
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci., 19 May 2020
Sec. Veterinary Epidemiology and Economics
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00282
This article is part of the Research TopicAfrican Swine FeverView all 11 articles
African Swine Fever (ASF) is a viral disease that affects animals of the Suidae family, and soft ticks from the genus Ornithodoros can also be infected by the ASF virus (ASFV). The disease was first described in Africa at the beginning of the twentieth century as an acute disease characterized by high mortality and fatal hemorrhages. ASF has caused outbreaks in numerous countries and it continues to be devastating nowadays for the porcine sector in those countries affected, and a massive threat for those free of the disease. ASF can follow clinical courses from peracute to chronic in domestic pigs (Sus scrofa) depending on a variety of factors, including the immune status of the animals and the virulence of the ASFV strain. The key features of the pathogenesis of the disease in domestic swine are a) a severe lymphoid depletion including lymphopenia and a state of immunodeficiency, and b) hemorrhages. However, African wild swine like bushpigs (Potamochoerus larvatus), red river hogs (Potamochoerus porcus), and warthogs (Phacochoerus africanus) can be infected by ASFV showing no clinical signs of disease and acting as natural reservoir hosts. In this article we review the key features of the gross and microscopic pathology together with a description of the pathogenesis of ASFV infection in domestic pigs following the different clinical courses. The pathogenesis of ASF in wild and domestic swine is also described, what can provide important information for the design of control strategies, such as vaccines.
African swine fever (ASF) is the most important infectious disease of swine and has proven to be devastating for the pork industry worldwide. ASF was first observed in the early 1900's in East Africa, when European domestic pig breeds were introduced in the Kenya Colony and animals developed a form of hemorrhagic disease with high morbidity and mortality (1). ASF was confined to African countries until 1957 when it reached Portugal via contaminated waste containing infected pork products that were used to feed local pigs. This outbreak was quickly controlled, but ASF re-entered Portugal in 1960 and spread rapidly to the Iberian peninsula (2) and produced sporadic outbreaks in several European countries, including Belgium, the Netherlands, Italy, Malta, and France (3–6). ASF spread to the Americas, with sporadic outbreaks in Brazil, the Dominican Republic, Haiti, and Cuba (7–11). ASF was eradicated from all these countries out of Africa, except the Italian island of Sardinia, where the disease has persisted since 1978 (2, 12–14). The disease continued to persist and spread within Africa (15) and entered the Republic of Georgia in 2007 through the port of Poti (16), most likely via contaminated food used to feed domestic pigs (17). ASF spread rapidly within the Caucasian region and neighboring countries and continues to spread to West, including European Union countries (18, 19) and to the East, with the disease causing abundant outbreaks and affecting dramatically the pork industry in China, Vietnam, Cambodia, Philippines, Laos, and East Timor (20–23).
ASF is caused by a large, complex, enveloped DNA virus (ASFV), from the family Asfarviridae (24). ASFV is composed of more than 50 structural proteins and can produce more than 150 proteins in the infected cells (17, 25–27), many of which are highly immunogenic. The main target cell for ASFV is the monocyte/macrophage in both domestic and wild swine (28–30), but infection in lymphocytes has not been reported (30). ASFV may also replicate in other cell types, including hepatocytes, renal tubular epithelial cells, neutrophils, and endothelial cells (31–33). The ASFV replication and the immune responses from the host induce different clinical courses and pathology in swine species. ASFV can also replicate in soft ticks from the genus Ornithodoros, including O. moubata in Africa and O. erraticus in the Iberian peninsula (34–37), which are involved in the epidemiological cycles of ASF (38, 39). Other soft tick species have also been reported to be susceptible to ASFV infection and may play a role in the epidemiology of ASF in other countries.
ASF has produced a high economic cost to the pork industry and it is the most important porcine disease nowadays, mostly due to the difficult prevention and control as no vaccine is available and other strategies must be used to control the disease from different territories. In this review article, we describe the different clinical and pathological features of ASF in domestic and wild suids together with the key pathogenic mechanisms that induce the disease in the host species.
The clinical presentation and the gross pathological lesions of ASF in domestic pigs may vary depending on the virulence of the virus isolate, the route, and dose of infection and host characteristics (17). ASFV isolates can be classified as highly virulent, moderately virulent, and low virulent (40). The clinical courses observed in ASF in domestic pigs can be described as peracute (or hyperacute), acute, subacute, or chronic.
Highly virulent strains are typically responsible for this clinical course, characterized by a very rapid clinical course, with high fever (up to 42°C), anorexia, lethargy, and sometimes sudden death without signs of disease. This is often observed when the virus enters a naïve farm causing death of some animals before the explosion of clinical cases. Some animals can show respiratory distress due to the high fever, but no gross lesions are usually found at the post mortem examination.
This clinical form is cause by highly or moderately virulent isolates, and it is the typical course observed in naïve farms very quickly after the first fatal cases are reported. The clinical course is characterized by high fever, with temperatures of 40–42°C, lethargy, anorexia, and inactivity (Figure 1A). The affected animals tend to bunch up together. Many affected animals show a centripetal cyanosis, easily found in the ears (Figure 1B), snout (Figure 1C), limbs (Figure 1D), abdomen, tail, and perianal area. Respiratory distress is usually observed, with severe pulmonary oedema in animals affected by highly pathogenic isolates (41, 42). Skin lesions are frequent, with presence of petechial hemorrhages or ecchymosis (Figure 1E). Other clinical signs may include nasal discharges, sometimes stained with blood (epistaxis), vomiting, and diarrhea, that can be also blood-stained (melaena) (17, 43–45), causing black-colored stains in the perianal area of the animal (Figure 1F). Abortions may occur in pregnant sows and the mortality rates may reach up to 100% in affected farms within 7 days of the onset of the disease.
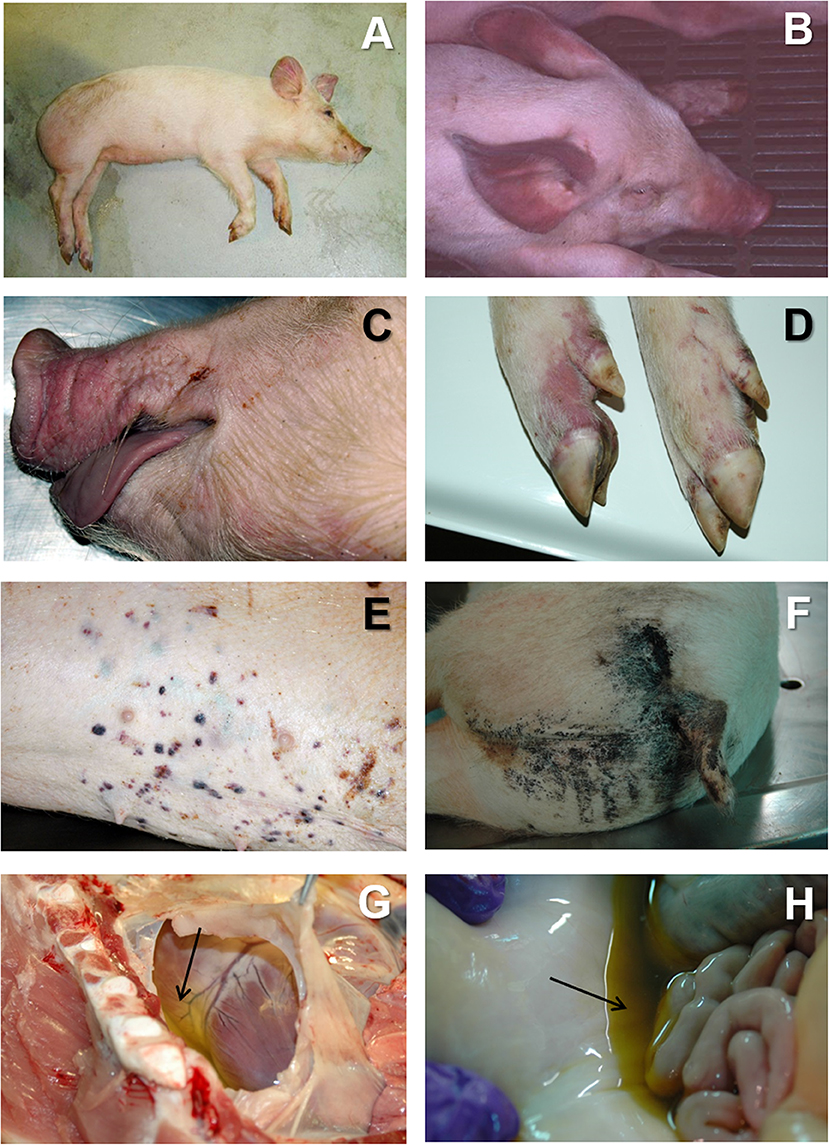
Figure 1. (A) Lethargic animal in acute ASF. The animal show cyanosis ion the ears abdomen and limbs. (B) Severe cyanosis in an animal suffering from acute ASF, associated to very high hyperthermia (41–42°C). (C) Cyanosis in the snout and lips in acute ASF. (D) Cyanosis in the limbs in acute ASF. (E) Multifocal petechiae and ecchymosis in the skin in acute ASF. (F) Blood-stained perianal area in a pig affected by subacute ASF. (G) Severe hydropericardium (arrow) in subacute ASF. (H) Moderate to severe ascites (arrow) in subacute ASF.
