- 1Chubu University Academy of Emerging Sciences, Kasugai-shi, Japan
- 2Wildlife Research Center, Kyoto University, Sakyo, Kyoto, Japan
- 3Japan Monkey Centre, Inuyama, Japan
- 4Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia
- 5Sabah Wildlife Department, Wisma Muis, Kota Kinabalu, Malaysia
- 6School of Sociology and Anthropology Department, Sun Yat-sen University, Guang Zhou, China
- 7Singapore Zoo, Wildlife Reserve Singapore, Singapore, Singapore
- 8Zoorasia, Yohohama Zoological Gardens, Yokohama, Japan
- 9Department of Animal Science and Resources, Nihon University, Kameino, Fujisawa, Japan
- 10ETH Zurich, Institute of Agricultural Sciences, Zurich, Switzerland
- 11Clinic for Zoo Animals, Exotic Pets and Wildlife, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland
Understanding the natural diet of species may provide useful information that can contribute to successful captive maintenance. A common problem experienced with captive foregut-fermenting primate (colobine) diets is that they are deficient in fiber and therefore highly digestible. This may contribute to gastrointestinal disorders often observed in zoos. An approach to obtain information relevant for the improvement of diets is to compare the nutrient composition of feces from free-ranging and captive individuals. In theory, fecal material can be considered a proxy for diet intake integrated over a certain period of time. We collected fecal samples from eight free-ranging proboscis monkey (Nasalis larvatus, a highly endangered colobine species) groups from a secondary forest along the Kinabatangan River and four from a mixed mangrove-riverine forest along the Garama River, Sabah, Borneo, Malaysia. We also collected fecal samples from 12 individual captive adult/sub-adult proboscis monkeys from three different zoos. We confirmed that feces from free-ranging monkeys contained more fiber and less metabolic fecal nitrogen than those from captive specimens, indicating a less digestible diet in the wild. Modifying the diets of captive colobines to include more fiber, comparable to those of free-ranging ones, may contribute to their health and survival.
Introduction
Today, habitat destruction and poaching threaten nearly half of the world’s free-ranging primate species with extinction (1). Hence, conservation programs have become integral aspects of zoological management. An important issue in ex situ animal management is to determine the nutritional requirements of animals, to ensure that an appropriate diet is made available, and to facilitate their breeding (2). Foregut-fermenting primates, i.e., colobines, were historically difficult to maintain healthy in captivity, and they had shorter lifespans compared to free-ranging individuals (3–5). Free-ranging wild colobine monkeys are highly folivorous (6, 7); however, in captivity they have often been fed diets similar to those fed to frugivorous and/or omnivorous primates [e.g., Ref. (5, 8, 9)], which may lead to gastrointestinal disorders probably due to a less fibrous or too well-digestible diet (10–12), given that commercial fruits typically have high nutrient density compared to wild fruits (13–15).
Proboscis monkeys (Nasalis larvatus), endangered and endemic to Borneo, are the largest foregut-fermenting colobines. They consume leaves, fruits, and flowers in various proportions, although leaves generally dominate their diet [representing 38–92% of their diets: (16–20)]. Even compared to other colobines, these monkeys are notoriously difficult to maintain and breed in captivity, with the only notable successful long-term husbandry (1998–present) being at the Wildlife Reserves Singapore (21). Several other attempts to breed them have been made at zoos in non-tropical regions (5, 10, 22), and Yokohama Zoo, Japan, is the only non-tropical zoo that currently holds the species [2009–present: (23)].
