
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 26 November 2018
Sec. Animal Behavior and Welfare
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00299
Surgical castration of piglets is performed routinely on commercial pig farms, to prevent boar taint and minimize aggression. While this procedure is known to be painful, piglets are generally not provided any analgesic for pain relief, leading to welfare concerns. The objectives of this study were to assess the efficacy of two non-steroidal anti-inflammatory drugs (NSAIDs), meloxicam (MEL) (0.4 mg/kg or 1.0 mg/kg) and ketoprofen (KET) (6.0 mg/kg) in reducing behavioral indicators of pain in castrated piglets. This study also examined the utility of the Piglet Grimace Scale (PGS) as a pain assessment tool. Nineteen litters of 5-days-old male piglets (n = 120) were used and piglets within a litter were randomly assigned to one of eight possible treatments: 0.4 mg/kg MEL-castrated or uncastrated, 1.0 mg/kg MEL-castrated or uncastrated, 6.0 mg/kg KET-castrated or uncastrated, saline (castrated control), or sham (uncastrated control). Treatments were administered intramuscularly (IM) 20 min prior to surgical castration. Piglets were video recorded for 1 h pre-procedure, for 8 h immediately post-castration and for another hour, 24 h post-procedure. Twenty-one behaviors and postures were scored continuously for the first 15 min of each hour and 1,156 still images of piglet faces were collected and scored using the PGS. Within each treatment group post-castration, castrated piglets displayed significantly more pain-related behaviors than uncastrated piglets (0.4 mg/kg MEL: p = 0.0339, 1.0 mg/kg MEL: p = 0.0079, 6.0 mg/kg KET: p = 0.0034, Controls: p < 0.0001). Castrated piglets also grimaced significantly more post-procedure than uncastrated piglets (p = 0.0061). Compared to the castrated control, none of the NSAID treatments significantly reduced piglet pain behaviors (0.4 mg/kg MEL: p = 1.385334, 1.0 mg/kg MEL: p = 0.9995, 6.0 mg/kg KET: p = 0.4163) or facial grimacing. Piglets demonstrated significantly more pain behaviors 24 h post-castration than at all other time points (p < 0.0001). The PGS was a less effective measure to detect acute pain; however, our findings suggest it does have utility as a pain assessment tool in neonatal pigs. Our findings also indicate that the use of these NSAIDs were ineffective at alleviating castration-associated pain in piglets.
Piglets are surgically castrated on commercial pig production farms to prevent boar taint and reduce aggression (1). This is known to be a painful procedure, based on specific behavioral and physiologic indicators, including rump scratching, increased blood cortisol levels, and high-frequency vocalizations (2–4), yet piglets are generally not provided any analgesia or anesthesia for pain relief. This has been recognized as a significant welfare concern in pig production, with guidelines in the EU and Canada now requiring analgesia administration prior to surgical castration (5, 6). Non-steroidal anti-inflammatory drugs (NSAIDs), such as meloxicam (MEL) and ketoprofen (KET), are the most common type of analgesic given to food animals and are currently being recommended for use in piglets to alleviate pain. The label dose of meloxicam (0.4 mg/kg) has had variable success in significantly reducing surgical castration pain (4, 7). There is limited research on the use of ketoprofen for pain control in piglets following castration.
A Piglet Grimace Scale (PGS) was developed by our research group at the University of Guelph to rapidly assess pain based on piglet facial expressions (8). How well piglet grimacing corresponds to expression of pain behaviors is important to determine the accuracy of this novel pain assessment tool.
The objectives of this study were to assess the effectiveness of meloxicam at the label dose (0.4 mg/kg) and a high dose (1.0 mg/kg), as well as, ketoprofen (6.0 mg/kg) in reducing pain resulting from surgical castration of piglets. We hypothesized that piglets receiving 1.0 mg/kg meloxicam would show the greatest reduction in pain behaviors. This study also aimed to determine if the PGS could be used as a pain assessment tool by comparing it against castration-related pain behaviors.
All animal use and procedures were approved by the University of Guelph Animal Care Committee (Animal Utilization Protocol #3350). The institution is registered under the Animals for Research Act of Ontario and holds a Good Animal Practice certificate issued by the Canadian Council on Animal Care.
A total of 120 Yorkshire-Landrace × Duroc male piglets (5-days-old, 1.15–2.95 kg BW) from 19 different litters were used in this study. Cross-fostering of piglets did occur on-farm when necessary, but litters of piglets were selected for this study that remained with their biological sow. Sows and piglets were housed in farrowing pens at the University of Guelph Arkell Swine Research Station. The floor space for each pen was 1.8 × 2.4 m and the farrowing crate was 0.8 × 2.3 m. The farrowing rooms were maintained at ambient temperature (23 ± 0.5°C) with lights on/off at 07:00/21:00, and natural light was provided by windows in each room. Sows were fed ab libitum beginning 4 days after farrowing. The creep areas for piglets were heated to approximately 30–35°C by means of a heating pad or lamp (the farrowing pens in this study were equipped with heat lamps).
Eight treatments were used, and each treatment group was identified by a unique symbol (a T, V, X, ∞, #, diamond, heart, or square) that was marked on the piglet's forehead and back with a black permanent marker prior to castration. This was to ensure that individuals involved in most aspects of the study (e.g., scoring behavior, pulling images of piglet faces, statistical analysis) were unaware of animal treatment. Marking the piglet's forehead was necessary to maintain animal-treatment group identification when only observing piglet faces. Numbers were also written on the back leg of piglets for individual identification. Fifteen piglets were assigned to each treatment group. Group size was based on a sample size estimate, using α = 0.05, population σ = 0.1 (determined from a pilot study) and 5% precision (8, 9). Within each litter, piglets were randomly assigned to one of the following treatments: 0.4 mg/kg meloxicam-castrated, 0.4 mg/kg meloxicam-uncastrated, 1.0 mg/kg meloxicam-castrated, 1.0 mg/kg meloxicam-uncastrated, 6.0 mg/kg ketoprofen-castrated, 6.0 mg/kg ketoprofen-uncastrated, saline (castrated control), or sham (uncastrated control). Meloxicam (MEL) (Metacam 20 mg/mL; Boehringer Ingelheim Ltd., Burlington, ON, Canada) was administered as an intramuscular (IM) injection at the label dose (0.4 mg/kg) and a higher, semi-log increment dose (1.0 mg/kg). Metacam was diluted to 4 mg/mL for administration of 0.4 mg/kg and 10 mg/mL for administration of 1.0 mg/kg, to give an average volume of ~0.2 mL to all piglets (range: 0.1–0.3 mL/piglet). Ketoprofen (KET) (Anafen 100 mg/mL; Merial Canada Inc., Baie-D'Urfé, QC, Canada; extra-label use) was administered IM and diluted to 80 mg/mL to administer ~0.2 mL to all piglets (range: 0.1–0.2 mL/piglet). Ketoprofen dose was derived from the literature (10). Saline was given IM at 0.2 mL/piglet. The sham treatment group was only handled and did not receive an injection.
