- Department of Small Animal Medicine, University of Leipzig, Leipzig, Germany
Diagnosing necrotizing encephalitis, with its subcategories of necrotizing leukoencephalitis and necrotizing meningoencephalitis, based on magnetic resonance imaging alone can be challenging. However, there are breed-specific imaging characteristics in both subcategories that allow establishing a clinical diagnosis with a relatively high degree of certainty. Typical breed specific imaging features, such as lesion distribution, signal intensity, contrast enhancement, and gross changes of brain structure (midline shift, ventriculomegaly, and brain herniation) are summarized here, using current literature, for the most commonly affected canine breeds: Yorkshire Terrier, French Bulldog, Pug, and Chihuahua.
Introduction
Necrotizing encephalitis (NE) includes several breed specific inflammatory brain diseases within the group of meningoencephalitis of unknown etiology/etiology (MUA/MUE). Several synonyms exist for MUA (non-infectious encephalitis, immune mediated encephalitis) reflecting the fact that even though these diseases have been known for decades, the underlying pathology is still not completely understood and achieving a definite diagnosis intra vitam is usually difficult. Based on histopathological features MUA can be subdivided into granulomatous meningoencephalomyelitis (GME) and NE, with the latter comprising Necrotizing Leukoencephalitis (NLE) and necrotizing meningoencephalitis (NME). These subtypes of NE are seen in specific breeds with NLE affecting Yorkshire Terriers (1–9) and French Bulldogs (10, 11), whereas NME affects Pugs (12–21), Maltese (22–24), Chihuahuas (24–33), Pekingese (25, 34), Shih Tzus (24, 25, 35), West Highland White Terriers (36), Papillons (23, 35), Coton de Tulears (35), and Brussels Griffons (35).
The often applied practice of using the rather unspecific term of MUA instead of the exact subclassification (GME, NME, or NLE) in imaging diagnostics is due to the fact, that differentiation based on imaging alone can be difficult. This situation is further complicated by the fact that magnetic resonance imaging (MRI) may generally have only moderate sensitivity in detecting inflammatory intracranial pathology. Abnormalities in brain MRI were found in 76% of dogs with inflammatory cerebrospinal fluid (CSF) (37). Therefore, imaging findings should be interpreted under consideration of the CSF analysis results. However, normal CSF was found in 28.6% of dogs with histologically confirmed NLE and in 14.3% of Pugs with histologically confirmed NME (25). Similarly, a normal CSF nucleated cell count was reported in 12.5% of dogs with NE (38). Therefore, a definite diagnosis of encephalitis may often require histological confirmation based on a brain biopsy (31).
Nevertheless, common MRI features for NME and NLE that allow diagnosing these diseases with a relatively high degree of certainty are known. The typical breed specific MRI characteristics of both diseases in the most frequently affected breeds will be presented. All images provided for illustration are obtained from dogs with histologically confirmed diagnoses. The images can illustrate only some features of the specific disease in a given breed. Illustration of all possible MRI characteristics is beyond the scope of this publication.
NLE in Yorkshire Terriers
Uni- or bilateral asymmetrical lesions are seen in the telencephalon and diencephalon. The brainstem is often less severely affected. The cerebellum and spinal cord are usually unaffected on magnetic resonance (MR) images even though in rare cases histopathological examination also shows inflammatory foci here (6, 7). Lesion distribution based on MRI has been described in 27 Yorkshire Terrier cases: forebrain (n = 25), thalamus (n = 10), midbrain (n = 8), and caudal brainstem (n = 10; 9). Additional syringohydromyelia was seen in 7/27 (26%) dogs. Lesions were multifocal in about 78% of cases. However, diagnosis in these Yorkshire Terriers was described as meningoencephalitis of unknown origin and not as NLE. Therefore, it cannot be ruled out that some of these dogs were affected by other types of encephalitis.
Telencephalic lesions usually affect the periventricular and subcortical white matter, often leaving the overlying cortical gray matter untouched (2, 5). The shape of the lesion may vary from finger-like, following the shape of subcortical white matter, to round-shaped in deep-seated lesions (Figure 1). Therefore, asymmetric thalamic lesions are usually round-shaped and well demarcated. They need to be differentiated from bilateral symmetric round lesions in the telencephalon (including basal nuclei), mid-thalamus and brainstem seen in subacute necrotizing encephalopathy (SNE) of Yorkshire Terriers. Signal intensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences are similar in both NLE and SNE, but symmetry of lesions should clearly point toward SNE [(39, 40); Figure 2].