To obtain information relevant for the improvement of diets of captive proboscis monkeys, one approach is to compare the nutrient composition of feces in free-ranging and captive individuals. To our knowledge, this approach has not yet been undertaken in primates, although in theory, it should be applicable to primates based on the accumulation of considerable knowledge regarding such nutrient analyses derived mostly from studies on grazing ruminant livestock (24). A notable exception is a study by Chapman et al. (25), that compared fecal nitrogen content (but not other nutrients) of free-ranging and captive colobines, suggesting that quantifying fecal nitrogen levels may be useful for assessing their habitat quality. However, their study did not differentiate fecal nitrogen derived from indigestible plant protein [neutral detergent fiber (NDF)-bound protein: (26)] and metabolic fecal nitrogen (MFN), a distinction of particular relevance in browsing animals (27). MFN consists predominantly of microbial nitrogen that is derived either from the degradation of plant protein or the incorporation of endogenous proteins (e.g., digestive enzyme residues) into microbial matter.
Here, we compared the fecal nutrient concentrations of free-ranging and captive proboscis monkeys and hypothesized that more fiber and lower levels of MFN would be found in the free-ranging specimens. A higher fiber content of the feces would result either from a higher proportion of fiber in the diet or from a lower digestibility/higher lignification of the fiber, both consistent with a lower energy density and digestibility of the diet. Without knowing the quantity and composition of food consumed and the quantity of feces defecated, fecal composition alone cannot be considered conclusive evidence for the composition and digestibility of a diet, because theoretically, different combinations of diet nutrient composition, intake, and digestibility can lead to the same fecal nutrient composition. Nevertheless, the use of fecal nitrogen [total fecal nitrogen (TFN)] as an indicator of diet quality has a long-standing tradition. A traditional view in herbivore ecology is that TFN can serve as a proxy for the protein content of the ingested diet. However, fecal protein levels represent, to a large proportion, microbial protein, and processes resulting in microbial growth do not directly reflect dietary protein levels, but rather overall diet digestibility (to which dietary protein levels are only one of many contributory factors); therefore, TFN should rather be considered as a proxy for the overall diet digestibility (28–30). In folivorous species that ingest a substantial amount of plant secondary compounds, TFN is compromised as an indicator by high proportions of indigestible N in the diet, and therefore, MFN is considered a better proxy (27).
Materials and Methods
Fecal samples were collected from eight different free-ranging proboscis monkey groups between June and July 2015—four from monkeys inhabiting a secondary forest along the Kinabatangan River (118°30′E, 5°30′N) and four from monkeys present in a mixed mangrove-riverine forest along the Garama River (115°30′E, 5°21′N), Sabah, Malaysia. The samples were collected in the early morning (06:00–09:00 h) after the group left their sleeping trees (located the previous evening); all samples from within a group were pooled at the same point in time. Only fecal samples presumed to be (based on sample size) from adult individuals (31) were selected. Individual fecal samples were collected from 12 adult/sub-adult proboscis monkeys at three different zoos—four (male, one and females, three) from the Singapore Zoo (Singapore) in April 2014, four (males, three and female, one) from Lok Kawi Wildlife Park (Sabah, Malaysia) in July 2015 and four (males, two and females, two) from Yokohama Zoological Gardens Zoorasia (Yokohama, Japan) in September 2015. Several defecations of one individual were pooled until a sufficient sample volume for nutrient analysis was obtained (dry weight, ~15 g).
All fecal samples were sealed in plastic bags and stored at −20°C until oven-dried at 60°C for 60 h in the laboratory in state of Sabah, Malaysia, Singapore, and Japan, respectively. The dried samples were then milled and analyzed for total ash (TA), nitrogen/crude protein (CP), NDF, acid detergent fiber (ADF), acid detergent lignin (ADL), and acid-insoluble ash (AIA) using standard methods (32). Detergent fiber data are presented without residual ash. The MFN content of feces was calculated as TFN—undigested N from the diet quantified by analyzing the N content of the NDF fraction (NDF-N). Only TA, CP, and NDF were analyzed in Singapore Zoo samples.
Data were tested for normality (Kolmogorov–Smirnov test), followed by parametric t-tests for variables that were normally distributed, or nonparametric Mann–Whitney U-tests for variables that were not normally distributed, using SPSS (SPSS 23.0, IBM, Armonk, New York, NY, USA) to compare the fecal nutritional measurement between free-ranging and captive populations. Additionally, nonparametric correlations were tested using Spearman’s rank coefficient. The significance level was set at 0.05.