Twenty-four hours prior to the trial, piglets were weighed and marked with the symbol that corresponded to their treatment group (treatments were not piglet weight-balanced; mean piglet weight in each treatment group is presented in Table 2). On the day of castration, male piglets within a pen were separated from their littermates, placed in a transport cart, and administered their assigned treatments 20 min prior to castration. Piglets were surgically castrated using two vertical incisions and tearing of the spermatic cord (11) and then returned to their pen. Separation from the sow and littermates lasted ~30–40 min. All castrations occurred between 08:00 and 10:00 and were conducted by one individual (AVV). Piglets in the sham treatment group were positioned on the leg of the castrator as if they were about to undergo the procedure, the handle of the scalpel was used to simulate the incision and the scrotum was manipulated to resemble a surgical castration. Uncastrated-treatment piglets were given an IM injection only and did not undergo a simulated castration.
Piglets were video recorded pre-procedure for 1 h using a high definition video camera (JVC GZ-E200 full HD Everio Camcorder, Yokohama, Japan) mounted on a tripod outside of the farrowing pens. Immediately post-castration, piglets were video recorded continuously for 8 h, and again for 1 h at 24 h post-procedure (i.e., 10 h of video data were collected in total for each litter of pigs). The behavior of each piglet was scored continuously by four trained observers for the first 15 min of every hour of data collected using the Observer XT program (Version 12.0: Noldus Information Technology, Wageningen, The Netherlands) according to a detailed ethogram adapted from Hay et al. (2) (Table 1). The observers were blinded as to time point, litter, and piglet treatment; however, they could observe which piglets had been castrated and which had not. Two observers scored two pens each, one observer scored six pens, and one observer scored nine pens. The scoring reliability between the four observers was assessed at three points during the behavior scoring period, by having all participants score the same piglet in a video. The intraclass correlation coefficient (ICC) was calculated to ensure behaviors were being scored consistently (all interobserver reliability tests produced an ICC above 0.9, indicating excellent agreement between scorers). A total of 18,000 min (300 h) of behavior recordings were scored and analyzed for this study (2.5 h per piglet).
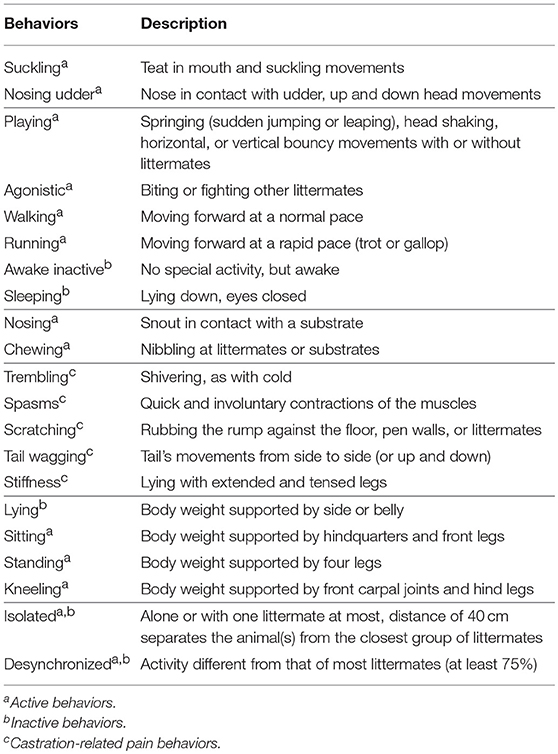
Table 1. Ethogram used to score piglet behavior, grouped into feeding, locomotion, non-specific behaviors, castration-related pain behaviors, posture, and social cohesion [adapted from Hay et al. (2)].
Piglet behaviors were analyzed individually and then grouped into “active,” “inactive,” and “pain” categories, to assess the activity level of piglets across the observation period and the total proportion of pain behaviors displayed. Active behaviors included playing, walking, suckling, nosing, chewing, and running. Inactive behaviors included awake inactive and sleeping. Postures were used for this behavioral analysis; piglets that were sitting or standing were scored as demonstrating an “active” behavior and piglets that were lying were scored as exhibiting an “inactive” behavior. Sitting was placed in the active category because most piglets assumed this posture when suckling or scratching the rump and these were considered active behaviors. Pain behaviors included trembling, stiffness, spasms, tail wagging, and rump scratching (2).
Still images of piglet faces were captured from the first 30 min of every hour of video data collected by an individual blinded as to animal treatment and time point. Videos were uploaded to the Everio MediaBrowser 4 program (Pixela Corporation, Osaka, Japan) and whenever a piglet face was in view, the video was paused, and the still image was captured (excluding times when piglets were lying with their head down or sleeping). Taking at least one facial image of each piglet per time point in this study was attempted. A total of 1,156 facial images were captured (Table 2). Prior to scoring, the images were uploaded to Photoshop (Adobe Systems Incorporated, San Jose, CA) and the symbol marked on each piglet's forehead was blurred to ensure volunteer scorers were blind to treatment. Faces were then randomized into files using a random number generator (random.org).
The preliminary PGS (8) was modified for this study (Figure 1). Ear position, which was originally placed on a 3-point scale (0–2), was expanded to a 4-point scale (0–3). Images of piglets with upright and floppy ears were included to make scoring ear position easier. Both front-facing piglets and profile images were added to the cheek tightening/nose bulge category and descriptive text was provided to explain the facial feature changes in detail. The maximum pain score using the revised PGS was 6. These changes were made to make the PGS more sensitive to pain expression, allowing for better reliability and to make the scale easier to use.
