- 1USDA-ARS, U.S. National Poultry Research Center, Egg Safety and Quality Research Unit, Athens, GA, United States
- 2Center for Food Safety, Food Science Department, University of Arkansas, Fayetteville, AR, United States
- 3Department of Biological Sciences, University of Southern Mississippi, Hattiesburg, MS, United States
Foodborne pathogens such as Salmonella, Campylobacter, Escherichia coli, and Listeria are a major concern within the food industry due to their pathogenic potential to cause infection. Of these, Listeria monocytogenes, possesses a high mortality rate (approximately 20%) and is considered one of the most dangerous foodborne pathogens. Although the usual reservoirs for Listeria transmission have been extensively studied, little is known about the relationship between Listeria and live poultry production. Sporadic and isolated cases of listeriosis have been attributed to poultry production and Listeria spp. have been isolated from all stages of poultry production and processing. Farm studies suggest that live birds may be an important vector and contributor to contamination of the processing environment and transmission of Listeria to consumers. Therefore, the purpose of this review is to highlight the occurrence, incidence, and potential systemic interactions of Listeria spp. with poultry.
Introduction
Microorganisms such as Salmonella, Campylobacter, and Listeria represent a considerable concern within the food industry due to their pathogenic properties and their potential to establish infections in humans. It is imperative that intervention strategies be established to reduce risk of foodborne illness to consumers, specifically Listeria monocytogenes. However, little is known about the prevalence of Listeria spp. throughout the poultry production and processing continuum. Listeria species are Gram-positive, non-spore forming, rod-shaped bacteria that are naturally found in the environment, including soil, sewage, feces from animals and birds, and surface water (1, 2, 3, 4). Listeria are persistent facultative anaerobes that ideally proliferate in temperatures of 30 to 37°C, but can withstand temperatures between 0 and 43°C (4, 5). In addition to being able to survive a wide range of temperatures, Listeria spp. can grow in a variety of salt concentrations, high osmotic pressure, and low pH environments, but succumb to pasteurization (6). There are at least six known species: Listeria grayi, Listeria seeligeri, Listeria welshimeri, Listeria ivanovii, Listeria innocua, and L. monocytogenes. Of the six species, L. ivanovii is pathogenic to animals and L. monocytogenes is the only species pathogenic to humans. While Listeria commonly colonize multiple mammalian hosts, it remains unclear the exact relationship of Listeria with avian species. While a frequent contaminant of ready to eat meats, the relationship with the live bird production aspect is much less clear. In this review, the occurrence, incidence, and potential systemic interaction of Listeria spp. with chickens and other avian species will be discussed.
Listeria and Foodborne Disease
Listeria monocytogenes can be subdivided into different phylogenic evolutionary lineages (I, II, III, and IV) based on ecology, genomic content, and recombination rates (7). Phylogenetic serotypes are based on cell wall antigen expression and thus can be used to identify variations among all 13 serotypes (7). Lineage I consists of serotypes 1/2b, 3b, 3c, and 4b strains, while lineage II includes serotypes 1/2a, 1/2c, and 3a (8, 9). Phylogenetic evidence has indicated that rare serotypes may have evolved recently, or multiple times, from one of the major serotypes (10). Lineage III belongs to two groups, formerly lineages IIIA/C and IIIB/C known as lineages III and IV, with serotypes 4a and 4c strains (11). However, limited knowledge exists for lineage IV due to rarity and low strain variability, which contributes to seven unclear serotypes. Similarly, little is known about the lineage status of serotype 7 due to lack of availability of such strains (10, 12, 13).
Serotypes that are most commonly associated with human cases are 11 (1/2b) in chicken meat, C1-056 (1/2a) in human, sporadic case, N1-225 (4b) causing human epidemic, and N1-227 (4b) (14). Variations in strains of L. monocytogenes are characterized according to their physiological properties and phenotypic characteristics, such as growth behavior, acid tolerance, and resistance to various stresses (14). There has also been identification of strain variance due to heat resistance (14), which could be directly related to those involved in food contamination that carries over to the consumer due to resistance to heat treatments.
Previous reports have shown an increase in the prevalence of L. monocytogenes in ready-to-eat (RTE), vacuum packaged, sliced meat products where 95% of all L. monocytogenes belonged to Lineage II, serotype 1/2a, with the remaining 5% varying between serotypes 1/2b, 3b, and 4b (15). Kramarenko et al. (16) reported that 93% of all L. monocytogenes isolates obtained from meat products belonged to serotype 1/2a and 1/2c (16). Therefore, this suggests that variations between stress and exposure influence which lineage, serotype, and strain is ultimately responsible for contamination.
Listeria monocytogenes is the causative agent of listeriosis, where those considered to be most susceptible include the elderly, immunocompromised, and pregnant women (1, 13). Although one of the less common foodborne illnesses, it frequently requires hospitalization (17–20) and has a mortality rate of 20 to 30% (6). The Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report states that of the 123 cases that occurred in 2013, 91% of reported cases resulted in hospitalization (21). There are a variety of symptoms that may arise upon infection, including septicemia, meningitis, and gastroenteritis (22). In pregnant women, it may cause spontaneous abortion, premature labor, and neonatal disease (23). Serotypes 1/2a (lineage II) and 1/2b and 4b (lineage I) are responsible for a majority of the L. monocytogenes hospitalized cases (10, 12, 13). Lineage I is responsible for cases among outbreaks of human clinical listeriosis (10, 13), while Lineage II strains exhibit a significantly higher prevalence among food isolates, the environment and animal clinical cases (9). Lineage III and IV strains account for approximately 1% of human listeriosis cases in humans but are more prominent in animals (24). Most sporadic human listeriosis cases appear to be caused by serotype-4b and -1/2a strains, while most human listeriosis outbreaks have been linked to serotype-4b strains (11). Outbreaks have rarely occurred because of non-4b serotypes but do happen (8)). For example, a serotype-1/2a outbreak of gastrointestinal listeriosis was linked to sliced turkey in the United States (8).
