- 1Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, CA, USA
- 2California Animal Health and Food Safety Laboratory System, School of Veterinary Medicine, University of California, Davis, CA, USA
- 3VCA Sacramento Veterinary Referral Center, Sacramento, CA, USA
Contamination of recreational waters with cyanobacterial toxins continues to increase and presents a risk to animals and humans. Although cases of acute hepato- and neurotoxicoses in dogs following cyanotoxin exposure exist, no reports of skin-related reactions in dogs exist. A 5-year-old female spayed 34 kg Bracco Italiano was initially presented for rapid onset of severe pruritus and urticaria. Marked excoriation and erythema were noted over the chest and neck, while urticaria was noted in the inguinal regions and ventral abdomen. Initial basic dermatology work-up excluded parasitic, fungal, and bacterial organisms. Due to the severity and progression of urticaria, the dog received IV dexamethasone and IM diphenhydramine. Improvement of the urticaria and the dog’s clinical status was noted over the next 45 min. Assessment of the dog’s environment revealed access to a lake on the property with visible algal bloom. Water from the lake was submitted for toxicology testing and revealed the presence of debromoaplysiatoxin. Access to the lake was discontinued and follow-up evaluation over the next few weeks revealed a complete resolution of the skin irritation. To the authors’ knowledge, this is the first case report of debromoaplysiatoxin exposure in a dog after swimming in cyanobacteria-contaminated water. Veterinarians should recognize the potential harm that contaminated waters may cause in terms of dermal, hepatic, and neurological conditions. In addition, more prudent oversight of contaminated recreational waters is recommended for animals and humans to prevent adverse events and intoxications.
Introduction
Cyanobacteria poisoning in dogs is not a new phenomenon. Most reports document exposure to hepatotoxic microcystins (1–4) and neurotoxic anatoxin-a (5, 6). In contrast, documentation of the irritant and allergenic effects of cyanotoxins is limited with most reports referring to studies in laboratory animals (7, 8) or epidemiological studies in humans (9–12). Although skin irritation after exposure to recreational waters is routinely mentioned as a health risk, a majority of effort has been focused on the association between total coliform, fecal coliform, and E. coli and skin-related symptoms (13) and not cyanobacteria. Dermatotoxins produced by cyanobacteria have been linked to outbreaks of skin irritation and include aplysiatoxins, debromoaplysiatoxins, and lyngbyatoxins. However, cyanobacterial dermatotoxins were not widely recognized until the late 1950s when an epidemic of acute contact dermatitis was reported in bathers along the beaches of Oahu, Hawaii (14). Exposure to Lyngbya majuscula was suspected and studies using purified extracts from L. majuscula positively identified exposure to this cyanobacterium as the inciting cause (15). Nevertheless, it would take more than a decade before the exact toxin, debromoaplysiatoxin, was isolated and identified (16). Unfortunately our understanding of cyanobacteria-associated dermatologic illnesses from exposure to freshwater is lacking with most acute dermatitis studies focusing on marine cyanobacteria such as the Hawaii epidemic.
A 2006 review of anecdotal and case reports and epidemiological studies of recreational and occupational exposure to freshwater cyanobacteria found that the true incidence of acute cyanobacteria-associated illness is unknown, likely due to under-recognition and under-diagnosis by healthcare providers (10). As our understanding of the toxins associated with cyanobacteria increase and our ability to detect the various toxins in biologic samples expand, linking exposure to illness will become easier. In addition, given the association between increasing temperatures and increased frequency of cyanobacteria blooms around the world, the incidence of cyanobacteria-associated illness will likely increase (17). Changing climates and anthropogenic activities are also likely to have an impact; therefore, healthcare providers and veterinarians will have to be vigilant when it comes to evaluating all aspects of adverse outcomes to cyanotoxins from recreational waters.
