
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 12 December 2019
Sec. Veterinary Infectious Diseases
Volume 6 - 2019 | https://doi.org/10.3389/fvets.2019.00463
This article is part of the Research Topic Poultry Coccidiosis: Strategies to Understand and Control View all 9 articles
Poultry coccidiosis is a costly intestinal disease that leads to considerable tissue damage, inefficient nutrient absorption, increased mortality, and predisposition to secondary infections. This study evaluated the effects of a direct feed microbial (DFM) dietary additive on performance, intestinal morphology, and immune response of broilers during a mixed coccidiosis challenge. In total, 840 Cobb500 male broilers were randomly allocated to 3 treatments (7 replicates, 40 birds/pen) including negative control (NC) fed basal diet; positive control (PC) fed basal diet with coccidiosis challenge; and DFM supplemented diet, with coccidiosis challenge. At 15 days of age, all birds except for the NC treatment were orally gavaged with live oocysts of a commercial vaccine. On d 21 (6 days post challenge), 4 birds/pen were randomly selected and euthanized for scoring of coccidia-caused lesions in the duodenum, jejunum, and ceca. Body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were recorded on d 7, 14, 28, and 42. Jejunal and ileal tissue samples were taken for histomorphological assessment from 2 birds/pen on d 21. Ileal samples were also taken for mRNA expression analysis on d 14 and d 21. The DFM birds had significantly greater BWG than PC birds during d 0–21 (P < 0.05). No differences were observed among the treatment groups in terms of FI and FCR. Dietary DFM supplementation significantly reduced lesion scores in the duodenum and jejunum when compared with PC group (P < 0.05). The coccidia challenge significantly reduced (P < 0.05) ileal villus height when compared to the non-challenged group on d 21. Conversely, dietary DFM supplementation alleviated the negative effects of coccidiosis by increasing ileal villus area on d 21 (P < 0.05). The challenged birds had significantly greater expression of IFN-γ and IL-1β in the ileum on d 21. Based on these findings, dietary DFM supplementation may help restore broiler performance during the starter and early grower periods during coccidiosis, likely by maintaining gut integrity via improving intestinal morphology and also by reducing disease severity as manifested by lower lesion scores.
Avian coccidiosis is an important parasitic disease that leads to considerable intestinal tissue damage, inefficient nutrient absorption, and increased mortality resulting in millions of dollars in economic losses to the world poultry industry every year (1, 2). This disease is caused by several species of Eimeria of the phylum Apicomplexa (3), which are ubiquitous pathogens in the environment of poultry farms making it difficult to control (2). However, some of the anticoccidials commonly used to control these pathogens have been under scrutiny (4). Despite the treatment and prevention ability of these chemotherapeutic agents against intestinal diseases, increased public concerns over potential drug residues in poultry products and the emergence of drug-resistant pathogens have put restrictions on the use of certain agents (2, 5). Therefore, there is an increasing demand in the poultry industry for new alternative strategies to improve performance and disease resistance including means of establishing a favorable gut microbiota.
Direct fed microbials (DFMs), also known as probiotics, influence the host's health by maintaining balanced gut microbiota, preventing the growth of pathogenic microorganisms, promoting intake and digestion of feed, and enhancing the immune system (6, 7). Dietary use of DFMs significantly influenced broiler performance (8–10), intestinal morphology (11, 12), and the colonization of beneficial microorganisms in the intestine (13). In addition, DFMs are also found to be suitable for chickens to reduce pathogen colonization and invasion in the intestinal tract to prevent several enteric infections such as Salmonella Enteritidis (14), E. coli (15), and Clostridium perfringens (16). Among the DFMs, Bacillus-based products have become more popular for potential use in broiler diets as alternatives to antibiotic growth promoters to improve both performance and health (17). Due to their spore-forming ability, these bacteria can withstand harsh environmental conditions including during feed processing and pelleting, as well as survive and germinate under conditions of the gastrointestinal tract (18, 19). Bacillus amyloliquefaciens is a spore-forming probiotic bacterium that produces a variety of extracellular enzymes including α-amylases, proteases, and phytase which could improve digestion and absorption of certain nutrients. Studies with Bacillus amyloliquefaciens have reported improved growth performance and villus morphology (20), modified cecal microbiota and metabolites (17), and increased serum IgG and IgA concentrations of healthy broilers (21). Li et al. (22) suggested that dietary supplementation of Bacillus amyloliquefaciens downregulated mRNA abundance of TLR-4, INF- γ, and IL-1β, and improved intestinal barrier junction in LPS-challenged broilers. Moreover, dietary Bacillus subtilis-based DFMs reduced the severity of coccidiosis challenge and improved the immune response in broilers (23). Similarly, a recent study showed that Bacillus amyloliquefaciens administration reduced coccidial symptoms as evidenced by reduced intestinal lesions and improved villus height (24).