All research was conducted in compliance with guidelines for care and use of non-human primates by the Japan Monkey Centre and applicable Japan, Malaysian and Singaporean laws.
Results
There were significant differences in all fecal fiber, nitrogen, and TA contents between free-ranging and captive proboscis monkeys (Table 1). Generally, the levels of fiber, TFN, and NDF-N were higher in the feces of free-ranging animals. In contrast, MFN and TA were significantly higher in the feces of captive individuals. Although higher AIA levels were observed in the feces of captive individuals, differences were not significant; the SD for AIA was very high for captive individuals. When plotting fecal NDF-N against fecal NDF, there was a significant correlation in the data of captive monkeys (R = 0.71, P = 0.047, n = 8) but no correlation in the data of free-ranging specimens (R = −0.24, P = 0.570, n = 8) (Figure 1).
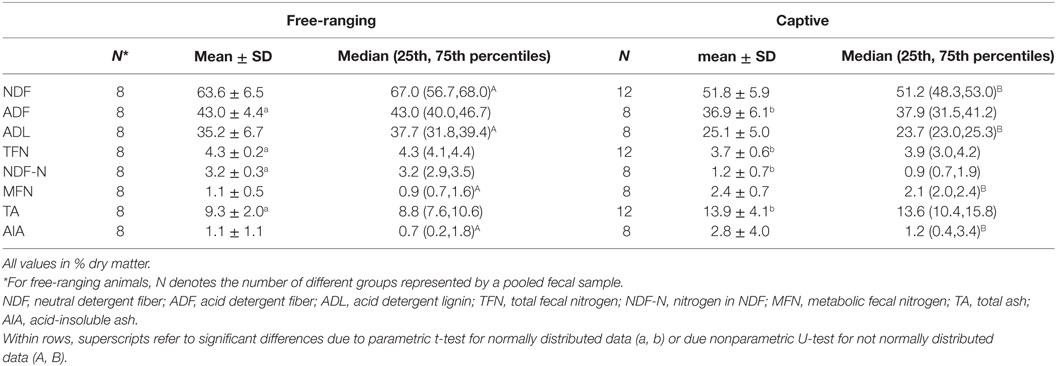
Table 1. Mean ± SD, median (25th, 75th percentiles) with number of observation of the contents of different constituents (% dry matter) in feces from free-ranging proboscis monkey groups (Nasalis larvatus) and captive proboscis monkey individuals.
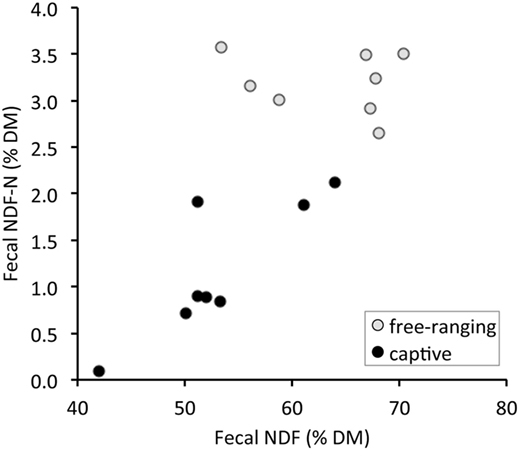
Figure 1. Relationship between total fecal nitrogen bound to neutral detergent fiber (NDF-N) and the concentration of neutral detergent fiber (NDF) in the feces of proboscis monkey groups (Nasalis larvatus) and captive proboscis monkey individuals.
