
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 02 August 2018
Sec. Veterinary Pharmacology and Toxicology
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00177
This article is part of the Research Topic Phytotherapeutic Alternatives in Veterinary Medicine View all 8 articles
 Daniel Buldain1,2*
Daniel Buldain1,2* Andrea V. Buchamer1
Andrea V. Buchamer1 María L. Marchetti1
María L. Marchetti1 Florencia Aliverti1
Florencia Aliverti1 Arnaldo Bandoni3
Arnaldo Bandoni3 Nora Mestorino1
Nora Mestorino1The emergence of resistance to antibiotics has been favored by abuse in the application of antimicrobials in human and animal medicine. Essential oils are a great resource to deal with this crisis. Melaleuca armillaris belongs to the family of Myrtaceae, rich in species with essential oils. Plant extracts has shown antimicrobial activity in many investigations. Cloxacillin (CLOX) is an antibiotic widely used in veterinary medicine against Staphylococcus aureus. Our aim was to assess pharmacodynamic interaction established by combining essential oil of M. armillaris (EO) with CLOX in search of a synergistic effect that maximizes the antibacterial activity against S. aureus. The EO was obtained by steam distillation and its composition was analyzed by a GC–FID–MS. The most abundant components in the EO were 1.8 cineole (72.3%), limonene (7.8%). and α-pinene (6%). We worked with wild type S. aureus strains (n = 3) isolated from Holstein cows, and S. aureus ATCC 29213 as the reference strain. The Minimum Inhibitory Concentration (MIC) of CLOX, EO and the combination was determined by microdilution in broth at pH 7.4; 6.5 and 5.0. The checkerboard method was applied to evaluate the interaction between CLOX and EO. The Fractional Inhibitory Concentration index (FIC) was established. From those combinations that yielded the lowest FIC values, we evaluated the index of antibacterial activity (E), established as the difference between the Log10 values of the number of viable bacteria at the initial (nt0) and at the end of the test (nt24). So, time-killing curves with CLOX and EO/CLOX combination at 0.5, 1, 2, 4, and 8 fold the MIC in broth at pH 7.4; 6.5 and 5.0 were prepared. We considered Bacteriostatic effect (E = 0) Bactericidal effect (E = −3) and Effect of virtual eradication of bacteria (E = −4). A clear synergic activity between the EO and the CLOX was demonstrated, which allows reducing the MIC of β-lactam against S. aureus. This interaction was favored by acidification of the medium, where lower concentrations of CLOX achieved a bactericidal effect, close to virtual eradication, in the presence of small amounts of EO.
Bovine mastitis is one of the most prevalent problem which affects dairy herd production worldwide, and antimicrobial therapy is the primary tool for the treatment. This disease is responsible for a negative effect in the economy of several countries, because of the decrease results in the production level and quality of milk. The most frequent pathogen which caused subclinical mastitis in dairy cows is Staphylococcus aureus (1). This microorganism is characterized by its capacity of select resistance against traditional antimicrobials and some virulence factors as its ability to form biofilms and to invade and survive inside epithelial cells (2).
The emergence of resistance to multiple antibiotics has been favored by abuse in the application of antimicrobials in several areas: medicine, veterinary, agriculture.
At present, the growing number of methicillin-resistant S. aureus (MRSA) strains isolated from both groups, humans, and animals, hazards the efficacy of traditional antimicrobial treatments. The excessive and irrational usage of antimicrobials favored the emergence of resistance to multiple antibiotic families.
It is probably that the introduction of antimicrobials resistant to β-lactamases in the 1960s led to the emergence of MRSA (3). In veterinary medicine literature, the isolation of MRSA in animals has been reported since 1975 (4). This has been generating a dissemination of resistant and multi-resistant strains transmitted between humans and animals (5).
Cloxacillin (CLOX) is a semisynthetic antimicrobial derivate of penicillin that resists being breakdown by the enzyme penicillinase. CLOX is an antibiotic widely used in veterinary medicine against S. aureus (1). It has bactericidal activity against β-lactamase producing S. aureus. However, the increasing occurrence of methicillin-resistance and the consequent failure in therapy induces the search of new therapeutic alternatives. An alternative to face the problem of bacterial resistance is the use of products derived from plant extracts. In the past, natural and phytochemical products were used in the treatment and prevention of infectious diseases. Plants naturally synthesize aromatic chemical compounds and secondary metabolites which serve as a defense mechanism against microbes (6). The biological activity of a medicinal plant is usually based on the presence of one or a set of chemical components located in the tissues of the plant. The compounds with antimicrobial activity are mostly present in essential oils, which can occur in one or more organs depending on the species (7). Essential oils turn out to be the final product of the secondary metabolism of aromatic plants (8).
