- 1Department of Animal Biosciences, University of Guelph, Guelph, ON, Canada
- 2Department of Integrative Biology, University of Guelph, Guelph, ON, Canada
Maternal stress can affect the offspring of birds, possibly due to hormone deposition in the egg. Additionally, phenotypic diversity resulting from domestication and selection for productivity has created a variety of poultry lines that may cope with stress differently. In this study, we investigated the effects of maternal stress on the behavior of different strains of laying hens and the role of corticosterone as its mediator. For this, fertilized eggs of five genetic lines—two brown (Brown 1 and 2), two white (White 1 and 2), and one pure line White Leghorn—were reared identically as four flocks of 27 birds (24F: 3M) per strain. Each strain was equally separated into two groups: Maternal Stress (“MS”), where hens were subjected to a series of daily acute psychological stressors for 8 days before egg collection, and “Control,” which received routine husbandry. Fertile eggs from both treatments were collected at three different ages forming different offspring groups that were treated as replicates; additional eggs from Control were injected either with corticosterone diluted in a vehicle solution (“CORT”) or just “Vehicle.” Eggs from each replicate were incubated and hatched, and offspring (N = 1,919) were brooded under identical conditions. To measure the effects of maternal stress on anxiety and fear-like behavior, offspring were subjected to a social isolation test (SI) between 5 and 10 days of age and a tonic immobility test (TI) at 9 weeks of age. Compared to Control, MS decreased the number of distress vocalizations emitted by White 2 in SI. No effects of MS were observed in TI, and no effects of CORT were observed in any tests. Overall, brown lines vocalized more in SI and remained in TI for a longer duration than white strains, suggesting genetic differences in fear behavior. Females vocalized more than males in TI and showed a trend toward significance for the same trait in SI. Overall, results suggest that the effects of maternal stress on fearfulness are not directly mediated by corticosterone. Moreover, it highlights behavioral differences across various strains of laying hens, suggesting that fear responses are highly dependent on genotype.
Introduction
Maternal stress can impact offspring physiology, behavior, and cognition (1–3). Its effects are highly dependent on the intensity, timing of exposure, and type of stressor experienced by the mother (4–6). More specifically, impacts on offspring behavior are evident across taxa [avian (7); mammals (1, 8); reptiles (9)], and at the neurological scale, maternal stress has been linked to structural and functional changes in the limbic system and prefrontal cortex of rats (1), and to changes in gene expression in the hypothalamus of chickens (10). These brain areas are involved in the mediation of fear and anxiety, social and cognitive processes, and working memory of mammals and birds (11, 12). Maternal stress may have long-term impacts on how an animal responds to its environment. For example, in laying hens, females subjected to an unpredictable food restriction schedule had chicks that stayed longer in tonic immobility (TI), a measure of fearfulness, and were less competitive for access to food in a novel environment than the offspring of control birds (13). Similarly, the offspring of female quails stressed during egg production displayed more anxiety-like behaviors, such as an increased occurrence of distress calls during emergence and open field tests, and when isolated from conspecifics (14).
Cottrell and Seckl (15) proposed two major hypotheses to explain the association between maternal stress and postnatal effects on offspring: fetal malnutrition and overexposure to glucocorticoid hormones. More recently, studies in avian species have shown that maternal stress can also be linked to the increase in other biological components in the egg, such as androgens (16), thyroid hormones (17), antioxidants (18), and immunoglobulins (19). Nevertheless, although glucocorticoid hormones are not a synonym for “stress” (20), corticosterone remains as one of the most analyzed mediators of maternal stress in the literature due to their pleiotropic role in regulating physiological responses to the environment and in the development and maturation of vital organs [reviewed in (6, 16, 21)]. Moreover, the hypothalamus–pituitary–adrenal (HPA) axis of chickens, responsible for corticosterone production, becomes functional between the 14 and 16th day of incubation and might also be affected by maternal hormone deposition (22). The effects of corticosterone on the behavior of the offspring are, however, inconsistent and appear to depend on delivery method and species, possibly being related only to metabolic and developmental processes (23). For example, although corticosterone injections into fertile eggs and implants to female chickens were linked to an increase in the duration of TI in the offspring (24, 25), injections in yellow-legged gulls had no effects in the same test (26). Moreover, corticosterone injections decreased the offspring's ability to learn (27), compete for a wormlike object (24), and increased aggressiveness (28) in layer chickens.
As evidenced above, two experimental models are commonly used to increase corticosterone levels in the egg: a maternal model in which the adult female is exposed to stressors (either directly or through corticosterone injections or implants) and a pharmacological model that manipulates the egg. The maternal model might be considered more holistic as hormone or stress treatments integrate with other maternal elements that might also affect embryonic development (29). However, it precludes a specific control of the quantity of hormone reaching the embryo (30). Conversely, egg manipulation allows the study of exposure to an exact dose of specific hormone but relies on the use of an invasive injection procedure that can be harmful to the embryo (31, 32). Furthermore, hormonal responses are generally dose-dependent, and the actual concentrations deposited by the mother into the egg during development remain unknown (16, 33).
Similarly unknown is the relationship between maternal stress and genetics. Although no previous studies have tested multiple strains of commercial layers simultaneously, the levels of susceptibility to maternal stress may vary across different genotypes. A positive correlation between the concentration of corticosterone in layer breeders and the occurrence of an anxiety-like behavior in the offspring was observed in a white genetic hybrid but not in a brown hybrid (34). Furthermore, it has been found that adult brown and white strains of laying hens have distinct behavioral and physiological responses to stress (34–36); and comparisons between offspring of White Leghorns and their ancestor, the red jungle fowl, revealed that in response to maternal stress, only the White Leghorn chickens displayed decreased learning abilities and differences in gene expression in the hypothalamus and pituitary, suggesting that genetic selection may have increased maternal stress susceptibility (37).
The main goal of this study was to investigate whether the effects of maternal stress on offspring fear- and anxiety-like behavior differ across genetic lines of laying hens. For this, five strains of breeder hens were subjected to two stress models: one that involved subjecting the breeders to acute psychological stressors and another that involved egg injections of corticosterone. Using these treatments, we sought to decouple the role of corticosterone from the broader maternal milieu during maternal stress. We predicted that injections would affect all strains, acting as a positive control treatment regardless of genetics, and that the effects of Maternal Stress would vary according to the natural stress susceptibility of each strain.
Materials and Methods
The birds used in this study were treated in accordance with the Canadian Council on Animal Care, and all procedures were approved by the University of Guelph Animal Care Committee (Animal Utilization Protocol #1946). All the strains presented herein were anonymized as required by the genetics companies that donated the parent stock.
Parent Stock: Management
A total of 2,600 fertilized eggs of five strains of parent stock were provided by two commercial genetics companies (Brown 1 and White 1 from company 1; Brown 2 and White 2 from company 2; each company donated 360 female line eggs and 64 male eggs per strain) and the University of Guelph's Arkell Poultry Research Station (pure line White Leghorn). To guarantee similar experiences, eggs from all strains were collected from grandparent hens that were between 40 and 50 weeks of age. Eggs and chicks were subjected to identical incubation and husbandry conditions, as previously described (38). Chicks were wing banded at hatch, and each strain was equally distributed into 4 parent flocks that were placed in 2 rooms containing 10 pens of 27 birds (24 females and 3 males) each (see Supplementary Figure 1). Pens (3.7 m2) were enriched with pine shavings, one elevated perch and one lower perch, totaling a perch space of 12.8 cm/bird/pen. At 18 weeks, five nest boxes were added to each one of the pens. Chickens from different pens were visually separated from each other and did not interact at any moment. Apart from routine husbandry, all human interaction was avoided to prevent possible habituation.