At the post mortem examination, the most characteristic lesion of acute ASF is the hemorrhagic splenomegaly (28, 46, 47), with a very enlarged spleen, dark in color and friable at sectioning, occupying a large space within the abdominal cavity (Figures 2A,B). The second most important lesion described in acute ASF is a multifocal hemorrhagic lymphadenitis. Lymph nodes can have multifocal or extensive hemorrhages that can produce a marbled appearance (Figure 2D). The most affected lymph nodes are the gastrohepatic (Figure 2E), renal (Figure 2F), and other abdominal lymph nodes as ileocaecal (Figure 2G), and mesenteric (Figure 2H). Hemorrhages may also be observed with less frequency in other lymph nodes, such as submandibular, retropharyngeal, or inguinal. Petechial hemorrhages are often observed in the kidney surface (Figure 3A) and at sectioning. Other lesions can also be observed, mostly hemorrhages in the mucosa or the serosa of other organs, as the large (Figure 3E) and small intestine (Figure 3F), the epicardium in the heart (Figure 3G), or the urinary bladder (Figure 3H) (17, 43, 44, 48–51).
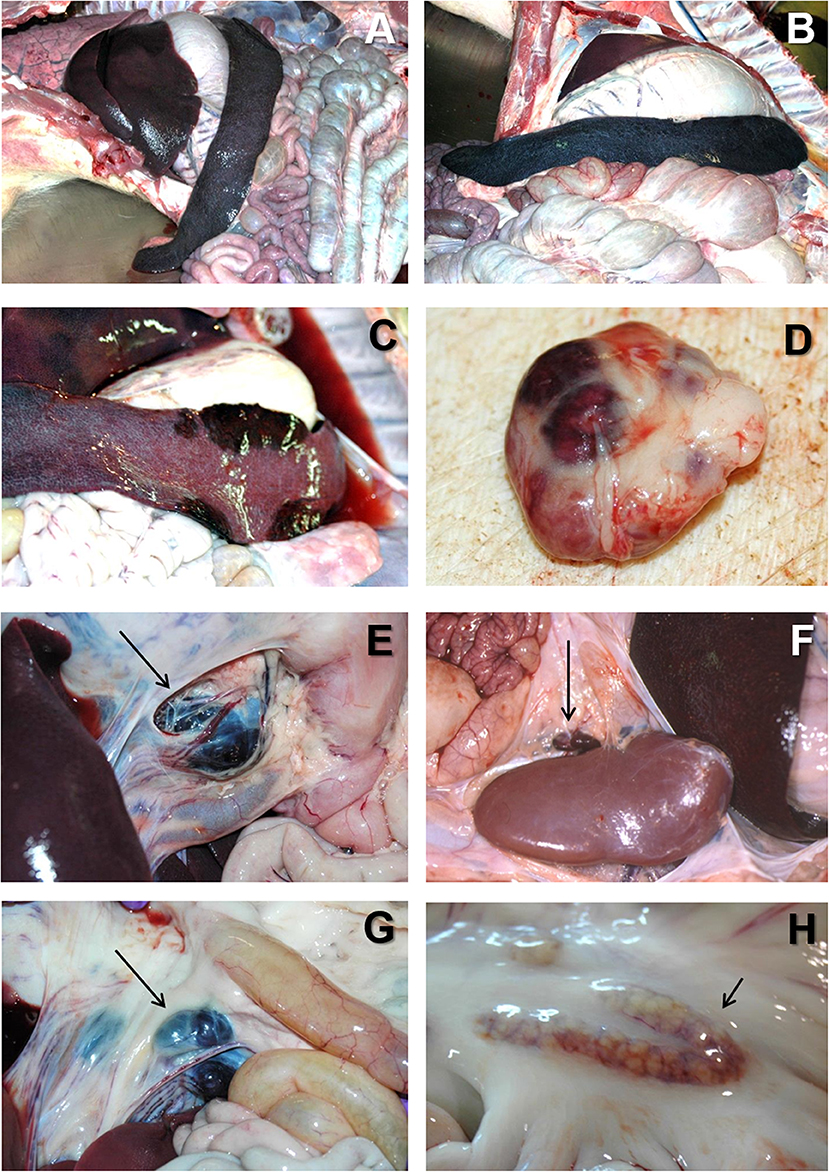
Figure 2. (A) Severe hemorrhagic splenomegaly observed at the opening of the abdominal cavity of an animal with acute ASF. The liver is severely congested. (B) Very large, dark colored spleen with rounded edges (hemorrhagic splenomegaly), and occupying a large volume of the abdominal cavity in acute ASF. (C) Multiple areas of partial hemorrhagic splenomegaly in the spleen from an animal with subacute ASF. (D) Multifocal hemorrhages in a lymph node with a marbled appearance in acute ASF. (E) Severe hemorrhagic lymphadenopathy in the gastrohepatic lymph node (arrow) in acute ASF. (F) Severe hemorrhagic lymphadenopathy in the renal lymph node (arrow) in acute ASF. (G) Severe hemorrhagic lymphadenopathy in the ileocaecal lymph node (arrow) in acute ASF. (H) Moderate hemorrhagic lymphadenopathy in the mesenteric lymph node (arrow) in acute ASF.
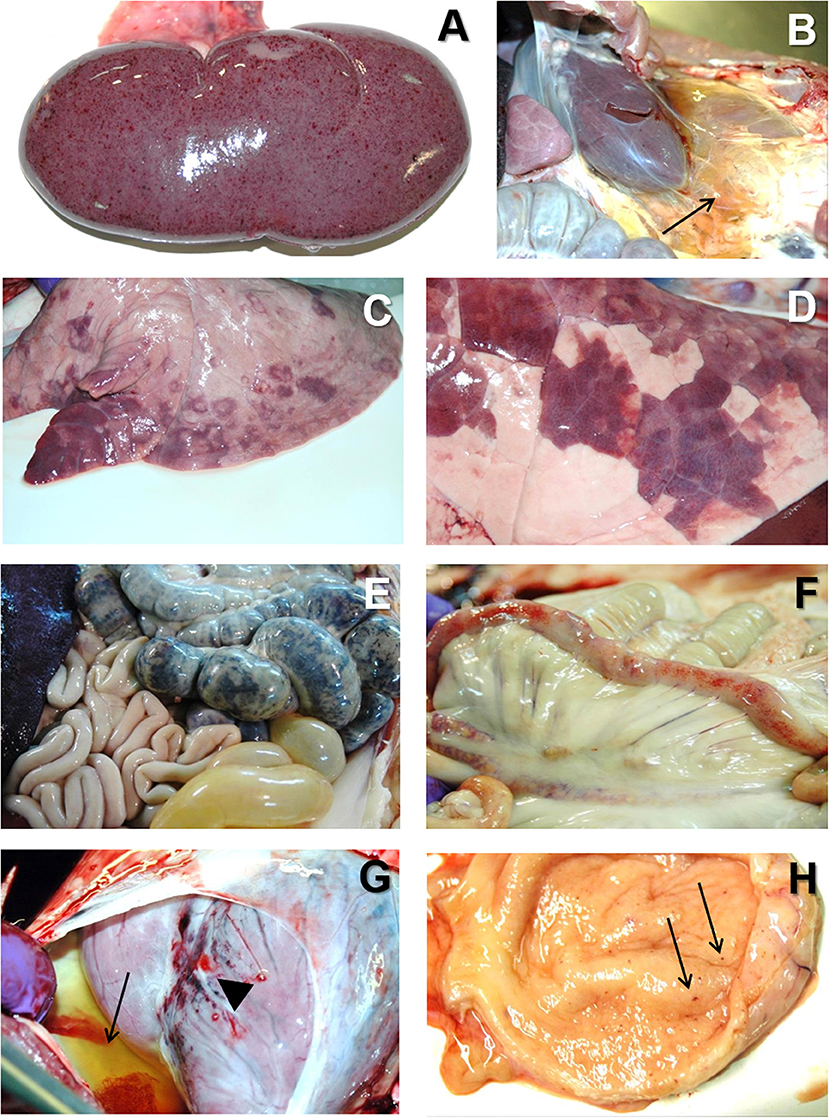
Figure 3. (A) Multiple petechial hemorrhages in the cortical surface of the kidney in acute ASF. (B) Severe perirenal oedema (arrow) in a pig with subacute ASF. (C) Multifocal areas of lung consolidation and pulmonary oedema in subacute ASF. (D) Multifocal pneumonia with dark color areas in the diaphragmatic lobe of the lung in subacute ASF. (E) Severe extensive hemorrhagic colitis in subacute ASF. (F) Multiple petechial hemorrhages in the serosa of the small intestine in acute ASF. (G) Multiple petechial ad ecchymotic hemorrhages in the epicardium (arrowhead) together with severe hydropericardium (arrow) in subacute ASF. (H) Multiple petechial hemorrhages in the mucosa of the urinary bladder in acute ASF.