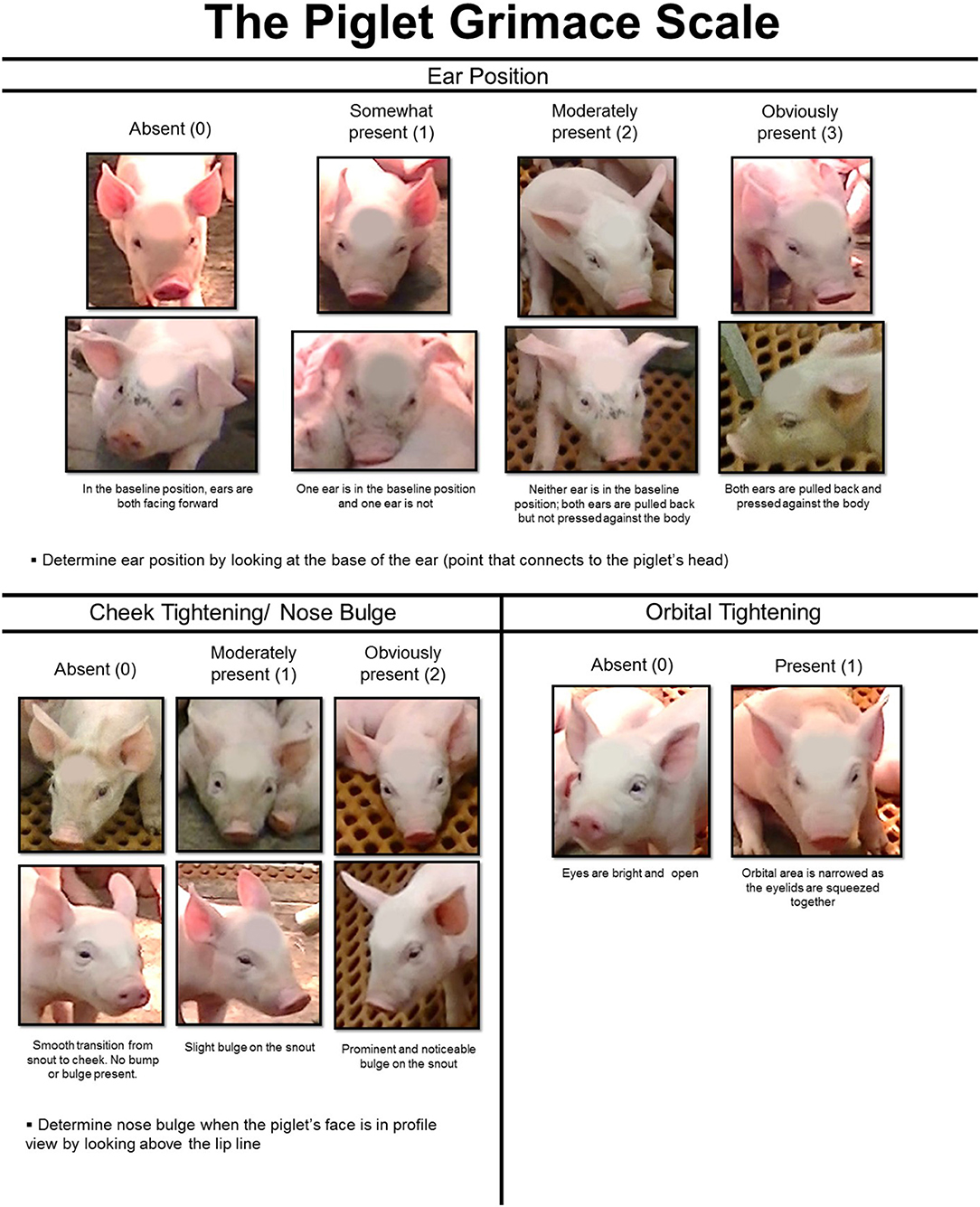
Figure 1. The Piglet Grimace Scale (PGS) is based on scoring three facial action units (FAUs): ear position, cheek tightening/nose bulge, and orbital tightening.
Eight individuals blinded as to piglet treatment, litter, and time point used the PGS to score each image. If an image could not be scored reliably, for example, due to poor image quality, the volunteers were instructed to exclude it from scoring (15 images were removed in total because of reported quality issues). The PGS score for each image was calculated by summing the scores given to the facial action units (ear position, cheek tightening/nose bulge, and orbital tightening). If more than one image was pulled for a piglet at the same time point, the PGS scores were averaged across images prior to analysis, to prevent pseudo-replication. The final PGS score of each piglet per time point was calculated as a mean of the scores from the eight individuals.
The total duration of behaviors was converted into proportion of time a piglet engaged in each behavior prior to analysis (to account for periods of time when the piglet was not in view and could not be scored). Normality was evaluated using the univariate procedure in SAS (Statistical Analysis System 9.4, SAS Institute Inc., NC). Data were analyzed using a GLIMMIX procedure with a beta distribution, including treatment, time, litter, and time x treatment interaction. Litter was included as a random effect and time was a repeated measure with piglet as the experimental unit. Post hoc tests were conducted using the Tukey-Kramer adjustment, to control the false-positive rate (i.e., incidence of Type I error) for multiple comparisons (12). Statistical significance was set at p < 0.05.
The grimace scale scores were analyzed using a mixed procedure, including litter, time, treatment, and time x treatment interaction. Litter was included as a random effect and time was a repeated measure with piglet as the experimental unit. A post hoc Tukey's test was conducted for significant outcomes.
The treatment variable was first set as each NSAID and control treatment administered to the piglets. When no significant effect of treatment and treatment × time interaction were found on any behavioral variable between 0.4 mg/kg MEL-castrated, 1.0 mg/kg MEL-castrated, 6.0 mg/kg KET-castrated, and saline piglets, data was pooled into a “castrated” group for further analysis. Similarly, when no significant effect of treatment and time x treatment interaction were found on any behavioral variable between 0.4 mg/kg MEL-uncastrated, 1.0 mg/kg MEL-uncastrated, 6.0 mg/kg KET-uncastrated, and sham piglets, data was pooled into a “uncastrated” group. These castrated and uncastrated groups were assessed for treatment and time × treatment effects. A final analysis was conducted on NSAID-castrated (0.4 mg/kg MEL-castrated, 1.0 mg/kg MEL-castrated, and 6.0 mg/kg KET-castrated) and NSAID-uncastrated (0.4 mg/kg MEL-uncastrated, 1.0 mg/kg MEL-uncastrated, and 6.0 mg/kg KET-uncastrated) piglet groups. Both behavioral and PGS data were used to assess the effectiveness of NSAID treatment in reducing surgical castration pain.
Nine individual behaviors were significantly affected by time across the observation period: awake inactive (p < 0.0001), lying (p < 0.0001), nosing (p < 0.0001), sleeping (p < 0.0001), standing (p < 0.0001), suckling (p < 0.0001), tail wagging (p < 0.0001), walking (p < 0.0001), and chewing (p = 0.0129) (Table 3). Across all treatment groups, piglets spent significantly more time walking and standing and less time lying at 0 and 24 h post-castration compared to all other time points (p < 0.05). At 24 h post-castration, they spent significantly more time nosing and wagging their tails (p < 0.05). At 0 h post-castration, all piglets spent significantly less time sleeping and were more awake inactive than at all other time points, except at 24 h (p < 0.05). Compared to pre-castration and 24 h post-castration, piglets slept significantly more at 7 h (p < 0.05). All piglets spent significantly more time suckling 5 h post-castration than at all other time points, except at 3 and 7 h (p < 0.05). Piglets demonstrated more chewing behaviors at 5 h post-castration than pre-castration (p = 0.0186). There were no significant behavioral differences between any of the treatment groups pre-castration (p > 0.05).