Discussion
To determine an appropriate diet for captive animals, the nutrient composition of the diets of captive and free-ranging individuals is typically compared (14, 15, 33). However, this approach requires the sampling and analysis of a large number of food items in the wild, coupled with observations of the respective feeding frequency and quantity consumed to determine their overall dietary contribution (2). In contrast, fecal material represents an integrated sample over a certain period of diet intake, is easier to obtain, and requires fewer samples. In terms of nutritional information, a comparison of nutrient contents, particularly fiber, may be of more immediate relevance to the design of diets than alternative measurements like microbiome composition or hormone levels (34–37).
We confirmed the prediction that the feces from free-ranging monkey groups contained more fiber (higher NDF, ADF, ADL) and less MFN, suggesting a lower diet digestibility than those of captive individuals. Although in theory, different combinations of dietary fiber levels, amounts of food intake, and fecal excretion can lead to the same fecal nutrient concentrations, this theoretical range of possibilities is in reality confined by the fact that across a broad range of dietary fiber levels, higher fiber levels are typically associated with lower digestibility (38, 39), also in colobine monkeys (11). The results therefore indicate that free-ranging monkeys consume food items of lower digestibility than do captive monkeys. Although free-ranging proboscis monkeys carefully select leaves containing less fiber and more protein with higher in vitro digestibility (40–42), the nutritive quality of commercial fruits and vegetables fed to captive individuals is higher than that of the foods accessible to the free-ranging monkeys (14, 15, 33). Higher NDF levels in the feces of captive animals might be achieved by feeding more browse, concomitantly leading to higher NDF-N, as evident in Figure 1. In contrast, in free-ranging individuals whose diet exclusively comprises wild leaves and fruits, no comparable relationship between these two measures (TFN vs. NDF-N) was observed (Figure 1) because the diet items selected by free-ranging animals most likely varied in their NDF-N contents at concomitantly high NDF contents (26). This also supports the presence of differences in nutritional characteristics of the diets between free-ranging and captive individuals.
Previous studies have demonstrated that the production of well-shaped (healthy) solid feces in captive colobines requires an appropriate dietary intake of fiber (e.g., NDF) (10, 43). We suggest that modifying captive colobine diets so that the fiber intake is more similar to that of free-ranging individuals, may putatively enhance their health and survival in captivity. The captive proboscis monkeys in our study had fecal NDF contents (42–64% in dry matter) that were lower than those of free-ranging conspecifics (53–70%), but still far higher than those reported for other captive colobines—proboscis monkeys, 17% [mean of two different values: (22)]; Javan langurs (Trachypithecus auratus), 37% [mean of six different values: (10)], François langur (T. françoisi), 31% [mean of three different values: (43)] and 28–44%, Black-and-white colobus monkey (Colobus guereza), 28–51%, Northern douc langur (Pygathris nemaeus), 34–49% [based on experiments with a low-fiber and a high-fiber pelleted food: calculated from Ref. (44)]. This difference is most likely due to the feeding regime, which includes a higher proportion of browse than reported for other colobines (21). The experiments of Edwards and Ullrey (44) demonstrate that including high levels of fiber in the pelleted food compound is a factor that can contribute to achieve fecal fiber levels closer to free-ranging conditions than traditional, low-fiber primate pellets. Although differences in fecal fiber levels are likely to occur within a species, due to factors related to habitat, season, sex or reproductive status, the general magnitude of differences can serve as a convenient proxy of the appropriateness of any particular diet in captivity. Long-term feeding trials will be necessary to test whether more fibrous foods can truly reinforce the health and reproductive success in captive proboscis monkeys.
The diets of captive browsing ungulates are thought to contain higher AIA than those in the wild (45, 46). We observed a similar but not significant trend (given the large SD) in the feces of captive monkeys. However, because AIA is related to animal tooth wear affecting body condition, reproductive success and longevity in ungulates (47, 48), this trend might be worth considering for captive colobines, in general, for future studies. Because neither browse nor fruits and vegetables contain significant amounts of AIA, the most likely source of high AIA levels are compound feeds (45). Controlling AIA in such feeds may be a relevant future objective in the manufacturing of zoo foods for non-grazing species.
