
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 13 March 2020
Sec. Animal Nutrition and Metabolism
Volume 7 - 2020 | https://doi.org/10.3389/fvets.2020.00124
 Ahmed A. Al-Sagan1*
Ahmed A. Al-Sagan1* Abdullah H. AL-Yemni2
Abdullah H. AL-Yemni2 Abdulaziz A. Al-Abdullatif3
Abdulaziz A. Al-Abdullatif3 Youssef A. Attia4*
Youssef A. Attia4* Elsayed O. S Hussein3
Elsayed O. S Hussein3This study aimed to investigate the effects of different dietary levels of blue lupine (Lupinus angustifolius) seed meal with or without probiotics (Bacillus subtilis) in broiler diets on the growth performance, carcass characteristics, internal and immune organs, and gut morphology. Three experimental diets containing 0, 20, and 30% of blue lupine, with or without probiotics, were formulated and fed to 144 day (d)-old Ross 308 broiler chickens. Overall, chicks fed blue lupine meal diets, especially at the 30% rate, showed improved growth, feed performance parameters, and carcass characteristics in comparison to chicks fed a soybean meal-based diet. For example, a 30% blue lupine diet resulted in a significant increase in the duodenum length percentage of 35 d-old broilers; the addition of probiotics had no—effects on the dressing, thigh, and leg percentages of 21- and 35 d-old broilers and the drumstick and leg percentages of 35 d-old broilers. In conclusion, a 30% blue lupine seed diet with the addition of probiotics could provide a cheap source of protein without negative effects on the growth performance, carcass characteristics, immune organs and gut morphology of broilers.
Poultry production necessitates the provision of adequate protein and amino acids to chicks for normal growth and maximum production (1). The protein requirements of broiler chick with adequate growth performance and body weight are high (2, 3). The cost of importing feed ingredients such as soybean meal and corn to formulate poultry diets is one of the most important impediments to achieving high productivity. Soybean meal, preferred for its high nutritional value, is the main source of protein for broilers. However, the soybean meal price fluctuates worldwide with a tendency to increase with decreasing supply during certain periods of the year (4). The increase in the cost of broiler diets is mainly due to the volatility of the feed market and the stiff competition for food resources between human and animal diets and the biofuel-producing industries (5, 6). Thus, there is a need for local feed resources that will provide alternatives to soybean meal, thereby decreasing feed costs.
One of the most important strategies for the poultry industry is to develop domestic dietary formulations, which will allow the use of local ingredients as substitutes for imported feed ingredients and, subsequently, reduce diet costs. Legume seeds such as the blue lupine (Lupinus angustifolius), which belongs to the family Fabaceae, are important sources of protein for monogastric animals and are considered as alternatives to the soybean meal (7, 8). Blue lupine is characterized by its year-round availability and its low price compared to soybean meal. The use of lupine seeds in the formulation of broiler diets is justified primarily because of their high protein content (≈40.08%) (9). Also, lupine is a good source of nutrients such as lipids, fiber, minerals, and vitamins after the ban of use soybean meal in organic farming (10–12).
The main anti-nutritional factors in lupins are alkaloids, phytates, protease inhibitors, and lectins, which cause retarded growth and poor feed utilization (13). Lupine has a high non-starch polysaccharide (NSP) content (14). The main NSP in lupine seeds is galactan that consists of different amounts of arabinose and galactose monosaccharides. NSPs impair gut ecology and reduce the digestibility of poultry diets (2, 15, 16). Reduced feed intake and growth rates are noted in birds that are fed lupine-based diets (2, 7); these adverse effects were noted during the first week and lasted up until the end of the experiments. However, lupine species such as L. angustifolius that have low-alkaloid content are increasingly used in both layers' and broilers' diets (17). Blue lupine can be incorporated in broilers' diets at 10% and yield similar results to the soybean meal diet, but the increasing level to 20% decreased performance while increasing wet droppings (18). Lupine flour can constitute up to 30% of soybean meal protein of broilers' diets while maintaining their production performance in good levels, but increasing blue lupine to 40% (18% in the diet) and 80% (~30% in the diet) of soybean meal protein decreased growth performance (19). Lupines can constitute up to 15% of layers' diets without any negative effects on their production performance and health (8). Thus, overcoming the anti-nutritional effects of lupine and improving the utilization of lupine NSPs require further research (20–22).
Probiotics are well-known microorganisms that have a positive effect on the performance of the host bird by improving the ecology of the gut (23–25). Growth performance and feed conversion rate (FCR) are improved in broiler chickens supplemented with probiotics (26–28). Probiotics improve gut ecology, immunity and eliminate toxic effects on animals (29–31). In literature, there were rare studies used probiotic as a tool to improve the use of blue lupine in chickens' feeding due to the negative effect of blue lupine on gut eco-system as evident by increasing wet dropping (18). Furthermore, the use of probiotics in the literature to improve animal performance and gut ecology has received great attention with some success (23–28). Thus, we hypothesized that probiotics supplementation to broilers' diets containing 30% blue lupine might improve growth performance and carcass traits due to improving gut ecosystem. Hence, the current study aimed to evaluate the effects of lupine (Lupinus angustifolius L. “Boltensia”) seed meal inclusion in broiler diets, with or without probiotics (Bacillus subtilis), on the performance, carcass quality, internal organs, immune system, and gut morphology of Ross 308 broiler chickens.
The L. angustifolius cultivar Boltensia, a low-alkaloid variety, was used in the present broiler study. Blue lupine seeds were milled in a hammer mill, sieved through a 3 mm screen, and mixed with the other ingredients. The chemical composition of blue lupine was determined according to (29) and used in diet formulation. The metabolizable energy value was calculated by using the equation published by (32):
AMEn = Nitrogen corrected metabolizable energy, CP = Crude protein, NFE = Nitrogen free extract (starch + sugar).
The composition of the experimental diets is presented in Tables 1, 2. Blue lupine was included in the experimental diets at 0, 20, and 30% with similar calorific and nitrogenous values. The experimental diets were formulated to meet or exceed the minimum broiler requirements (33). The broilers were fed diets with or without probiotics [the Bacillus subtilis, PB6 based-probiotic that was used in this experiment was CS (CloSTAT® brand, Kemin Industries Inc., Des Moines, IA, USA). The commercially available product (product no. 017176) contains live viable ≥1 × 1011 cfu/g B. subtilis PB6 according to the manufacturer (1.5 g of the product to 1 ton of feed), https://www.kemin.com/na/en-us/products/clostat. Thus, the experimental design included 3 concentrations of blue lupine × 2 two levels of B. subtilis (0 and 0.05 g/kg diet) in a factorial arrangement. The probiotics were used as an ideal agent for improving gut ecology due to excepted negative effects of blue lupine in the gut ecosystem. The probiotic products were mixed with a small amount of corn in a small mixer before being transferred to a larger mixer with the remaining components of the diet, to ensure homogeneity. Feed-in a mashed form and water were available ad libitum.
A total of 144 unsexed, 1 d-old Ross 308 broiler chicks were obtained from the Al-Wadi Company and were distributed randomly to 24 pens in 4 three-tier batteries. Six unsexed chickens were housed per cage (28 cm × 48 cm × 48 cm) at the Poultry Research Centre, King Abdulaziz City for Science and Technology (KACST), Al- Muzahimiyah, Saudi Arabia. Each pen had a 1 cm squared wire mesh bottom for waste collection and was equipped with a feeding trough placed outside and two water cups inside the pen. The batteries were placed in a windowless room equipped with forced ventilation. The light was continuous during the experiment, and the temperature was gradually reduced from 33 to 23°C until the termination of the study. Veterinary care was provided, and vaccination programs were implemented according to the husbandry practice for broiler chickens; both were carried out under the supervision of a veterinarian.
Chick performance was measured in terms of feed consumption, body weight, feed conversion rate, and survival rate using the replicate as the experimental unit. Chickens were weighed at 1, 21, and 35 d of age before being offered the feeds and sexed at 35 days by comb size to correct for sex differences within replicates and among treatments. At 21 and 35 d of age, feed intake was calculated. The feed intake and body weight gain data were used for the calculation of FCR. Mortality was recorded daily, and the survival rate was calculated for the complete experimental period. The survival rate was the number of live broilers at 35 d of age divided by the number of 1 day-old broilers. The European production efficiency index (EPEI) was calculated according to (15).
