
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 27 March 2020
Sec. Parasitology
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00134
This article is part of the Research Topic An Overview of Current Knowledge on Ticks and Tick-borne Diseases View all 8 articles
This is the first comprehensive review of the literature pertaining to Babesia species reported from domestic cats. Description of the four species (Babesia felis, Babesia cati, Babesia herpailuri, and Babesia pantherae) named based on morphology and/or host specificity is documented. Feline babesiosis is of major veterinary concern only in South Africa. Reports of the rare occurrence of feline babesiosis cases in Europe (France, Germany, Poland, and Spain) and Asia (Israel, India, and Pakistan) are documented. Molecular characterization has revealed that cats can harbor a variety of Babesia species. The previous practice of referring to all piroplasms, especially small ones, seen on feline blood smears as B. felis is therefore no longer tenable. The near-full-length 18S rRNA gene sequences entered into GenBank in 2001 (accession no. AF244912) are designated as definitive for B. felis sensu stricto. All published literature relating to molecular characterization of feline Babesia species that could be traced was critically assessed. Four Babesia species are now known to be involved in causing feline babesiosis in South Africa: the closely related B. felis s.s. and Babesia leo (clade I), Babesia lengau (clade II), and Babesia species cat Western Cape (clade VI, Babesia s.s.). Clade VI also includes Babesia canis presentii and Babesia hongkongensis reported from cats in Asia. Six other Babesia species have been reported from domestic cats: the dog-associated B. canis s.s., Babesia gibsoni, and B. vogeli, as well as Babesia lohae, Babesia microti, and Babesia vulpes. Phylogenetic relationships of all named species were assessed and are presented as trees. The relatively high prevalence of B. vogeli in clinically healthy cats (16% in Brazil, 13% on St Kitts, and 8.1% in Portugal) suggests that immunocompetent cats can harbor the infection with no discernible untoward effects. Reports of occurrence of B. felis and other Babesia species in domestic cats should be accepted only if they are supported by credible molecular provenance.
In the first reference to feline babesiosis, published in 1904, it was claimed that “spontaneous pyroplasmosis” occurred in tame and wild cats in India (1), but no supporting information was provided. This record is dubious, especially since the authors also include humans and chickens in their list of affected hosts. Published literature on feline babesiosis is rather meager, compared to the wealth of papers on babesiosis (or piroplasmosis) in other domestic animals. This is not surprising because babesiosis of domestic cats seems to be of major veterinary concern only in a restricted area of South Africa (2). Sporadic cases diagnosed elsewhere are deemed of sufficient interest to warrant documentation as case reports. All cases documented to date occurred in the Old World.
Before the advent of molecular characterization of species, one approach to identifying Babesia species was based on descriptions, detailed or otherwise, of morphology (including dimensions) of the organism. This led to the notion that Babesia species can be classified either as “large” or “small.” This approach had its pitfalls because piroplasms undergo marked morphological changes during their development in erythrocytes. In addition, there can be substantial overlap between measurements of “large” and “small” babesias. A second approach was to assume that Babesia species are more or less host specific. The logical outcome of such reasoning was that any Babesia seen on a blood smear of a wild felid should, by definition, be the same species as that found in domestic cats. Neither approach was satisfactory, and caution should be exercised when quoting names of Babesia species used in such publications.
The advent of molecular characterization has led to the description of various new Babesia species from domestic cats. A similar situation exists in African great ape populations, for example, where molecular characterization is revealing an unexpected diversity of Plasmodium species that had not been detected previously (3, 4).
This is the first comprehensive review of the literature pertaining to Babesia species reported from domestic cats, either from clinical cases or as incidental findings. Articles on piroplasms of wild felids are included only if there is a direct connection with domestic cats. In the first section, feline Babesia species named based on morphology and/or host-specificity are documented, in chronological order. In the second section reports of clinical feline babesiosis from Southern Africa, Europe and Asia are documented. In the third section, Babesia species reported from domestic cats based on molecular characterization are critically evaluated. In this evaluation, the near-full-length 18S rRNA gene sequences, entered into GenBank in 2001 (accession no. AF244912), were regarded as definitive for Babesia felis sensu stricto (5). In addition to B. felis s.s., four Babesia species and one subspecies have been described from domestic cats based on gene characterization. Various other Babesia species have also been reported from domestic cats. Data on all of these Babesia species were assembled and phylogenetic trees prepared.
The four Babesia species involved are discussed in chronological order, based on the date of their description. In 1929, Davis (6) described and named B. felis from a wild cat (Felis ocreata, presumably Felis sylvestris) in Sudan. Trophozoites varied in diameter from less than 1 μm to 2.25 μm, the majority being about 1.25 μm; the four pear-shaped merozoites were arranged in a cruciform manner (6). In the course of a thorough investigation, 22 domestic cats were artificially infected with a few drops of infected blood in 2% sodium citrate solution. Irrespective of route of infection (intravenous, intraperitoneal, or subcutaneous), all cats became parasitemic, but none developed overt clinical signs, even after undergoing splenectomy. Infection with the piroplasm did not precipitate overt clinical signs even after serial passage through five cats.
In February 1933, two cougars (Puma concolor) shipped from San Francisco arrived at the Cairo Zoological Gardens, Egypt, where they were housed in close proximity to other large felids (7). Within 2 to 3 weeks, both animals developed clinical signs of babesiosis and succumbed to the disease. Trophozoites resembled those of the canid-associated Babesia gibsoni (8), with an obtuse angle between pear-shaped merozoites joined at their tips. These piroplasms were named Babesiella felis by Carpano (7), who also argued that the piroplasm described by Davis (6) should be called Nuttallia felis. In this, Carpano (7) followed the classification proposed by du Toit (9). Although this case is not specifically linked to domestic cats, it is included because the name chosen for the piroplasm by Carpano (7) could lead to confusion.
A few years later, in 1937, Mangrulkar (10) reported finding small intraerythrocytic piroplasms on blood smears made during an autopsy on a somewhat anemic cat in India. Because merozoite tetrads (Maltese crosses) were not observed, Mangrulkar (10) concluded that the piroplasms concerned were different from B. felis and Babesiella felis. (Babesiella felis is not referred to further in this review; B. felis henceforth implies Babesia felis.) Since Mangrulkar (10) lacked suitable material for further study, he refrained from naming a new species. In 1950, a similar small piroplasm (0.5–2.5 μm), seen on blood smears made from an apparently healthy Indian wild cat, was named Babesia cati (11). Attempts at transmitting this piroplasm to domestic cats were unsuccessful.