Based on previous findings that suggest the benefits of dietary Bacillus-based DFM administration, the current study hypothesized that dietary supplementation of three strains of Bacillus amyloliquefaciens may be an effective method to maintain broiler performance and health by influencing intestinal morphology and immune system of broiler chickens during a coccidiosis challenge.
This project was approved and conducted under the guidelines of the Virginia Tech Institutional Animal Care and Use Committee. On day of hatch, 840 male Cobb500 broiler chicks were acquired from a commercial hatchery and transported to the Virginia Tech research facilities. The birds were randomly allocated to three experimental groups each comprising 7 replicate floor pens with 40 birds per pen raised to 42 days (d). The three treatment groups were (1) negative control (NC) fed a basal diet without challenge, (2) positive control (PC) fed basal diet with coccidiosis challenge, and (3) direct feed microbial (DFM)-supplemented basal diet with coccidiosis challenge. The DFM (Enviva® PRO, Animal Nutrition, DuPont Nutrition & Biosciences, DE, USA) consists of three strains of Bacillus amyloliquefaciens at 1:1:1 ratio and added to provide 1.5 × 105 CFU/g of feed. The birds had ad libitum access to water and a non-medicated corn/soybean-based starter diet (d 0–14) in mash form, and grower (d 15–28) and finisher (d 29–42) in pellet form. All diets were formulated to meet or exceed National Research Council nutrient recommendations (25). Birds were housed in a controlled environment with the ambient temperature thermostatically controlled and gradually reduced from 34°C on the first day to 22°C at 3 weeks, then maintained at 22°C thereafter. The light cycle was 20 h light and 4 h dark throughout the experimental period.
At 15 days of age, all birds except for the NC group (which were given 1 mL sterile water) were orally gavaged with 10X the commercial vaccine Advent® (1 mL per bird) containing live oocysts of Eimeria acervulina, E. maxima, and E. tenella as previously described by Ritzi et al. (4). On d 21 (6 days post challenge), 28 birds per treatment (4 birds/pen with average pen weight) were randomly selected and euthanized for scoring of coccidia-induced lesions in the duodenum, jejunum, and ceca by personnel blinded to the treatments. Scoring was performed according to the method of Johnson and Reid (26) based on scores ranging from 0 (no gross lesions) to 4 (most severe lesions).
Body weight (BW) and feed intake (FI) were recorded on per pen basis at d 14, 21 28, and 42. Body weight gain (BWG) was then calculated for the three feeding phases as well as at the end of the challenge period (d 21). Daily bird mortality and weights were recorded and feed conversion ratios (FCR) corrected accordingly.
Jejunal (10 cm distal from the bile duct) and ileal (10 cm proximal to the ileocecal junction) tissue samples were taken from 2 birds/pen on d 21. Histological samples were rinsed with ice-cold PBS, preserved in 10% neutral buffered formalin, and shipped to Histo-Scientific Research Laboratories (HSRL, Inc., Mt. Jackson, VA, USA) for slide preparation. The intestinal samples were embedded in paraffin and serially cut into 5 μm sections. Four sections from each jejunum and ileum were mounted on each slide, which were stained using routine procedures for hematoxylin and eosin. Histological measurements and calculations including villus length, mid-point villus width, crypt depth, villus area, and villus height to crypt depth ratio were performed using an Olympus BX50 microscope and SigmaScan Pro 5 software (Olympus America, Melville, NJ, USA) as previously described (27).
On d 22, one bird from each pen (21 birds/treatment) was selected with body weight close to pen average, weighed, euthanized and samples collected to assess gut strength. Sections of the jejunum and ileum were excised, rinsed with sterile PBS, and immediately tested for tensile strength using an Instron Universal Materials Testing Machine (Instron Corp., Norwood, MA, USA) (28).
Intestinal tissue samples were taken from 2 birds/pen on each d 14 and d 21 (from birds with average pen weight). Immediately following euthanasia by cervical dislocation, sections were aseptically excised, rinsed in cold PBS, minced on ice-cold surface, snap-frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted with Trizol reagent following the manufacturer's instructions (ZYMO Research, Direct-zol RNA MiniPrep). Total RNA concentration was determined at optical density (OD) 260 (NanoDrop-1000, Thermo Fisher Scientific, Waltham, MA, USA), and RNA purity was verified by evaluating the ratio of OD 260 to OD 280. After extraction, 2 μg of total RNA were reverse-transcribed into cDNA using the high capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA) following the manufacturer's protocol, and the cDNA was stored at −20°C.