After being fasted overnight, four males chickens from each treatment group, i.e., one chicken per replicate, were randomly slaughtered at 21 and 35 d of age according to the Islamic method (34, 35). The carcass, abdominal fat, breast muscles, unskinned right and left thighs, and thigh muscles were weighed and expressed in relation to living body weight. Besides, the gizzard, heart, liver, bursa, thymus, spleen, duodenum (pancreatic loop), jejunum (from the pancreatic loop to Meckel's diverticulum), ileum (from Meckel's diverticulum to the ileocecal junction), and ceca were separated, measured, weighed, and expressed in relation to body weight. The intestinal organs were cleaned before being weighed. The weight : length ratios of the 3 segments (duodenum, jejunum, and ileum) were calculated as indicators of intestinal density (36). All of the data regarding organ weight and length were expressed per 100 g of body weight.
The power analyses were run to estimate the number of replicates using 0.10 difference in FCR of broilers chickens at market age, i.e., 1.3 vs. 1.5 kg/kg, the standard division of 0.10 kg/kg, two sides test, P-value of 0.05 and the desired power of 80. The estimated number of replicates was 4 replicates according to [https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html]. The differences in FCR was considered to be 0.2 based in published resulted by (2, 37, 38).
The data were analyzed using the SAS software package (39), SAS Institute, Cary, NC, the USA with two-way variance analysis. In preliminary statistical analyses, sex differences within treatments and replicates were tested and it was not significant. Thus, we run the statistical analyses based on the replicate for body weight gain, feed intake, FCR and European production index. The data were analyzed using the GLM procedure of SAS using the replicate as the experimental unit according to the following model:
Where: Y is a single observation, m is the general mean, Ti is the effect of dietary lupine concentrations, Bj is the effect of supplements, Yij is the interaction between lupine concentration and supplements, and eijk is the experimental error.
Before the analysis, all percentages were subjected to logarithmic transformation (log10 x + 1) to normalize data distribution. Mean differences were tested at P ≤ 0.05 by all possible differences (39). The data were presented based on mean and SEM.
The chemical composition of blue lupine was 92.5% dry matter (DM), 30.4% (CP), 5.39% fat, 2.51% ash, 16.2% crude fiber (CF), and 38.0% nitrogen-free extract (NFE). The published values for blue lupine are 35.5% CP, 5.45% fat, 16.5% CF, 4.01% ash, and 38.5% NFE (32). The calculated metabolizable energy value of the feed basis (92.5% DM) was 7.41 MJ/kg. The results of the present study showed that blue lupine might be a good source of nutrients such as lipids, fiber, minerals, and vitamins (10, 12). In addition, (40) found that white lupine beans contain 44% CP, 10.7% crude fat, 16.1% CF, 4.00% ash, and 13.9 MJ/kg of metabolizable energy. Moreover, narrow-leaved lupine and yellow lupine consist of 89.1 and 87.1% DM, 35.4, and 41.2% CP, 5.96, and 5.45% crude fat, 17.9 and 15.5% CF, 3.71 and 5.45% ash, and 37.1 and 31.1% (soluble carbohydrate) NFE, respectively (16). The differences between our values and those mentioned in the literature regarding the chemical composition of lupine can be attributed to the variety of lupine strains (10, 16).
In addition, lupine proteins are superior to and more degradable than proteins of other legumes, e.g., soybean (41, 42). Moreover, blue lupine seeds are rich in lysine (43). Mukisira (44) indicated that lupine protein has relatively high amounts of threonine, lysine, and tryptophan (45), but low methionine content. The sulfur-containing amino acids are primarily limited in lupine protein (46). However, the lupine amino acid balance compares positively with that of soybean (47). The superiority of blue lupine protein to soybean meal may be due to its lower phytic acid and saponin levels as well as its lower lectin and protease inhibitor concentrations (48).
The effects of the dietary addition of blue lupine and/or probiotics on growth performance are shown in Tables 3, 4. The blue lupine and/or probiotics did not significantly affect feed intake, body weight gain (Table 3), and the EPEI (Table 4) during different experimental periods or the FCR from 1 to 21 and 1 to 35 d of age. Nonetheless, the FCR of broilers that were fed the 30% blue lupine diet during the 22–35 d period was significantly impaired compared with the control group. This may be due to a low essential amino acids concentration, particularly that of arginine, lysine, threonine, tryptophan, and valine when blue lupine was fed at 30%. In addition, this may also be due to the high fiber content of this diet and thus, the influence of blue lupine on the gut ecosystem (18). Both the deficiency of essential amino acids and the higher fiber content can impair feed use for growth (21, 22). However, the FCR was similar among different blue lupine groups for the entire period we examined. Broilers showed improved tolerance to amino acid deficiency and high crude fiber concentration, especially the older ones (15), due to gut maturation (27). There was no significant difference in the FCR among broilers that were fed the 20% blue lupine diet and those that were fed the control or 30% blue lupine diets (Table 4). In the literature, blue lupine can be incorporated in broilers' diets in up to 25% of soybean meal protein and yield similar results to the soybean meal (16, 18) and white lupine diets (21, 22). Other researchers found that when blue lupine is fed at 30% of soybean meal protein (19, 40, 49) and 15% it does not have any negative influences on the production performance and health of layers (8), but increasing blue lupine to 40 and 80% of soybean meal protein decreased growth and impaired gut ecosystem and increased wet dropping (18, 19). Similar results were obtained with the inclusion of 25–30% of blue lupine in turkey diets (40, 49). In one study (2) it was observed that raw lupine fed at 40% and dehulled lupine seed meal fed at 35% significantly decreased the feed intake and growth of broiler chickens; this was visible during the first week of age and continued up to 21 d of age. These response differences to dietary lupine could be attributed to the type and dietary concentration of lupine and the negative impact of blue lupine on gut ecology (18, 19, 21, 50).
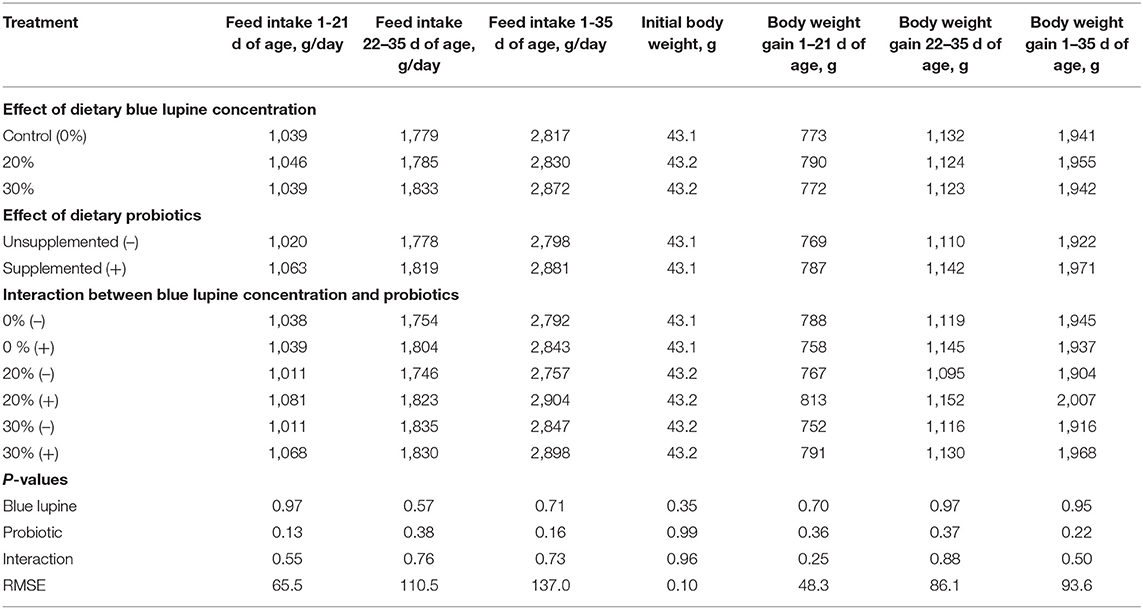
Table 3. Effect of blue lupine and probiotics on the growth performance parameters of broiler chicks at 1–35 d of age.