Only three individual behaviors, tail wagging (p < 0.0001), walking (p = 0.0042), and kneeling (p = 0.0261), were affected by treatment across all time points (Table 4). Within each treatment group, castrated piglets wagged their tails significantly more than uncastrated piglets (0.4 mg/kg MEL: p = 0.0432, 1.0 mg/kg MEL: p = 0.0228, 6.0 mg/kg KET: p = 0.0098, Controls: p < 0.0001). Piglets in the 0.4 mg/kg MEL-castrated group walked significantly less than piglets in the 1.0 mg/kg MEL-castrated group (p = 0.0095). Piglets in the 0.4 mg/kg MEL-uncastrated group spent significantly more time kneeling than piglets in the 1.0 mg/kg MEL-uncastrated group (p = 0.0235). Castrated piglets also displayed significantly more pain behaviors than uncastrated piglets within each treatment group (0.4 mg/kg MEL: p = 0.0339, 1.0 mg/kg MEL: p = 0.0079, 6.0 mg/kg KET: p = 0.0034, Controls: p < 0.0001) (Figure 2). None of the NSAID treatment groups significantly reduced piglet pain behaviors post-castration (0.4 mg/kg MEL: p = 1.0000, 1.0 mg/kg MEL: p = 0.9995, 6.0 mg/kg KET: p = 0.4163).
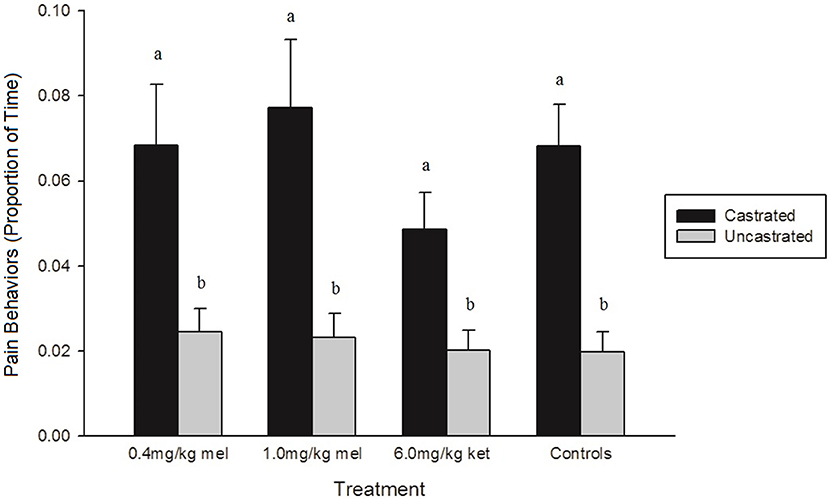
Figure 2. The proportion of time (± SE) piglets demonstrated pain-related behaviors (trembling, stiffness, spasms, tail wagging, and rump scratching) in each treatment group. The control groups were saline-castrated and sham-uncastrated piglets (n = 15 piglets/treatment group). Observers (n = 4) were unaware of piglet treatment, litter, and time point when scoring. Different letters (a, b) indicate significant differences between treatments (p < 0.05).
There was no significant effect of treatment on piglet activity level (p = 0.8557) but there was a significant time effect, with piglets being more active at 0 h and 24 h post-castration compared to all other time points (p < 0.0001) (Figure 3).
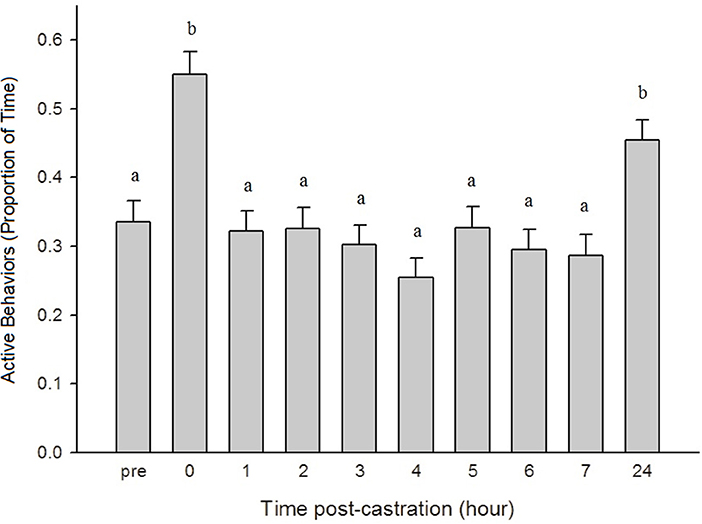
Figure 3. The proportion of time (± SE) piglets engaged in active behaviors (playing, walking, suckling, nosing, chewing, and running) throughout the observation period (n = 120 piglets/time point). Observers (n = 4) were unaware of piglet treatment, litter, and time point when scoring. Different letters (a, b) indicate significant differences between time points (p < 0.05).
After analyzing the effect of each NSAID treatment on behavior and identifying no significant treatment-related effects, data were pooled into two groups: piglets that were castrated and those that were uncastrated. Only two behaviors, tail wagging (p < 0.0001) and pain (p < 0.0001), were significant across the entire observation period, with castrated piglets displaying significantly more tail wagging and pain-related behaviors than uncastrated piglets. There were also time x treatment interactions found for lying (p = 0.0027), sleeping (p = 0.0037), standing (p = 0.0024), tail wagging (p < 0.0001), walking (p < 0.0001), isolated (p = 0.0018), activity (p = 0.0045), and pain (p < 0.0001). At 0 h, castrated piglets spent significantly less time lying and more time standing, walking, and engaged in active behaviors than castrated piglets at 3, 4, and 7 h, and uncastrated piglets at 4 h (p < 0.05). At 0 h, uncastrated piglets were also significantly more active, spending more time standing and walking and less time lying and sleeping than both castrated and uncastrated piglets from 1 to 7 h post-castration (p < 0.05). At 24 h post-procedure, uncastrated piglets were significantly more active, spending less time lying and more time standing and walking than castrated piglets at 3, 4, and 7 h, and uncastrated piglets at 4 and 6 h (p < 0.05). Castrated piglets spent significantly more time isolated from their littermates at 0 h than castrated pigs from 2 to 5 h, 7, and 24 h and uncastrated piglets from 0, 1, 3 h to 24 h post-procedure (p < 0.05). Castrated piglets at 0–3 h, 6 and 7 h demonstrated significantly more tail wagging and pain-related behaviors than uncastrated piglets at 2 and 5 h (p < 0.05). At 24 h post-castration, castrated piglets were observed engaging in significantly more tail wagging and pain-related behaviors than both castrated and uncastrated piglets from 0 to 7 h (p < 0.0001) (Figure 4).