Quantitative real-time PCR (qRT-PCR) was performed using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). The cDNA was diluted 1:30 in nuclease-free water, and 1 μL of the diluted cDNA was added to each well of a 96-well plate. Next, 9 μL of real-time PCR master mix containing 5 μL of Fast SYBR Green Master Mix (Applied Biosystems), 0.5 μL each of 2 μM forward and reverse primers, and 3 μL of sterile nuclease-free water per reaction were added to each well for a final volume of 10 μL. During the PCR reaction, samples were subjected to an initial denaturation phase at 95°C for 20 s followed by 40 cycles of denaturation at 95°C for 3 s and annealing and extension at 60°C for 30 s. mRNA expression for interferon (IFN)-γ and interleukin (IL)-1β was analyzed using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control. Each reaction was run in duplicate. Primers were designed (Table 1) using the Primer Express 3.0 software (Applied Biosystems). Results from qRT-PCR were analyzed using the 7500 Real-Time PCR software (Applied Biosystems). Average mRNA abundance relative to the GAPDH endogenous control for each sample was calculated using the 2−ΔΔCt method (29).
All data were subjected to one-way analysis of variance (ANOVA) using SAS (2004). When significant differences were noted, Tukey's test was performed to separate means and significance accepted at P ≤ 0.05.
Body weight gain (g/bird) is presented in Figure 1. No significant differences in BWG were found between the control groups (non-challenged or challenged) and treatment group from d 0 to d 14. Birds in the DFM group had significantly higher (P < 0.05) BWG than the challenged control birds (PC) between d 0 and d 21(NC: 763.5 g; PC: 747.2 g; DFM: 773.7 g). The coccidia challenge reduced the cumulative BWG over the overall experimental period (d 0–42) regardless of dietary DFM supplementation. Feed intake (g/bird) and FCR (g/g bird) are presented in Figures 2, 3, respectively. No significant differences were observed among the treatment groups during d 0–14, d 0–21, and d 0–42, in terms of feed intake and FCR. Although not significantly different, overall mortality rate was slightly higher in challenged (PC: 6.07%, DFM: 6.07%) birds in comparison to non-challenged (4.64%) birds.
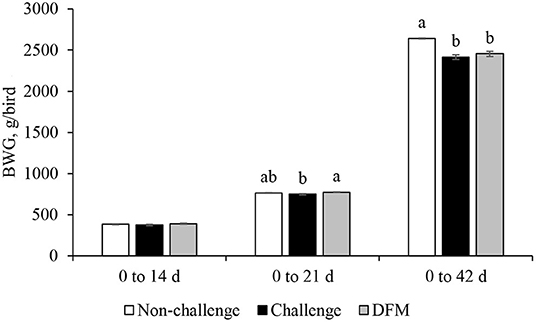
Figure 1. Body weight gain (BWG) of broiler chickens fed control and treatment diets. Each bar represents the mean ± SE (n = 7). a,bBars with different superscripts are significantly different (P < 0.05).
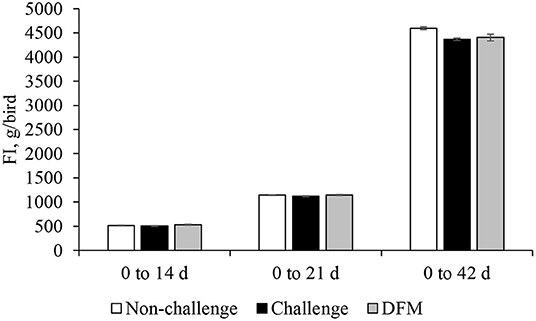
Figure 2. Feed intake (FI) of broiler chickens fed control and treatment diets. Each bar represents the mean ± SE (n = 7).
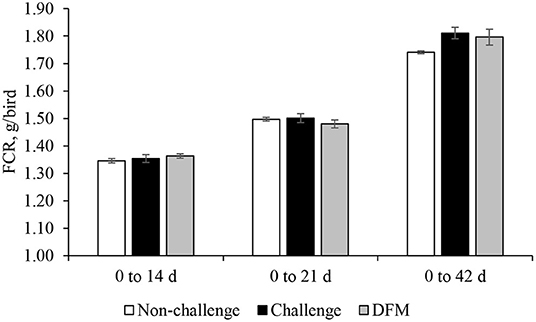
Figure 3. Feed conversion ratio (FCR) of broiler chickens fed control and treatment diets. Each bar represents the mean ± SE (n = 7).