In the United States, there is a zero-tolerance policy for L. monocytogenes in food that requires the recall of any adulterated food product (18). L. monocytogenes has been investigated in various food products such as seafood, dairy products, meats, and RTE products. In the past, there were listeriosis outbreaks in the United States linked to contaminated cantaloupes, soft cheeses, RTE turkey deli meat, ice cream, unpasteurized milk, candied apples, packages and frozen vegetables, and most recently, soft raw milk cheeses (25).
There has been limited focus on Listeria spp. related to poultry and poultry products. To date, there have been no poultry (chickens, specifically)-related listeriosis outbreaks; however, though uncommon, poultry flocks can be contaminated with L. monocytogenes and result in sporadic listeriosis cases (3, 5, 26). In the few studied cases where the disease was attributed to poultry sources, symptoms have included septicemia or localized encephalitis (5). Poultry flocks can serve as a reservoir and can contaminate the litter and surrounding environments (3, 5, 27–29). Although rare cases of human listeriosis from raw poultry meat has stemmed from contamination and unhygienic practices of processing environments (4, 5), little is known about the prevalence within poultry and poultry products. Therefore, the purpose of the remainder of this review is to take a farm-to-fork approach and highlight the possible issues and places of potential Listeria spp. contact during the production of poultry and the importance for continued research of Listeria spp. in poultry to reduce the potential of future outbreaks.
Live Poultry Production Practices
Consumers have repeatedly expressed concerns about animal welfare related to intensive chicken farming. They want to be sure that all animals being raised for food are treated with respect and are properly cared for during their lives. Farmers and companies share the public’s concern and recognize that they have an ethical obligation to make sure that the animals on their farms are well cared for (30). Guidelines for poultry management include hatchery operations, appropriate housing and space, proper nutrition and feeding, health care, and monitoring, and these guidelines are provided within handbooks to poultry breeders (31), industry association protocols (30), or legislative texts (32).
Housing type and management are dictated by the type of poultry being produced (broiler versus layers), economics and the preferences in a particular region and climate. Globally, poultry housing must provide comfortable and protective shelter for the birds and effective measures must be established to protect flock health and minimize any negative impact on bird welfare (30). Several housing systems are used including conventional cages, furnished/enriched cages, barn systems, or free-range systems which exist for layers (33). The most common housing systems around the world remain cages with a space allowance ranging from 300 to 750 cm2 according to the legislation of the country (34). Broilers are generally held in groups in environmentally controlled housing, open and naturally ventilated poultry houses or on free range (34). In grow-out houses, the minimum space should be one-half square foot per bird as stated by the Council for Agricultural Science and Technology (CAST) (30). Whatever the rearing system, poultry housing and equipment must be designed to protect the birds from environmental conditions. Appropriate ventilation and heater systems are needed to regulate seasonal temperatures to keep air moving throughout the house and to provide optimal air quality at any time (30, 31). The bedding material must be of good quality and the litter must be kept clean. Ammonia emissions must be monitored and appropriate measures be taken, if necessary to reduce to the minimum allowable level by appropriate measures, if necessary (30, 31, 35).
Nutrition and water requirements for poultry depend on a range of factors including the commercial goals of the poultry enterprise, type of bird, breed and age, or stage of development (30, 31, 36). A good-quality dietary feed will help the birds stay healthy and grow well. Birds should have unlimited access to clean, fresh, and good quality drinking water. All feeding and drinking systems must be checked for proper operation daily and must be adjusted in height as the birds grow. Precise and complete guidelines about the nutrition and water requirements, as well as the feeding and drinking systems, are given by poultry breeders (31). A good poultry health management is an important component of flock management and meat or egg production (37). Good hygienic conditions within the poultry house must be achieved through the implementation of correct biosecurity, cleaning, and vaccination programs. A working relationship with an avian veterinarian is an integral part of health management (30, 31).
Listeria spp. within the Hatchery Environment
Live poultry production includes the hatchery and the grow-out farm environments. These two steps of poultry production may contribute to the contamination of live birds with L. monocytogenes and potentially lead to the contamination in the food processing plant and the poultry meat. The hatchery is responsible for the incubation of fertile eggs obtained from parent breeders and the hatching of chicks. The hatchery is the first production stage where eggshell surfaces, embryos, and chicks can be contaminated by pathogenic bacteria. Very few studies have investigated the occurrence of Listeria spp. and L. monocytogenes in the hatchery environment, Over 200 samples collected in three commercial broiler hatcheries in northern Georgia, USA, only 1% of chick paper pads and 6% of eggshell fragments were positive for L. monocytogenes (38). In Thailand, 32 hatcheries were inspected for L. monocytogenes over a 5-year period. Incubator trays of equipment used in hatcheries were swabbed (548 samples) and meconium from 10-day-old chicks were collected (523 samples). L. monocytogenes was not detected in 1,071 samples over this 5-year period (39).
