
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 26 July 2018
Sec. Veterinary Epidemiology and Economics
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00173
This article is part of the Research Topic Bovine Tuberculosis – International Perspectives on Epidemiology and Management View all 28 articles
Bovine tuberculosis (bTB), mainly caused by Mycobacterium bovis, can affect domestic and wild animals as well as humans. Identifying the major transmission mechanisms in an area is necessary for disease control and management. In this study, we aimed to evaluate the involvement of different types of contact in M. bovis transmission between cattle farms of south-western France between 2007 and 2015. We analyzed an empirical contact network of cattle farms as nodes, with known infection status and molecular types (16 circulated during the study period of which 14 affected only cattle and two both badgers and cattle). Edges were based on cattle trade data (T-edges) and on spatial neighborhood relationships between farms, either direct (P-edges) or badger-mediated, when two farms neighbored the same badger home range (B-edges), or two distinct but neighboring badger home ranges (D-edges). Edge types were aggregated so that the contact network contained only unique edges labeled by one or several edge types. The association between the contact network structure and bTB infection status was assessed using a non-parametric test, each molecular type being considered a marker of an independent epidemic. Using a logistic regression model, we estimated the contribution of each edge type to the probability for an edge originating from an infected farm to end at another infected farm. A total number of 1946 cattle farms were included in the study and were linked by 54,243 edges. Within this contact network, infected farms (whatever the molecular type) always belonged to the same component, suggesting the contact network may have supported bTB spread among those farms. A significant association between the pattern of bTB-infected farms and the structure of the contact network was observed when all the molecular types were simultaneously considered. The logistic regression model showed a significant association between M. bovis infection in direct neighbors of infected farms and the connection by T-, B- and D-edges, with odds-ratios of 7.4, 1.9, and 10.4, respectively. These results indicate a multifactorial M. bovis transmission between cattle farms of the studied area, with varying implication levels of the trade, pasture and badger networks according to the molecular type.
Since its discovery by Theobald Smith in the late 1800's (1) Mycobacterium bovis, the main agent of bovine tuberculosis (bTB) has been found in a wide variety of domestic and wild animal hosts, as well as in humans (2, 3). In Europe, the main host of M. bovis is cattle (4–6), but sheep (7), pigs (8) and goats (9) can be affected too. Wildlife species found infected on this continent include red deer (Cervus elaphus) (10, 11), roe deer (Capreolus capreolus) (12), red fox (Vulpes vulpes) (13–16), wild boar (Sus scrofa) (17, 18) and badger (Meles meles) (19–21).
Different routes may allow M. bovis transmission between wild and domestic hosts. The largest part of M. bovis shedding seems to occur through aerosols (respiratory tract secretions) and to a lesser extent through saliva, urine, feces (20, 22, 23), milk in cattle (24) and even wound exudates in badgers (20). Therefore close contacts (e.g., nose to nose) between infected individuals and susceptible ones can allow the transmission of M. bovis. However, several studies have shown that M. bovis may survive outside a host in a favorable environment for several months (24–26), allowing transmission through indirect contacts. M. bovis transmission between cattle can also involve different susceptible species either wild (27) or domestic [although the implication of other domestic species than cattle remains unclear regarding cattle transmission (24)]. At the herd level, several risk factors of bTB have been identified such as larger herd sizes, neighborhood with other herds, cattle movements, farm management practices such as grazing, dispersion of slurry on pastures or the share of water points (24, 28–31). Environmental risk factors have also been studied, with certain environmental conditions favoring the survival and persistence of M. bovis (such as shade, moisture or even some soil types) that foster M. bovis transmission (24–26). A third category of risk factors involves wildlife interactions, especially with badgers, wild boars and deer. For the latter two species, the sharing of feed or water on pastures appears to be a risk factor of M. bovis indirect transmission (23, 32, 33). The transmission between badgers and cattle seems a bit more complex, with uncertain direct contacts on pastures (34–36) and/or inside farm buildings (37). This interspecies transmission could occur on pastures through the shedding of the mycobacteria in urine and feces of infected badgers (24), and in respiratory tract secretions and feces of infected cattle (6, 29).
BTB molecular types are stable (38, 39) and can be used to trace independent epidemics (4). In France, while the officially bTB-free status was obtained in 2000, M. bovis infection has persisted in several regions. In 2014, 46% of incident outbreaks were detected in south-western France, with a national number of 105 cattle herds newly detected infected (40). Molecular typing methods spoligotyping (39) combined to MLVA (Multiple Loci Variable Number of Tandem Repeats, VNTR Analysis) based on MIRU-VNTR [Mycobacterial Interspersed Repetitive Unit–VNTR; (4, 38)] have allowed identifying 16 molecular types in this area between 2007 and 2015 from cattle isolates, two of which were shared between cattle and wildlife (4). Because spoligotype and MIRU-VNTR are considered stable markers (at least at a time horizon of several years), these 16 molecular types allow identifying 16 independent epidemics spreading in the same area during the same time period.
An effective way of representing the structure of contacts between hosts of an infectious disease consists in building networks (41), with epidemiological units as nodes, to which an infection status is associated. Edges linking nodes represent the contacts between epidemiological units that may allow the transmission of the disease agent. Regarding M. bovis transmission between cattle in France and in light of the above, nodes can represent cattle farms and edges may represent direct or indirect contacts between them. Two types of direct contacts may be featured by edges between farms: (i) contacts due to the trade of live cattle (42, 43) and (ii) contacts due to pasture neighborhood between cattle belonging to different farms but with nose to nose contacts over the fence (31, 44, 45). Besides, indirect contacts between cattle farms due the presence of wildlife may also be represented by edges. Concerning the badger, a known susceptible species to M. bovis infection (21, 40), the spatial organization of social groups with stable home ranges around setts (46, 47) allows us to represent indirect contacts with cattle based on the spatial intersection between farm pastures and home ranges (48).
The aim of our study was to analyze M. bovis transmission between cattle farms in a south-western area of France using contact networks and molecular types as infection status information. We built different networks featuring possible direct and indirect contacts between cattle farms and analyzed the association between their structure and the observed pattern of infected farms.
The study population was made up of the 1946 farms having reported cattle between January 2007 and March 2016 (end of the 2015 herd skin-testing period) and owning at least one pasture included in a 2,735 km2 study area, an area straddling the border of Pyrénées-Atlantiques and Landes French departments (Figure 1). Pastures were defined as land parcels used by cattle for grazing according to the “Relevé Parcellaire Graphique” (RPG) of 2013 provided by the French Ministry of Agriculture. Two pastures were considered neighbors if the minimal distance between their borders was less than 3 m. Farm sizes (number of bovine females over two years old) and types (dairy, beef, fattening, mixed, small and other herds) were obtained from the French cattle tracing system (“Base de Données Nationale d'Identification” denoted below BDNI) (Table 1).