Lesion scores are presented in Figure 4. Dietary DFM supplementation significantly reduced lesion scores in the duodenum and jejunum when compared with the PC (challenged) group (P < 0.05). As expected, no lesions were observed in the NC (non-challenged) birds.
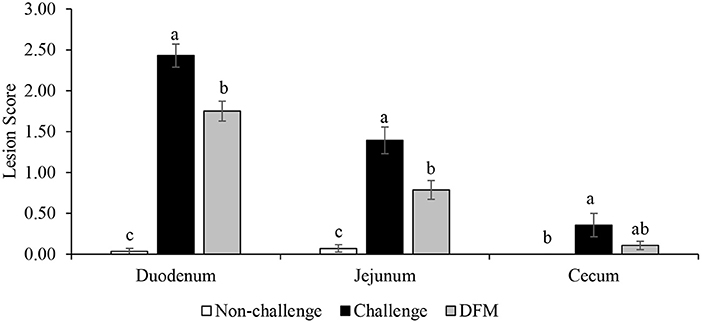
Figure 4. Lesion scores of duodenum, jejunum, and cecum on d 21. a–cBars with different superscripts are significantly different (P < 0.05).
Morphological measurements of jejunal and ileal tissues are presented in Table 2. At 6 days post challenge (d 21), jejunum villus height (P < 0.05) and villus area (P < 0.01) of the birds in the DFM group were significantly greater than birds in the challenge group (PC). Jejunum crypt depths were increased (P ≤ 0.001) with the dietary DFM supplementation when compared to no-challenge treatment on d 21. The coccidia challenge significantly reduced (P < 0.05) ileal villus height when compared to the non-challenged group on d 21. Conversely, dietary DFM supplementation alleviated the negative effects of coccidiosis by increasing villus area of the ileum on d 21 (P < 0.05).
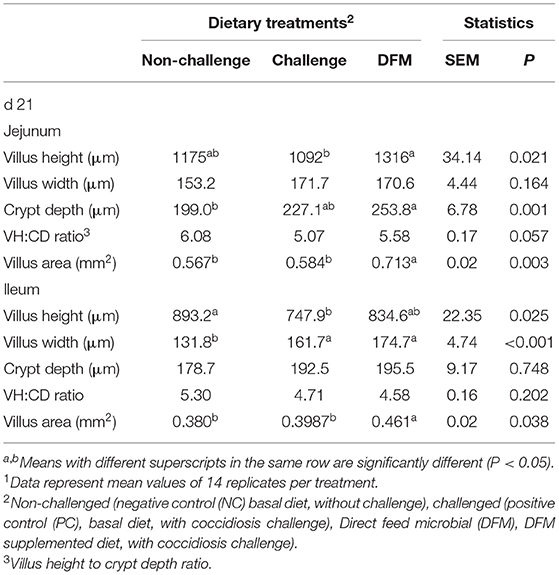
Table 2. Effect of dietary DFM supplementation on broiler jejunum and ileum histomorphology 6 days after post challenge (d 21)1.
The effect of dietary DFM supplementation on intestinal tensile strength is shown in Figure 5. No significant differences were observed among the treatment groups in terms of intestinal tensile strength on d 22.
Figure 5. Effect of dietary DFM supplementation on broiler gut tensile strength on d 22. Each bar represents the mean ± SE.
The effect of dietary DFM supplementation on the mRNA expression of IFN-γ and IL-1β in the ileum are shown in Figure 6. Ileal IFN-γ mRNA level was not influenced by dietary treatments before challenge (d 14), but was greater following the coccidia challenge (d 21) regardless of dietary DFM supplementation (P < 0.001). The challenge control group (PC) had greater IL-1β mRNA level when compared with the non-challenged (NC) group on d 21 (P < 0.01).
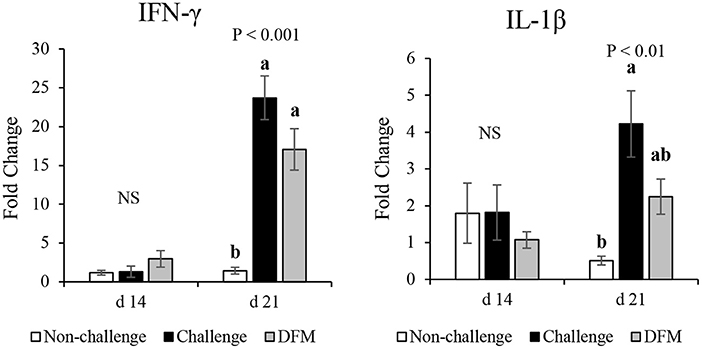
Figure 6. Effect of dietary DFM supplementation on IFN-γ and IL-1β mRNA expression in the ileum on d 21. Each bar represents the mean ± SE. a,bBars with different superscripts are significantly different (P < 0.05). NS, Not significant.