This study may shed light on the establishment of a constructive in situ and ex situ collaborative link to aid the management of dietary husbandry for captive colobines and possibly to provide necessary impetus for conservation and education initiatives, which will be beneficial for their long-term conservation. Further comparisons of fecal nutrient levels in other colobine species will be useful to establish targets for group- or species-specific fiber (and potentially other nutrients and minerals) supplementation.
Conclusion
Lower fecal fiber contents in captive foregut-fermenting proboscis monkeys than those of free-ranging conspecifics were found, but they were still far higher than those reported in the literature for other captive foregut-fermenting primates. In addition, the feces of free-ranging proboscis monkey groups contained less MFN, indicating that the free-ranging proboscis monkeys consumed foods of lower digestibility compared to captive ones. To reduce the occurrence of gastrointestinal disorders and enhance health and survival, it may be recommendable to alter diets of captive animal diets to closer replicate fecal fiber levels found in free-ranging specimens.
Author Contributions
IM and MC conceptualized the initial idea; IM, HB, IO, and SS performed the fecal sampling; AT, SKSSN, JCMS, RS, AW, and DARS arranged the sampling in the wild/zoo; IM, SA, and MK performed the nutritional analyses, IM and MC performed and interpreted the statistical analysis; IM, MC, and MK drafted the manuscript; and IM organized the projects. All authors contributed to the final version of the manuscript.
Conflict of Interest Statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
We thank the Sabah Biodiversity Center, the Sabah Wildlife Department, the Sabah Forestry Department, the Singapore Zoo, the Yokohama Zoo, the Lok Kawi Wildlife Park, MARDI Kota Kinabalu, and our colleagues for their assistance, especially S. Luz, A. Masis, Asnih, Jasrudy, and S. W. Chung.
Funding
This study was mainly supported by the Hibi Science Fundation (to IM) and partially funded by the JSPS KAKENHI (#21770261 to IM) and Pro Natura Foundation Japan (to HB).
References
1. Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, et al. Impending extinction crisis of the world’s primates: why primates matter. Sci Adv (2017) 3:e1600946. doi:10.1126/sciadv.1600946
2. Crissey SD, Pribyl LS. Utilizing wild foraging ecology information to provide captive primates with an appropriate diet. Proc Nutr Soc (2007) 56:1083–94. doi:10.1079/PNS19970112
3. Hill O. The maintenance of langurs (Colobinae) in captivity; experiences and some suggestions. Folia Primatol (1964) 2:222–31. doi:10.1159/000155018
4. Collins L, Roberts M. Arboreal folivores in captivity-maintenance of a delicate minority. In: Montgomery GG, editor. The Ecology of Arboreal Folivores. Washington, DC: Smithsonian Institution (1978). p. 5–12.
5. Hollihn UWE. Remarks on the breeding and maintenance of Colobus monkeys Colobus guereza, Proboscis monkeys Nasalis larvatus and douc langurs Pygathrix nemaeus in zoos. Int Zoo Yearbook (1973) 13:185–8. doi:10.1111/j.1748-1090.1973.tb02146.x
6. Fashing PJ. African colobine monkeys: their behavior, ecology, and conservation. 2nd ed. In: Campbell CJ, Fuentes A, Mackinnon KC, Bearder SK, Stumpf RM, editors. Primates in Perspective. Oxford: Oxford University Press (2011). p. 203–29.
7. Kirkpatrick RC. The Asian colobines: diversity among leaf-eating monkeys. 2nd ed. In: Campbell CJ, Fuentes A, Mackinnon KC, Bearder SK, Stumpf RM, editors. Primates in Perspective. Oxford: Oxford University Press (2011). p. 189–202.
8. Watkins BE, Ullrey DE, Whetter PA. Digestibility of a high-fiber biscuit-based diet by black and white colobus (Colobus guereza). Am J Primatol (1985) 9:137–44. doi:10.1002/ajp.1350090207
9. Edwards MS, Crissey SD, Oftedal OT. Leaf-eating primates: nutrition and dietary husbandry. NAG Handbook Fact Sheet 007 (1997).