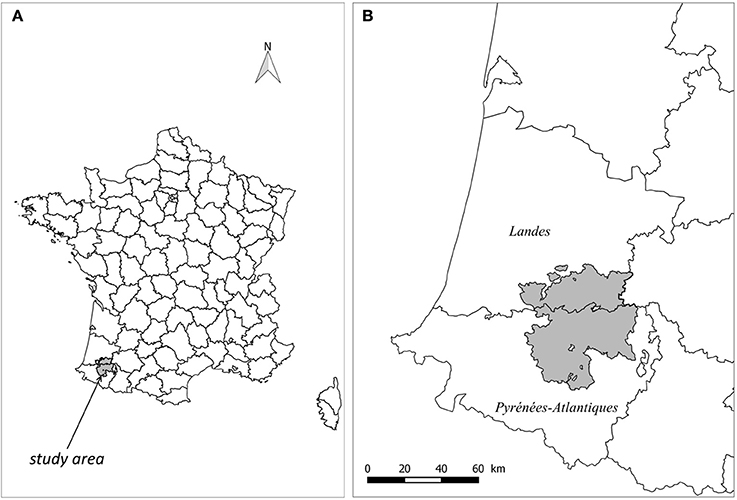
Figure 1. Location of the study area (A), at the border between Pyrénées-Atlantiques (south) and Landes (north) French departments (B).
BTB surveillance data were provided by the French Ministry of Agriculture. Herd skin-testing was performed each year in the study area in communes (the smallest French administrative subdivision) where infected farms had been detected the previous year, as well as in the neighboring ones, using either single intradermal comparative tuberculin tests (SICTT) (in all dairy farms or in farms located in the communes with confirmed infected farms) or single intradermal tuberculin tests (SITT) (in all the other situations), both performed in the cervical region. In the other communes of the study area, herd testing was biennial in Landes department, and triennial in Pyrénées-Atlantiques department. M. bovis infection was confirmed by polymerase chain reaction (PCR) and/or bacterial culture (either following a positive skin test or the detection of a suspect lesion during routine meat inspection at a slaughterhouse) (40) in 69 cattle farms of the study area during the study period; all the cattle of these farms were subsequently slaughtered and molecular typing was performed on each bovid found infected (with a mean of four cattle per farm detected infected during the study period). Molecular typing results were provided by the National Reference Laboratory (NRL) (Anses, Maisons-Alfort). The combination of spoligotyping and MLVA based on MIRU-VNTR allowed identifying 16 distinct molecular types (Table 2). A unique molecular type was identified in all of the 69 detected infected farms, except two where several molecular types were identified.
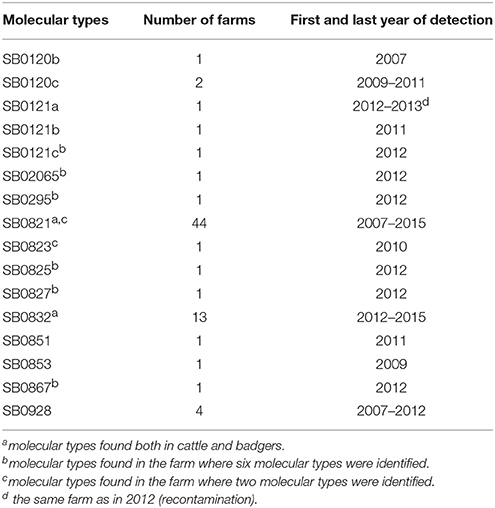
Table 2. Number of cattle farms detected infected per molecular type during the study period and within the study area.
A farm was classified infected by a given molecular type if this type had been detected at least once in the farm during the study period. Because of the geographic differences in the frequency of skin testing, having detected M. bovis earlier in a given farm than in another one does not imply that the former had been infected earlier than the latter. For this reason, the detection dates could not be taken into account.
Two thousand four hundred and 25 badger setts were identified and geolocalised by hunters in the study area, between 2013 and 2015. Around those setts, considered as main setts (i.e., hosting a social group), we defined badger home ranges using a two-step procedure: (i) a Dirichlet tessellation was first built around all setts [in which the perpendicular bisectors of each segment between two adjacent setts delineate the home range around one given sett, thus assuming that boundaries were located halfway between neighboring main setts (47)] and (ii) to avoid unrealistically home range large sizes, a home range was defined as the intersection of a tile with a 1,000 m-radius buffer area drawn around the setts (48). Two setts were considered neighbors if the corresponding home ranges were adjacent. A sett and a farm were considered neighbors if one of the farm pastures intersected with the badger home range.
BTB surveillance data were provided by the French Ministry of Agriculture. In the study area, bTB surveillance in badgers was performed according to the “Sylvatub” surveillance network, which started in 2012 in the study area (49). Surveillance protocol included badger trapping (i) within a 1.5 km-radius around confirmed infected farms, (ii) within a 2 km radius around setts with confirmed infected badgers and (iii) in communes at less than 5 km of communes where confirmed infected farms were located (one badger per sett). Trapping was performed using stopped restraints (https://www.plateforme-esa.fr/filedepot_download/35377/100) and snares were checked the morning after the day they were set up within the 2 h following sunrise, in order to limit the stress of trapped badgers. Trapped badgers were culled by head shot except in a minority of cases where they were found already dead (due to trap related injuries that sometimes occurred when snares were placed on sloping terrain, with no possible alternative). Road-killed badgers were also considered. Stopped restraints used for trapping were placed near sett entrances, those setts being considered as the sett of the trapped animals. Where badgers were found dead along roads, hunters reported the most probable sett according to their knowledge of the area (48). All the trapped and road-killed badgers were tested for M. bovis infection. Among 401 analyzed badgers (4.5% were road-killed badgers), 11.2% were detected infected (45 animals, one was a road-killed badger), of which 39 harbored the SB0821 molecular type and 6 the SB0832 molecular type, both molecular types having also been found in cattle (Table 2). All the badgers trapped could be attributed to 113 distinct setts, of which 33.6% hosted at least one infected badger (32 setts with at least one badger detected infected by SB0821 and 6 by SB0832). Road-killed badgers were attributed to five distinct setts. For four of these setts, the analysis of road-killed badgers did not provide additional information as they had also been subjected to trapping measures. For the fifth sett, the analysis of one road-killed badger allowed the detection of infection (SB0821 molecular type), not revealed by trapping. Setts with at least two badgers tested negative were considered as uninfected (n = 75). All the remaining setts, either with only one badger tested negative or without analyzed badger were considered of unknown status.
A contact network was built using farms of the study population as nodes, and four types of edges (Figure 2):
- A trade edge (denoted T-edge below) from farms i to farm j represented the sale of one or several cattle by farm i to farm j during the study period, at one or several occasions;
- A pasture neighborhood edge (denoted P-edge below) between farms i and j represented the fact that a pasture owned by i and another one owned by j were neighbors;
- A simple badger-mediated edge (denoted B-edge below) between farms i and j represented the fact that both farms were neighbors of a given sett;
- A second level badger-mediated edge (denoted D-edge below) between farms i and j represented the fact that (i) farm i was neighbor of a sett k1, (ii) farm j was neighbor of a sett k2, and (iii) the setts k1 and k2 were themselves neighbors.