Case Presentation
Within 24 h of swimming in a lake in Northern California, a 5-year-old, 34-kg, spayed female Bracco Italiano developed severe pruritus, urticaria, and malaise and presented to the VCA Sacramento Veterinary Referral Center (SVRC) for evaluation. On evaluation, a large area of excoriation with erythema was noted over the cranial chest and at the base of the neck. In addition, the pinnae were erythematous and there was frequent head shaking by the dog. On otoscopic examination, the tympanic membranes were intact with only a small amount of cerumin noted; no erythema was seen within the canals. The dog’s vulva was also thickened and erythematous with urticaria noted on the skin adjacent. Urticaria was evident in the inguinal regions and ventral abdomen and appeared to progress during the physical evaluation. A skin scraping of the cranial thorax revealed no evidence of parasites. An impression smear, also of the cranial thorax, revealed degenerate neutrophils, a few eosinophils, and no bacteria or fungal organisms. No fungal organisms or bacteria were found on cytology of the ear.
Due to the progression of urticaria over the inguinal region, ventral abdomen, and shoulders during the initial evaluation, a hypersensitivity reaction to an unknown stimulus was suspected. The dog was given dexamethasone SP (0.02 mg/kg IV) and diphenhydramine (2 mg/kg IM) and kept for observation for the next 45 min. During that time, the urticaria appeared to be resolving, although there was still some residual erythema along the cranial thorax and the head shaking continued. Concern by the owner for the possibility of blue-green algae intoxication prompted the collection of blood for selected chemistries, CBC, and venous blood gas analysis. Abnormalities were only identified on the CBC and consisted of a mild neutrophilia (12,940 cells/μL; ref range: 3,000–10,500 cells/μL). The dog was discharged on cephalexin (1000 mg PO q 12 h × 14 days), prednisone (30 mg PO q 12 h for 7 days, followed by tapering to q 48 h for 7 days), and diphenhydramine (75 mg PO q 8–12 h PRN × 5 days). Referral to a dermatology clinic was also recommended if the erythema and head shaking continued. Overnight, the dog vomited several times and developed diarrhea. She was presented to VCA SVRC the next morning and continued to vomit bile and was inappetent. On physical examination, the dog was alert, eupneic, dehydrated, and the abdomen palpated as non-painful. Due to the recent history of hypersensitivity reaction, GI shock was considered the most likely cause for vomiting and diarrhea; however, other underlying causes were also considered especially because of the dog’s previous history of eosinophilic and lymphocytic-plasmacytic enteritis. The owner elected inpatient care consisting of the administration of LRS (on a 5% rehydration rate over 6 h, IV), famotidine (1 mg/kg, IV, q 24 h), metronidazole (10 mg/kg, IV, q 12 h), dolasetron (0.6 mg/kg, IV, q 24 h), and diphenhydramine (2 mg/kg, IM, TID). She was also placed on NPO before being referred to the VCA critical care unit for further monitoring, where clinical improvement was noted over the next 48 h. The dog was sent home after 2 days of hospitalization. Follow-up evaluation after 1 week revealed a complete resolution of clinical signs.
Careful evaluation of the dog’s environment revealed access to several lakes with visible algal bloom (Figures 1 and 2). Water from the lake the dog had access to prior to developing illness was submitted for phycological and toxicological evaluation. Microscopic observation of the water sample (Green Water Laboratories/Cyanolab, Palatka, FL, USA) showed a dominant presence of a tiny vascular plant Wolffia columbiana (common name: watermeal), a high density of motile bacterial rods and bacterial filaments, and an abundant filamentous cyanophyte called Microchaete cf. uberrima. The water sample also contained green algae (Chlorophyta) and diatoms (Bacillariophyta). Specific to cyanobacteria, the water sample contained Microchaete cf. uberrima, Lyngbya sp. (Figure 3), Pseudanabaena sp., Anabaena/Trichormus sp. (Figure 4), Microchaete sp., Scytonema sp., Calothrix sp., and Aphanothece elabens. Amongst the cyanobacteria identified, only Anabaena/Trichormus sp. and Lyngbya sp. were of toxicological significance. Unfortunately, positive species identification for Anabaena/Trichormus sp. and Lyngbya sp. could not be achieved from collected specimens.

Figure 2. Lake surface covered with a dense growth of Wolffia columbiana. W. columbiana is a small, free-floating aquatic plant commonly known as watermeal and native to California. Watermeal is non-toxic and thrives in nutrient-rich water, conditions suitable for cyanbacteria growth and toxin production.