Parent Stock: Experimental Design
Treatments and egg collection were performed at 32, 52, and 72 weeks of age. To form the offspring groups, equal numbers of fertile eggs (sampled over time, preference given to recent over old) from all parent flocks were incubated 1 day after the end of stressors, and the offspring flocks from each maternal age were treated as replicates (Figure 1). This experimental design allowed us to work with a larger sample size, but it also resulted in replicates confounded with incubatory settings, chick transfer and placement from the incubator to pens, and egg composition, since the nutritional value of the egg changes as a hen ages (39).
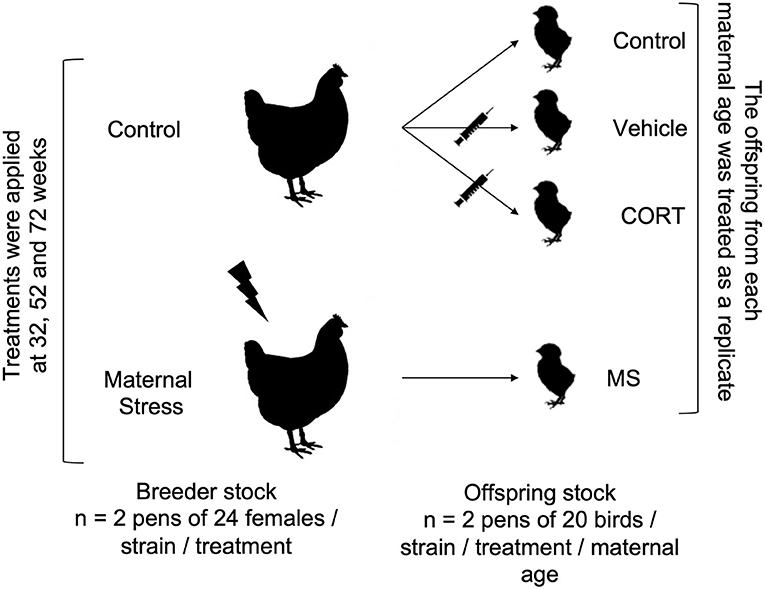
Figure 1. Experimental design. Treatments (Control and Maternal Stress) were applied to each strain (Commercial Brown 1 and 2, Commercial White 1 and 2, and Pure Line White Leghorn) of the breeder flocks at three different ages (32, 52, and 72 weeks). The offspring of each maternal age were statistically treated replicates.
Parent Stock: Control and Maternal Stress Treatments
Each flock of breeders was randomly assigned to either Control or Maternal Stress (“MS”) treatments with two replicate flocks per strain and treatment. Regular husbandry was strictly adhered to for the Control groups, while the females of the MS flocks were subjected to daily sessions of acute psychological stress procedures that were selected based on their ability to increase plasma corticosterone concentration in avian species (see references for each test and species below). Since the average time window for egg production from the beginning of vitellogenesis until laying is 8 days, each MS flock received a minimum of 8 consecutive days of stressors before the beginning of egg collection.
Hens from the MS flocks were subjected to each of the following procedures: (1) Hens were equally distributed into two plastic crates (89 cm long × 60 cm wide × 26 cm high; 12 hens/crate), followed by 15 min of transportation [Figure 2A, laying hen: (40)]. (2) Hens were individually removed from their home pens and placed inside a cloth bag located in a nearby room for 10 min of physical restraint [Figure 2B, laying hen: (41)]. (3) Hens were crated into two groups of 12 birds, transported to an empty room 400 m away from their home pen and transferred to a test arena (100 cm long × 100 cm wide × 200 cm high) constructed of solid white panels with two doors located on opposite walls and two LED lights on the ceiling for 30 min. In the arena, hens were exposed to three simulations of a predator attack (30 s/each) using the silhouette of a sparrow-hawk made of black cardboard (35 cm long × 50 cm wide) [Figures 2C,D, great tit: (42)]. (4) Hens were crated and transported to the test arena for 15 min. An air horn was blown for 3 s at 5-min intervals [Japanese quail: 14; European starling: (43)]. (5) Hens were crated and transported to the test arena for 30 min with a different strain [laying hen: (44)]. All birds were immediately returned to their home pens after each stress session. Overall, sessions respected the following criteria: (1) Flocks were subjected to one stressor a day. (2) Stressors and egg collection were performed until the total number of eggs necessary for incubation had been collected. (3) To avoid a decrease in the physiological response to stressors due to repeated exposure, the minimum interval between the application of the same stressor was 4 days. (4) Sessions ran randomly from 9:00 to 16:00 h.

Figure 2. Stressors used in the MS treatment. (A) Breeder hens were crated and transported around the research facility for 15 min. (B) Physical restraint in a cloth bag. (C) The silhouette of a sparrow-hawk made of black cardboard (35 cm long × 50 cm wide). (D) Layer breeders were transferred to a test arena (100 cm long × 100 cm wide × 200 cm high); three simulations of a predator attack were performed (All photos used with permission of subjects).
Parent Stock: Vehicle and CORT Treatments
The CORT treatment aimed to increase the concentration of corticosterone in fertilized eggs from breeder hens. According to previous studies, the basal level of corticosterone in laying hens ranges from 0.3 to 5 ng/ml (45), reaching 30 ng/ml in response to stress (46). The concentration of corticosterone in egg yolks has been previously reported to range from 0.77 to 2.8 ng/g in Hy-Line Brown (47–49) to an average of 1.6 ng/g in Hy-Line White (47) and 2.13 ng/g in Bovan White (50) under control conditions. The mean concentration of corticosterone in eggs from unstressed birds has been previously reported as 1.17 in yolk and 1.55 ng/ml albumen (51). However, analytical validation of enzyme- and radio-immunoassay techniques showed the presence of cross-reactive substances that hamper the quantification of corticosterone in the yolk and albumen of eggs (52). Furthermore, recent work has shown that even when more precise techniques such as Celite or HPLC are conducted, they may not be sufficient [reviewed in (16)]. Therefore, since the exact concentration of corticosterone in eggs remains unknown, we followed the methodology proposed by Janczak et al. (32) and modified by Peixoto et al. (38), which was based on plasma corticosterone concentration of hens. Injections of 10 ng/ml cortisol diluted in sesame (CORT treatment) or sesame oil alone (Vehicle treatment) were used. In preparation for this procedure, a layer of approximately 0.5 mm of silicone sealant (General Electric, Boston, MA) was smeared on the basal tip of the shell (2 cm long × 1 cm wide) of a subsample of Control eggs 1 day before egg incubation; this sealant would help prevent gas exchange and contamination following perforation and injection through the shell. On the morning of each incubation day, Vehicle and CORT solutions were prepared. The average weight of egg content, which is estimated to be 90% of total egg weight (53), was 50, 50, and 59 g per hen age group; thus, a volume of 50 μl of either CORT or Vehicle solutions were injected into eggs from 32- to 52-week-old breeders, while 60 μl was injected into eggs from 72-week-old breeders. Injections were performed using a sterile 23-gage needle through a small hole that was perforated through the silicone layer using an egg piercer. Eggs from all treatments were immediately incubated.
Offspring Stock: Management and Data Collection
Egg collection, incubation, and hatch occurred under similar conditions for all offspring groups. Chicks from each maternal age were individually wing-banded at hatch. The placement of chicks from each strain and treatment was randomized across rooms 40 pens equally distributed in four rooms (see Supplementary Table 1 and Supplementary Figures 2–4). Each pen (3.72 m2) was enriched with a perch (length: 155 cm) and litter floor. Each replicate in time aimed to comprise two pens with 20 birds each (10 female: 10 male) per treatment and strain; however, final densities varied due to lower hatchability of injected eggs (38). The test orders for the procedures described below were balanced across the period of the day for all flocks, strains, and treatments to minimize the effects of time and circadian rhythm on the results.
