- 1Arid Land Agriculture Department, Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Animal and Poultry Production Department, Faculty of Agriculture, Damanhur University, Damanhur, Egypt
- 3Environment and Life Sciences Research Center, Kuwait Institute for Scientific Research, Kuwait City, Kuwait
- 4Poultry and Fish Diseases Department, Faculty of Veterinary Medicine, Damanhour University, Damanhur, Egypt
This study was executed to investigate the effect of supplementing three multienzyme levels (0, 0. 1, and 0.2%) with two types of diet [standard diet (SD) vs. low-density diet (LDD)] on immune response, blood hematology and biochemistry, antioxidant status, and organ histology of broilers during 1–38 days of age. A total of 216 unsexed 1-day-old Arbor Acres broiler chicks were randomly distributed, on a factorial design (2 × 3), to six treatments each with six replicates. There were six chicks per replicate. Results showed that LDD significantly decreased body weight gain (BWG) of broilers, but did not affect the European Production Efficiency Index (EPEI). Addition of multienzymes at both levels (0.1 and 0.2%) significantly increased BWG and improved EPEI, compared to the control diet. Alanine aminotransferase (ALT), aspirate aminotransferase (AST), malondialdehyde (MDA), lymphocyte, lymphocyte transformation test (LTT), and phagocyte activity (PA) were significantly higher for LDD than the SD, but eosinophil was lower. Supplementation of multienzymes significantly decreased ALT, AST, and MDA, compared to the control group, but increased packed cell volume (PCV), hemoglobin (Hgb), lymphocytes, and monocytes. Immune organs, such as spleen, thymus, and the bursa of Fabricius were significantly increased with multienzyme supplementation. It could be concluded that multienzyme supplementation at either 0.1 or 0.2% to SD or LDD improved EPEI and immune status of broiler chicks.
Introduction
The cost of poultry feed ingredients represent about 60–70% of the total production cost, and hence, feed formulation is a critical approach in poultry industry. Feed utilization can be met with inclusion of enzymes, antimicrobials, probiotics, or prebiotic or natural products (1–9).
The immunomodulatory effect of supplementing poultry feed with multienzymes has been well-documented in the literature. Hosseindoust et al. (10) concluded that β-mannanase has a potential to improve the gut health of broiler chickens fed with a corn and soybean meal (SBM)–based diet. In the same study, growth performance and the total tract retention of nutrients were also improved. Liu et al. (11) investigated the effect of multienzymes containing phytase, protease, and xylanase at 1,000, 2,000, and 2,000 U/kg of broiler feed, respectively. The authors showed that multienzymes significantly improved feed intake, body weight gain, polymeric Ig receptor (pIgR), secretary IgA (sIgA), and ileal counts of Lactobacilli and Bifidobacteria and significantly reduced lesions in the intestine, serum a-toxin antibodies, mucin 2 expression, and ileal count of Clostridium perfringens. However, the strength of the multienzyme effect depends on the protein content in the diet. The authors pointed that high non-conventional protein in the diet can lead to increased occurrence of subclinical necrotic enteritis, while multienzyme supplementation can reduce this effect in broiler chickens by enhancing the gut immunity (11).
Supplementing poultry feed rations with enzymes can enhance feed utilization and eliminate the negative effect of non-starch polysaccharides (NSPs) in broiler performance (12–14). Multienzyme supplementation, such as amylase, xylanase, and protease is claimed to stimulate disintegration of starch, cell walls, and endogenous proteins, and thus improves energy utilization in corn-SBM and sorghum-SBM diets (15). Attia et al. (16) revealed that supplementing poultry diet with multienzyme enhanced the economic cost of the diets. However, the composition and the type of enzyme mixture determine the effect of the multienzyme supplementation on poultry production performance (17–19).
The use of enzyme complex containing carbohydrases and proteases is claimed to improve utilization of energy, protein, P, and Ca by broilers, laying hens, ducks, and Japanese quail (16, 17, 20, 21). However, there are still conflicting results about the beneficial effect of multienzymes on the poultry performance, and this could be attributed to dietary composition, age, and type of chicks, suggesting the need for further research (17, 22).
The use of low-density diet (LDD) with or without enzyme supplementation in relation to physiological and immunological responses and antioxidant status biochemistry of broilers is a new concept in broilers' nutrition. Most of previous research focused on productive performance and carcass traits as economic criteria (18, 22, 23). In literature, enzyme supplementation, particularly low-energy diets or feedstuffs containing high-fiber and/or antinutritional factors, was found to improve energy use for growth (12–18, 21, 23–26). On the other hand, immunity and antioxidant status and development of the inner organs may need more nutrients than those needs for growth and carcass quality and can perfectly reflect the nutritional status of animals (24, 25). Thus, this study focuses on nutritional intervention between LDD and enzyme supplementation from the point of view of biochemistry, physiological and immunological responses, and antioxidant status and organs histology.
Materials and Methods
Chicks, Diet, and Experimental Design
The department committee of Animal and Poultry Production accepts all the procedures done in the current study. These procedures suggest minimal stress to animal to ensure rights and welfare by eliminating harm or suffering to animals according to official decrees of the Ministry of Agriculture in Egypt regarding animal welfare [Decree No. 27 (1967) that enforces the humane treatment of animals generally].
A total of 216 1-day-old Arbor Acres broiler chicks were purchased from Cairo Poultry Company, wing-banded and distributed in random manner, regardless of their sex, with equal initial BW (45.8 ± 1.42) in 36 cages of 6 birds per cage. Each treatment consists of six replicates, with six chicks per replicate.
The experiment was designed with two diets [standard diet (SD) vs. LDD] and three multienzyme levels (unsupplemented, 0.1 and 0.2%) in a factorial design (2 × 3) during 1–38 days of age. Galzym-M are products of Tex Biosciences, UK. Galzym® is a multienzyme containing cellulase 100,000,000 U/kg, 4-β-xylanase 1,500,000 U/kg, lipase 6,500 U/g, α-amylase 250,000 U/kg, protease 40,000 U/kg, pectinase 30,000 U/kg, and sodium benzoate (preservative) 50 mg/kg. The chemical composition of the experimental basal diets fed during the experiment stages is shown in Table 1 (27). The starter diet was fed from hatch until 7 days of age (1 week); a grower diet was fed from 8 to 21 days of age (2–3 weeks); and a finisher diet was fed from 22 to 38 days of age.
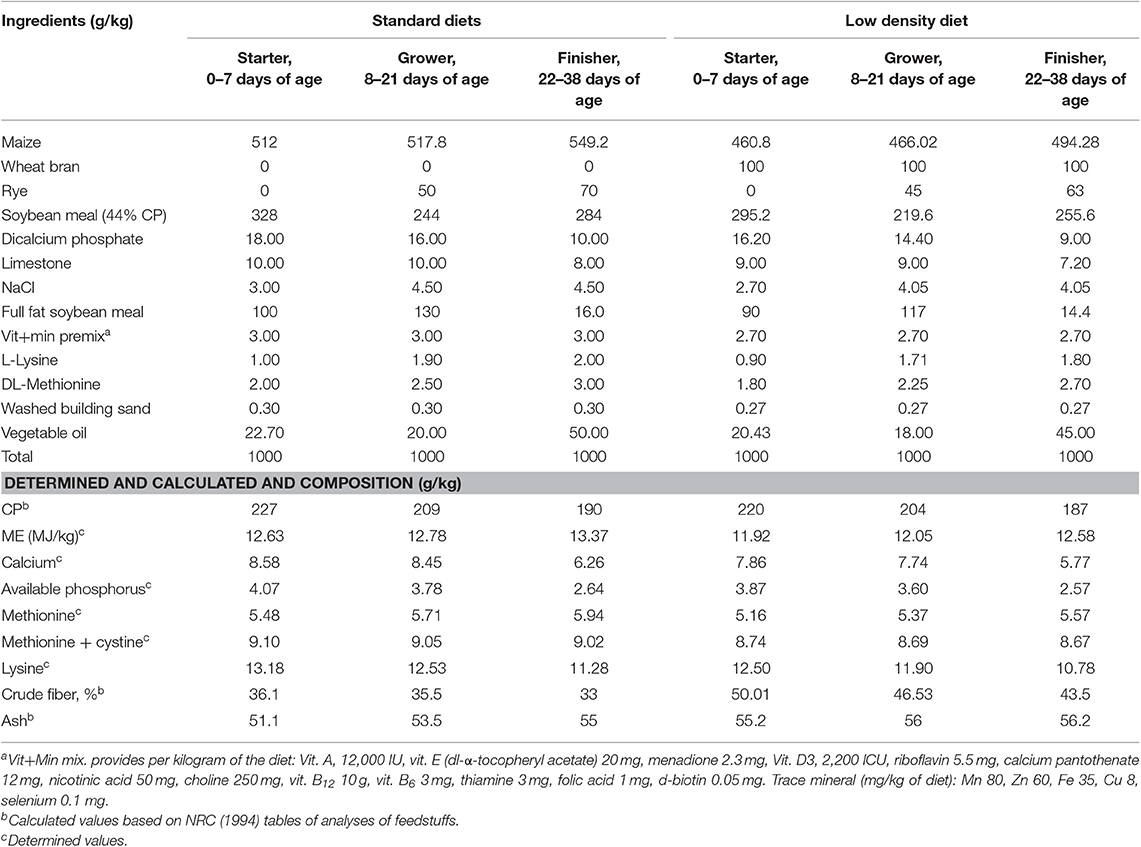
Table 1. Ingredients and chemical composition of the experimental basal diets fed during the experiment stages.
Animal Housing
Chicks were distributed in battery cages in a semi-opened room. The feed rations were fed ad libitum and water was freely accessed. A commercial light schedule was followed until the 7th day of 23-h light followed by 20-h light from the 8th day through the rearing phase until the 38th day of age. The outside minimum and maximum temperatures and relative humidity during the rearing phase were averaged as 22.9 and 28.3°C and 60.1 and 62.2%, respectively. The indoor temperature was 33.2, 28.3, and 26.7°C during 1–7, 8–18, and 15–20 days of age, respectively. The vaccination program involves clone 30 on day 8, dual injection of dead Influenza H5 N2 and Newcastle disease virus (NDV) under the skin of the neck on day 10, and clone 30 and Gumboro on day 21.