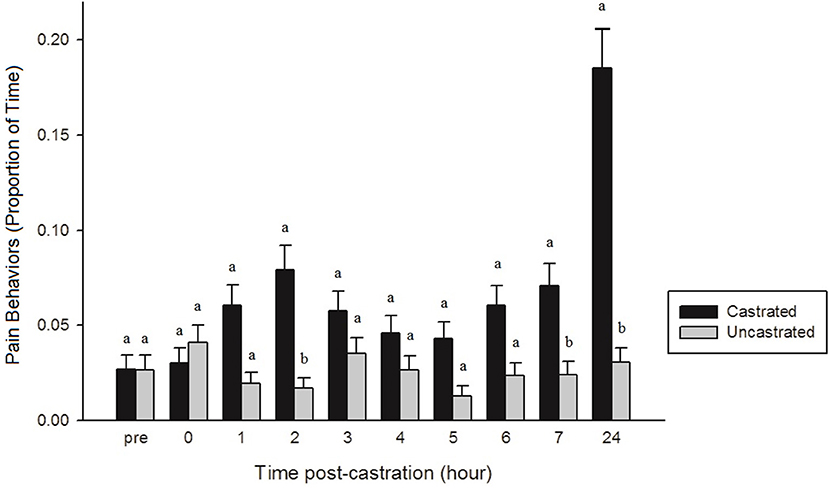
Figure 4. The proportion of time (± SE) castrated and uncastrated piglets demonstrated pain-related behaviors (trembling, stiffness, spasms, tail wagging, and rump scratching) throughout the observation period (n = 60 piglets/group). Observers (n = 4) were unaware of piglet treatment and time point when scoring. Different letters (a, b) indicate significant differences between groups within each time point (p < 0.05).
Data were also collapsed into two groups: NSAID-castrated piglets and NSAID-uncastrated piglets (after no behavior variables were found to be significant). The pre-treatment time point and the control piglets were removed from this analysis. The results were very similar to the above comparison between castrated and uncastrated piglets (Table 5).
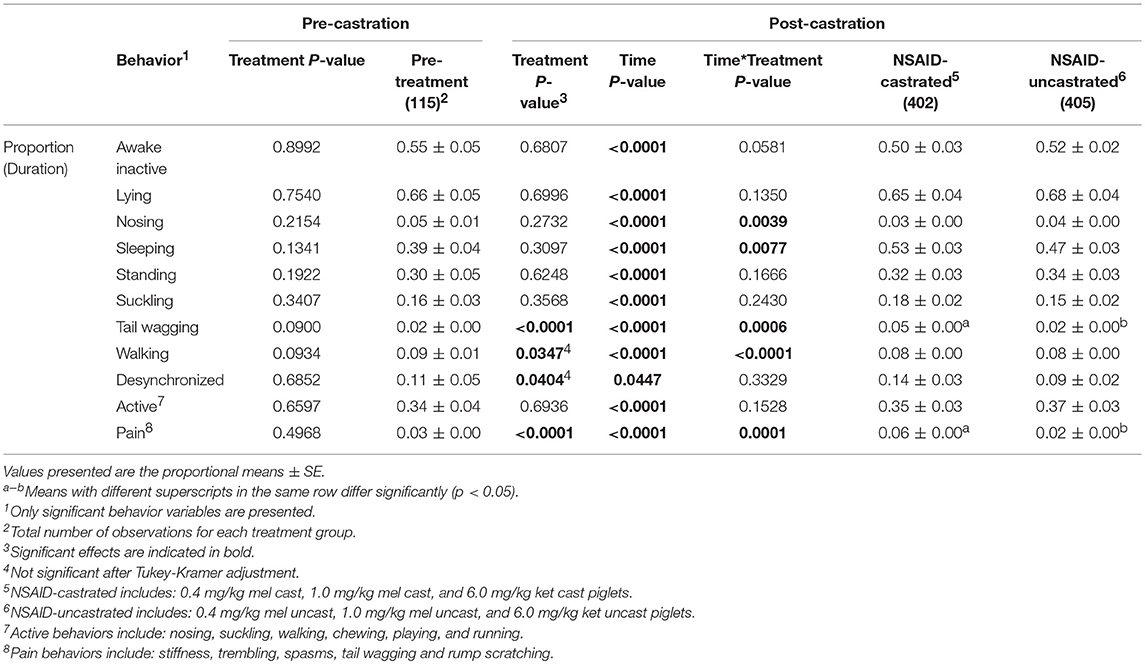
Table 5. Behavioral analysis of piglets (n = 120) pre-treatment and post-treatment across all litters, replicates, and time points.
There was a significant treatment effect on PGS score (p = 0.0019) (Figure 5). Piglets in the sham treatment group grimaced significantly less than those in the 1.0 mg/kg MEL-castrated and 6.0 mg/kg KET-castrated piglets (p = 0.0101 and p = 0.0491, respectively), with a trend toward significance found between sham and 0.4 mg/kg MEL-castrated piglets (p = 0.0724). Castrated piglets treated with 1.0 mg/kg MEL grimaced significantly more than 1.0 mg/kg MEL-uncastrated and 0.4 mg/kg MEL-uncastrated (p = 0.0366 and p = 0.0256, respectively). None of the NSAID treatments significantly reduced facial grimacing in castrated piglets.
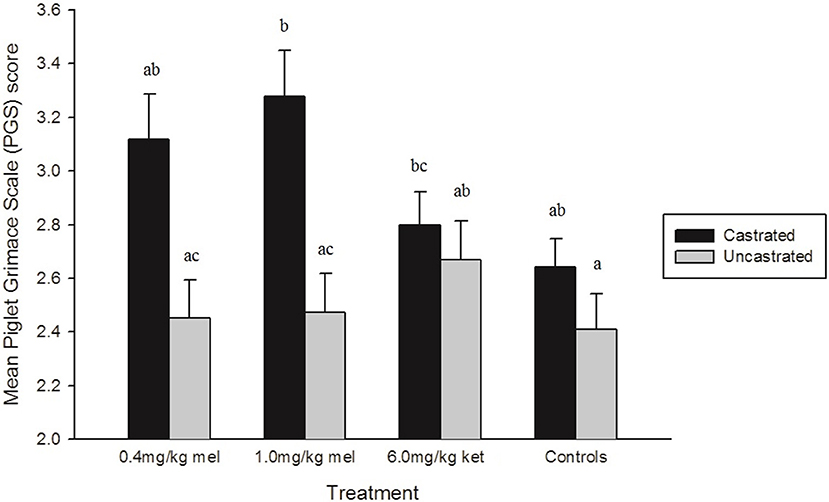
Figure 5. Mean Piglet Grimace Scale (PGS) scores (± SE) in each treatment group. Higher PGS scores indicate increased pain expression. Volunteers (n = 8) were unaware of piglet treatment, litter, and time point when scoring. Different letters (a, b, c) indicate significant differences between treatments (p < 0.05).
Collapsing data into castrated and uncastrated groups found, across the entire observation period, castrated piglets displayed significantly more facial grimacing than uncastrated piglets (p = 0.0061).