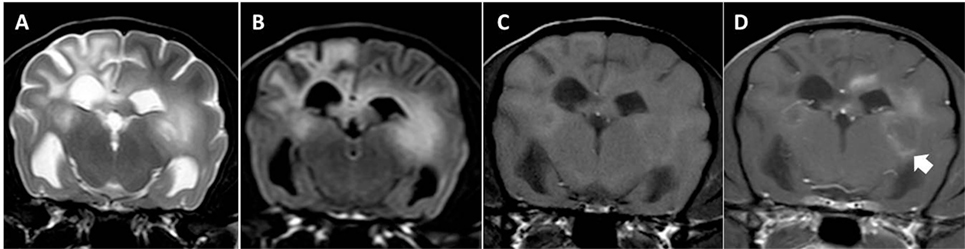
Figure 1. Transverse magnetic resonance images (3 T) of a Yorkshire Terrier (2 years, female) with necrotizing leukoencephalitis. T2 (A) and fluid-attenuated inversion recovery (B) hyperintense finger-like lesions affecting the subcortical white matter, the corona radiata, and the diencephalon. Right-sided ventricular enlargement, focal widening of sulci, and mild midline shift to the right are most likely secondary to white matter loss in the right prosencephalon. Contrast enhancing lesions as on the left side [(C) T1-weighted native; (D) T1 weighted after contrast injection] and non-contrast enhancing lesions (right side) may coexist in one patient, which most likely reflects different stages of the disease. Enhancement may be patchy or it may be seen around necrotic areas (ring-like, arrow head). Meningeal contrast enhancement is deficient.
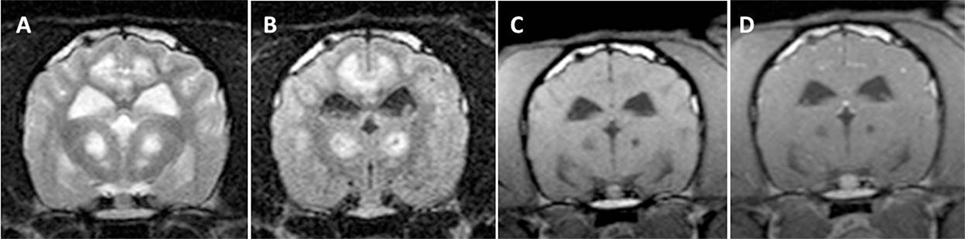
Figure 2. Transverse magnetic resonance images (0.5 T) of a Yorkshire Terrier (3 years, male) with subacute necrotizing encephalopathy. Bilateral symmetric hyperintensities in the cingulate gyrus and in the thalamus on T2-weighted (A) and fluid-attenuated inversion recovery (B) images. These lesions have a hypointense center on T1-weighted (C) images and they do not enhance after a contrast injection (D).
Lesions in NLE are usually hyperintense on T2-weighted and FLAIR images and hypointense to isointense on T1-weighted sequences. These signal intensity characteristics may represent multiple areas of cystic necrosis (41). The secondary white matter edema beyond the boundaries of the primary inflammatory lesions is rather mild. On positron emission tomography (PET) imaging, areas of necrosis and cavitation correspond to areas of glucose hypometabolism (30, 42).
In few cases (12%), lesions might be hyperintense on T1 and hypo- or hyperintense on T2-weighted sequences, which most likely correspond to areas of intraparenchymal hemorrhage. The latter could be verified by identification of an intralesional signal void in a T2* sequence. However, studies confirming this assumption in Yorkshire Terriers with NLE are deficient. Midline shift and ventriculomegaly are seen in 50 and 36% of dogs, respectively (25). Both are less likely to be caused by a mass effect of the inflammatory foci, but by a loss of white matter on the more severely affected hemisphere (3, 5). Brain herniation is not a feature of NLE in Yorkshire Terriers.
Lesion enhancement after a contrast injection is usually mild to moderate (5, 7, 25). It can be patchy and inhomogeneous within parenchymal lesions (87%) or ring-like in the periphery of a necrotic lesion corresponding to perinecrotic inflammation (7, 25). The degree of contrast enhancement appears to be related to the degree of lymphohistiocytic inflammation on histological examination (7). Lesions with moderate enhancement and those without enhancement may coexist in one patient (7). The first may reflect active inflammation whereas areas without enhancement may be reflecting burnt out lesions and therefore indicating a more chronic stage of the same disease (5, 7). Meningeal enhancement is lacking in NLE (25). A few Yorkshire Terriers with NLE (7%) may not show any contrast enhancement at all (6, 25).
In addition to NLE, Yorkshire Terriers can also be affected by GME. It may even be seen as a combination of NLE, NME, and GME in the same dog, as described in two cases (43). One displayed typical MRI features of NLE in the prosencephalon, whereas the other had a lesion compatible with GME. Histopathological examination, however, revealed characteristics of NLE, NME, and GME in both dogs.
