
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 15 March 2018
Sec. Parasitology
Volume 5 - 2018 | https://doi.org/10.3389/fvets.2018.00048
This article is part of the Research Topic Parasites at the Wildlife-Domestic Animal Interface View all 9 articles
A study was carried out to investigate the presence of Echinococcus multilocularis in red foxes (Vulpes vulpes) in two regions of Turkey—central Anatolia (in Asia Minor) and Thrace (in the European part of Turkey). A total of 405 putative fox feces were collected from central Anatolia (186 specimens in 59 locations) and from Thrace (219 specimens in 114 locations). All samples were examined by the flotation and sieving method for taeniid eggs, and positive and putative samples were further analyzed by multiplex PCR. In seven samples from three locations in central Anatolia (5.1%) and in one (0.9%) from Thrace, E. multilocularis DNA was amplified, and this result was confirmed with another PCR specific for E. multilocularis. In addition, Echinococcus granulosus s.l. was found in two (0.5%) of the samples. Although alveolar echinococcosis (AE) is known as a serious zoonosis in Turkey, this is the first field study detecting E. multilocularis in collected fecal samples documenting the environmental contamination with eggs of this zoonotic parasite.
Echinococcus multilocularis is a taeniid parasite (Taeniidae, Cestoda) with a wild animal cycle that includes foxes and other canids as definitive hosts and rodents (particularly Arvicolidae) as intermediate hosts. The parasite is asymptomatic in definitive hosts but can cause a fatal disease, alveolar echinococcosis (AE), in intermediate and accidental hosts. E. multilocularis is a zoonotic cestode, and humans become infected by consumption of parasite eggs (1).
The geographic distribution of E. multilocularis depends on the presence of definitive hosts predating potential intermediate hosts. Central and eastern Europe, northern Asia, and North America are endemic for E. multilocularis, while some of these regions are regarded as highly endemic, namely, Central Europe, the Baltic countries, China, Japan, Mongolia, Kyrgyzstan, Russia, Turkey, and the western part of Alaska (2).
Although Turkey is considered a highly endemic region for human AE (2–4), there is little information available on the epidemiology of E. multilocularis in animal hosts. So far, two E. multilocularis infections in foxes, one from Trace (5) and the other from the eastern part of minor Asia (6), have been reported.
The aim of this study is to demonstrate the presence of E. multilocularis in field samples in two regions of Turkey: Central Anatolia where AE in humans is known to occur (7) and Thrace, where there is only historical evidence of possible endemicity.
Turkey is located on 36°–42° North and 26°–45° East longitude and consists of two parts—Anatolia (Asia Minor) in Asia and Thrace in Europe. The study was performed in five cities—two (Kayseri and Nevşehir) in central Anatolia and three (Kırklareli, Edirne, and Tekirdağ) in Thrace (Figure 1). There are no records on the fox population in Turkey. Consequently, fox feces were collected randomly from different locations, each at least 1 km distance from the other. All locations were situated in rural areas, including villages and fields with some locations around fox holes.
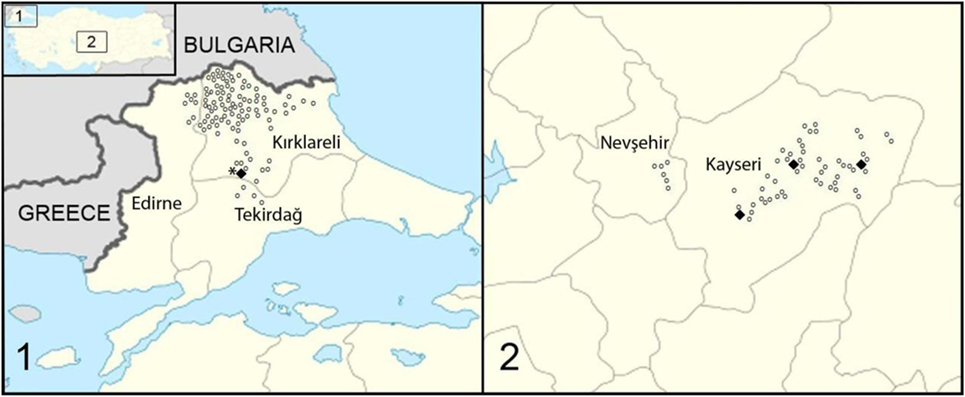
Figure 1. Study area and locations where fecal samples were collected in Turkey, (1) in Thrace and (2) in central Anatolia (Asia Minor). Open dots represent the location of samples negative for Echinococcus multilocularis; bold dots represent samples with positive E. multilocularis PCR result. The star localizes the origin of the fox infection with E. multilocularis found in the sixties (5).