In 1967, a “large” Babesia from a neotropical jaguarundi (Puma yagouaroundi, previously assigned to the genus Herpailurus) was established artificially in spleen-intact domestic cats, which did not develop overt clinical signs (12). In 1969, this species was described and named Babesia herpailuri (13). In 1972, Babesia pantherae from leopards (Panthera pardus), a piroplasm smaller than B. herpailuri, was established in splenectomized domestic cats (14, 15). A small piroplasm matching the morphological description of B. felis was also transmitted artificially from leopards to domestic cats (14).
Clinical cases of feline babesiosis were first reported from the vicinity of Cape Town, South Africa, in 1937. In 1972, Dennig and Brocklesby (14) confirmed that clinical cases had not been reported outside of South Africa.
The first two reports of babesiosis in domestic cats appeared in 1937. In the first paper, a case report, the cat was pyrexic and anemic and recovered after administration of quinuronium sulfate (16). Piroplasms had an average diameter of approximately 1.5 μm, but rare pyriforms measured up to 4 μm long. Noting that cats artificially infected with B. felis by Davis (6) had not developed overt clinical signs and following the classification of du Toit (9), Jackson and Dunning (16) named the organism incriminated in this case N. felis var. domestica. The second article was a general report on babesiosis occurring in free-ranging cats (17). Clinical signs included pyrexia and anemia; treatment with quinuronium sulfate or trypan blue affected clinical cure (17). Since the disease only occurred in cats that had access to undisturbed natural vegetation, McNeil (17) speculated that the wild cat (Felis caffra; syn. F. sylvestris) may be a reservoir of infection. The disease was subsequently reported from Knysna, a coastal town approximately 430 km east of Cape Town (18). Here the cats also tended to be pyrexic.
The first systematic investigation into the disease included 70 cats presented at veterinary clinics and 20 artificially infected cats (19). The causative organism was called B. felis. Blood from a sick cat whose owners lived in what is now Table Mountain National Park was used for the 20 artificial infections. Clinical signs in reacting cats included lethargy, anorexia, and anemia, but pyrexia was not a feature of the disease (19). The absence of pyrexia may indicate that the pathogen involved here was not the same as that reported in the earlier clinical cases.
In contrast to earlier reports, trypan blue and quinuronium sulfate were not effective in treating cats artificially infected with B. felis sensu lato (19). This is a further indication that more than one piroplasm species may have been involved in earlier reports.
Babesiosis was subsequently shown to occur fairly commonly in cats along the eastern and southern coast of South Africa and adjacent inland areas, primarily in summer (2). It also occurs along the eastern escarpment in Mpumalanga and Limpopo Provinces (20).
A large piroplasm was observed on blood smears of a sick cat from the suburbs of Harare, Zimbabwe (21). The cat had a temperature of 40°C. Treatment with diminazene affected clinical cure. In line with dogma acceptable at the time and based on morphology and measurements, the organism was tentatively identified as B. herpailuri.
Feline babesiosis is rare in Europe. The first clinical case, reported in 1992, was a somewhat anemic 8-year-old cat in France (22). A low parasitemia of small piroplasms (1.5–2 μm) was found. The report mentioned large piroplasms (3.5–5.5 μm in diameter) on a blood smear of a cat from Paris; no further detail was given. Bourdeau (23) hypothesized that the small organisms were Babesia divergens or Babesia microti, whereas the large one was B. canis or Babesia vogeli. A subsequent case from France was attributed to Babesia vulpes (referred to as Babesia annae) (24).
The only clinical case in Germany, reported in 1997 (25), was a 10-month-old cat imported from northern Sweden. Clinical signs included pyrexia (39.6°C) and anemia. The cat responded well to treatment with imidocarb. Blood smears indicated a large Babesia, with mean dimensions 3.6 × 2.3 μm. In Poland, a 10-year-old cat showing weakness, anemia, fever, and hematuria recovered fully after administration of imidocarb (26). Piroplasms on blood smear resembled B. canis, but polymerase chain reaction (PCR) sequencing of a 559-bp fragment of the 18S rRNA gene showed only 95% homology with B. canis s.s.
On the Iberian Peninsula, B. canis s.s. was incriminated in one feline babesiosis case in Spain (27). Subsequently, B. canis s.s. was reported from 1.3% and B. vogeli from 8.1% of 320 cats presented at 30 veterinary medical centers in northern and central Portugal (28).
As mentioned previously, an autopsy on a somewhat anemic cat in India indicated the presence of small piroplasms (10). Also in India, small amoeboid, oval, or rounded parasites of varying sizes resembling B. felis were seen in blood smears from a pyrexic, lethargic, anorexic kitten that made an uneventful recovery after administration of primaquine phosphate (29). A large babesia, incriminated in causing disease in a cat in India, was successfully treated with diminazene (30).
In Lahore, Pakistan, babesiosis was diagnosed in 163 (3.1%) of cats presented at a university clinic (31). Blood smears from 50 cats in Mosul, Iraq, were examined for presence of hemoparasites (32). It is not clear whether the cats were healthy. Based on morphology of piroplasms, the author claimed that 26% of the cats were positive for Babesia species and 22% for Cytauxzoon felis.
Babesia canis subspecies presentii was described and named from two cats in Israel; one was sick, and the other a subclinical carrier (33). The sick cat, which was coinfected with feline immunodeficiency virus and “Candidatus Mycoplasma haemominutum,” showed icterus and anemia. It responded rapidly to treatment with imidocarb and doxycycline and made a complete recovery.
The 18S locus is a conservative marker among eukaryotes and evolves more slowly than other barcoding loci. Because of the unreliability of morphological characters for delimiting most hemoparasites, 18S is the most widely used locus to delimit hemoparasite species (34). Variation in the 18S rRNA gene has been very widely used as a taxonomic and phylogenetic tool for classifying Babesia species. There is, however, no universally used criterion (% sequence identity) for classifying organisms to species level based on this variation. Greay et al. (34) recently stated that the genetic distances between proposed novel apicomplexans and their closest relatives (described to date) will be greater than the genetic distances between the next two most closely related species.