NLE in French Bulldogs
Reports on specific MRI features of NLE in French Bulldogs are limited to three publications regarding four dogs with histologically confirmed disease, with one scan being performed post mortem (10, 11, 25). Lesions may be uni- or multifocal and can be round-shaped and clearly delineated or they may be diffuse. They are T2 and FLAIR hyperintense and can be found in the telencephalon, diencephalon and brainstem, whereas the cerebellum seems to be unaffected. These lesions are iso- to hypointense on T1-weighted images. They may represent necrotic areas in a similar way (as described earlier) for Yorkshire Terriers. However, the primary inflammatory focus is frequently masked by a secondary white matter edema and therefore it may be indistinguishable from secondary edema without a contrast injection. The primary inflammatory focus can often be identified by a mild to moderate peripheral or even ring-like enhancement on T1-weighted images after a contrast injection (Figure 3). More diffuse lesions may commonly show a varying degree of contrast enhancement ranging from a moderate to strong uniform pattern. However, lesions without contrast enhancement can also be seen in the same patient. Meningeal involvement is minimal and therefore it cannot be identified on MRI. Accordingly, the nucleated cell count in CSF is usually only mildly elevated (25). Midline shift is not commonly seen or is only mild. In general, lesions seem to be less severe in French Bulldogs than in Yorkshire Terriers.
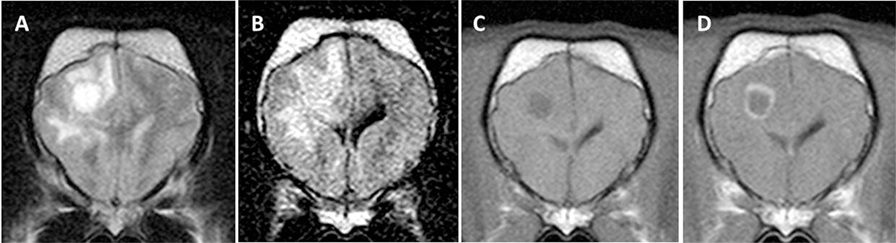
Figure 3. Transverse magnetic resonance images (0.5 T) of a French Bulldog (2 years, female) with necrotizing leukoencephalitis. T2 (A) and fluid-attenuated inversion recovery (FLAIR) (B) hyperintense round-shaped lesion accompanied by secondary white matter edema in the right frontal lobe. The T2 signal of the round center is partially suppressed on the FLAIR sequence indicating a liquid-like texture being consistent with necrosis. There is a ring-like contrast enhancement around the necrotic center after contrast injection [(C) T1-weighted native; (D) T1 weighted after contrast injection].
One report described inflammatory changes affecting both the optic nerves and retina on histopathology (in addition to those found in the forebrain), which went unnoticed on a post mortem MR image (11).
NME in Pugs
The majority of Pugs with NME (94%) have multifocal or diffuse, asymmetrical prosencephalic lesions with at least one lesion being located in the telencephalon (21). Lesions are more common in the middle and caudal prosencephalon, being most severe in occipital and parietal lobes, tapering off to rostral with the frontal lobes being less frequently affected (20, 21). About one-third of dogs have additional diencephalic lesions (21).
Brainstem (17%) and cerebellar (22%) involvement are much less common and lesions here are less severe than those in the prosencephalon (21, 25). However, lesion distributions may reflect the limited sensitivity of MRI to detect inflammatory lesions, especially if those are relatively mild, since it is known from a pathological study that 40% of Pugs with NME also have cerebellar lesions (19). In addition, one Pug with NME has been reported with a single brainstem lesion (21). In general, lesion burden in NME can be more dramatic for Pugs compared with other types of NE. However, no correlation could be demonstrated so far between MRI visible lesion severity and prognosis (21).
Lesions being hyperintense on T2-weighted and FLAIR images and mildly hypo- to isointense on T1 images may dominate in the cerebral white matter (about 30% of lesions) or they may more commonly involve gray and white matter to a similar extent. White and gray matter distinction is often missing altogether and corresponds to anatomical disruptions of those structures on histopathological examination (Figure 4). Focal, well-demarcated hyperintensities on T2-weighted images, which follow the white-gray matter boundaries, correlate histologically with white matter edema rather than with areas of macroscopic necrosis of brain parenchyma. Therefore, the latter is not a typical feature of NME in Pugs. Hyperintensity on T2-weighted and FLAIR images in the hippocampus and piriform lobe is mainly consistent with excitotoxic edema caused by long-lasting seizure activity, whereas similar imaging features in other forebrain regions correspond to areas of inflammation or microscopic liquefaction on histopathology (25).
