Bioequivalence Study of Two Long-Acting Formulations of Oxytetracycline Following Intramuscular Administration in Bovines
- Faculty of Veterinary Medicine, Department of Pharmacology and Toxicology, Ankara University, Ankara, Turkey
The pharmacokinetics of conventional and long-acting (LA) oxytetracycline (OTC), widely used broad-spectrum antibacterial drugs in veterinary medicine, were evaluated in Kilis goats at single dosage of 20 mg/kg body weight (bw). A total of 21 goats were divided into three groups: intravenous (Group I) and intramuscular (IM) (Group II) administration of the conventional formulation and IM administration of the LA formulation (Group III). Blood samples were taken at 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, and 96 h; and OTC analysis was performed by HPLC. For Group III and Group II, time to reach maximal plasma drug concentration (Tmax) was 0.6 ± 0.28 and 0.46 ± 0.09 h and maximal plasma drug concentrations (Cmax) were 8.72 ± 2.47 μg/ml and 13.57 ± 5.83 μg/ml, respectively. In Group I, C0 concentration was found to be 63.51 ± 11.59 μg/ml. The elimination times (T1/2) were 10.84 ± 3.20, 27.96 ± 11.66, and 10.47 ± 1.30 h; and AUC were 115 ± 29.12, 96.44 ± 9.49, and 80.86 ± 12.76 μg/ml/h for Group I; Group II, and Group III, respectively. Bioavailability by IM administration were 69.71% for the conventional OTC and 83.15% for the LA OTC.
Introduction
Kilis Goat is a hybrid of hair goat and goat of Aleppo. The hair is usually black, but can also be brown, tan, gray, or tawny. As a trait, they are large with a long body; where the females have a developed mammary gland along with good dairy performance (1), corresponding to the highest milk yield among the other domestic breeds. Production of the Kilis Goats is officially carried out in Gaziantep, Hatay, and particularly in Kilis. It is estimated that their number is between 60,000 and 70,000 (2, 3).
Oxytetracycline (OTC) is a broad-spectrum antibiotic, frequently used in veterinary medicine for gastrointestinal and respiratory system diseases, for mainly aerobic microorganisms including Gram-positive/negative bacteria, Rickettsia, Mycoplasma, and Chlamydia species. Long-acting (LA) formulation of the drug is available in different solvent. Those formulations are used considering a dose interval of 3 to 5 days. Furthermore, OTC, as an HCl salt, can be administered by different routes. It can be easily absorbed by the mammalian intestine, while the absorption is limited in poultry. After oral administration in ruminants, OTC loses its effect because of the front gut flora. Following oral administration, the value of t1/2 was previously described as 3.6 h in sheep and goats (4). Therefore, the parenteral route is preferred for the systemic effect (5). OTC, passes into intracellular fluid quickly when administered systemically. The drug can also pass the blood–brain barrier and the placenta as well (6). It accumulates in the internal organs, including liver, kidney, spleen, and the lungs, and also in developing parts of the bones (4). It passes to the eyes, synovial fluid, milk, and eggs (4). OTC is metabolized in the liver, where it is converted into tetracycline and then it is mostly excreted through the bile and urine (7–10). OTC is removed from the body by being filtered by glomerulae of the kidney. However, it remains long in the body when dispersed in excess proportion to the tissues and gets into circulation of the liver–intestine (11–14).
Antimicrobial agents are classified according to their pattern of antimicrobial activity and successful pharmacotherapy should integrate both the pharmacokinetic and the pharmacodynamic properties of antimicrobial agents (e.g., MIC, AUC, PAE). For this purpose, long-term formulations have been developed (4, 15). OTC is delivered via intramuscular (IM) and intravenous (IV) routes to sheep and goats at 2–10 mg/kg as a daily dose. LA preparations are administered to cattle, sheep, and goats at 20 mg/kg for 2–4 day intervals or 30 mg/kg for six daily intervals. LA formulations can provide adequate and well controlled level of drug for the systemic treatment or control for 3–4 days (16–19). These formulations are used in the treatment of acute diseases as well as chronic diseases (20). The aim of this study was to investigate the pharmacokinetic parameters of OTC in Kilis Goats, after IM and IV administration of conventional and LA formulations.