10. Hollihn KU. Das Verhalten von Guerezas (Colobus guereza und Colobus polykomos), Nasenaffen (Nasalis larvatus) und Kleideraffen (Pygathrix nemaeus) bei der Nahrungsaufnahme und ihre Haltung. Mammalian Biol (1971) 36:65–95.
11. Nijboer J, Clauss M. The digestive physiology of colobine primates. In: Nijboer J, editor. Fibre Intake and Faeces Quality in Leaf-Eating Monkeys (2006). PhD thesis, University of Utrecht, The Netherlands, 9–28.
12. Clauss M, Dierenfeld ES. The nutrition of browsers. 6 ed. In: Fowler ME, Miller RE, editors. Zoo and Wild Animal Medicine: Current Therapy. St. Louis: Saunders Elsevier (2008). p. 444–54.
13. Nijboer J, Dierenfeld ES. Comparison of diets fed to southeast Asian colobines in North American and European zoos, with emphasis on temperate browse composition. Zoo Biol (1996) 15:499–507. doi:10.1002/(SICI)1098-2361(1996)15:5<499::AID-ZOO6>3.0.CO;2-6
14. NRC. Nutrient Requirements of Nonhuman Primates, Second Revised Edition. Washington, DC: National Academies Press (2003).
15. Schwitzer C, Polowinsky SY, Solman C. Fruits as foods – common misconceptions about frugivory. In: Clauss M, Fidgett AL, Hatt JM, Huisman T, Hummel J, Nijboer J, Plowman A, editors. Zoo Animal Nutrition IV. Fürth: Filander Verlag (2009). p. 131–68.
16. Bennett EL, Sebastian AC. Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. Int J Primatol (1988) 9:233–55. doi:10.1007/BF02737402
17. Yeager CP. Feeding ecology of the proboscis monkey (Nasalis larvatus). Int J Primatol (1989) 10:497–530. doi:10.1007/BF02739363
18. Boonratana R. Feeding ecology of proboscis monkeys (Nasalis larvatus) in the Lower Kinabatangan, Sabah, Malaysia. Sabah Parks Nat J (2003) 6:1–6.
19. Matsuda I, Tuuga A, Higashi S. The feeding ecology and activity budget of proboscis monkeys. Am J Primatol (2009) 71:478–92. doi:10.1002/ajp.20677
20. Bernard H, Matsuda I, Hanya G, Phua M-H, Oram F, Ahmad AH. Feeding ecology of the proboscis monkey in Sabah, Malaysia, with special reference to plant species-poor forests. In: Barnett AA, Matsuda I, Nowak K, editors. Primates in Flooded Habitats: Ecology and Conservation. Cambridge: Cambridge University Press (2018).
21. Sha JC, Alagappasamy S, Chandran S, Cho KM, Guha B. Establishment of a captive all-male group of proboscis monkey (Nasalis larvatus) at the Singapore Zoo. Zoo Biol (2012) 32(3):281–90. doi:10.1002/zoo.21020
22. Dierenfeld ES, Koontz FW, Goldstein RS. Feed intake, digestion and passage of the proboscis monkey (Nasalis larvatus) in captivity. Primates (1992) 33:399–405. doi:10.1007/BF02381201
23. Inoue E, Ogata M, Seino S, Matsuda I. Sex identification and efficient microsatellite genotyping using fecal DNA in proboscis monkeys (Nasalis larvatus). Mammal Study (2016) 41:141–8. doi:10.3106/041.041.0304