Meloxicam and ketoprofen are commonly used analgesics in food animals. Both NSAIDs have demonstrated efficacy in treating lameness in sows (13–15) and in reducing blood cortisol levels, heart and respiration rate in dehorned calves (16, 17). Meloxicam has previously been suggested to provide at least some analgesic effects after surgical castration in piglets (7, 18, 19); yet other studies have also found that it had no beneficial effect (4, 8). The contradictory results following NSAID use for castration have made recommendations for piglet pain control difficult (20). Two studies that found meloxicam effectively reduced behavioral indices of castration pain had a number of study design limitations that we identified. Keita et al. (7) observed piglet behavior “for a few minutes” at each time point in their study (0.5, 1, 2, 4, and 24 h post-castration) and used a very simplistic ethogram, only scoring piglets on the presence (1) or absence (0) of prostration, tremors, tail movements, and isolation. Kluivers-Poodt et al. (19) used a more detailed ethogram but employed a scan sampling method of data collection at two periods in the day (once in the morning and once in the afternoon). Both of these study methods may have resulted in a large amount of pain behaviors displayed by analgesia-treated piglets being missed and inappropriate conclusions being made with regards to drug efficacy. While both studies were sufficiently blinded to treatment, they made behavioral assessments through direct observation in the farrowing rooms, with no indication as to whether sows and piglets were habituated to the presence of an observer (7, 19). This may have impacted the behaviors observed. To address these limitations, we employed a much more comprehensive behavior scoring method (continuous observation of each piglet for 15 min per hour, at 10 time points, for a total of 2.5 h scored per piglet), and used video cameras to reduce the observer effect on animal behavior (21). We also used female researchers for all handling and technical procedures, to eliminate any potential risk of increased stress and an altered pain response in animals exposed to male researchers, as has been shown in mice (22). Our results determined that meloxicam (at either dose) and ketoprofen were ineffective in preventing or alleviating castration-associated pain in piglets. The treatment controls (piglets that were given an NSAID but were not castrated) confirmed that there are no negative behavioral side effects associated with either meloxicam or ketoprofen administration. These groups did not undergo a simulated castration because we wanted to observe the potential behavioral side effects caused by drug administration only; the stress resulting from a simulated castration may have confounded these behavioral results.
NSAIDs do not address the acute painful aspects of the castration procedure, such as the scrotal incision and tearing of the spermatic cord (23). They primarily provide analgesia by suppressing synthesis of prostaglandins responsible for inflammation post-procedure (24). Lidocaine, a local anesthetic, has been shown to decrease the pain response of piglets during castration when injected directly into the testicle (4, 18, 25, 26); however, this route of administration may be painful, and it provides minimal peri-operative analgesia (27). A multi-modal approach to pain management (meloxicam and lidocaine) is effective in reducing castration pain in piglets and calves (18, 28), but would greatly increase the castration time for each piglet and thus, may have limited practicality on-farm. A more potent drug class, such as opioids, may be required to sufficiently reduce pain; however, the feasibility of administering a controlled drug on-farm is low.
Castrated piglets demonstrated significantly more pain behaviors at 0–3 h, 6 and 7 h post-castration compared to uncastrated piglets, with castrated piglets exhibiting significantly more pain behaviors at 24 h post-castration compared to all other time points observed. This may be due to progression of the inflammatory processes, causing an increase in pain (29). Previous work found behaviors indicative of castration pain can persist in piglets beyond 24 h and some were still present 4 days after the procedure (2). 0.4 mg/kg meloxicam, administered to piglets IV, has a half-life of 2.7 h and 6.0 mg/kg ketoprofen, administered to piglets IV, has a half-life of 3.4 h (30, 31). This suggests appropriate pain management for piglets may involve more than one dose of analgesic drug following castration.
Increased activity (or restlessness) has been observed in animals experiencing pain (2, 32). This was evident in our study, with piglets at 24 h post-castration having both a significant increase in activity level and pain behaviors. The significant increase in activity 0 h post-castration may be attributed to pain from the castration procedure itself or, for piglets in the uncastrated groups, the repeated handling, IM injection or prolonged separation from the sow prior to the observation. It is possible that working in the farrowing room and castrating piglets in nearby pens may have also caused piglets already castrated (with cameras recording their behavior for scoring at 0 h) to be more alert and alter their natural behavior. Castrated piglets immediately post-procedure spent significantly more time isolated from their littermates. This behavior has previously been observed in piglets after castration as a response to pain (2, 33). An increase in tail wagging after castration has been reported in lambs, calves and piglets (2, 34–36). It has been speculated that tail wagging may signal nociceptive pain from the rear part of the body (37); however, tail wagging has also been shown to increase after dehorning calves (38). This suggests that it may serve as a less localized pain signal. It is worth noting that piglets in this study were not previously tail docked. An increase in tail wagging has been observed in piglets long after the tail docking procedure, which is thought to be attributed to tail stump hyperalgesia (39). This would not have been a cause for the significant increase in tail wagging noted here. Future work should examine tail wagging behavior as a potential indicator of pain.
Facial analysis is a novel approach to assessing pain in animals and humans. Species-specific grimace scales have been developed for mice, rats, rabbits, horses, sheep, and lambs (40–45), and involve characterizing and quantifying facial features that change in response to pain. Previous research has also described changes to piglet facial expression after a painful event (46). A cow pain face has been described (47); however, this has not been associated with a grimace scale to date. This type of pain assessment tool is non-invasive and has the potential to permit rapid detection of pain, leading to improved animal welfare if an appropriate intervention occurs promptly (48). For facial grimace scales to be validated, they must correspond well to known indicators of pain, such as behavior. In this study, we compared the PGS to pain behaviors of piglets. There was excellent agreement between the PGS and pain behaviors when assessing castrated and uncastrated pigs. When castrated and uncastrated piglets were separated into their initial treatment groups (0.4 mg/kg MEL-castrated, 0.4 mg/kg MEL-uncastrated, 1.0 mg/kg MEL-castrated, 1.0 mg/kg MEL-uncastrated, 6.0 mg/kg KET-castrated, 6.0 mg/kg KET-uncastrated, saline, and sham), the relationship between these two pain assessment tools decreased; however, every significant treatment effect found using the PGS was supported by the pain behavior results. The eight volunteers who used the PGS to score piglet faces came from various backgrounds and most had little animal experience. A more robust training session prior to having volunteers score could have been beneficial and may have resulted in stronger PGS results. Future work will focus on better training and include volunteers with greater animal experience. Overall, an increase in facial grimacing in castrated piglets corresponded to an increase in pain behaviors. This is a significant first step toward validation of the PGS as a pain assessment tool. The PGS, once validated, may be used clinically and on-farm to rapidly detect piglet pain and provide intervention. For piglets used in research, the PGS may allow for tighter endpoints (e.g., if facial grimacing moves beyond a predetermined threshold), while also providing a non-invasive measured outcome for pain assessments. In studies that assess pain interventions, reduced facial grimacing could be used as a criteria to demonstrate drug efficacy.