The first molecular characterization of a Babesia species from a domestic cat was that of B. felis s.s., when near-full-length 18S rRNA gene sequences were entered into GenBank in 2001 (5). In this section, claims of occurrence of B. felis s.s. are documented and evaluated first, followed by the other Babesia species reported from domestic cats in alphabetical order.
The near-full-length 18S rRNA gene sequences entered into GenBank in 2001 (accession no. AF244912) are regarded as definitive for B. felis s.s. (5). This specimen was obtained from the isolate used for artificial infection of cats to test efficacy of various drugs in treating feline babesiosis (35, 36). Blood from three experimental cats at the Agricultural Research Council-Onderstepoort Veterinary Institute (ARC-OVI) known to harbor B. felis was pooled, and 4 mL injected subcutaneously into a susceptible cat. A stabilate was prepared from this cat's blood when parasitemia was approximately 12% (37); 1-mL aliquots were prepared and stored in the gas phase of a liquid nitrogen refrigerator.
One (2.9%) of 34 healthy cats in Qatar was positive for B. felis (99% nucleotide identity; AY452707) (38). Because there are many expatriates working in Qatar, the possibility of this cat becoming infected elsewhere cannot be ruled out.
In a study done by Salim et al. (39) to develop and optimize a loop-mediated isothermal amplification (LAMP) assay for the diagnosis of babesiosis in cats in Lahore, Pakistan, piroplasms were seen on blood smears of 45 of 100 domestic cats showing signs of anemia and lethargy. The positive samples were subjected to conventional PCR and LAMP; the LAMP assay was found to be more sensitive in detecting Babesia species (11/45) than the conventional PCR (5/45). The authors claimed that the piroplasms involved were B. felis. These finding cannot be verified, however, because close inspection of the conventional and LAMP PCR primers used in the study indicated that they could amplify any Babesia species Furthermore, no sequencing was done to confirm the PCR and LAMP results.
Babesia canis subspecies presentii was described from two cats in Israel (GenBank accession nos. AY272047 and AY272048) (33). The relatively short fragment (395 bp; GenBank AY15057) of the 18S rRNA gene sequenced when B. canis s.s. was identified in cats in Spain and Portugal (27) showed 99.5% identity when compared with the similar fragment of B. canis presentii. In a subsequent report from Portugal (28), specimens from 4 (1.3%) of 320 cats showed 100% identity with a B. canis s.s. sequence in GenBank (accession no. HQ662634.1).
The only report of B. gibsoni in cats was from St Kitts in the Caribbean, where B. gibsoni was found in 5 (4%) of 119 apparently healthy cats (40). The partial 18S rRNA sequences (538 bp) found (GenBank accession no. KY073362) were identical and showed 100% identity to 37 B. gibsoni sequences in GenBank (e.g., accession no. JX962780).
An apparently new species, genetically and geographically distinct from other previously described Babesia species and tentatively named B. hongkongensis, was found in blood and kidney specimens of a free-ranging cat in Hong Kong (41). Its mitochondrial cytochrome b gene sequences (GenBank accession no. JQ867357) had 90.4% identity with those of B. gibsoni (AB499087.1). The near-full-length 18S rRNA gene sequence (1 612 bp) (GenBank accession no. JQ867356) had 96.7% nucleotide identity to various Babesia sequences found in feral raccoons (Procyon lotor) and dogs. Phylogenetic analysis of the 18S rRNA gene sequence data showed that B. hongkongensis falls into a distinct branch of the Babesiidae.
Babesia lengau was described from cheetahs (Acinonyx jubatus) in South Africa (near-full-length 18S rRNA gene sequences were deposited in GenBank, accession nos. GQ411405–GQ411417) (42). Sequences for the ITS2 region were deposited in GenBank, accession numbers GQ411418 to GQ411430 (42). Babesia lengau was incriminated in two feline babesiosis cases in South Africa, one being the first report of cerebral babesiosis in a cat (GenBank accession nos. KC790443 and KC833036) (43).
Babesia leo (GenBank accession no. AF244911) was described from lions (Panthera leo) in Kruger National Park, South Africa (5). Babesia leo has been incriminated in causing disease in five cats: four in KwaZulu-Natal, South Africa and one in Maputo, Mozambique (44). Sequences from four of these cats were 100% identical to those of B. leo, whereas one sequence (1,520 bp) was 99% similar to B. leo (44).
Babesia lohae (GenBank MG593272 and MG593273) was identified in a female Ixodes holocyclus tick recovered from a cat, as well as in an Ixodes tasmani collected from a brushtail possum (Trichosurus vulpecula) in Queensland, Australia (45). Phylogenetically, it grouped within the Babesia s.s. clade and with other Babesia species from Australian marsupials and ticks from marsupials. It is possible, therefore, that brushtail possums are a native reservoir host of B. lohae.
In a survey of stray cats in Milan, Italy, two (0.8%) of 260 cats tested positive for B. microti DNA using conventional PCR (46). Both cats were healthy. Subsequent 18S rDNA sequencing of a 261-bp fragment confirmed these two samples as B. microti–positive with 100% identity (over a 199 bp region) to B. microti previously described in Ixodes ricinus ticks removed from dogs in Warsaw, Poland (GenBank accession no. EU882727). In Sicily, 6 of 23 cats were PCR positive for B. microti (47).
One clinically affected cat in Cape Town, South Africa, was infected with both B. felis and B. microti (44). This B. microti (MK095343) had 100% sequence identity with B. microti Otsu strain (AB119446) from Japan, and 99% and 98% sequence identity with B. microti Gray (AY693840) and B. microti Munich (AB071177) strains, respectively.
In a survey in Pakistan, 21 (13.2%) of 159 cats presented at veterinary clinics were reported to be infected with B. microti (48). Polymerase chain reaction amplified a 238-bp amplicon specific for 18S rRNA gene of B. microti in which sequences were 99 to 100% identical to those deposited in GenBank (no detail given). Two partial 18S rRNA gene sequences of B. microti from the Pakistani cats are in GenBank (accession nos. MF401440 and MF401441). These sequences were marked as “UNVERIFIED” in GenBank (i.e., GenBank staff was unable to verify the sequences and/or annotation provided by the submitter). A BLASTn analysis showed that although MF401440 had 100% sequence identity to B. microti (KX758442), it had only 75% sequence coverage.