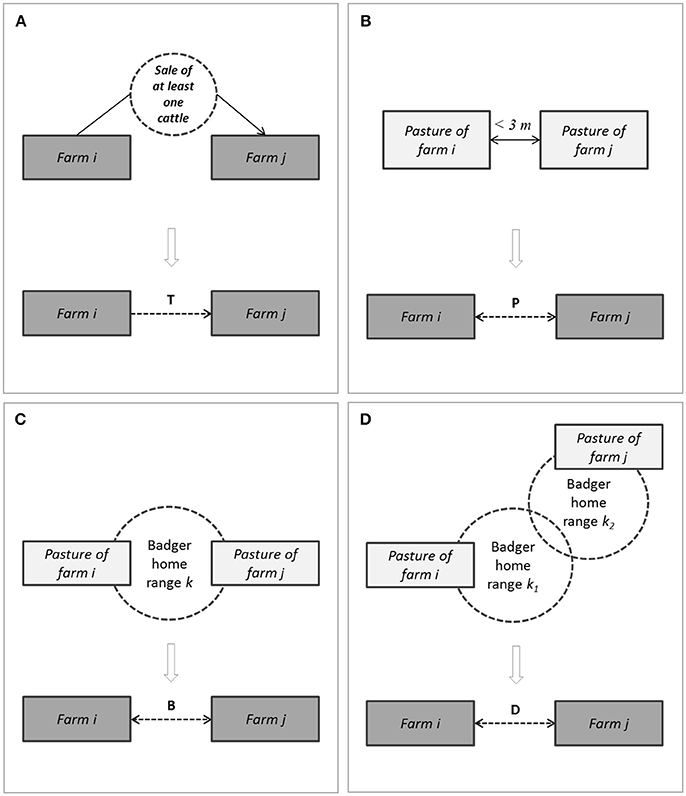
Figure 2. Schematic representation of the four types of edges between cattle farms in the contact network (A), trade edge (noted T); (B), pasture neighborhood edge (noted P); (C), simple badger-mediated edge (noted B); (D), second level badger-mediated edge (noted D).
To avoid duplicated edges, the types of edges (T, P, B and D) were aggregated at the edge level. The full contact network thus contained only unique edges labeled by one or several edge types (Table 3). Because the T-edges are directed, each undirected P-, B- and D-edge was transformed into two symmetric directed edges. The full contact network was thus a directed network.
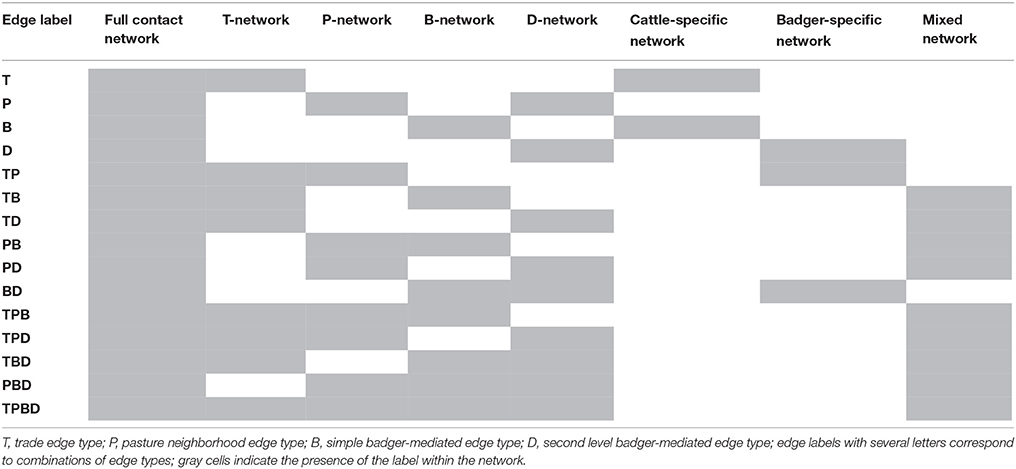
Table 3. Label of edges in the different networks of contacts between cattle farms in the study area.
Subnetworks were extracted from the full contact network by restricting the edges to those of specific types (Table 3). These subnetworks are termed below T-network, P-network, B-network and D-network. Similarly, we used edge types to split the full contact network in three non-overlapping subnetworks:
- the cattle-specific network incorporated edges labeled T, P or T-P, thus representing only contacts induced by cattle breeding practices;
- the badger-specific network incorporated edges labeled B, D or B-D, thus representing only badger-mediated contacts;
- the mixed network incorporated all the remaining edges, thus representing the co-occurrence of cattle-specific and badger-mediated contacts.
Each of the 16 molecular types of M. bovis identified in the study area was considered as a marker of an independent epidemic. For a given molecular type, the contact network may be considered as supporting M. bovis transmission between two farms only if a path exists in the network between these farms. The transmission tree rooted on a detected infected farm should then be entirely located in a single component of the network. The contact network may then be considered as supporting the spread of a given molecular type if most of the farms infected by this molecular type are located in the same component of the contact network. We thus first computed, for each molecular type identified in more than one farm, the number of components in which these infected farms were located (50). For the same subset of molecular types, we also computed, for each infected farm, the length of the shortest path to another farm where the same molecular type was detected.
To evaluate whether the observed pattern of bTB infected farms may have resulted from transmission processes in the contact network, we used the k-test proposed by VanderWaal et al. (51). This permutation-based test is based upon the calculation of the k-statistic: the mean number of infected cases among the neighbors of an infected node (the approach is easily extended to neighborhoods of order >1). The observed value of this statistic is then compared to the distribution of the same statistic obtained by randomly reallocating the location of cases, thus simulating a possible pattern of cases under the null hypothesis of an absence of association between bTB case location and network structure. The empirical p-value of the k-test is then the proportion of permutations for which the k-statistic is greater than the observed one. We adapted this test to a multi-type epidemic by redefining the k-statistic as the mean number of cases among the neighbors of a node, which were infected by the same molecular type as that node.
The k-test was first performed on the full contact network. It was then applied on the cattle-specific, badger-specific and mixed subnetworks; and this, for two groups of molecular types: those observed in cattle only and those observed in cattle and in badgers. Seven tests were thus performed and the Bonferroni correction was applied. Ten thousand permutations were used to compute the empirical p-value.
To further analyse the association between edge types and bTB occurrence, we focused on edges originating from infected farms. A binary status was assigned to each of these edges, with a value of 1 when the destination node was infected by the same molecular type as the originating node, and 0 otherwise. The association between this status and the edge type was then assessed using a case-control design: cases were edges having a status of 1, and controls the edges having the status 0. Four binary explicative variables were defined, based on the types labeling the edge: T, P, B, and D. In addition, we took into account the size (number of bovine females over the age of 2 years) of the edge originating and destination farms, herd size being a well-known risk factor for bTB detection in cattle farms (24). We thus modeled the probability for an edge starting from a detected infected farm to end at a farm detected infected by the same molecular type, using a logistic regression model including six independent variables: four binary variables (presence/absence of the T, P, B and D edge type) and two quantitative variables (sizes of the originating and destination farms). We checked the absence of multicollinearity using variance inflation factors (VIF) with a threshold of 10 (52). Odds ratios (OR) and their associated 95% confidence intervals were computed. Finally, attributable risk fractions (AF) were computed for each edge type.
