Corrigendum: Effects of in ovo inoculation of multi-strain lactobacilli on cytokine gene expression and antibody-mediated immune responses in chickens
- 1Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
- 2Department of Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Beni Suef, Egypt
- 3Department of Pathology and Molecular Medicine, McMaster Immunology Research Centre, M. G. DeGroote Institute for Infectious Disease Research, McMaster University, Hamilton, ON, Canada
- 4Department of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, NC, United States
This study was conducted to investigate the effects of various doses of a multi-strain lactobacilli mixture (Lactobacillus salivarius, Lactobacillus reuteri, Lactobacillus crispatus, and Lactobacillus johnsonii) on the innate and adaptive immune responses in broiler chickens. At embryonic day eighteen, 200 eggs were injected with PBS, or three different doses of a multi-strain lactobacilli mixture (1 × 105, 1 × 106, and 1 × 107 CFU/egg, P1, P2, and P3 respectively) along with a group of negative control. On days 5 and 10 post-hatch, cecal tonsil, bursa of fabricius, and spleen were collected for gene expression and cellular analysis. On days 14 and 21 post-hatch, birds were immunized intramuscularly with both sheep red blood cells (SRBC) and keyhole limpet hemocyanin (KLH). Serum samples were collected on days 0, 7, 14, and 21 after primary immunization. The results demonstrated that lactobacilli inoculation increased the splenic expression of cytokines, including interferon (IFN)-α, IFN-β, IFN-γ, interleukin (IL)-8, and IL-12 on day 5 post-hatch compared to the control group (PBS). However, in cecal tonsils, lactobacilli treatment downregulated the expression of IL-6 on day 5 post-hatch and IL-2 and IL-8 on day 10 post-hatch. No significant differences were observed in the expression of cytokine genes in the bursa except for IL-13 which was upregulated in lactobacilli-treated groups P2 and P3 on days 5 and 10 post-hatch. Flow cytometry analysis showed that the percentage of KUL01, CD4+ and CD8+ splenocytes was not affected by treatments. In addition, no significant differences were observed for antibody titers against SRBC. However, lactobacilli treatment (P1, P2, and P3) was found to increase IgM titers on day 21 post-primary immunization compared to controls. Furthermore, in ovo injection of the highest dose of probiotics (1 × 107, P3) increased serum IgG titers against KLH on day 7 post-primary immunization. In conclusion, this study demonstrated that that in ovo administration of lactobacilli can improve antibody-mediated immune responses and differentially modulate cytokine expression in mucosal and systemic lymphoid tissues of chickens.
Introduction
In the poultry industry, it is common for newly hatched chickens to experience delayed access to feed and water due to the time spent in the hatchery and during transportation to the production farm (1). This delay in feed and water intake may negatively influence post-hatch immune system function and bird performance (2). In addition, in broiler chickens, parents do not contribute to egg incubation, and development of the embryo occurs independently of its mother reducing parental influence on gut microbial development (3). Gut microbiota provides essential health benefits to the host by enhancing immune system development and maintaining and regulating intestinal immune homeostasis (4, 5). Recent studies have suggested that dysbiosis in gut microbiota is linked to the pathogenesis of a variety of intestinal disorders (6, 7). In chickens, the establishment of the gut microbiota occurs within 3 days post-hatch and the microbial composition remains relatively unchanged until 30 days of age (8). This indicates that early establishment of beneficial bacteria is very important and can further impact gut microbiota colonization and the development of barrier functions of the gastrointestinal tract (9–11). Therefore, pre-hatch colonization of chickens' gastrointestinal tracts with beneficial bacteria through in ovo technology may prevent pathogen colonization via competitive exclusion in addition to accelerating intestinal and immune system development (10). Different studies have reported the beneficial effects of probiotic bacteria on broiler growth performance, gut microbiota composition and immune system development (12–15). Among these probiotics, Lactobacillus bacteria have received considerable attention because of their immunomodulatory activities and intestinal health benefits (16–18). Lactobacilli are considered autochthonous residents in the chicken gastrointestinal tract and may contribute to the host gut health and immune system function through different mechanisms such as enhancement of the epithelial barrier, competitive exclusion of pathogenic microorganisms, production of antimicrobial substances, and interaction with immune system cells via stimulation of pattern recognition receptors (19, 20). Considering the vulnerability of newly hatched chicks toward various pathogens, pre-hatch administration of Lactobacillus bacteria via in ovo technology can be used as a strategy to strengthen immune responsiveness of chickens and reduce their susceptibility toward pathogens. Many studies suggest that different strains of lactobacilli can modulate multiple aspects of immune response including cytokine and chemokine expression, T lymphocyte populations and systemic antibody-mediated responses (21–23). In the present study, we hypothesized that one-time in ovo administration of a mixture of four Lactobacillus spp. (L. salivarius, L. reuteri, L. crispatus, and L. johnsonii) can modulate innate responses and thus, can accelerate the maturation of the immune system leading to enhanced antibody-mediated responses against thymus-dependent antigens. Therefore, this study was aimed at investigating the potential immunomodulatory effects of in ovo administration of lactobacilli on innate and antibody-mediated immune response in chickens.
Materials and Methods
Chickens and Housing
Embryonated chicken eggs were obtained from the Arkell Poultry Research Hatchery (University of Guelph, ON, Canada). Newly hatched commercial broiler chicks housed in a separated floor pens per each treatment group, on clean wood shavings with free access to water and feed at Arkell Poultry Research.
Experimental Design
In this experiment, the selected Lactobacillus spp. including L. salivarius, L. reuteri, L. crispatus, and L. johnsonii were isolated from the intestinal contents of healthy broiler chickens as previously described (16). Two hundred embryonated broiler chicken eggs were incubated at 37°C at Arkell Research Station (Guelph, ON). On day 18 of incubation, 40 embryonated eggs were injected with one of three different doses of a selected mixture of Lactobacillus bacteria, including 1 × 105 CFU (P1), 1 × 106 CFU (P2), and 1 × 107 CFU (P3) of bacteria or phosphate buffered saline (PBS), all injections were 100 μL total volume. Each lactobacillus was grown separately and prepared at the certain dose from 1 × 105 to 1 × 107 cfu/ml in PBS and the strains were associated in equal amount within the multi-strain cocktail designated for this study. The remaining eggs (24) served as a non-injected untreated negative control, creating 5 groups. The lactobacilli cocktail was delivered precisely to amniotic fluid, where the negative pressure in abdominal cavity facilitates the passage of the intestinal content via peristaltic movement. Lactobacilli used in the present study have been recovered from the intestines of newly hatched chickens (unpublished data). This was assessed using a culture-based method and would be relevant to use in the future to use tagged bacteria for tracking them in the intestine.