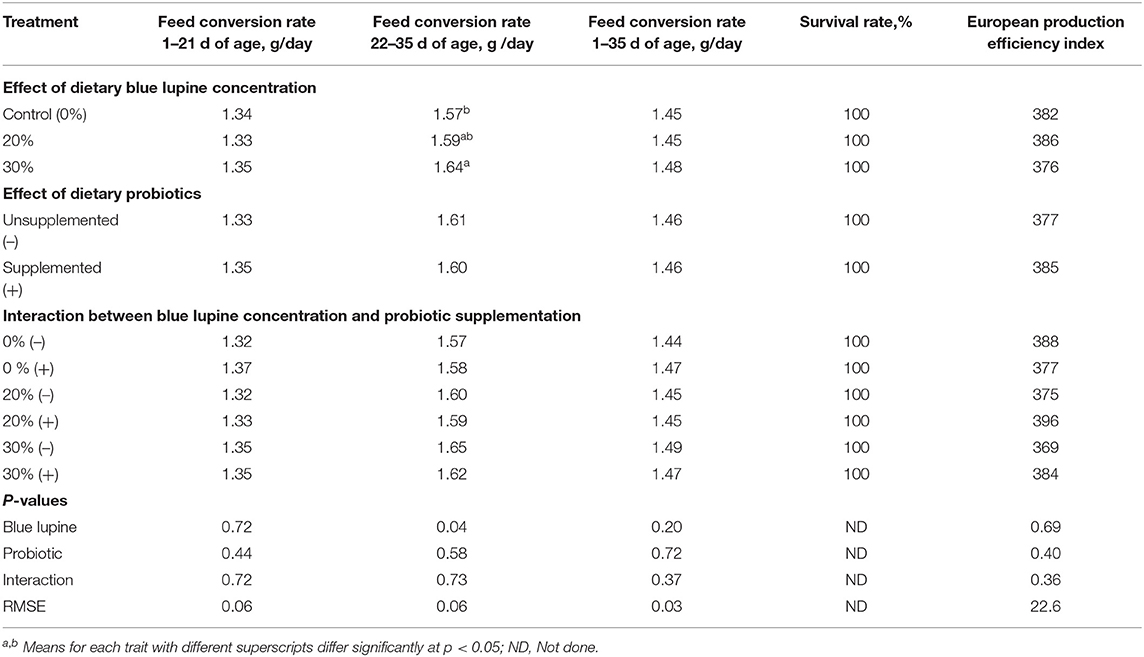
Table 4. Effect of blue lupine and probiotics on feed conversion rate, European production efficiency index, and economic efficiency of broiler chickens from 1 to 35 d of age.
The addition of probiotics had no significant effect on the growth performance of broilers (growth rate, feed intake, FCR, and EPEI) during different experimental periods (Tables 3, 4). These results are in agreement with those reported by (23–25, 51–53) that indicate that the dietary supplementation of probiotics has insignificant influence on the performance of broilers during the grower, finisher, and the whole periods (1–35 d- of age).
No significant interaction between blue lupine and probiotics on the growth performance and EPEI was observed. These results indicate that blue lupine could be included in broiler diets up to 30%, with or without the addition of probiotics, and lead to production performance and EPEI similar to those of the control group. However, probiotic addition seemed to be beneficial, particularly when added to a 30% blue lupine diet, which exhibited higher crude fiber and low amino acid concentrations, causing an improvement of 2.71, 1.34, and 4.07% in growth rate, FCR, and EPEI, respectively. These results are similar to those reported by (24, 25, 35, 54, 55), who found that probiotics containing Bacillus spp. and Saccharomyces boulardii benefit the production performance of broilers from 1 to 28 d of age. The positive effect of probiotics could be attributed to their ability to modulate gut ecology toward beneficial microflora, thus improving gut health and absorption capacity, thereby improve productive performance (31, 54, 56). Even, the present results supported the previous studies in literature, the results of growth performance found herein may be confounded by the number of replicates (4 per treatment) and thus, further experiments were suggested.
The effects of different levels of blue lupine and/or the addition of probiotics on the carcass characteristics of broilers at 21 and 35 d of age are shown in Table 5. The inclusion of 20% and 30% of blue lupine in broiler diets with or without the addition of probiotics at 21 d, had no significant effect on the dressing and breast muscle percentages. The inclusion of 20 and 30% of blue lupine in broiler diets at 35 d showed significant differences between the 20% and the 30% level (Table 5). The legs of 35 d-old broilers were not affected by dietary blue lupine and its interaction with probiotics (Table 5). Their breast muscle percentage tended to decrease (P = 0.07) with the inclusion of 30% of blue lupine in their diet. The decrease in breast muscle may be due to the low essential amino acid concentrations of the 30% blue lupine diet that is essential for muscle protein deposition.
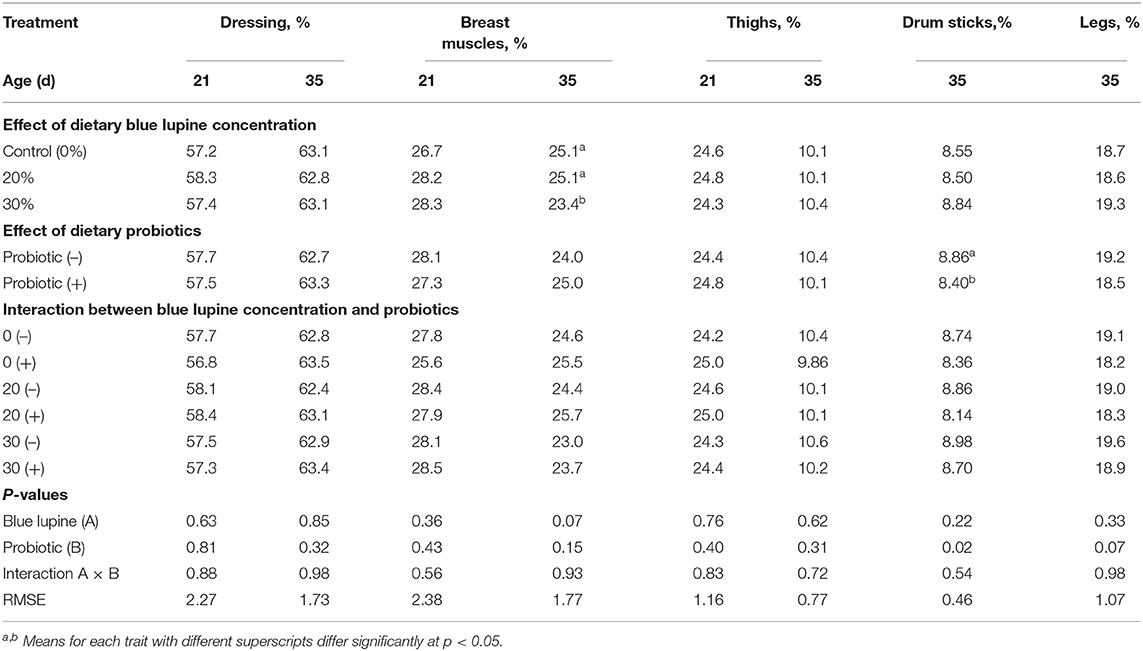
Table 5. Effect of blue lupine and probiotics on carcass traits of broiler chickens at 21 and 35 d of age.
There was no significant effect of the interaction between the blue lupine and probiotic addition on carcass traits at 21 and 35 d (Table 5). A similar trend was observed at 35 d of age with the exception of probiotic addition that significantly decreased the drumstick percentage compared to the unsupplemented control groups (Table 5). This drumstick percentage decrease (5.19%) concurred with numerical increases in the breast muscle percentage (4.17%). The present results demonstrated that blue lupine and probiotics had no adverse effect on carcass traits. These results are in agreement with those reported by (40) who observed that 14.0–25.7% of white lupine in broilers' and turkeys' diets has no adverse effect on slaughter yield and carcass quality. In addition, (49) noticed that 25–30% of blue lupine does not significantly affect the dressing percentage, edible parts, and abdominal fat of turkeys. On the other hand, (55) demonstrated that probiotics at 100 and 150 g/ton feed containing Bacillus spp. and Saccharomyces boulardii, reduced the dressing percentages, breast muscle values, liver weights, and abdominal fat weights; a 50 g/ton diet had no effect.