Data Collection
The BWG (g/bird) was calculated based on broiler weights (g) at 1, 21, and 38 days of age. Feed conversion rate (FCR) (g feed/g gain) was calculated based on the feed intake (g/bird), and the survival rate (SR, 100 – mortality rate) during 1–21, 22–38, and 1–38 days of age was calculated. European Production Efficiency Index (EPEI) was calculated using the equation of Hubbard Broiler Management Guide (28) as follows:
where PP = Production Period (days).
Collection of Blood Samples
Blood samples (n = 6) were randomly withdrawn from each experimental group on day 8 post-vaccination (29 days of age) as well as on day 0 (just before vaccination). Blood samples were withdrawn from the branchial vein by using a vacutainer tube using heparinized and non-heparinized tubes. Serum and plasma were separated by centrifugation at 1,500 × g for 15 min. Blood samples used for analyses were collected before the start of all vaccinations (day 0) and after the end of the last vaccination (day 29 of age).
Hematological Traits
The packed cell volume (PCV %) was determined by centrifuging the blood samples for 20 min at 2,000 × g using Wintrobe hematocrit tubes (Jiangdu Sunflower Glass Instrument Factory, Jiangdu, China). The cyanomethemoglobin method was used to determine hemoglobin concentration (Hgb) (29). Red blood cell count was determined using the method of (30), and red cell indices were calculated as described by Jain (31) according to the following equations:
Mean Corpuscular Volume (MCV) (μm3) = PCV × 10/RBC's
Mean Corpuscular Hemoglobin (MCH) (Pg) = Hgb × 10/RBC's
Mean Corpuscular Hemoglobin Concentration (MCHC) (g/dl) = Hgb × 100/PCV.
Immune Indices
Phagocytic activity and index was determined according to Kawahara et al. (32). Phagocytic activity (PA) = percentage of phagocytic cells containing yeast cells. Phagocytic index (PI) = number of yeast cells phagocytized/number of phagocytic cells. This test indicates the activity of the white blood cells that phagocytose harmful foreign particles, bacteria, and dead or dying cells. It is an important immune index, which indicates strongly and reflects the immune status. These phagocytes are mainly monocytes and macrophages, granulocytes, and dendritic cells. The phagocytic index indicates the strength of the phagocytic cell, represented by the number of the ingested bacteria. Phagocytic activity is considered as the first line of defense against antigens and pathogenic agents (33, 34).
Total antibody production specific for NDV vaccine was determined in serum using commercial ELISA kits (35). Antibody response was determined by hemagglutination inhibition (HI) test according to King and Seal (36). The assay is designed to measure IBD antibody bound to influenza antigen-coated plates (37). The Takatsy and Hamar method (38) was used to determine HI against NDV and avian influenza. Lymphocyte transformation test was determined following the method by Balhaa et al. (39). This test measures the T cell proliferation to a drug in vitro, from which one can simulate a previous in vivo reaction against a drug sensitization. Lymphocyte transformation test has been widely implemented for medical diagnosis of immunodeficiency, pathogenic diseases, and type IV allergy (40, 41). The Rainger and Rowley (42) was used to determine the serum bactericidal activity to Aeromonas hydrophila strain, and the results were expressed as survival index. This test is used to measure the bactericidal activity of the serum during antimicrobial treatment against bacteria isolated from the same patient. This test has been widely used in patients with infective endocarditis, osteomyelitis, bacteremia, or other serious bacterial infections (43, 44). The turbidimetric method of Engstad et al. (45) was used to measure the serum lysozyme activity. This test represents the monocyte/macrophage activity and has been widely used to measure the activity of various diseases (46, 47). The reduction in absorbency of 0.001/min refines the result of this test, which is expressed as one unit of lysozyme activity. Lysozyme activity = (A0 – A)/A.
Biochemical Traits
Commercial kits produced by Diamond diagnostics (23 EL-Montazah St. Heliopolis, Cairo, Egypt, http://www.diamonddiagnostics.com) were used to measure the biochemical traits of the blood. The methods of Armstrong and Carr (48), Doumas et al. (49), and Doumas and Peters (50) were used to measure the total serum protein and albumin concentrations. The method of Cole (51) was used to estimate the globulin concentration through subtraction of albumin concentration from serum total protein. The method of Bossuyt et al. (52) was used to measure the different types of globulin (α-β- and γ-globulin).
The method of Reitman and Frankel (53) was used to determine the activities of alanine aminotransferase (ALT, U/L) and aspartate aminotransferase (AST, U/L). The activities of alkaline phosphatase (ALKP) enzymes were assayed in samples by the method of McComb and Bowers (54). Serum total antioxidant capacity (TAC) and malondialdehyde (MDA) were determined according to Erel (55).
Histopathological Study
Intestine (i.e., ileum), the bursa of Fabricius, thymus, and spleen specimens were harvested at 38 days of age from six birds from each dietary treatment, n = 6, and directly fixed in 10% buffered formalin saline (BFS) for at least 24 h. Conventional paraffin embedding technique was used to process the fixed specimens. This technique involves dehydrating via ascending grades of ethanol, clearing in chloroform, and embedding in paraffin wax at 60C. The resulted paraffin blocks were sliced into slices of 5 μm thick and stained by hematoxyline and eosin (H&E) following the method described by Culling (56).
For morphometrical quantitative measurement of the longitudinal axis of large bursal follicle, five sections per replicate per treatment were examined using Optika imaging analyzer mounted on an Optika binocular microscope. Also, thymus cortical to medullary ratio was qualitatively measured. Spleen was examined to determine lymphoblastic cells hyperplasia presence where (–) means few; (+) means moderate; and (++) means severe.
Statistical Analysis
The General Linear Model procedure of the Statistical Analysis Software of SAS Institute (57) was applied using two-way factorial design (two types of feeds by three levels of multienzyme) as follows:
yijk = μ + Ai + βj + (Aβ)ij + eijk
Here, μ = general mean, Ai = effect of types of feeds, βj = effect of levels of multienzyme, (Aβ)ij = interaction between feeds and multienzymes, and eijk = random error.
Before analysis, arcsine transformation was done to normalize the data distribution. To confirm the homogeneity (normality test) of the data, the Kolmogorov–Smirnov (K-S) test was used (57). Means are considered different at P ≤ 0.05 using Student–Newman–Keuls test.
Results
Growth Performance
Table 2 shows the effect of different types of duets and levels of multienzymes on growth of broiler chicks fed with SD and LDD during days 1–38 of age. Results in Table 2 show that the LDD significantly decreased BWG of broilers compared to the SD, but did not affect EPEI during the experimental period. The interaction between the multienzyme level and the type of diet on BWG and EPEI was not significant. Results also indicated that multienzyme supplementation at 0.1 and 0.2% significantly and similarly increased BWG and improved EPEI, as compared to the control diet.
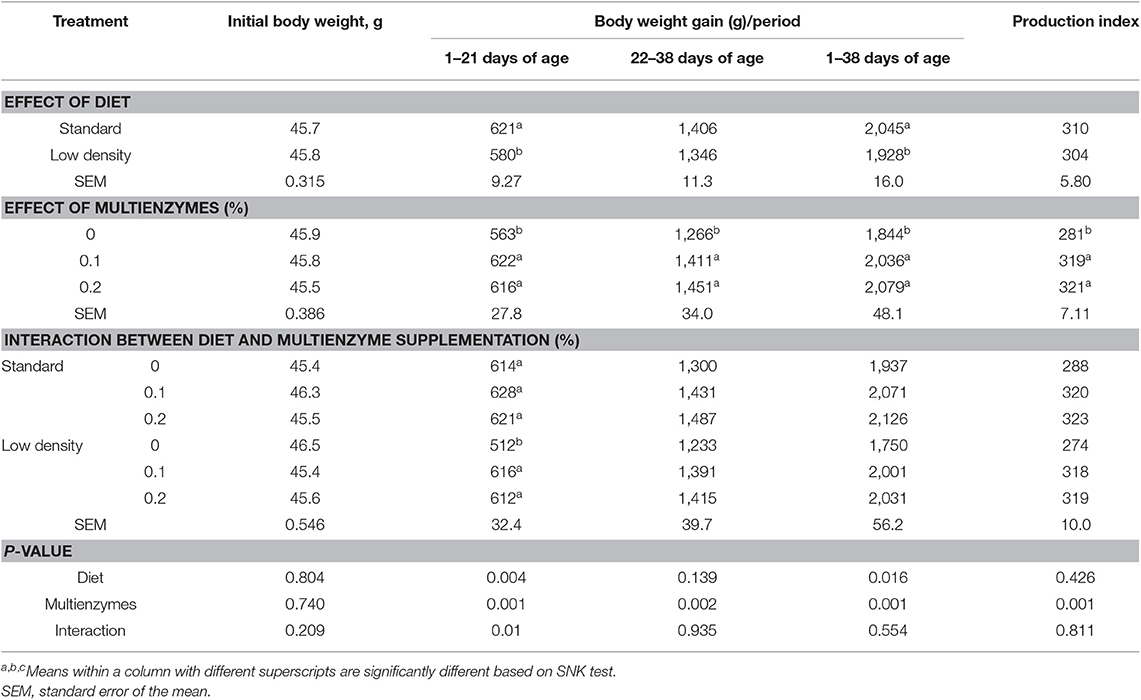
Table 2. Effect of different levels of multienzymes on growth of broiler chicks fed with standard and low-density diets during days 1–38 of age.