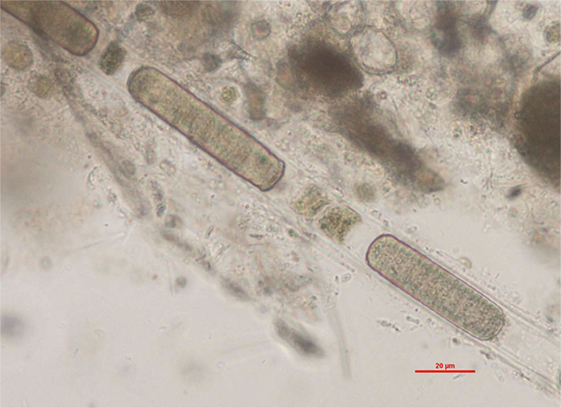
Figure 3. Lyngbya sp. identified in the lake water. Filamentous cyanobacteria with a thin, colorless sheath. Wet mount of lake water, ×400 (scale bar = 20 μm).
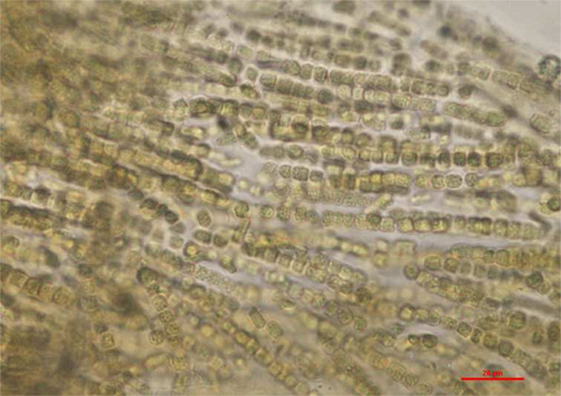
Figure 4. Anabaena/Trichormus sp. identified in the lake water. Only one colony of filaments was observed in the sample. Akinetes, important characteristics morphological features were not present which prevented further identification to the lowest possible taxonomic level. Wet mount of lake water, ×400 (scale bar = 20 μm).
The water samples were also analyzed for cyanotoxins by LC-MS/MS (Green Water Laboratories/Cyanolab, Palatka, FL, USA and the California Animal Health and Food Safety Laboratory, University of California, Davis, CA, USA). The water contained 3.8 μg/g debromoaplysiatoxin [limit of detection (LOD) 0.3 μg/g]. The water also contained a low concentration of anatoxin-a that was estimated to be 1 μg/L. The water contained no aplysiatoxin (LOD 0.5 μg/g), lyngbyatoxin-a (LOD 0.2 μg/g), or cylindrospermopsin (LOD 1 μg/L).
Discussion
The current case study confirms a case of debromoaplysiatoxin poisoning in a dog following access to contaminated lake water and highlights the potential for serious skin irritation after cyanotoxin exposure. While most of the toxic cyanobacteria are freshwater species, the first documentation of algal toxin-induced dermatitis dates back to 1958 when swimmers along the beaches of Oahu, Hawaii, developed acute, vesicular contact dermatitis (14). A similar outbreak in 1976 again on the shores of Oahu, Hawaii was referred to as “swimmers’ itch” (18). In the past decade, numerous outbreaks in swimmers have occurred in Australia and Hawaii (19, 20). Clinical findings in people include gradual itching and burning progressing to reddening and swelling of the skin with blister formation especially in areas covered by a bathing suit, where the seaweed/toxins are trapped. Desquamation of blisters can lead to skin erosions. The dermatitis typically resolves completely within 1 week. In more severe cases, symptomatic treatment with analgesics, antihistamine, and steroids is provided. Lyngbya dermatitis can develop after only minutes of exposure to contaminated waters; airborne contact dermatitis on faces has also been noted during weather patterns with strong winds. The dog in this case developed severe pruritus and urticaria within 24 h of swimming in contaminated lake water. Prompt treatment with dexamethasone and diphenhydramine prevented progression to a more severe dermatitis. Within 1 week, the dog’s skin condition had resolved completely.