The inclusion of 20 and 30% of blue lupine had no significant effects on the bursa, thymus, and spleen percentages at 21 and 35 d of age (Table 6). These results indicated that blue lupine had no negative influence on lymphoid organs and immune index of broilers. This suggests that blue lupine diets without or with probiotics provide adequate nutrients for the growth of immune organs.
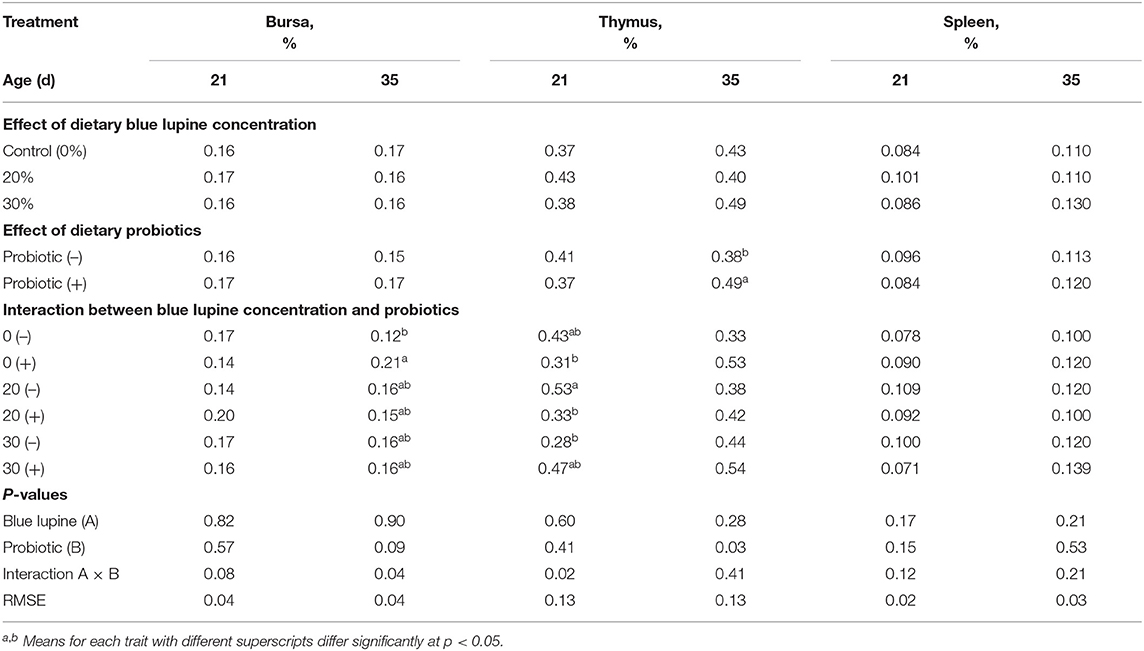
Table 6. Effect of blue lupine and probiotics on the internal body organs of broiler chickens at 21 and 35 d of age.
There was a significant effect of the interaction between the blue lupine and probiotics on the bursa percentage at 35 d of age, as well as the thymus percentage at 21 d of age (Table 6). At 21 d of age, the bursa percentage of broilers that were fed a 20% blue lupine diet had the highest increase due to probiotic addition, compared to probiotic-supplemented control groups and probiotic-unsupplemented 20% blue lupine groups; however, the increase did not reach a level of significance (P = 0.08). At 35 d of age, the bursa percentage of broilers that were fed the control diet supplemented with probiotics, significantly increased when compared to the unsupplemented control group. This trend was different from that observed at 21 d and indicated that the effect of probiotic addition on bursa measurements depends on the age of broilers and the level of blue lupine in their diet (Table 6).
Probiotic addition had no significant influence on the lymphoid organs percentage at 21 d of age (Table 6). The thymus percentage of 35 d-old broilers increased significantly (29.3%) in the probiotic-supplemented group, compared to the unsupplemented group. These results indicate that a prolonged probiotic feeding period until 35 d of age significantly increased the thymus percentage in the probiotic-supplemented group as compared to the unsupplemented group; however, these results are in contrast to what was obtained at 21 d of age. Probiotic addition to a 20% blue lupine diet significantly decreased the thymus percentage (0.33%) as compared to the effects of the same diet without the addition of probiotics on the thymus percentage (0.53%). In addition, a 30% blue lupine diet without the addition of probiotics, significantly decreased the thymus percentage (0.28%) compared to a 20% blue lupine diet without probiotic addition (0.53%). These results indicate that an unsupplemented, 30% blue lupine diet may have a negative effect on the thymus percentage. The addition of probiotics may have caused a complete recovery of the thymus percentage, suggesting a beneficial effect of probiotics on the lymphoid organs of broilers fed 30% blue lupine due to improving gut ecosystem and thus increasing nutrients available for growth of immune organs. Thus, 30% of blue lupine, supplemented with probiotics, could be included in broiler diets without any negative effects on immunity. It is well-known that thymus has an important role in the preparation and development of T-lymphocytes or T cells, extremely important leukocyte types (30). In general, probiotic addition had a beneficial effect on the bursa, thymus, and spleen percentages of broilers that were not fed blue lupine at 35 d of age. These results are similar to those reported by (55, 57), who found that the addition of probiotics improved the immune index of broiler chickens and beneficially modulated gut microflora, particularly in the ceca area and resulted in enhanced growth (23–25).
The 20 and 30% of blue lupine with the addition of probiotics and the interaction between the two variables did not significantly affect the percentages of the liver, heart, and abdominal fat at 35 d of age (Table 7). However, the gizzard percentage at 21 d was significantly reduced (14.5%) by the inclusion of 20% of lupine, compared to the control group. The inclusion of 20 and 30% of blue lupine in broiler diets increased the gizzard percentage by 16.8 and 21.1%, respectively, at 35 d of age. The increase in the gizzard observed at 35 d reflected the increase in the CF percentage of the tested diets and indicated that a prolonged feeding period might be needed for blue lupine to exert an effect.
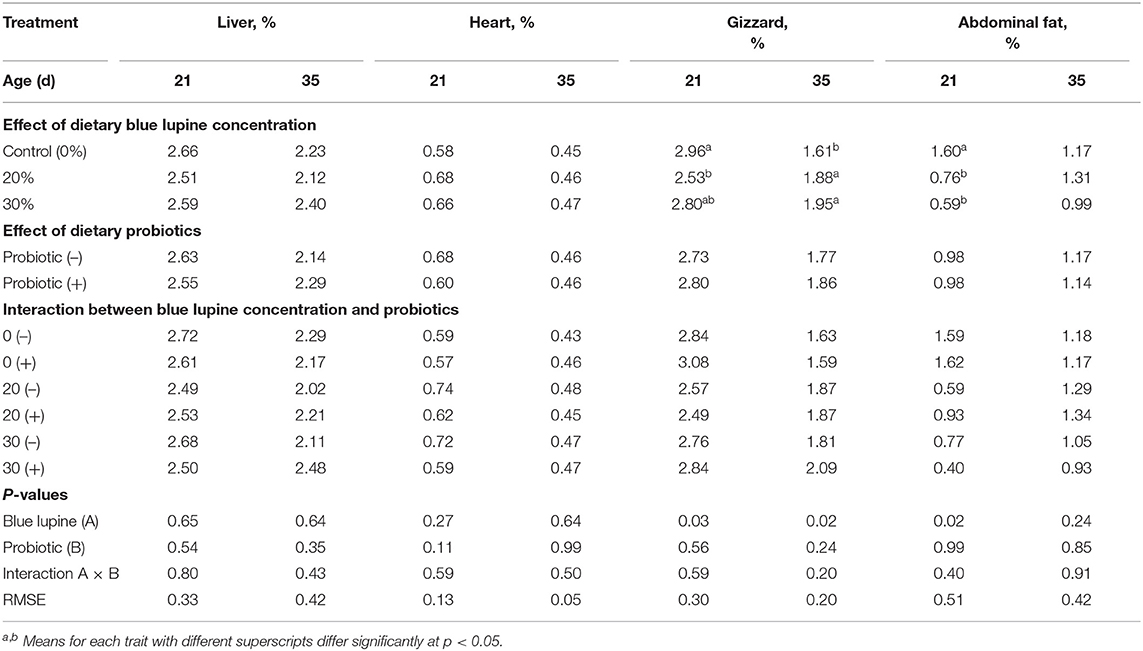
Table 7. Effect of blue lupine and probiotics on internal body organs of broiler chickens at 21 and 35 d of age.