Overall, limited data are available on the occurrence of Listeria spp. and L. monocytogenes in the hatchery environment. The vertical transmission of L. monocytogenes from the parent flock to the day-old chicken leaving the hatchery has not fully been investigated, contrary to other foodborne pathogens such as Salmonella (40, 41) and Campylobacter (42, 43). Since L. monocytogenes contamination of poultry products has focused on the processing stage (44, 45) and RTE products (1, 6), the hatchery environment has not been viewed as important to poultry meat contamination processes. However, there is no evidence to exclude the hatchery environment in the early contamination of chicks and consequently the final product.
Listeria spp. within the Grow-Out Farm Environment
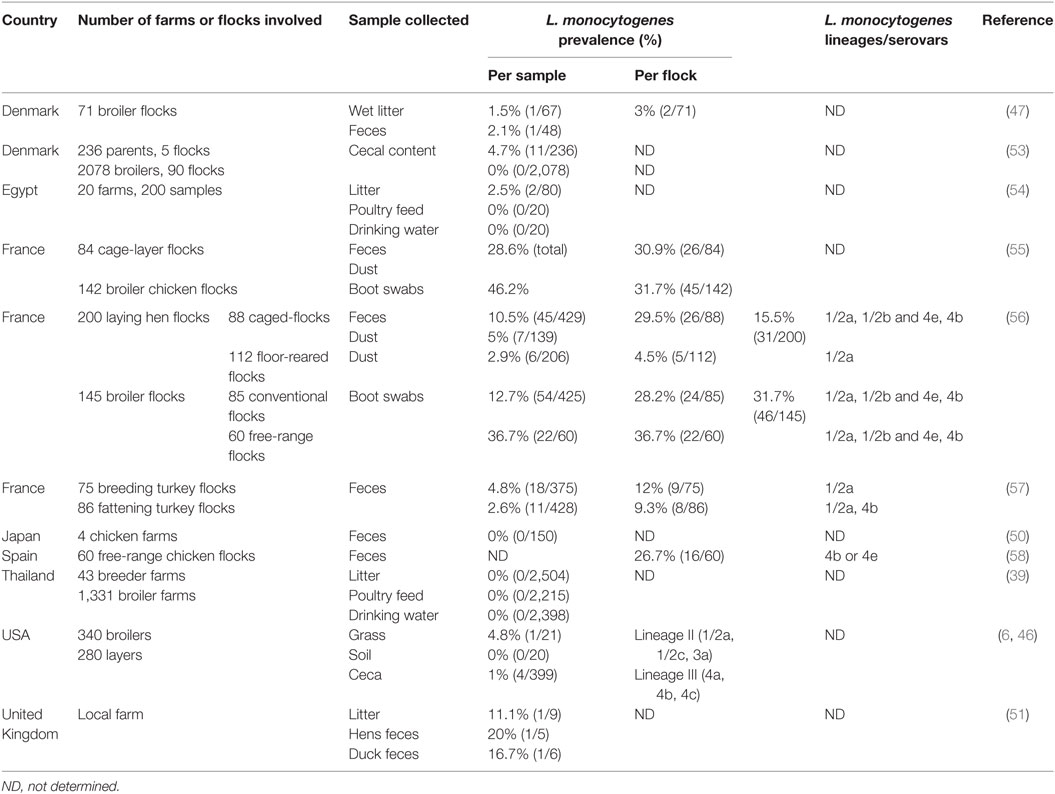
Once the 1-day-old chicks leave the hatchery environment, they are shipped to the grow-out farms to reach pre-determined size/weight based on the processing/final product requirements. Studies reporting the prevalence of Listeria spp. and L. monocytogenes in the grow-out farm environment vary according to the country, the number of farms and flocks examined, the breed of bird, and the type of samples collected (Table 1). To evaluate the contamination rate of Listeria spp. and L. monocytogenes on grow-out farms, studies have either investigated its prevalence in environmental samples surrounding or within the production area (soil, grass, dust, litter, feed, drinking water, layer egg shells, nest boxes) or in bird ceca and feces. In studies focusing on environmental samples, Listeria spp. prevalence ranged from as low as 1.4 to 53% (5, 6, 46–49). According to the sample type, 9.8 to 52.5, 70, 10, 30, and 6 to 42.8% of the samples were positive for Listeria spp. in broiler litter, farm feed, farm drinking water, soil, and grass samples, respectively (5, 46–48). Milillo et al. (46) demonstrated that environmental samples collected from the pasture before broiler introduction were rarely positive for Listeria spp. (5%), whereas samples collected after broiler exposure were significantly more likely to contain Listeria spp. (53%) (46). These findings implicate poultry as a source of the Listeria spp. being found in these environmental samples. Not only Listeria spp. have been isolated from environmental samples from grow-out farms but also they have also been isolated directly from the poultry. An investigation in Danish broilers and the broiler house environment of 71 flocks. Listeria spp. was identified in 9.8% of litter samples and 17% of fecal samples yielding an overall prevalence of 14% (10/71) in broiler flocks (47). A lower prevalence of Listeria spp. (4.7%) was found in 150 fresh fecal droppings collected at four chicken farms in the suburbs of Tokyo (50). Listeria spp. were more likely to be isolated from young broilers, suggesting that as the birds’ intestinal microbiota develop, their levels of Listeria spp. decline (46). L. innocua is the predominant species found on grow-out farms, representing ≤78% of all isolated Listeria spp. (5, 46, 47, 50, 51). L. innocua is important because it is closely related to L. monocytogenes and both are genetically similar (6, 52). Other Listeria species, such as L. ivanovii, L. welshimeri, and L. seeligeri, have also been identified in environmental farm samples or chicken feces but their detection remain infrequent (5, 50).