Materials and Methods
Oxytetracycline preparations were used conventional (OTC hydrochloride salt 0.1 g/ml) and LA (OTC dihydrate salt preparations of milliliters of 0.2 g) formulations. Twenty-one Kilis male goats, around 12–13 months and 20–40 kg weight range were used in this study, and the parasitic load was previously tested. The animals were allowed to acclimate for a week before the study. Animals were given access to water ad libitum. Feed (without antibiotics contributes to dry weed, straw, and barley) requirements was met, twice per day. Animals were numbered using spray paint for a clear distinguishment. To perform the pharmacokinetic studies, the animals were divided into three groups of seven animals. Compliance with the principles of operation of the ethics committee was approved by the Ministry of Food Agriculture and Livestock Adana Veterinary Control Institute Ethics Board of the date 22.05.2014 and decision No. 29.
Group I, received 20 mg/kg via vena jugularis by IV route; Group 2 received the same dose by IM route through administration at musculus semitendinosus at 100 mg/ml concentrated formulation. Group 3 received LA formulation at 200 mg/ml at the same dose as 20 mg/kg by IM route.
Blood samples were taken and transferred to Ca EDTA tubes at 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, and 96 h after drug administration. Samples were kept refrigerated until they arrived to the laboratory. Tubes were centrifuged (Hettich U320R) at 5,000 rpm for 15 min on the same day, and the plasma were separated to the labeled plastic tubes. For plasma samples, the method of Chamberlain (21) was adopted for the HPLC analysis of OTC determination. The mobile phase consisted of acetonitrile and oxalic acid (20:80 v/v) and was delivered (Thermo Finnigan Surveyor HPLC) at a flow rate of 0.7 ml/min. A nucleosil C18 analytical column (5 µm, 150 mm × 4.6 mm, Phenomenex synergi Max RP) was used with photo diode array detection at an excitation wavelength of 365 nm.
Pharmacokinetic parameters were calculated using the individual plasma concentration–time curve graphs plotted pharmacokinetic software program (WinNonlin, 5.0, Pharsight, USA). The peak plasma concentration (Cmax) and the time to reach the peak plasma concentration (Tmax) were determined from the concentration–time curve for each animal. The area under the plasma concentration–time curve (AUC) and the area under the first-torque curve was calculated by the trapezoidal method, the average length of stay. OTC bioavailability was calculated after administration intramuscularly calculated AUC and the ratio of the calculated AUC after IV route, using the following formula (%)F = (AUCIM/AUCIV) × 100.
The average pharmacokinetic parameters were evaluated by comparing the differences between OTC IV, OTC LA IM, and OTC IM Groups by Duncan test using a statistically one-way analysis of variance. The average value under p < 0.05 was considered as statistically different.
Results
Retention time were found as 6.4 min; with a limit of detection (LOD) as 0.0125 g/ml and LOD as 0.025 µg/ml. Average recovery rate was 91.12%. The average plotted regression line for OTC concentration standards were calculated from 0.0125, 0.025, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8, 25, and 50 µg/ml concentrations with correlation coefficient as r2 = 0.9964 using y = 49,239x formula.
Kinetic parameters are shown in Table 1 for Groups 1, 2, and 3.
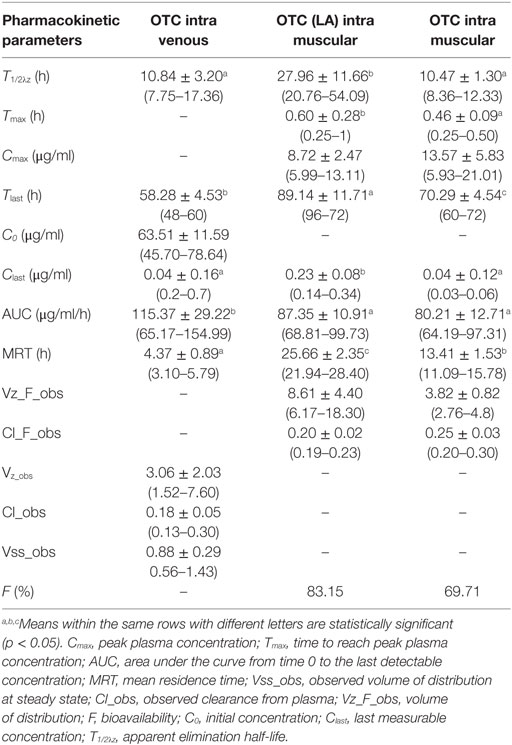
Table 1. Pharmacokinetic parameters of OTC in Kilis Goat following administration of conventional and long-acting formulations at a single dose of 20 mg/kg.