24. Van Soest PJ. Nutritional Ecology of the Ruminant. Ithaca, United States: Comstock Publishing Associates (1994).
25. Chapman CA, Webb T, Fronstin R, Wasserman MD, Santamaria AM. Assessing dietary protein of colobus monkeys through faecal sample analysis: a tool to evaluate habitat quality. Afr J Ecol (2005) 43:276–8. doi:10.1111/j.1365-2028.2005.00575.x
26. Rothman JM, Chapman CA, Pell AN. Fiber-bound nitrogen in gorilla diets: implications for estimating dietary protein intake of primates. Am J Primatol (2008) 70:690–4. doi:10.1002/ajp.20540
27. Steuer P, Südekum K-H, Tütken T, Müller DWH, Kaandorp J, Bucher M, et al. Does body mass convey a digestive advantage for large herbivores? Funct Ecol (2014) 28:1127–34. doi:10.1111/1365-2435.12275
28. Lukas M, Südekum KH, Rave G, Friedel K, Susenbeth A. Relationship between fecal crude protein concentration and diet organic matter digestibility in cattle. J Anim Sci (2005) 83:1332. doi:10.2527/2005.8361332x
29. Wang CJ, Tas BM, Glindemann T, Rave G, Schmidt L, Weißbach F, et al. Fecal crude protein content as an estimate for the digestibility of forage in grazing sheep. Anim Feed Sci Technol (2009) 149:199–208. doi:10.1016/j.anifeedsci.2008.06.005
30. Gálvez-Cerón A, Gassó D, López-Olvera JR, Mentaberre G, Bartolomé J, Marco I, et al. Gastrointestinal nematodes and dietary fibre: two factors to consider when using FN for wildlife nutrition monitoring. Ecol Indic (2015) 52:161–9. doi:10.1016/j.ecolind.2014.11.020
31. Matsuda I, Tuuga A, Hashimoto C, Bernard H, Yamagiwa J, Fritz J, et al. Faecal particle size in free-ranging primates supports a ’rumination’ strategy in the proboscis monkey (Nasalis larvatus). Oecologia (2014) 174:1127–37. doi:10.1007/s00442-013-2863-9
32. AOAC. Official Methods of Analysis of AOAC International. Gaithersburg, MD: Association of Official Analytical Chemists International (2012).
33. Nijboer J, Clauss M, Olsthoorn M, Noordermeer W, Huisman TR, Verheyen C, et al. Effect of diet on the feces quality in javan langur (Trachypithecus auratus auratus). J Zoo Wildlife Med (2006) 37:366–72. doi:10.1638/05-113.1
34. Fujita S, Kageyama T. Polymerase chain reaction detection of Clostridium perfringens in feces from captive and wild chimpanzees, Pan troglodytes. J Med Primatol (2007) 36:25–32. doi:10.1111/j.1600-0684.2006.00191.x
35. Rangel-Negrín A, Alfaro JL, Valdez RA, Romano MC, Serio-Silva JC. Stress in Yucatan spider monkeys: effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim Conserv (2009) 12:496–502. doi:10.1111/j.1469-1795.2009.00280.x
36. Amato KR, Metcalf JL, Song SJ, Hale VL, Clayton J, Ackermann G, et al. Using the gut microbiota as a novel tool for examining colobine primate GI health. Global Ecol Conserv (2016) 7:225–37. doi:10.1016/j.gecco.2016.06.004
37. Clayton JB, Vangay P, Huang H, Ward T, Hillmann BM, Al-Ghalith GA, et al. Captivity humanizes the primate microbiome. Proc Natl Acad Sci U S A (2016) 113:10376–81. doi:10.1073/pnas.1521835113
38. Demment MW, Van Soest PJ. A nutritional explanation for body size patterns of ruminant and nonruminant herbivores. Am Nat (1985) 125:641–72. doi:10.1086/284369