Listeria monocytogenes has been isolated on grow-out farms from litter (51, 59), dust (56), grass (46), feed (60), feces (56, 61, 62), and cecal (46, 53) samples, with an overall contamination rate ranging from 0 to 46.2% in those samples. The prevalence of L. monocytogenes in poultry ceca and feces is generally low but can be highly variable, ranging from 0 to 32% (39, 47, 50, 51, 53, 56). Moreover, only a small percentage of birds may be long-term carriers of the organism (63). Intestinal carriage of L. monocytogenes may be transient, most likely resulting from ingestion of Listeria-contaminated feed, soil, and/or drinking water. Indeed, it has rarely been proven that poultry feed and drinking water can be contaminated with Listeria spp. generally (54) or L. monocytogenes specifically (60, 64). Broiler flock contamination rates for L. monocytogenes can range from 3 to 32% (47, 55, 56, 58), with similar contamination rates for layer hens and turkey flocks (55–57). The number of positive samples within positive flocks is generally low, with most of the positive flocks (32 to 55.6%) represented by only one positive sample (55–57). These results suggest that poultry may not represent a common reservoir for L. monocytogenes in the grow-out farm environment, although variability in its prevalence among broiler flocks makes poultry a potential source of L. monocytogenes that should be further investigated.
Poultry can shed Listeria spp. and L. monocytogenes in fecal material and contaminate elements of the grow-out farm environment, including the poultry house where studies have shown that ≤52.5 and ≤25% of litter samples were positive for Listeria spp. and L. monocytogenes, respectively (51, 54, 59). Chemaly et al. (56) showed that L. monocytogenes detection within samples collected from caged laying hen flocks was dependent on sample type. When only L. monocytogenes-positive flocks were considered, the difference between dust and fecal samples were strongly significant, with a greater detection in feces than in dust samples (56). This may be attributed to dust samples being contaminated by contact with fecal materials shed on the floor. A potential transfer of Listeria spp. between broilers and their environment has been shown by Milillo et al. (46). The circulation of Listeria spp., especially L. monocytogenes, between animals and farm environment has also been observed in ruminant farming systems (65, 66).
Poultry farm characteristics and management practices also have an influence on the prevalence of L. monocytogenes in bird flocks. These risk factors are mostly related to the hygienic status of the house and sanitary measures applied to the flocks. Aury et al. (55) identified six risk factors significantly associated with L. monocytogenes contamination at the end of the broiler flocks production cycle (55). The risk of L. monocytogenes contamination was increased when (i) farmers did not respect the principle of two areas (clean and dirty) at the poultry house entrance, (ii) disinfection was not carried out between flocks by spraying, (iii) there was an absence of pest control of the poultry house before the arrival of the next flock, (iv) litter was not protected during storage, (v) farm staff cared for other broiler houses, and (vi) the watering system did not consist of nipples with cups. Within the same study, three risk factors significantly increased L. monocytogenes contamination in caged hen flocks at the end of the laying period: (i) presence of pets in the production site, (ii) type of feed (use of meal rather than crumbs/crumbles), and (iii) insufficient or incomplete removal of fecal droppings (e.g., conveyor belt with deep pit storage or deep pit only methods not used) (55).
The prevalence of L. monocytogenes contamination may also be dependent of the type of production system. No significant differences in the prevalence of Listeria spp. were found on and within eggs and in the environment of a sister flock of conventional cage and free-range laying hens. In this study, L. monocytogenes represented 28.5% (2/7) of the Listeria spp. isolated (48). This result is supported by Schwaiger et al. (49) who compared cloacal swabs from 20 conventional and organic egg farms in Germany and found no significant difference in Listeria spp. between production methods (49). Conversely, Chemaly et al. (56) showed a significant difference between caged- and floor-reared hens with a greater detection of L. monocytogenes in dust samples from floor-reared hens, in L. monocytogenes-positive flocks (56). Alternatively raised broilers (e.g., all natural, pastured) represent management systems with unknown food safety implications, considering these poultry are raised in less controlled environments than conventionally raised birds (67). Poultry farms frequently have other animals (beef cattle, sheep, goats, or swine) and pets present on the production site (67). These animals can be reservoirs for and play a role in the multiplication and excretion of L. monocytogenes into the environment. The presence of other animals during grow-out was found to increase the risk of L. monocytogenes contamination in laying hen flocks (55). Because L. monocytogenes is commonly associated with other farm animals and is a natural saprophyte, the occurrence of L. monocytogenes in alternatively raised poultry is of particular interest.
Few studies have identified L. monocytogenes from poultry farms at the serogroup level. From these, serovar 1/2a represented a dominant proportion of the isolates regardless of the bird species (46, 56, 57). To a lesser extent, serovars 1/2b and 4e/4b were also identified in laying hen, broiler and turkey flocks (56–58). Although serovar 1/2a did not differ between caged- and floor-reared hens, or between standard and free-range systems for broilers, serovars 1/2b and 4e/4b were significantly more prevalent in broilers (56). Although rarer in poultry than other important foodborne pathogens (Salmonella, Campylobacter), the above discussions show that Listeria spp., and specifically L. monocytogenes, can be present both within the birds (ceca and feces), and the grow-out farm environment. These data highlight the potential for the live birds to be a vector for this pathogen to enter the processing environment, which is the side most commonly viewed as the greatest risk for L. monocytogenes contamination.
