter M, Pepper MS. Making the switch: alternatives to fetal bovine serum for adipose-derived stromal cell expansion. Front Cell Dev Biol. (2016) 4:115. doi: 10.3389/fcell.2016.00115
81. Gstraunthaler G. The Bologna statement on good cell culture practice (GCCP) e 10 years later. Altex. (2010) 27:141–6.
82. Schuster FL. Cultivation of Babesia and Babesia-like blood parasites: agents of an emerging zoonotic disease. Clin Microbiol Reviews. (2002) 15:365–73. doi: 10.1128/CMR.15.3.365-373.2002
83. Sanchez C, Campos E, Oliva AG. Babesia bovis: effect of Albumax II and orotic acid in a low-serum in vitro culture. Exp Parasitol. (2009) 121:274–8. doi: 10.1016/j.exppara.2008.11.017
84. Schrevel J, Grellier P, Rigomier D. New approaches in vitro cultures of Plasmodium falciparum and Babesia divergens by using serum-free medium based on human high-density lipoproteins. Mem Inst Oswaldo Cruz. (1992) 87:71–5. doi: 10.1590/S0074-02761992000700009
85. Zintl A, Skerrett HE, Gray JS, Bropy PO, Mulcahy G. Babesia divergens (Phylum Apicomplexa) in vitro growth in the presence of calf serum. Vet Parasitol. (2004) 122:127–30. doi: 10.1016/j.vetpar.2004.03.014
86. Zweygarth E, van Niekerk CJ, de Wal DT. Continuous in vitro cultivation of Babesia caballi in serum- free medium. Parasitol Res. (1999) 85:413–6. doi: 10.1007/s004360050568
87. Iscove NN, Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide reactive B lymphocytes. J Exp Med. (1978) 147:923–33. doi: 10.1084/jem.147.3.923
88. Ikadai H, Nagasawa H, Fujisaki K, Suzuki N, Mikami T, Kudo N, et al. Analysis of a growth- promoting factor for Babesia caballi cultivation. J Parasitol. (2001) 87:1484–6. doi: 10.1645/0022-339520010871484:AOAGPF2.0.CO;2
89. Neves L, Cross HF, Loureiro L, Akca A, Hommel M, Trees AJ. Addition of hypoxanthine to culture media allows in vitro cultivation of Babesia bovis and Babesia bigemina at reduced serum concentrations. Parasitol. (2001) 123:357–63. doi: 10.1017/S0031182001008502
90. Sheldon EB Jr, Sharon MP. The Case for Serum-Free Media. Baltimore, MD: Athena Environmental Sciences, Inc. (2003). p. 56–8.
91. Rojas-Martinez C, Rodriguez-Vivas RI, Figueroa-Millan JV, Acosta-Viana KY, Gutierrez-Ruiz EJ, Alvarez-Martinez JA. In vitro culture of Babesia bovis in a bovine serum-free culture medium supplemented with insulin, transferrin, and selenite. Exp Parasitol. (2016) 170:214–9. doi: 10.1016/j.exppara.2016.10.002
92. Rojas-Martinez C, Rodriguez-Vivas RI, Figueroa-Millan JV, Acosta-Viana KY, Gutierrez-Ruiz EJ, Alvarez-Martinez JA. Putrescine: essential factor for in vitro proliferation of Babesia bovis. Exp Parasitol. (2017) 175:79–84. doi: 10.1016/j.exppara.2017.01.010
93. Rojas-Martinez C, Rodriguez-Vivas RI, Figueroa-Millan JV, Acosta-Viana KY, Gutierrez-Ruiz EJ, Bautista-Garfias CR, et al. Babesia bigemina: advances in continuous in vitro culture using serum free medium, supplemented with insulin, transferrin, selenite and putrescine. Parasitol Intl. (2018) 67:294–301. doi: 10.1016/j.parint.2017.11.003
94. Jensen MD, Conley M, Helstowski LD. Culture of Plasmodium falciparum. The role of pH, glucose and lactate. J Parasitol. (1983) 69:1060–7. doi: 10.2307/3280864
95. Ball EG, McKee RW, Anfinsen CB, Cruz WO, Geiman QM. Studies on malarial parasites: IX Chemical and metabolic changes during growth and multiplication in vivo and in vitro. J Biol Chem. (1948) 175:547–71.