The first report of B. vogeli infection in cats was from Bangkok, Thailand, where 21 (1.4%) of 1,490 stray cats tested positive (49). Sequenced target amplicons (330 bp) from 21 samples were identical (GenBank accession no. EU697608). The authors reported that a BLASTn search indicated 98% identity with a B. vogeli from dogs in Brazil (GenBank accession no. not given) (50). BLASTn analysis now indicates 98.59% identity to various B. vogeli and B. canis sequences currently deposited in GenBank, but with only 86% gene coverage. Unidentified piroplasms had previously been reported from cats in Bangkok (51).
In a survey in north and central Portugal, 26 (8.1%) of 320 cats were positive for B. vogeli; specimens from three of these cats that were sick showed 100% identity with B. vogeli accession no. JX871885 in GenBank (28). In a separate study, eight cats were positive for B. vogeli (GenBank accession nos. AB896788–AB896795) (52). In a survey in southern Portugal, 43 (6.6%) of 649 cats were positive for Babesia species, possibly B. vogeli (53).
In Brazil, six (16%) of 37 free-ranging cats in a zoo in Sao Paolo were positive for Babesia (54). Their sequences (GenBank accession nos. KF970926–KF970929) (499–650 bp) showed 99% identity to B. vogeli (GenBank accession no. HM590440). Also in Brazil, specimens from 2 (4%) of 30 cats in Rio Grande do Sul State were positive, their sequences (GenBank accession nos. KT323932 and KT323933) (764–766 bp) showing 100% identity with B. vogeli (GenBank accession no. HM590440) (55).
On the island of St Kitts in the Caribbean, B. vogeli was found in 15 (13%) of 119 apparently healthy cats (40). All sequences were identical (GenBank accession no. KY073363) (545 bp) and showed 100% identity to 19 B. vogeli sequences in GenBank (e.g., accession no. HQ148664). In Qatar, one (2.9%) of 34 healthy cats was positive for B. vogeli (100% nucleotide identity; KT333456) (38).
Two clinically normal cats in Portugal that had immunosuppressive viral infections were positive for B. vulpes (then referred to as Theileria annae) (27, 56). The sequence found (AY150068) had 100% similarity with a dog isolate (AF188001). Babesia vulpes (referred to as B. annae) was also incriminated in a clinical case in a cat in France (24). Cats were not mentioned as hosts when T. annae was formally renamed B. vulpes (57).
A pathogenic piroplasm from the Babesia s.s. clade was recently discovered in South Africa (44). Seven clinical cases were reported: six from the vicinity of Cape Town and one from Durban, KwaZulu-Natal. Because of the lack of an appropriate-type specimen, a new species was not described, and the novel organism is referred to as Babesia species cat Western Cape (GenBank KR611133–KR611159). All previously reported feline babesiosis cases in South Africa had been caused by B. felis, B. lengau, or B. leo.
In recent years, many novel Babesia 18S rRNA gene sequences have been deposited in the public DNA sequence databases. Apart from a limited number of 5S rRNA gene and internal transcribed spacer sequences, only 18S rRNA gene sequence data are available for the phylogenetic analysis of feline Babesia species of these, very few are full or near-full-length sequences; the majority are partial 18S rDNA sequence data.
In determining the phylogenetic relationships of the Babesia species reported from domestic cats, we used near-full-length 18S rRNA Babesia gene sequences available in GenBank to perform a multiple sequence alignment using ClustalX (version 1.81 for Windows) (58). None of the B. canis, B. vogeli, or B. gibsoni sequences described from cats could be included in our analysis because of the very short 18S rRNA gene fragment sizes. The alignment was truncated to the size of the smallest sequence. The Hasegawa–Kishino–Yano (HKY + G + I) substitution model (59), determined as the best-fit model using MEGA 7 (60), was used to infer a maximum likelihood phylogenetic tree. The 18S rDNA sequences of Hepatozoon felis (AY628681) and C. felis (AF399930) were included as outgroup. All positions containing gaps and missing data were eliminated. The final data set comprised a total of 1,351 positions. Evolutionary analyses were conducted in MEGA7.
The Babesia species from cats fell into three distinct clades (Figure 1), in concordance with Schnittger et al. (61). Clade I also includes rodent-infecting B. microti and Babesia rodhaini and feline-infecting B. leo and B. felis. Clade II also includes Babesia duncani isolated from humans, canine Babesia conradae, and B. lengau described from cheetahs in South Africa. The recently described pathogenic Babesia species cat Western Cape (44) fell into clade VI (Babesia s.s.). This clade also includes dog-infecting B. gibsoni, B. canis s.s., Babesia rossi, and B. vogeli, the human-infecting Babesia venatorum, as well as species infecting ungulates (such as B. divergens and Babesia odocoilei), and Babesia species infecting other carnivores such as bears, cougars, and raccoons, as well as field rodents.