Oxytetracycline LA (IM) compared to the OTC conventional formulations (IM and IV) showed statistically significant differences (p < 0.05) (Table 1). OTC goats in Group 2 (LA)’s half-life (T1/2: 27.96 ± 11.66 h) were significantly longer than the other Groups (p < 0.05). In terms of half-life, OTC IV (10.84 ± 3:20 h) and IM (10.47 ± 1.30 h) applications did not show any significant differences statistically (Table 1). Peak plasma concentration (Cmax) in goats treated with OTC via the IM route (13.57 ± 5.83 μg/ml) and OTC (LA), IM (8.72 ± 2.47 μg/ml) was shown in Table 1. The Tmax was higher (p < 0.05) in Group 2 compared to other two Groups (Group 1 Tmax: 0.28 ± 0.09 h; Group 3 Tmax: 0.46 ± 0.09 h) (Table 1). The latest time that drug was determined plasma was (Tlast) 58.28 ± 4.53 h in Group 1, 89.14 ± 11.71 h in Group 2, and 70.29 ± 4.54 h in Group 3. OTC concentration in plasma stayed the longest for Group 3 application (Table 1). The area under the curve (AUC) was much higher (p < 0.05) in Group 1 compared to Group 2 and 3 (Table 1). Bioavailability of OTC LA (IM route) and conventional OTC (IM route) at 20 mg/kg dose were 83.15 and 69.71% (Table 1). The plasma OTC concentrations of some animals were not been able to be determined (one animal for OTC IV at 12th hour; and two for IM applications; 12th and 8th hour). Following 48th hour of OTC LA IM administration, no drug was detected in the plasma.
Plasma concentrations of OTC and OTC LA is given in Figure 1.
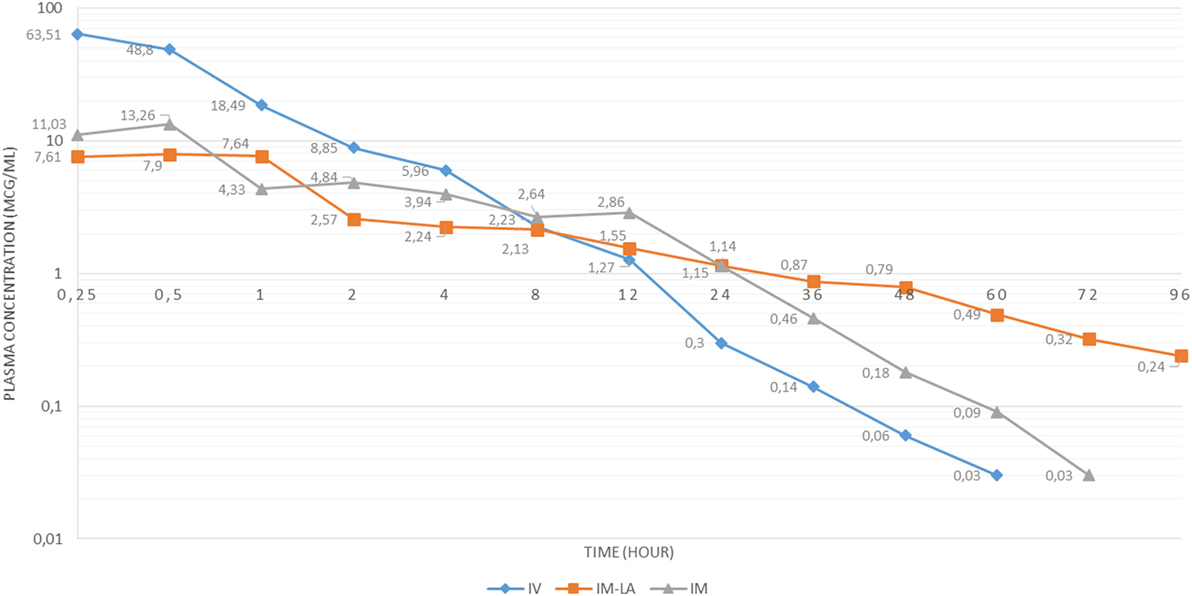
Figure 1. Plasma concentration-time curve for oxytetracycline (OTC) intravenous (IV), OTC long-acting (LA) intramuscular (IM), and OTC IM following administration of 20 mg/kg dose in Kilis Goat.