Hematological Traits of Blood
Tables 3, 4 show the effect of experimental diets on hematological traits of blood and differential white blood cells, respectively. PCV, Hgb, RBCs, MCV, MCH, MCHC, WBCs, and different types of WBC were not affected by the type of diet. Lymphocytes had significantly higher LDD while the opposite trend was shown in the case of eosinophils (Table 3). Supplementation of multienzymes significantly increased Hgb, PCV (Table 3), lymphocyte, and monocyte (Table 4) compared to control diet. There was a significant interaction between type of diet and multienzyme concentrations on Hgb, PCV (Table 3), and WBCs (Table 4), showing that multienzyme increased Hgb and PCV of the SD in a stepwise manner, but did not affect the LDD, showing that the impact of multienzyme on Hgb and PCV depends on the type of diet. The lymphocyte response to multienzyme dose was in a stepwise manner (Table 4).
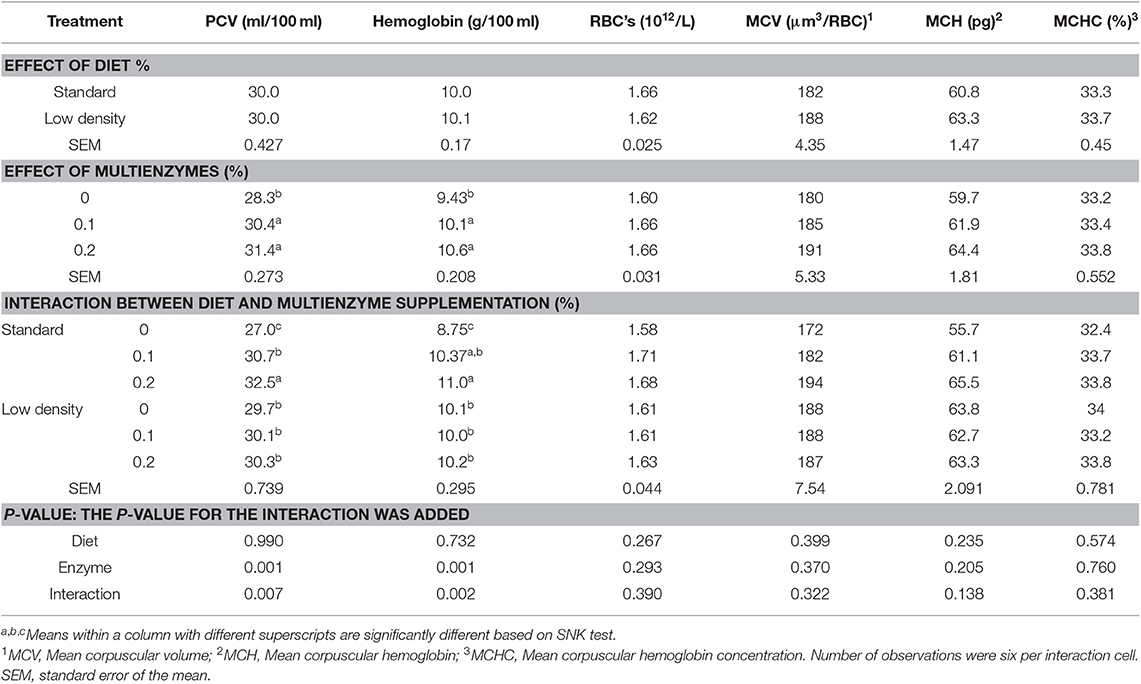
Table 3. Effect of different concentrations of enzyme cocktail on blood hematological traits of broiler chicks fed with standard and low-density diets.
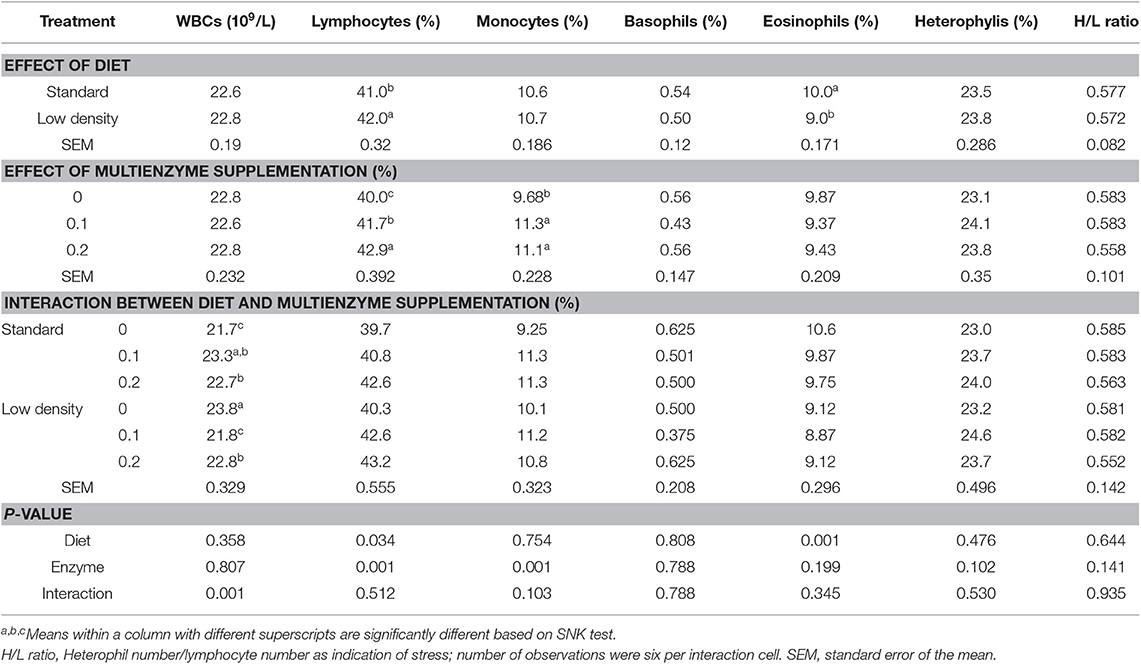
Table 4. Effect of different concentrations of enzyme cocktail on white blood cell and its fractions of broiler chicks fed with standard and low-density diet.
There was a significant interaction between the type of diet and the multienzyme level only on WBCs, showing that EC supplementation to SD significantly increased WBCs but decreased LDD, showing that the impact of multienzymes on WBCs depends on the type of diet.
Biochemical Constituents of Blood
Data for biochemical constituents of blood are shown in Tables 5, 6. The ALT, AST, and MAD were significantly higher in LDD than in SD, but TAC/MDA (antioxidants balance) was lower (Table 5). The total protein, albumin, α-, β-, and γ-globulin, globulin, and globulin/albumin ratio were not significantly influenced by the type of diet (Table 6).
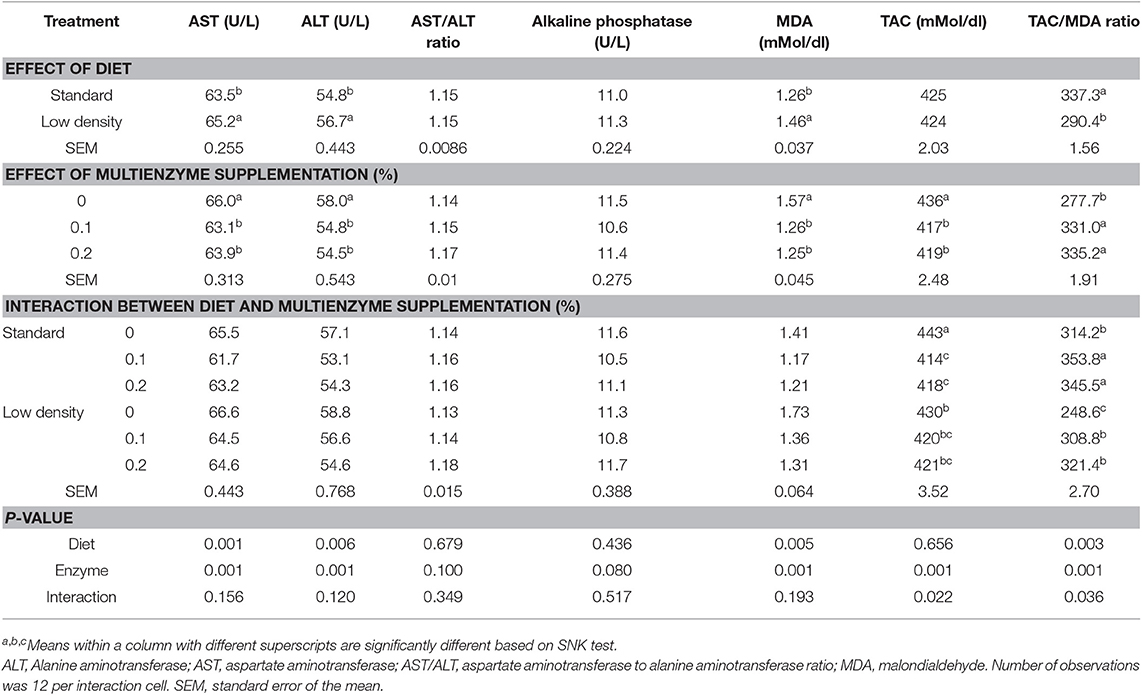
Table 5. Effect of different concentrations of enzyme on liver enzymes, blood serum malondialdehyde, and total antioxidant capacity of broiler chicks fed with standard and low-density diet.