Isolation of the actual dermatotoxins from L. majuscula and from the digestive gland of the sea hare Stylocheilus longicauda occurred in the early 1970s (21, 22). Subsequent toxicity studies with debromoaplysiatoxin in laboratory animals and human volunteers confirmed this compound to be a potent primary skin irritant (23). Mice and rabbits developed erythema, edema, and sloughing of skin within 6 h of topical application of a 0.05% debromoaplysiatoxin solution. Human volunteers described tingling and burning sensation after application and developed an irritant pustular folliculitis between 6 and 12 h of topical administration that required up to 10 days to resolve. Debromoaplysiatoxin was subsequently isolated from other tropical marine cyanobacteria including Lyngbya gracilis, Oscillatoria nigroviridis, and Schizothrix calcicola (16). In the freshwater environment, the filamentous, benthic cyanobacterium Lyngbya wollei is now the cause for common proliferations in lakes and rivers from the St. Lawrence/Great Lakes basin to Florida (24). In the lake sample collected for this case work-up, the presence of Lyngbya sp. was confirmed by phycological evaluation. Unfortunately, only one colony of filaments was observed in the presence, which did not allow for species identification. The finding of Lyngbya sp. in the lake water is not surprising, considering L. wollei has been recorded in three sites of California streams (25). L. wollei has the ability to produce a wide range of toxins including paralytic shellfish toxins, and hepatotoxic cylindrospermopsin and its derivatives (26). Currently, it is unclear whether L. wollei is capable of producing dermatotoxins. Interestingly, most recent review articles provide in-depth information on the neuro- and hepatotoxicity of cyanotoxins but highlight the need for further examination of dermatotoxins because they bear major potential public health consequences for recreational users, while there are very limited toxicity and geographic distribution data available (27–29). In addition to debromoaplysiatoxin, aplysiatoxin and lyngbyatoxins have been associated with skin irritation (30). A debromoaplysiatoxin-producing Lyngbya bloom that occurred in the Homosassa River in 2008 was hypothesized as the cause for ulcerative dermatitis in manatees (31). Thus, while debromoaplysiatoxin is predominantly detected in marine water, one report documents its occurrence in freshwater ecosystems in Florida. Our report adds additional occurrence data of debromoaplysiatoxin in freshwater and is the first documentation of debromoaplysiatoxin in freshwater from a Northern California lake resulting in skin irritation in a dog. Debromoaplysiatoxin produced dermatitis on the murine ear at 0.005 nmol (equivalent to 2.7 ng) per ear (32). Considering an estimated biomass of 30 mg/L dry weight in the lake water, 1 L of water would have contained 114 ng of debromoaplysiatoxin. Based on the limited toxicity data available for topical exposure, the concentrations found are considered to be capable of resulting in dermal irritation. The lake water contained no detectable amounts of aplysiatoxin or lyngbyatoxin-a. Debromoaplysiatoxin, aplysiatoxin, and lyngbyatoxins exert their toxic effects by binding and activating protein kinase C isozymes (33). PKC belongs to a family of serine/threonine kinases and plays important roles in cellular signaling transduction for proliferation, differentiation, and apoptosis (34); activation of PKC can induce cutaneous inflammation (35). Debromoaplysiatoxin and its brominated analog aplysiatoxin can also cause diarrhea in animals and humans (36, 37). The dog developed diarrhea and vomiting approximately 48 h after swimming in the lake. These gastrointestinal signs could have been a direct toxic insult of debromoaplysiatoxin on the intestine similar to what has been confirmed after oral administration of aplysiatoxin to mice (38). However, because of the dog’s previous history of eosinophilic and lymphocytic-plasmacytic enteritis, other underlying causes were also considered.