This clinical form is usually observed in animals infected by moderately virulent isolates, with similar clinical signs as those observed in acute ASF, although normally less marked (17). Affected pigs show moderate to high fever and the mortality rate ranges from 30 to 70% (17), with pigs dying at 7–20 after infection.
The vascular changes, mostly hemorrhages and oedema, in the subacute form of the disease can be more intense than the acute form (45, 52).
The death of affected animals may happen at two different stages: (a) during an initial thrombocytopenia and leukopenia (53–55), or (b) during a “recovery” phase, observed in young animals, causing erythrodiapedesis induced by vasodilation (53, 56).
At the post mortem examination, animals show hydropericardium (Figure 1G), ascites (Figure 1H), and multifocal oedema, very characteristic in the wall of the gall bladder or in the perirenal fat (Figure 3B) (17). Some animals may show hemorrhagic splenomegaly as described for the acute form of the disease, but many animals will show partial splenomegaly, with patches of spleen affected and other areas unaffected (Figure 2C). A multifocal hemorrhagic lymphadenitis can also be observed with multiple lymph nodes in all areas of the body showing the hemorrhages and the “marble” appearance (45). Petechial hemorrhages can also be observed in the kidney (50, 51). Multifocal pneumonia is also observed with patches of consolidation and dark color in the lung (Figures 3C,D). This lesion can also be attributed to secondary infections due to the state of immunosuppression induce by ASFV (45, 57, 58).
This clinical form is caused by the infection of low virulence isolates and has been observed, quite infrequently, in the Iberian Peninsula and the Dominican Republic (17, 54). It has been hypothesized that this low virulence isolates, and the associated chronic form, has evolved from ASFV isolates employed in early vaccine trials carried out in the Iberian Peninsula in the 1960's (17). The evolution of highly and moderately virulent isolates in other areas where the virus has been present for long periods of time has not produced this chronic form of the disease (17, 59).
This clinical form is characterized by multifocal necrosis in the skin and arthritis, growth retardation emaciation, respiratory distress and abortion (60, 61). No vascular changes are observed in the chronic form of ASF, and many observed lesions are associated with bacterial secondary infections, inducing fibrinous polyserositis, necrotic, or chronic pneumonia, necrosis of the skin, tongue, and tonsils (17, 43, 60).
ASF is characterized by severe leukopenia, mostly associated with lymphopenia, and a general state of immunodeficiency (58, 62). Initially, the virus enters the pigs following an oral-nasal route of after the bite of an infected soft tick. The virus replicates initially in the tonsils or regional lymph nodes (63, 64), spreading through the lymph and blood to secondary organs of replication within 2–3 days (65), and then spreading to the rest of the organs, where virus can replicate in a variety of cells (56, 66).
Monocytes and macrophages are the main target cell for ASFV (28, 42, 45). ASFV is a DNA virus, but the replication occurs within the cytoplasm and not in the nucleus (67–69). The infected monocyte-macrophage appears swollen, with margination of the nuclear chromatin (Figures 4A,B) and showing an intracytoplasmic juxtanuclear inclusion body, identifiable by its pale color when semithin (1-micron) sections are stained with toluidine blue dye (Figure 4A). These inclusion bodies show viral factories when studied under transmission electron microscopy (Figure 4B). The virus replication induce necrosis in the infected cells and virions are released by budding, and can be observed free in the blood, lymph, and the interstitial tissue (31, 70–72).
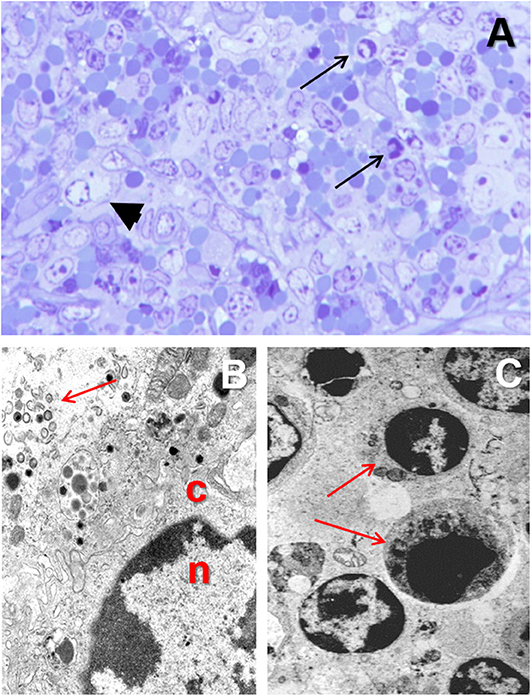
Figure 4. (A) Toluidine blue stained semithin (1 μm) section showing a macrophage with margination of the nuclear chromatin and a juxtanuclear clear intracytoplasmic inclusion body (arrowhead) in the spleen from a pig experimentally infected with acute ASF (3 dpi). (B) Transmission electron microscopy image of the nucleus (n) and cytoplasm (c) of a macrophage in the spleen from a pig infected with ASFV showing margination of the nuclear chromatin and a viral factory within the cytoplasm (arrow). (C) Apoptosis of lymphocytes (arrows) in the spleen of from a pig experimentally infected with acute ASF (5 dpi).
The destruction of monocytes-macrophages in ASF has been attributed to apoptosis (73) or necrosis (74) due to the action of ASFV (75). ASFV genome contain genes involved un programmed cell death both in an inhibitory or an inducing manner (64, 76–85). Some of these genes may promote the survival of the infected cells, and apoptosis has been described as the less likely cause of cell death in the infected monocyte-macrophage population (52, 58, 86).
ASF is characterized by a massive destruction of the lymphoid organs and tissues, including spleen, lymph nodes, thymus, and tonsils (58, 86, 87). There is a large proportion of B and T lymphocytes and macrophages undergoing cell death in acute ASFV infection (58, 78, 86, 88).
The virus replication in the monocyte-macrophages (Figures 5F–H) induces an activation in this cell population and an increase in the secretion of proinflammatory cytokines have been observed at the early stages of the disease (28, 42, 58). The upregulation in the expression of proinflammatory cytokines, including IL-1, TNF-α, and IL-6, and described as a “cytokine storm” (89), is the responsible mechanism for the massive induction of apoptosis in lymphocytes (Figure 4C) neighboring the activated/infected monocyte-macrophages in tissues (58).