Immunization, Serum Collection, and Tissue Sampling
To evaluate antibody-mediated immune responses, on days 14 and 21 post-hatch, birds were immunized intramuscularly with 0.25 mL of 2% SRBC (PML Microbiologicals, Mississauga, ON, Canada) in PBS and subsequently with 0.25 ml of PBS containing 100 μg keyhole limpet hemocyanin (KLH) (Sigma, Oakville, ON, Canada). The untreated, unimmunized group was injected with PBS. Blood samples (1–2 ml) were collected from the wing vein of 12 birds per treatment on days 0, 7, 14, and 21 post primary immunization. Blood samples were kept at room temperature for 2 h and then centrifuged at 580 × g for 10 min to isolate serum. Serum samples were stored at –20°C for antibody analysis. On days 5 and 10 post-hatch 6 birds per treatment were euthanized and bursa of Fabricius, cecal tonsils, and spleen tissues were collected, kept in RNA later and stored at −80°C for gene expression analysis. Spleen tissue was also kept on ice in 1 X Hanks' balanced salt solution (HBSS) (Gibco, Grand Island, NY) for analysis of splenocytes with flow cytometry.
Isolation of Spleen Mononuclear Cells and Flow Cytometry Analysis
Single-cell suspensions of mononuclear cells were prepared according to the procedure of Taha-Abdelaziz et al. (25). Briefly, spleen samples from 6 chickens per treatments were rinsed three times in HBSS and filtered through a 40-μm nylon cell strainer using the flat end of a 1 ml syringe plunger. Cells were resuspended in 5 ml RPMI (Invitrogen, Burlington, Ontario, Canada) containing 10% fetal bovine serum, 2.5% HEPES (Sigma Aldrich, St Louis, MO), 1% Penicillin-Streptomycin (Gibco, Grand Island, NY), 0.5% Gentamicin (Gibco, Grand Island, NY), and 0.05% 2-Mercaptoethanol (Sigma Aldrich, St Louis, MO) and they were overlaid on 4 ml Histopaque-1077 (Sigma, Oakville, ON) for density gradient separation, and mononuclear cells at the interface were harvested and washed twice in RPMI (Gibco, Grand Island, NY) media. Cells were counted using automated cell counter MOXI Z (Orflo, Ketchum, ID, USA) and 100 μL of each cell suspension was seeded in round bottom 96 well plates at density of 1 × 106 /ml in RPMI medium. Subsequently, cells were washed twice in FACS buffer (PBS containing 1% BSA) and stained for 30 min at 4°C in the dark with fluorescent monoclonal antibodies including mouse anti-chicken CD3-PB [CT-3], mouse anti-chicken CD4-PE [CT-4], mouse anti-chicken CD8-APC [CT-8], and mouse anti-chicken monocyte/macrophage-FITC [KUL01] (Southern Biotechnology Associates, Inc., Burlington, ON). The cells were washed twice in FACS buffer, fixed in 2% paraformaldehyde (PFA) and transferred to 5 ml polystyrene round-bottom tubes for analysis. Flow cytometry was performed using a FACS Canto II flow-cytometer (BD Bioscience, San Jose, CA, USA) and data were analyzed using FlowJo Software (v.10).
Serological Analysis
Detection of the total antibody responses to SRBC in sera was performed by a direct hemagglutination assay according to the procedure of Haghighi et al. (26). Serum samples were heat-treated at 56°C for 30 min. Then, 50 μL of PBS containing 0.05% of bovine serum albumin (BSA) was added into each well of a round-bottomed 96-well microplate, and 2-fold serial dilutions of serum samples were generated in duplicate. Subsequently, 50 μL of 1% SRBC in PBS was added to each well and the plates were shaken for 1 min followed by incubation for 24 h at 37°C. Positive result were recorded when at least 50% of SRBC agglutination was observed.
Detection of KLH-specific IgG and IgM titers in sera was performed by indirect enzyme-linked immunosorbent assay (ELISA). Briefly, each well of a flat-bottomed 96-well Maxisorp high binding microplate was coated overnight at 4°C with 100 μL of 1 μg/ml KLH in coating buffer (0.1 M NaHCO3, pH 9.6) containing BSA (30 μg /ml). Wells were then washed 4 times with 200 μL of PBS with 0.05% Tween 20 (P137 Sigma Aldrich Inc., St. Louis, MO) (PBST) and were completely decanted between each washing step. Subsequently, 100 μL of blocking buffer (PBST containing 0.25% of gelatine) was added to each well and the plate was incubated for 2 h at room temperature. Washing was repeated and was followed by addition of 100 μL of chicken serum (diluted 1:200 v/v in blocking buffer) to each well. Plates were incubated 2 h at room temperature and then were washed 4 times with the washing solution. One hundred μL of detection antibody (goat anti-chicken IgG-Fc and IgM-Fc, Bethyl laboratories) conjugated with horseradish peroxidase (diluted in 1/5,000 of blocking buffer) was added to each well and incubated for 1 h at room temperature. Washing was repeated and was followed by addition of 100 μL ABTS [2,2_-azinobis (3 ethylbenzthiazolinesulfonic acid)] peroxidase substrate system (Mandel Scientific, Guelph, ON, Canada) to each well. Plates were incubated for 30 min at room temperature in the dark and absorbance was measured at 405 nm using the micro plate reader (Epoch, BioTek Instruments Inc., Winooski, VT). Positive and negative-control serum (fetal bovine serum) were included in each plate to justify the plate-to-plate variations. Sample/positive (Sp) ratios were calculated according to the following formula: (mean of test sample—mean of negative control)/(mean of positive control—mean of negative control).
RNA Extraction and Reverse Transcription
Total RNA was extracted from spleen, bursa of Fabricius and cecal tonsil tissues using Trizol as described by the manufacturer (Invitrogen Canada Inc., Burlington, ON, Canada). Total RNA was treated with DNase (DNA-free kit, Ambion, Austin, TX) and the quantity and purity of the RNA samples was measured by using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). Reverse-transcription to cDNA was performed by using Superscript® II First Strand Synthesis kit (Invitrogen) according to the manufacturer's protocol.