Essential oils are an interesting kind of herbal extract composed by complex mixtures with high antimicrobial activities. It is useful to take advantage of this property and transfer it to the treatment of bacteria resistant to antibiotics. There are many works that establish the restoration of antimicrobial activity of antibiotics, which have diminished their effectiveness against microorganisms, when they are combined with essential oils (9–12).
The exploitation of essential oils in the prevention of bacterial resistance is promising because those are multi-components with different targets of action, compared to many conventional antimicrobial agents that only have a single target site. Depending on the composition of the essential oils, different mechanisms of action can be attributed, including damage to essential proteins of the pathogen, blocking of membrane enzymes with which the microorganisms can pump out the active principle, changes in the metabolism, permeabilization of their membranes. These oils contain wide ranges of polyphenols and terpenoids, which have strong binding affinity to different molecular structures such as membranes, due to their great lipophilicity, presenting a high potential to penetrate through cell walls and disorganize them, leading to leakage cellular content (13).
Several authors are researching about the application of essential oils as adjuvant to increase the effect of antimicrobials against bacterial species. This is a new concept with high potential. For example Pelargonium graveolens essential oil reduces the minimum effective dose of norfloxacin against Bacillus cereus, Bacillus subtilis, Escherechia coli, and S. aureus (10). Rodrígues et al. (11) reported that the essential oil of leaves of Croton zehntneri is able to enhance the activity of gentamicin by 42.8% against Pseudomonas aeruginosa by gaseous contact, which suggests that the oil has a potential to be used as an adjuvant in antimicrobial therapy (11). Gram positive bacteria as S. aureus are more susceptible to essential oils than Gram negative ones (14), because these last one have an outer layer with lipopolysaccharide that cover the peptidoglycan and limiting the diffusion of hydrophobic compounds through it (15).
The antimicrobial properties of essential oils promote scientific interest in the study of them as a new group of pharmacological compounds. They possess a huge chemical and structural variety, converting them into functionally versatile mixtures (9). Although their activity can be associated with the major components, all of them influence in the pharmacological effect. It is probably that the high concentration of the major compounds masks the effects of the others. However, in terms of antimicrobial activity, every isolated component shows less antibacterial effect than the complete essential oil (16). It has also been shown that the function of the main compounds is regulated by the minority molecules, contributing to the synergic effect (17). Considering the large number of chemical structures that make up the essential oils it is likely that antibacterial properties cannot be attributed to a single chemical compound, and therefore there could be several targets in the microbial cells where these would act resulting in an enhancing influence. That is why it is reasonable to study the essential oils as a whole and take advantage of the synergy of all the components (18).
Melaleuca armillaris (Soland. ex Gaertn.) Sm. is one of the most widely cultivated Melaleuca plants. It is commonly known as Honey Bracelet Myrtle and grows in the form of a large bush or as a small tree. Reports of the literature about M. armillaris essential oil (EO) remain scarce. Investigations by GC-MS of its EO revealed the presence of 1.8-cineole as the major component (19–21). Several activities have been determined for this EO. Rizk et al. (22) obtained positive results in vivo using this EO in the treatment against the parasitoid Schistosoma mansoni, responding to the oxidant activity generated by the pathogen. Inhibitory activity was also found, in vitro, against some bacterial species such as B. subtilis, S. aureus, S. epidermidis, E. coli, and P. aeruginosa (22, 23). The activities observed for the EO of M. armillaris in pure form allow supposing that the good results obtained by themselves can be increased in synergistic systems for the same purpose, such as the improvement of antibiotics to treat infections caused by strains resistant to conventional treatment. No bibliography has been found indicating the use of the essential oil of M. armillaris as an enhancer of antibiotics used in the treatment of microbial infections, particularly against strains of S. aureus. This pathogen is very resistant to antibiotics, and is responsible for high percentage persistent infections. The indiscriminate use of antimicrobials is generating microorganisms resistant to multiple drugs, becoming a serious problem for global health (24).