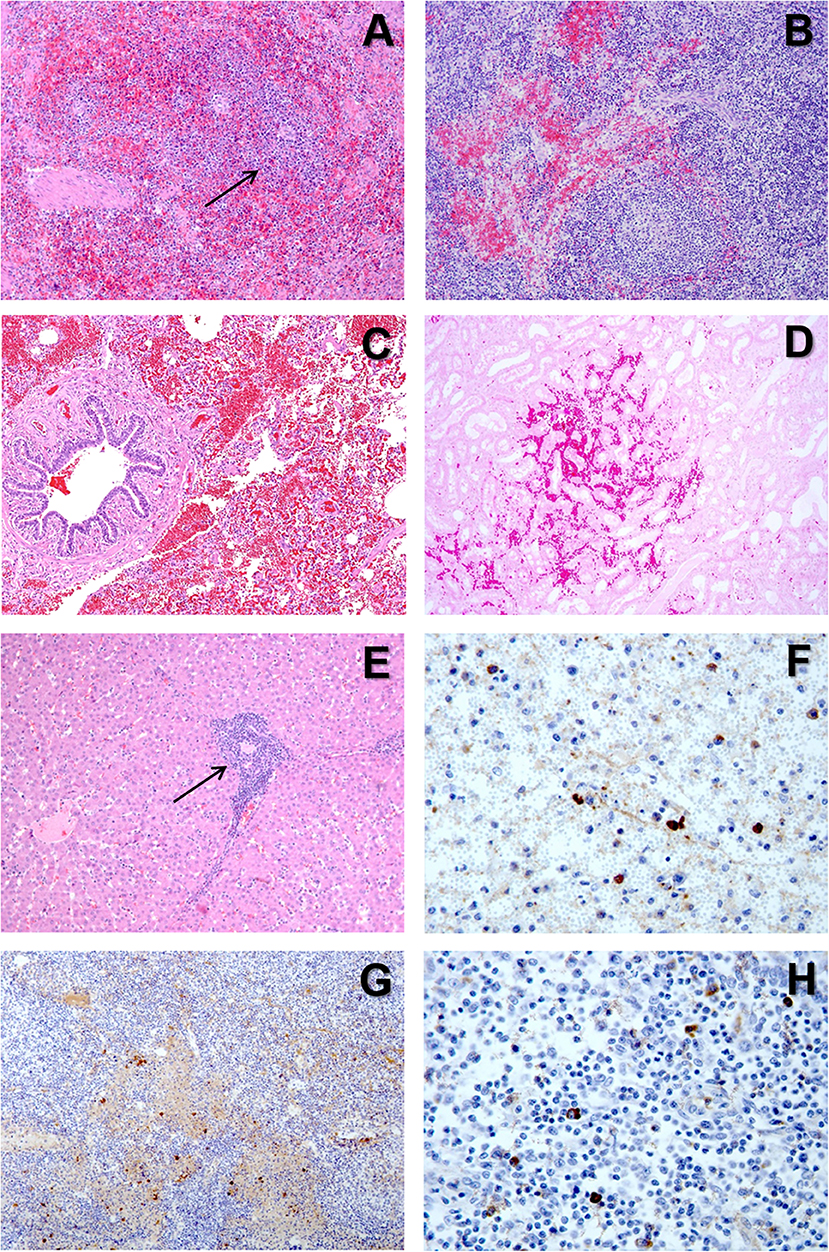
Figure 5. (A) H&E stain of the spleen from a pig with acute ASF showing abundant red blood cells within the red pulp and severe lymphoid depletion, with very small lymphoid follicles (arrow) in the white pulp. (B) H&E stain of the gastrohepatic lymph node from a pig with subacute ASF showing hemorrhages in the perifollicular lymphoid tissue and the medulla, together with a moderate lymphoid depletion. (C) H&E stain of the lung from a pig with subacute ASF showing severe hemorrhages in the septa and the alveolar spaces. (D) H&E stain of the kidney from a pig with acute ASF showing interstitial hemorrhages within the renal cortex. (E) H&E stain of the liver from a pig with acute ASF showing periportal inflammatory infiltrates (arrow) composed of lymphocytes, macrophages and plasma cells. (F) IHC detection of ASFV p72 in the spleen showing strong positive reaction in macrophages in the red pulp and cell debris within the necrotic areas. (G) IHC detection of ASFV p72 in the gastrohepatic lymph node showing strong positive reaction in macrophages within the perifollicular areas and the medulla. (H) IHC detection of ASFV p72 in the tonsil showing strong positive reaction in macrophages within the perifollicular areas.
ASF can be considered a hemorrhagic fever, with some pathogenic mechanisms similar to those described for hemorrhagic fevers affecting humans, as Ebola or Marburg filovirus infection (90, 91). Among the typical vascular changes observed in acute ASF, we can include petechial and ecchymotic hemorrhages in multiple organs, hemorrhagic, or hyperaemic splenomegaly, pulmonary oedema, and disseminated intravascular coagulopathy (D.I.C.). In subacute ASF, we can also observe these vascular changes together with a more marked oedema, ascites, and hydropericardium.
The most typical lesion in ASF is the hemorrhagic or hyperaemic splenomegaly (44, 46). The severity of this lesion will vary depending on the virulence of the isolate. The histopathological appearance of the spleen will include a hyperaemic red pulp, that can be completely filled with red blood cells (Figure 5A), platelet thrombi and cell debris, producing a disruption of the normal architecture of the organ (47, 58). The porcine splenic red pulp contains a mesh of fibers and smooth muscle cells surrounded by a population of macrophages fixed in the splenic cords (92). The necrosis of the macrophages in the red pulp is followed by a loss of intercellular junctions with the smooth muscle cells and the exposure of the basal lamina, inducing the activation of the coagulation cascade, platelet aggregation, and fibrin deposition, giving rise to the accumulation of red blood cells within the splenic cords (56, 93).
Hemorrhages are very common in the late phases of the disease, mostly in organs without a fixed vascular macrophage population, as the renal and gastrohepatic lymph nodes or the kidney (Figures 5B,D) (56). Even though ASFV can replicate in endothelial cells, this phenomenon has not been observed in all the organs showing hemorrhages (Figure 5C), and more importantly, this virus replication has only been reported in endothelial cells in the last phases of the disease, while hemorrhages may occur at earlier stages (33, 48). A different pathogenic mechanism has been observed and proposed as one of the main factors contributing to the hemorrhages in the early phases of the disease: the phagocytic activation of capillary endothelial cells, followed by endothelial cell hypertrophy that may lead to the total occlusion of the capillary lumen and a severe increase in the intravascular pressure (56). The subsequent loss of endothelial cells results in the exposure of the capillary basal membrane to which platelets can adhere, prompt the activation of the coagulation system and induce the D.I.C. (54–56).
An intense transient thrombocytopenia is frequently observed during subacute ASF, when hemorrhages are very frequent and severe (54, 55). This phenomenon may play an important role in the development of hemorrhages in the middle stages of the disease and is associated to structural changes of megakaryocytes in the bone marrow, with the presence of frequent denuded megakaryocytes (94), a feature also observed in relationship to hemorrhages in Classical swine fever (95).
The pathogenesis of the pulmonary oedema starts with the severe infection of pulmonary intravascular macrophages (PIMs), that is the main target cell for ASFV in the lung (31). Infected and non-infected PIMs tend to be enlarged and show signs of secretory activation. The production of proinflammatory cytokines such as IL-1α and TNF-α induce chemotactic activity and increase the endothelial permeability, leading to the leakage of fluid into the interalveolar septa and the alveolar spaces (42).
The marked anorexia in infected animals reduces dramatically the food/protein intake and accelerate the presence of hypo-oncotic oedema leading to internal fat consumption, ascites, hydrothorax, and hydropericardium, very typical in subacute ASF. Moreover, the liver of infected animals show a marked congestion, but also histopathological lesions, including multifocal periportal inflammatory infiltrates (Figure 5E), infection of Kupffer cells, which show severe secretory activation, and hepatocytes in the late stages of the disease (32, 49, 70, 96, 97). Hepatic malfunction may also contribute to the development of the multifocal oedema.
The Eurasian wild boar (Sus scrofa) is a native suid species of most of Europe and Asia and Northern Africa, but has also been introduced in other continents, including many islands. It is considered the natural ancestor of the domestic pig and both are classified as the same species. At present, the wild boar play a very significant role in the spread of ASF infection in Europe, and probably also in Asia, being also considered the main source of infection in the recent outbreaks in Central and Eastern Europe (98–102).
Due to the close taxonomic relationship between Eurasian wild boar and domestic pigs, many similarities in terms of immune responses to infections can be observed. However, even though they are the same species (Sus scrofa), they belong to different subspecies (101). Moreover, domestic pigs, and in some instances also wild boar, are managed with a close control on the health, reproduction and nutrition, whereas free-ranging wild boar are subjected to many natural variations on reproductive, sanitary, and nutritional conditions (101).
Before the outbreak of ASF in Georgia in 2007 and its further expansion, several studies were conducted to study the pathology and pathogenesis of ASFV infection wild boar, both in natural and experimental conditions [reviewed by Sanchez-Cordon et al. (101)]. No significant differences were found in the clinical presentation of ASF in wild boar compared with the domestic pig, with very similar acute, and subacute clinical courses, and associated lesions (17, 24, 103, 104). After 2007, a major emphasis has been put on the study of ASF in wild boar after the reports of infected individuals in relationship to the spread of the virus (105–109).
Several studies have been carried out in wild boars with low and high virulent isolates, in different settings and conditions. Highly pathogenic isolates from genotype II (110) induce hemorrhagic/hyperaemic splenomegaly, hemorrhagic lymphadenitis, pulmonary oedema, and multifocal petechial hemorrhages (64, 107, 111), sometimes described as even more severe than in the domestic pig (101). The mortality in is also very high (90–100%) in these infected animals. However, there are attenuated variants of the genotype II circulating in some parts of Europe (112–114). Infected wild boar with low virulent isolates and surviving the infection may transmit the virus to naïve contact animals for months, although current non-haemadsorbing genotype II isolates do not induce long-term carriers as a major outcome for recovery pigs isolates (111).