Quantitative Real-Time PCR
Quantitative real-time (qRT) PCR was performed using the LightCycler® 480 II system (Roche Diagnostics GmbH, Mannheim, DE). Each qRTPCR reaction consisted of 10 μl of 2X SYBR Green Master mix (Roche Diagnostics), 1 μl of forward- and 1 μl of reverse-primer (5 μM), 3 μl PCR-grade water and 5 μl of target cDNA (1:10, diluted in nuclease free-water). The PCR cycling protocol included an initial denaturation step at 95°C, followed by amplification for 40–50 cycles consisting of 95°C for 10 s, an annealing step at a temperature described in Table 1 for each of the primer pairs, and extension at 72°C for 10 s. The primers used were synthesized by Sigma-Aldrich (Oakville, ON), and their specific sequences and accession numbers are presented in Table 1.
Statistical Analysis
The expression levels of all genes were calculated relative to the housekeeping gene (β-actin) using the LightCycler® 480 software (Roche Diagnostics) and data were analyzed by using GLM procedure of SAS (SAS Institute Inc., Cary, NC). Differences among treatment means were determined using Tukey's multiple comparison test after log transformation when error deviations did not have homogenous variance across the treatments. P-value of <0.05 was considered statistically significant.
Results
Hatchability
Hatchability was recoded on the day of the hatch. The results showed that in ovo inoculation of either PBS or lactobacilli did not influence hatchability of the chickens and 99.38% of eggs were hatched following in ovo injection.
Cytokine Gene Expression in Cecal Tonsils, Spleen, and Bursa of Fabricius
The results for gene expression of cytokines are presented in Figures 1–3. In the spleen (Figure 1), the expression of IL-2, IL-6, and IL-13 was not altered by treatment (P > 0.05). However, expression of IFN-α, IFN-γ, and IL-12 on day 5 and IL-8 on day 10 post-hatch was upregulated in the spleen of birds that received 105 CFU of lactobacilli (P1) (P< 0.05). In addition, lactobacilli-treatment of 106 CFU (P2) significantly upregulated the expression of IFN-γ and IL-12 on day 5 and IFN-β on day 10 post-hatch. In the cecal tonsils (Figure 2), expression of IFN-α, IFN-β, IFN-γ, and IL-13 was not affected by lactobacilli administration (P > 0.05) however, it led to downregulation of IL-6 on day 5 and IL-2 and IL-8 on day 10 post-hatch. In contrast, expression of IL-12 was upregulated in lactobacilli-treated groups on days 5 (P1 and P2) and day 10 (P3) post-hatch in the cecal tonsils. No significant differences were observed in cytokine gene expression in the bursa of Fabricius, except for IL-13, which was upregulated on day 5 (P1 and P2) and on day 10 (P2) post-hatch (Figure 3).
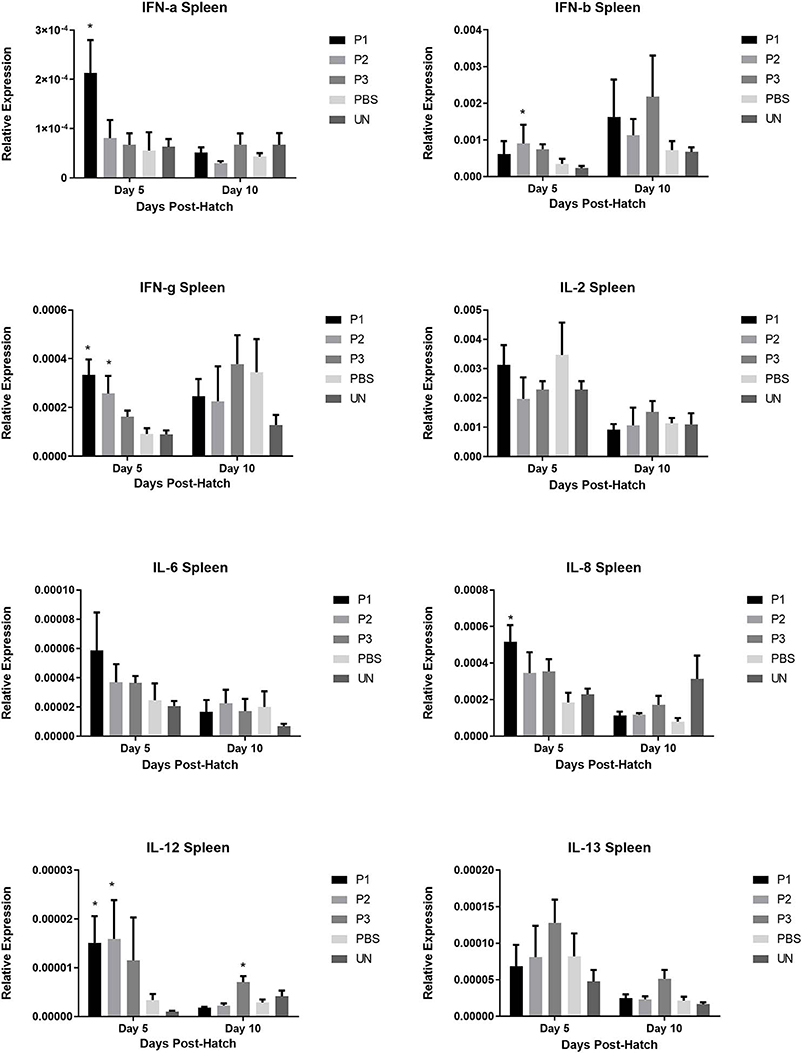
Figure 1. Relative gene expression of IFN-α, IFN-β, IFN-γ, IL-2, IL-6, IL-8, IL-12, and IL-13 in the spleen of chickens at days 5 and 10 post-hatch. Samples collected from 6 birds per treatment. Treatment groups were as follows: P1, P2, and P3 received 1 × 105, 1 × 106, 1 × 107 CFU/egg of a selected mixture of Lactobacillus bacteria (Lactobacillus salivarius, Lactobacillus reuteri, Lactobacillus crispatus, and Lactobacillus johnsonii), respectively (PBS, phosphate-buffered saline group; and UN, non-injected eggs). The reference gene (Beta-actin) was used for relative gene expression. Statistical significance among treatment groups was calculated using one-way ANOVA followed by Tukey's comparison test. Error bars represent standard errors of the mean. Results were considered statistically significant from the control group if P < 0.05. *Bars with asterisks differ significantly from control (PBS) group.