Our aim was to assess the pharmacodynamic interaction established by combining EO of M. armillaris with CLOX emulating an extracellular and intracellular pH conditions (pH 7.4, 6.5, and pH 5) in search of a synergistic effect that maximizes the activity of the antibiotic against S. aureus.
Leaves and herbaceous branches of M. armillaris were collected in the month of July in the outskirts of Coronel Brandsen town, near La Plata city, Buenos Aires, Argentina. This region is located at latitude 35°06′18.9″S and longitude 58°10′57.0″W, and the climate is warm and humid with very rainy winters. The plant material was deposited in the LPAG Herbarium of the Faculty of Agrarian and Forestry Sciences, UNLP (25).
Vegetal material collection and extraction of the EO were carried out in the month of July during the morning. The extraction of EO was done by steam distillation with a distillation equipment of 385 liters of capacity. We used 38.5 liters of water for steam distillation and the amount of leaves and herbaceous branches was 44.750 kg. The EO obtain was dried with anhydrous Sodium Chloride, filtered and stored in amber glass bottle in a refrigerator at 4°C until use.
The EO was analyzed by a GC–FID–MS Agilent (Agilent Technologies, Santa Clara, CA, USA) 7890A/5975C, equipped with one injector (split ratio 1:100), connected by a flow splitter to two capillary columns (HPWAX and DB-1-MS, both 60 m × 0.25 mm with 0.25 μm of fixed phase). The polar column was connected to a FID, whereas the non-polar column was connected to a quadrupolar mass detector (HP 5975C; 70 eV). Helium was used as gas carrier, at 1.8 mL/min. The injector temperature was set at 250°C. Injection volume was 0.3 μL. The column temperature was programmed according to the following gradient: 100°C, increasing at 2°C/min to 240°C and kept constant for 15 min. FID temperature was 260°C, and temperatures for the transference line and the ionic source were set at 280 and 230°C, respectively. Mass range (m/z) was 40–500 Da. Data acquisition, processing and instrument control was performed using the Agilent ChemStation (Agilent Technologies, Santa Clara, CA, USA) software. The identification of the compounds was achieved by analyzing the retention indexes (relative to C8–C24 n-alkanes) obtained in both columns and compared with those of reference compounds identified in chemically well-known essential oils and from bibliography (26, 27). Additionally, each mass spectra obtained was compared to those from the literature libraries (26, 28, 29) and mass spectra obtained from reference compounds. Relative percentage contribution of the compounds was calculated from the FID response by a computerized integration assuming all of the responses factors were 1.
The study was carried out using 3 wild strains of S. aureus and the S. aureus ATCC 29213 as reference strain for quality control. Wild strains were isolated from milk samples obtained of Holstein cows with subclinical mastitis. They were identified by colony morphology and routine biochemical tests, Gram stain, coagulase, and catalase test, development of β-hemolysis, glucose fermentation and growth in saline medium (7.5%).
A standard of CLOX, pontency 96.4% (Sigma-Aldrich, chemical Company, St. Louis, USA) was used to performed this assay.
To determine the MIC of CLOX and EO, we used 96-well polystyrene microtiter plates with Mueller Hinton broth (MHB) supplemented with 0.5% of Tween 80 (to enhance the EO dissolution). The broth was adjusted to pH 7.4, 6.5, or 5.0 with hydrochloric acid 1N (Anedra, Argentina), in order to emulate the conditions of pH to extracellular and intracellular level. The range of CLOX concentrations (applying a scheme of two-fold serial dilution) evaluated was from 256 to 0.007 μg/mL. The concentrations of EO tested were from 50 μL/mL to 0.1 μL/mL. In both cases each well was inoculated with a final bacterial concentration of 5 × 105 CFU/mL. The suspension was adjusted to match 0.5 Mc Farland (1 × 108 CFU/mL). The plates were incubated at 35°C for 18–24 h. The MIC was established as the lowest concentration which inhibits the bacterial growth. All determinations were carried out in triplicate and in all cases positive and negative controls were used with MHB containing 0.5% Tween 80.