Potential for Listeric Infections in Poultry
Although poultry can be an asymptomatic carrier of L. monocytogenes, they can also develop, in rare cases, listeric infections (27, 62). Only a few sporadic clinical outbreaks have been described. An outbreak of listeriosis was reported in a backyard poultry flock in Washington State was attributed to serotype 4b the source of the infection (26). L. monocytogenes serovar 4b was also involved in an outbreak of listeriosis in a pheasant breeder farm of Jingzhou, Hubei Province, China (68). There is greater evidence for potential systemic Listeria infection in other avian species, such as turkeys. Huff et al. (69) demonstrated in young turkey poults inoculated with a high or low dose of L. monocytogenes Scott A in the air sac that the high dosed birds reached 100% mortalities in 2 weeks, and the Listeria challenge strain could be isolated from the liver, pericardium, brain, both knee joints, suggesting that L. monocytogenes Scott A could be invasive through the respiratory system of susceptible turkey poults. In a follow-up challenge study comparing oral or oculonasal routes, Huff et al. (70) demonstrated that the oculonasal route led to greater mortalities and lower body weights than orally challenged birds. Stress may be a factor as well. When Huff et al. (71) exposed 13-week-old male turkeys to an immunosuppressive treatment and stress associated with transport, they observed an increase Listeria colonization in older birds. There are no comparable studies conducted with commercial poultry but a recent study by Jarvis et al. (72) demonstrated that L. monocytogenes strains could infect HD11 chicken macrophage-like cells and that infection leads to an initial halt in growth of the HD11 cells for at least 11 h before the HD11 cells begin to lyse. The authors used this as evidence to suggest there could be sufficient time for Listeria infected macrophages to circulate in the blood and potentially infect other tissues in the chicken. Therefore, it would be interesting to compare cellular mechanisms of these Listeria infected HD11 cells with other avian species such as turkeys as well non-avian macrophages such as those from mice.
Conclusion
Listeria is considered one of the major bacterial foodborne pathogens but it is often not considered epidemiologically important in poultry production, although there is nothing about the poultry production and processing environments that preclude the survival and persistence of Listeria spp. While sporadic and very isolated cases of listeriosis have been attributed to poultry, Listeria spp., and specifically L. monocytogenes have been isolated from all stages of the poultry production and processing continuum. Grow-out farm studies show that live birds are an important potential vector for Listeria contamination of the processing environment. Different studies have described factors related to the survival of Listeria within processing facilities, but there is a paucity of evidence linking live production and processing environments. The following food safety-related question must be asked: Does Listeria contamination of poultry meat come from poultry or its environment? To address this question, there is a need to better understand the genetics of Listeria spp. and L. monocytogenes isolated from poultry environments as compared to other sources and listeriosis outbreaks attributed to poultry. If the epidemiological and genetic factors related to Listeria prevalence and pathogenicity can be elucidated, there is an opportunity to better assess the potential public health effects of Listeria from the poultry industry and develop management practices or treatments to mitigate these effects.
Author Contributions
The authors declare that there is no conflict of interest and contribution was equally distributed among authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with several of the authors MR, AL, and TM and states that the process nevertheless met the standards of a fair and objective review.
References
2. Shivaprasad HL, Kokka R, Walker RL. Listeriosis in a cockatiel (Nymphicus hollandicus). Avian Dis (2007) 51(3):800–4. doi:10.1637/0005-2086(2007)51[800:LIACNH]2.0.CO;2
3. Wesley IV. Listeriosis in animals. 3rd ed. In: Ryser ET, Marth EH, editors. Listeria, Listeriosis, and Food Safety (Vol. 160), Boca Raton: CRC Press (2007). 873 p.