The percentages of abdominal fat at 21 d of age (Table 7) significantly decreased due to feeding 20 and 30% blue lupine diets, by 52.3 and 63.5%, respectively, compared to the control, but this effect diminished at 35 d of age. These beneficial effects indicate that the meat of broilers fed blue lupine diets may be leaner and contain less fat; thus, maybe healthier (1). Similarly, (15, 21, 22) observed that 14.0–25.7% of white lupine in broiler and turkey diets improves the meat quality of carcasses, decreases saturated fatty acids and increases polyunsaturated n-3 fatty acids, thus, making the meat more nutritious and heather.
In general, our results indicate that blue lupine and probiotics had no adverse effects on the liver and the heart. In partial agreement with our results, other research has shown that the addition of 10% of lupine in broiler diets during the first 1–14 d of age and the addition of 15 or 25% from 15 to 35 d of age significantly increases the liver and gizzard weights but does not affect abdominal fat (16). Regarding the effect of probiotics, other authors have demonstrated that probiotics at 100 and 150 g/ton feed containing Bacillus spp. and Saccharomyces boulardii decrease liver weights and abdominal fat (31, 35, 55).
The effect of blue lupine with or without probiotics on duodenum morphology at 21 and 35 d of age are displayed in Tables 8, 9, respectively. Broilers fed a 30% blue lupine diet had a significantly increased duodenum percentage and length at 21 and 35 d of age, respectively, when compared to broilers fed the control diet and the 20% blue lupine diet. The duodenum weight percentage at 35 d of age, the duodenum length percentage at 21 d of age, and the duodenum weight/length ratio at 21 and 35 d of age were not significantly affected by the blue lupine. The duodenum percentage and length increase could be due to the NSP content in lupine, particularly in the groups on the 30% blue lupine diet. The duodenum is involved in feed digestion (16, 50, 58). Lupine has high CF and NSP contents (14). The main NSP in lupine seeds is galactan; this consists of different amounts of arabinose and galactose monosaccharides. NSPs impair gut ecology and reduce the digestibility of poultry diets (2, 6, 15, 16). Thus, pectinase supplementations to 10% lupine diet improve chicken' performance and eliminate anti-nutritional factors, thus enhance nutrient availability. The results of the present study are similar to those reported by (2), who observed that all sections of the intestinal canal as well as the duodenum, jejunum, ileum, and ceca lengths significantly increase in lupine fed groups compared to control groups; there were no effects on mucosa, submucosa, and serosa morphology.
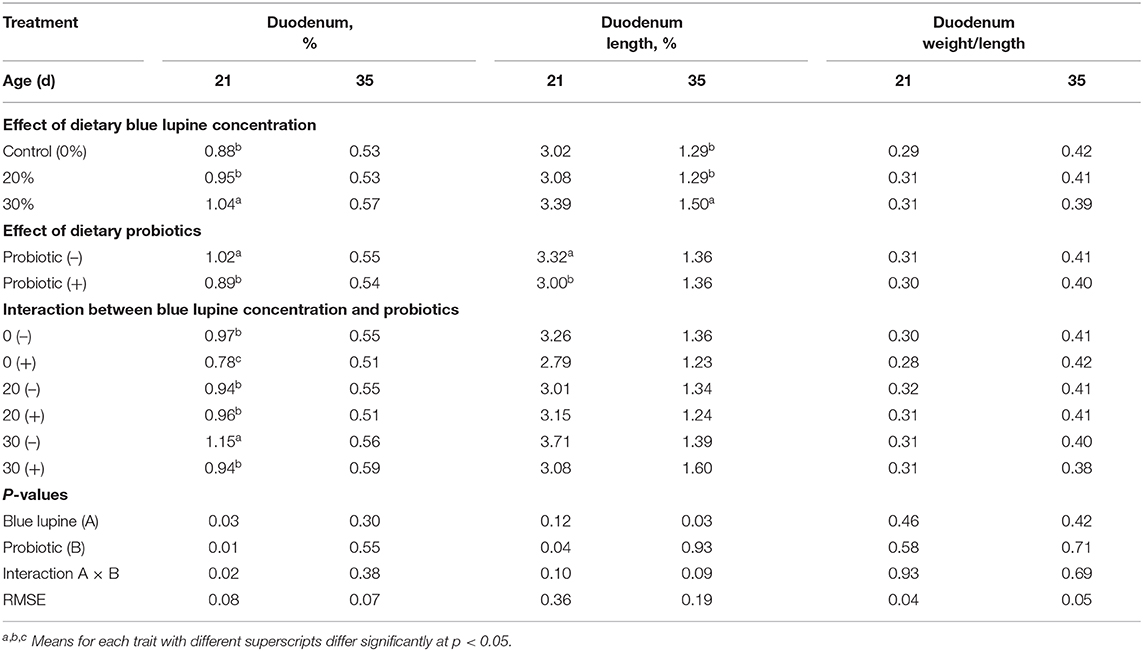
Table 8. Effect of blue lupine, probiotics, and age of chickens on internal body organs of broiler chickens at 21 and 35 d of age.
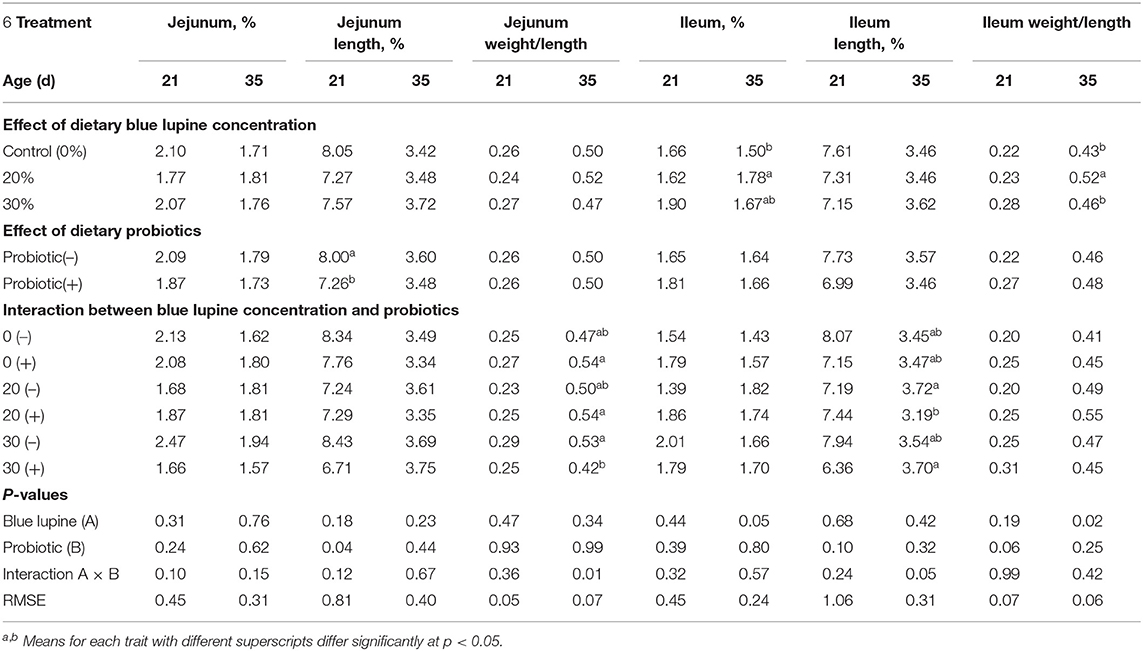
Table 9. Effect of blue lupine and probiotics on jejunum and ileum measurements of broiler chickens at 21 and 35 d of age.
At 21 d of age, a significant reduction in duodenum percentage (12.8%) and duodenum length percentage (9.64%) was observed in chicks that received probiotics, compared to those that were on diets without probiotics (Table 8); this effect disappeared at 35 d of age (Table 8), suggesting that the effect of probiotics may depend on the age of chickens and be more prominent in early age (24, 25).