The MIC of the mixture was performed as previously described by CLOX and EO. The combinations of the antibiotic and the plant extract were prepared according to checkerboard technique (30) against the strains selected. The microtiter plate consisted of a row with two-fold serial dilution of EO and a column with two-fold serial dilution of CLOX (as MIC control). The intermediate wells presented EO/CLOX combinations. The results were interpreted as for MIC of individual antimicrobials, but considering this parameter as a mix. The bacterial inoculums of S. aureus wild type and the reference strain used was 5 × 105 CFU/mL per well. Incubation was carried out at 35°C for 18–24 h. The fractional inhibitory concentration index (FIC) was determined according to the following equation:
Where (A): CLOX MIC in combination with EO; (B): EO MIC in combination with CLOX; (MIC)a and (MIC)b: MIC of the antimicrobial and EO alone, respectively. Synergism (S) was considered if FIC ≤ 0.5; partial or low synergism (PS) if 0.5 < FIC < 1; indifference or addition (I) if 1 ≤ FIC <2 and antagonism (A) when FIC ≥ 2.
The antibacterial activity index (E) was evaluated from those combinations that showed the lowest FIC values, established as the MIC value of the mixture. For this purpose we carried out time-kill assays for different concentrations (0.5MIC; 1MIC; 2MIC; 4MIC; 8MIC) of EO, CLOX and the combination. For the last one, 1MIC correspond to the lower FIC value blending and the others (0.5MIC, 2MIC, 4MIC, 8MIC) keep the proportion of both compounds (diluting or concentrating as the case may be). We prepared 7 tubes in each case (considering control positive and negative) with 1 mL of final volume containing MHB 0.5 Tween 80, antimicrobial (or AE/CLOX combination) and the inoculums. All tubes had a bacterial concentration of approximately 5 × 105 CFU/mL (except the negative control). They were incubated at 35°C and samples were obtained at 0, 2, 4, 8, 12, and 24 h in order to construct survival curves by plate count. Those were performed for all strains at pH 7.4; 6.5 and 5.0.
The E index was quantified as the difference between the Log10 values of the number of viable bacteria (CFU/mL) at initial time (nt-0) and at the end of the test (nt-24) according to the following equation: E = nt-24-nt-0. To evaluate E, three theoretical cut points were applied (31): (a) Bacteriostatic effect: E = 0; there are no changes in the value of nt-0; (b) Bactericidal effect: E = −3; there is a reduction of ≥3 log10 of nt-0 and (c) Effect of virtual eradication of bacteria: E = −4; there is a reduction of ≥4 Log10 (99.99%) respect to Log of nt-0. The results obtained were plotted using the GraphPad Prism 6 program in order to obtain E vs. Log10 (concentration of CLOX) curves. The assays were performed in triplicate and the wild strains were grouped obtaining an n = 3.
The whole distillation process lasted 5 h and 550 mL of essential oil were extracted. The analysis of composition revealed the presence of 1.8 cineole (72.3%), limonene (7.8%) and α-pinene (6%) as the most abundant components in the EO (Table 1, Figure 1).
The strains used in this study were identified as members of S. aureus specie after characterizing them biochemically, resulting in Gram positive, coagulase positive, catalase positive, β-hemolytic, glucose fermenting, and growth in saline (7.5%) strains.
The MIC of the CLOX obtained for S. aureus ATCC 29213 at pH 7.4 was 0.125 μg/mL, which is in accordance with the recommendations of the CLSI 2009 (32). The 3 wild strains (named SA13, SA96, and SA139) presented a MIC of 0.5 μg/mL at pH 7.4. In all cases, a decrease in this concentration was observed when acidifying the culture medium (Table 2).
Respect the M. armillaris EO, for S. aureus ATCC 29213 the MIC was 25 μL/mL, while for the 3 wild strains it was 12.5 μL/mL. These values decreased slightly with the acidity of the culture medium (Table 3).