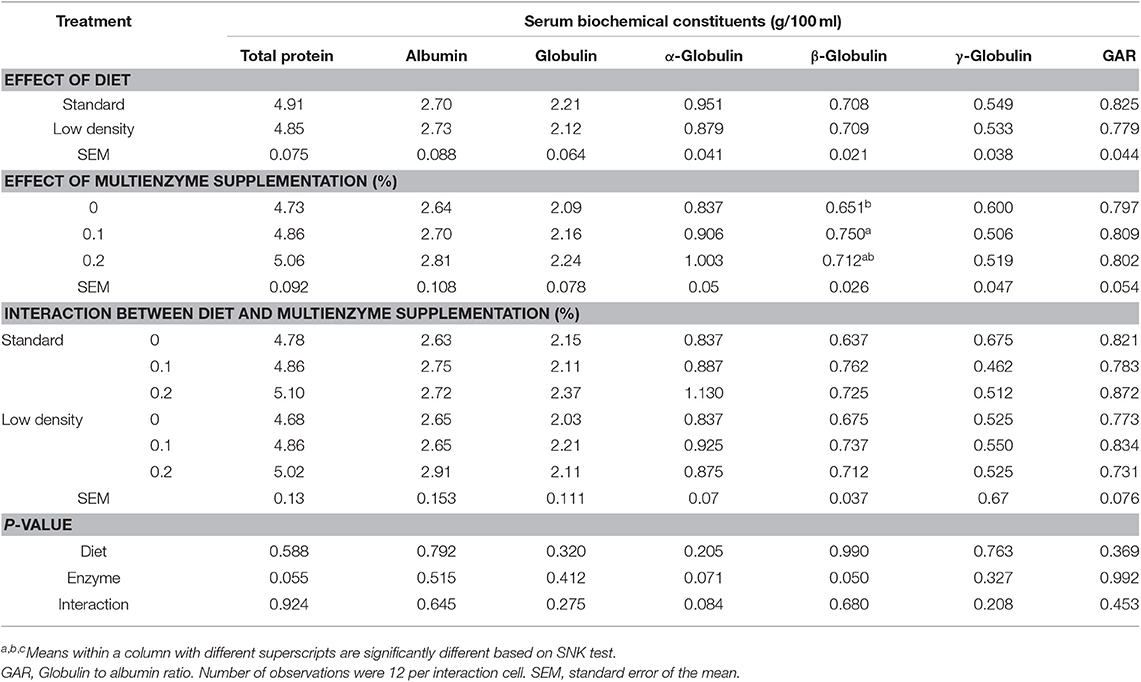
Table 6. Effect of different concentrations of enzyme cocktail on biochemical constituents of blood serum of broiler chicks fed with standard and low-density diet.
Supplementation of multienzymes at 0.1 and 0.2% significantly decreased the ALT, AST, MDA, and TAC compared to the control group (Table 5), but increased antioxidant balance (TAC/MDA). Liver enzyme ratio (ALT/AST) and alkaline phosphatase were not significantly affected by multienzyme supplementation. There was a significant effect of multienzymes on β-globulin showing greater values of chicks on diet with 0.2 and 0.1% multienzymes than those on the control diet without multienzyme supplementation (Table 6). The multienzyme effect on the total protein was moderate (P = 0.057).
There was no significant influence of the interaction between multienzyme supplementation and type of diet on blood MDA, protein, and liver functions as reflected by blood enzymes (ALT, AST, ALT/AST, and alkaline phosphatase). However, a significant effect of the interaction was observed in the TAC and antioxidant balance (TAC/MDA). The results indicated an increase in the TAC/MDA ratio of broilers supplemented with enzymes of SD and LDD.
Lymph Organs and Antibody Titer
Table 7 shows the effect of different concentrations of multienzymes on immune organs and antibody titer of broilers fed with SD and LDD. The type of diet had no significant effect on lymph organs, such as spleen, thymus, and the bursa of Fabricius and HI for NDV. However, HI for AI was significantly higher in LDD than that in SD. These organs were also significantly higher for broilers on diet supplemented with either 0.1 or 0.2% multienzymes than those on diet without EC supplementations.
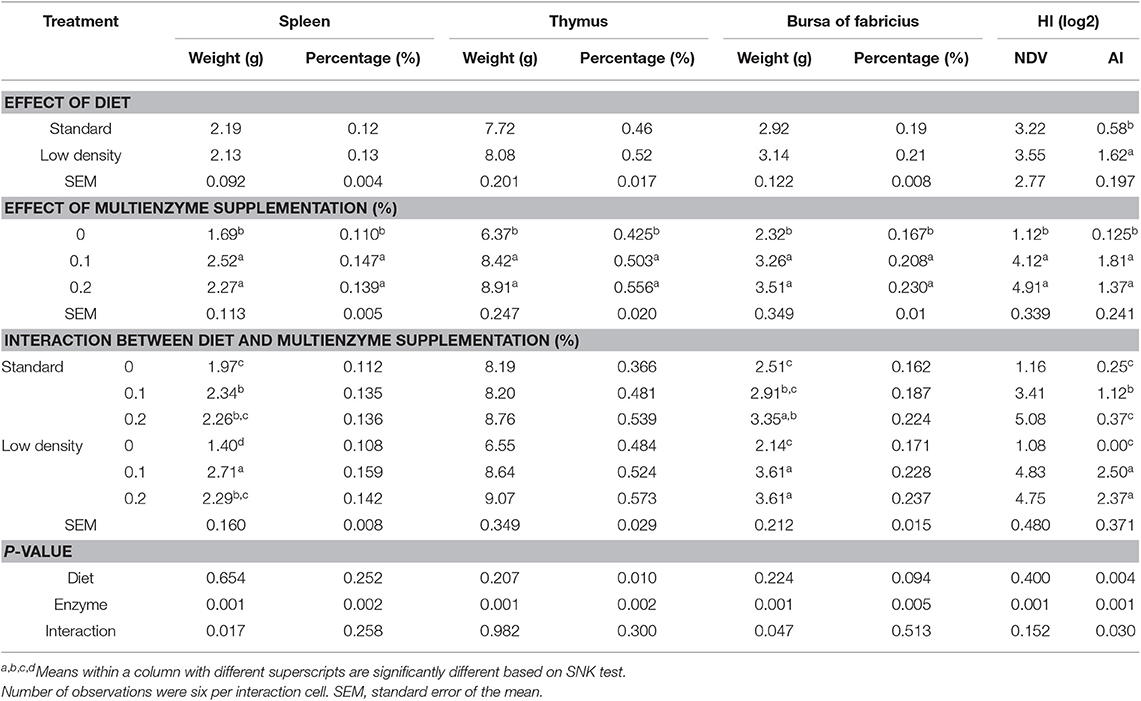
Table 7. Effect of different concentrations of enzyme cocktail on immune organs and antibody titer of broiler chicks fed with standard and low-density diet.
There were significant interactions between multienzyme supplementation and the type of diet on the absolute weight of spleen and the bursa of Fabricius and HI for AI. The results showed that multienzyme supplementation significantly increased the weight of spleen and the bursa of Fabricius and HI for AI. However, multienzyme supplementation at 0.1% significantly increased the weight of spleen compared to the unsupplemented control, whereas 0.2% multienzyme increased the absolute weight of the bursa of Fabricius.
Immune Indices
Table 8 shows the effect of the different experimental diets on the immune indices. There was a significant effect of the type of diet on LTT, BACT, and PA, showing the enhancing effect of LDD on the immune response (Table 8).
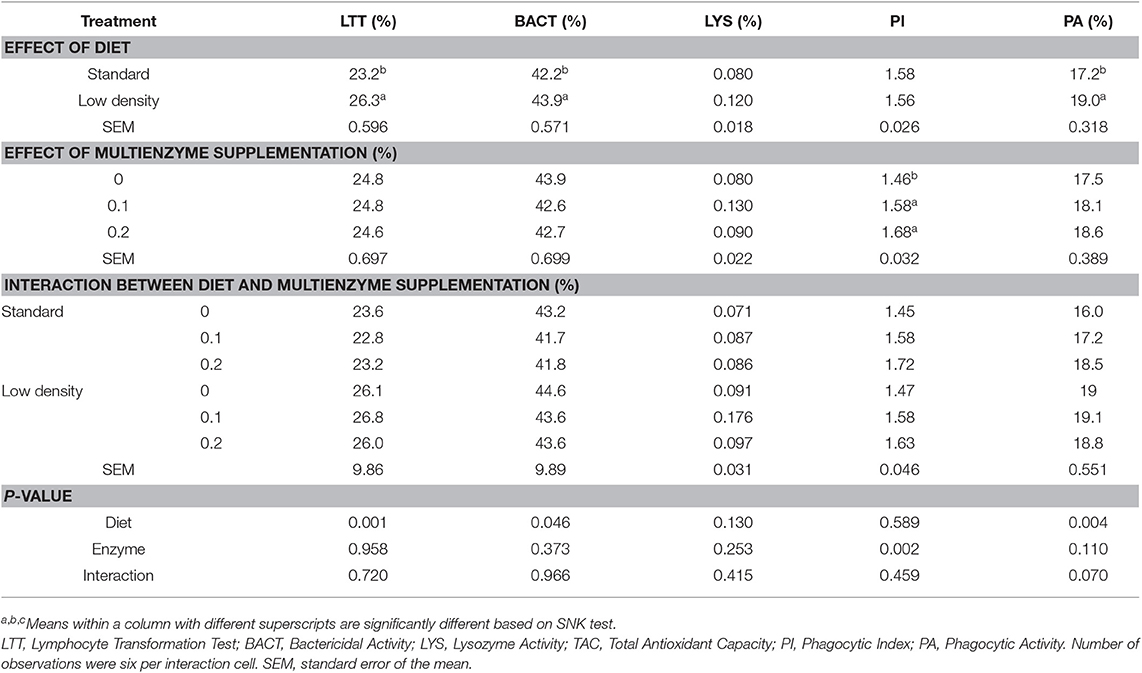
Table 8. Effect of different concentrations of enzyme cocktail on immune index of broiler chicks fed with standard and low-density diet.
Supplementation of multienzymes significantly increased PI compared to the control group and had no effect on the other immune indices, such as LTT, BACT, LYS, and PA.
There was no significant influence of the interaction between the dose of multienzymes and the type of diet on LTT, BACT, LYS, PI, and PA.
Histology Study
The effect of the type of diet and multienzyme supplementation on the morphology of the intestine and the bursa of Fabricius is shown in Table 9. The type of diet had no significant effect on the diameter of the large follicle of the bursa of Fabricius. There was a significant increase in the diameter of the large follicle of bursa of Fabricius (Figures 1, 2; Table 9) due to supplementation of 0.1% multienzyme, as compared to the other multienzyme concentrations. The increase in the bursa of Fabricius amounted to 16.6%, respectively. There was also insignificant increase due to supplementation of 0.2% multienzymes.