In East Africa, ASFV is maintained in an ancient sylvatic cycle involving the common warthog (Phacochoerus africanus) and the arthropod vector (soft tick), Ornithodoros moubata, that inhabit their burrows (24, 85).
Since very early experimental studies, it was demonstrated that warthogs were very resistant to ASFV infection (1, 115), showing no clinical signs of the disease, except in young animals, which develop a transient viremia (116, 117). Viremia in adult warthogs is very rare with infectious virus mostly restricted to lymph nodes (85). The infectious ASFV may persist in warthog tissues for up to 25 weeks post infection, but is cleared by 56 weeks (118), what could explain the repeated re-infection of warthogs by ticks with the same virus strain (85).
Several genetics differences have been described between warthogs and domestic pigs (85). A difference between tolerance to infection and severe pathology may be due to a polymorphic RELA (p65; v-rel reticuloendotheliosis viral oncogene homolog A) variant found in warthogs (119).
ASFV has also been isolated from bushpigs (Potamochoerus larvatus) and red river hogs (Potamochoerus porcus), wild suid species found in sub-Saharan West and Central Africa (85, 116, 120, 121). ASFV infection does not induce clinical signs in these species, but moderate viremia can be observed (118, 120). ASFV can replicate in tissues without causing histological lesions, and mostly restricted to the B cell areas of the lymph nodes (85). Infected animals may transmit ASFV to feeding ticks but also to in-contact domestic pigs, although the role in the epidemiological maintenance of ASFV as a reservoir in unclear since these species do not inhabit burrows like warthogs and they are not in close contact with the Ornithodoros spp. ticks (85).
ASF is spreading very rapidly worldwide, and current control strategies rely on rapid detection, strict biosecurity, and implementation of quarantine and slaughter policies, in the absence of a commercial secure, and efficacious vaccine. These measures are not always implemented correctly or are insufficient, leading to culling large numbers of animals. The rapid detection is very important when ASF enters a new territory, and education, and communication are crucial tools to detect the first cases of the disease and follow up the official measures implemented to control the outbreaks. The clinical course and associated lesions of the disease may vary, and farmers and veterinarians must be always aware of the different presentations of ASF.
The pathogenesis of this disease is very complex, and more research is required to understand some of the pathogenic mechanisms, including how ASFV modulates the host immune responses and the role of the multiple proteins encoded by the virus. Several research groups are developing prototype vaccines mostly based on subunits or live attenuated isolates. More information is also needed to understand the correlates of protection to help with the development of these vaccines.
Finally, the presence of wild suids in the epidemiological cycles in Africa and Eurasia, makes the control of the disease very complicated, with the added problem of soft tick species as potential arthropod reservoirs in different countries. Moreover, the population of wild boar is increasing dramatically in Europe, but also in some parts of Africa and America, adding more problems to the control of ASF when outbreaks are reported. The rapid expansion of ASF in South Asia also raises the concern about the possibility of transmission into local wild suid species and the establishment of potential new epidemiological cycles in this and other areas of the world.
FS is the sole author of this manuscript, and conceived the idea of this review article after discussing ASF pathology with many colleagues in Asia during 2019, trying to produce a review focused on the pathology of ASF that could be useful to support veterinarians working in government and academic institutions, with abundant images and briefly discussing the main features of the disease in wild suids.
FS has been supported by internal funding at Public Health England (PHE).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
FS would like to acknowledge many colleagues from the ASF scientific community, including the University of Cordoba and Centro de Investigacion en Sanidad Animal (CISA-INIA) in Spain, the Vietnam National University of Agriculture (Hanoi, Vietnam), and The Pirbright Institute (United Kingdom). The images shown in this article come from past animal experiments carried out at CISA-INIA, in Valdeolmos, Madrid, from a variety of research projects.
1. Montgomery RE. On a form of swine fever occurring in British East Africa (Kenya Colony). J Comp Pathol Ther. (1921) 34:159–91. doi: 10.1016/S0368-1742(21)80031-4
2. Sanchez-Vizcaino JM, Mur L, Martinez-Lopez B. African swine fever: an epidemiological update. Transbound Emerg Dis. (2012) 1(Suppl.59):27–35. doi: 10.1111/j.1865-1682.2011.01293.x
3. Biront P, Castryck F, Leunen J. An epizootic of African swine fever in Belgium and its eradication. Vet Rec. (1987) 120:432–4. doi: 10.1136/vr.120.18.432
4. Terpstra C, Wensvoort G. African swine fever in the Netherlands. Tijdschr Diergeneeskd. (1986) 111:389–92.
5. Swaney LM, Lyburt F, Mebus CA, Buonavoglia C, Orfei A. Genome analysis of African swine fever virus isolated in Italy in 1983. Vet Microbiol. (1987) 14:101–4. doi: 10.1016/0378-1135(87)90001-0
6. Wilkinson PJ, Lawman MJ, Johnston RS. African swine fever in Malta, 1978. Vet Rec. (1980) 106:94–7. doi: 10.1136/vr.106.5.94
7. Preliminary report on the African swine fever epizootic in Cuba. Methods of diagnosis and control. Bull Off Int Epizoot. (1971) 75:367–437.
8. Alexander FC. Experiences with African swine fever in Haiti. Ann N Y Acad Sci. (1992) 653:251–6. doi: 10.1111/j.1749-6632.1992.tb19654.x
9. Reichard RE. African swine fever in the Americas. In: Proceedings, Annual Meeting of the United States Animal Health Association. (1978). p. 226–31.
10. Mebus CA, Dardiri AH, Hamdy FM, Ferris DH, Hess WR, Callis JJ. Some characteristics of African swine fever viruses isolated from Brazil and the Dominican Republic. Proc Annu Meet U S Anim Health Assoc. (1978) 1978:232–6.
11. Lyra TM. The eradication of African swine fever in Brazil, 1978-1984. Rev Sci Tech. (2006) 25:93–103. doi: 10.20506/rst.25.1.1652
12. Costard S, Jones BA, Martinez-Lopez B, Mur L, de la Torre A, Martinez M, et al. Introduction of African swine fever into the European Union through illegal importation of pork and pork products. PLoS ONE. (2013) 8:e61104. doi: 10.1371/journal.pone.0061104
13. Costard S, Mur L, Lubroth J, Sanchez-Vizcaino JM, Pfeiffer DU. Epidemiology of African swine fever virus. Virus Res. (2013) 173:191–7. doi: 10.1016/j.virusres.2012.10.030
14. Laddomada A, Rolesu S, Loi F, Cappai S, Oggiano A, Madrau MP, et al. Surveillance and control of African Swine fever in free-ranging pigs in Sardinia. Transbound Emerg Dis. (2019) 66:1114–9. doi: 10.1111/tbed.13138
15. Penrith ML, Vosloo W. Review of African swine fever: transmission, spread and control. J S Afr Vet Assoc. (2009) 80:58–62. doi: 10.4102/jsava.v80i2.172
16. Rowlands RJ, Michaud V, Heath L, Hutchings G, Oura C, Vosloo W, et al. African swine fever virus isolate, Georgia, 2007. Emerg Infect Dis. (2008) 14:1870–4. doi: 10.3201/eid1412.080591
17. Sanchez-Vizcaino JM, Mur L, Gomez-Villamandos JC, Carrasco L. An update on the epidemiology and pathology of African swine fever. J Comp Pathol. (2015) 152:9–21. doi: 10.1016/j.jcpa.2014.09.003
18. Nurmoja I, Motus K, Kristian M, Niine T, Schulz K, Depner K, et al. Epidemiological analysis of the 2015-2017 African swine fever outbreaks in Estonia. Prev Vet Med. (2018). doi: 10.1016/j.prevetmed.2018.10.001. [Epub ahead of print].