With the evaluation of the antibacterial activity index we could compare the incidence of EO in the CLOX activity, in addition to the effect caused by the pH variation. This is visualized in Figures 2, 3. It is observed how the acidification of the medium enhances the antimicrobial activity of CLOX against S. aureus (for the reference strain and the wild strains). Lower pH values requires a less amount of antibiotic to obtain an E-value lesser than −3 indicating a bactericidal effect. In the graphs it can be seen how the presence of the EO favors the action of the CLOX, since lower E-values are reached with lower concentrations of the antibiotic in comparison with the results obtained for CLOX alone. For the reference strain ATCC 29213, it was achieved a bactericidal effect very close to virtual eradication, since the E-values are close to −4. This was observed from concentrations of 2 the MIC of CLOX in the mixture, at the 3 pHs evaluated (Figure 2). In wild isolates the behavior was similar to that observed for the reference strain (Figure 3).
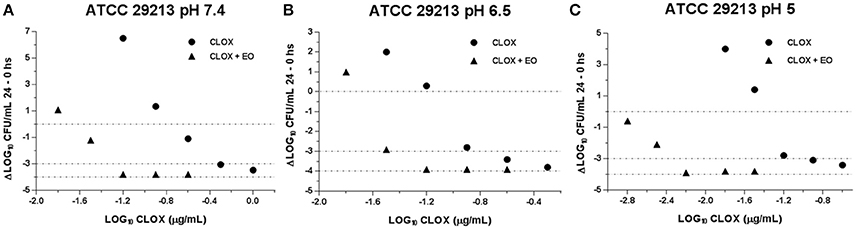
Figure 2. Graphic representation of the antibacterial effect (E: Δ Log CFU/mL 24–0 h) of Cloxacillin against S. aureus ATCC 29213 (n = 3) at pH 7.4 (A); 6.5 (B) and 5.0 (C) in the presence and absence of EO.
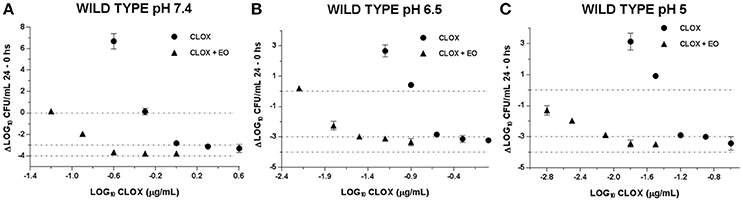
Figure 3. Graphic representation of the antibacterial effect (E: Δ Log CFU/mL 24–0 h) of Cloxacillin against WILD S. aureus strains (n = 3) at pH 7.4 (A); 6.5 (B) and 5.0 (C) in the presence and absence of EO.
The chromatographic analysis of our EO revealed the presence of 1.8 cineole as major component (72.3%) and in lesser magnitude limonene (7.8%) and α-pinene (6.0%). Those are commonly present in effective antimicrobial essential oils. The 1.8 cineole is a monocyclic monoterpene with high antimicrobial activity. In several studies (19, 22, 33, 34), the main component reported was also 1.8 cineole, but this result disagree with other authors like Amri et al. (23) because the concentration of this one was markedly low (3.6%). There is evidence that the differences found in the concentration of the components in essential oils, extracted from different plants, would be affected by environmental conditions (35). The main compound of the mixture generally exerts the strongest activity, but can be influenced by the other molecules (36).
In our study, EO alone, without the addition of the antimicrobial, have demonstrated a strong antibacterial activity against S. aureus, this result agree with the research carried out by Amri et al. (23). The MIC against S. aureus ATCC 29213 was 25 μL/mL at pH 7.4 and it decreased 2-fold at pH 5. This result was also observed in wild strains.
A clear synergic effect was observed for the combination EO/CLOX, since FIC values obtained were ≤1 (Table 4). When we faced the EO/CLOX combination against the S. aureus strains, there was a great decreased in the concentration of antibiotic necessary to inhibit the bacterial growth. In EO/CLOX combination the bactericidal effect was maintained even when the pH of the broth was reduced from 7.4 to 5.0, as we have mentioned above when EO was studied without CLOX. And in the same way, when the culture medium was acidified, the concentration of CLOX, in the mixture EO/CLOX, was reduced even up to 10-fold to inhibit the microorganism. As we have mentioned in the introduction, there is still no published works where the pharmacological interaction between the M. armillaris EO and CLOX has been studied. In the bibliography another Melaleuca sp., M. alternifolia (tree of tea), has been widely investigated using the checkerboard technique against S. aureus. However, the results obtained when combining its EO with different antibiotics such as vancomycin (37), tobramycin (38), and ciprofloxacin (39) were indifference or antagonism effect.