It is important to consider the role of anatoxin-a in the clinical presentation of this dog since anatoxin-a was detected in the lake water sample. Anatoxin-a is a potent cholinergic agonist at nicotinic acetylcholine receptors in neurons and at the neuromuscular junctions and can lead to muscle tremors and, in severe cases, seizures and respiratory paralysis (6, 39). The toxin is produced by many different cyanobacteria including as Anabaena, Plantkothrix, Oscillatoria, Microcystis, Aphanizomenon, Cylindrospermum, and Phormidium sp. Anatoxin-a has been found in rivers and lakes in CA (39). Anabaena/Trichormus sp. was identified in the water sample; due to lack of akinetes in the sample, further identification to the species level was not possible. However, the fact that anatoxin-a was identified in the water sample is a clear indication of the presence of a toxin producer. Fortunately, the dog did not develop neurologic signs, which is likely attributable to exposure to a dose below the toxic threshold. In other reported anatoxin-a poisoning cases, values of anatoxin-a ranging from 0.01 mg/L to 1 mg/L were detected (6, 39). In contrast, an estimated concentration of 1 μg/L was found in this case. This finding highlights the importance of toxicological evaluation of suspect water sample. While phycological examination of a water sample aids in the diagnostic approach of a suspect cyanotoxins exposure, it is important to note that the production of toxins by cyanobacteria is strain specific, and morphological observations alone cannot predict the hazard level. Thus, detection of toxins is needed to confirm a suspect intoxication.
Concluding Remarks
This study adds an additional toxin, debromoaplysiatoxin, to the list of freshwater toxins resulting in acute illness in animals. The presence of debromoaplysiatoxin in recreational waters has health implications for pets and humans using environments containing high concentrations of this cyanotoxin. Our investigation offers insight into the dermatotoxic risk from cyanotoxin exposure in companion animals, as well as the risk for co-exposure to multiple toxins and a complex clinical presentation. To the authors’ knowledge, this is the first documentation of debromoaplysiatoxin-induced skin irritation in a dog. Owners and veterinarians should recognize the potential harm in allowing dogs to access cyanobacteria-contaminated waters. If adverse effects are noted, access to water must be denied, and supportive care should be initiated. In addition, more prudent oversight of recreational waters is recommended to prevent adverse events/intoxications not only for companion animals but also for humans.
Author Contributions
AB was responsible for the toxicology case work-up and interpretation of test results. CW performed the clinical work-up and care of the dog. BP drafted the manuscript, mentored AB with the toxicological work-up, and acquired photographic materials. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that they have no competing interests. All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors would like to thank Mrs. Sue Watkins for providing photographs and video material, Dr. Steven Gallego for assistance during the case investigation, and Ms. Elizabeth Tor for anatoxin-a analysis.
Abbreviations
IM, intramuscular; IV, intravenous; NPO, nil per os, withhold oral intake of food and fluids; PO, orally; PRN, pre re nata, when necessary; Q, every.
References
1. DeVries SE, Galey FD, Namikoshi M, Woo JC. Clinical and pathologic findings of blue-green algae (Microcystis aeruginosa) intoxication in a dog. J Vet Diagn Invest (1993) 5(3):403–8. doi: 10.1177/104063879300500317
2. Sebbag L, Smee N, van der Merwe D, Schmid D. Liver failure in a dog following suspected ingestion of blue-green algae (Microcystis spp.): a case report and review of the toxin. J Am Anim Hosp Assoc (2013) 49(5):342–6. doi:10.5326/jaaha-ms-5913
3. Bautista AC, Moore CE, Lin Y, Cline MG, Benitah N, Puschner B. Hepatopathy following consumption of a commercially available blue-green algae dietary supplement in a dog. BMC Vet Res (2015) 11:136. doi:10.1186/s12917-015-0453-2
4. Moore CE, Juan J, Lin Y, Gaskill CL, Puschner B. Comparison of protein phosphatase inhibition assay with LC-MS/MS for diagnosis of microcystin toxicosis in veterinary cases. Mar Drugs (2016) 14(3):E54. doi:10.3390/md14030054
5. Gugger M, Lenoir S, Berger C, Ledreux A, Druart JC, Humbert JF, et al. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon (2005) 45(7):919–28. doi:10.1016/j.toxicon.2005.02.031
6. Puschner B, Pratt C, Tor ER. Treatment and diagnosis of a dog with fulminant neurological deterioration due to anatoxin – a intoxication. J Vet Emerg Crit Care (San Antonio) (2010) 20(5):518–22. doi:10.1111/j.1476-4431.2010.00578.x
7. Torokne A, Palovics A, Bankine M. Allergenic (sensitization, skin and eye irritation) effects of freshwater cyanobacteria—experimental evidence. Environ Toxicol (2001) 16(6):512–6. doi:10.1002/tox.10011.abs
8. Stewart I. Recreational Exposure to Freshwater Cyanobacteria: Epidemiology, Dermal Toxicity and Biological Activity of Cyanobacterial Lipopolysaccharides. St. Lucia, QLD: The University of Queensland, School of Population Health (2005). 417 p.