The definition of badger-mediated edges was based upon the neighborhood between pastures and one (B-edges) or two (D-edges) badger home ranges. For some of the corresponding setts, the trapping results allowed defining an infection status: setts were considered as (i) infected when at least one trapped badger had been found infected with an identified molecular type and (ii) uninfected when at least two trapped badgers had been tested negative and no occupant badger had been found infected [for more details, see (48)]. Based on these data, we finally used a Fisher exact test to analyze the association between the status of B- or/and D-edges and the infection status of the corresponding setts.
Dirichlet tessellations were computed using the deldir package (53) and buffers using the sp package (54). Network analyses were carried out using the igraph package (55) and variance inflation factors were computed using the car package (56). Attributable risk fractions were finally computed using the AF package (57). All those cited packages were used in R 3.3.2 (58).
Within the full contact network, the most frequent edge type was the combination of B- and D-edges, followed by single D-, T-, and B-edges. The P-edge type was less frequent alone than in combination with the other types (Figure 3).
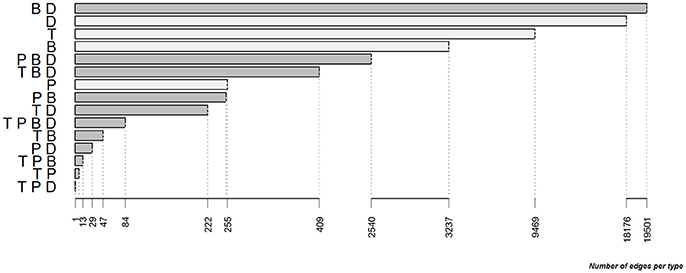
Figure 3. Distribution of the different types and combinations of types for the edges of the full contact network between cattle farms in the study area between 2007 and 2015 (T, trade edge; P, pasture neighborhood edge; B, simple badger-mediated edge; D, second level badger-mediated edge; edges having only one type are in light gray and combinations of several types are in gray).
The largest weak component of the full contact network incorporated 99.8% of the study population. Regarding the four edge-type-specific networks, the proportion of nodes included in the largest component was higher in trade and badger related networks (94.4% for the T-network, 94.7% for the B-network and 93.6% for the D-network) than in the pasture network (50.4%) (Table 4) (a more detailed analysis of networks topology is given in Supplementary Tables 2–4 and Supplementary Figures 1, 2).
For each of the 16 molecular types, the farms where the type had been observed were always located in the same component of the full contact network. This was also the case for the B-network, but not for the T-, P-, and D- networks (Table 5).
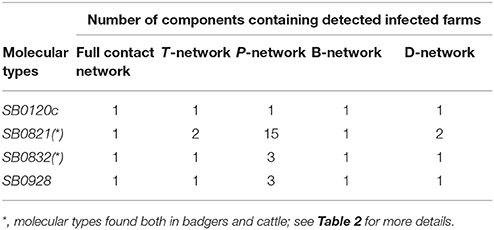
Table 5. Distribution of detected infected farms in the components of the full contact network and in the four edge-type-specific networks for the molecular types identified in more than one farm.
Four molecular types were observed in at least two detected infected farms (Table 2). For 87% of these farms, the path to the closest farm detected infected by the same molecular type was made of a single edge. It included one intermediary cattle farm in 11% of cases (Figure 4 and Supplementary Table 2). This result suggests a prominence of M. bovis transmission between an infected farm and its direct neighbors in the full contact network.
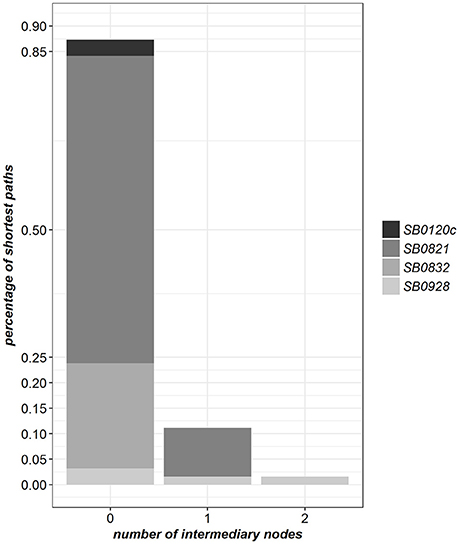
Figure 4. Distribution of the shortest path lengths in the full contact network between pairs of farms detected infected by the same molecular type (only the four molecular types found in at least two farms are considered).
We computed the proportion of shortest paths made of a single edge between farms infected (i) by molecular types found only in cattle and (ii) by molecular types found both in badgers and cattle. The difference between these two proportions was not significant (Fisher exact test: p = 0.13).
Using k-tests, a significant association was observed between the pattern of bTB detected infected farms and the structure of the full contact network (observed k-statistic: 2.3; distribution obtained by randomly reallocating the location of cases: mean = 0.39, SD = 0.12; p < 7.14*10−3, threshold after Bonferroni correction) (Figure 5). No significant association was observed for the cattle-specific network, neither for the molecular types observed in cattle only, nor for those found both in cattle and badgers. Conversely, a significant association was observed between the pattern of farms detected infected by molecular types shared between badgers and cattle and the structure of the badger-specific network (p < 7.14*10−3). Finally, the structure of the mixed network was significantly associated with the pattern of bTB-infected farms for both groups of molecular types (p = 0.006 and p < 7.14*10−3 respectively) (Table 6).
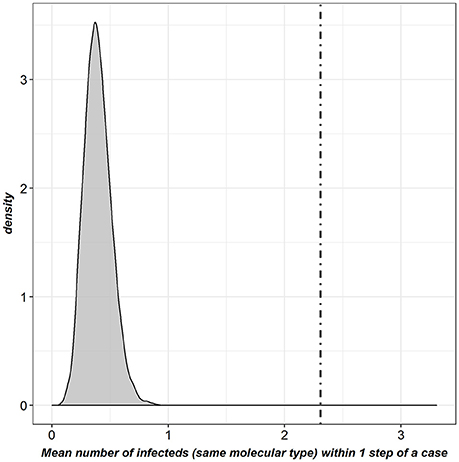
Figure 5. Graphical representation of the k-test results for the full contact network [dot-dashed line: k-statistic computed in the observed network; gray density plot: distribution obtained by randomly reallocating the location of cases; this last distribution was clearly lower than the k-statistic observed (p < 7.14*10−3, threshold after Bonferroni correction)].
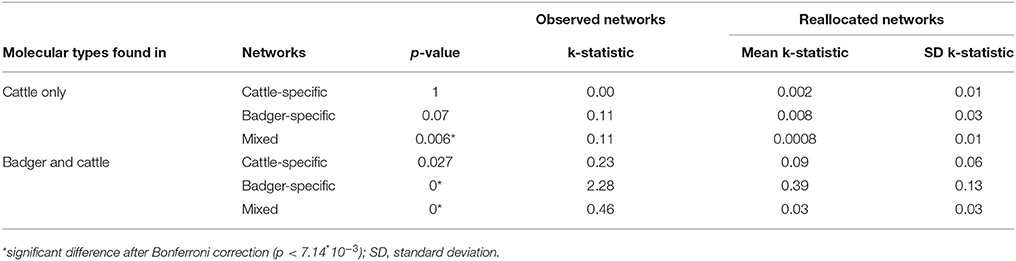
Table 6. Results of the k-tests for the cattle-specific, the badger-specific and for the mixed subnetworks of the full contact network, for the molecular types only found in cattle only and for those found both in badgers and cattle.