19. Roelandt S, Van der Stede Y, D'Hondt B, Koenen F. The assessment of african swine fever virus risk to belgium early, 2014 using the quick and semiquantitative pandora screening protocol. Transbound Emerg Dis. (2017) 64:237–49. doi: 10.1111/tbed.12365
20. Le VP, Jeong DG, Yoon SW, Kwon HM T, Trinh BN, Nguyen TL, et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg Infect Dis. (2019) 25:1433–35. doi: 10.3201/eid2507.190303
22. Zhou X, Li N, Luo Y, Liu Y, Miao F, Chen T, et al. Emergence of African swine fever in China, 2018. Transbound Emerg Dis. (2018) 65:1482–4. doi: 10.1111/tbed.12989
23. Smith D, Cooper T, Pereira A, Jong J. Counting the cost: the potential impact of African swine fever on smallholders in Timor-Leste. One Health. (2019) 8:100109. doi: 10.1016/j.onehlt.2019.100109
24. Dixon LK, Sun H, Roberts H. African swine fever. Antiviral Res. (2019) 165:34–41. doi: 10.1016/j.antiviral.2019.02.018
25. Dixon LK, Chapman DA, Netherton CL, Upton C. African swine fever virus replication and genomics. Virus Res. (2013) 173:3–14. doi: 10.1016/j.virusres.2012.10.020
26. Salas ML, Andres G. African swine fever virus morphogenesis. Virus Res. (2013) 173:29–41. doi: 10.1016/j.virusres.2012.09.016
27. Alejo A, Matamoros T, Guerra M, Andres G. A proteomic atlas of the african swine fever virus particle. J Virol. (2018). doi: 10.1128/JVI.01293-18. [Epub ahead of print].
28. Salguero FJ, Ruiz-Villamor E, Bautista MJ, Sanchez-Cordon PJ, Carrasco L, Gomez-Villamandos JC. Changes in macrophages in spleen and lymph nodes during acute African swine fever: expression of cytokines. Vet Immunol Immunopathol. (2002) 90:11–22. doi: 10.1016/S0165-2427(02)00225-8
29. Malmquist WA, Hay D. Hemadsorption and cytopathic effect produced by African Swine Fever virus in swine bone marrow and buffy coat cultures. Am J Vet Res. (1960) 21:104–8.
30. Minguez I, Rueda A, Dominguez J, Sanchez-Vizcaino JM. Double labeling immunohistological study of African swine fever virus-infected spleen and lymph nodes. Vet Pathol. (1988) 25:193–8. doi: 10.1177/030098588802500302
31. Carrasco L, de Lara FC, Gomez-Villamandos JC, Bautista MJ, Villeda CJ, Wilkinson PJ, et al. The pathogenic role of pulmonary intravascular macrophages in acute African swine fever. Res Vet Sci. (1996) 61:193–8. doi: 10.1016/S0034-5288(96)90062-4
32. Gomez-Villamandos JC, Hervas J, Mendez A, Carrasco L, Villeda CJ, Sierra MA, et al. A pathological study of the perisinusoidal unit of the liver in acute African swine fever. Res Vet Sci. (1995) 59:146–51. doi: 10.1016/0034-5288(95)90049-7
33. Gomez-Villamandos JC, Hervas J, Mendez A, Carrasco L, Villeda CJ, Wilkinson PJ, et al. Ultrastructural study of the renal tubular system in acute experimental African swine fever: virus replication in glomerular mesangial cells and in the collecting ducts. Arch Virol. (1995) 140:581–9. doi: 10.1007/BF01718433
34. Plowright W, Parker J, Peirce MA. African swine fever virus in ticks (Ornithodoros moubata, murray) collected from animal burrows in Tanzania. Nature. (1969) 221:1071–3. doi: 10.1038/2211071a0
35. Plowright W, Perry CT, Peirce MA, Parker J. Experimental infection of the argasid tick, Ornithodoros moubata porcinus, with African swine fever virus. Arch Gesamte Virusforsch. (1970) 31:33–50. doi: 10.1007/BF01241664
36. Basto AP, Nix RJ, Boinas F, Mendes S, Silva MJ, Cartaxeiro C, et al. Kinetics of African swine fever virus infection in Ornithodoros erraticus ticks. J Gen Virol. (2006) 87:1863–71. doi: 10.1099/vir.0.81765-0
37. Penrith ML, Vosloo W, Jori F, Bastos AD. African swine fever virus eradication in Africa. Virus Res. (2013) 173:228–46. doi: 10.1016/j.virusres.2012.10.011
38. Pietschmann J, Mur L, Blome S, Beer M, Perez-Sanchez R, Oleaga A, et al. African swine fever virus transmission cycles in Central Europe: Evaluation of wild boar-soft tick contacts through detection of antibodies against Ornithodoros erraticus saliva antigen. BMC Vet Res. (2016) 12:1. doi: 10.1186/s12917-015-0629-9
39. Haresnape JM, Mamu FD. The distribution of ticks of the Ornithodoros moubata complex (Ixodoidea: Argasidae) in Malawi, and its relation to African swine fever epizootiology. J Hyg. (1986) 96:535–44. doi: 10.1017/S0022172400066341
40. Pan IC, Hess WR. Virulence in African swine fever: its measurement and implications. Am J Vet Res. (1984) 45:361–6.
41. Sierra MA, Carrasco L, Gomez-Villamandos JC, Martin de las Mulas J, Mendez A, Jover A. Pulmonary intravascular macrophages in lungs of pigs inoculated with African swine fever virus of differing virulence. J Comp Pathol. (1990) 102:323–34. doi: 10.1016/S0021-9975(08)80021-7
42. Carrasco L, Nunez A, Salguero FJ, Diaz San Segundo F, Sanchez-Cordon P, Gomez-Villamandos JC, Sierra MA. African swine fever: Expression of interleukin-1 alpha and tumour necrosis factor-alpha by pulmonary intravascular macrophages. J Comp Pathol. (2002) 126:194–201. doi: 10.1053/jcpa.2001.0543
43. Moulton J, Coggins L. Comparison of lesions in acute and chronic African swine fever. Cornell Vet. (1968) 58:364–88.
44. Mebus CA, Dardiri AH. Additional characteristics of disease caused by the African swine fever viruses isolated from Brazil and the Dominican Republic. Proc Annu Meet U S Anim Health Assoc. (1979) 1979:227–39.
45. Gomez-Villamandos JC, Carrasco L, Bautista MJ, Sierra MA, Quezada M, Hervas J, et al. African swine fever and classical swine fever: a review of the pathogenesis. Dtsch Tierarztl Wochenschr. (2003) 110:165–9.
46. Konno S, Taylor WD, Hess WR, Heuschele WP. Spleen pathology in African swine fever. Cornell Vet. (1972) 62:486–506.
47. Carrasco L, Bautista MJ, Gomez-Villamandos JC, Martin de las Mulas J, Chacon LF, Wilkinson PJ, et al. Development of microscopic lesions in splenic cords of pigs infected with African swine fever virus. Vet Res. (1997) 28:93–9.
48. Carrasco L, Chacon LF, Martin de Las Mulas J, Gomez-Villamandos JC, Sierra MA, Villeda CJ, et al. Ultrastructural changes related to the lymph node haemorrhages in acute African swine fever. Res Vet Sci. (1997) 62:199–204. doi: 10.1016/S0034-5288(97)90190-9
49. Salguero FJ, Gil S, Revilla Y, Gallardo C, Arias M, Martins C. Cytokine mRNA expression and pathological findings in pigs inoculated with African swine fever virus (E-70) deleted on A238L. Vet Immunol Immunopathol. (2008) 124:107–19. doi: 10.1016/j.vetimm.2008.02.012
50. Hervas J, Gomez-Villamandos JC, Mendez A, Carrasco L, Sierra MA. The lesional changes and pathogenesis in the kidney in African swine fever. Vet Res Commun. (1996) 20:285–99. doi: 10.1007/BF00366926
51. Gomez-Villamandos JC, Hervas J, Mendez A, Carrasco L, Villeda CJ, Wilkinson PJ, et al. Pathological changes in the renal interstitial capillaries of pigs inoculated with two different strains of African swine fever virus. J Comp Pathol. (1995) 112:283–98. doi: 10.1016/S0021-9975(05)80081-7
52. Gomez-Villamandos JC, Hervas J, Mendez A, Carrasco L, Martin de las Mulas J, Villeda CJ, et al. Experimental African swine fever: apoptosis of lymphocytes and virus replication in other cells. J Gen Virol. (1995) 76:2399–405. doi: 10.1099/0022-1317-76-9-2399
53. Gomez-Villamandos JC, Bautista MJ, Carrasco L, Chacon-Manrique de Lara F, Hervas J, Wilkinson PJ, et al. Thrombocytopenia associated with apoptotic megakaryocytes in a viral hemorrhagic syndrome induced by a moderately virulent strain of African swine fever virus. J Comp Pathol. (1998) 118:1–13. doi: 10.1016/S0021-9975(98)80023-6
54. Villeda CJ, Williams SM, Wilkinson PJ, Vinuela E. Haemostatic abnormalities in African swine fever a comparison of two virus strains of different virulence (Dominican Republic '78 and Malta '78). Arch Virol. (1993) 130:71–83. doi: 10.1007/BF01318997
55. Villeda CJ, Williams SM, Wilkinson PJ, Vinuela E. Consumption coagulopathy associated with shock in acute African swine fever. Arch Virol. (1993) 133:467–75. doi: 10.1007/BF01313784
56. Gomez-Villamandos JC, Bautista MJ, Sanchez-Cordon PJ, Carrasco L. Pathology of African swine fever: the role of monocyte-macrophage. Virus Res. (2013) 173:140–9. doi: 10.1016/j.virusres.2013.01.017
57. Moulton JE, Pan IC, Hess WR, DeBoer CJ, Tessler J. Pathologic features of chronic pneumonia in pigs with experimentally induced African swine fever. Am J Vet Res. (1975) 36:27–32.