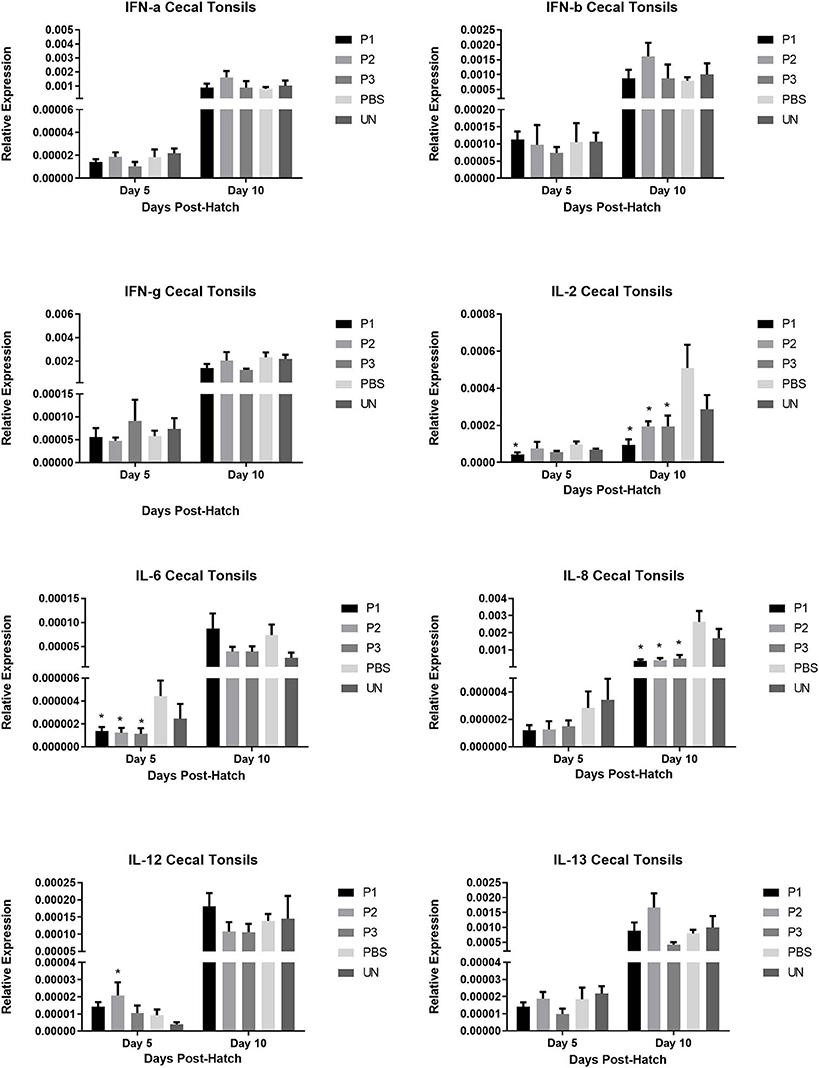
Figure 2. Relative gene expression of IFN-α, IFN-β, IFN-γ, IL-2, IL-6, IL-8, IL-12, and IL-13 in the bursa of Fabricius of chickens on days 5 and 10 post-hatch. Samples collected from 6 birds per treatment. Treatment groups were as follows: P1, P2, and P3 received 1 × 105, 1 × 106, 1 × 107 CFU/egg of a selected mixture of Lactobacillus bacteria (L. salivarius, L. reuteri, L. crispatus, and L. johnsonii) respectively (PBS, phosphate-buffered saline group; and UN, non-injected eggs). The reference gene (Beta-actin) was used for relative gene expression. Statistical significance among treatment groups was calculated using one-way ANOVA followed by Tukey's comparison test. Error bars represent standard errors of the mean. Results were considered statistically significant from the control group if P < 0.05. *Bars with asterisks differ significantly from control (PBS) group.
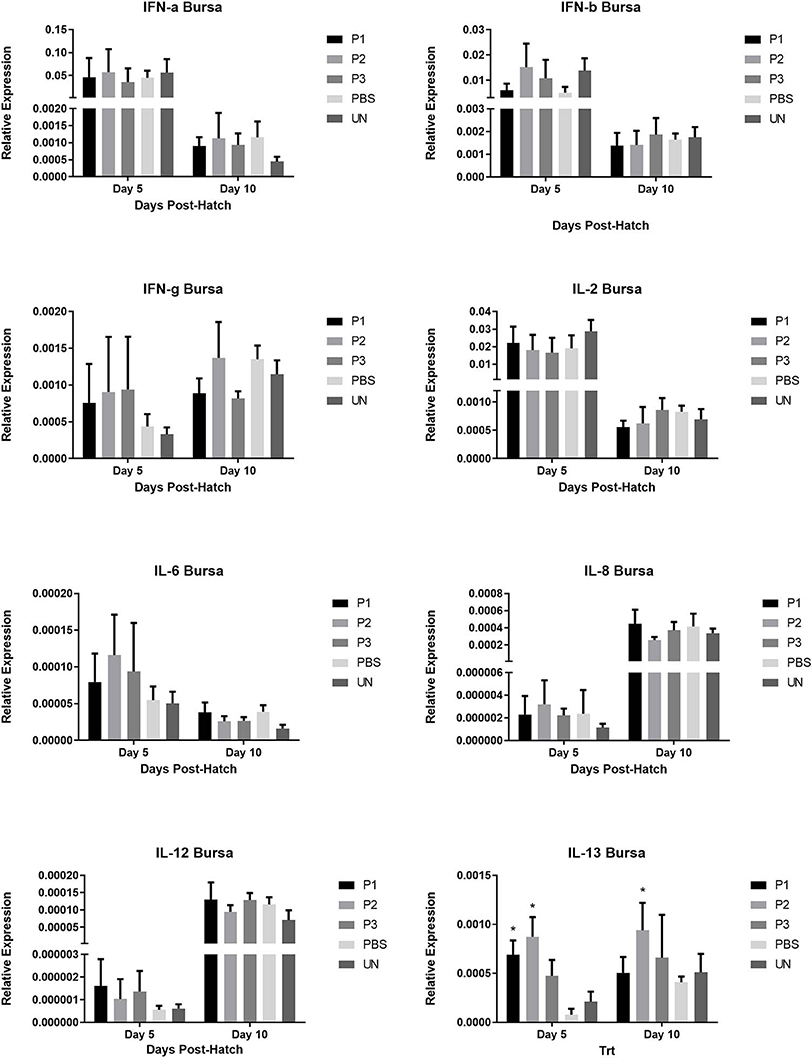
Figure 3. Relative gene expression of IFN-α, IFN-β, IFN-γ, IL-2, IL-6, IL-8, IL-12, and IL-13 in the cecal tonsils of chickens on days 5 and 10 post-hatch. Samples collected from 6 birds per treatment. Treatment groups were as follows: P1, P2, and P3 received 1 × 105, 1 × 106, 1 × 107 CFU/egg of a selected mixture of Lactobacillus bacteria (L. salivarius, L. reuteri, L. crispatus, and L. johnsonii) respectively (PBS, phosphate-buffered saline group; and UN, non-injected eggs). The reference gene (Beta-actin) was used for relative gene expression. Statistical significance among treatment groups was calculated using one-way ANOVA followed by Tukey's comparison test. Error bars represent standard errors of the mean. Results were considered statistically significant from the control group if P < 0.05. *Bars with asterisks differ significantly from control (PBS) group.