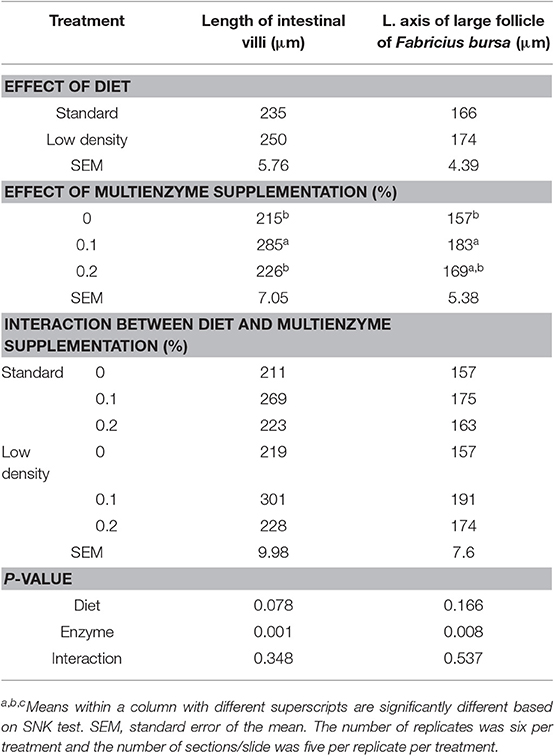
Table 9. Effect of type of diet and enzyme cocktail supplementation of morphology of the intestinal and Fabricius bursa.
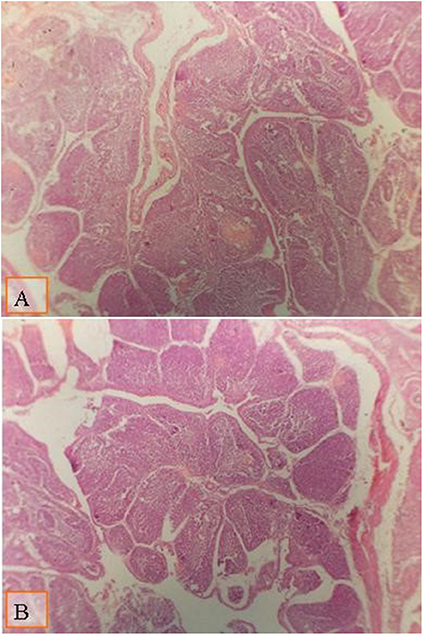
Figure 1. Micrograph of the bursa of Fabricius of broiler at day 28 of age stained with H&E (×40) to compare the follicle diameter in different groups; the distance between two follicular polar as shown all groups by lines: (A) broiler fed with a standard diet supplemented with 0.1% ml enzyme cocktail; (B) broiler fed with a diet supplemented with 0.2% of multienzyme. Moderate increase in the follicular diameter was noticed in broiler fed with diet supplemented with 0.1% of multienzyme (A).
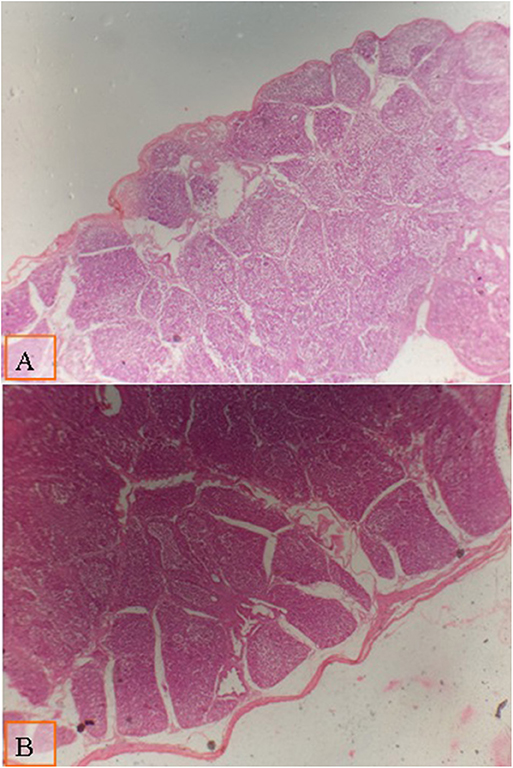
Figure 2. Micrograph of the bursa of Fabricius of broiler at day 28 of age stained with H&E (×40) to compare the follicle diameter in different groups; the distance between two follicular polar as shown all groups by lines: (A) broiler fed with low density diet supplemented with 0.1% of multienzyme; (B) broiler fed with a diet supplemented with 0.2% of multienzyme. Moderate increase in the follicular diameter was noticed in broiler fed with a diet supplemented with 0.1% of multienzyme (A).
There were no significant changes in spleen and thymus due to type of diet and multienzyme supplementation and their interaction (Figures 3 and 4). However, cortical to medullary ratio in thymus was decreased with increasing age of chicks. With time, the thymus began to atrophy and the decrease the cortical to medullary ratio became physiological.
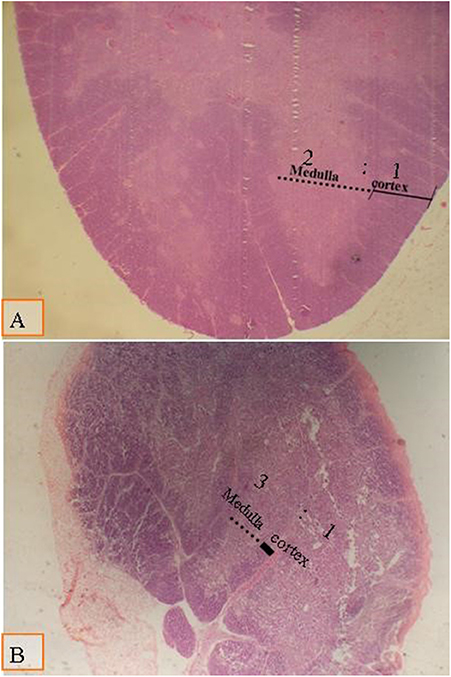
Figure 3. Micrograph of the thymus stained with H&E (×40) to compare the thymic cortical/medullary ratio: (A) the cortic/medullary ratio is 2:1 as shown in all groups at day 28 of age; (B) the cortic/medullary ration is 3:1 as shown in all groups at day 38 of age, which associated with physiological aged thymic atrophy.
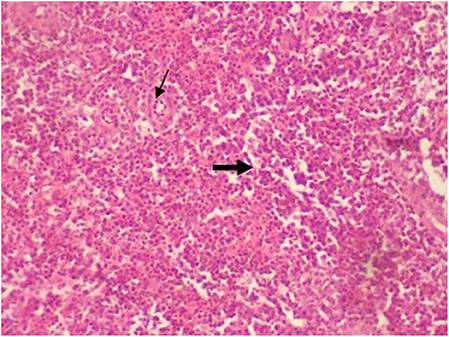
Figure 4. Micrograph of the spleen stained with H&E (×40) of control group shown with normal splenic histology featuring splenic arteriole (thin arrow) with white and red pulp (thick arrow). All groups showed the normal splenic histology as control.
Discussion
This work was executed to study the effect of supplementing three multienzyme levels (0, 0.1, and 0.2%) with two types of diet (SD vs. LDD) on immune response, blood hematology and biochemistry, antioxidant status, and organ histology of broilers during 1–38 days of age. The trend in using LDD in feeding broiler chicks has been recently discussed and may be a possible approach to decrease growth pressure on the skeletal system of birds, and reduce feed cost and environmental pollution (23, 24, 58). Data showed that inclusion of wheat bran in broiler diets at 10% induced a significant growth depression in BWG and FCR only during the early growth period, reaching 6.61 and 6.74%, respectively, which persisted in only BWG during the whole period, reaching 5.6%. This showed that LDD did not provide adequate nutrients for muscle growth during different experiment periods. However, broiler tolerance to dietary composition increased with increasing age of chicks (17, 59). The current results are in agreement with those reported by Al-Harthi (60) and Attia (16, 17). The enhanced BW and BWG as a result of multienzyme supplementation may be due to the increased nutrient availability and absorption as a result of increased digestibility of the ingested diets, as suggested by Choct (22), Attia et al. (18), and El-Kelawy (61). However, the effects of multienzyme depend on the composition of the diet and the enzyme type (17, 62). It was previously reported that multienzyme supplementation increased BWG and FCR while reducing feed intake. Multienzyme significantly increased the growth during 7–21 days of age, and this is explained by the existence of amylase and NSPs degrading enzymes (23, 60, 63, 64). Adding exogenous enzymes that hydrolyze the NSP of vegetable ingredients in the diets for monogastric enhances the energy availability and use of nutrients, and thus enhances feed conversion ratio (65). The increase in the release of nutrient due to enzyme supplementation resulted in higher nutrient available for absorption, as demonstrated by the increase in intestinal villi length, and thus for biochemical reaction in favor of anabolic reaction and muscle buildup (18) and for immune function as well (21, 25).
The use of LDD enhanced LTT and PA, indicating higher immunity. This enhancement in immunity could be attributed to redistribution of nutrients toward immunity rather than growth. Growth was decreased due to feeding LDD and thus the availability of nutrients increased for physiological response, immunity, and antioxidant utilization for eliminating free radical resulting from both non-enzymatic and enzymatic reactions due to decreasing nutrient demands for growth. For example, the TAC/MDA ratio (antioxidant balance) was significantly increased due to feeding enzyme-supplemented diet. This increase reflected the significant decrease in the antioxidants (TAC) for use in protection of cell from free radicals (7, 25).