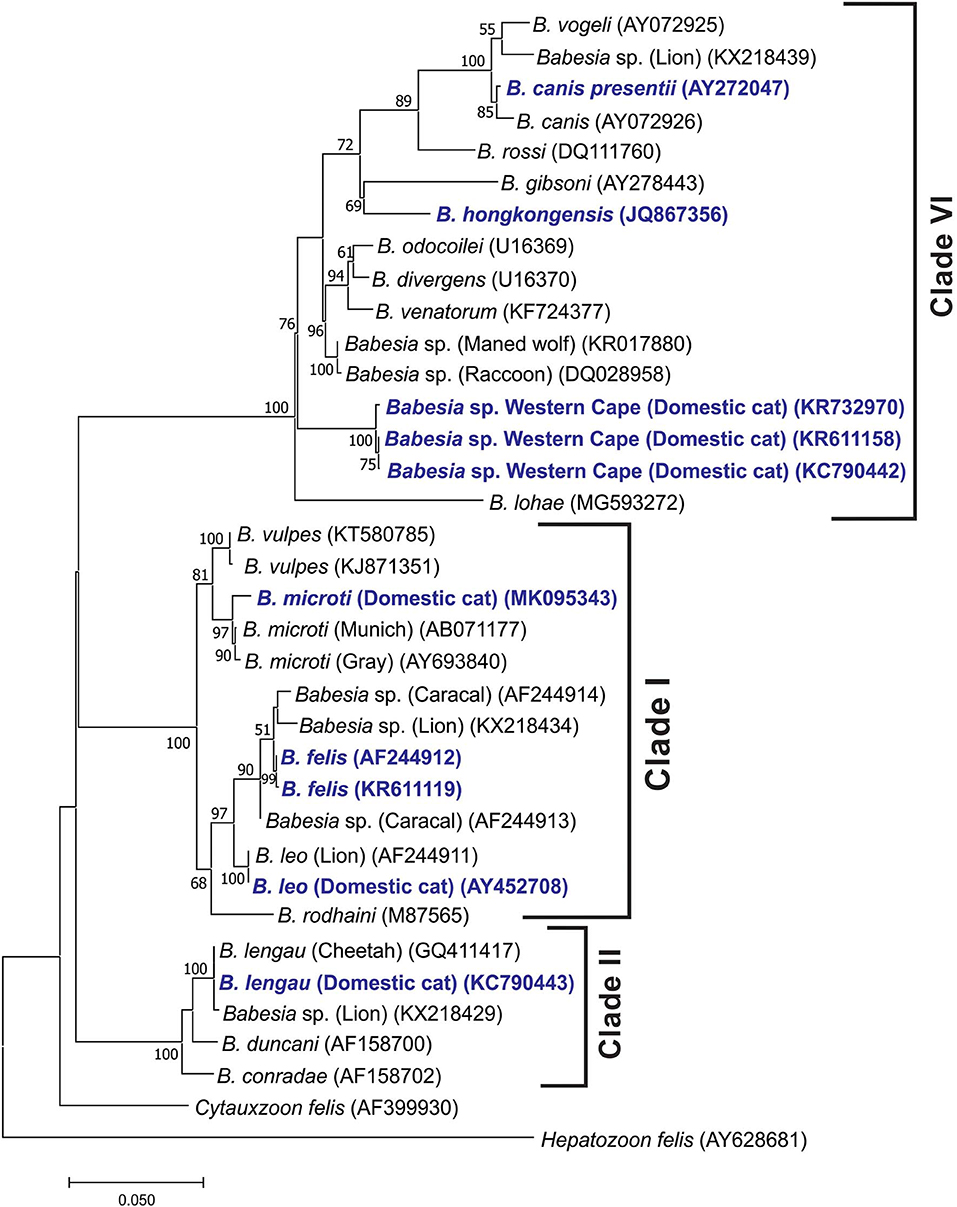
Figure 1. Maximum likelihood tree showing the evolutionary relationships of the published near-full-length Babesia 18S rDNA sequences. Babesias described from domestic cats are indicated in dark blue. Sequence accession numbers are shown in parentheses. The evolutionary history was inferred by using the maximum likelihood method based on the Hasegawa–Kishino–Yano model (HKY + G + I) substitution model (59). The tree with the highest log likelihood (−6,143.13) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood approach and then selecting the topology with superior log likelihood value. A discrete gamma distribution was used to model evolutionary rate differences among sites [five categories (+G, parameter = 0.4871)]. The rate variation model allowed for some sites to be evolutionarily invariable [(+I), 55.35% sites]. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 1,351 positions in the final data set. Evolutionary analyses were conducted in MEGA7 (60).
Our analysis placed B. hongkongensis in clade VI [also referred to as the “carnivore/rodent clade” by Schnittger et al. (61)], confirming the findings of Wong et al. (41). The feline genotype of B. canis described as a new subspecies B. canis presentii (33), grouped with B. canis within the Babesia s.s. clade. Babesia lohae grouped within the Babesia s.s. clade as described by Greay et al. (45). Babesia vulpes grouped within the B. microti group.
When a phylogenetic tree was generated from partial 18S rDNA sequence data (312 bp) to include the B. canis s.s., B. vogeli, and B. gibsoni sequences described from cats, similar groupings were found, although the branching of the clades was slightly different (Figure 2). Note that the B. canis sequences identified in cats in Spain and Portugal (27) could not be found in GenBank under the accession no. AY15057 and were therefore not included in the analysis.
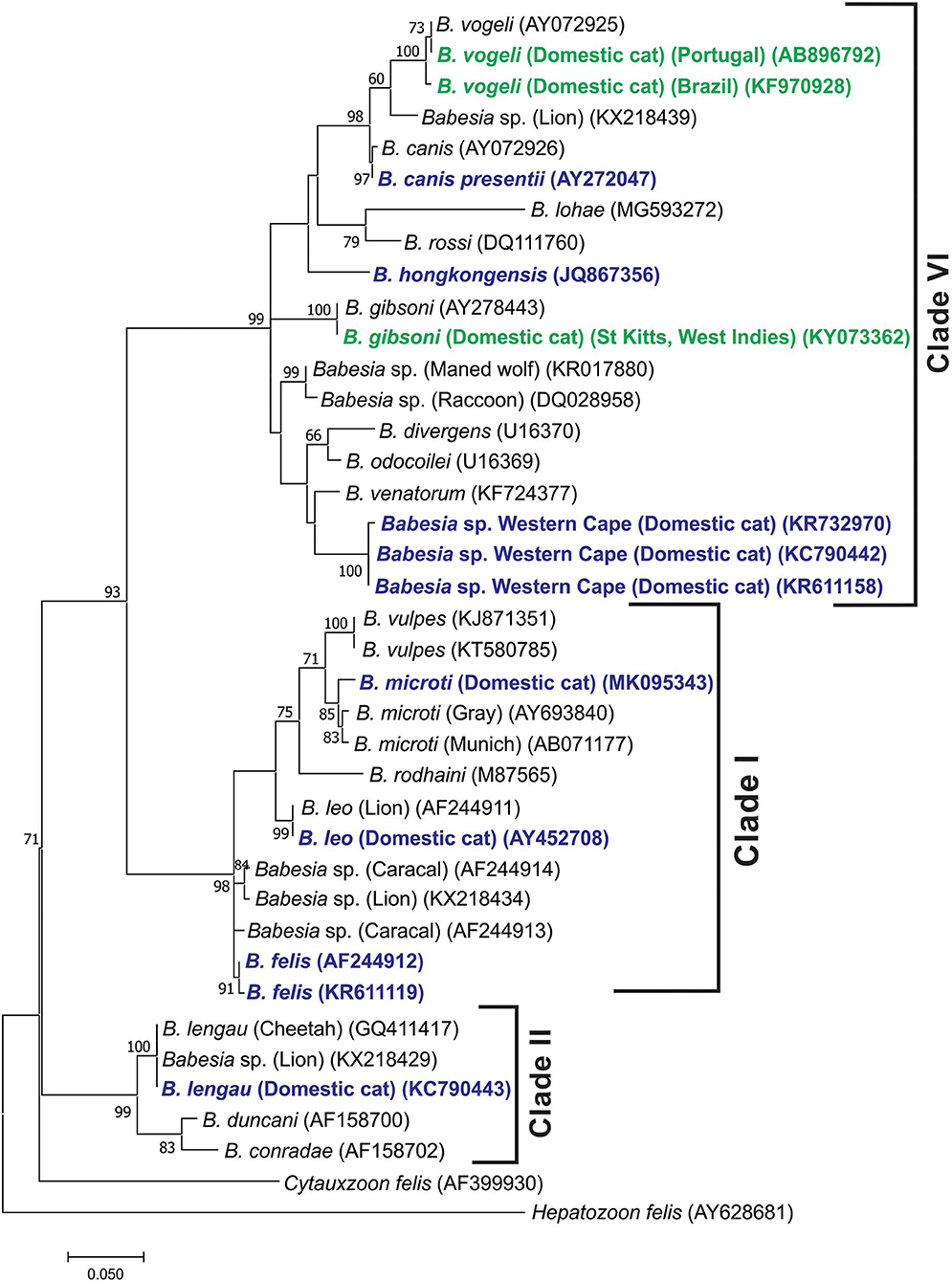
Figure 2. Molecular phylogenetic analysis showing relationships between representative B. vogeli and B. gibsoni partial 18S RNA gene sequences described from cats with the published Babesia 18S rDNA sequences. Babesias described from domestic cats are indicated in dark blue. The B. vogeli and B. gibsoni sequences described from domestic cats are indicated in dark green. Sequence accession numbers are shown in parentheses. The evolutionary history was inferred by using the maximum likelihood method based on the Tamura 3-parameter model (62). The tree with the highest log likelihood (−1,898.16) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. A discrete gamma distribution was used to model evolutionary rate differences among sites [five categories (+G, parameter = 0.3970)]. The rate variation model allowed for some sites to be evolutionarily invariable [(+I), 38.96% sites]. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. There were a total of 312 positions in the final data set. Evolutionary analyses were conducted in MEGA7 (60).
The four Babesia species known to cause disease in cats in South Africa fall in three distinct clades (61). The closely related B. felis s.s. and B. leo fall in clade I, B. lengau in clade II, and Babesia species cat Western Cape in clade VI (Babesia s.s.). In retrospect, differences in clinical manifestation, for example, presence or absence of pyrexia and response to specific treatment, reported in South African literature (1937 to 1980) suggest that more than one Babesia species was involved.