9. Williamson M, Corbett S. Investigating health risks from riverine blooms of blue green algae. NSW Public Health Bull (1993) 4(3):27–9. doi:10.1071/NB93013
10. Stewart I, Webb PM, Schluter PJ, Fleming LE, Burns JW, Gantar M, et al. Epidemiology of recreational exposure to freshwater cyanobacteria–an international prospective cohort study. BMC Public Health (2006) 6(1):1. doi:10.1186/1471-2458-6-93
11. Lévesque B, Gervais M-C, Chevalier P, Gauvin D, Anassour-Laouan-Sidi E, Gingras S, et al. Prospective study of acute health effects in relation to exposure to cyanobacteria. Sci Total Environ (2014) 466:397–403. doi:10.1016/j.scitotenv.2013.07.045
12. Lin CJ, Wade TJ, Sams EA, Dufour AP, Chapman AD, Hilborn ED. A prospective study of marine phytoplankton and reported illness among recreational beachgoers in Puerto Rico, 2009. Environ Health Perspect (2016) 124(4):477–83. doi:10.1289/ehp.1409558
13. Yau V, Wade TJ, de Wilde CK, Colford JM. Skin-related symptoms following exposure to recreational water: a systematic review and meta-analysis. Water Qual Expo Health (2009) 1(2):79–103. doi:10.1007/s12403-009-0012-9
15. Grauer FH, Arnold HL Jr. Seaweed dermatitis. First report of a dermatitis-producing marine alga. Arch Dermatol (1961) 84:720–32. doi:10.1001/archderm.1961.01580170014003
16. Mynderse JS, Moore RE, Kashiwagi M, Norton TR. Antileukemia activity in the Oscillatoriaceae: isolation of debromoaplysiatoxin from Lyngbya. Science (1977) 196(4289):538–40. doi:10.1126/science.403608
17. Paerl HW, Gardner WS, Havens KE, Joyner AR, McCarthy MJ, Newell SE, et al. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae (2016) 54:213–22. doi:10.1016/j.hal.2015.09.009
18. Serdula M, Bartolini G, Moore RE, Gooch J, Wiebenga N. Seaweed itch on windward Oahu. Hawaii Med J (1982) 41(7):200–1.
19. Osborne NJ, Shaw GR. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol (2008) 8:5. doi:10.1186/1471-5945-8-5
20. Werner KA, Marquart L, Norton SA. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol (2012) 51(1):59–62. doi:10.1111/j.1365-4632.2011.05042.x
21. Moikeha SN, Chu GW, Berger LR. Dermatitis-producing alga Lygnbya majuscula Gomont in Hawaii. I. Isolation and chemical characterization of the toxic factor 1,2. J Phycol (1971) 7(1):4–8. doi:10.1111/j.0022-3646.1971.00004.x
22. Kato Y, Scheuer PJ. Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk Stylocheilus longicauda. J Am Chem Soc (1974) 96(7):2245–6. doi:10.1021/ja00814a041
23. Solomon AE, Stoughton RB. Dermatitis from purified sea algae toxin (debromoaplysiatoxin). Arch Dermatol (1978) 114(9):1333–5. doi:10.1001/archderm.114.9.1333
24. Hudon C, De Sève M, Cattaneo A. Increasing occurrence of the benthic filamentous cyanobacterium Lyngbya wollei: a symptom of freshwater ecosystem degradation. Freshwater Science (2014) 33(2):606–18. doi:10.1086/675932
25. Fetscher AE, Howard MDA, Stancheva R, Kudela RM, Stein ED, Sutula MA, et al. Wadeable streams as widespread sources of benthic cyanotoxins in California, USA. Harmful Algae (2015) 49:105–16. doi:10.1016/j.hal.2015.09.002
26. Foss AJ, Phlips EJ, Aubel MT, Szabo NJ. Investigation of extraction and analysis techniques for Lyngbya wollei derived Paralytic shellfish toxins. Toxicon (2012) 60(6):1148–58. doi:10.1016/j.toxicon.2012.07.009
27. van Apeldoorn ME, van Egmond HP, Speijers GJ, Bakker GJ. Toxins of cyanobacteria. Mol Nutr Food Res (2007) 51(1):7–60. doi:10.1002/mnfr.200600185
28. Merel S, Walker D, Chicana R, Snyder S, Baurès E, Thomas O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ Int (2013) 59:303–27. doi:10.1016/j.envint.2013.06.013
29. Corbel S, Mougin C, Bouaïcha N. Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere (2014) 96:1–15. doi:10.1016/j.chemosphere.2013.07.056
30. Rzymski P, Poniedziałek B. Dermatotoxins synthesized by blue-green algae (Cyanobacteria). Post Dermatol Alergol (2012) 29:47–50.