T Lymphocyte and Monocyte/Macrophage Populations
Results for the flow cytometric analysis KUL01 and T lymphocyte subpopulations in the spleen (CD4+ and CD8+) are presented in Figure 4. Inoculation of eggs with lactobacilli did not change the population of monocyte/macrophage and T cell subsets (single positive CD3+CD4+ and CD3+CD8+) in the spleen (P > 0.05).
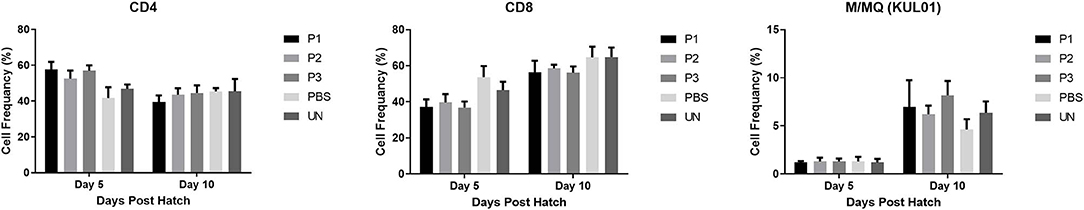
Figure 4. T cell subsets and monocyte/macrophage (%) in the spleen of chickens following in ovo inculcation of Lactobacillus bacteria at days 5 and 10 post-hatch. Samples collected from 6 birds per treatment. Treatment groups were as follows: P1, P2, and P3 received 1 × 105, 1 × 106, 1 × 107 CFU/egg of a selected mixture of Lactobacillus bacteria (L. salivarius, L. reuteri, L. crispatus, and L. johnsonii) respectively (PBS, phosphate-buffered saline group; and UN, non-injected eggs). Statistical significance among treatment groups was calculated using one-way ANOVA followed by Tukey's comparison test. Error bars represent standard errors of the mean. Results were considered statistically significant from the control group if P < 0.05.
Anti-SRBC and Anti-KLH Antibody Titres
The results for antibody-mediated immune responses against SRBC are presented in Figure 5. At 7, 14, and 21 days post-primary immunization, higher antibody titers against SRBC were observed in all immunized group compared to the non-immunized control group (P < 0.05). Nevertheless, inoculation of eggs with Lactobacillus bacteria did not affect serum anti-SRBC antibody titers (P > 0.05).
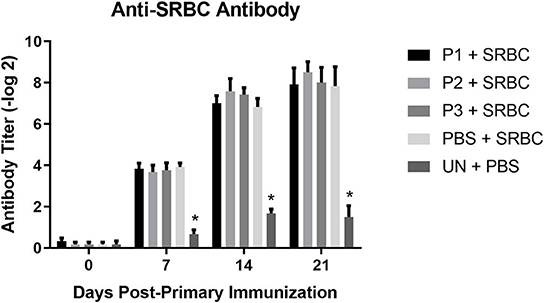
Figure 5. Serum anti-SRBC antibody titers as determined by direct hemagglutination assay. Treatment groups were as follows: P1, P2, and P3 received 1 × 105, 1 × 106, 1 × 107 CFU/egg of a selected mixture of Lactobacillus bacteria (L. salivarius, L. reuteri, L. crispatus, and L. johnsonii) respectively and immunized with SRBC (P1 + SRBC, P2 + SRBC, and P3 + SRBC); chickens received 100 μl of phosphate-buffered saline/egg and were immunized with SRBC (PBS + SRBC); and chickens from non-injected eggs that were injected with PBS served as a control group (PBS). Serum samples collected from 12 birds per treatment. Statistical significance among treatment groups was calculated using one-way ANOVA followed by Tukey's comparison test. Error bars represent standard errors of the mean. Results were considered statistically significant from the control group if P < 0.05. *Bars with asterisks differ significantly from control (PBS) group.
The results for antibody-mediated immune responses against KLH are presented in Figure 6. At 7, 14, and 21 days post-primary immunization, higher antibody titers against KLH were observed in all immunized groups compared with the non-immunized control group (P < 0.05). In addition, lactobacilli treatment at a dose of 107 CFU (P3) significantly enhanced serum IgG and IgM titers against KLH on day 7 and day 21 post-primary immunization, respectively.
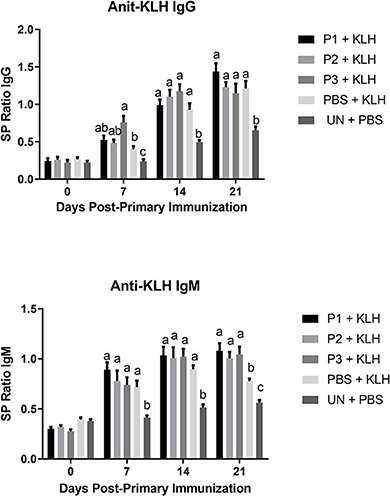
Figure 6. Serum anti-KLH IgG and IgM titers as determined by indirect ELISA. Treatment groups were as follow: P1, P2, and P3 received 1 × 105, 1 × 106, 1 × 107 CFU/egg of a selected mixture of Lactobacillus bacteria (L. salivarius, L. reuteri, L. crispatus, and L. johnsonii), respectively and immunized with KLH (P1 + KLH, P2 + KLH, and P3 + KLH); chickens received 100 μl of phosphate-buffered saline/egg and were immunized with KLH (PBS + KLH); and chickens from non-injected eggs that were injected with PBS served as control group (PBS). Serum samples collected from 12 birds per treatment. Statistical significance among treatment groups was calculated using one-way ANOVA followed by Tukey's comparison test. Error bars represent standard errors of the mean. Results were considered statistically significant from the control group if P < 0.05. (a–c) Means with no common superscripts differ significantly.