4. Goh SG, Kuan CH, Loo YY, Chang WS, Lye YL, Soopna P, et al. Listeria monocytogenes in retailed raw chicken meat in Malaysia. Poult Sci (2012) 91(10):2686–90. doi:10.3382/ps.2012-02349
5. Dhama K, Verma AK, Rajagunala S, Kumar A, Tiwari R, Chakrabort S, et al. Listeria monocytogenes infection in poultry and its public health importance with special reference to food borne zoonoses. J Biol Sci (2013) 16(7):301–8. doi:10.3923/pjbs.2013.301.308
6. Milillo SR, Friedly EC, Saldivar JC, Muthaiyan A, O’Bryan C, Crandall PG, et al. A review of the ecology, genomics, and stress response of Listeria innocua and Listeria monocytogenes. Crit Rev Food Sci Nutr (2012) 52(8):712–25. doi:10.1080/10408398.2010.507909
7. Zhu M, Du M, Cordray J, Ahn DU. Control of Listeria monocytogenes contamination in ready-to-eat meat products. Compr Rev Food Sci Food Saf (2005) 4(2):34–42. doi:10.1111/j.1541-4337.2005.tb00071.x
8. Gray MJ, Zadoks RN, Fortes ED, Dogan B, Cai S, Chen Y, et al. Listeria monocytogenes isolates from foods and humans form distinct but overlapping populations. Appl Environ Microbiol (2004) 70(10):5833–41. doi:10.1128/aem.70.10.5833-5841.2004
9. Nightingale KK, Windham K, Wiedmann M. Evolution and molecular phylogeny of Listeria monocytogenes isolated from human and animal listeriosis cases and foods. J Bacteriol (2005) 187(16):5537–51. doi:10.1128/jb.187.16.5537-5551.2005
10. Ward TJ, Usgaard T, Evans P. A targeted multilocus genotyping assay for lineage, serogroup, and epidemic clone typing of Listeria monocytogenes. Appl Environ Microbiol (2010) 76(19):6680–4. doi:10.1128/aem.01008-10
11. Liu D, Lawrence ML, Wiedmann M, Gorski L, Mandrell RE, Ainsworth AJ, et al. Listeria monocytogenes subgroups IIIA, IIIB, and IIIC delineate genetically distinct populations with varied pathogenic potential. J Clin Microbiol (2006) 44(11):4229–33. doi:10.1128/JCM.01032-06
12. Nadon CA, Woodward DL, Young C, Rodgers FG, Wiedmann M. Correlations between molecular subtyping and serotyping of Listeria monocytogenes. J Clin Microbiol (2001) 39(7):2704–7. doi:10.1128/JCM.39.7.2704-2707.2001
13. Tsai Y-HL, Maron SB, McGann P, Nightingale KK, Wiedmann M, Orsi RH. Recombination and positive selection contributed to the evolution of Listeria monocytogenes lineages III and IV, two distinct and well supported uncommon L. monocytogenes lineages. Infect Genet Evol (2011) 11(8):1881–90. doi:10.1016/j.meegid.2011.08.001
14. Lianou A, Stopforth JD, Yoon Y, Wiedmann M, Sofos JN. Growth and stress resistance variation in culture broth among Listeria monocytogenes strains of various serotypes and origins. J Food Prot (2006) 69(11):2640–7. doi:10.4315/0362-028x-69.11.2640
15. Berzins A, Terentjeva M, Korkeala H. Prevalence and genetic diversity of Listeria monocytogenes in vacuum-packaged ready-to-eat meat products at retail markets in Latvia. J Food Prot (2009) 72(6):1283–7.
16. Kramarenko T, Roasto M, Meremäe K, Kuningas M, Põltsama P, Elias T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control (2013) 30(1):24–9. doi:10.1016/j.foodcont.2012.06.047
17. McCollum JT, Cronquist AB, Silk BJ, Jackson KA, O’Connor KA, Cosgrove S, et al. Multistate outbreak of listeriosis associated with cantaloupe. N Engl J Med (2013) 369(10):944–53. doi:10.1056/NEJMoa1215837
18. Tompkin RB. Control of Listeria monocytogenes in the food-processing environment. J Food Prot (2002) 65(4):709–25. doi:10.4315/0362-028X-65.4.709
19. Vongkamjan K, Fuangpaiboon J, Jirachotrapee S, Turner MP. Occurrence and diversity of Listeria spp. in seafood processing plant environments. Food Control (2015) 50:265–72. doi:10.1016/j.foodcont.2014.09.001
20. Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis (1999) 5(5):607–25. doi:10.3201/eid0505.990502
21. Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, et al. Incidence and trends of infection with pathogens transmitted commonly through food – Foodborne Diseases Active Surveillance Network, 10 US sites, 2006–2013. MMWR Morb Mortal Wkly Rep (2014) 63(15):328–32.
22. Silk BJ, Date KA, Jackson KA, Pouillot R, Holt KG, Graves LM, et al. Invasive listeriosis in the Foodborne Diseases Active Surveillance Network (FoodNet), 2004-2009: further targeted prevention needed for higher-risk groups. Clin Infect Dis (2012) 54(Suppl 5):S396–404. doi:10.1093/cid/cis268
23. Ferreira V, Wiedmann M, Teixeira P, Stasiewicz MJ. Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J Food Prot (2014) 77(1):150–70. doi:10.4315/0362-028X.JFP-13-150
24. Jeffers GT, Bruce JL, McDonough PL, Scarlett J, Boor KJ, Wiedmann M. Comparative genetic characterization of Listeria monocytogenes isolates from human and animal listeriosis cases. Microbiology (2001) 147(5):1095–104. doi:10.1099/00221287-147-5-1095
25. Centers for Disease Control and Prevention. Listeria Outbreaks (2017). Available from: https://www.cdc.gov/listeria/outbreaks/index.html
26. Crespo R, Garner MM, Hopkins SG, Shah DH. Outbreak of Listeria monocytogenes in an urban poultry flock. BMC Vet Res (2013) 9(1):1. doi:10.1186/1746-6148-9-204
27. Gray ML, Killinger A. Listeria monocytogenes and listeric infections. Bacteriol Rev (1966) 30(2):309.
28. Njagi LW, Mbuthia PG, Bebora LC, Nyaga PN, Minga U, Olsen JE. Carrier status for Listeria monocytogenes and other Listeria species in free range farm and market healthy indigenous chickens and ducks. East Afr Med J (2004) 81(10):529–33. doi:10.4314/eamj.v81i10.9236
29. Kurazono M, Nakamura K, Yamada M, Yonemaru T, Sakoda T. Pathology of listerial encephalitis in chickens in Japan. Avian Dis (2003) 47(4):1496–502. doi:10.1637/7066
30. National Chicken Council. Animal Welfare Guidelines and Audit Checklist for Broilers. Washington, DC: NCC (2014).
32. European Commission. Commissioner Kyprianou Welcomes Council Agreement on Animal Welfare Rules for Broilers. Brussels: European Commission, DG Consumer Protection and Health (2007).