31. Harr KE, Szabo NJ, Cichra M, Phlips EJ. Debromoaplysiatoxin in Lyngbya-dominated mats on manatees (Trichechus manatus latirostris) in the Florida King’s Bay ecosystem. Toxicon (2008) 52(2):385–8. doi:10.1016/j.toxicon.2008.05.016
32. Kaneshima H, Hiai H, Fujiki H, Oguro YB, Iijima S, Sugimura T, et al. Tumor promoter-dependent mouse leukemia cell line. Cancer Res (1983) 43(10):4676.
33. Shimomura K, Mullinix MG, Kakunaga T, Fujiki H, Sugimura T. Bromine residue at hydrophilic region influences biological activity of aplysiatoxin, a tumor promoter. Science (1983) 222(4629):1242–4. doi:10.1126/science.6316505
34. Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer (2007) 7(7):554–62. doi:10.1038/nrc2168
35. Cataisson C, Joseloff E, Murillas R, Wang A, Atwell C, Torgerson S, et al. Activation of cutaneous protein kinase Cα induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol (2003) 171(5):2703–13. doi:10.4049/jimmunol.171.5.2703
36. Centers for Disease Control and Prevention (CDC). Outbreak of gastrointestinal illness associated with consumption of seaweed – Hawaii, 1994. MMWR Morb Mortal Wkly Rep (1995) 44(39):724.
37. Nagai H, Yasumoto T, Hokama Y. Aplysiatoxin and debromoaplysiatoxin as the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. Toxicon (1996) 34(7):753–61. doi:10.1016/0041-0101(96)00014-1
38. Ito E, Nagai H. Morphological observations of diarrhea in mice caused by aplysiatoxin, the causative agent of the red alga Gracilaria coronopifolia poisoning in Hawaii. Toxicon (1998) 36(12):1913–20. doi:10.1016/S0041-0101(98)00113-5
Keywords: dog, cyanobacteria, cyanotoxins, debromoaplysiatoxin, Lyngbya sp., skin irritation, toxicosis
Citation: Puschner B, Bautista AC and Wong C (2017) Debromoaplysiatoxin as the Causative Agent of Dermatitis in a Dog after Exposure to Freshwater in California. Front. Vet. Sci. 4:50. doi: 10.3389/fvets.2017.00050
Received: 18 February 2017; Accepted: 24 March 2017;
Published: 06 April 2017
Edited by:
Ramesh Chandra Gupta, Murray State University, USAReviewed by:
Begum Yurdakok Dikmen, Ankara University, TurkeyCristina Cortinovis, Università degli Studi di Milano, Italy
Copyright: © 2017 Puschner, Bautista and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birgit Puschner, YnB1c2NobmVyQHVjZGF2aXMuZWR1
 Birgit Puschner
Birgit Puschner Adrienne C. Bautista
Adrienne C. Bautista Chris Wong3
Chris Wong3