58. Salguero FJ, Sanchez-Cordon PJ, Nunez A, Fernandez de Marco M, Gomez-Villamandos JC. Proinflammatory cytokines induce lymphocyte apoptosis in acute African swine fever infection. J Comp Pathol. (2005) 132:289–302. doi: 10.1016/j.jcpa.2004.11.004
59. Giammarioli M, Gallardo C, Oggiano A, Iscaro C, Nieto R, Pellegrini C, et al. Genetic characterisation of African swine fever viruses from recent and historical outbreaks in Sardinia (1978-2009). Virus Genes. (2011) 42:377–87. doi: 10.1007/s11262-011-0587-7
60. Arias ML, Escribano JM, Rueda A, Sanchez-Vizcaino JM, La peste porcina africana. Med Veterinaire. (1986) 3:333–50.
62. Sanchez-Vizcaino JM, Slauson DO, Ruiz-Gonzalvo F, Valero F. Lymphocyte function and cell-mediated immunity in pigs with experimentally induced African swine fever. Am J Vet Res. (1981) 42:1335–41.
63. Greig A. Pathogenesis of African swine fever in pigs naturally exposed to the disease. J Comp Pathol. (1972) 82:73–9. doi: 10.1016/0021-9975(72)90028-X
64. Blome S, Gabriel C, Beer M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. (2013) 173:122–30. doi: 10.1016/j.virusres.2012.10.026
65. Colgrove GS, Haelterman EO, Coggins L. Pathogenesis of African swine fever in young pigs. Am J Vet Res. (1969) 30:1343–59.
66. Heuschele WP Studies on the pathogenesis of African swine fever. I. Quantitative studies on the sequential development of virus in pig tissues. Arch Gesamte Virusforsch. (1967) 21:349–56. doi: 10.1007/BF01241735
67. Alcami A, Carrascosa AL, Vinuela E. Interaction of African swine fever virus with macrophages. Virus Res. (1990) 17:93–104. doi: 10.1016/0168-1702(90)90071-I
68. Wardley RC, Wilkinson PJ. The growth of virulent African swine fever virus in pig monocytes and macrophages. J Gen Virol. (1978) 38:183–6. doi: 10.1099/0022-1317-38-1-183
69. Martins CL, Scholl T, Mebus CA, Fisch H, Lawman MJ. Modulation of porcine peripheral blood-derived macrophage functions by in vitro infection with African swine fever virus (ASFV) isolates of different virulence. Viral Immunol. (1987) 1:177–90. doi: 10.1089/vim.1987.1.177
70. Sierra MA, Bernabe A, Mozos E, Mendez A, Jover A. Ultrastructure of the liver in pigs with experimental African swine fever. Vet Pathol. (1987) 24:460–2. doi: 10.1177/030098588702400516
71. Gomez-Villamandos JC, Hervas J, Moreno C, Carrasco L, Bautista MJ, Caballero JM, et al. Subcellular changes in the tonsils of pigs infected with acute African swine fever virus. Vet Res. (1997) 28:179–89.
72. Gomez-Villamandos JC, Bautista MJ, Carrasco L, Caballero MJ, Hervas J, Villeda CJ, et al. African swine fever virus infection of bone marrow: lesions and pathogenesis. Vet Pathol. (1997) 34:97–107. doi: 10.1177/030098589703400202
73. Ramiro-Ibanez F, Ortega A, Brun A, Escribano JM, Alonso C. Apoptosis: a mechanism of cell killing and lymphoid organ impairment during acute African swine fever virus infection. J Gen Virol. (1996) 77:2209–19. doi: 10.1099/0022-1317-77-9-2209
74. Sierra MA, Quezada M, Fernandez A, Carrasco L, Gomez-Villamandos JC, Martin de las Mulas J, et al. Experimental African swine fever: evidence of the virus in interstitial tissues of the kidney. Vet Pathol. (1989) 26:173–6. doi: 10.1177/030098588902600211
75. Mebus CA. African swine fever. Adv Virus Res. (1988) 35:251–69. doi: 10.1016/S0065-3527(08)60714-9
76. Brun A, Rivas C, Esteban M, Escribano JM, Alonso C. African swine fever virus gene A179L, a viral homologue of bcl-2, protects cells from programmed cell death. Virology. (1996) 225:227–30. doi: 10.1006/viro.1996.0592
77. Afonso CL, Neilan JG, Kutish GF, Rock DL. An African swine fever virus Bc1-2 homolog, 5-HL, suppresses apoptotic cell death. J Virol. (1996) 70:4858–63. doi: 10.1128/JVI.70.7.4858-4863.1996
78. Oura CA, Powell PP, Parkhouse RM. African swine fever: a disease characterized by apoptosis. J Gen Virol. (1998) 79:1427–38. doi: 10.1099/0022-1317-79-6-1427
79. Yanez RJ, Rodriguez JM, Nogal ML, Yuste L, Enriquez C, Rodriguez JF, et al. Analysis of the complete nucleotide sequence of African swine fever virus. Virology. (1995) 208:249–78. doi: 10.1006/viro.1995.1149
80. Neilan JG, Lu Z, Afonso CL, Kutish GF, Sussman MD, Rock DL. An African swine fever virus gene with similarity to the proto-oncogene bcl-2 and the Epstein-Barr virus gene BHRF1. J Virol. (1993) 67:4391–4. doi: 10.1128/JVI.67.7.4391-4394.1993
81. Zsak L, Neilan JG. Regulation of apoptosis in African swine fever virus-infected macrophages. Sci World J. (2002) 2:1186–95. doi: 10.1100/tsw.2002.214
82. Hernaez B, Diaz-Gil G, Garcia-Gallo M, Ignacio Quetglas J, Rodriguez-Crespo I, Dixon L, et al. The African swine fever virus dynein-binding protein p54 induces infected cell apoptosis. FEBS Lett. (2004) 569:224–8. doi: 10.1016/j.febslet.2004.06.001
83. Dixon LK, Islam M, Nash R, Reis AL. African swine fever virus evasion of host defences. Virus Res. (2019) 266:25–33. doi: 10.1016/j.virusres.2019.04.002
84. Nogal ML, Gonzalez de Buitrago G, Rodriguez C, Cubelos B, Carrascosa AL, Salas ML, et al. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J Virol. (2001) 75:2535–43. doi: 10.1128/JVI.75.6.2535-2543.2001
85. Netherton CL, Connell S, Benfield TO, Dixon LK. The genetics of life and death: virus-host interactions underpinning resistance to African swine fever, a viral hemorrhagic disease. Front Genet. (2019) 10:402. doi: 10.3389/fgene.2019.00402
86. Salguero FJ, Sanchez-Cordon PJ, Sierra MA, Jover A, Nunez A, Gomez-Villamandos JC. Apoptosis of thymocytes in experimental Africa Swine Fever virus infection. Histol Histopathol. (2004) 19:77–84. doi: 10.14670/HH-19.77
87. Fernandez de Marco M, Salguero FJ, Bautista MJ, Nunez A, Sanchez-Cordon PJ, Gomez-Villamandos JC. An immunehistochemical study of the tonsils in pigs with acute African swine fever virus infection. Res Vet Sci. (2007) 83:198–203. doi: 10.1016/j.rvsc.2006.11.011
88. Oura CA, Powell PP, Parkhouse RM. Detection of African swine fever virus in infected pig tissues by immunocytochemistry and in sity hybridisation. J Virol Methods. (1998) 72:205–17. doi: 10.1016/S0166-0934(98)00029-9
89. Gomez del Moral M, Ortuno E, Fernandez-Zapatero P, Alonso F, Alonso C, Ezquerra A, et al. African swine fever virus infection induces tumor necrosis factor alpha production: implications in pathogenesis. J Virol. (1999) 73:2173–80. doi: 10.1128/JVI.73.3.2173-2180.1999
90. Smither SJ, Nelson M, Eastaugh L, Laws TR, Taylor C, Smith SA, et al. Experimental respiratory Marburg virus hemorrhagic fever infection in the common marmoset (Callithrix jacchus). Int J Exp Pathol. (2013) 94:156–68. doi: 10.1111/iep.12018
91. Smither SJ, Nelson M, Eastaugh L, Nunez A, Salguero FJ, Lever MS. Experimental respiratory infection of marmosets (Callithrix jacchus) with ebola virus Kikwit. J Infect Dis. (2015) 212(Suppl.2):S336–45. doi: 10.1093/infdis/jiv371
92. Carrasco L, Bautista MJ, Martin de las Mulas J, Gomez-Villamandos JC, Espinosa de los Monteros A, Sierra MA. Description of a new population of fixed macrophages in the splenic cords of pigs. J Anat. (1995) 187:395–402.