96. Francis SE, Sullivan DJ Jr, Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol. (1957) 51:97–123. doi: 10.1146/annurev.micro.51.1.97
97. Müller S, Coombs GH, Walter RD. Targeting polyamines of parasitic protozoa in chemotherapy. Trends Parasitol. (2001) 17:242–9. doi: 10.1016/S1471-49220101908-0
98. Jortzik E, Becker K. Thioredoxin and glutathione systems in Plasmodium falciparum. Int J Med Microbiol. (2012) 302:187–94. doi: 10.1016/j.ijmm.2012.07.007
99. Asahi H, Kanazawa T, Kajihara Y, Takahashi K, Takahashi T. Hypoxanthine: a low molecular weight factor essential for growth of erythrocytic Plasmodium falciparum in serum-free medium. J Parasitol. (1996) 113:19–23. doi: 10.1017/S0031182000066233
100. Tuvshintulga B, AbouLaila M, Davaasuren B, Ishiyama A, Sivakumar T, Yokoyama N, et al. Clofazimine inhibits the growth of Babesia and Theileria parasites in vitro and in vivo. Antimicrob Agents Chemother. (2016) 60:2739–46. doi: 10.1128/AAC.01614-15
101. Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Path. (2007) 3:1401–13. doi: 10.1371/journal.ppat.0030148
102. Grande N, Precigout E, Ancelin ML, Moubri K, Carcy B, Lemesre JL, et al. Continuous in vitro culture of Babesia divergens in a serum-free medium. Parasitol. (1997) 115:81–9. doi: 10.1017/S0031182097008937
103. de Castro JJ. Sustainable tick and tick-borne disease control in livestock improvement in developing counties. Vet Parasitol. (1997) 71:77–97. doi: 10.1016/S0304-40179700033-2
104. Buening GM, Rodriguez SD, Carson CA. Evaluation of a cloned Babesia organism as a live immunogen. Vet Parasitol. (1986) 22:235–42. doi: 10.1016/0304-4017(86)90110-X
105. Yunker CE, Kuttler KL, Johnson LW. Attenuation of Babesia bovis by in vitro cultivation. Vet Parasitol. (1987) 24:7–13. doi: 10.1016/0304-40178790125-7
106. Kuttler KL, Levy MG, Ristic M. Cell culture-derived Babesia bovis vaccine: sequential challenge exposure of protective immunity during a 6-month postvaccination period. Am J Vet Res. (1983) 44:1456–9.
107. Kuttler KL, Zaugg JL, Yunker CE. The pathogenicity and immunologic relationship of a virulent and a tissue-culture-adapted Babesia bovis. Vet Parasitol. (1988) 27:239–44. doi: 10.1016/0304-40178890038-6
108. Hernandez OR, Alvarez MJA, Buening GM, Canto AG, Monroy M, Ramos JA, et al. Diferencias en la virulencia y en la induccion de proteccion de aislamientos de Babesia bigemina derivados de cultivo in vitro. Tec Pecu Mex. (1990) 28:51.
109. Canto GJ, Figueroa JV, Ramos JA, Alvarez JA, Mosqueda JJ, Vega CA. Evaluacion de la patogenicidad y capacidad protectora de un inmunogeno fresco combinado de Babesia bigemina y Babesia bovis. Vet Mex. (1999) 30:215–20.
110. Vega CA, Figueroa MJV, Rojas RE, Ramos AJA, Canto AG. Insuficiente inmunidad cruzada en bovinos por Babesia bigemina y/o Babesia bovis derivadas del cultivo in vitro. Tec Pecu Mex. (1999) 37:13–22.
111. Bautista-Garfias CR, Lozano AR, Rojas-Martinez C, Alvarez JA, Figueroa JV, García GR, et al. Co-immunization of cattle with a vaccine against babesiosis and Lactobacillus casei increases specific IgG1 levels to Babesia bovis and B. bigemina. Parasitol Int. (2015) 64:319–23. doi: 10.1016/j.parint.2015.04.005
112. Ojeda JJ, Orozco Flores R, Rojas C, Figueroa JV, Alvarez JA. Validation of an attenuated live vaccine against babesiosis in native cattle in an endemic area. Transboun Emer Dis. (2010) 57:84–6. doi: 10.1111/j.1865-1682.2010.01123.x
113. Rojas-Martinez C, Rodriguez-Vivas RI, Figueroa-Millan JV, Bautista-Garfias CR, Castaneda-Arriola RO, Lira-Amaya JJ, et al. Bovine babesiosis: Cattle protected in the field with a frozen vaccine containing Babesia bovis and Babesia bigemina cultured in vitro with a serum-free medium. Parasitol Intl. (2018) 67:190–5. doi: 10.1016/j.parint.2017.11.004
114. Bock RE, de Vos AJ, Kingston TG, Shields IA, Dalgliesh RJ. Investigations of breakdowns in protection provided by living Babesia bovis vaccine. Vet Parasitol. (1992) 43:45–56. doi: 10.1016/0304-40179290047-D
115. Callow LL, Dalgliesh RJ, de Vos AJ. Development of effective living vaccines against bovine babesiosis. The longest field trial? Int J Parasitol. (1997) 27:747–67. doi: 10.1016/S0020-75199700034-9
116. De Vos AJ, Combrink MP, Bessenger R. Babesia bigemina vaccine: Comparison of the efficacy and safety of Australian and South African strains under experimental conditions in South Africa. Onderstepoort J Vet Res. (1982) 49:155–8.