Discussion
Plasma concentrations at different time points following a conventional OTC formulation via IV route were homogeneous. The results of conventional OTC IV administered goats showed similar values with Kaya et al. (15) study of sheep administered by conventional OTC IV.
In a pharmacokinetic study with goats that is given 10 mg/kg dose IV, the effective plasma concentration of the conventional oxytetracycline were reached at fourth hour and the Co value was measured as 34.50 ± 1.65 μg/ml. In the study by Mandal et al. (22), half-life (t1/2β) were found as 1.11 ± 0.05 h, with area under the curve (AUC) 27.69 ± 3.01 μg/ml/h, Vc 0.28 ± 0.03 l/kg, Vdarea 0.59 ± 0.06 l/kg, steady state volume of distribution Vdss 0.51 ± 0.05 l/kg, body clearance Cl 369.67 ± 35.21 ml/kg/h. In the Nubian goats after 5 mg/kg dose of conventional OTC IV administration, the pharmacokinetic parameters were calculated as the half-life t1/2β 3.99 h, the area under the curve AUC 8.23 ± 0.08 μg/ml/h, Vdss 3088 ± 491.50 ml/kg, and Cltotal 608.18 ± 5.96 ml/kg/h (23). Elsheikh et al. (23) and Mandal et al. (22) indicated that the possible reason for the differences between pharmacokinetic parameters were the difference in the application concentrations. The correlation between the concentration and the kinetic parameters are seen in these studies along with our study.
In a study carried out on camels, sheep, and goats; OTC was administered at 5 mg/kg IV, where the calculated kinetic parameters, respectively, are, t1/2β, 2.8, 3.4, and 3.2 h, Cmax 10.2, 850, and 780 µg/ml, Vdarea 1.41, 13.4, and 12.1 l/kg. t1/2 and Vd values for goat and sheep were similar (24). In another study, OTC at 5 mg/kg IV resulted with t1/2β as 3.89 and 6.30 h for Nubian goats and desert sheep, AUC 12.08 ± 1.50 and 18.37 ± 1.68 μg/ml/h, Vdarea 2.53 ± 0.29 and 2.67 ± 0.39 L/kg, CI 436.99 ± 47.94 and 281.31 ± 25.01 ml/kg/h, respectively. Pharmacokinetic differences of OTC for sheep and goat are thought to be caused by protein bindings and changes in the renal extractions (25). The differences in terms of kinetic parameters with studies of Al-Nazawi (24) and Elsheikh et al. (25) are related to the applied concentration differences; while a correlation is evident.
In the study by Payne et al. (18), OTC LA was administered to goats by IV route (a single dose of 20 mg/kg; Liquamyc L-200); where the plasma density reached to the peak at 0.77 ± 0.83 h (tmax) and Cmax was measured as 8.59 ± 7,47 μg/ml. T1/2 value was calculated as 14.4 ± 4.92 h, AUC as 67.4 ± 21.7 μg/ml/h, Vdss as 4.10 ± 1.65 l/kg, and Cl as 0.333 ± 0.117 l/kg/h. The pharmacokinetic parameters, in the current study, were found to be similar to the study by Payne et al (18).
In another study (10), the same OTC LA preparations (Liquamyc L-200) were administered to calves and sheep through IM route at 20 mg/kg; where the tmax was 3.5 ± 1.2 h, and average Cpeak was 6.1 ± 1.3 μg/ml in sheep. AUC for calves (168 ± 14.6 μg/ml/h) was found to be significantly lower than that for sheep (209 ± 43 μg/ml/h). Vdss was 3.3 ± 0.49 and 3.08 ± 0.82 l/kg and Cl was 1.88 ± 0.12 and 1.65 ± 0.30 ml/min/kg for calves and sheep, respectively. The difference in pharmacokinetic parameters was related to the differences in species. Compared to the current study, Kilis goats were found to have shorter tmax, higher Cpeak, and lower AUC values compared to the calf and sheep.