Offspring Stock: Social Isolation (SI)
The social separation of young chicks from their conspecifics produces an increase in distress vocalizations and stress-induced analgesia (54), allowing for the measurement of anxiety-related behaviors. Following the methodology proposed by Sufka et al. (55), chicks between 5 and 10 days of age (N = 701; Table 1) were individually placed into a squared soundproof box (63.5 cm high × 63.5 cm deep × 63.5 cm wide) where their vocalizations were recorded. The box was constructed of solid panels, covered with acoustic fabric, and equipped with five LED lights and a microphone taped on the ceiling for recordings (Figures 3A,B). SI lasted 5 min and was conducted from 08:00 to 12:00 h and from 14:00 to 18:00 h in a quiet room nearby the chicks' home pen. Distress calls were recorded, saved as an MPEG-4 file using the Voice Memos application (Apple, Cupertino, USA). The total number of distress calls emitted by the chicks were counted by three observers blind to treatment using WavePad (NCH Software, Greenwood Village, USA).
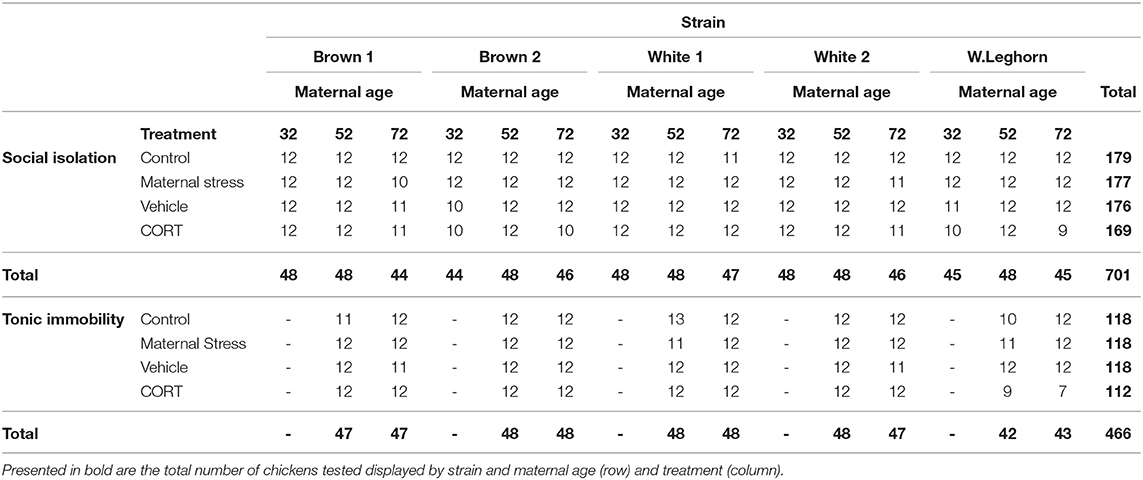
Table 1. Number of chickens tested by treatment, strain, and maternal age (weeks) in the social isolation and the tonic immobility tests.
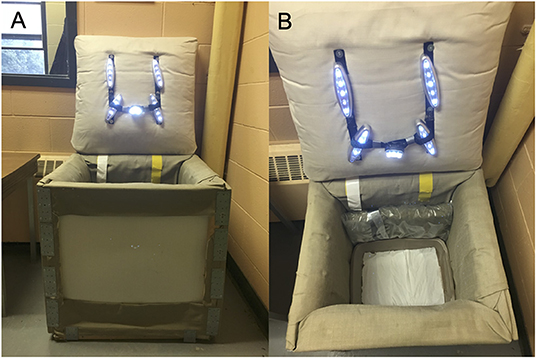
Figure 3. (A) Soundproof box (63.5 cm high × 63.5 cm deep × 63.5 cm wide) used in the social isolation test. (B) The box was constructed of wood panels, covered with acoustic fabric, and equipped with five LED lights and a microphone taped on the ceiling.
Offspring Stock: Tonic Immobility (TI)
A modified version of the TI methodology proposed by Jones (56) was used to measure fear in chickens at 9 weeks of age (N = 466; Table 1). Chickens were individually caught, moved into a quiet nearby room, and placed on their back in a V-shaped cradle, where the experimenter gently applied pressure on their sternum (57). If immobility lasted a minimum of 10 s, it was considered a successful induction. If not, up to three consecutive attempts at induction were performed. Each test lasted 10 min or until the bird stood up. Data were collected only from the offspring of hens of 52 and 72 weeks. Testing was conducted from 09:00 to 12:00 h and 13:00 to 16:00 h, and the procedure was recorded using a camcorder (Panasonic HC-V180K) that had been positioned perpendicularly to the cradle. Behavior was analyzed from videos and included duration until the bird rights itself up, the number of vocalizations emitted during the test, and the number of inductions needed to attain a successful induction. Data were analyzed by two trained observers blind to treatment.
Although the term TI implies in a state of reduced responsiveness that includes suppressed vocal behavior and intermittent periods of eye closure and muscle tremors in the extremities (58), different responses can be observed throughout the test (e.g., vocalization and head movement). As described by Rovee and Luciano (59), TI can be classified in three stages: In stages 1 and 2, distress calls can be emitted and eyes are either open or with occasional fluttering eyelids. Whereas, in stage 3, complete eye closure, no vocalizations, head bobbing, and occasional generalized body twitches are observed. Since these behaviors may vary in response to different methodologies [which can affect the validity of the test (57, 60)], data for the duration of stage 3 of TI, which will specifically be referred to as “3rd stage of TI” throughout the text, were separately recorded and analyzed.
Statistical Analyses
The Glimmix procedure of SAS 9.4 (SAS Institute, Cary, NC) was used to perform all statistical analyses. The basic statistical model in ANOVA included fixed effects of treatment (Control, MS, Vehicle and CORT), strain (Brown 1 and 2, White 1 and 2, and White Leghorn), sex, and a treatment by strain interaction. Random effects included maternal age (32, 52, and 72 weeks) and pen (10 pens) nested in room (4 rooms), with offspring bird as the experimental unit. Further pre-planned comparisons included treatment (Control vs. MS, Control vs. Vehicle, and Control vs. CORT) and white vs. brown strains. Tests for normality included Shapiro–Wilk and Anderson Darling measurements in conjunction with visual plots. When a significant strain by treatment interaction was found, analyses controlled for the multiple testing error using the percentage of false positives, which estimates the false discovery rate [FDR (61)]. Significance was declared at P < 0.05. Reliability between observers (all blind to treatment) was calculated using Kendall's Tau-b coefficient. Kendall's τ score of 1.0 is considered a perfect relationship, and a score of 0.7 is considered acceptable (62). Consequently, scores reported for SI (Kendall's τ = 0.93; P < 0.001) and duration of TI (Kendall's τ = 0.82; P < 0.001) indicate agreement among observers.
Social Isolation
The SI data were subjected to the basic model and log-normally transformed to meet the assumption of a normal distribution of residuals. Significance post-FDR correction was set at P < 0.005 and followed by a power analysis (alpha = 0.005). Least square (LS-) means and standard error of means (SEM) were back-transformed and are presented in the results as the average of distress vocalizations.