Inclusion of wheat bran in the diet and particularly polysaccharides has been reported to be antioxidants, immunomodulators, anti-inflammatory, antitussive, anticancerous, and antimutagenic (1, 66–68). In addition, wheat bran and arabinoxylans have been shown to improve phagocytosis of macrophages in animal models. They also stimulate humoral response in chickens. It improved production of total IgS, IgG, and IgM anti-SRBC antibody titers on days 7 and 14 PPI and PSI of SRBCs, relative to the standard (69, 70). Administration of arabinoxylans in the diet significantly increased anti-SRBC antibody response in arabinoxylans-administered chickens and suggested increased humoral immune response, which may be attributed to an improved animal's potential to encounter disease agents (71). In addition, wheat bran is rich in phytase enzyme, which can improve nutrient availability by chickens, such as protein, energy, and minerals (17, 72).
Interestingly, the current results showed that EC improved the EPEI of broiler chicks. This result is in line with those shown by Al-Harthi (60) and Attia (16, 17). The improved EPPI due to EC supplementation was concurred with greater villi length for the group on 0.1% multienzyme supplementation; this is in line with the findings of Choct (22) and Attia et al. (18). However, the effect of multienzyme depends on diet composition and the type of enzyme (17, 62). The effect of enzyme supplementation on animal performance could be a result of the effect of amylase and NSPs degrading enzymes (23, 60, 63, 64). The addition of exogenous enzymes that hydrolyze the NSP of vegetable ingredients in the feeds for monogastric enhances the availability of energy and use of nutrients and thus enhances FCR (65). In addition, Gao et al. (73) reported that xylanase supplementation of wheat-based diets for cockerels significantly improved the production of serum antibody response to NDV as a mean of humoral response. On the contrary, Basmacioglu Malayoglu et al. (74) revealed that NSP-degrading enzyme supplementation had no significant effect on immune response represented by titers of IgG and IgM. Also, Khaksar et al. (75) found that the relative weight of immune organs (thymus, spleen, and the bursa of Fabricius) was not influenced by enzyme supplementation.
In addition, dietary composition intervention with enzymes was recently studied by Head et al. (76) who suggested that if broilers are supplemented with flaxseed, the nutrient digestibility and the availability of n-3 FA will be limited due to the presence of non-starch polysaccharides (NSP), by affecting the genetic process during lipid metabolism in the liver. The results revealed that enzyme addition to diets supplemented with 10% flaxseed decreased arachidonic acid and total long chain n-6 FA. Dietary flaxseed and enzyme treatments upregulated PPARα target genes CPT1A and ACOX1 while reducing the expression of de novo FA synthesis-related genes (76). In addition, Seidavi et al. (77) demonstrated that supplementing the diet with probiotic/enzyme mixture had no effect on the humoral response against AI and NDV on day 42. There was a significant difference of IgG after the second challenge with SRBC's (P = 0.003). A lower body weight of the birds was found in most of the treated groups, relative to the standard (P = 0.031). The absolute and relative weight of the spleen was significantly different compared to the control group (P = 0.003 and P = 0.001, respectively). The weights of the thymus and the bursa of Fabricius were not different (77). Further study was conducted by Abdel-Hafeez et al. (78) who found that using potato peels at 15% and sugar beet pulp at 7.5% decreased the body weight. Enzyme inclusion significantly improved the body weight in potato peels and sugar beet pulp. The total cholesterol and low-density lipoprotein cholesterol serum levels were reduced in all experimental groups. Also, the carcass fat content was reduced using the potato peels and sugar beet pulp, with or without enzyme (78). This indicates that enzymes supplementation affects blood biochemistry of broilers (61) and thus meat quality improved because of decreasing carcass fat (18).
These results suggest that it is possible to dilute nutrient profiles of broiler diets during the growing and finishing phases without negative effect on EPEI and EC while improving immune response and economic efficiency. These results are in agreement with those reported by Abudabos (23).
The use of LDD in the current study did not negatively affect villi length. The relationship between gut morphology and the type of diet was reported in the literature; the use of cereal with a high NSP level may increase the size of the gastrointestinal tract (72, 79). A significant positive correlation between arabinoxylan level in wheat and the relative weights of the duodenum, jejunum, and ileum has been reported (80). It is known that diet makeup may induce microscopic changes in the intestinal mucosa, and it is possible that dietary NSP levels may also affect the morphology of the gastrointestinal tract (81). Iji (82) reported that the crypt depth of both the jejunum and ileum was improved due to supplementation with guar gum and xanthan gum, indicating that NSP may stimulate gastrointestinal tract cell turnover. The increased crypt depth indicated increased villus cell stimulation and thus an increase in nutrients being absorbed and thus being utilized by the gastrointestinal tract. This indicates that cereal type affects the gastrointestinal tract size and the morphology of the intestine (83).
It is well-known that increasing the cortex in relation to the medulla ratio increased the number of T-lymphoblasts and increased the T-lymphocyte, which reflects on stimulating cellular immunity (84–86).
A recent study by Woyengo et al. (87) showed that using amylase, NSPase, or a combination of amylase and NSPase in the phytase-supplemented basal diet further improved (P < 0.05) ileum digestibility to 63.4, 69.9, and 67.3%, respectively. However, the dietary nitrogen corrected apparent metabolizable energy value was not affected by the addition of phytase, amylase, or a combination of amylase and NSPase. Woyengo et al. (87) concluded that the addition of amylase and NSPase to broiler phytase-supplemented diets is beneficial to improve apparent metabolizable energy corrected by nitrogen. In another study by Sateri et al. (88), they showed no significant effects of olive meal and enzymes on growth performance and on cecum microflora. Antibody titers against infectious bronchitis virus (IBV) and Gumboro disease were higher in birds fed with 4% olive meal. Supplementing the diet with enzymes did not affect the aforementioned parameters (88).
The present results indicate that EC supplementations improved the immunity of broiler chicks as measured by organ changes. This was concurred with an increasing diameter of large follicle of the bursa of Fabricius for the group supplemented with 0.1% multienzymes. The increase in the follicular diameter of the bursa of Fabricius indicates an increase in the number of B-lymphoblasts that leads to the formulation of B-lymphocytes that internally form antibodies. This reflects stimulation of the humoral immunity due to increased nutrient available for antibody formation (89, 90). In addition, the phagocytic index was similarly increased with supplementation of 0.1 and 0.2% multienzyme concentrations. The mechanism(s) by which wheat bran and/or enzyme supplementation positively affected chick's immunity may involve an improvement in gut health due to the decrease in harmful microbiota in the hind gut, increase in the intestinal villi length, and/or increase in the nutrient available for immune function (21, 24, 25, 73, 89, 90). In this regard, Gao et al. (73) reported that feeding cockerels on wheat-based diets supplemented with xylanase significantly enhances serum antibodies to NDV, indicating that enzyme supplementations enhance the humoral response due to increased nutrient utilization for immune function. But in another study, there was no significant effect on humoral response (IgG and IgM) with NSP-degrading enzyme supplementation (74). Also, Khaksar et al. (75) found that the relative weight of immune organs (thymus, spleen, and the bursa of Fabricius) was not influenced by enzyme supplementation. This contraindication in published literature indicated that immunity of birds is a complex concept that is affected by diet composition, age, and stress of animals (24, 25, 84).
In conclusion, multienzyme supplementation at either 0.1 or 0.2% to SD or LDD improved the production index while enhancing the immune response of broilers during age of 1–38 days.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Department Committee of Animal and Poultry Production, official decrees of the Ministry of Agriculture in Egypt relevant to animal welfare are No. 27 (1967).
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This article was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah; the authors, therefore, acknowledge with thanks DSR technical and financial support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors YA.
Acknowledgments
The authors extended their appreciation to the management of Kuwait Institute for Scientific Research (KISR) for kind help and support.
References
1. Al-Khalaifah HS. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult Sci. (2018) 97:3807–15. doi: 10.3382/ps/pey160
2. Kutlu HR, Saber SN, Kutay H, Celik L, Uzun Y, Toy N, et al. Effect of multi-enzyme produced by a single fungus on growth performance and some carcass parameters of broiler chicks fed on maize-soya based diets. Kafkas Üniv Vet Fakült Dergisi. (2019) 25:221–30. doi: 10.9775/kvfd.2018.20765
3. Sun HY, Kim IH. Effects of multi-enzyme on production performance, egg quality, nutrient digestibility, and excreta noxious gas emission of early phase Hy-line brown hens. Poult Sci. (2019) 98:4889–95. doi: 10.3382/ps/pez237
4. Attia YA, Addeo NF, Abd Al-Hamid AA-HE, Bovera F. Effects of phytase supplementation to diets with or without zinc addition on growth performance and zinc utilization of white pekin ducks. Animals (Basel). (2019) 9:280. doi: 10.3390/ani9050280
5. Al-Khalaifa H, Al-Nasser A, Al-Surayee T, Al-Kandari S, Al-Enzi N, Al-Sharrah T, et al. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult Sci. (2019) 98:4465–79. doi: 10.3382/ps/pez282
6. Al-Khalifa H. Immunological techniques in avian studies. Worlds Poult Sci J. (2016) 72:573–84. doi: 10.1017/S0043933916000532
7. Al-Khalifa H. Enrichment of poultry diets with polyunsaturated fatty acids (PUFA) for human consumption. Approach Poult Dairy Vet Sci. (2017) 1:APDV.000523. doi: 10.31031/APDV.2017.01.000523
8. Al-Khalifa H, Al-Nasser A, Al-Bahouh M, Ragheb G, Al-Qalaf S, Al-Omani N, et al. The effect of polyunsaturated fatty acids on avian immune cell subpopulations in peripheral blood, spleen, and thymus. Worlds Poult Sci J. (2016) 72:531–4. doi: 10.1017/S0043933916000428
9. Al-Khalifa H. Production of added-value poultry meat: enrichment with n-3 polyunsaturated fatty acids. Worlds Poult Sci J. (2015) 71:319–26. doi: 10.1017/S004393391500032X
10. Hosseindoust A, Lee S, Gook Nho W, Song YH, Shin JS, Laxman Ingale S, et al. A dose–response study to evaluate the effects of pH-stable β-mannanase derived from Trichoderma citrinoviride on growth performance, nutrient retention, and intestine morphology in broiler chickens. Ital J Anim Sci. (2019) 18:147–54. doi: 10.1080/1828051X.2018.1500872
11. Liu N, Wang JQ, Gu KT, Deng QQ, Wang JP. Effects of dietary protein levels and multienzyme supplementation on growth performance and markers of gut health of broilers fed a miscellaneous meal based diet. Anim Feed Sci Technol. (2017) 234:110–7. doi: 10.1016/j.anifeedsci.2017.09.013
12. Douglas MW, Parsons CM, Bedford MR. Effect of various soybean meal sources and avizyme on chick growth performance and ileal digestible energy. J Appl Poult Res. (2000) 9:74–80. doi: 10.1093/japr/9.1.74
13. Suresh G, Santos DU, Rouissi T, Brar SK, Mehdi Y, Godbout S, et al. Production and in-vitro evaluation of an enzyme formulation as a potential alternative to feed antibiotics in poultry. Process Biochem. (2019) 80:9–16. doi: 10.1016/j.procbio.2019.01.023
14. Bedford MR. New enzyme technologies for poultry feeds. Br Poult Sci. (2003) 44:14–6. doi: 10.1080/713655277
15. Aftab U, Bedford MR. The use of NSP enzymes in poultry nutrition: myths and realities. Worlds Poult Sci J. (2018) 74:277–86. doi: 10.1017/S0043933918000272
16. Attia YA, El-Razak A, El-Din ET, Zeweil HS, Hussein AS, Qota ESM, et al. The effect of supplementation of enzyme on laying and reproductive performance in Japanese quail hens fed nigella seed meal. J Poult Sci. (2008) 45:110–5. doi: 10.2141/jpsa.45.110
17. Attia YA. Value of rice bran, its maximal utilization, and upgrading by phytase and other enzymes and diet-formulation based on available amino acid for broiler chicks. Archiv Geflugelk. (2003) 67:157–66.