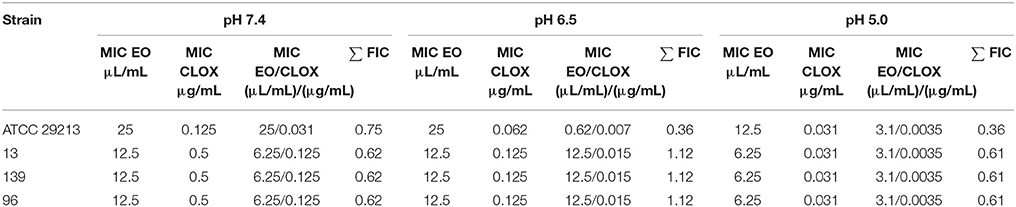
Table 4. Fractional concentration indexes (FIC) obtained for the EO/CLOX combination under different pH conditions vs. individual MICs.
The combined activity of EO and CLOX, used in our study, was also evidenced in the bactericidal effect, established by the E index. The decrease in the initial bacterial inoculum -Log10 (CFU/mL)- in a factor of 3 in 24 h marked bactericidal activity observed in both for the CLOX alone and for the mixture. However, the concentration of the antimicrobial in the presence of EO was clearly lower and the E-value obtained was closer to the virtual eradication effect (E = −4) than the concentration needed when applying β-lactam alone. In a study conducted by Nascimento et al. (40) the essential oil of Eremanthus erythropappus was evaluated in combination with ampicillin (β-lactam antibiotic) against S. aureus achieving a synergistic bactericidal effect after 24-h incubation (40).
In conclusion, it was possible to reduce the concentration of the antibiotic needed to inhibit S. aureus by combining CLOX with EO in vitro. Considering that CLOX is an antimicrobial of the group of β-lactams with good activity against S. aureus and with wide use in veterinary medicine, the EO enhance CLOX antibacterial effect even when the intracellular pH were more acid than the extracellular medium. This is important for the treatment of intracellular infections where S. aureus is internalized inside of phagolysosomes because the probability of therapeutic success would be really increased. Our results suggest an increase of the susceptibility to β-lactams due to the acidic pH prevailing in the vacuoles where S. aureus live and prosper, which would be facilitated by the action of M. armillaris EO. The acid pH causes a conformational change of the target protein of action (PBP2a), increasing the affinity of its catalytic center for β-lactam (41). So the synergy we found between CLOX and EO in acid conditions could take place in the inner cell and have an important effect against S. aureus when it is refractory to immunological mechanisms. These findings become into a valuable alternative for the treatment of persistent staphylococcal infections. M. armillaris EO needs to be considered in the design of future formulations to evaluate in vivo effects, in order to maximize the efficacy of current and future antimicrobials.
NM conceived and designed the experiments. DB performed all the experimental assay and statistical analysis. AVB, MM, and FA contributed with experimental assays. AB performed the EO quality assay. All authors contributed to the redaction, revision, and approved the final manuscript.
This work is partially financed by the Laboratory of Pharmacological and Toxicological Studies (LEFyT) and the National Agency for Scientific and Technical Promotion (ANPCyT) (PICT 15-831).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank to National Scientific and Technical Research Council (CONICET) for their collaboration in granting the 2016-2021 Ph.D Scholarship.
CLOX, Cloxacillin; EO, Melaleuca armillaris essential oil; MHB, Mueller Hinton broth.
1. Mestorino N, Errecalde J. “Pharmacokinetic-Pharmacodynamic considerations for bovine mastitis treatment.” In: Pérez Marín CC, editor. A Bird's-Eye View of Veterinary Medicine. Rijeka: InTech. (2012). p. 423–72. doi: 10.5772/31721
2. Rich M. Staphylococci in animals: prevalence, identification and antimicrobial susceptibility, with an emphasis on methicillin-resistant Staphylococcus aureus. Br J Bio Sci. (2005) 62:98–105. doi: 10.1080/09674845.2005.11732694
3. Nami TS, Le Dell KH, Sabetti K, Borchadt SM, Boxrud DJ, Elianne J. Comparison of community and health care associated methicillinresistant Staphylococcus aureus infection. JAMA (2003) 290:2976–84. doi: 10.1001/jama.290.22.2976
4. Devriese LA, Hommez J. Epidemiology of methicillin-resistant Staphylococcus aureus in dairy herds. Res Vet Sci. (1975) 19:23–7.