Tonic Immobility
The duration of immobility and number of vocalizations were subjected to the basic statistical model in ANOVA. To meet the assumption of a normal distribution of residuals, data for duration were subjected to a log-normal transformation, while vocalization data were transformed by the arcsine of the square root. Random effects were grouped by strain. LS-Means and standard deviation (SD) of both tests were back-transformed and are presented in the results as the average duration of TI in seconds and the average number of calls emitted during the test. The number of attempts needed for induction is presented as a percentage of birds; data were subjected to a Poisson transformation but were not normally distributed when the model included a strain by treatment interaction. Thus, a simpler statistical model containing only treatment as the fixed effect was used. Differences between LS-means were tested using a chi-square test. Due to the small number of birds induced into stage 3 of TI (n = 41), residuals for measurements of duration were not normally distributed when the model included a strain by treatment interaction. Therefore, a simpler statistical model containing only strain, treatment, and sex as fixed effects was used.
Results
Social Isolation
The number of distress calls expressed by the offspring of layer breeders was affected by strain and stress treatment (P < 0.001; Table 2). Chicks of the White 2 strain vocalized less when their mothers were subjected to MS compared to Control (P < 0.001). Similarly, MS breeders from the Brown 1, Brown 2, and White Leghorn strains produced chicks that vocalized more than White 2. Overall, brown chicks vocalized more than white (P < 0.001), and sex displayed a trend toward significance (P = 0.066), with females (125.7 ± 39.6 calls) vocalizing more than males (99.4 ± 31.3 calls).
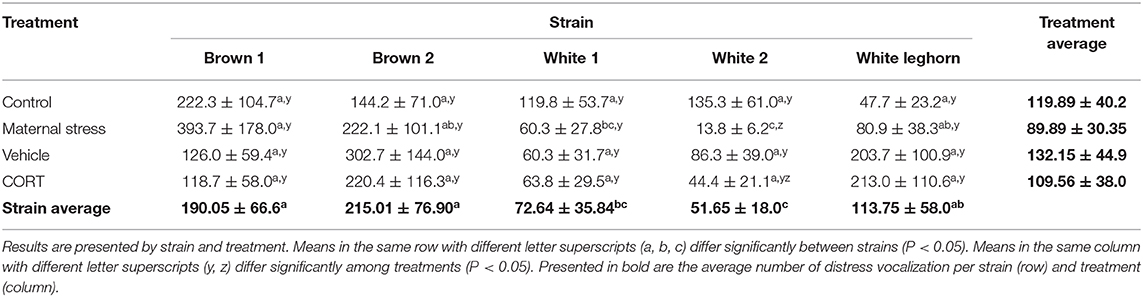
Table 2. Average number of distress vocalizations (± SEM) performed by chicks between 5 and 10 days of age during the social isolation test.
Tonic Immobility
The duration of TI in 9-week-old offspring of layer breeders was not affected by an interaction of strain by treatment (P = 0.105), treatment (P = 0.924), or sex (P = 0.643); but brown chickens stayed longer (P < 0.001) in TI than white (Figure 4). The duration of the third stage of TI was not affected by treatment (P = 0.863), strain (P = 0.701), or sex (P = 0.089).
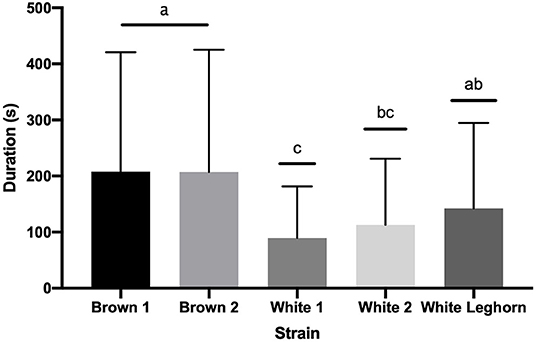
Figure 4. Duration (s) of tonic immobility displayed by strain (+ SD). Means with different letter superscripts(a−c) differ (P < 0.05).
The number of vocalizations expressed by offspring in TI was also not affected by an interaction of strain by treatment (P = 0.580) or treatment (P = 0.325). However, chickens of brown strain vocalized more (P < 0.003) than white (Figure 5), and pullets (10.2 ± 1.2 calls) vocalized more (P < 0.001) than cockerels (3.05 ± 0.7 calls).
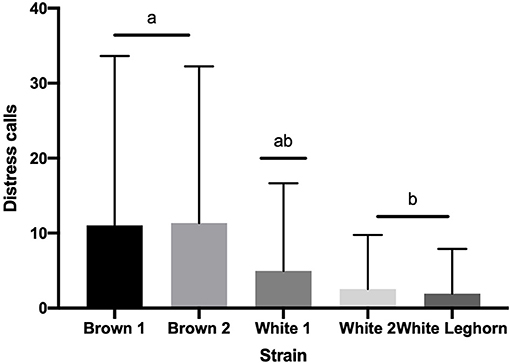
Figure 5. Number of distress calls (+SD) emitted during tonic immobility displayed by strain. Means with different letter superscripts(a, b) differ (P < 0.05).
The number of attempts needed to induce a chicken into TI was not affected by treatment (P = 0.892). More chickens from the Brown 1 strain needed a second attempt to reach TI compared to the White 1 strain (P = 0.015) (Figure 6).
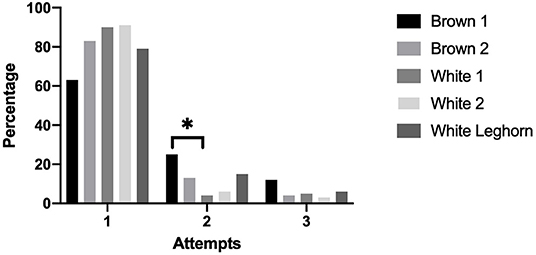
Figure 6. Percentage of chickens that were induced into tonic immobility after 1, 2, or 3 attempts displayed by strain (*P < 0.05).
Discussion
Limitations and Effects of the Stress Treatments
This study aimed to determine the effects of maternal stress on the behavior of different strains of laying hens. We hypothesized that the CORT treatment would show a clear response acting as a positive control treatment, while MS would highlight genetic differences among strains. In contrast to our hypothesis, the CORT treatment showed no effects on the behavior of the offspring and MS decreased the number of distress calls expressed by the offspring of White 2 mothers during SI but showed no differences in TI.
One limitation of this study is that the acute stressors used in the MS treatment were based on reports in the literature and not validated in our population of layer breeders, with the exception of the physical restraint test. The HPA axis activation of a subsample of layer breeders from all strains and treatment groups was tested at 75 weeks of age (N = 119). Breeders from both MS and Control treatments produced elevated concentrations of corticosterone in response to the restraint test [baseline control: 2.37 ± 0.49 ng/ml; baseline MS: 2.97 ± 0.47 ng/ml (P = 0.822); stress response control: 5.24 ± 0.55; stress response MS: 5.73 ± 0.55 (P = 0.841)] confirming that layers from the MS treatment were still physiologically responsive to restraint after repeated exposure (unpublished data). Nevertheless, we were unable to measure if this transient increase in plasma corticosterone was enough to alter the egg composition. Lastly, corticosterone has a short lifetime in chickens [~22 min (63)], and each stressor used in the study lasted a maximum of 30 min from catching until layers were returned to their home pen. Chronic stress is likely more important to signal the offspring than the short-term, acute stressors used in our experiment.