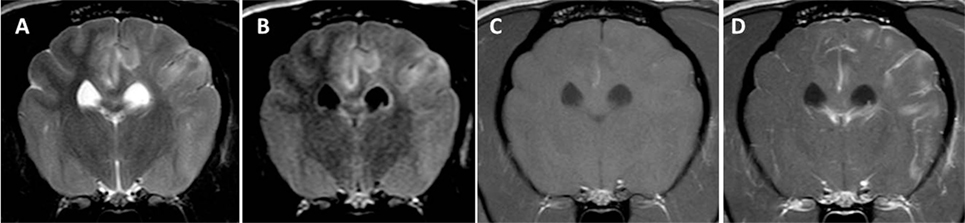
Figure 4. Transverse magnetic resonance images (3 T) of a Pug (3 years, male) with necrotizing meningoencephalitis. Diffuse T2 (A) and fluid-attenuated inversion recovery (B) hyperintensities predominantly on the left in the prosencephalon resulting in the complete loss of cortical gray and white matter distinction. There is a patchy parenchymal and a leptomeningeal enhancement after a contrast injection [(C) T1-weighted native; (D) T1 weighted after contrast injection]. The midline is slightly shifted to the right side caused by the expansion of the prosencephalon on the left-hand side.
Lesion enhancement after a contrast injection is varying but is usually mild to moderate with an inhomogeneous and patchy intraparenchymal pattern. Meningeal enhancement is seen in slightly more than half of the dogs involving the dura mater and more commonly the leptomeninges (21, 25). The enhancement pattern, corresponding to inflammatory infiltrates found histopathologically in sulci, explains why Pugs are more likely to experience elevated nucleated cell counts on CSF analysis than dogs with NLE (25) and why mean nucleated cell counts are higher in Pugs (250 cell/μl; SD: 172) than in Yorkshire Terriers (19 cells/μl; SD: 21) (25). Between 22 and 43% of Pugs may not show any contrast enhancement at all (21, 25). However, lack of contrast enhancement does not rule out meningitis or encephalitis (37, 44).
Midline shift away from the more severely affected brain hemisphere is seen in 25–61% of dogs (21, 25). Different types of brain herniation were detected in about one-third of cases with a caudal transtentorial herniation being most common [(20, 21, 25); Figure 5]. A foramen magnum herniation of the cerebellum is seen in up to 12% of dogs and may contribute to the sudden death observed in some cases (25). Uni- or bilateral ventricular dilation can be identified in about one-third of dogs, with some experiencing signs of intraventricular hypertension such as periventricular edema and dilatation of the olfactory recess. Ventricular dilatation and/or asymmetry may be attributed to the space-occupying effect of inflammatory foci and secondary edema. However, one should keep in mind that it might be just an incidental finding in some cases since ventricular asymmetry can be seen in up to 38% of normal dogs (45).
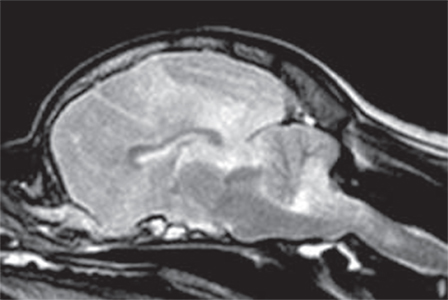
Figure 5. Sagittal T2-weighted magnetic resonance image (0.5 T) of a Pug (9 months, female) with necrotizing meningoencephalitis (NME). Caudal transtentorial herniation of the occipital lobes and foramen magnum herniation of the cerebellum caused by significant swelling of the prosencephalon. This may explain the sudden death of some Pugs with NME.
NME in Chihuahuas
Magnetic resonance imaging of two dogs brains demonstrated multifocal loss of cortical gray/white matter demarcation and marked thinning and collapse of the right parietal, temporal, and occipital cortices (29). In contrast to NME in Pugs, a small rim of overlying cortical gray matter might be spared resulting in an MRI that may resemble NLE in Yorkshire Terriers (Figure 6). Involvement of deep white matter such as the capsula interna and diencephalon is also described [(25, 30, 32); Figure 7]. The lesions were hyperintense on T2-weighted images, with T2-weighted hypointensity and slight post-contrast enhancement (29). Brainstem and cerebellum seem to be largely unaffected (29). Lesions correspond to intensely cellular, non-suppurative meningoencephalitis, often with cystic necrosis in subcortical white matter (29). On fluorine-18 fluorodeoxyglucose-PET scans the lesions correspond to areas of hypometabolism (30). CSF analysis can be normal in some dogs and hinders establishing a definite intra vitam diagnosis (29).
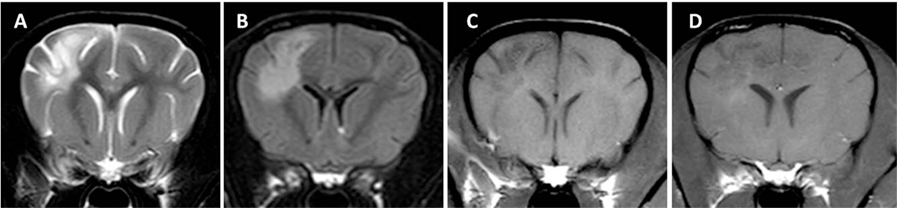
Figure 6. Transverse magnetic resonance images (3 T) of a Chihuahua (11 months, female) with necrotizing meningoencephalitis. T2 (A) and fluid-attenuated inversion recovery (B) hyperintense finger-like lesions affecting the subcortical white and gray matter in the right prosencephalon. These characteristics may resemble those of Yorkshire Terriers with necrotizing leucoencephalitis since there might be a thin rim of intact cortical gray matter. However, involvement of gray matter can be visualized in most cases. The lesion is hypointense on T1-weighted images (C) and is only mildly enhanced in the periphery of the lesion (D).