Fox feces were collected in October 2014 in central Anatolia and in March 2015 in Thrace. They were identified based on their size, shape, and the presence of food residues such as hair, fruit, and feathers. The samples were picked up off the ground, put into sterile fecal containers and numbered, and the coordinates of each sample were recorded. In total, 405 samples were collected from 173 different locations; 186 specimens in 59 locations from central Anatolia and 219 specimens in 114 locations from Thrace.
All fecal samples were stored at −80°C for at least a week before analysis as safety precautions. Diagnosis of E. multilocularis consisted of three steps; determination of the presence of taeniid eggs in the samples; isolation of taeniid eggs; and molecular analysis of them.
All fecal samples were examined and taeniid eggs were concentrated by the flotation and sieving method, modified by Mathis et al. (8). Samples with identifiable taeniid eggs or with particles with similar size and shape as taeniid eggs and six negative samples collected in Thrace, where an E. multilocularis positive fox was identified in the 50 years previously (5), were further investigated by PCR. DNA was extracted by alkaline lysis (9) and amplified using the multiplex-PCR protocol according to Trachsel et al. (10). All positive samples for E. multilocularis were confirmed with the PCR protocol according to Stieger et al. (11).
In samples with Echinococcus spp. DNA amplification, the fox origin was confirmed by multiplex PCR as described by Nakao et al. (12). Positive control DNA extracted by tissue from the fox’s tongue and dog’s blood. Size of PCR products were 165 bp for fox and 355 bp for dog samples.
DNA was extracted from 60 of 405 putative fox fecal samples. E. multilocularis DNA was amplified in eight samples in both areas investigated (Table 1; Figure 1). From Anatolia, seven samples were found to be positive for E. multilocularis. Six of these samples contained other Cestoda spp. DNA. In one sample, E. multilocularis, Echinococcus granulosus, and non-Echinococcus spp. cestode DNA was amplified.
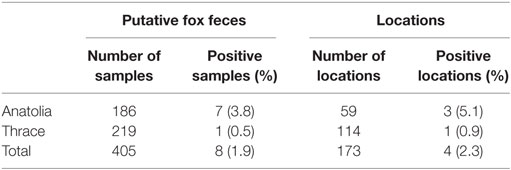
Table 1. Detection of Echinococcus multilocularis DNA in putative fox fecal samples collected in the environment in Turkey.
In Thrace, E. multilocularis DNA was amplified in one of six egg negative samples. Furthermore, E. granulosus DNA was identified in one sample, and 25 samples contained DNA of non-Echinococcus cestodes.
All samples with Echinococcus spp. DNA amplification were identified by PCR as being of fox origin.