There was a significant reduction in the duodenum percentage at 21 d of age due to probiotic addition both in control and the 30% blue lupine diet group that amounted to 19.6 and 18.3%, respectively. Probiotic supplementation increased the duodenum length at 35 d of age in broilers that were fed the 30% blue lupine diet (Table 8). Meanwhile, there were no significant effects of the interaction between the blue lupine and probiotics on the duodenum length percentage and the duodenum weight/length ratio (Table 8).
Probiotics decreased the duodenum percentage and duodenum length percentage at 21 d of age; this effect depended on the blue lupine level, but it diminished at 35 d of age (Table 8). These results indicate a reduction in duodenum percentage at 21 d of age in control and 30% blue lupine groups in response to the probiotic addition in the broilers' diet. This temporary adaptive effect shown at 21 d of age may indicate an increase in the absorption surface area that had been diminished after feeding a blue lupine diet for a prolonged period of time (24, 25). These changes indicate that the effect of probiotics on the weight percentage and length percentage of the duodenum depends on the diet profile, age of broilers, and the measurement of the duodenum (6, 15, 23).
Table 9 shows the influence of the blue lupine and/or probiotics on the jejunum and ileum characteristics of broilers at 21 and 35 d of age, respectively. The results show a significant effect of probiotics on the jejunum length percentage, indicating a significant decrease (9.25%) when compared to groups that were not supplemented with probiotics (Table 9). However, the influence of probiotics on the jejunum length percentage at 21 d of age (Table 9) was diminished at 35 d of age (Table 9). These results indicate that the addition of blue lupine and probiotics had no adverse effects on the lower small intestine parts' (jejunum and ileum) morphology; an exception to this was the increase in the ileum percentage and the ileum weight/length ratio of the 20% blue lupine diet group observed at 35 d of age. This effect was not observed at 21 d of age; this indicates that prolonged feeding of a 20% blue lupine diet may exert an effect on the ileum length percentage and weight/length ratio.
There were significant effects of the interaction between blue lupine and probiotics on the jejunum weight/length ratio and ileum length at 35 d. These results indicate that probiotics may have decreased the jejunum weight/length ratio of the 30% blue lupine diet group and the ileum length percentage of the 20% blue lupine diet group.
An increase in the ileum length may reflect an adaptive effect in the absorption site in response to feeding blue lupine at a 20% rate. It is well-known that the lower small intestine is mainly involved in nutrient absorption (58). The results of the present study indicate that broiler chickens could be fed a diet containing up to 30% of blue lupine without adverse effects on their lower small intestine parts' (jejunum and ileum) morphology and function.
Table 10 displays the influences of blue lupine and/or probiotics on the small intestine and ceca morphology at 21 and 35 d of age, respectively. At 21 d of age, the effect of blue lupine and probiotics was not significant (P < 0.05) on the small intestine percentage, small intestine weight /length ratio, and ceca weight/length ratio. However, the addition of probiotics significantly decreased the small intestine length percentage compared to the control group at 21 d of age. There was no significant influence of either the blue lupine or probiotics on the small intestine percentage or the ceca percentage, length, and weight/length ratio at 35 d of age.
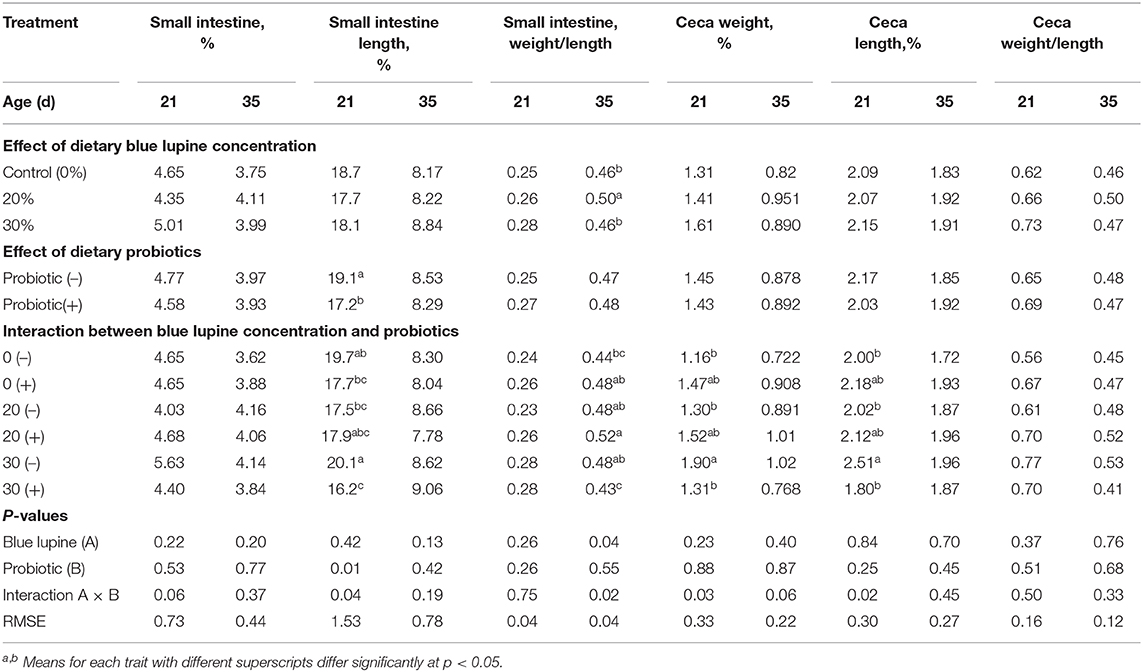
Table 10. Effect of blue lupine and probiotics on small intestine parts of broiler chickens at 21 and 35 d of age.
There was a significant effect of the interaction between the blue lupine level and the probiotics on the small intestine length percentage at 21 d of age, the small intestine weight/length ratio at 35 d of age, and the ceca weight and length percentage at 21 d (Table 10).
At 21 d of age, the small intestine length percentage of broilers fed a 30% blue lupine diet without probiotics, increased significantly compared to that of broilers fed a 20% blue lupine diet without probiotics and the control group with probiotics, reflecting the CF content of the diet. At 35 d of age, the addition of probiotics to a 30% blue lupine diet significantly decreased the small intestine weight/length ratio compared to the other groups.
Generally, broilers offered a 30% blue lupine diet without probiotics, had significantly increased ceca weight and length percentages compared to groups on 0 and 20% blue lupine diets without probiotics. The addition of probiotics to a 30% blue lupine diet diminished the negative effect (enlargement) of blue lupine on the ceca weight and length percentages and the small intestine percentage. This reflected the CF content of the diet and the role of probiotics in improving gut ecology and health (23–25). The main function of ceca is water and electrolyte absorption and fermentation (58). Additionally, it is, to a very large extent, affected by diet, and it enlarges as a consequence of an increased amount of fermentable material, particularly fiber, in the diet (59). The beneficial effects of probiotic supplementation to a 30% blue lupine diet at 21 d of age are similar both in the ceca and the small intestine length percentages (Table 10). Previous research showed also similar results regarding the positive effect of probiotics on gut health (23–25).
The addition of blue lupine (L. Angustifolius) seeds at a 30% rate in broiler diets supplemented with DL-methionine, L-lysine, L-threonine, and probiotics (B. Subtilis) could be a suitable protein source for broilers, without any adverse effects on growth performance, carcass characteristics, immune organs, and gut morphology of broilers. Probiotics also maintain gut enhanced gut health and immune organs and improved growth of broilers on 20 and 30% blue lupine diets. The limitation of this experiment is the small number of replicates (4) which may confound the growth performance results and its application, and thus the number of replicates could be increased in the further experiment.
The datasets generated for this study are available on request to the corresponding author.
The experimental procedures were approved by King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia that recommends animal rights, welfare and minimal stress and did not cause any harm or suffering to animals according to the Royal Decree number M59 in 14/9/1431H.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This article was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah. Therefore, the authors appreciate the technical and financial support of the DSR.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to extend their sincere appreciation to KACST for rendering its facilities for conducting this study.
FCR, feed conversion rate; AMEn, nitrogen corrected metabolizable energy; CP, Crude protein; NFE, Nitrogen free extract (starch + sugar); KACST, King Abdulaziz City for Science and Technology; d, days; EPEI, European production efficiency index; SAS, statistical analyses software; GLM, general linear model; SEM, standard error or mean; CF, crude fiber; CFU, colony-forming unit; NSP, non-starch polysaccharide.