Promoting the colonization of beneficial bacteria via non-drug feed additives such as probiotics, to modify the intestinal microbiome and subsequently improve gut barrier function and immune response is becoming an accepted alternative strategy in modern poultry production. However, seeking effective non-drug alternatives to reduce or prevent intestinal pathogens is still under development. In this context, the current work investigated the effects of dietary DFM supplementation on broiler performance, intestinal integrity and immune response of broiler chickens challenged with coccidia.
Coccidiosis is a prevalent intestinal disease characterized by epithelial damage, malabsorption and reduced performance. As an expected outcome, coccidia-challenged birds fed a basal diet displayed retarded growth performance compared with non-challenged control birds. The present study showed that dietary DFM supplementation alleviated the growth suppression effect of coccidiosis by improving BWG at the end of week three when pathology was assessed. Similarly, dietary addition of Bacillus-based probiotics restored performance loss compared with coccidia-infected control (no probiotic) birds (23). Moreover, Giannenas et al. (30) noted that birds fed multi-species probiotic mix performed better than infected control birds. The growth promoting effects of DFMs could be related to several modes of action, such as competitive exclusion of pathogens at the epithelial attachment sites, improved intestinal integrity in terms of villi health, or increased concentration of beneficial bacteria in the intestinal tract (31). However, contrary to the observed improvement trend in performance during the grower period, challenged birds, both PC and DFM, had similar BW at the end of the study. These findings are likely due to the compensatory growth potential of fast-growing broilers (32). Further, growth promoting effects of the DFM may be more efficient and pronounced at industrial standards compared to controlled trial as commercial birds are typically exposed to more stressors than a single challenge (33).
Broiler performance and intestinal lesion scores are important parameters used to evaluate the severity of enteric diseases such as coccidiosis (34) and necrotic enteritis (35). Eimeria spp. are responsible for mild-to-severe intestinal lesions and these lesions differ across intestinal infection sites depending on the species (3, 36). As expected, no coccidia-induced lesions were found in the intestinal tissues of the non-challenged control birds while coccidial lesions were observed in the challenge control birds without probiotic supplementation (PC). However, birds in the DFM group had less severe duodenal and jejunal lesions compared to PC. These results are in agreement with previous studies in which supplementations of several probiotics were reported to reduce the severity of the intestinal lesions associated with coccidiosis (4, 23). Similarly, Abdelrahman et al. (2) observed significant reduction in oocyst shedding and intestinal lesion scores. Lower lesion scores are indicative of healthier and more functional intestinal epithelium and such changes can be directly correlated with more efficient nutrient utilization and absorption (34, 37). The observed improvements could be attributed to the direct and/or indirect effect of DFMs on colonization and replication of this intracellular parasite in the epithelial tissues, either by competitive exclusion mechanism or possible immune modulatory effects (2, 38). The present study demonstrated that dietary DFM supplementation reduced intestinal lesions under coccidiosis challenge conditions.
Structural changes in the small intestinal architecture can reveal important information about bird performance and gut health (39). Intestinal infections such as coccidiosis, induce villus atrophy (40, 41) and thickening of the lamina propria (41, 42), which retard digestion and nutrient absorption and induce subsequent reduction of growth performance (24). The current findings showed that the coccidiosis challenge significantly influenced jejunal and ileal morphology by decreasing villus height and surface area. Conversely, dietary DFM supplementation alleviated the negative effects of coccidiosis. These results also coincide with reduced intestinal lesion scores. In agreement with the current study, Giannenas et al. (30) concluded that Bacillus subtilis-supplemented birds had greater villus height compared to E. tenella infected birds. Similarly, Tsukahara et al. (24) reported that birds fed a diet containing Bacillus amyloliquefaciens (TOA5001) had larger villi than those fed a control diet under a coccidiosis challenge. It is apparent that alimentary- and bacteria-related antigens in the digesta impair the absorptive functions and intestinal integrity (43). However, the use of dietary non-drug alternatives, such as probiotics, may help to maintain intestinal integrity through several possible mechanisms. According to our results, observed improvements in intestinal integrity might be related to the inhibitory effects of DFM against the invasion and proliferation of Eimeria parasites. These enhancements also can be attributed to the stimulation of the colonized beneficial microbiota and increased abundance of bacterial metabolites such as butyrate, which could induce enterocyte differentiation and proliferation.