Turkey is a highly endemic region for E. multilocularis. Since 1939, when first reported, more than 750 human cases of AE have been recorded (3, 13–15), but the true incidence is estimated to be much higher, for example, Torgerson et al. proposed that there must be at least 100 cases of AE per year in Turkey (4). However, little information is available about the epidemiology of E. multilocularis in Turkey. There are no convincing reports on infections of wild rodents as intermediate hosts. In definitive hosts, only two cases have been published to date. One reports an E. multilocularis infection dating back to 1963 in which E. multilocularis was identified in a single necropsied red fox (Vulpes vulpes) from Thrace (5). Interestingly, further north, E. multilocularis has been described in rodents in Bulgaria and in foxes in Romania (16). In total, in this study, eight fox feces (1.9%) in four locations (2%) were positive for E. multilocularis. Three locations were from the central Anatolia and one from Thrace. Our finding of a positive fecal sample in Thrace, although negative for taeniid eggs, was confirmed by two PCRs targeting different mitochondrial genes. This therefore confirms that E. multilocularis is still endemic in Thrace in the same area where it was identified in 1960s. Interestingly, however, no cases of AE are known from this region, except possibly three suspect cases (17). By contrast, Anatolia (Asia Minor) is well known to be an endemic area for AE in humans, and recently the first infected fox has been documented (6). Between 1980 and 2010, 13 AE cases were reported from the city of Kayseri and one from the city of Nevşehir (7). The number of AE cases in humans increases gradually in an eastward direction in Turkey, with most cases recorded from East Turkey (17–19). In this study, we documented E. multilocularis in definitive hosts feces in central Anatolia for the first time. We amplified E. multilocularis DNA in 7 (4.4%) of 159 fox feces in Kayseri, but we found no evidence of infection in Nevşehir. This adds to information from neighboring countries. For example, in Iran, where only 40 AE cases have been reported in humans (20–22) even though E. multilocularis infection rates of 22.9% in foxes and of 20.9% in other carnivores (dog, jackal, hyena, and wolf) have been documented (23). In Iraq, by contrast, only two AE cases have been published, and no reports on definitive host infections with E. multilocularis are available (24).
In this preliminary study, we focused on the environmental contamination with E. multilocularis eggs based on fecal samples. However, we cannot exclude that the morphological identification of the samples was 100% specific for fox samples. Poulle et al. documented that the morphological assessment of fecal samples has a sensitivity of around 83% (95% confidence intervals, 61–95%) (25). Therefore, for all Echinococcus positive samples, the fox origin was confirmed by PCR even in the two samples with E. granulosus-specific amplification. Turkey is a well-known endemic area of E. granulosus (2), and E. granulosus prevalence in foxes ranged up to 50% in Australian foxes (26).
AG and PD developed the study protocol and oversaw all procedures. AG, FG, CB, MA, and ŞU organized to collection and investigation of the materials. AG and PD drafted the manuscript, and the final version was approved by all authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Our pilot study was supported by Institute of Parasitology, University of Zurich.
1. Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, et al. Chapter five – Ecology and life cycle patterns of Echinococcus species. Adv Parasitol (2017) 95:213–314. doi:10.1016/bs.apar.2016.11.002
2. Deplazes P, Rinaldi L, Alvarez-Rojas CA, Torgerson P, Majid F, Harandi MF, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol (2017) 95:315–493. doi:10.1016/bs.apar.2016.11.001
4. Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis (2010) 4:722. doi:10.1371/journal.pntd.0000722
5. Merdivenci A. Türkiye’de tilki (Vulpes vulpes)’lerde ilk helmintolojik araştırma ve ilk Echinococcus multilocularis (Leuckart, 1864), Vogel, 1935 olayı. Türk Vet Hek Dern Derg (1963) 33:290–6. (In Turkish, with English abstract.).
6. Avcioğlu H, Guven E, Balkaya I, Kirman R, Bia MM, Gulbeyen H. First molecular characterization of Echinococcus multilocularis in Turkey. Vector Borne Zoonotic Dis (2016) 16:627–9. doi:10.1089/vbz.2016.1983
7. Deniz K, Nazlım S, Patıroğlu TE, Deniz E, Artış T, Karaman A, et al. Retrospective evaluation of the alveolar echinococcosis cases between 1980-2010 in Erciyes University Hospital. Acta Parasitol Turcica (2012) 36:33–6. (In Turkish, with English abstract.). doi:10.5152/tpd.2012.08
8. Mathis A, Deplazes P, Eckert J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J Helminthol (1996) 70:219–22. doi:10.1017/S0022149X00015443
9. Stefanic S, Shaikenov BS, Deplazes P, Dinkel A, Torgerson PR, Mathis A. Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol Res (2004) 92:347–51. doi:10.1007/s00436-003-1043
10. Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology (2007) 134:911–20. doi:10.1017/S0031182007002235
11. Stieger C, Hegglin D, Schwarzenbach G, Mathis A, Deplazes P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology (2002) 124:631–40. doi:10.1017/S0031182002001749
12. Nakao N, Sano T, Inoue T, Armua MT, Fukui D, Katakura K, et al. Multiplex PCR system for identifying the carnivore origins of faeces for an epidemiological study on Echinococcus multilocularis in Hokkaido, Japan. Parasitol Res (2009) 106:75–83. doi:10.1007/s00436-009-1629-0
13. Canda MŞ, Canda T. The echinococcosis map and an index of the literature of Turkey. T Ekopatol Derg (1995) 1:59–69. (In Turkish, with English abstract.).