1. Attia YA, Bovera F, Abd-El-Hamid E, Abd-Elrazk E, EL-Din T, Al-Harthi MA, et al. Effect of dietary protein concentrations, amino acids and conjugated linoleic acid supplementations on productive performance and lipid metabolism of broiler chicks. Ital J Anim Sci. (2017) 16:563–72. doi: 10.1080/1828051X.2017.1301228
2. Olkowski AA, Olkowski BI, Amarowicz R, Classen HL. Adverse effects of dietary lupine in broiler chickens. Poult Sci. (2001) 80:621–5. doi: 10.1093/ps/80.5.621
3. Steenfeldt S, González E, Bach Knudsen KE. Effects of inclusion with blue lupins (Lupinus angustifolius) in broiler diets and enzyme supplementation on production performance, digestibility and dietary AME content. Anim Feed Sci Technol. (2003) 110:185–200. doi: 10.1016/S0377-8401(03)00218-9
4. Shi SR, Lu J, Tong HB, Zou JM, Wang KH. Effects of graded replacement of soybean meal by sunflower seed meal in laying hen diets on hen performance, egg quality, egg fatty acid composition, and cholesterol content. J Appl Poult Res. (2012) 21:367–74. doi: 10.3382/japr.2011-00437
5. Pimentel D, Marklein A, Toth MA, Karpoff M, Paul GS, McCormack R, et al. Biofuel impacts on world food supply: use of fossil fuel, land and water resources. Energies. (2008) 1:41–78. doi: 10.3390/en1010041
6. Attia YA, El-Tahawy WS, Abd El-Hamid AE, Nizza A, El-Kelway MI, Al-Harthi MA, et al. Effect of feed form, pellet diameter and enzymes supplementation on carcass characteristics, meat quality, blood plasma constituents and stress indicators of broilers. Arch Tierz. (2014) 57:1–14. doi: 10.7482/0003-9438-57-030
7. Naveed A, Acamovic T, Bedford MR. The influence of carbohydrase and protease addition on amino acid digestibility of lupin-based diets for broiler chicks. Proc Aust Poult Sci Symp. (1999) 11:93–6.
8. Lee MRF, Parkinson S, Fleming HR, Theobald VJ, Leemans DK, Burgess T. The potential of blue lupins as a protein source, in the diets of laying hens. Vet Anim Sci. (2016) 1–2:29–35. doi: 10.1016/j.vas.2016.11.004
9. Mierlita D. Studies on cultivation suitability and nutritional characterization of lupine alkaloid-free varieties. Anal Univ Oradea Ecotox Zootehnie. (2012) 21:501–7.
10. Roth-Maier DA, Paulicks BR. Feeding and nutritional value of sweet blue and yellow lupin seed (Lupinus angustifolius L. and Lupinus luteus L.) for broiler chicks. Arch Geflügelk. (2003) 67:175–8.
11. Palander S, Laurinen P, Perttila S, Valaja J, Partanen K. Protein and amino acid digestibility and metabolizable energy value of pea (Pisum sativum), faba bean (Vicia faba), and lupine (Lupinus angustifolius) seeds for turkeys of different age. Anim Feed Sci Technol. (2006) 127:89–100. doi: 10.1016/j.anifeedsci.2005.07.003
12. Suchý P, Eva S, Ivan H, Ladislav S, Josef V, Leo K. Effect of replacing soybean meal with lupin seed-based meal in chicken diet on performance, carcass value and meat quality. Acta Vet Brno. (2010) 79:195–202. doi: 10.2754/avb201079020195
13. Feeding Lupins to Poultry - eXtension. Available online at: http://articles.extension.org/pages/67050/feeding-lupins-to-poultry (accessed May 20, 2018).
14. Petterson DS. Composition and food uses of lupins. Chapter 12. In: Gladstones JS, Atkins CA, Hamblin J, editors. Lupins as Crop Plants: Biology, Production and Utilization. Wallingford: CAB International (1998). pp. 353–84.
15. Attia YA, El-Tahawy WS, Abd El-Hamid AE, Nizza A, Bovera F, Al-Harthi MA, El-Kelway MI. Effect of feed form, pellet diameter and enzymes addition on growth performance and nutrient digestibility of broiler during days 21–37 of age. Arch Tierz. (2014) 57:1–11. doi: 10.7482/0003-9438-57-034
16. Smulikowska S, Konieczka P, Czerwinski J, Mieczkowska A, Jankowiak J. Feeding broiler chickens with practical diets containing lupin seeds (L. angustifolius or L. luteus): effects of incorporation level and mannanase addition on growth performance, digesta viscosity, microbial fermentation and gut morphology. J Anim Feed Sci. (2014) 23:64–72. doi: 10.22358/jafs/65718/2014
17. Hughes RJ, Choct M, Kocher A, Van Barneveld RJ. Effect of food enzymes on AME and composition of digesta from broiler chickens fed on diets containing non-starch polysaccharides isolated from lupin kernel. Br Poult Sci. (2000) 41:318–23. doi: 10.1080/713654936
18. Williams I, Ali A, Sipsas S. Lupine for Poultry: Mechanical and Enzymatic Improvement of Lupine for Broiler and Layers. Rural Industries Research and Development Corporation. RIRDC Project No WAU-1A. (2005).
19. Mierlita D, Popovici D. Effect of partial substitution of soybean meal with lupine seeds on growth and economic efficiency of broilers. Lucrări Stiintifice-Seria Zootehnie. (2013) 59:60–5.
20. Knudsen KE. Carbohydrate and lignin contents of plant material used in animal feeding. Anim Feed Sci Technol. (1997) 67:319–38. doi: 10.1016/S0377-8401(97)00009-6
21. Tufarelli V, Demauro R, Laudadio V. Dietary micronized-dehulled white lupin (Lupinus albus L.) in meat-type guinea fowls and its influence on growth performance, carcass traits and meat lipid profile. Poult Sci. (2015) 94:2388–94. doi: 10.3382/ps/pev218.
22. Laudadio V, Tufarelli V. Dehulled-micronised lupin (Lupinus albus L. cv. Multitalia) as the main protein source for broilers: influence on growth performance, carcass traits and meat fatty acid composition. J Sci Food Agric. (2011) 91:2081–7. doi: 10.1002/jsfa.4426
23. Vase-Khavari K, Mortezavi SH, Rasouli B, Khusro A, Salem AZM, Seidavi AR. The effect of three tropical medicinal plants and superzist probiotic on growth performance, carcass characteristics, blood constitutes, immune response, and gut microflora of broiler. Trop Anim Health Pro. (2019) 51:33–42. doi: 10.1007/s11250-018-1656-x
24. Hasibuan AS, Silalahi J, Masfria M. The effect of herbal extracts and probiotic feeding on productivity and quality of broilers. Asian J Pharm Clin Res. (2019) 7:5–9. doi: 10.22270/ajprd.v7i3.521
25. Baghban-Kanani P, Hosseintabar-Ghasemabad B, Azimi-Youvalari S, Seidavi AR, Ragni M, Laudadio F, et al. Effects of using artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. J Poult Sci. (2019) 56:120–127. doi: 10.2141/jpsa.0180050
26. Kabir SML, Rahman MM, Rahman MB, Rahman MM, Ahmed SU. The dynamics of probiotics on growth performance and immune response in broilers. Int J Poult Sci. (2004) 3:361–4. doi: 10.3923/ijps.2004.361.364
27. Kabir SML, Rahman MM, Rahman MB. Potentiation of probiotics in promoting microbiological meat quality of broilers. J Bangl Soc Agri Sci Technol. (2005) 2:93–6.
28. Mountzouris KC, Tsistsikos KPE, Nitsh S, Schatzmayr G, Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci. (2007) 86:309–17. doi: 10.1093/ps/86.2.309
29. Official Method of Analysis of the Association of Official Analytical Chemists, 11th edn. Washington, DC: Association of Official Agricultural Chemists (2004).