33. Tauson R. Management and housing systems for layers – effects on welfare and production. Worlds Poult Sci J (2005) 61(03):477–90. doi:10.1079/WPS200569
34. Van Horne P, Achterbosch T. Animal welfare in poultry production systems: impact of EU standards on world trade. Worlds Poult Sci J (2008) 64(01):40–52. doi:10.1017/S0043933907001705
35. Carey J, Lacey R, Mukhtar S. A review of literature concerning odors, ammonia, and dust from broiler production facilities: 2. Flock and house management factors. J Appl Poult Res (2004) 13(3):509–13. doi:10.1093/japr/13.3.509
36. National Research Council; Subcommittee on Poultry Nutrition. Nutrient Requirements of Poultry. 9th ed. Washington, DC: National Academies (1994).
38. Cox N, Bailey J, Berrang M. The presence of Listeria monocytogenes in the integrated poultry industry. J Appl Poult Res (1997) 6(1):116–9.
39. Kanarat S, Jitnupong W, Sukhapesna J. Prevalence of Listeria monocytogenes in chicken production chain in Thailand. Thai J Vet Med (2011)41(2):155. doi:10.1.1.998.9679
40. Heyndrickx M, Vandekerchove D, Herman L, Rollier I, Grijspeerdt K, De Zutter L. Routes for Salmonella contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect (2002) 129(02):253–65. doi:10.1017/S0950268802007380
41. Cox NA, Bailey JS, Mauldin JM, Blankenship LC. Presence and impact of Salmonella contamination in commercial broiler hatcheries. Poult Sci (1990) 69(9):1606–9. doi:10.3382/ps.0691606
42. Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I, De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect (2003) 131(03):1169–80. doi:10.1017/S0950268803001183
43. Petersen L, Nielsen EM, On SL. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Vet Microbiol (2001) 82(2):141–54. doi:10.1016/S0378-1135(01)00382-0
44. Berrang ME, Lyon CE, Smith DP, Northcutt JK. Incidence of Listeria monocytogenes on pre-scald and post-chill chicken. J Appl Poult Res (2000) 9(4):546–50. doi:10.1093/japr/9.4.546
45. Verghese B, Lok M, Wen J, Alessandria V, Chen Y, Kathariou S, et al. comK Prophage junction fragments as markers for Listeria monocytogenes genotypes unique to individual meat and poultry processing plants and a model for rapid niche-specific adaptation, biofilm formation, and persistence. Appl Environ Microbiol (2011) 77(10):3279–92. doi:10.1128/aem.00546-11
46. Milillo S, Stout J, Hanning I, Clement A, Fortes E, Den Bakker H, et al. Listeria monocytogenes and hemolytic Listeria innocua in poultry. Poult Sci (2012) 91(9):2158–63. doi:10.3382/ps.2012-02292
47. Petersen L, Madsen M. Listeria spp. in broiler flocks: recovery rates and species distribution investigated by conventional culture and the EiaFoss method. Int J Food Microbiol (2000) 58(1):113–6. doi:10.1016/S0168-1605(00)00258-0
48. Jones D, Anderson K, Guard J. Prevalence of coliforms, Salmonella, Listeria, and Campylobacter associated with eggs and the environment of conventional cage and free-range egg production. Poult Sci (2012) 91(5):1195–202. doi:10.3382/ps.2011-01795
49. Schwaiger K, Schmied E, Bauer J. Comparative analysis on antibiotic resistance characteristics of Listeria spp. and Enterococcus spp. isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, Germany. Zoonoses Public Health (2010) 57(3):171–80. doi:10.1111/j.1863-2378.2008.01229.x
50. Iida T, Kanzaki M, Maruyama T, Inoue S, Kaneuchi C. Prevalence of Listeria monocytogenes in intestinal contents of healthy animals in Japan. J Vet Med Sci (1991) 53(5):873–5. doi:10.1292/jvms.53.873
51. Fenlon D, Wilson J, Donachie W. The incidence and level of Listeria monocytogenes contamination of food sources at primary production and initial processing. J Appl Bacteriol (1996) 81(6):641–50. doi:10.1111/j.1365-2672.1996.tb01966.x
52. Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, et al. Comparative genomics of Listeria species. Science (2001) 294(5543):849–52. doi:10.1126/science.1063447
53. Ojeniyi B, Wegener HC, Jensen N, Bisgaard M. Listeria monocytogenes in poultry and poultry products: epidemiological investigations in seven Danish abattoirs. J Appl Bacteriol (1996) 80(4):395–401. doi:10.1111/j.1365-2672.1996.tb03234.x
54. Dahshan H, Merwad A, Mohamed TS. Listeria species in broiler poultry farms: Potential public health hazards. J Microbiol Biotechnol (2016) 26(9):1551–6. doi:10.4014/jmb.1603.03075