Besides its main function of digestion and absorption, the intestine also plays an important role in protection against pathogens by activation of both adaptive and innate immune responses (44). As an intracellular enteric parasite, Eimeria spp. cause epithelial damage and induce inflammation, depending on the severity of the infection, by invading the intestinal mucosa (45). Penetration and invasion of the pathogen triggers a cascade of signaling events that lead to production of various cytokines such as IL-1β, IFN-γ, IL-17, TGF-β, and IL-10 (46, 47). IL-1β and IFN-γ mRNA levels were upregulated in the ileum of the challenged birds, indicating that coccidiosis infection induced inflammation (48). Coccidia-induced upregulation of IFN-γ and/or IL-1β mRNA levels were also documented in previous studies (41, 49–51). Production of these pro-inflammatory Th1 cytokines induces cell mediated immune responses during coccidiosis. As reported herein, no differences were observed between challenge control (PC) and DFM groups in terms of IFN-γ and IL-1β expression levels, except only numerical downregulation in the DFM group compared to PC birds. Dietary use of Lactobacillus-based probiotic elevated the intestinal IFN-γ level 3 days post-challenge; however, no differences were observed thereafter (38). This time-dependent difference in the IFN-γ level might be a possible reason in the current study as mRNA levels were evaluated 6 days post-challenge. It should be noted that severity of the infection and the probiotic strain(s) as well as dosage are important considerations when alternative growth stimulators are applied. Therefore, minor differences between PC and DFM birds in terms of IFN-γ and IL-1β mRNA levels might be related to the time of sampling, severity of infection, or probiotic type and dose.
Based on the presented findings, it can be concluded that dietary DFM supplementation may help restore broiler performance during the starter and grower periods, after coccidian challenge by improving intestinal morphology and reducing lesion severity. Due to its potential beneficial effects on broiler performance and health, this DFM may be used in the broiler industry as a promising alternative when antibiotic growth promoters are not used. Additionally, it would be useful to further assess the effects of this probiotic on specific intestinal tight junction proteins, gut microbiota, and short chain fatty acid composition under similar disease challenge conditions.
All datasets generated for this study are included in the article/supplementary material.
This project was approved and conducted under the guidelines of the Virginia Tech Institutional Animal Care and Use Committee (VA Tech IACUC).
AC, IO, and MW conducted the study and supervised all analyses. AC drafted the manuscript. WL contributed to research design and manuscript revisions. RD was the principal investigator overseeing all aspects of the study. All authors read and approved the final manuscript.
This work was supported in part by the USDA National Institute of Food and Agriculture Hatch funds to the Virginia Agricultural Experiment Station.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AC was a recipient of the postdoctoral fellowship of the Scientific and Technological Research Council of Turkey (TUBITAK). The authors wish to thank Dr. Justin Barone for his technical support.
1. Ott C, Omara I, Persia M, Dalloul R. The impact of β-glucans on performance and response of broiler chickens during a coccidiosis challenge. Poult Sci. (2018) 97:2713–21. doi: 10.3382/ps/pey148
2. Abdelrahman W, Mohnl M, Teichmann K, Doupovec B, Schatzmayr G, Lumpkins B, et al. Comparative evaluation of probiotic and salinomycin effects on performance and coccidiosis control in broiler chickens. Poult Sci. (2014) 93:3002–8. doi: 10.3382/ps.2014-04212
3. Quiroz-Castañeda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. BioMed Res Int. (2015) 2015:430610. doi: 10.1155/2015/430610
4. Ritzi MM, Abdelrahman W, Mohnl M, Dalloul RA. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult Sci. (2014) 93:2772–8. doi: 10.3382/ps.2014-04207
5. Calik A, Ergun A. Effect of lactulose supplementation on growth performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult Sci. (2015) 94:2173–82. doi: 10.3382/ps/pev182
6. Kim KM, Kim MJ, Kim DH, Park YS, Kang JS. Characterization of Bacillus polyfermenticus KJS-2 as a probiotic. J Microbiol Biotechnol. (2009) 19:1013–8. doi: 10.4014/jmb.0903.113