The four edge types were included in the logistic regression model as no significant multicollinearity was detected. T-, B-, and D-edge types were significantly associated to the probability of being a case with an OR of 7.13 for the T-edge type (95% CI: [3.39–15.06]), 1.89 for the B-edge type (95% CI: [1.32–2.76]) and 10.44 for the D-edge type (95% IC: [4.38–26.66]). The size of the destination farm of the edge was also significantly associated to the probability of being a case. Regarding edge types, attributable risk fractions were 84% for the D edge type, 32% for the B edge type, and 12% for the T edge type (Table 7).
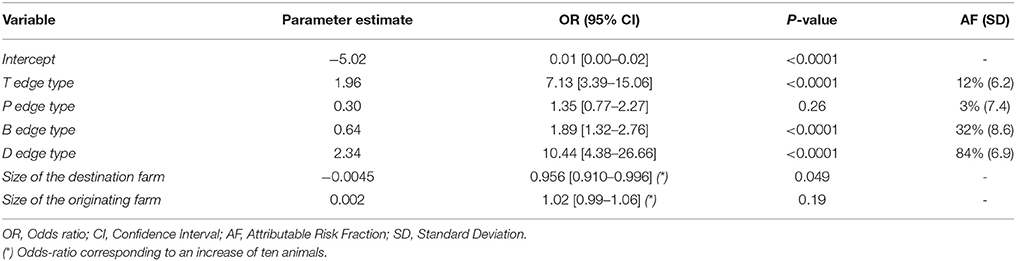
Table 7. Logistic model of the probability of an edge starting from a detected infected cattle farm to join another detected infected cattle farm and with the same molecular type according to the type of edge.
Among edges representing badger-mediated transmission (i.e., B- and D-edges), the infection status of badger setts involved (one sett regarding B-edges and at least one of the two setts regarding D-edges) was known for 264 edges (5%) originating from a farm infected by one of the two molecular types shared between badgers and cattle. Among them, 44 were case edges (i.e., the destination farm had also been found infected by the same molecular type) of which 38 (86%) were supported by positive badger setts; and 220 were control edges of which 102 were supported by positive badger setts (46%). These differences were significant (Fisher exact test: p < 0.0001) with an associated OR of 7.3 [95% CI: (2.9–21.9)].
The objective of this study was to provide a better understanding of M. bovis transmission mechanisms between cattle farms in south-western France using networks which represented the direct and indirect contacts that may allow M. bovis transmission among farms of this area between 2007 and 2015.
Four types of edges were represented because of their potential involvement in M. bovis transmission between cattle farms and we assumed that they represented the main transmission mechanisms in the study area. Cattle movements due to trade are a known M. bovis transmission route in Great Britain (59, 60), but also in France (42). The neighborhood with an infected farm through adjoining pastures (allowing over the fence contacts between herds) has also been identified as a potential risk factor for the M. bovis transmission between French cattle farms (31). The intersection of badger home ranges with cattle pastures and between each other's was considered a proxy for badger-mediated transmission, considering the territoriality of badgers (36) and the ability of M. bovis to survive in the soil (25, 26). BTB surveillance measures in badgers were not homogeneous among setts of the study area, as they were dependent on bTB detection in the cattle farms in their vicinity. For this reason, although the location of setts was known, we did not model badger setts as nodes in the contact network (we would have been unable to attribute an infection status to each of them). Instead of that, sett location data were used to represent badger-mediated contacts between farms by specific edges, based on neighboring badger home ranges. Two types of badger-mediated contacts were thus modeled by edges. B-edges represented a situation in which two farms neighbored the same badger home range: farm to farm M. bovis transmission through such edges thus only assumed cattle to badger and badger to cattle transmission. Conversely, D-edges represented a situation in which two farms neighbored two distinct but neighboring badger home ranges: farm to farm transmission through such edges thus also assumed badger to badger transmission in animals from neighboring setts. Because the epidemiological unit of this study was the farm, P-, B-, and D-edges were built based on the aggregation of pastures of each cattle farm. In the study area, cattle are often moved from one pasture to another one belonging to the same farm, e.g., when rotational grazing is used, we thus assumed that this simplification was meaningful.
The frequency of testing cattle was different in the different parts of the study area and this could have biased our results. However, testing was performed each year in communes where infected herds had been detected, and was also performed reactively in farms identified by contact tracing from these herds, based on cattle trade data and on pasture neighborhood. For these reasons, farms directly connected (in the full contact network) to a herd detected infected were considered having been submitted to similar testing regimens, both for B and D edge types (as in most cases the connected farms were located in the same commune), and for the T and P edge types (because of contact tracing). As only edges originating from herds detected infected were considered in the k-tests and in the logistic regression model, the corresponding results should not have been biased by geographic variations of the frequency of testing in the study area.
Taking into account the molecular types of isolates allowed considering 16 independent epidemics, of which 12 appeared restricted to a single farm, and 14 to less than 10 farms. All of these 14 molecular types affected only cattle. This predominance of molecular types found in a single cattle farm (75%) was in line with a previous study carried out in France between 1979 and 2000 in which a large majority of molecular types (84%) were found at a low frequency (less than 10 farms). This result has been interpreted as the sign of a poor spread of these strains (61), which could be traces of older epidemics that would have spread prior to 2007, but without significant transmission afterwards. Indeed, in our study, the 14 molecular types found in less than 10 farms were all detected not later than 2012 (Table 2).
Farms detected infected by a given molecular type were always located in the same large weak component of the full contact network that contained 99.8% of farms, whereas it was not the case for three of the edge-type-specific networks: the T-, P-, and D- networks. This indicated that, although the T-, P-, and D-edge-type-specific networks could not alone have supported the spread of bTB infection within the study area (contrary to the B-network), the strong connectivity resulting from the union of the four networks into the full contact network provided a structure that might enable the spread of the M. bovis infection in the study area. This result is in line with multifactorial mechanisms of bTB spread previously suggested by other studies (24, 29). As an example in Great Britain, dynamic modeling of cattle taking into account farm environment helped understanding M. bovis transmission routes (62). Prominent identified routes of M. bovis transmission were moving infected cattle between farms and reinfection from an environmental reservoir. The conclusion of this study was that control measures should simultaneously address several transmission routes to be effective.