As a good therapeutic option, OTC is widely used by the veterinarians for the treatment of the diseases of Kilis goats. Since the trait differences have an effect for the optimization of the therapeutic doses of the drugs and no data was available for the pharmacokinetic parameters in Kilis goats, this study provided valuable information for the practicioners in the field. As veterinarians should consider diseases, animal welfare, treatment type, time, place, accessibility to the drugs, and access to the patient for the proper drug (OTC) administration, this paper would expected to contribute for the selection of the administration route and the formulation of the drug for the practitioners as well.
Having a long-time effect and long administration intervals, long effective (LA) OTC preparations are considered as convenient options by veterinarians (4–6, 26). IV infusion which should be administered in every 12 h to ensure the effective concentration is difficult in terms of both animals and practitioners. To provide effective plasma concentration and for ease of administration, IM preparations were found more appropriate. Such studies highlight the necessity of pharmacokinetic studies to adjust the therapeutic dose in the target animal.
Author Contributions
EY conceived the idea of the study and evaluated pharmacokinetic parameters. İA has given drugs for animals, collected data, analyzed drug, and did the statistical analyses. The manuscript was prepared, edited, and approved by both authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Prof. Dr. Cengiz Gokbulut for assistance with pharmacokinetics analysis and comments that greatly improved the manuscript.
References
1. Alizadehasl M, Ünal N. Some morphological traits of Kilis, Norduz and Honamlı indigenous goats breeds. Lalahan Hay Araş Enst Derg (2011) 51:81–92.
2. Paksoy N, Iriadam M. An investigation of selenium levels in Kilis goats. Harran Univ J Fac Vet Med (2012) 1:6–8.
3. Aksoy AM, Kaya S. Pharmacokinetics of amoxicillin in Kilis goats. Vet J Ankara Univ (2007) 54:171–6. doi:10.1501/Vetfak_0000000276
4. Kaya S. Kemoterapötikler. 2nd ed. In: Kaya S, Pirinçci İ, Bilgili A, editors. Veteriner Farmakoloji. (Vol. 2), Ankara: Medisan Press (2007). p. 327–488.
5. Özdemir N. Kimi uzun etkili (LA) veteriner tetrasiklin formülasyonlarının biyoyararlanım ve biyoeşdeğerlilik yönünden incelenmesi [PhD Thesis]. Istanbul: İstanbul Üniversitesi Sağlık Bilimleri Enstitüsü (2003).
6. Achenbach TE. Physiological ve Classical Pharmacokinetic Models of Oxytetracycline in Cattle [Master of Science in the Department of Biological Sciences]. Canada: Simon Fraser University (2000).
7. Ebadi M. Desk Reference of Clinical Pharmacology. New York: CRC Press, Taylor & Francis Group (2008).
8. Reiche R. Drug disposition in the newborn. In: Ruckebusch Y, Toutain PL, Koritz GD, editors. Veterinary Pharmacology and Toxicology. Lancaster, England: MTP Press Limited (1983). p. 49–55.
9. Bowden BC. Pharmacokinetic Profiles of Oxytetracycline in Yellow Perch (Perca flavescens) as Determined by Plasma Concentration Following Different Routes of Administration [Master of Science in Veterinary Medical Sciences]. Blacksburg, Virginia: Virginia-Maryland Regional College of Veterinary Medicine, Virginia Polytechnic Institute and State University (2001).