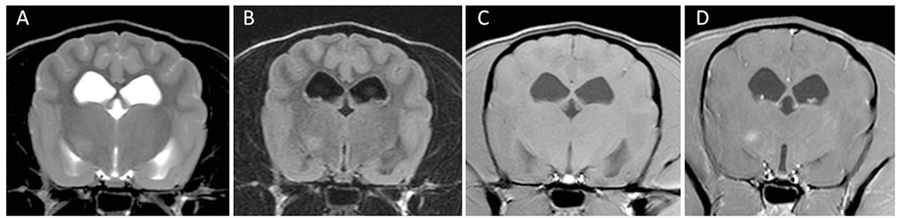
Figure 7. Transverse magnetic resonance images (3 T) of a Chihuahua (5 years, female) with necrotizing meningoencephalitis. Single T2 (A) and fluid-attenuated inversion recovery (B) hyperintense round-shaped lesion in the right diencephalon. There is a small uniform contrast enhancement in the center of the lesion on the post-contrast T1-weighted image (D) compared to the pre-contrast T1-weighted image (C).
In the case of Chihuahuas, the situation is further complicated by the fact that GME can also be seen in this breed and therefore has to be differentiated from NME (25, 46).
NE in Other Canine Breeds
Imaging characteristics of the four breeds described here are summarized in Table 1. For the other breeds, having been reported to be affected by either NLE or NME, none or only single case reports of MRI characteristics are available and therefore generalizations do not seem feasible.
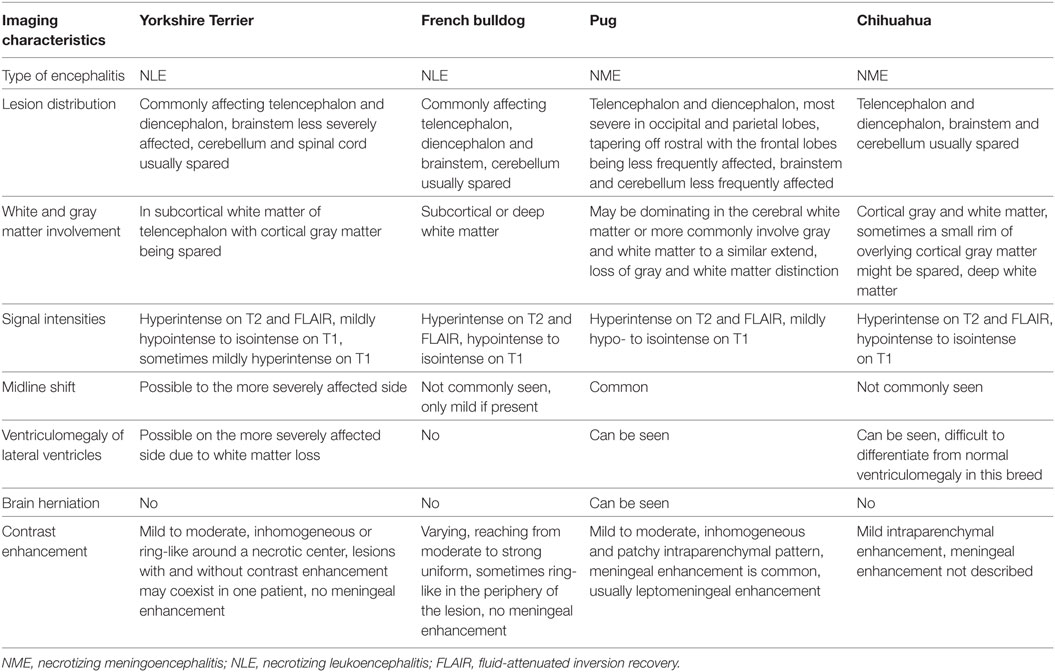
Table 1. Summary of typical imaging characteristics of necrotizing encephalitis in Yorkshire Terriers, French bulldogs, Pug dogs, and Chihuahuas.
Future Developments
Despite the fact that imaging characteristics of NME and NLE have been outlined earlier, diagnosis is usually presumptive if based on imaging alone. Often, histological confirmation based on a brain biopsy is required to establish a definite diagnosis (20). However, performing brain biopsies is invasive and it is associated with certain morbidity. Therefore, non-invasive methods of imaging immunology are being investigated in human medicine. Inflammatory cells can be labeled with intravenously injected paramagnetic or superparamagnetic compounds (47, 48). Alternatively, cellular labeling can be achieved using other dipoles that are less common in normal tissue than hydrogen such as 19F, 13C, or 15N (47). Cells labeled this way may potentially be tracked by MRI in inflammatory and ischemic intracranial lesions allowing cellular characterization of encephalitis without the need for a histological examination.