18. Attia YA, El-Tahawy WS, Abd El-Hamid AE-HE, Hassan SS, Nizza A, El-Kelaway MI. Effect of phytase with or without multienzyme supplementation on performance and nutrient digestibility of young broiler chicks fed mash or crumble diets. Ital J Anim Sci. (2012) 11:e56. doi: 10.4081/ijas.2012.e56
19. Hussein EOS, Suliman GM, Abudabos AM, Alowaimer AN, Ahmed SH, Abd El-Hack ME, et al. Effect of a low-energy and enzyme-supplemented diet on broiler chicken growth, carcass traits and meat quality. Arch Anim Breed. (2019) 62:297–304. doi: 10.5194/aab-62-297-2019
20. Cowieson AJ, Singh DN, Adeola O. Prediction of ingredient quality and the effect of a combination of xylanase, amylase, protease and phytase in the diets of broiler chicks. 1. Growth performance and digestible nutrient intake. Br Poult Sci. (2006) 47:477–89. doi: 10.1080/00071660600830603
21. Yang X, Zhang B, Guo Y, Jiao P, Long F. Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult Sci. (2010) 89:254–60. doi: 10.3382/ps.2009-00234
22. Choct M. Enzymes for the feed industry: past, present and future. Worlds Poult Sci J. (2006) 62:5–16. doi: 10.1079/WPS200480
23. Attia YA. Effects of microbial phytase without or with cell wall splitting enzymes on the performance of broilers fed marginal levels of dietary protein and metabolizable energy. Egypt Poult Sci. (2001) 21:521–47.
24. Abudabos AM. Effect of enzyme supplementation to normal and low density broiler diets based on corn-soybean meal. Asian J Anim Vet Adv. (2012) 7:139–48. doi: 10.3923/ajava.2012.139.148
25. Attia YA, Al-Khalaifah H, Ibrahim MS, Al-Hamid AEA, Al-Harthi MA, El-Naggar A. Blood hematological and biochemical constituents, antioxidant enzymes, immunity and lymphoid organs of broiler chicks supplemented with propolis, bee pollen and mannan oligosaccharides continuously or intermittently. Poult Sci. (2017) 96:4182–92. doi: 10.3382/ps/pex173
26. Cooper EL, Ma MJ. Understanding nutrition and immunity in disease management. J Tradit Complement Med. (2017) 7:386–91. doi: 10.1016/j.jtcme.2016.12.002
29. Eilers RJ. Notification of final adoption of an international method and standard solution for hemoglobinometry specifications for preparation of standard solution. Am J Clin Pathol. (1967) 47:212–4. doi: 10.1093/ajcp/47.2.212
31. Jain SK. Membrane lipid peroxidation in erythrocytes of the newborn. Clin Chim Acta. (1986) 161:301–6. doi: 10.1016/0009-8981(86)90014-8
32. Kawahara E, Ueda T, Nomura S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. (1991) 26:213–4. doi: 10.3147/jsfp.26.213
33. Hannum LG, Chen T. Advanced age does not diminish phagocytic activity or NET production in zebrafish (Danio rerio) kidney leukocytes. J Immunol. (2018) 200:46.22.
34. Grasse M, Rosenkrands I, Olsen A, Follmann F, Dietrich J. A flow cytometry-based assay to determine the phagocytic activity of both clinical and nonclinical antibody samples against Chlamydia trachomatis. Cytometry A. (2018) 93:525–32. doi: 10.1002/cyto.a.23353
35. Jeffery N, Sanderson P, Sherrington E, Newsholme E, Calder P. The ratio of n-6 to n-3 polyunsaturated fatty acids in the rat diet alters serum lipid levels and lymphocyte functions. Lipids. (1996) 31:737–45. doi: 10.1007/BF02522890
36. King DJ, Seal BS. Biological and molecular characterization of newcastle disease virus (NDV) field isolates with comparisons to reference NDV strains. Avian Dis. (1998) 42:507–16. doi: 10.2307/1592677
37. Cosgrove A. An apparently new disease of chickens: avian nephrosis. Avian Dis. (1962) 6:385–9. doi: 10.2307/1587909
38. Takatsy GY. The use of spiral loops in serological and virolegical micromethods. Acta Microbiol Acad Sci. Hung. (1956) 3:197.
39. Balhaa RL, Hinz HH, Luders H, Siegmann O. Clinical experiences with the drugs for lymphocyte transformation in chickens and turkey flocks. Tierarztliche Umschau. (1985) 43:507–8.
40. Azoury ME, Filì L, Bechara R, Scornet N, de Chaisemartin L, Weaver RJ, et al. Identification of T-cell epitopes from benzylpenicillin conjugated to human serum albumin and implication in penicillin allergy. Allergy. (2018) 73:1662–72. doi: 10.1111/all.13418
41. Mori F, Fili L, Barni S, Giovannini M, Capone M, Novembre EM, et al. Sensitization to amoxicillin/clavulanic acid may underlie severe rashes in children treated for infectious mononucleosis. J Allergy Clin Immunol Pract. (2019) 7:728–31.e1. doi: 10.1016/j.jaip.2018.06.022
42. Rainger G, Rowley A. Antibacterial activity in the serum and mucus of rainbow trout, Oncorhynchus mykiss, following immunisation with Aeromonas salmonicida. Fish Shellfish Immunol. (1993) 3:475–82. doi: 10.1006/fsim.1993.1046
43. Loose M, Naber KG, Hu Y, Coates A, Wagenlehner FME. Serum bactericidal activity of colistin and azidothymidine combinations against mcr-1-positive colistin-resistant Escherichia coli. Int J Antimicrob Agents. (2018) 52:783–9. doi: 10.1016/j.ijantimicag.2018.08.010
44. Nahm MH, Yu J, Weerts HP, Wenzel H, Tamilselvi CS, Chandrasekaran L, et al. Development, interlaboratory evaluations, and application of a simple, high-throughput Shigella serum bactericidal assay. mSphere. (2018) 3:e00146–18. doi: 10.1128/mSphere.00146-18
45. Engstad RE, Robertsen B, Frivold E. Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol. (1992) 2:287–97. doi: 10.1016/S1050-4648(06)80033-1
46. Ceballos-Francisco D, Guardiola FA, Cordero H, Cuesta A, Esteban MÁ. Humoral immune parameters in serum of gilthead seabream (Sparus aurata L.) after induced skin injury. Fish Shellfish Immunol. (2018) 75:291–4. doi: 10.1016/j.fsi.2018.02.017
47. Luo C, Gwekwe B, Choto P, Miao W, Chen M, Xue C, et al. Bitter peptides from enzymatically hydrolyzed protein increase the number of leucocytes and lysozyme activity of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. (2018) 81:130–4. doi: 10.1016/j.fsi.2018.07.013
48. Armstrong WD, Carr CW. Estimation of serum total protein. In: Physiological Chemistry Laboratory Directions, 3rd ed. Minneapolis, MN: Burges Publishing Co. (1964). 153 p.
49. Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. (1997) 258:21–30. doi: 10.1016/S0009-8981(96)06447-9
50. Doumas BT, Peters T. Serum and urine albumin: a progress report on their measurement and clinical significance. Clin Chim Acta. (1997) 258:3–20. doi: 10.1016/S0009-8981(96)06446-7
51. Cole DF. Action of bradykinin on intraocular pressure and pupillary diameter. Ophthal Res. (1974) 6:308–14. doi: 10.1159/000264717
52. Bossuyt X, Lissoir B, Mariën G, Maisin D, Vunckx J, Blanckaert N, et al. Automated serum protein electrophoresis by Capillarys®. Clin Chem Lab Med. (2003) 41:704–10. doi: 10.1515/CCLM.2003.107
53. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. (1957) 28:56–63. doi: 10.1093/ajcp/28.1.56
54. McComb RB, Bowers GN. Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin Chem. (1972) 18:97–104.
55. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. (2004) 37:277–85. doi: 10.1016/j.clinbiochem.2003.11.015
56. Culling CF. Handbook of Histopathological and Histochemical Staining Techniques. 3rd ed. London: Butterworth (1983).
58. Martínez Y, Carrión Y, Rodríguez R, Valdivié M, Olmo C, Betancur C, et al. Growth performance, organ weights and some blood parameters of replacement laying pullets fed with increasing levels of wheat bran. J Braz J Poult Sci. (2015) 17:347–54. doi: 10.1590/1516-635X1703347-354
59. Jeroch H, Dänicke S, Brufau J. The influence of enzyme preparations on the nutritional value of cereals for poultry. A review. J Anim Feed Sci. (1995) 4:263–85. doi: 10.22358/jafs/69800/1995
60. Al-Harthi MA. Impact of supplemental feed enzymes, condiments mixture or their combination on broiler performance, nutrients digestibility and plasma constituents. Int J Poult Sci. (2006) 5:764–71. doi: 10.3923/ijps.2006.764.771
61. El-Kelawy MI. Effect of feed form, pellet diameter and enzymes supplementation on productive and physiological performance of broiler chicks (Ph.D. thesis), Faculty of Agriculture Damanhour University, Damanhur, Egypt (2012).
62. Zanella I, Sakomura N, Silversides F, Fiqueirdo A, Pack M. Effect of enzyme supplementation of broiler diets based on corn and soybeans. Poult Sci. (1999) 78:561–8. doi: 10.1093/ps/78.4.561
63. Cowieson AJ, Acamovic T, Bedford MR. Supplementation of diets containing pea meal with exogenous enzymes: effects on weight gain, feed conversion, nutrient digestibility and gross morphology of the gastrointestinal tract of growing broiler chicks. Br Poult Sci. (2003) 44:427–37. doi: 10.1080/00071660310001598292
64. Ghazalah AA, Abd EI-Gawad AH, Soliman MS, Youssef A, W. Effect of enzyme preparation on performance of broilers fed corn-soybean meal based diets. Egypt Poult Sci J. (2005) 25:295–316.
65. Shirmohammad F, Mehr M. Effects of dietary supplementation of multi-enzyme complex on the energy utilization in rooster and performance of broiler chicks. Afr J Biotechnol. (2011) 10:7541–7. doi: 10.5897/AJB10.2260
66. Zhou X, Wang Y, Gu Q, Li W. Effect of dietary probiotic, Bacillus coagulans, on growth performance, chemical composition, and meat quality of Guangxi Yellow chicken. Poult Sci. (2010) 89:588–93. doi: 10.3382/ps.2009-00319
67. Al-Khalaifah H, Al-Nasser A, Ragheb G, Al-Qalaf S, Al-Omani N, Aneesh N, et al. The Effect of Dietary Probiotics and Prebiotics on the Performance of Broiler Chickens in Kuwait. Technical Report No 2 (November 1–January 31, 2017), Kuwait Institute for Scientific Research (2017).
68. Akhtar M, Tariq AF, Awais MM, Iqbal Z, Muhammad F, Shahid M, et al. Studies on wheat bran Arabinoxylan for its immunostimulatory and protective effects against avian coccidiosis. Carbohydr Polym. (2012) 90:333–9. doi: 10.1016/j.carbpol.2012.05.048
69. Cao Y, Xu Y, Auchoybur ML, Chen W, He S, Qin W, et al. Regulatory role of IKK? in myocardial ischemia/reperfusion injury by the determination of M1 versus M2 polarization of macrophages. J Mol Cell Cardiol. (2018) 123:1–12. doi: 10.1016/j.yjmcc.2018.08.021
70. Cao L, Liu X, Qian T, Sun G, Guo Y, Chang F, et al. Antitumor and immunomodulatory activity of arabinoxylans: a major constituent of wheat bran. Int J Biol Macromol. (2011) 48:160–4. doi: 10.1016/j.ijbiomac.2010.10.014
71. Korte J, Fröhlich T, Kohn M, Kaspers B, Arnold GJ, Härtle S. 2D DIGE analysis of the bursa of Fabricius reveals characteristic proteome profiles for different stages of chicken B-cell development. Proteomics. (2013) 13:119–33. doi: 10.1002/pmic.201200177
72. Attia YA. Nutritive value of undehulled sunflower meal as affected by multienzymes supplementation to broiler diets. Archiv Geflugelk. (2003) 67:97–106.
73. Gao F, Jiang Y, Zhou GH, Han ZK. The effects of xylanase supplementation on growth, digestion, circulating hormone and metabolite levels, immunity and gut microflora in cockerels fed on wheat-based diets. Br Poult Sci. (2007) 48:480–8. doi: 10.1080/00071660701477320
74. Basmacioglu Malayoglu H, Baysal S, Misirlioglu Z, Polat M, Yilmaz H, Turan N. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat–soybean meal diets. Br Poult Sci. (2010) 51:67–80. doi: 10.1080/00071660903573702
75. Khaksar V, Golian A, Kermanshahi H. Immune response and ileal microflora in broilers fed wheat-based diet with or without enzyme Endofeed W and supplementation of thyme essential oil or probiotic PrimaLac®. Afr J Biotechnol. (2012) 11:14716–23. doi: 10.5897/AJB12.1237
76. Head B, Bionaz M, Cherian G. Flaxseed and carbohydrase enzyme supplementation alters hepatic n-3 polyunsaturated fatty acid molecular species and expression of genes associated with lipid metabolism in broiler chickens. Vet Sci. (2019) 6:25. doi: 10.3390/vetsci6010025
77. Seidavi A, Dadashbeiki M, Alimohammadi-Saraei M, Hoven R, Payan-Carreira R, Laudadio V, et al. Effects of dietary inclusion level of a mixture of probiotic cultures and enzymes on broiler chickens immunity response. Environ Sci Pollut Res. (2017) 24:4637–44. doi: 10.1007/s11356-016-8206-8
78. Abdel-Hafeez HM, Saleh ESE, Tawfeek SS, Youssef IMI, Abdel-Daim ASA. Utilization of potato peels and sugar beet pulp with and without enzyme supplementation in broiler chicken diets: effects on performance, serum biochemical indices and carcass traits. J Anim Physiol Anim Nutr. (2018) 102:56–66. doi: 10.1111/jpn.12656
79. El-Ghamry AA, Al-Harthi MA, Attia YA. Possibility to improve rice polishing utilisation in broiler diets by enzymes or dietary formulation based on digestible amino acids. Archiv Geflügelk. (2005) 69:49–56.
80. Steenfeldt S. The dietary effect of different wheat cultivars for broiler chickens. Br Poult Sci. (2001) 42:595–609. doi: 10.1080/00071660120088416
81. Yamauchi K-E. Review on chicken intestinal villus histological alterations related with intestinal function. J Poult Sci. (2002) 39:229–42. doi: 10.2141/jpsa.39.229
82. Iji PA. The impact of cereal non-starch polysaccharides on intestinal development and function in broiler chickens. World Poult Sci J. (1999) 55:375–87. doi: 10.1079/WPS19990026
83. Thomas DV, Ravindran V. Effect of cereal type on the performance, gastrointestinal tract development and intestinal morphology of the newly hatched broiler chick. J Poult Sci. (2008) 45:46–50. doi: 10.2141/jpsa.45.46
84. Luskin MR, DeAngelo DJ. T-cell acute lymphoblastic leukemia: current approach and future directions. Adv Cell Gene Ther. (2019) 2:e70. doi: 10.1002/acg2.70
85. Maddu N, Raghavendra PB. Review of lithium effects on immune cells. Immunopharmacol Immunotoxicol. (2015) 37:111–25. doi: 10.3109/08923973.2014.998369
86. Robert J, Sung M, Cohen N. In vitro thymocyte differentiation in MHC class I-negative Xenopus larvae. Dev Compar Immunol. (2001) 25:323–36. doi: 10.1016/S0145-305X(00)00066-5
87. Woyengo TA, Bogota KJ, Noll SL, Wilson J. Enhancing nutrient utilization of broiler chickens through supplemental enzymes. Poult Sci. (2018) 98:1302–9. doi: 10.3382/ps/pey452
88. Sateri S, Seidavi A, Bouyeh M, Neumann P, Kutzler M, Laudadio V, et al. Effect of olive meal and supplemental enzymes on performance traits, blood biochemistry, humoral immunity response and caecal microbiota of broilers. South Afr J Anim Sci. (2017) 47:804–12. doi: 10.4314/sajas.v47i6.8
89. Hashem NM, Soltan YA, El-Desoky NI, Morsy AS, Sallam SMA. Effects of Moringa oleifera extracts and monensin on performance of growing rabbits. Livest Sci. (2019) 228:136–43. doi: 10.1016/j.livsci.2019.08.012
Keywords: broilers, nutrient density, multienzymes, immune response, supplementation
Citation: Attia YA, Al-Khalaifah H, Abd El-Hamid HS, Al-Harthi MA and El-shafey AA (2020) Effect of Different Levels of Multienzymes on Immune Response, Blood Hematology and Biochemistry, Antioxidants Status and Organs Histology of Broiler Chicks Fed Standard and Low-Density Diets. Front. Vet. Sci. 6:510. doi: 10.3389/fvets.2019.00510
Received: 14 September 2019; Accepted: 23 December 2019;
Published: 04 February 2020.
Edited by:
Kyung-Woo Lee, Konkuk University, South KoreaReviewed by:
Alireza Seidavi, Islamic Azad University, Rasht Branch, IranNesrein M. Hashem, Alexandria University, Egypt
Copyright © 2020 Attia, Al-Khalaifah, Abd El-Hamid, Al-Harthi and El-shafey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Y. A. Attia, eWFhdHRpYUBrYXUuZWR1LnNh; H. Al-Khalaifah, aGtoYWxpZmFAa2lzci5lZHUua3c=
 Y. A. Attia
Y. A. Attia H. Al-Khalaifah
H. Al-Khalaifah H. S. Abd El-Hamid
H. S. Abd El-Hamid M. A. Al-Harthi1
M. A. Al-Harthi1