Once viewed as a successful model for testing the effects of maternal stress (6), the largely unnatural and invasive aspects of the egg injection methodology should be carefully considered. Firstly, the actual concentration of corticosterone transferred from mother to egg remains unknown (52, 64), may differ across strains (47), and can potentially overwhelm the embryo if outside of the physiological range of eggs. Indeed, as published in Peixoto et al. (38), the average hatchability for the control treatment of this study was 83%, whereas hatchability for the vehicle and control treatments were 38 and 25%, respectively. The decrease in hatchability in the vehicle treatment suggests that mechanical damage such as puncturing and disrupting eggshell membranes (which might increase the chances of pushing eggshell particles into the albumen) or the chemical composition of the vehicle affected the progeny. It is also possible that the silicone layer used to seal the hole was applied ineffectively, leaving an open hole in the shell that facilitated contamination. Lastly, the high levels of embryonic mortality in the injected groups may have created a subset of birds that were more resistant to the adverse effects of the injection, limiting the generalization of the results presented herein. Until a precise method for quantifying corticosterone in the egg and less invasive procedures are available, the efficacy of this methodology and the biological relevance of the corticosterone dosage used in the present experiment are debatable.
SI is a well-validated test that has been used as an in vivo preclinical screening of anxiolytic drugs (65, 66), which were shown to reverse distress vocalizations and pain-related behavior in chicks (67). Moreover, birds tested with and without the presence of a mirror confirmed the assumption that vocalizations increased due to an absence of conspecifics (68). In the present study, the offspring of the White 2 MS breeders vocalized less than the Control treatment of the same strain. To our knowledge, this is the first study that evaluated the effects of maternal stress on anxiety-like behavior through the SI test, and the results are congruent with those observed in quails tested in an open field test (14). Interestingly, the eggs from stressed quails showed higher concentrations of testosterone compared to control groups. Androgenic hormones such as testosterone are known to be important mediators of maternal effects on the behavior of the offspring (69, 70), possibly more than corticosterone [reviewed in (16, 71, 72)]. In addition, genetic differences across strains display a higher susceptibility of the White 2 strain to maternal stress compared to the other strains used in this study. Nevertheless, only minimal outcomes were observed in the progeny of the MS breeders, suggesting a higher resiliency to stressors than expected.
Tonic immobility is a state of reduced responsiveness thought to be a defense strategy used to decrease the predator's interest in the prey (73). It is induced by physical restraint, and its duration is considered a measure of fearfulness in birds (56, 57). Our lack of treatment effects in TI corroborates with Rubolini et al. (26), who injected corticosterone into fertile eggs of yellow-legged gulls. Contrary to our findings, the offspring of hens subjected to an unpredictable feeding schedule stayed longer in TI (13). This stressor, however, is not necessarily associated with increased levels of corticosterone in the egg and may be translated to the offspring via different pathways (e.g., nutrition). Also using a single egg injection of corticosterone prior to incubation, Janczak et al. (74) observed that chicks from injected eggs stayed longer in TI but only if they had been previously handled, suggesting that life experiences influence this behavioral effect of maternal stress. Interestingly, physiological studies on maternal stress and the HPA axis activation of the offspring showed that treatment effects are only observed when the offspring is also subjected to stressors (75–77). Therefore, a combination of maternal stress and life experience might be essential to trigger behavioral and physiological responses in the offspring. Our lack of treatment effects in TI might, thus, be related to a natural preservation of the phenotype of the offspring, since behavioral changes can easily become detrimental. This has important consequences for predicting and managing maternal effects in both breeder and commercial flocks, which may be regularly exposed to stressful events.
Analyses of the duration of the 3rd stage TI failed to display any effects of treatment or strain. Although the description of a bird in the 3rd stage (i.e., complete eye closure, no vocalizations, head bobbing, and occasional generalized body twitches) seems more similar to the original description of TI by Nash et al. (58), it is possible that the rigorousness of the method (which excludes birds with their eyes open and vocalizing, common behaviors during TI) may have reduced the test's ability to detect subtle behavioral differences, and therefore, it is not recommended.
Effect of Strain
Strain effects were found in both behavior tests. Contrast statements showed that the differences were primarily associated with the phylogenetically distant brown and white strains. The brown strains vocalized more in SI and TI and showed longer durations of immobility during TI, suggesting a higher occurrence of anxious and fearful behaviors compared to the white lines. This variability might be due to the intense genetic selection for productive traits in the domestic layer or by the phylogenetic, behavioral, and physiological differences across strains (34, 36, 47, 78, 79), which might be explained by evolution and domestication. Population studies exploring genetic diversity showed that brown lines originally came from African and Mediterranean genetic clustering, whereas white lines originated from the European cluster [reviewed by (78)]. Moreover, commercial brown lines are based from the Rhode Island Red, an originally dual-purpose breed (selected for both meat and eggs) with medium genetic diversity, whereas commercial white lines are based from White Leghorn, a low genetic diversity breed (80).
Genetic selection for production traits may have also affected the behavior of chickens if the traits are correlated or genetically linked. Several quantitative trait loci (QTL) related to fear response, for example, have been found on different chromosomes in White Leghorns. More specifically, TI was associated with three different QTLs on chromosome 1 that coincide with the position of two major QTLs for growth and bodyweight (81, 82). Therefore, genetic selection for body weight may have simultaneously affected fearfulness in White Leghorn. However, data for these studies were obtained exclusively from one strain, and it would be important to measure if this is also valid for lines expressing different genetics, such as brown strains. An early study of genetic differences and behavior showed that White Leghorns chicks displayed longer duration of TI than a Production Red strain, and when the two strains were crossbred, offspring showed intermediate durations (83), supporting the hypothesis that behavioral differences between brown and white strains are genetically dependent.
Contrary to our findings on vocalization (in both SI and TI) and duration of TI, the measurement of the number of attempts to attain a successful induction in TI showed that Brown 1 needed more second attempts than White 1, therefore suggesting that for this particular trait, a brown strain was less fearful than a white strain. Overall, results on anxiety and fearfulness found in the literature are often inconsistent, and a bird's motivation to engage in certain behaviors remains unclear. For example, some studies have found that brown strains lasted longer in TI (35, 36, 84, 85) and vocalized less than white strains in an open field test (86). The interpretation of these tests is thus difficult, with a multitude of factors such as genetic selection (87), hormones (88), the environment (89), and test methodology simultaneously affecting the behavior of layers.
Effect of Sex
In accordance with previous research (25, 41, 90), the present study did not show an interaction between treatment and sex to affect measures of anxiety and fearfulness in the offspring. Nevertheless, the current study suggests that female chickens are more anxious than males, displaying a higher frequency of distress calls during TI and a similar trend pattern in SI. These findings corroborate with Jones (86), who observed that hens were more active and vocalized more than cockerels in an open field test.
The development of sexual dimorphism in behavior is mostly related to the influence of gonadal hormones, androgens, and estrogens on the nervous system (91). Individually and combined, these hormones can organize and reorganize the neuronal circuitry involved in neuroendocrine and behavioral functions, including the serotonin system (91, 92) that is responsible for anxiety traits (93, 94). Moreover, the environment can also interact with sex to affect behavior. For example, Vallorttgara and Zanforlin (95) found that social isolation from cage companions was more stressful for female than male chickens. In the current study, birds were separated from conspecifics at both tests. Consequently, the hens might have vocalized more due to an intensified emotional response experienced during the tests.
Conclusion
Our findings suggest that the effects of maternal stress on measures of anxiety and fearfulness were contingent on genetic strain, but only when stressors are applied directly to the mother. The lack of CORT treatment effect suggests that maternal stress may not be mediated by corticosterone. Additionally, genetic strains responded differently to both behavior tests, with brown birds displaying higher levels of fearfulness in comparison to white strains, suggesting genetic differences in fear behavior across the genetic lines of commercial layers. These findings have important implications, since behavioral variations can be decisive to determine the overall adaptability of a strain to a specific production system. Moreover, in research settings, researchers must take into consideration behavioral differences when assessing different strains of laying hens, since generalization might be misleading.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by University of Guelph Animal Care Committee (Animal Utilization Protocol #1946).