Author Contributions
TF has gathered all information and wrote the manuscript.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Funding
The funding does apply to the publication fee only.
Abbreviations
CSF, cerebrospinal fluid analysis; FDG-PET, fluorine-18 fluorodeoxyglucose; FLAIR, fluid-attenuated inversion recovery; GME, granulomatous meningoencephalomyelitis; MUE, meningoencephalitis of unknown etiology; NE, necrotizing encephalitis; NLE, necrotizing leucoencephalitis; NME, necrotizing meningoencephalitis; MR, magnetic resonance; MRI, magnetic resonance imaging; PET, positron emission tomography; SNE, subacute necrotizing encephalopathy.
References
1. Tipold A, Fatzer R, Jaggy A. Necrotizing encephalitis in Yorkshire terriers. J Small Anim Pract (1993) 34:623–8. doi:10.1111/j.1748-5827.1993.tb02598.x
2. Sawashima Y, Sawashima K, Taura Y, Shimada A, Umemura T. Clinical and pathological findings of a Yorkshire terrier affected with necrotizing encephalitis. J Vet Med Sci (1996) 58:659–61. doi:10.1292/jvms.58.659
3. Jull BA, Merryman JI, Thomas WB, McArthur A. Necrotizing encephalitis in a Yorkshire terrier. J Am Vet Med Assoc (1997) 211:1005–7.
4. Ducote JM, Johnson KE, Dewey CW, Walker MA, Coates JR, Berridge BR. Computed tomography of necrotizing meningoencephalitis in 3 Yorkshire terriers. Vet Radiol Ultrasound (1999) 40:617–21. doi:10.1111/j.1740-8261.1999.tb00888.x
5. Lotti D, Capucchio MT, Gaidolfi E, Merlo M. Necrotizing encephalitis in a Yorkshire terrier: clinical, imaging, and pathologic findings. Vet Radiol Ultrasound (1999) 40:622–6. doi:10.1111/j.1740-8261.1999.tb00889.x
6. Kuwamura M, Adachi T, Yamate J, Kotani T, Ohashi F, Summers BA. Necrotizing encephalitis in the Yorkshire terrier: a case report and literature review. J Small Anim Pract (2002) 43:459–63. doi:10.1111/j.1748-5827.2002.tb00014.x
7. Von Praun F, Matiasek K, Grevel V, Alef M, Flegel T. Magnetic resonance imaging and pathologic findings associated with necrotizing encephalitis in two Yorkshire terriers. Vet Radiol Ultrasound (2006) 47:260–4. doi:10.1111/j.1740-8261.2006.00137.x
8. Higginbotham MJ, Kent M, Glass EN. Noninfectious inflammatory central nervous system diseases in dogs. Compend Contin Educ Vet (2007) 29:488–97.
9. Wrozek M, Nicopon J, Giza E, Niedzwiedz A, Sotysiak Z. Meningoencephalitis of unknown origin in a cohort of 27 Yorkshire terriers in Poland: clinical manifestation, topographic magnetic resonance imaging characteristics, clinical outcome. 24th Symposium ESVN-ECVN, Trier, Germany. J Vet Intern Med (2012) 26:842.
10. Timmann D, Konar M, Howard J, Vandevelde M. Necrotising encephalitis in a French bulldog. J Small Anim Pract (2007) 48:339–42. doi:10.1111/j.1748-5827.2006.00239.x
11. Spitzbarth I, Schenk HC, Tipold A, Beinecke A. Immunohistochemical characterization of inflammatory and glial responses in a case of necrotizing leucoencephalitis in a French bulldog. J Comp Pathol (2010) 142:235–41. doi:10.1016/j.jcpa.2009.08.158
12. Cordy DR, Holliday TA. A necrotizing meningoencephalitis of pug dogs. Vet Pathol (1989) 26:191–4. doi:10.1177/030098588902600301
13. Kobayashi Y, Ochiai K, Umemura T, Goto N, Ishida T, Itakura C. Necrotizing meningoencephalitis in pug dogs in Japan. J Comp Pathol (1994) 110:129–36. doi:10.1016/S0021-9975(08)80184-3
14. Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: a retrospective study. J Vet Intern Med (1995) 9:304–14. doi:10.1111/j.1939-1676.1995.tb01089.x
15. Hinrichs U, Tobias R, Baumgärtner W. Ein Fall von nekrotisierender Menigoenzephalitis beim Mops (pug dog encephalitis–PDE). Tierärztl Prax (1996) 24:489–92.