Discussion
In ovo technology was first introduced to the poultry industry several decades ago for vaccination against Marek's disease virus (27). This technique enables the delivery of various pharmaceuticals and biological supplements to chicken embryos during embryonation (28). One candidate supplement that can be administered in ovo to provide health benefits to the chickens are probiotics. It has been reported that the gut microbiota plays a critical role in development and regulation of the immune system (29). Probiotics may enhance immune responses and control pathogen infections in chickens by improving and restoring gut microflora (30). Several studies have reported the immunomodulatory activities of probiotics in chickens (16, 26, 31, 32). Therefore, the present study was conducted to evaluate the effects of in ovo inoculation of lactobacilli on innate and adaptive immune responses of chickens.
In the current study, expression of IL-2 was down-regulated in the cecal tonsils of lactobacilli-treated birds. IL-2 is mainly produced by activated T lymphocytes and is involved in the proliferation and activation of both T helper and cytotoxic T cells (33). Downregulation of IL-2 in lactobacilli-treated birds suggests immunomodulatory properties of these bacteria in the absence of an infection. This suggestion can be supported by our observation that there was also a downregulation of IL-6 and IL-8 in the cecal tonsils of lactobacilli-treated birds, thus indicating that Lactobacillus bacteria might help maintaining immune homeostasis in the chicken intestine.
The results of previous studies indicate that dysbiosis of gut microbiota caused by a microbial challenge or an infectious disease is often associated with an activation of the immune system and upregulation of cytokines in secondary lymphoid organs in chickens (34, 35). Probiotics are thought to play a key role in maintaining the normal intestinal microbiota by reducing the population of pathogenic microorganisms though different processes, including competitive exclusion, inhibition of pathogen adhesion, and production of anti-pathogenic substances (19). In the present study, lactobacilli treatment downregulated the expression of cytokines (especially inflammatory cytokines) in cecal tonsils which are considered an intestinal lymphoid organs. This indicates that Lactobacillus bacteria might maintain microbial balance in the intestinal ecosystem by decreasing the population of pathogenic bacteria, thus preventing activation of the immune system. Decreased inflammatory responses to commensal bacteria within gut-associated lymphoid tissues (GALT) has been reported in previous studies, suggesting that although immune system cells in GALT can mount an inflammatory response toward pathogenic bacteria, they also remain slightly responsive to commensal bacteria (36).
Unlike in the cecal tonsils, the expression of cytokines was upregulated in the spleen, suggesting that lactobacilli might differentially modulate cytokine expression profiles in systemic (spleen) and local (cecal tonsils) secondary lymphoid organs. Gene expression in the bursa of Fabricius demonstrated that among all cytokines, only the expression of IL-13 was upregulated in lactobacilli-treated groups. Bursa of fabricius is considered as the primary lymphoid organs for B cell development and differentiation in newly hatched chick-s (37); and IL-13 is a T helper type 2 anti-inflammatory cytokine with the function closely related to IL-4 including stimulation of activated B cells, and differentiation of B cells into plasma cells (38). Therefore, higher expression of IL-13 in the bursa of Fabrocius of lactobacilli-treated birds suggests the role of lactobacilli as beneficial commensal bacteria in B cell development. It has been previously reported that germ-free animals show impaired immune responses against different antigens suggesting the critical role of commensal bacteria in immune system development (39). In chickens, diversification of immunoglobulin mostly occurs during embryonic development, challenging the role of microbiota in pre-hatch B cells development and Ig diversification. However, it is reported that shortly after hatch, gut microbiota appears to influence the B-lymphocyte repertoire in bursa through transepithelial pinocytotic flow of intestinal contents into bursal follicles that occurred by M cell-like follicle-associated epithelium (24, 40). To this end, our observation of augmented IL-13 expression in the bursa can imply that in ovo administration of probiotic lactobacilli can influence bursal development of B cells.
In this study, we evaluated the effects of a mixture of Lactobacillus bacteria on CD4+ and CD8+ cell populations in chicken splenocytes. T helper cells (CD4+) are involved in various immune system processes such as activation of B cells, macrophages and cytotoxic T cells (41). In addition, they play a key role in generating adaptive immune responses through interaction with major histocompatibility complex (MHC) class II molecules on antigen presenting cells (42). Inoculation of embryonated eggs with lactobacilli did not change the percentage of CD4+ splenocytes on days 5 and 10 post-hatch. In contrast, Dalloul et al. (43) demonstrated that feeding lactobacilli to chickens increased the percentage of CD4+ intestinal intraepithelial lymphocytes. Similarly, Noujaim et al. (22) showed that administration of a mixture of Lactobacillus bacteria including L. acidophilus and L. reuteri increased the number of CD4+ cells in the small intestine of chickens. The percentage of CD8+ T cells in the current study was not significantly affected by lactobacilli treatment. Asgari et al. (44) also observed no significant differences in CD8+ cell counts in immune system organs (cecal tonsil and bursa of fabricius) of chickens treated with lactobacilli. However, Noujaim et al. (22) demonstrated that oral treatment of L. reuteri and L. acidophilus increased the number of CD8+ cells in the epithelium and in the intestinal lamina propria of chickens. The inconsistent results observed in these studies could be attributed to the different types and dosages, including regimens of Lactobacillus bacteria in addition to differences in the route of administration used in different studies. The present results demonstrated that in ovo inoculation of eggs with lactobacilli enhanced serum IgG and IgM responses against KLH when a dose of 107 CFU was administered. In agreement with this result, previous studies have demonstrated that dietary/oral administration of probiotic bacteria enhances antibody responses against KLH, infectious bursal disease virus and avian influenza virus (16, 44, 45). Unlike KLH, lactobacilli treatment did not affect antibody production against SRBC. Similarly, Qorbanpour et al. (46) showed that dietary supplementation with multi-strain probiotics did not change antibody production against SRBC. In contrast, other studies demonstrated that dietary or oral administration of probiotic bacteria improves antibody response to SRBC (26, 47). In another study, Brisbin et al. (16) demonstrated that oral treatment of chickens with L. salivarius significantly increased serum antibody responses against SRBC compared to the control group; however, no such effect was observed when chickens were treated with L. reuteri and L. acidophilus. The conflicting results regarding the effects of lactobacilli on antibody-mediated immune response observed in different studies suggests that the immunomodulatory activities of Lactobacillus bacteria likely cannot be generalized at this point due to a number of factors such as the strain and dose of Lactobacillus bacteria, administration route, immunization regimen, timing of administration and experimental conditions.