Using k-tests, a significant association was observed between the pattern of bTB-infected farms and the structure of the full contact network. Moreover, the structures of the badger-specific and mixed networks were significantly associated with the pattern of farms detected infected by molecular types shared between badgers and cattle. This result was expected and confirmed that badger-mediated edges could be viewed as paths for the interspecies M. bovis transmission. In addition, the structure of the mixed network was significantly associated with the pattern of bTB-infected farms for molecular types found only in cattle, whereas it was not the case for the cattle-specific network. We could assume that the spread of cattle molecular types would be more efficient when direct contact (trade and/or pasture neighborhood) are associated with indirect badger-mediated contacts. In addition, we should be cautious about the cattle specificity of these molecular types, as these molecular types may be (or have been) present in the badger population without being observed, because of the relatively low sensitivity of bTB surveillance in the badger population.
Considering edges originating from detected infected farms, we used a case-control design and a logistic model to analyse the relationship between the types of an edge and the detection of the same molecular type at the originating and destination farm of the edge (case edges) or at the originating farm only (control edges). Because the detection dates could not be considered in the study to infer dates of infection, the co-occurrence of the same molecular type at both ends of case edges does not model the transmission of M. bovis through the edge, although the edges of the full contact network represent possible transmission paths for the bacteria and case edges thus represent possible transmission events. The largest odds-ratio was attributed to the D edge type, followed by the T edge type. This predominance of badger-mediated edges reflects the specific situation of the study area, where molecular types shared between badgers and cattle were predominant (84% of detected infected farms Table 2), and the predominant effect of the D edge type suggests a probable spread of M. bovis between badgers from neighboring setts, and not only between badgers and cattle. However, B and D edges were defined based on a geographic representation of home ranges, with a maximal distance of 1,000 m to the sett. This distance threshold, the Dirichlet tessellation used to model home ranges, and the fact that some setts may have been unoccupied, are three elements that may have led to an underestimation of home range size, and to an overestimation of the role of the D edge type.
The T edge type was also associated with a putative transmission of M. bovis (AF = 12%). This result is in agreement with a previous French study conducted at the national scale, according to which the population attributable risk fraction of bTB infection had been estimated at 12% [5–18%] for cattle trade (42), often allowing long distance bTB spread.
In a previous study conducted in France, pasture neighborhood was found significantly associated with the farm infection status (31). However, in the present study, the P edge type was not significantly associated with M. bovis transmission when using the case-control design. This may be first explained by the fact that some of farmers of the study area use rotational grazing, with some pastures left unoccupied for grass re-growth. Furthermore, P-edges were defined based on a direct neighborhood between pastures (<3 m). This short distance does not allow other opportunities of direct contacts between cattle, such as the wandering of livestock, to be represented.
The badger-specific edges (B and D edge types) were defined based on sett locations, one or two setts being associated to each. For some of these setts, an infection status could be determined based on bTB surveillance data. We showed that this infection status was significantly associated with the fact that the sett as well as the originating and the destination farms had all been found infected by isolates of the same molecular type (OR = 7.3; 95% CI:[2.9-21.9]). This result supports an actual badger-mediated transmission through these types of edges. Nevertheless, wild boars have also been found infected with M. bovis within the study area. Indeed, among 548 analyzed wild boars between 2011 and 2015, 15 (2.7%) were found infected. The corresponding molecular types found in these wild boars were the two molecular types shared between badgers and cattle. Therefore we cannot exclude the role of this wild species that we could not consider in this study because of a lack of field data that would have allowed its spatial organization (captured through radio tracking, for example) to be represented. Not considering wild boars in our analyses could have led to an over-estimate of the role of B and D edge types in M. bovis transmission between cattle farms.
Other indirect contacts through herd practices could also have contributed to the predominance of the D edge type. Indeed, this type of edge created links between farms without direct contacts at pasture but being in a kind of vicinity. As examples, the sharing of material or the loan of animals could create links between farms that may overlap the D edges. However, no data were available to investigate this assumption. Its confirmation or refutation would require supplementary investigation.
In conclusion, this study supports the multifactorial nature of M. bovis transmission between cattle farms within the Pyrénées-Atlantiques–Landes area, France from 2007 to 2015. The largest part of bTB spread seemed to be due to badger-mediated contacts, however cattle trade played a significant role. Consequently, to be truly effective, control measures should not focus on a single type of contact but ought to act on the different mechanisms we raised.
MB-Z, AC, and BD conceived and designed the study. MB-Z prepared the data for the analysis. MB-Z and BD performed the analysis. MB-Z wrote the manuscript. MB-Z, AC, and BD revised the manuscript. All the authors approved the submitted version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the French Ministry of Food, Agriculture and Forest, Directorate General for Food (DGAl) and by the University of Paris-Sud, which both funded MB-Z's PhD grant. The authors also warmly thank Pierre Jabert (DGAl), Christian Peboscq (Pyrénées-Atlantiques Departmental Federation of Hunters-FDC 64), and all the hunters of the Pyrénées-Atlantiques and Landes for the census of badger setts in the study area.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2018.00173/full#supplementary-material
1. Malone KM, Gordon SV. “Mycobacterium tuberculosis complex members adapted to wild and domestic animals.” ln: Strain Variation in the Mycobacterium Tuberculosis Complex: its Role in Biology, Epidemiology and Control Advances in Experimental Medicine and Biology. Cham: Springer (2017). p. 135–54.