117. Brizuela CM, Ortellado CA, Sanabria E, Torres O, Ortigosa D. The safety and efficacy of Australian tick-borne disease vaccine strains in cattle in Paraguay. Vet Parasitol. (1998) 76:27–44. doi: 10.1016/S0304-40179700047-2
118. Echaide IE, de Echaide A, Guglielmone A. Live and soluble antigens for cattle protection to Babesia bigemina. Vet Parasitol. (1993) 51:35–40. doi: 10.1016/0304-40179390193-Q
119. Mangold A, Vanzini VR, Echaide IE, de Echaide ST, Volpogni MM, Guglielmone AA. Viability after thawing and dilution of simultaneously cryopreserved vaccinal Babesia bovis and Babesia bigemina strains cultured in vitro. Vet Parasitol. (1996) 61:345–8. doi: 10.1016/0304-40179500839-X
120. Timms P, Dalgliesh RJ, Barry DN, Dimmock CK, Rodwell BJ. Babesia bovis: comparison of culture-derived parasites, non-living antigen and conventional vaccine in the protection of cattle against heterologous challenge. Vet J. (1983) 60:75–7. doi: 10.1111/j.1751-0813.1983.tb05874.x
121. Fish L, Leibovich B, Krigel Y, MaElwain T, Shkap V. Vaccination of cattle against B. bovis infection with live attenuated parasites and non-viable immunogens. Vaccine. (2008) 26S:G29–33. doi: 10.1016/j.vaccine.2008.09.070
122. Timms P, Stewart NP. Growth of Babesia bovis parasites in stationery and suspension cultures and their use in experimental vaccination of cattle. Res Vet Sci. (1989) 47:309–14. doi: 10.1016/S0034-52881831252-9
123. Suarez CE, McElwain TF. Transient transfection of purified Babesia bovis merozoites. Exp Parasitol. (2008) 118:498–504. doi: 10.1016/j.exppara.2007.10.013
124. Suarez CE, McElwain TF. Stable expression of a GFP-BSD fusion protein in Babesia bovis merozoites. Int J Parasitol. (2009) 39:289–97. doi: 10.1016/j.ijpara.2008.08.006
125. Suarez CE, Laughery JM, Schneider DA, Sondgerot KS, Mc Elwain TF. Acute and persistent infection by a transfected Mo7 strain of Babesia bovis. Mol Biochem Parasitol. (2012) 185:52–7. doi: 10.1016/j.molbiopara.2012.05.003
126. Lau AO, Kalyanaraman A, Echaide I, Palmer G, Bock R, Pedroni MJ, et al. (2011). Attenuation of virulence in an apicomplexan hemoparasite results in reduced genome diversity at the population level. BMC Genomics. (2011) 12:410. doi: 10.1186/1471-2164-12-410
Keywords: Babesia bovis, Babesia bigemina, in vitro cultivation, production, live attenuated vaccines
Citation: Alvarez JA, Rojas C and Figueroa JV (2020) An Overview of Current Knowledge on in vitro Babesia Cultivation for Production of Live Attenuated Vaccines for Bovine Babesiosis in Mexico. Front. Vet. Sci. 7:364. doi: 10.3389/fvets.2020.00364
Received: 30 November 2019; Accepted: 26 May 2020;
Published: 26 June 2020.
Edited by:
Guiquan Guan, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Monica Florin-Christensen, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaCopyright © 2020 Alvarez, Rojas and Figueroa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julio V. Figueroa, ZmlndWVyb2EuanVsaW9AaW5pZmFwLmdvYi5teA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.