14. Miman Ö, Yazar S. Alveolar echinococcosis in Turkey: in the light of the literature. Acta Parasitol Turcica (2012) 36:116–20. (In Turkish, with English abstract.). doi:10.5152/tpd.2012.28
16. Siko SB, Deplazes P, Ceica C, Tivadar CS, Bogolin I, Popescu S, et al. Echinococcus multilocularis in south-eastern Europe (Romania). Parasitol Res (2011) 108:1093–7. doi:10.1007/s00436-010-2150
17. Altıntaş N, Yazar S, Yolasığmaz A, Şakru N, Gödekmerdan A, Suay A, et al. Alveolar echinococcosis cases detected between 1980-1998 in Turkey. Acta Parasitol Turcica (1998) 23:133–6.
18. Emre A, Özden I, Bilge O, Arıcı C, Alper A, Ökten A, et al. Alveolar echinococcosis in Turkey. Dig Surg (2002) 20:301–5. doi:10.1159/000071695
19. Gündoğdu C, Arslan R, Arslan MÖ, Gıcık Y. Evaluation of cystic and alveolar echinococcosis cases in people in Erzurum and surrounding cities. Acta Parasitol Turcica (2005) 29:163–6. (In Turkish, with English abstract.).
20. Geramizadeh B, Nikeghbalian S, Malekhosseini SA. Alveolar echinococcosis of the liver: report of three cases from different geographic areas of Iran. Hepat Mon (2012) 12:6143. doi:10.5812/hepatmon.6143
23. Beiromvand M, Akhlaghi L, Massom SHF, Mobedi I, Meamar AR, Oormazdi H, et al. Detection of Echinococcus multilocularis in carnivores in Razavi Khorasan Province, Iran using mitochondrial DNA. PLoS Negl Trop Dis (2011) 5:1379. doi:10.1371/journal.pntd.0001379
24. Benyan AKZ, Mahdi NAK, Amir FA, Ubaid O. Second reported case of multilocular hydatid disease in Iraq. Qatar Med J (2013) 1:28–9. doi:10.5339/qmj.2013.5
25. Poulle ML, Bastien M, Richard Y, Josse-Dupuis E, Aubert D, Villena I, et al. Detection of Echinococcus multilocularis and other foodborne parasites in fox, cat and dog faeces collected in kitchen gardens in a highly endemic area for alveolar echinococcus. Parasite (2017) 24:29. doi:10.1051/parasite/2017031
Keywords: alveolar echinococcus, echinococcus multilocularis, fox, feces, Turkey
Citation: Gürler AT, Gori F, Bölükbas¸ CS, Umur Ş, Açıcı M and Deplazes P (2018) Investigation of Echinococcus multilocularis in Environmental Definitive Host Feces in the Asian and the European Parts of Turkey. Front. Vet. Sci. 5:48. doi: 10.3389/fvets.2018.00048
Received: 17 November 2017; Accepted: 26 February 2018;
Published: 15 March 2018
Edited by:
David Modrý, University of Veterinary and Pharmaceutical Sciences Brno, CzechiaReviewed by:
Gad Baneth, Hebrew University of Jerusalem, IsraelCopyright: © 2018 Gürler, Gori, Bölükbaş, Umur, Açıcı and Deplazes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Tümay Gürler, dGd1cmxlckBvbXUuZWR1LnRy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.