2. de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberc Edinb Scotl. (2006) 86:77–109. doi: 10.1016/j.tube.2005.05.002
3. Good M, Bakker D, Duignan A, Collins DM. The history of in vivo tuberculin testing in bovines: tuberculosis, a “One Health” issue. Front Vet Sci. (2018) 5:59. doi: 10.3389/fvets.2018.00059
4. Hauer A, De Cruz K, Cochard T, Godreuil S, Karoui C, Henault S, et al. Genetic evolution of Mycobacterium bovis causing tuberculosis in livestock and wildlife in France since 1978. PLoS ONE (2015) 10:e0117103. doi: 10.1371/journal.pone.0117103
5. Guta S, Casal J, Napp S, Saez JL, Garcia-Saenz A, Perez de Val B, et al. Epidemiological investigation of bovine tuberculosis herd breakdowns in Spain 2009/2011. PLoS ONE (2014) 9:e104383. doi: 10.1371/journal.pone.0104383
6. Phillips CJC, Foster CRW, Morris PA, Teverson R. The transmission of Mycobacterium bovis infection to cattle. Res Vet Sci. (2003) 74:1–15. doi: 10.1016/S0034-5288(02)00145-5
7. Muñoz Mendoza M, Juan L, de Menéndez S, Ocampo A, Mourelo J, Sáez JL, et al. Tuberculosis due to Mycobacterium bovis and Mycobacterium caprae in sheep. Vet J. (2012) 191:267–9. doi: 10.1016/j.tvjl.2011.05.006
8. Bailey SS, Crawshaw TR, Smith NH, Palgrave CJ. Mycobacterium bovis infection in domestic pigs in Great Britain. Vet J. (2013) 198:391–7. doi: 10.1016/j.tvjl.2013.08.035
9. Napp S, Allepuz A, Mercader I, Nofrarías M, López-Soria S, Domingo M, et al. Evidence of goats acting as domestic reservoirs of bovine tuberculosis. Vet Rec. (2013) 172:663. doi: 10.1136/vr.101347
10. Queirós J, Vicente J, Alves PC, de la Fuente J, Gortázar C. Tuberculosis, genetic diversity and fitness in the red deer, Cervus elaphus. Infect Genet Evol. (2016) 43:203–12. doi: 10.1016/j.meegid.2016.05.031
11. Zanella G, Bar-Hen A, Boschiroli M-L, Hars J, Moutou F, Garin-Bastuji B, et al. Modelling transmission of bovine tuberculosis in red deer and wild boar in Normandy, France. Zoonoses Public Health (2012) 59(Suppl. 2):170–8. doi: 10.1111/j.1863-2378.2011.01453.x
12. Lambert S, Hars J, Réveillaud E, Moyen J-L, Gares H, Rambaud T, et al. Host status of wild roe deer in bovine tuberculosis endemic areas. Eur J Wildl Res. (2017) 63:15. doi: 10.1007/s10344-016-1071-4
13. Martín-Atance P, Palomares F, González-Candela M, Revilla E, Cubero MJ, Calzada J, et al. Bovine tuberculosis in a free ranging red fox (Vulpes vulpes) from Doñana National Park (Spain). J Wildl Dis. (2005) 41:435–6. doi: 10.7589/0090-3558-41.2.435
14. de Lisle GW, Mackintosh CG, Bengis RG. Mycobacterium bovis in free-living and captive wildlife, including farmed deer. Rev Sci Tech Int Off Epizoot. (2001) 20:86–111. doi: 10.20506/rst.20.1.1262
15. Millán J, Jiménez MA, Viota M, Candela MG, Peña L, León-Vizcaíno L. Disseminated bovine tuberculosis in a wild red fox (Vulpes vulpes) in southern Spain. J Wildl Dis. (2008) 44:701–6. doi: 10.7589/0090-3558-44.3.701
16. Zanella G, Durand B, Hars J, Moutou F, Garin-Bastuji B, Duvauchelle A, et al. Mycobacterium bovis in wildlife in France. J Wildl Dis. (2008) 44:99–108. doi: 10.7589/0090-3558-44.1.99
17. Richomme C, Boadella M, Courcoul A, Durand B, Drapeau A, Corde Y, et al. Exposure of wild boar to Mycobacterium tuberculosis complex in France since 2000 is consistent with the distribution of bovine tuberculosis outbreaks in cattle. PLoS ONE (2013) 8:e77842. doi: 10.1371/journal.pone.0077842
18. Gortázar C, Vicente J, Gavier-Widén D. Pathology of bovine tuberculosis in the European wild boar (Sus scrofa). Vet Rec. (2003) 152:779–80. doi: 10.1136/vr.152.25.779
19. Balseiro A, Rodríguez O, González-Quirós P, Merediz I, Sevilla IA, Davé D, et al. Infection of Eurasian badgers (Meles meles) with Mycobacterium bovis and Mycobacterium avium complex in Spain. Vet J. (2011) 190:e21–5. doi: 10.1016/j.tvjl.2011.04.012
20. Corner LAL, Murphy D, Gormley E. Mycobacterium bovis infection in the Eurasian badger (Meles meles): the disease, pathogenesis, epidemiology and control. J Comp Pathol. (2011) 144:1–24. doi: 10.1016/j.jcpa.2010.10.003
21. Payne A, Boschiroli ML, Gueneau Eric, Moyen J-L, Rambaud T, Dufour B, et al. Bovine tuberculosis in “Eurasian” badgers (Meles meles) in France. Eur J Wildl Res. (2013) 59:331–9. doi: 10.1007/s10344-012-0678-3
22. Neill SD, Bryson DG, Pollock JM. Pathogenesis of tuberculosis in cattle. Tuberculosis (2001) 81:79–86. doi: 10.1054/tube.2000.0279
23. Naranjo V, Gortázar C, Vicente J, de la Fuente J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet Microbiol. (2008) 127:1–9. doi: 10.1016/j.vetmic.2007.10.002
24. Broughan JM, Judge J, Ely E, Delahay RJ, Wilson G, Clifton-Hadley RS, et al. A review of risk factors for bovine tuberculosis infection in cattle in the UK and Ireland. Epidemiol Infect. (2016) 144:2899–926. doi: 10.1017/S095026881600131X
25. Barbier E, Rochelet M, Gal L, Boschiroli ML, Hartmann A. Impact of temperature and soil type on Mycobacterium bovis survival in the environment. PLoS ONE (2017) 12:e0176315. doi: 10.1371/journal.pone.0176315
26. Fine AE, Bolin CA, Gardiner JC, Kaneene JB. A study of the persistence of Mycobacterium bovis in the environment under natural weather conditions in Michigan, USA. Vet Med Int. (2011) 2011:765430. doi: 10.4061/2011/765430
27. Gortázar C, Ruiz-Fons JF, Höfle U. Infections shared with wildlife: an updated perspective. Eur J Wildl Res. (2016) 62:511–25. doi: 10.1007/s10344-016-1033-x
28. Griffin JM, Martin SW, Thorburn MA, Eves JA, Hammond RF. A case-control study on the association of selected risk factors with the occurrence of bovine tuberculosis in the Republic of Ireland. Prev Vet Med. (1996) 27:75–87. doi: 10.1016/0167-5877(95)00548-X
29. Humblet M-F, Boschiroli ML, Saegerman C. Classification of worldwide bovine tuberculosis risk factors in cattle: a stratified approach. Vet Res. (2009) 40:50. doi: 10.1051/vetres/2009033
30. Kaneene JB, Bruning-Fann CS, Granger LM, Miller R, Porter-Spalding BA. Environmental and farm management factors associated with tuberculosis on cattle farms in northeastern Michigan. J Am Vet Med Assoc. (2002) 221:837–42. doi: 10.2460/javma.2002.221.837
31. Marsot M, Béral M, Scoizec A, Mathevon Y, Durand B, Courcoul A. Herd-level risk factors for bovine tuberculosis in French cattle herds. Prev Vet Med. (2016) 131:31–40 doi: 10.1016/j.prevetmed.2016.07.006
32. Palmer MV, Thacker TC, Waters WR, Gortázar C, Corner LAL. Mycobacterium bovis: a model pathogen at the interface of livestock, wildlife, and humans. Vet Med Int. (2012) 2012:236205. doi: 10.1155/2012/236205
33. Delahay RJ, Smith GC, Barlow AM, Walker N, Harris A, Clifton-Hadley RS, et al. Bovine tuberculosis infection in wild mammals in the South-West region of England: a survey of prevalence and a semi-quantitative assessment of the relative risks to cattle. Vet J. (2007) 173:287–301. doi: 10.1016/j.tvjl.2005.11.011