Meloxicam at the recommended dose (0.4 mg/kg) and at a higher dose (1.0 mg/kg) and ketoprofen (6.0 mg/kg) were ineffective at alleviating surgical castration pain in piglets. NSAIDs are the most widely used drugs in Europe to reduce piglet pain and are currently being recommended by the Canadian Pork Council to comply with the recent Codes of Practice (6, 49, 50). Post-operative pain persists after castration of piglets and significantly increases at 24 h. Future work should assess a more potent drug class or drug combination to treat pain. The PGS did not detect pain as strongly as behavioral assessment, but it did correspond well with castration pain behaviors and may become a useful tool to assess piglet pain. Future validation of the PGS is needed.
AV and PT conceived and designed the experiments. AV conducted the studies and analyzed the data. The manuscript was prepared and edited by AV and PT.
Funding was provided by Ontario Pork (052367) and National Pork (052457).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to thank Brianne Mercer, Julia Whatley, Hailey Hoffman, Tim Thalen, and personnel at the Arkell Swine Research Station for technical assistance.
1. Sutherland MA. Welfare implications of invasive piglet husbandry procedures, methods of alleviation and alternatives: a review. N Z Vet J. (2015) 63:52–7. doi: 10.1080/00480169.2014.961990
2. Hay M, Vulin A, Genin S, Sales P, Prunier A. Assessment of pain induced by castration in piglets: behavior and physiological responses over the subsequent 5 days. Appl Anim Behav Sci. (2003) 82:201–18. doi: 10.1016/S0168-1591(03)00059-5
3. Prunier A, Mounier AM, Hay M. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J Anim Sci. (2005) 83:216–22. doi: 10.2527/2005.831216x
4. Kluivers-Poodt M, Houx BB, Robben SR, Koop G, Lambooij E, Hellebrekers LJ. Effects of a local anaesthetic and NSAID in castration of piglets, on the acute pain responses, growth and mortality. Animal (2012) 6:1469–75. doi: 10.1017/S1751731112000547
5. European Commission. European Declaration on Alternatives to Surgical Castration of Pigs (2010). Available online at: https://ec.europa.eu/food/animals/welfare/practice/farm/pigs/castration_alternatives_en (accessed October 15, 2017).
6. National Farm Animal Care Council. Code of Practice for the Care and Handling of Pigs (2014). Available online at: http://www.nfacc.ca/pdfs/codes/pig_code_of_practice.pdf (accessed October 15, 2017).
7. Keita A, Pagot E, Prunier A, Guidarini C. Pre-emptive meloxicam for postoperative analgesia in piglets undergoing surgical castration. Vet Anaesth Analg. (2010) 37:367–74. doi: 10.1111/j.1467-2995.2010.00546.x
8. Viscardi AV, Hunniford M, Lawlis P, Leach M, Turner PV. Development of a piglet grimace scale to evaluate piglet pain using facial expressions following castration and tail docking: a pilot study. Front Vet Sci. (2017) 4:51. doi: 10.3389/fvets.2017.00051
9. Suresh KP, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. (2012) 5:7–13. doi: 10.4103/0974-1208.97779
10. Fosse TK, Toutain PL, Spadavecchia C, Haga HA, Horsberg TE, Ranheim B. Ketoprofen in piglets: enantioselective pharmacokinetics, pharmacodynamics and PK/PD modelling. J Vet Pharm Therap. (2010) 34:338–49. doi: 10.1111/j.1365-2885.2010.01236.x
11. Rault JL, Lay DC, Marchant-Forde JN. Castration induced pain in pigs and other livestock. Appl Anim Behav Sci. (2011) 13:214–25. doi: 10.1016/j.applanim.2011.10.017
12. Ranganathan P, Pramesh CS, Buyse M. Common pitfalls in statistical analysis: the perils of multiple testing. Perspect Clin Res. (2016) 7:106–7. doi: 10.4103/2229-3485.179436
13. Hirsch AC, Philipp H, Kleemann R. Investigation on the efficacy of meloxicam in sows with mastitis-metritis-agalactia syndrome. J Vet Pharmacol Therap. (2003) 26:355–60. doi: 10.1046/j.1365-2885.2003.00524.x
14. Mustonen K, Ala-Kurikka E, Orro T, Peltoniemi O, Raekallio M, Vainio O, Heinonen M. Oral ketoprofen is effective in the treatment of non-infectious lameness in sows. Vet J. (2011) 190:55–9. doi: 10.1016/j.tvjl.2010.09.017
15. Pairis-Garcia MD, Johnson AK, Stalder KJ, Abell CA, Karriker LA, Coetzee JF, Millman ST. Behavioral evaluation of analgesic efficacy for pain mitigation in lame sows. Anim Welf. (2015) 24:93–9. doi: 10.7120/09627286.24.1.093
16. Stafford KJ, Mellor DJ, Todd SE, Bruce RA, Ward RN. Effects of local anaesthesia or local anaesthesia plus a non-steroidal anti-inflammatory drug on the acute cortisol response of calves to give different methods of castration. Res Vet Sci. (2002) 73:61–70. doi: 10.1016/S0034-5288(02)00045-0
17. Heinrich A, Duffield TF, Lissemore KD, Squires E, Millman ST. The impact of meloxicam on postsurgical stress associated with cautery dehorning. J Dairy Sci. (2009) 92:540–7. doi: 10.3168/jds.2008-1424
18. Hansson M, Lundeheim N, Nyman G, Johansson G. Effect of local anaesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet Scand. (2011) 53:34. doi: 10.1186/1751-0147-53-34
19. Kluivers-Poodt M, Zonderland JJ, Verbraak J, Lambooij E, Hellebrekers LJ. Pain behavior after castration of piglets; effect of pain relief with lidocaine and/or meloxicam. Animal (2013) 7:1158–62. doi: 10.1017/S1751731113000086
20. O'Connor A, Anthony R, Bergamasco L, Coetzee J, Gould S, Johnson AK, et al. Pain management in the neonatal piglet during routine management procedures. Part 2: grading the quality of evidence and the strength of recommendations. Anim Health Res Rev. (2014) 15:39–62. doi: 10.1017/S1466252314000073
21. Iredale SK, Nevill CH, Lutz CK. The influence of observer presence on baboon (Papio spp.) and Rhesus macaque (Macaca mulatta) behavior. Appl Anim Behav Sci. (2010) 122:53–7. doi: 10.1016/j.applanim.2009.11.002
22. Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods (2014) 11:629–32. doi: 10.1038/nmeth.2935
23. Taylor AA, Weary DM. Vocal responses of piglets to castration: identifying procedural sources of pain. Appl Anim Behav Sci. (2000) 70:17–26. doi: 10.1016/S0168-1591(00)00143-X
24. Papich MG. An update on nonsteroidal anti-inflammatory drugs (NSAIDs) in small animals. Vet Clin Small Anim. (2008) 38:1243–61. doi: 10.1016/j.cvsm.2008.09.002
25. White RG, DeShazer JA, Tressler CJ, Borcher GM, Davey S, Waninge A, et al. Vocalization and physiological response of pigs during castration with or without a local anesthetic. J Anim Sci. (1995) 73:381–6. doi: 10.2527/1995.732381x
26. Marx G, Horn T, Thielebein J, Knubel B, von Borell E. Analysis of pain-related vocalization in young pigs. J Sound Vib. (2003) 266:687–98. doi: 10.1016/S0022-460X(03)00594-7
27. Leidig MS, Hertrampf B, Failing K, Schumann A, Reiner G. Pain and discomfort in male piglets during surgical castration with and without local anaesthesia as determined by vocalisation and defense behavior. Appl Anim Behav Sci. (2009) 116:174–8. doi: 10.1016/j.applanim.2008.10.004
28. Meléndez DM, Marti S, Janzen ED, Moya D, Gellatly D, Pajor EA, Schwartzkopf-Genswein KS. 011 Effect of lidocaine and meloxicam on indicators of pain and distress after knife castration in weaned beef calves. J Anim Sci. (2017) 95(Suppl. 4):5–6. doi: 10.2527/asasann.2017.011