93. Gomez-Villamandos JC, Bautista MJ, Hervas J, Carrasco L, de Lara FC, Perez J, et al. Subcellular changes in platelets in acute and subacute African swine fever. J Comp Pathol. (1996) 115:327–41. doi: 10.1016/S0021-9975(96)80069-7
94. Bautista MJ, Gomez-Villamandos JC, Carrasco L, Ruiz-Villamor E, Salguero FJ, Sierra MA. Ultrastructural pathology of the bone marrow in pigs inoculated with a moderately virulent strain (DR'78) of African swine fever virus. Histol Histopathol. (1998) 13:713–20.
95. Gomez-Villamandos JC, Salguero FJ, Ruiz-Villamor E, Sanchez-Cordon PJ, Bautista MJ, Sierra MA. Classical Swine Fever: pathology of bone marrow. Vet Pathol. (2003) 40:157–63. doi: 10.1354/vp.40-2-157
96. Konno S, Taylor WD, Hess WR, Heuschele WP. Liver pathology in African swine fever. Cornell Vet. (1971) 61:125–50.
97. Sanchez-Cordon PJ, Romero-Trevejo JL, Pedrera M, Sanchez-Vizcaino JM, Bautista MJ, Gomez-Villamandos JC. Role of hepatic macrophages during the viral hemorrhagic fever induced by African Swine Fever Virus. Histol Histopathol. (2008) 23:683–91. doi: 10.14670/HH-23.683
98. Cadenas-Fernandez E, Sanchez-Vizcaino JM, Pintore A, Denurra D, Cherchi M, Jurado C, et al. Free-ranging pig and wild boar interactions in an endemic area of African Swine Fever. Front Vet Sci. (2019) 6:376. doi: 10.3389/fvets.2019.00376
99. Cwynar P, Stojkov J, Wlazlak K. African swine fever status in Europe. Viruses. (2019) 11:310. doi: 10.3390/v11040310
100. Eble PL, Hagenaars TJ, Weesendorp E, Quak S, Moonen-Leusen HW, W.Loeffen LA. Transmission of African Swine Fever Virus via carrier (survivor) pigs does occur. Vet Microbiol. (2019) 237:108345. doi: 10.1016/j.vetmic.2019.06.018
101. Sanchez-Cordon PJ, Nunez A, Neimanis A, Wikstrom-Lassa E, Montoya M, Crooke H, et al. African swine fever: disease dynamics in wild boar experimentally infected with ASFV isolates belonging to genotype I and II. Viruses. (2019) 11:852. doi: 10.3390/v11090852
102. Schulz K, Conraths FJ, Blome S, Staubach C, Sauter-Louis C. African swine fever: fast and furious or slow and steady? Viruses. (2019) 11:866. doi: 10.3390/v11090866
103. Perez J, Fernandez AI, Sierra MA, Herraez P, Fernandez A, Martin, de las Mulas J Serological and immunohistochemical study of African swine fever in wild boar in Spain. Vet Rec. (1998) 143:136–9. doi: 10.1136/vr.143.5.136
104. Sanchez-Cordon PJ, Montoya M, Reis AL, Dixon LK. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet J. (2018) 233:41–8. doi: 10.1016/j.tvjl.2017.12.025
105. Rahimi P, Sohrabi A, Ashrafihelan J, Edalat R, Alamdari M, Masoudi M, et al. Emergence of African swine fever virus, northwestern Iran. Emerg Infect Dis. (2010) 16:1946–8. doi: 10.3201/eid1612.100378
106. Gabriel C, Blome S, Malogolovkin A, Parilov S, Kolbasov D, Teifke JP, et al. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg Infect Dis. (2011) 17:2342–5. doi: 10.3201/eid1712.110430
107. Blome S, Gabriel C, Dietze K, Breithaupt A, Beer M. High virulence of African swine fever virus caucasus isolate in European wild boars of all ages. Emerg Infect Dis. (2012) 18:708. doi: 10.3201/eid1804.111813
108. Sanchez-Vizcaino JM, Mur L, Martinez-Lopez B. African swine fever (ASF): five years around Europe. Vet Microbiol. (2013) 165:45–50. doi: 10.1016/j.vetmic.2012.11.030
109. Li L, Ren Z, Wang Q, Ge S, Liu Y, Liu C, et al. Infection of African swine fever in wild boar, China, 2018. Transbound Emerg Dis. (2019) 66:1395–98. doi: 10.1111/tbed.13114
110. Bastos AD, Penrith ML, Cruciere C, Edrich JL, Hutchings G, Roger F, et al. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch Virol. (2003) 148:693–706. doi: 10.1007/s00705-002-0946-8
111. Pikalo J, Zani L, Huhr J, Beer M, Blome S. Pathogenesis of African swine fever in domestic pigs and European wild boar - Lessons learned from recent animal trials. Virus Res. (2019) 271:197614. doi: 10.1016/j.virusres.2019.04.001
112. Gallardo C, Nurmoja I, Soler A, Delicado V, Simón A, Martin E, et al. Evolution in Europe of African swine fever genotype II viruses from highly to moderately virulent. Vet Microbiol. (2018) 219:70–9. doi: 10.1016/j.vetmic.2018.04.001
113. Gallardo C, Soler A, Rodze I, Nieto R, Cano-Gomez C, Fernandez-Pinero J, et al. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia (2017). Transbound Emerg Dis. (2019) 66:1399–404. doi: 10.1111/tbed.13132
114. Nurmoja I, Petrov A, Breidenstein C, Zani L, Forth JH, Beer M, et al. Biological characterization of African swine fever virus genotype II strains from north-eastern Estonia in European wild boar. Transbound Emerg Dis. (2017) 64:2034–41. doi: 10.1111/tbed.12614
115. Heuschele WP, Coggins L. Epizootiology of African swine fever virus in warthogs. Bull Epizoot Dis Afr. (1969) 17:179–83.
116. Jori F, Bastos AD. Role of wild suids in the epidemiology of African swine fever. Ecohealth. (2009) 6:296–310. doi: 10.1007/s10393-009-0248-7
117. Thomson GR. The epidemiology of African swine fever: the role of free-living hosts in Africa. Onderstepoort J Vet Res. (1985) 52:201–9.
118. Anderson EC, Hutchings GH, Mukarati N, Wilkinson PJ. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet Microbiol. (1998) 62:1–15. doi: 10.1016/S0378-1135(98)00187-4
119. Palgrave CJ, Gilmour L, Lowden CS, Lillico SG, Mellencamp MA, Whitelaw CB. Species-specific variation in RELA underlies differences in NF-kappaB activity: a potential role in African swine fever pathogenesis. J Virol. (2011) 85:6008–14. doi: 10.1128/JVI.00331-11
120. Oura CA, Powell PP, Anderson E, Parkhouse RM. The pathogenesis of African swine fever in the resistant bushpig. J Gen Virol. (1998) 79:1439–43. doi: 10.1099/0022-1317-79-6-1439
Keywords: African swine fever, pathology, pathogenesis, virus, swine
Citation: Salguero FJ (2020) Comparative Pathology and Pathogenesis of African Swine Fever Infection in Swine. Front. Vet. Sci. 7:282. doi: 10.3389/fvets.2020.00282
Received: 30 November 2019; Accepted: 27 April 2020;
Published: 19 May 2020.
Edited by:
Jose Manuel Sanchez-Vizcaino, Complutense University of Madrid, SpainReviewed by:
Sandro Rolesu, Istituto Zooprofilattico Sperimentale della Sardegna (IZS), ItalyCopyright © 2020 Salguero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco J. Salguero, SmF2aWVyLnNhbGd1ZXJvQHBoZS5nb3YudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.