55. Aury K, Le Bouquin S, Toquin MT, Huneau-Salaün A, Le Nôtre Y, Allain V, et al. Risk factors for Listeria monocytogenes contamination in French laying hens and broiler flocks. Prev Vet Med (2011) 98(4):271–8. doi:10.1016/j.prevetmed.2010.11.017
56. Chemaly M, Toquin MT, Le Nôtre Y, Fravalo P. Prevalence of Listeria monocytogenes in poultry production in France. J Food Prot (2008) 71(10):1996–2000. doi:10.4315/0362-028X-71.10.1996
57. Aury-Hainry K, Le Bouquin S, Labbé A, Petetin I, Chemaly M. Listeria monocytogenes contamination in French breeding and fattening turkey flocks. J Food Prot (2011) 74(7):1096–103. doi:10.4315/0362-028X.JFP-10-540
58. Esteban JI, Oporto B, Aduriz G, Juste RA, Hurtado A. A survey of food-borne pathogens in free-range poultry farms. Int J Food Microbiol (2008) 123(1):177–82. doi:10.1016/j.ijfoodmicro.2007.12.012
59. Roberts B, Bailey R, McLaughlin M, Miles D, Brooks J. Spatial and temporal analysis of microbial populations in production broiler house litter in the southeastern United States. J Appl Poult Res (2013) 22(4):759–70. doi:10.3382/japr.2012-00688
60. Whyte P, McGill K, Collins J. A survey of the prevalence of Salmonella and other enteric pathogens in a commercial poultry feed mill. J Food Saf (2003) 23(1):13–24. doi:10.1111/j.1745-4565.2003.tb00348.x
61. Skovgaard N, Morgen C-A. Detection of Listeria spp. in faeces from animals, in feeds, and in raw foods of animal origin. Int J Food Microbiol (1988) 6(3):229–42. doi:10.1016/0168-1605(88)90015-3
62. Wesley IV. Listeriosis in animals. Listeria, Listeriosis, and food safety. 3rd ed. Boca Raton: CRC Press (2007). p. 55–84.
63. Husu J, Beery J, Nurmi E, Doyle M. Fate of Listeria monocytogenes in orally dosed chicks. Int J Food Microbiol (1990) 11(3):259–69. doi:10.1016/0168-1605(90)90019-2
64. Blank G, Savoie S, Campbell LD. Microbiological decontamination of poultry feed – evaluation of steam conditioners. J Sci Food Agric (1996) 72(3):299–305. doi:10.1002/(SICI)1097-0010(199611)72:3<299::AID-JSFA656>3.0.CO;2-A
65. Nightingale K, Schukken Y, Nightingale C, Fortes E, Ho A, Her Z, et al. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl Environ Microbiol (2004) 70(8):4458–67. doi:10.1128/AEM.70.8.4458-4467.2004
66. Fox EM, Leonard N, Jordan K. Molecular diversity of Listeria monocytogenes isolated from Irish dairy farms. Foodborne Pathog Dis (2011) 8(5):635–41. doi:10.1089/fpd.2010.0806
67. Rothrock MJ Jr, Hiett KL, Guard JY, Jackson CR. Antibiotic resistance patterns of major zoonotic pathogens from all-natural, antibiotic-free, pasture-raised broiler flocks in the southeastern United States. J Environ Qual (2016) 45(2):593–603. doi:10.2134/jeq2015.07.0366
68. Gu Y, Liang X, Huang Z, Yang Y. Outbreak of Listeria monocytogenes in pheasants. Poult Sci (2015) 94(12):2905–8. doi:10.3382/ps/pev264
69. Huff GR, Huff WE, Beasley JN, Rath NC, Johnson MG, Nannapeneni R. Respiratory infection of turkeys with Listeria monocytogenes Scott A. Avian Dis (2005) 49:551–7. doi:10.1637/7375-05040R.1
70. Huff GR, Huff WE, Dutta V, Johnson MG, Nannapaneni R. Pathogenicity of Listeria monocytogenes Scott A after oral and oculonsal challenges of day-old turkey poults. Avian Dis (2008) 52:444–50. doi:10.1637/8244-013008-Reg.1
71. Huff GR, Dutta V, Huff WE, Johnson MG, Nannapaneni R, Saylor R. Co-infection of market-age turkeys with Escherichia coli and Listeria monocytogenes in two stress models. Avian Dis (2009) 54:495–501. doi:10.1637/8675-030309-Reg.1
Keywords: Listeria, poultry, live production, isolation, detection
Citation: Rothrock MJ Jr., Davis ML, Locatelli A, Bodie A, McIntosh TG, Donaldson JR and Ricke SC (2017) Listeria Occurrence in Poultry Flocks: Detection and Potential Implications. Front. Vet. Sci. 4:125. doi: 10.3389/fvets.2017.00125
Received: 27 March 2017; Accepted: 25 July 2017;
Published: 11 August 2017
Edited by:
Michael Kogut, Agricultural Research Service (USDA), United StatesReviewed by:
Jo Stevens, University of Edinburgh, United KingdomChuck Czuprynski, University of Wisconsin-Madison, United States
Copyright: © 2017 Rothrock, Davis, Locatelli, Bodie, McIntosh, Donaldson and Ricke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven C. Ricke, c3JpY2tlQHVhcmsuZWR1
 Michael J. Rothrock Jr.
Michael J. Rothrock Jr. Morgan L. Davis
Morgan L. Davis Aude Locatelli
Aude Locatelli Aaron Bodie
Aaron Bodie Tori G. McIntosh
Tori G. McIntosh Janet R. Donaldson
Janet R. Donaldson Steven C. Ricke
Steven C. Ricke