29. Kumar V, Abbas A, Aster J. Robbins & Cotran Pathologic Basis of Disease. 9th ed. Amsterdam: Elsevier (2015).
30. Fosse TK, Haga HA, Hormazabal V, Haugejorden G, Horsberg TE, Ranheim B. Pharmacokinetics and pharmacodynamics of meloxicam in piglets. J Vet Pharm Ther. (2008). 31:246–52. doi: 10.1111/j.1365-2885.2008.00958.x
31. Fosse TK, Horsberg TE, Haga HA, Hormazabal V, Ranheim B. Enantioselective pharmacokinetics of ketoprofen in piglets: the significance of neonatal age. J Vet Pharm Ther. (2011) 34:153–59. doi: 10.1111/j.1365-2885.2010.01205.x
32. Mellor DJ, Cook CJ, Stafford KJ. Quantifying some responses to pain as a stressor. In: Moberg, GP, Mench JA, editors. The Biology of Animal Stress. Amsterdam: CAB International (2000). pp. 173–98
33. Gottardo F, Scollo A, Contiero B, Ravagnani A, Tavella G, Bernardini D, et al. Pain alleviation during castration of piglets: a comparative study of different farm options. J Anim Sci. (2016) 94:5077–88. doi: 10.2527/jas.2016-0843
34. Robertson IS, Kent JE, Molony V. Effect of different methods of castration on behavior and plasma cortisol in calves of three ages. Res Vet Sci. (1994) 56:8–17. doi: 10.1016/0034-5288(94)90189-9
35. Rault JL, Lay DC. Nitrous oxide by itself is insufficient to relieve pain due to castration in piglets. J Anim Sci. (2014) 89:3318–25. doi: 10.2527/jas.2011-4104
36. Jongman EC, Borg S, Hemsworth PH. Assessment of pain responses associated with castration of 10-week-old lambs using the Callicrate ‘WEE Bander’ compared with a standard elastrator. Appl Anim Behav Sci. (2016) 179:46–52. doi: 10.1016/j.applanim.2016.03.014
37. Prunier A, Mounier L, Le Neindre P, Leterrier C, Mormède P, Paulmier V, et al. Identifying and monitoring pain in farm animals: a review. Animal (2013) 7:998–1010. doi: 10.1017/S1751731112002406
38. Graf B, Senn M. Behavioral and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Appl Anim Behav Sci. (1999) 62:153–71. doi: 10.1016/S0168-1591(98)00218-4
39. Di Giminiani P, Edwards SA, Malcolm EM, Leach MC, Herskin MS, Sandercock DA. Characterization of short- and long-term mechanical sensitisation following surgical tail amputation in pigs. Sci Rep. (2017) 7:4827. doi: 10.1038/s41598-017-05404-y
40. Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods (2010) 7:447–9. doi: 10.1038/nmeth.1455
41. Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain (2011) 7:55. doi: 10.1186/1744-8069-7-55
42. Keating SCJ, Thomas AA, Flecknell PA, Leach MC. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioral and facial expression responses. PLoS ONE (2012) 7:e44437. doi: 10.1371/journal.pone.0044437
43. Costa ED, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the horse grimace scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE (2014) 9:e92281. doi: 10.1371/journal.pone.0092281
44. Guesgen MJ, Beausoleil NJ, Leach M, Minot EO, Stewart M, Stafford KJ. Coding and quantification of a facial expression for pain in lambs. Behav Proc. (2016) 132:49–56. doi: 10.1016/j.beproc.2016.09.010
45. McLennan KM, Rebelo CJB, Corke MJ, Holmes MA, Leach MC, Constantino-Casas F. Development of a facial expression scale using footrot and mastitis as models of pain in sheep. Appl Anim Behav Sci. (2016) 176:19–26. doi: 10.1016/j.applanim.2016.01.007
46. Di Giminiani P, Brierley VL, Scollo A, Gottardo F, Malcolm EM, Edwards SA, Leach MC. The assessment of facial expressions in piglets undergoing tail docking and castration: toward the development of the piglet grimace scale. Front Vet Sci. (2016) 3:100. doi: 10.3389/fvets.2016.00100
47. Gleerup KB, Andersen PH, Munksgaard L, Forkman B. Pain evaluation in dairy cattle. Appl Anim Behav Sci. (2015)171:25–32. doi: 10.1016/j.applanim.2015.08.023
48. Miller AL, Leach MC. The mouse grimace scale: a clinically useful tool? PLoS ONE (2015) 10:e0136000. doi: 10.1371/journal.pone.0136000
49. Briyne ND, Berg C, Blaha T, Temple D. Pig castration: will the EU manage to ban pig castration by 2018? Porcine Health Manag. 2:29. doi: 10.1186/s40813-016-0046-x
50. Canadian Pork Council. Swine Health Ontario. Pain control information for swine. (2016). Available online at: http://www.swinehealthontario.ca/About-Us/Latest-News-View/ArticleId/2563/Pain-Control-Information-for-Swine (accessed January 25, 2018).
Keywords: analgesia, animal welfare, castration, piglet, pain assessment, NSAID
Citation: Viscardi AV and Turner PV (2018) Use of Meloxicam or Ketoprofen for Piglet Pain Control Following Surgical Castration. Front. Vet. Sci. 5:299. doi: 10.3389/fvets.2018.00299
Received: 26 July 2018; Accepted: 07 November 2018;
Published: 26 November 2018.
Edited by:
Jeremy N. Marchant-Forde, Livestock Behavior Research Unit (USDA-ARS), United StatesReviewed by:
Kenny Rutherford, Scotland's Rural College, United KingdomCopyright © 2018 Viscardi and Turner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abbie V. Viscardi, YXZpc2NhcmRpQHZldC5rc3UuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.