16. Kuwabara M, Tanaka S, Fujiwara K. Magnetic resonance imaging and histopathology of encephalitis in a pug. J Vet Med Sci (1998) 60:1353–5. doi:10.1292/jvms.60.1353
17. Uchida K, Hasegawa T, Ikeda M, Yamaguchi R, Tateyama S. Detection of an autoantibody from pug dogs with necrotizing encephalitis (pug dog encephalitis). Vet Pathol (1999) 36:301–7. doi:10.1354/vp.36-4-301
18. Hasegawa T, Uchida K, Sugimoto M. Long-term management of necrotizing meningoencephalitis in a pug dog. Canine Pract (2000) 25:20–2.
19. Levine JM, Fosgate GT, Porter B, Schatzberg SJ, Greer K. Epidemiology of necrotizing meningoencephalitis in pug dogs. J Vet Intern Med (2008) 22:961–8. doi:10.1111/j.1939-1676.2008.0137.x
20. Flegel T, Henke D, Boettcher IC, Aupperle H, Oechtering G, Matiasek K. Magnetic resonance imaging finding in histologically confirmed pug dog encephalitis. Vet Radiol Ultrasound (2008) 49:419–24. doi:10.1111/j.1740-8261.2008.00400.x
21. Young BD, Levine JM, Fosgate GT, de Lahunta A, Flegel T, Matiasek K, et al. Magnetic resonance imaging characteristics of necrotizing meningoencephalitis in pug dogs. J Vet Intern Med (2009) 23:527–37. doi:10.1111/j.1939-1676.2009.0306.x
22. Stalis IH, Chadwick B, Dayrell-Hart B, Summers BA, Van Winkle TJ. Necrotizing meningoencephalitis of Maltese dogs. Vet Pathol (1995) 32:230–5. doi:10.1177/030098589503200303
23. Suzuki M, Uchida K, Morozumi M, Hasegawa T, Yanai T, Nakayama H, et al. A comparative pathological study on canine necrotizing meningoencephalitis and granulomatous meningoencephalomyelitis. J Vet Med Sci (2003) 65:1233–9. doi:10.1292/jvms.65.1319
24. Jung DI, Kang BT, Park C, Yoo JH, Gu SH, Jeon HW, et al. A comparison of combination therapy (cyclosporine plus prednisolone) with sole prednisolone therapy in 7 dogs with necrotizing meningoencephalitis. J Vet Med Sci (2007) 69:1303–6. doi:10.1292/jvms.69.1303
25. Flegel T. Diagnostics and Therapy of Granulomatous and Necrotizing Encephalitis in Dogs. Habilitation Theses. Leipzig: University of Leipzig (2010).
26. Matsuki N, Fujiwara K, Tamahara S, Uchida K, Matsunaga S, Nakayama H, et al. Prevalence of autoantibody in cerebrospinal fluids from dogs with various CNS diseases. J Vet Med Sci (2004) 66:295–7. doi:10.1292/jvms.66.295
27. Kube SA, Dickinson PJ, Affolter TW, Higgins RJ. Necrotizing meningoencephalitis in Chihuahua dogs. J Vet Intern Med (2005) 19:461. doi:10.1111/j.1939-1676.2005.tb02714.x
28. Zarfoss M, Schatzberg S, Venator K, Cutter-Schatzberg K, Cuddon P, Pintar J, et al. Combined cytosine arabinoside and prednisone therapy for meningoencephalitis of unknown aetiology in 10 dogs. J Small Anim Pract (2006) 47:588–95. doi:10.1111/j.1748-5827.2006.00172.x
29. Higgins RJ, Dickinson PJ, Kube SA, Moore PF, Couto SS, Vernau KM, et al. Necrotizing meningoencephalitis in five Chihuahua dogs. Vet Pathol (2008) 45:336–46. doi:10.1354/vp.45-3-336
30. Eom KD, Lim CY, Gu SH, Kang BT, Kim YB, Jang DP, et al. Positron emission tomography features of canine necrotizing meningoencephalitis. Vet Radiol Ultrasound (2008) 49:595–9. doi:10.1111/j.1740-8261.2008.00437.x
31. Flegel T, Oevermann A, Oechtering G, Matiasek K. Diagnostic yield and adverse effects of MRI-guided free-hand brain biopsies through a mini-burr hole in dogs with encephalitis. J Vet Intern Med (2012) 26:969–76. doi:10.1111/j.1939-1676.2012.00961.x
32. de Lahunta A, Glass E. Visual system. In: de Lahunta A, Glass E, editors. Veterinary Neuroanatomy and Clinical Neurology. St Louis, MO: Saunders Elsevier (2009). p. 411–2.