39. Hagen KB, Tschudin A, Liesegang A, Hatt J-M, Clauss M. Organic matter and macromineral digestibility in domestic rabbits (Oryctolagus cuniculus) as compared to other hindgut fermenters. J Anim Physiol Anim Nutr (2015) 99:1197–209. doi:10.1111/jpn.12323
40. Yeager CP, Silver SC, Dierenfeld ES. Mineral and phytochemical influences on foliage selection by the proboscis monkey (Nasalis larvatus). Am J Primatol (1997) 41:117–28. doi:10.1002/(SICI)1098-2345(1997)41:2<117::AID-AJP4>3.0.CO;2-#
41. Matsuda I, Tuuga A, Bernard H, Sugau J, Hanya G. Leaf selection by two Bornean colobine monkeys in relation to plant chemistry and abundance. Sci Rep (2013) 3:1873. doi:10.1038/srep01873
42. Matsuda I, Clauss M, Tuuga A, Sugau J, Hanya G, Yumoto T, et al. Factors affecting leaf selection by foregut-fermenting proboscis monkeys: new insight from in vitro digestibility and toughness of leaves. Sci Rep (2017) 7:42774. doi:10.1038/srep42774
43. Nijboer J, Becher F, Van Der Kuilen J, Beynen AC. Chemical analysis and consistency of faeces produced by captive monkeys (Francois langurs, Trachypithecus francoisi) fed supplemental fibre. Vet Q (2001) 23:76–80. doi:10.1080/01652176.2001.9695086
44. Edwards MS, Ullrey DE. Effect of dietary fiber concentration on apparent digestibility and digesta passage in non-human primates. II. Hindgut- and foregut-fermenting folivores. Zoo Biol (1999) 18:537–49. doi:10.1002/(SICI)1098-2361(1999)18:6<537::AID-ZOO8>3.0.CO;2-F
45. Clauss M, Franz-Odendaal TA, Brasch J, Castell JC, Kaiser T. Tooth wear in captive giraffes (Giraffa camelopardalis): mesowear analysis classifies free-ranging specimens as browsers but captive ones as grazers. J Zoo Wildlife Med (2007) 38:433–45. doi:10.1638/06-032.1
46. Taylor LA, Müller DWH, Schwitzer C, Kaiser TM, Codron D, Schulz E, et al. Tooth wear in captive rhinos differs from that of free-ranging conspecifics. Contrib Zool (2014) 83:107–17. doi:10.5167/uzh-94211
47. Ozaki M, Kaji K, Matsuda N, Ochiai K, Asada M, Ohba T, et al. The relationship between food habits, molar wear and life expectancy in wild sika deer populations. J Zool (2010) 280:202–12. doi:10.1111/j.1469-7998.2009.00653.x
Keywords: colobine, fecal nutrient, captivity, folivore, foregut fermentation
Citation: Matsuda I, Bernard H, Tuuga A, Nathan SKSS, Sha JCM, Osman I, Sipangkui R, Seino S, Asano S, Wong A, Kreuzer M, Ramirez Saldivar DA and Clauss M (2018) Fecal Nutrients Suggest Diets of Higher Fiber Levels in Free-Ranging than in Captive Proboscis Monkeys (Nasalis larvatus). Front. Vet. Sci. 4:246. doi: 10.3389/fvets.2017.00246
Received: 11 November 2017; Accepted: 22 December 2017;
Published: 19 January 2018
Edited by:
Pietro Celi, DSM, United StatesReviewed by:
Barry Bradford, Kansas State University, United StatesMegan A. McCrory, Boston University, United States
Copyright: © 2018 Matsuda, Bernard, Tuuga, Nathan, Sha, Osman, Sipangkui, Seino, Asano, Wong, Kreuzer, Ramirez Saldivar and Clauss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ikki Matsuda, aWtraS1tYXRzdWRhQGlzYy5jaHVidS5hYy5qcA==, aWtraS5tYXRzdWRhQGdtYWlsLmNvbQ==
 Ikki Matsuda
Ikki Matsuda Henry Bernard
Henry Bernard Augustine Tuuga5
Augustine Tuuga5 John C. M. Sha
John C. M. Sha Rosa Sipangkui
Rosa Sipangkui Michael Kreuzer
Michael Kreuzer Marcus Clauss
Marcus Clauss