The dog-associated B. vogeli appears to be quite widespread in domestic cats, with reports from Brazil (54, 55) and St Kitts (40) in the Americas, Portugal (28, 52) in Europe, Qatar in the Middle East (38), and Thailand (49) in Asia. This is not surprising because its vector, Rhipicephalus sanguineus s.l., has a cosmopolitan distribution. Its relatively high prevalence in clinically healthy cats, for example, 16% in Brazil (54), 13% on St Kitts (40), and 8.1% in Portugal (28), suggests that immunocompetent cats can harbor the infection with no discernible untoward effects. None of 27 cats tested in South Africa was positive for B. vogeli (63). The European Dermacentor reticulatus–transmitted B. canis s.s., on the other hand, was found in only 4 (1.3%) of 320 cats in north and central Portugal (28).
Babesia rossi, which causes severe babesiosis in dogs in sub-Saharan Africa, has not been reported from cats. Specimens from 27 cats tested specifically for B. rossi were negative (63). Since Haemaphysalis elliptica, the only known vector of B. rossi, is the most prevalent and abundant tick on cats in the Western Cape Province of South Africa (64), where canine babesiosis is rife, it is not unlikely that B. rossi may be recorded in local cats. Three separate attempts at artificial transmission of B. rossi from dogs to cats were unsuccessful, however (65–67).
In the absence of easily discernible morphological differences, molecular characterization is indispensable in distinguishing between Babesia species and in identifying and describing new taxa. Molecular characterization has made it abundantly clear that referring to all piroplasms encountered in cats merely as B. felis is no longer acceptable. In view of the body of literature on the topic published since 2001, persisting in doing so is scientifically irresponsible. Reports of occurrence of B. felis s.s. and other Babesia species in domestic cats should only be accepted if they are supported by credible molecular provenance.
BP performed the literature searches and drafted the basic manuscript. MO performed the phylogenetic analyses and wrote the relevant sections.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the following for assisting us in providing references that were difficult to trace: Patrick Bourdeau, Gioia Capelli, Maria Grazia Pennisi and the staff of the Jotello F. Soga Library, Faculty of Veterinary Science, University of Pretoria.
1. Lingard A, Jennings E. A preliminary note on a piroplasmosis found in man and in some of the lower animals. Ind Med Gaz. (1904) 39:161–5.
2. Jacobson LS, Schoeman T, Lobetti RG. A survey of feline babesiosis in South Africa. JS Afr Vet Assoc. (2000) 71:222–8. doi: 10.4102/jsava.v71i4.719
3. Prugnolle F, Durand P, Neel C, Ollomo B, Ayala FJ, Arnathau C, et al. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc Nat Acad Sci USA. (2010) 107:1458–63. doi: 10.1073/pnas.0914440107
4. Boundenga L, Ollomo B, Rougeron V, Mouele LY, Mve-Ondo B, Delicat-Loembet LM, et al. Diversity of malaria parasites in great apes in Gabon. Mal J. (2015) 14:111. doi: 10.1186/s12936-015-0622-6
5. Penzhorn BL, Kjemtrup AE, López-Rebollar LM, Conrad PA. Babesia leo n. Sp. from lions in the Kruger National Park, South Africa, and its relation to other small piroplasms. J Parasitol. (2001) 87:681–5. doi: 10.1645/0022-3395(2001)087[0681:BLNSFL]2.0.CO;2
6. Davis LJ. On a piroplasm of the Sudanese wild cat (Felis ocreata). Trans R Soc Trop Med Hyg. (1929) 22:523–34. doi: 10.1016/S0035-9203(29)90042-0
7. Carpano M. Sur les Piroplasmoses des Carnassiers et sur un Nouveau Piroplasme des Félins Babesiella felis Chez le Puma: Felis concor (sic). Bull. 137. Cairo: Min Agric (1934).