In conclusion, the results of the current study demonstrated that in ovo inoculation of lactobacilli downregulated cytokine gene expression in the cecal tonsils, indicating the anti-inflammatory capacity of these bacteria in the intestine. However, elevated expression of cytokines observed in the spleen of Lactobacillus-treated birds suggested that lactobacilli may have different immunomodulatory activities in local and systemic secondary lymphoid organs. In addition, lactobacilli-treated groups, enhanced specific antibody-mediated immune responses against a highly immunogenic T cell-dependent antigen (KLH), suggesting the stimulatory effects these bacteria have on adaptive immunity. On the other hand, Lactobacillus bacteria did not have significant effects on T cell subsets in the spleen. Therefore, further studies are needed to investigate the effects of in ovo administration of lactobacilli on T and B cells population in the local and systemic immune system organs of chickens, in addition to further exploring the protecting effects of in ovo-inoculated lactobacilli against challenge with an infectious pathogen.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by Animal Care Committee, University of Guelph.
Author Contributions
MA conceived and designed the project, collected and analyzed the data, and prepared the manuscript. BS, JA, KT-A, SK, JB, and RK helped for sample collection and reviewed the manuscripts, and provided suggestion and comments. SS provided intellectual input, approved the protocol, reviewed the manuscript and provided critical thinking, suggestion and comments.
Funding
This research was supported with funds from the Canadian Poultry Research Council, Natural Sciences and Engineering Research Council and the Ontario Ministry of Agriculture, Food and Rural Affairs. This research was supported in part by the University of Guelph's Food from Thought initiative, thanks to funding from the Canada First Research Excellence Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge the support of Arkell Research Station and OVC Isolation Staff.
References
1. Wang Y, Li Y, Willems E, Willemsen H, Franssens L, Koppenol A, et al. Spread of hatch and delayed feed access affect post hatch performance of female broiler chicks up to day 5. Animal. (2014) 8:610–7. doi: 10.1017/S175173111400007X
2. Shinde Tamboli AS, Goel A, Mehra M, Rokade JJ, Bhadauria P, Yadav AS, et al. Delayed post-hatch feeding affects the performance and immunocompetence differently in male and female broiler chickens. J Appl Anim Res. (2018) 46:306–13. doi: 10.1080/09712119.2017.1299739
3. Taha-Abdelaziz K, Hodgins DC, Lammers A, Alkie TN, Sharif S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: a review. Vet Immunol Immunopathol. (2018) 201:1–11. doi: 10.1016/j.vetimm.2018.05.001
4. Peterson CT, Sharma V, Elmén L, Peterson SN. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. (2015) 179:363–77. doi: 10.1111/cei.12474
5. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut microbes. (2012) 3:4–14. doi: 10.4161/gmic.19320
6. Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. (2015) 26:26191. doi: 10.3402/mehd.v26.26191
7. Chang C, Lin H. Dysbiosis in gastrointestinal disorders. Best Pract Res Clin Gastroenterol. (2016) 30:3–15. doi: 10.1016/j.bpg.2016.02.001
8. Apajalahti J, Kettunen A, Graham H. Characteristics of the gastrointestinal microbial communities, with special reference to the chicken. Worlds Poult Sci J. (2004) 60:223–32. doi: 10.1079/WPS200415
9. Ding J, Dai R, Yang L, He C, Xu K, Liu S, et al. Inheritance and establishment of gut microbiota in chickens. Front Microbiol. (2017) 8:1967. doi: 10.3389/fmicb.2017.01967
10. Schokker D, Veninga G, Vastenhouw SA, Bossers A, de Bree FM, Kaal-Lansbergen LM, et al. Early life microbial colonization of the gut and intestinal development differ between genetically divergent broiler lines. BMC Genomics. (2015) 16:418. doi: 10.1186/s12864-015-1646-6
11. Lilburn MS, Loeffler S. Early intestinal growth and development in poultry. Poult Sci. (2015) 94:1569–76. doi: 10.3382/ps/pev104
12. An BK, Cho BL, You SJ, Paik HD, Chang H-I, Kim SW, et al. Growth performance and antibody response of broiler chicks fed yeast derived β-glucan and single-strain probiotics. Asian-Australasian J Anim Sci. (2008) 21:1027–32. doi: 10.5713/ajas.2008.70571
13. Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, Sanei B, et al. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. (2006) 13:975–80. doi: 10.1128/CVI.00161-06
14. Li YB, Xu QQ, Yang CJ, Yang X, Lv L, Yin CH, et al. Effects of probiotics on the growth performance and intestinal micro flora of broiler chickens. Pak J Pharm Sci. (2014) 27:713–7.