33. Vernau KM, Liu H, Higgins RJ, Dickinson PJ, Sturges BK, Vernau W, et al. Dog leucocyte antigen class II association in Chihuahuas with necrotizing meningoencephalitis. 2010 ACVIM Forum, Anaheim, USA. J Vet Intern Med (2010) 24:736. doi:10.1111/j.1939-1676.2010.0521.x
34. Cantile C, Chianini F, Arispici M, Fatzer R. Necrotizing meningoencephalitis associated with cortical hippocampal hamartia in a Pekingese dog. Vet Pathol (2001) 38:119–22. doi:10.1354/vp.38-1-119
35. Cooper JJ, Schatzberg SJ, Vernau KM, Summers BA, Porter BF, Siso S, et al. Necrotizing encephalomyelitis in atypical dog breeds: a case series and literature review. J Vet Intern Med (2014) 28:198–203. doi:10.1111/jvim.12233
36. Aresu L, D’Angelo A, Zannatta R, Valenza F, Capuchio MT. Canine necrotizing encephalitis associated with anti-glomerular basement membrane glomerulonephritis. J Comp Pathol (2007) 136:279–82. doi:10.1016/j.jcpa.2007.02.008
37. Lamb CR, Croson PJ, Capello R, Cherubini GB. Magnetic resonance imaging findings in 25 dogs with inflammatory cerebrospinal fluid. Vet Radiol Ultrasound (2005) 46:17–22. doi:10.1111/j.1740-8261.2005.00003.x
38. Granger N, Smith PM, Jeffery ND. Clinical findings and treatment of non-infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Vet J (2010) 184:290–7. doi:10.1016/j.tvjl.2009.03.031
39. Fischer A, Baiker K, Hofmann S, Medl S, Schmahl W, Bauer MF, et al. Subacute necrotizing encephalopathy in Yorkshire terriers is associated with combined respiratory chain complex I and IV defect. J Vet Intern Med (2008) 22:770. doi:10.1111/j.1939-1676.2008.0103.x
40. Baiker K, Hofmann S, Fischer A, Gödde T, Medl S, Schmahl W, et al. Leigh-like subacute necrotizing encephalopathy in Yorkshire terriers: neuropathological characterisation, respiratory chain activities and mitochondrial DNA. Acta Neuropathol (2009) 118:697–709. doi:10.1007/s00401-009-0548-6
41. Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract (2010) 51:138–49. doi:10.1111/j.1748-5827.2009.00823.x
42. Kang B-T, Kim S-G, Lim C-Y, Gu S-H, Jang D-P, Kim Y-B, et al. Correlation between fluorodeoxyglucose positron emission tomography and magnetic resonance imaging findings of non-suppurative meningoencephalitis in 5 dogs. Can Vet J (2010) 51:986–92.
43. Hoffmann MV, Iseringhausen M, Spitzbarth I, Gerhauser I, Hunkeler D, Oevermann A, et al. Concomitant granulomatous, necrotizing meningoencephalitis and necrotizing leukoencephalitis in two Yorkshire terriers. 24th Symposium ESVN-ECVN, Trier, Germany. J Vet Intern Med (2012) 26:842.
44. Keenihan EK, Summers BA, David FH, Lamb CR. Canine meningeal disease: associations between magnetic resonance imaging signs and histological findings. Vet Radiol Ultrasound (2013) 54:504–15. doi:10.1111/vru.12055
45. Pivetta M, De Risio L, Newton R, Dennis R. Prevalence of lateral ventricle asymmetry in brain MRI studies of neurologically normal dogs and dogs with idiopathic epilepsy. Vet Radiol Ultrasound (2013) 54:516–21. doi:10.1111/vru.12063
46. Luján Feliu-Pascal A, Matiasek L, de Stefani A, Beltrán E, De Risio L. Efficacy of mycophenolate mofetil for the treatment of presumptive granulomatous meningoencephalomyelitis: preliminary results. Abstract 20th Symposium ESVN-ECVN, Bern, Switzerland. J Vet Intern Med (2008) 22:508. doi:10.1111/j.1939-1676.2008.0047.x
47. Stoll G, Bendszus M. New approaches to neuroimaging of central nervous system inflammation. Curr Opin Neurol (2010) 23:282–6. doi:10.1097/WCO.0b013e328337f4b5
Keywords: necrotizing encephalitis, necrotizing leucoencephalitis, necrotizing meningoencephalitis, magnetic resonance imaging, breed specific magnetic resonance imaging
Citation: Flegel T (2017) Breed-Specific Magnetic Resonance Imaging Characteristics of Necrotizing Encephalitis in Dogs. Front. Vet. Sci. 4:203. doi: 10.3389/fvets.2017.00203
Received: 29 August 2017; Accepted: 17 November 2017;
Published: 04 December 2017
Edited by:
Andrea Tipold, University of Veterinary Medicine Hannover, GermanyReviewed by:
Marcin Adam Wrzosek, Wroclaw University of Environmental and Life Sciences, PolandCurtis Wells Dewey, Cornell University, United States
Copyright: © 2017 Flegel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Flegel, ZmxlZ2VsQGtsZWludGllcmtsaW5pay51bmktbGVpcHppZy5kZQ==
 Thomas Flegel
Thomas Flegel