Author Contributions
TW conceived the work and prepared the grants. TW and MP designed the study and prepared the manuscript. NK and AN contributed to the conception of the study. MP conducted the work and analyzed the data. All authors reviewed and approved the final manuscript.
Funding
This research was funded by Egg Farmers of Canada, the Ontario Ministry of Agriculture and Rural Affairs (OMAFRA), the Canadian Poultry Research Council, Hendrix Genetics, and Lohmann Tierzucht.
Conflict of Interest
TW holds the Egg Farmers of Canada Chair in Poultry Welfare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Andrew Janczak for his comments and suggestions; Daniel Rothschild, Priyanka Thavarajah, and Madeleine Browne for the assistance with data collection; and the personnel at the University of Guelph's Arkell Poultry Research Station for the technical assistance and maintenance of the birds.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2020.00128/full#supplementary-material
References
1. Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. (2008) 32:1073–86. doi: 10.1016/j.neubiorev.2008.03.002
2. Thompson WR. Influence of prenatal maternal anxiety on emotionality in young rats. Science. (1957) 126:73–4. doi: 10.1126/science.126.3263.73-a
3. Painter RC, Roseboom TJ, de Rooij SR. Long-term effects of prenatal stress and glucocorticoid exposure. Birth Defects Res Part C Embryo Today. (2012) 96:315–24. doi: 10.1002/bdrc.21021
4. Kapoor A, Matthews SG. Prenatal stress modifies behavior and hypothalamic-pituitary-adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology. (2008) 149:6406–15. doi: 10.1210/en.2008-0347
5. Pinson SE, Wilson JL, Navara KJ. Timing matters: corticosterone injections 4 h before ovulation bias sex ratios towards females in chickens. J Comp Physiol B. (2015) 185:539–46. doi: 10.1007/s00360-015-0897-5
6. Henriksen R, Rettenbacher S, Groothuis TGG. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci Biobehav Rev. (2011) 35:1484–501. doi: 10.1016/j.neubiorev.2011.04.010
7. Dixon LM, Sparks NHC, Rutherford KMD. Early experiences matter: a review of the effects of prenatal environment on offspring characteristics in poultry. Poult Sci. (2016) 95:489–99. doi: 10.3382/ps/pev343
8. Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev. (2008) 57:586–95. doi: 10.1016/j.brainresrev.2007.06.013
9. Belliure J, Meylan S, Clobert J. Prenatal and postnatal effects of corticosterone on behavior in juveniles of the common lizard, Lacerta vivipara. J Exp Zool A Comp Exp Biol. (2004) 301:401–10. doi: 10.1002/jez.a.20066
10. Nätt D, Lindqvist N, Stranneheim H, Lundeberg J, Torjesen PA, Jensen P. Inheritance of acquired behaviour adaptations and brain gene expression in chickens. PLoS ONE. (2009) 4:e6405. doi: 10.1371/annotation/4f90ac09-ae5e-469a-a2f3-21a5ac68dc31
11. Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. (1992) 13:35–41. doi: 10.1016/0165-6147(92)90014-W
12. Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. (2004) 28:771–84. doi: 10.1016/j.neubiorev.2004.09.006
13. Janczak AM, Torjesen P, Palme R, Bakken M. Effects of stress in hens on the behaviour of their offspring. Appl Anim Behav Sci. (2007) 107:66–77. doi: 10.1016/j.applanim.2006.09.016
14. Guibert F, Richard-Yris MA, Lumineau S, Kotrschal K, Bertin A, Petton C, et al. Unpredictable mild stressors on laying females influence the composition of Japanese quail eggs and offspring's phenotype. Appl Anim Behav Sci. (2011) 132:51–60. doi: 10.1016/j.applanim.2011.03.012
15. Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. (2009) 3:19. doi: 10.3389/neuro.08.019.2009
16. Groothuis TGG, Hsu B-Y, Kumar N, Tschirren B. Revisiting mechanisms and functions of prenatal hormone-mediated maternal effects using avian species as a model. Philos Trans R Soc B Biol Sci. (2019) 374:20180115. doi: 10.1098/rstb.2018.0115
17. Ruuskanen S, Hsu B-Y. Maternal thyroid hormones: an unexplored mechanism underlying maternal effects in an ecological framework. Physiol Biochem Zool. (2018) 91:904–16. doi: 10.1086/697380
18. Possenti CD, Secomandi S, Schiavon A, Caprioli M, Rubolini D, Romano A, et al. Independent and combined effects of egg pro- and anti-oxidants on gull chick phenotype. J Exp Biol. (2018) 221:jeb174300. doi: 10.1242/jeb.174300
19. Roth O, Beemelmanns A, Barribeau SM, Sadd BM. Recent advances in vertebrate and invertebrate transgenerational immunity in the light of ecology and evolution. Heredity. (2018) 121:225–38. doi: 10.1038/s41437-018-0101-2
20. MacDougall-Shackleton SA, Bonier F, Romero LM, Moore IT. Glucocorticoids and “Stress” are not synonymous. Integr Org Biol. (2019) 1:obz017. doi: 10.1093/iob/obz017
21. Ahmed AA, Musa HH, Sifaldin AZ. Prenatal corticosterone exposure programs growth, behavior, reproductive function and genes in the chicken. Asian Pac J Reprod. (2016) 5:271–8. doi: 10.1016/j.apjr.2016.06.013
22. Jenkins SA, Porter TE. Ontogeny of the hypothalamo-pituitary-adrenocortical axis in the chicken embryo: a review. Domest Anim Endocrinol. (2004) 26:267–75. doi: 10.1016/j.domaniend.2004.01.001
23. Garamszegi LZ, Rosivall B, Rettenbacher S, Markó G, Zsebok S, Szöllosi E, et al. Corticosterone, avoidance of novelty, risk-taking and aggression in a wild bird: no evidence for pleiotropic effects. Ethology. (2012) 118:621–35. doi: 10.1111/j.1439-0310.2012.02049.x
24. Janczak AM, Braastad BO, Bakken M. Behavioural effects of embryonic exposure to corticosterone in chickens. Appl Anim Behav Sci. (2006) 96:69–82. doi: 10.1016/j.applanim.2005.04.020
25. Henriksen R, Rettenbacher S, G.G. Groothuis T. Maternal corticosterone elevation during egg formation in chickens (Gallus gallus domesticus) influences offspring traits, partly via prenatal undernutrition. Gen Comp Endocrinol. (2013) 191:83–91. doi: 10.1016/j.ygcen.2013.05.028
26. Rubolini D, Romano M, Boncoraglio G, Ferrari RP, Martinelli R, Galeotti P, et al. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm Behav. (2005) 47:592–605. doi: 10.1016/j.yhbeh.2005.01.006
27. Rodricks CL, Miller SL, Jenkin G, Gibbs ME. The role of corticosterone in prehatch-induced memory deficits in chicks. Brain Res. (2006) 1123:34–41. doi: 10.1016/j.brainres.2006.09.028
28. Lay DC, Wilson ME. Development of the chicken as a model for prenatal stress. J Anim Sci. (2002) 80:1954–61. doi: 10.2527/2002.8071954x
29. Henriksen R, Groothuis TG, Rettenbacher S. Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects. PLoS ONE. (2011) 6:e23824. doi: 10.1371/journal.pone.0023824
30. von Engelhardt N, Henriksen R, Groothuis TGG. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen Comp Endocrinol. (2009) 163:175–83. doi: 10.1016/j.ygcen.2009.04.004
31. Heiblum R, Arnon E, Chazan G, Robinzon B, Gvaryahu G, Snapir N. Glucocorticoid administration during incubation: embryo mortality and posthatch growth in chickens. Poult Sci. (2001) 80:1357–63. doi: 10.1093/ps/80.9.1357
32. Janczak AM, Haung A, Bakken M. Evaluation of experimental methods for manipulating chicken egg hormone content using injections. J Anim Vet Adv. (2007) 6:500–4.