7. Lutful Kabir SM. The role of probiotics in the poultry industry. Int J Mol Sci. (2009) 10:3531–46. doi: 10.3390/ijms10083531
8. Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. (2014) 94:341–8. doi: 10.1002/jsfa.6365
9. Mountzouris KC, Tsirtsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci. (2007) 86:309–17. doi: 10.1093/ps/86.2.309
10. Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, et al. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci. (2010) 89:58–67. doi: 10.3382/ps.2009-00308
11. Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. (2009) 88:49–56. doi: 10.3382/ps.2008-00244
12. Sen S, Ingale SL, Kim YW, Kim JS, Kim KH, Lohakare JD, et al. Effect of supplementation of Bacillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res Vet Sci. (2012) 93:264–8. doi: 10.1016/j.rvsc.2011.05.021
13. Jacquier V, Nelson A, Jlali M, Rhayat L, Brinch KS, Devillard E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult Sci. (2019) 98:2548–54. doi: 10.3382/ps/pey602
14. Park J, Kim I. Supplemental effect of probiotic Bacillus subtilis B2A on productivity, organ weight, intestinal Salmonella microflora, and breast meat quality of growing broiler chicks. Poult Sci. (2014) 93:2054–9. doi: 10.3382/ps.2013-03818
15. Cao GT, Zeng XF, Chen AG, Zhou L, Zhang L, Xiao YP, et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci. (2013) 92:2949–55. doi: 10.3382/ps.2013-03366
16. Wang H, Ni X, Qing X, Liu L, Lai J, Khalique A, et al. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front Immunol. (2017) 8:1592. doi: 10.3389/fimmu.2017.01592
17. Cao GT, Zhan XA, Zhang LL, Zeng XF, Chen AG, Yang CM. Modulation of broilers' caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J Anim Physiol Anim Nutr. (2018) 102:E909–17. doi: 10.1111/jpn.12856
18. Shivaramaiah S, Pumford NR, Morgan MJ, Wolfenden RE, Wolfenden AD, Torres-Rodriguez A, et al. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult Sci. (2011) 90:1574–80. doi: 10.3382/ps.2010-00745
19. Amerah AM, Quiles A, Medel P, Sanchez J, Lehtinen MJ, Gracia MI. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim Feed Sci Tech. (2013) 180:55–63. doi: 10.1016/j.anifeedsci.2013.01.002
20. Lei XJ, Ru YJ, Zhang HF. Effect of Bacillus amyloliquefaciens-based direct-fed microbials and antibiotic on performance, nutrient digestibility, cecal microflora, and intestinal morphology in broiler chickens. J Appl Poult Res. (2014) 23:486–93. doi: 10.3382/japr.2014-00965
21. Ahmed ST, Islam MM, Mun HS, Sim HJ, Kim YJ, Yang CJ. Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult Sci. (2014) 93:1963–71. doi: 10.3382/ps.2013-03718
22. Li Y, Zhang H, Chen YP, Yang MX, Zhang LL, Lu ZX, et al. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim Feed Sci Tech. (2015) 208:119–31. doi: 10.1016/j.anifeedsci.2015.07.001
23. Lee KW, Lillehoj HS, Jang SI, Li G, Lee SH, Lillehoj EP, et al. Effect of Bacillus-based direct-fed microbials on Eimeria maxima infection in broiler chickens. Comp Immunol Microbiol Infect Dis. (2010) 33:e105–10. doi: 10.1016/j.cimid.2010.06.001
24. Tsukahara T, Inoue R, Nakayama K, Inatomi T. Inclusion of Bacillus amyloliquefaciens strain TOA5001 in the diet of broilers suppresses the symptoms of coccidiosis by modulating intestinal microbiota. Anim Sci J. (2018) 89:679–87. doi: 10.1111/asj.12980
26. Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. (1970) 28:30–6. doi: 10.1016/0014-4894(70)90063-9
27. Fasina YO, Hoerr FJ, Mckee SR, Conner DE. Influence of Salmonella enterica serovar Typhimurium infection on intestinal goblet cells and villous morphology in broiler chicks. Avian Dis. (2010) 54:841–7. doi: 10.1637/9055-090809-Reg.1
28. Miles RD, Butcher GD, Henry PR, Littell RC. Effect of antibiotic growth promoters on broiler performance, intestinal growth parameters, and quantitative morphology. Poult Sci. (2006) 85:476–85. doi: 10.1093/ps/85.3.476
29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
30. Giannenas I, Papadopoulos E, Tsalie E, Triantafillou E, Henikl S, Teichmann K, et al. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol. (2012) 188:31–40. doi: 10.1016/j.vetpar.2012.02.017
31. Wang X, Farnell YZ, Kiess AS, Peebles ED, Wamsley KGS, Zhai W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult Sci. (2019) 98:3839–49. doi: 10.3382/ps/pez096