15. Pereira R, Bortoluzzi C, Durrer A, Fagundes NS, Pedroso AA, Rafael JM, et al. Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy. J Anim Physiol Anim Nutr. (2019) 103:72–86. doi: 10.1111/jpn.13004
16. Brisbin JT, Gong J, Orouji S, Esufali J, Mallick AI, Parvizi P, et al. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. (2011) 18:1447–55. doi: 10.1128/CVI.05100-11
17. Forte C, Manuali E, Abbate Y, Papa P, Vieceli L, Tentellini M, et al. Dietary Lactobacillus acidophilus positively influences growth performance, gut morphology, and gut microbiology in rurally reared chickens. Poult Sci. (2017) 97:930–6. doi: 10.3382/ps/pex396
18. Stephenson DP, Moore RJ, Allison GE. Lactobacillus strain ecology and persistence within broiler chickens fed different diets: identification of persistent strains. Appl. Environ. Microbiol. (2010) 76:6494–503. doi: 10.1128/AEM.01137-10
19. Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. (2012) 61:160–74. doi: 10.1159/000342079
20. Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol. (2016) 69:187–203. doi: 10.1136/jclinpath-2015-202976
21. Brisbin JT, Gong J, Parvizi P, Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. (2010) 17:1337–43. doi: 10.1128/CVI.00143-10
22. Noujaim JC, Filho RA, Lima ET, Okamoto AS, Amorim RL, Neto RT. Detection of T lymphocytes in intestine of broiler chicks treated with Lactobacillus spp. and challenged with Salmonella enterica serovar Enteritidis. Poult Sci. (2008) 87:927–33. doi: 10.3382/ps.2007-00476
23. Wang Z, Yu Q, Fu J, Liang J, Yang Q. Immune responses of chickens inoculated with recombinant L actobacillus expressing the haemagglutinin of the avian influenza virus. J Appl Microbiol. (2013) 115:1269–77. doi: 10.1111/jam.12325
24. Luckheeram Sayegh CE, Demaries SL, Iacampo S, Ratcliffe MJ. Development of B cells expressing surface immunoglobulin molecules that lack V (D) J-encoded determinants in the avian embryo bursa of Fabricius. Proc Natl Acad Sci U S A. (1999) 96:10806–11. doi: 10.1073/pnas.96.19.10806
25. Taha-abdelaziz K, Alkie TN, Hodgins DC, Shojadoost B, Sharif S. Characterization of host responses induced by Toll-like receptor ligands in chicken cecal tonsil cells. Vet Immunol Immunopathol. (2016) 174:19–25. doi: 10.1016/j.vetimm.2016.04.002
26. Haghighi HR, Gong J, Gyles CL, Hayes MA, Sanei B, Parvizi P, et al. Modulation of antibody-mediated immune response by probiotics in chickens. Clin Diagn Lab Immunol. (2005) 12:1387–92. doi: 10.1128/CDLI.12.12.1387-1392.2005
27. Sharma JM, Burmester BR. Resistance of Marek's disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. (1982) 26:134–49. doi: 10.2307/1590032
28. Bakyaraj S, Bhanja SK, Majumdar S, Dash B. Modulation of post-hatch growth and immunity through in ovo supplemented nutrients in broiler chickens. J Sci Food Agric. (2012) 92:313–20. doi: 10.1002/jsfa.4577
29. Rhee KJ, Sethupathi P, Driks A, Lanning DK, Knight KL. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. (2004) 172:1118–24. doi: 10.4049/jimmunol.172.2.1118
30. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. (2013) 6:39–51. doi: 10.1177/1756283X12459294
31. Bai SP, Wu AM, Ding XM, Lei Y, Bai J, Zhang KY, et al. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult Sci. (2013) 92:663–70. doi: 10.3382/ps.2012-02813
32. Wang H, Ni X, Qing X, Liu L, Lai J, Khalique A, et al. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front Immunol. (2017) 8:1592. doi: 10.3389/fimmu.2017.01592
33. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. (2012) 12:180–90. doi: 10.1038/nri3156
34. De Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. (2017) 152:1–12. doi: 10.1111/imm.12765
35. Yu LCH. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. (2018) 25:79. doi: 10.1186/s12929-018-0483-8
36. Tanoue T, Umesaki Y, Honda K. Immune responses to gut microbiota-commensals and pathogens. Gut microbes. (2010) 1:224–233. doi: 10.4161/gmic.1.4.12613
37. Ko KH, Lee IK, Kim G, Gu MJ, Kim HY, Park BC, et al. Changes in bursal B cells in chicken during embryonic development and early life after hatching. Sci Rep. (2018) 8:16905. doi: 10.1038/s41598-018-34897-4
38. Wynn TA. IL-13 effector functions. Annu Rev Immunol. (2003) 21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142
39. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. (2012) 336:1268–73. doi: 10.1126/science.1223490
40. Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. (1973) 136:55–77. doi: 10.1002/aja.1001360406
41. Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+ T cells: differentiation and functions. Clin Dev Immunol. (2012) 2012:925135. doi: 10.1155/2012/925135
42. Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. (2000) 165:5558–65. doi: 10.4049/jimmunol.165.10.5558
43. Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult Sci. (2003) 82:62–6. doi: 10.1093/ps/82.1.62
44. Asgari F, Madjd Z, Falak R, Bahar MA, Nasrabadi MH, Raiani M, Shekarabi M. Probiotic feeding affects T cell populations in blood and lymphoid organs in chickens. Benef Microbes. (2016) 7:669–75. doi: 10.3920/BM2016.0014
45. Qorbanpour Naseem S, Rahman SU, Shafee M, Sheikh AA, Khan A. Immunomodulatory and growth-promoting effect of a probiotic supplemented in the feed of broiler chicks vaccinated against infectious bursal disease. Braz J Poult Sci. (2012) 14:109–13. doi: 10.1590/S1516-635X2012000200004
46. Qorbanpour M, Fahim T, Javandel F, Nosrati M, Paz E, Seidavi A, et al. Effect of dietary ginger (Zingiber officinale Roscoe) and multi-strain probiotic on growth and carcass traits, blood biochemistry, immune responses and intestinal microflora in broiler chickens. Animals (Basel). (2018) 8:117. doi: 10.3390/ani8070117
Keywords: lactobacilli, in ovo, chickens, cytokines, antibody
Citation: Alizadeh M, Shojadoost B, Astill J, Taha-Abdelaziz K, Karimi SH, Bavananthasivam J, Kulkarni RR and Sharif S (2020) Effects of in ovo Inoculation of Multi-Strain Lactobacilli on Cytokine Gene Expression and Antibody-Mediated Immune Responses in Chickens. Front. Vet. Sci. 7:105. doi: 10.3389/fvets.2020.00105
Received: 27 October 2019; Accepted: 11 February 2020;
Published: 28 February 2020.
Edited by:
Xin Zhao, McGill University, CanadaReviewed by:
Pascale Quéré, Institut National de la Recherche Agronomique, FranceChristi Swaggerty, United States Department of Agriculture (USDA), United States
Copyright © 2020 Alizadeh, Shojadoost, Astill, Taha-Abdelaziz, Karimi, Bavananthasivam, Kulkarni and Sharif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shayan Sharif, shayan@uoguelph.ca
 Mohammadali Alizadeh
Mohammadali Alizadeh Bahram Shojadoost1
Bahram Shojadoost1 Khaled Taha-Abdelaziz
Khaled Taha-Abdelaziz Seyed Hossein Karimi
Seyed Hossein Karimi Shayan Sharif
Shayan Sharif