8. Patton WS. Preliminary report on a new piroplasm (Piroplasma gibsoni sp. Nov.) Found in the blood of the hounds of the Madras Hunt and subsequently discovered in the blood of the jackal Canis aureus. Bull Soc Pathol Exot. (1910) 3:274–81.
10. Mangrulkar MV. On a piroplasm of the Indian cat (Felis domesticus). Ind J Vet Sc Anim Husb. (1937) 7:243–6.
11. Mudaliar SV, Achary G., Alwar VS. On a species of Babesia in an Indian wild cat (Felis catus). Ind Vet J. (1950) 26:391–5.
12. Dennig HK. An unknown babesia species in the Jaguarundi (Herpailurus yaguarondi). Small Animal Prac. (1967) 12:146–50.
13. Dennig HK. Babesia infections in exotic cats and the importance of these blood parasites for veterinary research. Acta Zool Pathol Antverp. (1969) 48:361–7.
14. Dennig HK, Brocklesby DW. Babesia pantherae sp. nov., a piroplasm of the leopard (Panthera pardus). Parasitol. (1972) 64:525–32. doi: 10.1017/S0031182000045595
15. Dennig HK, Hebel R. Light and electron microscopic investigations on two Babesia species of the felids. Z parasite. (1969) 32:95–111. doi: 10.1007/BF00259972
16. Jackson C, Dunning FJ. Biliary fever (Nuttalliosis) of the cat: a case in the Stellenbosch District. JS Afr Vet Med Assoc. (1937) 8:83–8.
19. Futter GJ, Belonje PC. Studies on feline babesiosis. Clinical observations. JS Afr Vet Assoc. (1980) 51: 143–6.
20. Penzhorn BL, Stylianides E, Coetzee MA, Viljoen JM, Lewis BD. A focus of feline babesiosis at Kaapschehoop on the Mpumalanga escarpment. JS Afr Vet Assoc. (1999) 70:60. doi: 10.4102/jsava.v70i2.755
21. Stewart CG, Hackett KJW, Collett MG. An unidentified Babesia of the domestic cat (Felis domesticus). JS Afr Vet Assoc. (1980) 51:219–22.
22. Leger N, Ferte H, Berthelot P, Nourry D, Brocvielle P. Un cas de babésiose féline en Haut-Saône, France. Sci Vet Méd Comp. (1992) 94:249–52.
24. Fritz D, Derré G. A case of babesiosis due to Babesia annae in a cat. Summ Anim Comp. (2011) 5:61–5.
25. Moik K, Gothe R. Babesia infections of the felids: a case description in a cat in Germany. Vet Prac. (1997) 25:532–5.
26. Adaszek L, Winiarczyk S, Lukaszewska J, Heile C. Feline babesiosis. Small Animal Prac. (2010) 55:624–34.
27. Criado-Fornelio A, Martinez-Marcosa A, Buling-Saraña A, Barba-Carretero JC. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. (2003) 93:307–17. doi: 10.1016/S0378-1135(03)00044-0
28. Vilhena H, Martinez-Díaz VL, Cardoso L, Vieira L, Altet L, Francino O, et al. Feline vector-borne pathogens in the north and center of Portugal. Par Vect. (2013) 6:99. doi: 10.1186/1756-3305-6-99
29. Bendangla C, Varshney JP. Babesiosis in a domestic kitten - a clinical report. J Vet Parasitol. (2006) 20:103–4.
30. Sabu L, Sreekrishnan R, Devada K, Rejitha TS, Lakshmanan B. Babesia species in a cat - a case report. J Vet Parasitol. (2013) 27:68–9.
31. Ahmad SS, Khan MS, Ahmad N. Prevalence of babesiosis in cats in Lahore, Pakistan. J. Anim. Plant Sci. (2011) 21 (Suppl. 2):354–7.
32. Suliman EG. Detection the infection with Babesia spp., Cytauxzoon felis and Haemobartonella felis in stray cats in Mosul. Iraqi J Vet Sci. (2009) 23 (suppl. 1):En49–En55.
33. Baneth G, Kenny MJ, Tasker S, Anug Y, Shkap V, Levy A, et al. Infection with a proposed new subspecies of Babesia canis, Babesia canis subsp. presentii, in domestic cats. J Clin Microbiol. (2004) 42:99–105. doi: 10.1128/JCM.42.1.99-105.2004
34. Greay TL, Zahedi A, Krige AS, Owens JM, Rees RL, Ryan UM, et al. Response to the Letter to the Editor by Harris. Par Vect. (2019) 12:178. doi: 10.1186/s13071-019-3439-2
35. Potgieter FT. Chemotherapy of B. felis infection: efficacy of certain drugs. JS Afr Vet Assoc. (1981) 52:289–93.
36. Penzhorn BL, Lewis BL, López-Rebollar LM, Swan GE. Screening of five drugs for efficacy against Babesia felis in experimentally infected cats. JS Afr Vet Assoc. (2000) 71:53–7. doi: 10.4102/jsava.v71i1.678
37. Potgieter FT, Els HJ. The fine structure of intra-erythrocytic stages of Babesia bigemina. Onderstepoort J Vet Res. (1977) 44:157–68.