34. O'Mahony DT. Badger-cattle Interactions in the Rural Environment - Implications for Bovine Tuberculosis Transmission. TB & Brucellosis Policy Branch, Department of Agriculture and Rural Development, Northern Ireland (2014). Available online at: https://www.dardni.gov.uk/publications/badger-cattle-interactions-rural-environment-implications-bovine-tuberculosis
35. Böhm M, Hutchings MR, White PCL. Contact networks in a wildlife-livestock host community: identifying high-risk individuals in the transmission of bovine TB among badgers and cattle. PLoS ONE (2009) 4:e5016. doi: 10.1371/journal.pone.0005016
36. Woodroffe R, Donnelly CA, Ham C, Jackson SYB, Moyes K, Chapman K, et al. Badgers prefer cattle pasture but avoid cattle: implications for bovine tuberculosis control. Ecol Lett. (2016) 19:1201–8. doi: 10.1111/ele.12654
37. Payne A, Chappa S, Hars J, Dufour B, Gilot-Fromont E. Wildlife visits to farm facilities assessed by camera traps in a bovine tuberculosis-infected area in France. Eur J Wildl Res. (2015) 62:33–42. doi: 10.1007/s10344-015-0970-0
38. Allix C, Walravens K, Saegerman C, Godfroid J, Supply P, Fauville-Dufaux M. Evaluation of the epidemiological relevance of variable-number tandem-repeat genotyping of Mycobacterium bovis and comparison of the method with IS6110 restriction fragment length polymorphism analysis and spoligotyping. J Clin Microbiol. (2006) 44:1951–62. doi: 10.1128/JCM.01775-05
39. Aranaz A, Liébana E, Mateos A, Dominguez L, Vidal D, Domingo M, et al. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. (1996) 34:2734–40. Available online at: http://jcm.asm.org/content/34/11/2734.short
40. Cavalerie L, Courcoul A, Boschiroli M-L, Réveillaud E, Gay P. Bovine tuberculosis in France in 2014: a stable situation. Bull Épidémiologique Anim Health Nutr. (2015) 71:4–11. Available online at: http://www.bovinetb.info/docs/bovine-tuberculosis-in-france-in-2014-a-stable-situation.pdf
41. Wang XF, Chen G. Complex networks: small-world, scale-free and beyond. IEEE Circuits Syst Mag. (2003) 3:6–20. doi: 10.1109/MCAS.2003.1228503
42. Palisson A, Courcoul A, Durand B. Role of cattle movements in bovine tuberculosis spread in France between 2005 and 2014. PLoS ONE (2016) 11:e0152578. doi: 10.1371/journal.pone.0152578
43. Dubé C, Ribble C, Kelton D, McNab B. Estimating potential epidemic size following introduction of a long-incubation disease in scale-free connected networks of milking-cow movements in Ontario, Canada. Prev Vet Med. (2011) 99:102–11. doi: 10.1016/j.prevetmed.2011.01.013
44. Palisson A, Courcoul A, Durand B. Analysis of the spatial organization of pastures as a contact network, implications for potential disease spread and biosecurity in livestock, France, 2010. PLoS ONE (2017) 12:e0169881. doi: 10.1371/journal.pone.0169881
45. Dommergues L, Rautureau S, Petit E, Dufour B. Network of contacts between cattle herds in a French area affected by bovine tuberculosis in 2010. Transbound Emerg Dis. (2012) 59:292–302. doi: 10.1111/j.1865-1682.2011.01269.x
46. Bodin C, Benhamou S, Poulle M-L. What do European badgers (Meles meles) know about the spatial organisation of neighbouring groups? Behav Processes (2006) 72:84–90. doi: 10.1016/j.beproc.2006.01.001
48. Bouchez-Zacria M, Courcoul A, Jabert P, Richomme C, Durand B. Environmental determinants of the Mycobacterium bovis concomitant infection in cattle and badgers in France. Eur J Wildl Res. (2017) 63:74. doi: 10.1007/s10344-017-1131-4
49. Sylvatub. Surveillance de la tuberculose bovine dans la faune sauvage en France : Dispositif SYLVATUB - Bilan fonctionnel et sanitaire 2014-2015. Plateforme ESA (2015). Available online at: https://www.plateforme-esa.fr/filedepot_download/36412/1100
50. Robinson SE, Everett MG, Christley RM. Recent network evolution increases the potential for large epidemics in the British cattle population. J R Soc Interface (2007) 4:669–74. doi: 10.1098/rsif.2007.0214
51. VanderWaal K, Enns EA, Picasso C, Packer C, Craft ME. Evaluating empirical contact networks as potential transmission pathways for infectious diseases. J R Soc Interface (2016) 13:20160166. doi: 10.1098/rsif.2016.0166
52. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2nd Edn. Charlottetown: VER Inc. (2009).
53. Turner R. deldir: Delaunay Triangulation and Dirichlet (Voronoi) Tessellation. R Package Version 0.1-14. Available online at: https://CRAN.R-project.org/package=deldir (2017).
54. Pebesma EJ, Bivand RS. Classes and methods for spatial data in R. R News 5 (2), Available online at: https://cran.r-project.org/doc/Rnews/. (2005).
55. Csardi G, Nepusz T. The igraph software package for complex network research. InterJ Complex Syst. (2006) 1695:1–9. Available online at: http://www.necsi.edu/events/iccs6/papers/c1602a3c126ba822d0bc4293371c.pdf
56. Fox J, Weisberg S. An {R} companion to applied regression, 2nd Edn. Thousand Oaks CA: Sage. Available online at: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. (2011).
57. Dahlqwist E, Sjölander A. AF: Model-Based Estimation of Confounder-Adjusted Attributable Fractions. R package version 0.1.4. Available online at: https://CRAN.R-project.org/package=AF. (2017).
58. R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna (2016). Available online at: http://www.R-project.org.
59. Clegg TA, Blake M, Healy R, Good M, Higgins IM, More SJ. The impact of animal introductions during herd restrictions on future herd-level bovine tuberculosis risk. Prev Vet Med. (2013) 109:246–57. doi: 10.1016/j.prevetmed.2012.10.005
60. Gopal R, Goodchild A, Hewinson G, Domenech R de la R, Clifton-Hadley R. Introduction of bovine tuberculosis to north-east England by bought-in cattle. Vet Rec. (2006) 159:265–71. doi: 10.1136/vr.159.9.265
61. Haddad N, Ostyn A, Karoui C, Masselot M, Thorel MF, Hughes SL, et al. Spoligotype diversity of Mycobacterium bovis strains isolated in France from 1979 to 2000. J Clin Microbiol. (2001) 39:3623–32. doi: 10.1128/JCM.39.10.3623-3632.2001
Keywords: bovine tuberculosis, network analysis, cattle herds, badger-cattle interface, cattle trade, pastures
Citation: Bouchez-Zacria M, Courcoul A and Durand B (2018) The Distribution of Bovine Tuberculosis in Cattle Farms Is Linked to Cattle Trade and Badger-Mediated Contact Networks in South-Western France, 2007–2015. Front. Vet. Sci. 5:173. doi: 10.3389/fvets.2018.00173
Received: 05 May 2018; Accepted: 04 July 2018;
Published: 26 July 2018.
Edited by:
Andrew William Byrne, Agri Food and Biosciences Institute, United KingdomReviewed by:
Joseph Crispell, University College Dublin, IrelandCopyright © 2018 Bouchez-Zacria, Courcoul and Durand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benoit Durand, YmVub2l0LmR1cmFuZEBhbnNlcy5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.