33. Rettenbacher S, Möstl E, Groothuis TGG. Gestagens and glucocorticoids in chicken eggs. Gen Comp Endocrinol. (2009) 164:125–9. doi: 10.1016/j.ygcen.2009.05.019
34. de Haas EN, Kemp B, Bolhuis JE, Groothuis T, Rodenburg TB. Fear, stress, and feather pecking in commercial white and brown laying hen parent-stock flocks and their relationships with production parameters. Poult Sci. (2013) 92:2259–69. doi: 10.3382/ps.2012-02996
35. Fraisse F, Cockrem JF. Corticosterone and fear behaviour in white and brown caged laying hens. Br Poult Sci. (2006) 47:110–9. doi: 10.1080/00071660600610534
36. Pusch EA, Bentz AB, Becker DJ, Navara KJ. Behavioral phenotype predicts physiological responses to chronic stress in proactive and reactive birds. Gen Comp Endocrinol. (2018) 255:71–7. doi: 10.1016/j.ygcen.2017.10.008
37. Nätt D, Nätt D, Rubin C-J, Wright D, Johnsson M, Beltéky J, et al. Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics. (2012) 13:59. doi: 10.1186/1471-2164-13-59
38. Peixoto MRLV, Karrow NA, Widowski TM. Effects of prenatal stress and genetics on embryonic survival and offspring growth of laying hens. Poult Sci. (2020) 99:1618–27. doi: 10.1016/j.psj.2019.10.018
39. Nielsen H. Hen age and fatty acid composition of egg yolk lipid. Br Poult Sci. (1998) 39:53–6. doi: 10.1080/00071669889394
40. Beuving G, Vonder GMA. Effect of stressing factors on corticosterone levels in the plasma of laying hens. Gen Comp Endocrinol. (1978) 35:153–9. doi: 10.1016/0016-6480(78)90157-0
41. Ericsson M, Henriksen R, Bélteky J, Sundman AS, Shionoya K, Jensen P. Long-term and transgenerational effects of stress experienced during different life phases in chickens (Gallus gallus). PLoS ONE. (2016) 11:e0153879. doi: 10.1371/journal.pone.0153879
42. Pitk M, Tilgar V, Kilgas P, Mänd R. Acute stress affects the corticosterone level in bird eggs: a case study with great tits (Parus major). Horm Behav. (2012) 62:475–9. doi: 10.1016/j.yhbeh.2012.08.004
43. Rich EL, Romero LM. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am J Physiol Regul Integr Comp Physiol. (2005) 288:R1628–36. doi: 10.1152/ajpregu.00484.2004
45. Scanes CG. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult Sci. (2016) 95:2208–15. doi: 10.3382/ps/pew137
46. Johnston PA, Liu H, O'Connell T, Phelps P, Bland M, Tyczkowski J, et al. Applications in in ovo technology. Poult Sci. (1997) 76:165–78. doi: 10.1093/ps/76.1.165
47. Navara KJ, Pinson SE. Yolk and albumen corticosterone concentrations in eggs laid by white versus brown caged laying hens. Poult Sci. (2010) 89:1509–13. doi: 10.3382/ps.2009-00416
48. Ahmed AA, Ma W, Ni Y, Wang S, Zhao R. Corticosterone in ovo modifies aggressive behaviors and reproductive performances through alterations of the hypothalamic-pituitary-gonadal axis in the chicken. Anim Reprod Sci. (2014) 146:193–201. doi: 10.1016/j.anireprosci.2014.02.013
49. Engel JM, Widowski TM, Tilbrook AJ, Butler KL, Hemsworth PH. The effects of floor space and nest box access on the physiology and behavior of caged laying hens. Poult Sci. (2019) 98:533–47. doi: 10.3382/ps/pey378
50. Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B Biol Sci. (2012) 279:1447–56. doi: 10.1098/rspb.2011.1913
51. Eriksen MS, Haug A, Torjesen PA, Bakken M. Prenatal exposure to corticosterone impairs embryonic development and increases fluctuating asymmetry in chickens (Gallus gallus domesticus). Br Poult Sci. (2003) 44:690–7. doi: 10.1080/00071660310001643660
52. Rettenbacher S, Groothuis TG, Henriksen R, Möstl E. Corticosterone in bird eggs: the importance of analytical validation. Wien Tierarztl Monatsschr. (2013) 100:283–90.
53. Beuving G, Vonder GMA. The influence of ovulation and oviposition on corticosterone levels in the plasma of laying hens. Gen Comp Endocrinol. (1981) 44:382–8. doi: 10.1016/0016-6480(81)90016-2
54. Sufka KJ, Weed NC. Construct validation of behavioral indices of isolation stress and inflammatory nociception in young domestic fowl. Physiol Behav. (1994) 55:741–6. doi: 10.1016/0031-9384(94)90054-X
55. Sufka KJ, Feltenstein MW, Warnick JE, Acevedo EO, Webb HE, Cartwright CM. Modeling the anxiety-depression continuum hypothesis in domestic fowl chicks. Behav Pharmacol. (2006) 17:681–9. doi: 10.1097/FBP.0b013e3280115fac
56. Jones RB. The tonic immobility reaction of the domestic fowl: a review. Worlds Poult Sci J. (1986) 42:82–96. doi: 10.1079/WPS19860008
57. Forkman B, Boissy A, Meunier-Salaün MC, Canali E, Jones RB. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol Behav. (2007) 91:531–65. doi: 10.1016/j.physbeh.2007.03.016
58. Nash RF, Gallup GG, Czech DA. Psychophysiological correlates of tonic immobility in the domestic chicken (Gallus gallus). Physiol Behav. (1976) 17:413–8. doi: 10.1016/0031-9384(76)90100-1
59. Rovee CK, Luciano DP. Rearing influences on tonic immobility in three-day-old chicks (Gallus gallus). J Comp Physiol Psychol. (1973) 83:351–4. doi: 10.1037/h0034429
60. Jones RB, Faure JM. Sex and strain comparisons of tonic immobility (“Righting time”) in the domestic fowl and the effects of various methods of induction. Behav Processes. (1981) 6:47–55. doi: 10.1016/0376-6357(81)90015-2
61. Garcia LV. Controlling the false discovery rate in ecological research. Trends Ecol Evol. (2016) 18:553–4. doi: 10.1016/j.tree.2003.08.011
62. Arndt S, Turvey C, Andreasen NC. Correlating and predicting psychiatric symptom ratings: Spearman's v versus Kendall's tau correlation. J Psychiatr Res. (1999) doi: 10.1016/S0022-3956(98)90046-2
63. Birrenkott GP, Wiggins ME. Determination of dexamethasone and corticosterone half-lives in male broilers. Poult Sci. (1984) 63:1064–8. doi: 10.3382/ps.0631064
64. Almasi B, Rettenbacher S, Müller C, Brill S, Wagner H, Jenni L. Maternal corticosterone is transferred into the egg yolk. Gen Comp Endocrinol. (2012) 178:139–44. doi: 10.1016/j.ygcen.2012.04.032
65. Smith KK, Dharmaratne HRW, Feltenstein MW, Broom SL, Roach JT, Nanayakkara NPD, et al. Anxiolytic effects of kava extract and kavalactones in the chick social separation-stress paradigm. Psychopharmacology. (2001) 155:86–90. doi: 10.1007/s002130100686
66. Sufka KJ, Roach JT, Chambliss WG, Broom SL, Feltenstein MW, Wyandt CM, et al. Anxiolytic properties of botanical extracts in the chick social separation-stress procedure. Psychopharmacology. (2001) 153:219–24. doi: 10.1007/s002130000571