38. Alho AM, Lima C, Latrofa MS, Colella V, Ravagnan S, Capelli G, et al. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Par Vect. (2017) 10:298. doi: 10.1186/s13071-017-2237-y
39. Salim MA, Akhtar R, Lateef M, Rashid MI, Akbar H, Shehzad W, et al. First report on optimization of loop-mediated isothermal amplification (LAMP) for the diagnosis of Babesia felis. Ind J Anim Res. (2018) 52:401–4. doi: 10.18805/ijar.B-567
40. Kelly PJ, Köster L, Li J, Zhang J, Huang K, Branford GM, et al. Survey of vector-borne agents in feral cats and first report of Babesia gibsoni in cats on St Kitts, West Indies. BMC Vet Res. (2017) 13:331. doi: 10.1186/s12917-017-1230-1
41. Wong SSY, Poon RSW, Hui JJY, Yuena KY. Detection of Babesia hongkongensis sp. nov in a free-roaming Felis catus cat in Hong Kong. J Clin Microbiol. (2012) 50:2799–803. doi: 10.1128/JCM.01300-12
42. Bosman AM, Oosthuizen MC, Peirce MA, Venter EH, Penzhorn BL. Babesia lengau sp. nov., a novel Babesia species in cheetah (Acinonyx jubatus, Schreber, 1775) populations in South Africa. J Clin Microbiol. (2010) 48:2703–2708. doi: 10.1128/JCM.02266-09
43. Bosman AM, Oosthuizen MC, Venter EH, Steyl JCA, Gous TA, Penzhorn BL. Babesia lengau associated with cerebral and haemolytic babesiosis in two domestic cats. Par Vect. (2013) 6:128. doi: 10.1186/1756-3305-6-128
44. Bosman AM, Penzhorn BL, Brayton KA, Schoeman T, Oosthuizen MC. A novel Babesia sp. associated with clinical signs of babesiosis in domestic cats in South Africa. Par Vect. (2019) 12:138. doi: 10.1186/s13071-019-3395-x
45. Greay TL, Zahedi A, Krige AS, Owens JM, Rees RL, Ryan UM, et al. Endemic, exotic and novel apicomplexan parasites detected during a national study of ticks from companion animals in Australia. Par Vect. (2018) 11:197. doi: 10.1186/s13071-018-2775-y
46. Spada E, Proverbio D, Galluzzo P, Perego R, Giorgi GBD, Roggero N, et al. Frequency of piroplasms Babesia microti and Cytauxzoon felis in stray cats from northern Italy. BioMed Res Int. (2014) 2014:943754. doi: 10.1155/2014/943754
47. Pennisi MG, Alongi A, Agnone A, Vitale F, Reale S, Torina A. Cats as reservoir of Babesia microti. Parassitologia. (2007) 49 (Suppl 1):100.
48. Akram IN, Parveen T, Abrar A, Mehmood AK, Iqbal F. Molecular detection of Babesia microti in dogs and cat blood samples collected from Punjab (Pakistan). Trop Biomed. (2019) 36:304–9.
49. Simking P, Wongnakphet S, Stich RW, Jittapalapong S. Detection of Babesia vogeli in stray cats of metropolitan Bangkok, Thailand. Vet Parasitol. (2010) 173:70–5. doi: 10.1016/j.vetpar.2010.06.025
50. Passos LMF, Geiger SM, Ribeiro MFB, Pfister K, Zahler-Rinder M. First molecular detection of Babesia vogeli in dogs from Brazil. Vet Parasitol. (2005) 127:81–5. doi: 10.1016/j.vetpar.2004.07.028
51. Jittapalapong S, Jansawan W. Preliminary survey on blood parasites of cats in Bangkhen District Area. Type of tape. J Nat Sci. (1993) 27:330-5.
52. Vilhena H, Tvarijonaviciute A, Ceron JJ, Vieira L, Pastor J, Silvestre-Ferreira AC. Acute phase proteins response in cats naturally infected with Hepatozoon felis and Babesia vogeli. Vet Clin Path. (2017) 46:72–6. doi: 10.1111/vcp.12451
53. Maia C, Ramos C, Coimbra M, Bastos F, Martins A, Pinto P, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Par Vect. (2014) 7:115. doi: 10.1186/1756-3305-7-115
54. André MR, Denardi NCB, de Sousa KCM, Goncalves LR, Henrique PC, Ontivero CRGR, et al. Arthropod-borne pathogens circulating in free-roaming domestic cats in a zoo environment in Brazil. Ticks Tick Borne Dis. (2014) 5:545–51. doi: 10.1016/j.ttbdis.2014.03.011
55. Malheiros J, Costa MM, do Amaral RB, de Sousa KCM, Andre MR, Machado RZ, et al. Identification of vector-borne pathogens in dogs and cats from Southern Brazil. Ticks Tick Borne Dis. (2016) 7:893–900. doi: 10.1016/j.ttbdis.2016.04.007
56. Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. (2000) 89:241–8. doi: 10.1016/S0304-4017(00)00202-8
57. Baneth G, Cardoso l, Brilhante - Simões P, Schnittger L. Establishment of Babesia vulpes n. Sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Par Vect. (2019) 12:129. doi: 10.1186/s13071-019-3385-z
58. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. (1997) 25:4876–82. doi: 10.1093/nar/25.24.4876
59. Hasegawa M, Kishino H, Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J Mol Evol. (1985) 22:160–74. doi: 10.1007/BF02101694
60. Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. (2016) 33:1870–4. doi: 10.1093/molbev/msw054
61. Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. (2012) 12:1788–809. doi: 10.1016/j.meegid.2012.07.004
62. Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol Biol Evol. (1992) 9:678–87.
63. Bosman AM, Venter EH, Penzhorn BL. Occurrence of Babesia felis and Babesia leo in domestic cats and various wild felid species in Southern Africa, based on reverse line blot analysis. Vet Parasitol. (2007) 144:33–8. doi: 10.1016/j.vetpar.2006.09.025
64. Horak IG, Matthee S. Parasites of domestic and wild animals in South Africa. XLIII. Ixodid ticks of domestic dogs and cats in the Western Cape Province. Onderstepoort J Vet Res. (2003) 70:187–95. Available online at: http://hdl.handle.net/2263/17746
65. Robertson W. Malignant jaundice in the dog. J Comp Path Therap. (1901) 14:327–36. doi: 10.1016/S0368-1742(01)80065-5
66. Nuttall GHF, Graham-Smith GS. Note on attempts to infect the fox and the jackal with Piroplasma canis. Parasitol. (1909) 2:212–4. doi: 10.1017/S0031182000001670
Keywords: Babesia felis, Babesia leo, Babesia lengau, Babesia canis presentii, Babesia hongkongensis, Babesia species cat Western Cape, Babesia vogeli, feline babesiosis
Citation: Penzhorn BL and Oosthuizen MC (2020) Babesia Species of Domestic Cats: Molecular Characterization Has Opened Pandora's Box. Front. Vet. Sci. 7:134. doi: 10.3389/fvets.2020.00134
Received: 19 December 2019; Accepted: 24 February 2020;
Published: 27 March 2020.
Edited by:
Simona Gabrielli, Sapienza University of Rome, ItalyReviewed by:
David Peterson, University of Georgia, United StatesCopyright © 2020 Penzhorn and Oosthuizen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barend L. Penzhorn, YmFuaWUucGVuemhvcm5AdXAuYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.