32. Voeten AC, Braunius WW, Orthel FW, Van Rijen MA. Influence of coccidiosis on growth rate and feed conversion in broilers after experimental infections with Eimeria acervulina and Eimeria maxima. Vet Q. (1988) 10:256–64. doi: 10.1080/01652176.1988.9694182
33. Timmerman HM, Veldman A, Van Den Elsen E, Rombouts FM, Beynen AC. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult Sci. (2006) 85:1383–8. doi: 10.1093/ps/85.8.1383
34. Ritzi MM, Abdelrahman W, Van-Heerden K, Mohnl M, Barrett NW, Dalloul RA. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet Res. (2016) 47:111. doi: 10.1186/s13567-016-0397-y
35. Jayaraman S, Thangavel G, Kurian H, Mani R, Mukkalil R, Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult Sci. (2013) 92:370–4. doi: 10.3382/ps.2012-02528
36. Kang Q, Vahl CI, Fan H, Geurden T, Ameiss KA, Taylor LP. Statistical analyses of chicken intestinal lesion scores in battery cage studies of anti-coccidial drugs. Vet Parasitol. (2019) 272:83–94. doi: 10.1016/j.vetpar.2018.12.002
37. Amerah AM, Ravindran V. Effect of coccidia challenge and natural betaine supplementation on performance, nutrient utilization, and intestinal lesion scores of broiler chickens fed suboptimal level of dietary methionine. Poult Sci. (2015) 94:673–80. doi: 10.3382/ps/pev022
38. Dalloul RA, Lillehoj HS, Tamim NM, Shellem TA, Doerr JA. Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis. (2005) 28:351–61. doi: 10.1016/j.cimid.2005.09.001
39. Awad WA, Bohm J, Razzazi-Fazeli E, Ghareeb K, Zentek J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci. (2006) 85:974–9. doi: 10.1093/ps/85.6.974
40. Assis RC, Luns FD, Beletti ME, Assis RL, Nasser NM, Faria ES, et al. Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeria acervulina. Vet Parasitol. (2010) 168:185–9. doi: 10.1016/j.vetpar.2009.11.017
41. Dersjant-Li Y, Gibbs K, Awati A, Klasing K. The effects of enzymes and direct fed microbial combination on performance and immune response of broilers under a coccidia challenge. J Appl Anim Nutr. (2016) 4:1–14. doi: 10.1017/jan.2016.2
42. Bozkurt M, Aysul N, Kucukyilmaz K, Aypak S, Ege G, Catli AU, et al. Efficacy of in-feed preparations of an anticoccidial, multienzyme, prebiotic, probiotic, and herbal essential oil mixture in healthy and Eimeria spp.-infected broilers. Poult Sci. (2014) 93:389–99. doi: 10.3382/ps.2013-03368
43. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. (2014) 14:141–53. doi: 10.1038/nri3608
44. Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev. (2008) 9:101–10. doi: 10.1017/S146625230800145X
45. Yun CH, Lillehoj HS, Lillehoj EP. Intestinal immune responses to coccidiosis. Dev Comp Immunol. (2000) 24:303–24. doi: 10.1016/S0145-305X(99)00080-4
46. Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F. T helper cells plasticity in inflammation. Cytometry A. (2014) 85:36–42. doi: 10.1002/cyto.a.22348
47. Fasina YO, Lillehoj HS. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult Sci. (2019) 98:188–98. doi: 10.3382/ps/pey390
48. Alcala-Canto Y, Ramos-Martinez E, Tapia-Perez G, Gutierrez L, Sumano H. Pharmacodynamic evaluation of a reference and a generic toltrazuril preparation in broilers experimentally infected with Eimeria tenella or E. acervulina. Br Poult Sci. (2014) 55:44–53. doi: 10.1080/00071668.2013.872770
49. Cox CM, Sumners LH, Kim S, Mcelroy AP, Bedford MR, Dalloul RA. Immune responses to dietary beta-glucan in broiler chicks during an Eimeria challenge. Poult Sci. (2010) 89:2597–607. doi: 10.3382/ps.2010-00987
50. Wils-Plotz EL, Jenkins MC, Dilger RN. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult Sci. (2013) 92:735–45. doi: 10.3382/ps.2012-02755
Keywords: broiler, coccidiosis, direct feed microbials, performance, immune response, cytokine
Citation: Calik A, Omara II, White MB, Li W and Dalloul RA (2019) Effects of Dietary Direct Fed Microbial Supplementation on Performance, Intestinal Morphology and Immune Response of Broiler Chickens Challenged With Coccidiosis. Front. Vet. Sci. 6:463. doi: 10.3389/fvets.2019.00463
Received: 26 August 2019; Accepted: 28 November 2019;
Published: 12 December 2019.
Edited by:
Michael Kogut, United States Department of Agriculture, United StatesReviewed by:
Christi Swaggerty, United States Department of Agriculture, United StatesCopyright © 2019 Calik, Omara, White, Li and Dalloul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rami A. Dalloul, cmRhbGxvdWxAdnQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.