10. Craigmill AL, Holland RE, Robinson D, Wetzlich S, Arndt T. Serum pharmacokinetics of oxytetracycline in sheep and calves and tissue residues in sheep following a single intramuscular injection of a long-acting preparation. J Vet Pharmacol Ther (2000) 23:345–52. doi:10.1046/j.1365-2885.2000.00292.x
11. Baggot JD. The Physiological Basis of Veterinary Clincal Pharmacology. Paris, France: Blackwell Science Ltd (2001).
12. IPCS. Oxytetracycline. International Programme on Chemical Safety. (2015). Available from: http://www.inchem.org/documents/jecfa/jecmono/v27je06.htm
13. IPCS. Tetracyclines: oxytetracycline, chlortetracycline, and tetracycline (addendum) toxicological evaluation of certain veterinary drug residues in food who food additives series 41. Prepared by: The 50th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva: World Health Organization (1998). Available from: http://www.inchem.org/documents/jecfa/jecmono/v041je07.htm
14. Rule R, Moreno L, Serrano JM, Garcia Roman A, Moyano R, Garcia J. Pharmacokinetics and residues in milk of oxytetracyclines administered parenterally to dairy goats. Aust Vet J (2001) 79:492–6. doi:10.1111/j.1751-0813.2001.tb13023.x
15. Kaya S, Yarsan E, Baydan E, Akkaya R, Aksoy A. Comparison of the pharmacokinetic of conventional and long-acting formulations of oxytetracycline in sheep. Turk J Vet Anim Sci (2001) 25:173–7.
17. Kikuvi GM, Mitema ES, Buoro IBJ. The pharmacokinetics of a long-acting oxytetracycline formulation in healthy dogs and in dogs infected with Ehrlichia canis. Vet Res Commun (2001) 25:391–400. doi:10.1023/A:1010646826963
18. Payne MA, Babish JG, Bulgin M, Lane M, Wetzlich S, Craigmill AL. Serum pharmacokinetics and tissue and milk residues of oxytetracycline in goats following a single intramuscular injection of a long-acting preparation and milk residues following a single subcutaneous injection. J Vet Pharmacol Ther (2002) 25:25–32. doi:10.1046/j.1365-2885.2002.00378.x
19. Yar M, Ahmad M, Bukhari NI, Khawaja MNA. Pharmacokinetics of oxytetracycline in sheep after various intravenous doses. Turk J Vet Anim Sci (2000) 24:135–8.
20. Hassan SAA, Shaddad SAI, Muddathir AK, El-Tayeb IB, Nour AOM, Alnazawi MH, et al. Pharmacokinetics of long-acting oxytetracycline formulation following ıntramuscular administration on desert sheep. J Anim Vet Adv (2008) 7:433–6.
21. Chamberlain J. The Analysis of Drugs in Biological Fluids. 2nd ed. Florida, USA: CRC Press Inc (1985).
22. Mandal TK, Sarkar S, Chakraborty AK, Ghose RK. Pharmacokinetics of oxytetracycline in presence of calcium gluconate in goats. Indian J Physiol Pharmacol (1992) 36:112–4.
23. Elsheikh HA, Osman Intisar AM, Eltayeb IB, Salam Abdullah A. Effect of dehydration on the pharmacokinetics of oxytetracycline hydrochloride administered intravenously in goats (Capra hircus). Gen Pharmacol (1998) 31:455–8. doi:10.1016/S0306-3623(98)00013-5
24. Al-Nazawi MH. Comparative pharmacokinetic studies on oxytetracycline in camels, sheep and goats. Pak Vet J (2003) 23:187–91.
25. Elsheikh HA, Osman IA, Ali BH. Comparative pharmacokinetics of ampicillin trihydrate, gentamicin sulphate and oxytetracycline hydrochloride in Nubian goats and desert sheep. J Vet Pharmacol Ther (1997) 20:262–6. doi:10.1046/j.1365-2885.1997.00061.x
Keywords: pharmacokinetics, Kilis goat, oxytetracycline, long-acting, conventional
Citation: Aktas İ and Yarsan E (2017) Pharmacokinetics of Conventional and Long-Acting Oxytetracycline Preparations in Kilis Goat. Front. Vet. Sci. 4:229. doi: 10.3389/fvets.2017.00229
Received: 05 September 2017; Accepted: 12 December 2017;
Published: 22 December 2017
Edited by:
Nora Mestorino, National University of La Plata, ArgentinaReviewed by:
Mariana Florencia Lucas, Universidad del Salvador, ArgentinaLloyd Reeve-Johnson, University of the Sunshine Coast, Australia
Copyright: © 2017 Aktas and Yarsan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ender Yarsan, eyarsan@gmail.com
 İbrahim Aktas
İbrahim Aktas Ender Yarsan
Ender Yarsan