67. Watson GS, Sufka KJ. Chlordiazepoxide reverses social-separation-induced distress vocalizations and analgesia in young domestic fowl. Exp Clin Psychopharmacol. (1996) 4:347–53. doi: 10.1037/1064-1297.4.4.347
68. Feltenstein MW, Lambdin LC, Webb HE, Warnick JE, Khan SI, Khan IA, et al. Corticosterone response in the chick separation-stress paradigm. Physiol Behav. (2003) 78:489–93. doi: 10.1016/S0031-9384(03)00030-1
69. Guibert F, Richard-Yris MA, Lumineau S, Kotrschal K, Möstl E, Houdelier C. Yolk testosterone levels and offspring phenotype correlate with parental age in a precocial bird. Physiol Behav. (2012) 105:242–50. doi: 10.1016/j.physbeh.2011.08.009
70. Niall Daisley J, Bromundt V, Möstl E, Kotrschal K. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm Behav. (2005) 47:185–94. doi: 10.1016/j.yhbeh.2004.09.006
71. Groothuis TGG, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos Trans R Soc B Biol Sci. (2008) 363:1647–61. doi: 10.1098/rstb.2007.0007
72. Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in Avian species. Neurosci Biobehav Rev. (2005) 29:329–52. doi: 10.1016/j.neubiorev.2004.12.002
73. Thompson RKR, Foltin RW, Boylan RJ, Sweet A, Graves CA, Lowitz CE. Tonic immobility in Japanese quail can reduce the probability of sustained attack by cats. Anim Learn Behav. (1981) 9:145–9. doi: 10.3758/BF03212037
74. Janczak AM, Heikkilä M, Valros A, Torjesen P, Andersen IL, Bakken M. Effects of embryonic corticosterone exposure and post-hatch handling on tonic immobility and willingness to compete in chicks. Appl Anim Behav Sci. (2007) 107:275–86. doi: 10.1016/j.applanim.2006.10.002
75. Carter AW, Bowden RM, Paitz RT. Evidence of embryonic regulation of maternally derived yolk corticosterone. J Exp Biol. (2018) 221:jeb.182600. doi: 10.1242/jeb.182600
76. Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica). Biol Lett. (2014) 10:20140502. doi: 10.1098/rsbl.2014.0502
77. Vassallo BG, Litwa HP, Haussmann MF, Paitz RT. In ovo metabolism and yolk glucocorticoid concentration interact to influence embryonic glucocorticoid exposure patterns. Gen Comp Endocrinol. (2019) 272:57–62. doi: 10.1016/j.ygcen.2018.11.013
78. Tixier-Boichard M, Bed'Hom B, Rognon X. Chicken domestication: from archeology to genomics. C R Biol. (2011) 334:197–204. doi: 10.1016/j.crvi.2010.12.012
79. Groothuis TGG, Carere C. Avian personalities: characterization and epigenesis. Neurosci Biobehav Rev. (2005) 29:137–50. doi: 10.1016/j.neubiorev.2004.06.010
80. Lyimo CM, Weigend A, Msoffe PL, Eding H, Simianer H, Weigend S. Global diversity and genetic contributions of chicken populations from African, Asian and European regions. Anim Genet. (2014) 45:836–48. doi: 10.1111/age.12230
81. Schütz KE, Kerje S, Jacobsson L, Forkman B, Carlborg Ö, Andersson L, et al. Major growth QTLs in fowl are related to fearful behavior: possible genetic links between fear responses and production traits in a red junglefowl x white leghorn intercross. Behav Genet. (2004) 34:121–30. doi: 10.1023/B:BEGE.0000009481.98336.fc
82. Kerje S, Carlborg Ö, Jacobsson L, Schütz K, Hartmann C, Jensen P, et al. The twofold difference in adult size between the red junglefowl and white leghorn chickens is largely explained by a limited number of QTLs. Anim Genet. (2003) 34:264–74. doi: 10.1046/j.1365-2052.2003.01000.x
83. Gallup GG, Ledbetter DH, Maser JD. Strain differences among chickens in tonic immobility: evidence for an emotionality component. J Comp Physiol Psychol. (1976) 90:1075–81. doi: 10.1037/h0078662
84. Albentosa MJ, Kjaer JB, Nicol CJ. Strain and age differences in behaviour, fear response and pecking tendency in laying hens. Br Poult Sci. (2003) 44:333–44. doi: 10.1080/00071660310001598085
85. Mahboub HDH, Müller J, von Borell E. Outdoor use, tonic immobility, heterophil/lymphocyte ratio and feather condition in free-range laying hens of different genotype. Br Poult Sci. (2004) 45:738–44. doi: 10.1080/00071660400014267
86. Jones R. Sex and strain differences in the open-field responses of the domestic chick. Appl Anim Ethol. (1977) 3:255–61. doi: 10.1016/0304-3762(77)90006-2
87. Dennis RL, Chen ZQ, Cheng HW. Serotonergic mediation of aggression in high and low aggressive chicken strains. Poult Sci. (2008) 87:612–20. doi: 10.3382/ps.2007-00389
88. Cockrem JF. Stress, corticosterone responses and avian personalities. J Ornithol. (2007) 148:169–78. doi: 10.1007/s10336-007-0175-8
89. Uitdehaag K, Komen H, Rodenburg TB, Kemp B, van Arendonk J. The novel object test as predictor of feather damage in cage-housed Rhode Island red and white leghorn laying hens. Appl Anim Behav Sci. (2008) 109:292–305. doi: 10.1016/j.applanim.2007.03.008
90. Goerlich VC, Nätt D, Elfwing M, Macdonald B, Jensen P. Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm Behav. (2012) 61:711–8. doi: 10.1016/j.yhbeh.2012.03.006
91. Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. (2001) 25:219–33. doi: 10.1016/S0149-7634(01)00010-0
92. Carlsson M, Carlsson A. A regional study of sex differences in rat brain serotonin. Prog Neuropsychopharmacol Biol Psychiatry. (1988) 12:53–61. doi: 10.1016/0278-5846(88)90061-9
93. Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5- HT(1A) receptor mutant mice. Proc Natl Acad Sci USA. (1998) 95:15049–54. doi: 10.1073/pnas.95.25.15049
94. Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT2C receptors regulate anxiety-like behavior. Genes, Brain Behav. (2007) 6:491–6. doi: 10.1111/j.1601-183X.2007.00316.x
Keywords: corticosterone, layer breeder, fear, anxiety, genetics, chicken
Citation: Peixoto MRLV, Karrow NA, Newman A and Widowski TM (2020) Effects of Maternal Stress on Measures of Anxiety and Fearfulness in Different Strains of Laying Hens. Front. Vet. Sci. 7:128. doi: 10.3389/fvets.2020.00128
Received: 10 December 2019; Accepted: 20 February 2020;
Published: 27 March 2020.
Edited by:
Dana L. M. Campbell, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaReviewed by:
Andrew M. Janczak, Norwegian University of Life Sciences, NorwayElske N. De Haas, Utrecht University, Netherlands
Copyright © 2020 Peixoto, Karrow, Newman and Widowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tina M. Widowski, dHdpZG93c2tAdW9ndWVscGguY2E=
 Mariana R. L. V. Peixoto
Mariana R. L. V. Peixoto Niel A. Karrow
Niel A. Karrow Amy Newman
Amy Newman Tina M. Widowski
Tina M. Widowski