30. Attia YA, Al-Harthi MA, El-Shafey AS, Rehab YA, Kim WK. Enhancing tolerance of broiler chickens to heat stress by addition with vitamin E, vitamin C and/or probiotics. Ann Anim Sci. (2017) 17:1–15. doi: 10.1515/aoas-2017-0012
31. Attia YA, Abd El-Hamid AE, Ismaiel AM, de Oliveira AMC, Al-Harthi MA, El-Naggar AS, et al. Nitrate detoxification using antioxidants and probiotics in the water of rabbits. Rev Colomb Cienc Pec. (2018) 31:130–8. doi: 10.17533/udea.rccp.v31n2a06
32. Janssen WMMA. European Table of Energy Values for Poultry Feedstuffs, 3rd ed. Beekbergen: Spelderholt Center for Poultry Research and Information Services (1989).
33. National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed. Washington, DC: National Academic Press (1994).
34. Attia YA, Bovera F, Abd El-Hamid AE, Tag El-Din AE, Al-Harthi MA, El-Shafy AS. Effect of zinc bacitracin and phytase on growth performance, nutrient digestibility, carcass and meat traits of broilers. J Anim Physiol Anim Nutr. (2016) 100:485–91. doi: 10.1111/jpn.12397
35. Attia YA, Abd Al-Hamid AE, Allakany HF, Al-Harthi MA, Mohamed NA. Necessity of continuing of addition of non-nutritive feed additive during day 21–42 of age following three weeks of feeding aflatoxin to broiler chickens. J Appl Anim Res. (2016) 44:87–98. doi: 10.1080/09712119.2015.1013964
36. Taylor RD, Jones GPD. The incorporation of whole grain into pelleted broiler chicken diets. Br Poult Sci. (2004) 45:237–46. doi: 10.1080/00071660410001715849
37. Hejdysz MM, Kaczmarek SA, Kubiś M, Jamroz D, Kasprowicz-Potocka M, Zaworska A, et al. Effect of increasing levels of raw and extruded narrow-leafed lupin seeds in broiler diet on performance parameters, nutrient digestibility and AMEN value of diet. J Anim Feed Sci. (2018) 27:55–64. doi: 10.22358/jafs/83015/2018
38. Diaz D, Morlacchini M, Masoero F, Moschini M, Fusconi G, Piva G. Pea seeds (Pisum sativum), faba beans (Vicia faba var. minor) and lupin seeds (Lupinus albus var. multitalia) as protein sources in broiler diets: effect of extrusion on growth performance. Ital J Anim Sci. (2006) 5:43–53. doi: 10.4081/ijas.2006.43
39. SAS. Propriety Software Release 8.1. Cary, NC: Statistical analysis Systems Institute, INC. (2000).
40. Mierlita D. The effect of lupine seed in broiler diet on animal performance and fatty acids profile of their meat. Bull UASVMed Anim Sci Biotechnol. (2015) 72:186–93. doi: 10.15835/buasvmcn-asb:11375
41. Guillaume J, Chenieux JC, Rideau M. Feeding value of Lupinus albus in chicken diets (with emphasis on the role of alkaloids). Nutr. Rep. Int. (1979) 20:57–65.
42. Monteiro MRP, Costa A, Campos SF, Silva MR, Silva C, Martino H et al. Evaluation of the chemical composition, protein quality and digestibility of lupin (Lupinus albus and Lupinus angustifolius). Mundo Saúde. (2014) 38:251–9. doi: 10.15343/0104-7809.20143803251259
43. Tomczak A, Zielinska-Dawidziak M, Piasecka-Kwiatkowska D, Eleonora L-S,. Blue lupine seeds protein content and amino acids composition. Plant Soil Environ. (2018) 64:147–55. doi: 10.17221/690/2017-PSE
44. Mukisira EA. The influence of alkaloids on voluntary intake and performance by ruminants fed diets containing lupin seed in kenya (M.Sc. thesis). Macdonald College of McGill University, Montreal, QC, Canada (1994).
45. King RH. Non Traditional Feed Sources for Use in Swine Production. Stoneham, MA: Butterworths (1990).
46. Wiseman J, Cole DXA. European legumes in diets for non-ruminants. In: Haresign W, Cole DJA, editors. Recent Advances in Animal Nutrition. London: Butterworths (1988) p. 13–37.
47. Aguilera JF, Molina E, Prieto C. Digestibility and energy value of sweet lupin seed (L. albus var.Multolupa) in pigs. Anim Feed Sci Technol. (1985) 12:171–8. doi: 10.1016/0377-8401(85)90010-0
48. Sujak A, Kotlarz A, Strobel W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. (2006) 98:711–19. doi: 10.1016/j.foodchem.2005.06.036
49. Juodka R, Nainiene R, Juskiene V, Juska R, Stuoge I, Leikus R. Effects of different amounts of blue lupine (L. Angustifolius L.). Rev Bras Cienc Avic. (2017) 19:117–24. doi: 10.1590/1806-9061-2016-0240
50. Kaczmarek, SA, Hejdysz, M, Kubis, M, Kasprowicz-Potocka M, Rutkowski A. The nutritional value of yellow lupin (Lupinus luteus L.) for broilers. Anim Feed Sci Tech. (2016) 222:43–53. doi: 10.1016/j.anifeedsci.2016.10.001
51. Yu B, Lin JR, Chiou MY, Hsu YR, Chiou PWS. The effects of probiotic Lactobacillus reuteri pg4 strain on intestinal characteristics and performance in broilers. asian-australas. J Anim Sci. (2007) 20:1243–51. doi: 10.5713/ajas.2007.1243
52. Shargh MSB, Dastar S, Zerehdaran M, Moradi KA. Effects of using plant extracts and a probiotic on performance, intestinal morphology, and microflora population in broilers. J Appl Poult Res. (2012) 21:201–8. doi: 10.3382/japr.2010-00145
53. Allahdo P, Ghodraty J, Zarghi H, Saadatfar Z, Kermanshi H, Dovom MRE. Effect of probiotic and vinegar on growth performance, meat yields, immune responses, and small intestine morphology of broiler chickens. Ital J Anim Sci. (2018) 17:675–85. doi: 10.1080/1828051X.2018.1424570
54. Park JH, Kim IH. Supplemental effect of probiotic bacillus subtilis B2A on productivity, organ weight, intestinal salmonella microflora, and breast meat quality of growing broiler chicks. Poult Sci. (2014) 93:2054–9. doi: 10.3382/ps.2013-03818
55. Manafi M, Hedayati MS. Probiotic bacillus species and saccharomyces boulardii improve performance, gut histology and immunity in broiler chickens. S Afr J Anim Sci. (2018) 48:379–89. doi: 10.4314/sajas.v48i2.19
56. Al-Owaimer AN, Suliman GM, Alyemni AH, Abudabos AM. Effect of different probiotics on breast quality characteristics of broilers under Salmonella challenge. Ital J Anim Sci. (2014) 13:450–4. doi: 10.4081/ijas.2014.3189
57. Ahmad I. Effect of probiotic (Protexin) on the growth of broilers with special reference to the small intestinal crypt cells proliferation (M.Phil thesis) Centre of Biotechnology, University of Peshawar, Peshawar (2004).
58. Svihus, B. Function of the digestive system. J Appl Poult Res. (2014) 23:306–14. doi: 10.3382/japr.2014-00937
Keywords: blue lupine, probiotics, broilers, carcass quality, intestinal morphology, immune organs
Citation: Al-Sagan AA, AL-Yemni AH, Al-Abdullatif AA, Attia YA and Hussein EOS (2020) Effects of Different Dietary Levels of Blue Lupine (Lupinus angustifolius) Seed Meal With or Without Probiotics on the Performance, Carcass Criteria, Immune Organs, and Gut Morphology of Broiler Chickens. Front. Vet. Sci. 7:124. doi: 10.3389/fvets.2020.00124
Received: 29 November 2019; Accepted: 19 February 2020;
Published: 13 March 2020.
Edited by:
Amlan Kumar Patra, West Bengal University of Animal and Fishery Sciences, IndiaReviewed by:
Vito Laudadio, University of Bari Aldo Moro, ItalyCopyright © 2020 Al-Sagan, AL-Yemni, Al-Abdullatif, Attia and Hussein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed A. Al-Sagan, YWJkZWVuQGthY3N0LmVkdS5zYQ==; Youssef A. Attia, eWFhdHRpYUBrYXUuZWR1LnNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.