5. Cervantes-García E, García-González R, Salazar-Schettino PM. Características generales del Staphylococcus Aureus. Rev Latinoam Patol Clin Med Lab. (2014) 61:28–40.
6. Zomorodian K, Moein M, Lori ZG, Ghasemi Y, Rahimi MJ, Bandegani A, et al. Chemical composition and antimicrobial activities of the essential oil from Myrtus communis leaves. J Essent Oil Bear Pl. (2013) 16:76–84. doi: 10.1080/0972060X.2013.764183
7. López-Malo A, Palou E, Alzamora S. “Naturally occurring compounds – plants sources.” In: Davidson PM, Sofos JN, Branen AL, editors. Antimicrobials in Food. Boca Raton, FL: CRC Press (2005). p. 428–51.
8. Vega-Portocarrero E, López-Malo A. Agentes antimicrobianos presentes en especies y hierbas. Temas selectos de ingeniería en alimentos (2009) 3–1:85–95.
9. Yap PS, Yiap BC, Ping HC, Lim SHE. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J. (2014) 8:6–14. doi: 10.2174/1874285801408010006
10. Rosato A, Vitali C, De Laurentis N, Armenise D, Milillo AM. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine (2007) 14:727–32. doi: 10.1016/j.phymed.2007.01.005
11. Rodrigues FF, Costa JG, Coutinho HD. Synergy effects of the antibiotics gentamicin and the essential oil of Croton zehntneri. Phytomedicine (2009) 16:1052–5. doi: 10.1016/j.phymed.2009.04.004
12. Orchard A, van Vuuren S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid Based Complement Alternat Med. (2017) 2017:1–92. doi: 10.1155/2017/4517971
13. Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (2013) 6:1451–74. doi: 10.3390/ph6121451
14. Azhdarzadeh F, Hojjati M. Chemical composition and antimicrobial activity of leaf, ripe and unripe peel of bitter orange (Citrus aurantium) essential oils. Nutr Food Sci Res. (2016) 3:43–50. doi: 10.18869/acadpub.nfsr.3.1.43
15. Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines (2017) 4:58. doi: 10.3390/medicines4030058
16. Burt S. Essential oils: their antibacterial properties and potential applications in foods -a review. Int J Food Microbiol. (2004) 94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022
17. Bassole IH, Lamien-Meda A, Bayala B, Tirogo S, Franz C, Novak J, et al. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules (2010) 15:7825–39. doi: 10.3390/molecules15117825
18. Bassolé IH, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules (2012) 17:3989–4006. doi: 10.3390/molecules17043989
19. Chabir N, Romdhane M, Valentin A, Moukarzel B, Marzoug H, Brahim N, Mars Mm, Bouajila J. Chemical study and antimalarial, antioxidant, and anticancer activities of Melaleuca armillaris (Sol Ex Gateau) Sm essential oil. J Med Food (2011) 14:1383–8. doi: 10.1089/jmf.2010.0168
20. Hayouni EA, Bouix M, Abedrabba M, Leveau J. Hamdi M. Mechanism of action of Melaleuca armillaris (Sol. Ex Gaertu) Sm. essential oil on six LAB strains as assessed by multiparametric flow cytometry and automated microtiter-based assay. Food Chem. (2008) 111:707–18. doi: 10.1016/j.foodchem.2008.04.044
21. Silva CJ, Barbosa LC, Maltha CR, Pinheiro AL, Ismail FM. Comparative study of the essential oils of seven Melaleuca (Myrtaceae) species grown in Brazil. Flavour Frag J. (2007) 22:474–478. doi: 10.1002/ffj.1823
22. Rizk M, Ibrahim N, El-Rigal N. Comparative in vivo antioxidant levels in Schistosoma mansoni infected mice treated with praziquantel or essential oil of Melaleuca armillaris leaves. Pak J Biol Sci. (2012) 15:971–8. doi: 10.3923/pjbs.2012.971.978
23. Amri I, Mancini E, De Martino L, Marandino A, Hamrouni L, Mohsen H, et al. Chemical composition and biological activities of the essential oils from three Melaleuca species grown in Tunisia. Int J Mol Sci. (2012) 13:16580-−91. doi: 10.3390/ijms131216580
24. WHO. Antimicrobial Resistance. Fact Sheet. January 2018. Available online at: http://www.who.int/mediacentre/factsheets/fs194/en/
25. Holmgren PK, Holmgren NH, Barnett LC. Index Herbariorum. Part I: The Herbaria of the World 8th edn. Bronx, New York, NY: New York Botanical Gardens (1990).
26. Joulain D, König WA. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. Hamburg: EB Verlag (1998).
27. Babushok VI, Linstrom PJ, Zenkevich IG. Retention indices for frequently reported compounds of plant essential oils. J Phys Chem. (2011) 40:1–47. doi: 10.1063/1.3653552
28. Adams RP. Identification of essential oil components by gas chromatography / mass spectrometry. 4th Ed. Allured: Carol Stream, IL (2007).
29. Wiley/NIST. (2008). The Wiley/NBS Registry of Mass Spectral Data. 8th Edn. New York, NY: J. Wiley & Sons, Inc; New York/NIST/EPA/NIH; Mass Spectral Library, vers. 2.0 (2005).
30. Eliopoulos GM, Moellering RC Jr. “Antimicrobial combinations.” In: Lorian V. Editor. Antibiotics in Laboratory Medicine, 4th ed. Baltimore, Md: The Williams & Wilkins Co (1996). p. 330–96.
31. Sidhu P, Landoni M, Aliabadi F, Lees P. PK-PD integration and modeling of marbofloxacin in sheep. Res Vet Sci. (2011) 88:134–41. doi: 10.1016/j.rvsc.2009.05.013
32. CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 19th Informational Supplement. M100-S19 Wayne, PA: Clinical and Laboratory Standards Institute (2009).
33. Farag RS, Shalaby AS, El-Baroty GA, Ibrahim NA, Ali MA, Hassan EM. Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytother Res. (2004) 18:30–35. doi: 10.1002/ptr.1348
34. Yvon Y, Raoelison EG, Razafindrazaka R, Randriantsoa A, Romdhane M, Chabir N, Mkaddem MG, Bouajila J. Relation between chemical composition or antioxidant activity and antihypertensive activity for six essential oils. J Food Sci. (2012) 7:H185–91. doi: 10.1111/j.1750-3841.2012.02812.x
35. Curado MA, Oliveira CBA, Jesus JG, Santos SC, Seraphin JC, Ferri PH. Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochemistry (2006) 67:2363–9. doi: 10.1016/j.phytochem.2006.08.002
36. Lang G, Buchbauer G. A review on recent research results (2008-2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr J. (2012) 27:13–39. doi: 10.1002/ffj.2082
37. LaPlante KL. In vitro activity of lysostaphin, mupirocin, and tea tree oil against clinical methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. (2007) 57:413–18. doi: 10.1016/j.diagmicrobio.2006.09.007
38. D'Arrigo M, Ginestra G, Mandalari G, et al. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine (2010) 17:317–22. doi: 10.1016/j.phymed.2009.07.008
39. Van Vuuren SF, Suliman S, Viljoen AM. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett Appl Microbiol. (2009) 48: 440–6. doi: 10.1111/j.1472-765X.2008.02548.x
Keywords: Staphyloccocus aureus, Melaleuca armillaris, essential oil, cloxacillin, resistance, antimicrobial, MRSA, synergism
Citation: Buldain D, Buchamer AV, Marchetti ML, Aliverti F, Bandoni A and Mestorino N (2018) Combination of Cloxacillin and Essential Oil of Melaleuca armillaris as an Alternative Against Staphylococcus aureus. Front. Vet. Sci. 5:177. doi: 10.3389/fvets.2018.00177
Received: 18 April 2018; Accepted: 11 July 2018;
Published: 02 August 2018.
Edited by:
Arturo Anadón, Complutense University of Madrid, SpainReviewed by:
Ayhan Filazi, Ankara University, TurkeyCopyright © 2018 Buldain, Buchamer, Marchetti, Aliverti, Bandoni and Mestorino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Buldain, ZGJ1bGRhaW5AZmN2LnVubHAuZWR1LmFy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.