- 1School of Laboratory Medicine and Medical Science, University of Kwazulu-Natal, Durban, Kwazulu-Natal, South Africa
- 2Basic and Translational Science, Africa Health Research Institute, Durban, Kwazulu-Natal, South Africa
- 3Centre for Clinical for Clinical Microbiology, Division of Infection and Immunity, University College London, London, United Kingdom
- 4Institute for Global Health, University College London, London, United Kingdom
Objective: Our objective was to conduct a review of host blood-derived biomarkers as potential diagnostic targets for pulmonary TB and as alternative tests to identify active tuberculosis in HIV co-infected individuals.
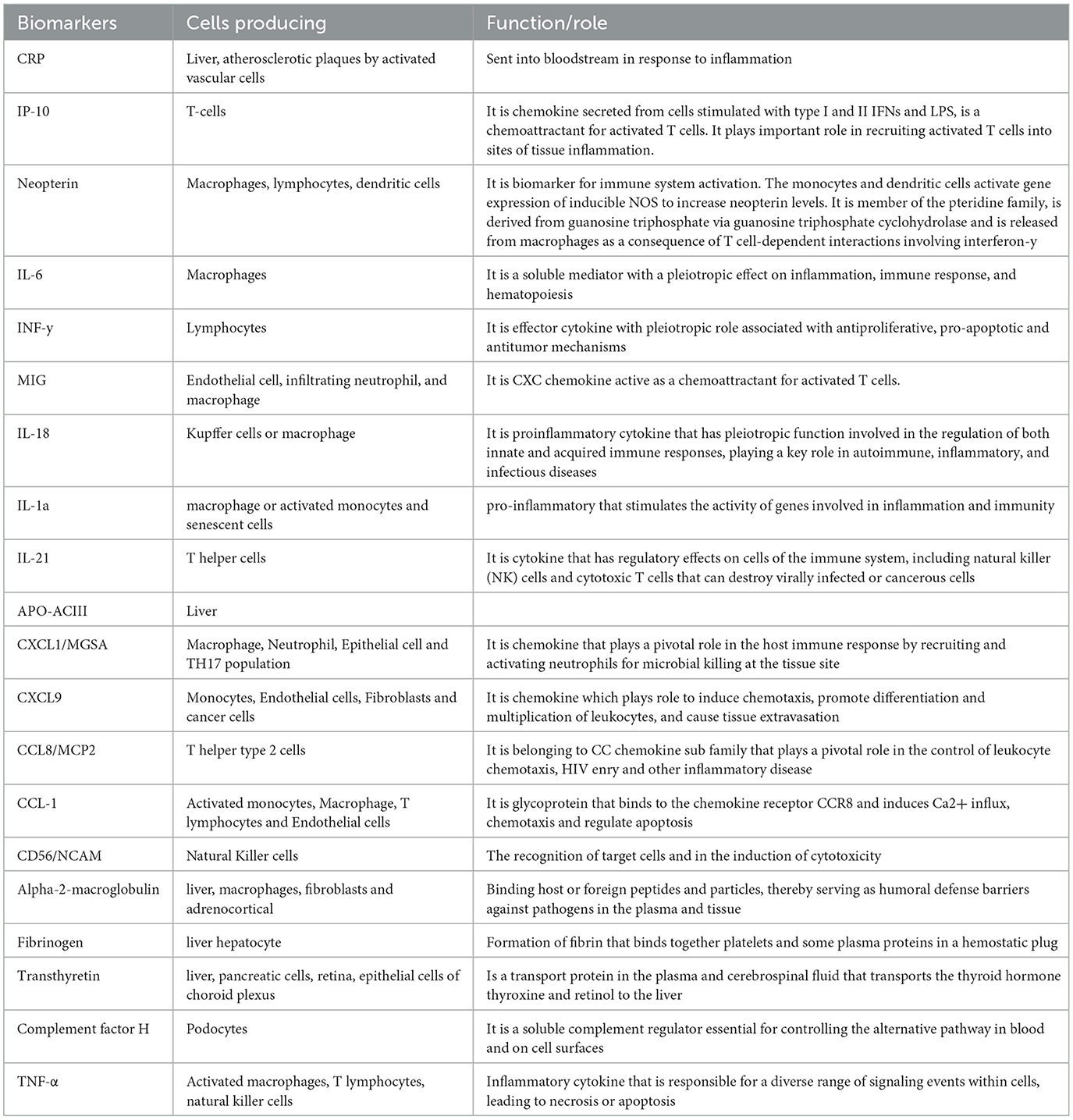
Methods: A systematic review and meta-analysis of host blood-derived biomarkers with potential for diagnosis of active tuberculosis in HIV co-infected individuals was conducted. Cochrane Library, Embase, MEDLINE, PubMed and Web of Science databases were searched up to 7 November 2023. A hierarchical summary receiver operating characteristic (HSROC) model was used to evaluate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR) of the following potential biomarkers: C-reactive protein (CRP), Interferon gamma induced protein-10 (IP-10), Neopterin, IGRA, Kynurenine to tryptophan (K/T) ratio and use of different panels of combined biomarkers; including 5 biomarker panel (IL-6, INF-y, MIG, CRP, and IL-18), 4 biomarker panel (IL-6, IL-21, INF-y, IL-1a), 6 biomarker panel (APO-ACIII, CXCL1, CXCL9, CCL8, CCL-1, and CD56), and 9 biomarker panel (Alpha-2-macroglobulin, fibrinogen, CRP, MMP-a, transthyretin, complement factor H, INF-y, IP-10, and TNF-α).
Results: Twenty-three studies were included. The pooled sensitivity of CRP, IP-10, Neopterin, combined biomarker signatures, IGRA and K/T ratio were 77% (60–88), 79% (72 - 84), 82% (43–96), 78% (64–88), 71% (65–76), 95% (90–98), respectively and the pooled specificity were 90% (80–96), 82% (59–93), 42% (22–66), 85% (73–92), 33% (18–54), and 95% (82–99), respectively.
Conclusion: CRP, IP-10, K/T ratio and the panels of multiple combined biomarkers that include the following cytokines, chemokines, and acute phase proteins IL-6, INF-y, MIG, CRP, IL-18, IL-21, IL-1a, APO-ACIII, CXCL1, CXCL9, CCL8, CCL-1, CD56, Alpha-2-macroglobulin, fibrinogen, MMP-a, transthyretin, complement factor H, IP-10, and TNF-α are potential blood biomarkers that can aid TB diagnosis in HIV co-infected individuals.
Introduction
The diagnosis of pulmonary tuberculosis (TB) in resource limited areas is mainly by chest X-ray, Acid Fast Bacilli (AFB) staining and molecular methods such as GeneXpert (1). The use of GeneXpert MTB/RIF as a first-line diagnostic test for TB is recommended by WHO (2); however the test has not been fully implemented across all sub-Saharan African countries (3). These countries carry a severe burden of TB infection which is aggravated by emergence of drug resistant strains, a high prevalence of HIV co-infection, poverty, and people living in remote areas without proper access to health facilities (4). In response to the burden of disease, the “End TB strategy” targets a 90% reduction in patients suffering from TB, and a 95% reduction in deaths from TB by 2035 (5). However, studies have shown that deaths among HIV-infected individuals as a result of co-infection with TB are significantly higher in sub-Saharan African low- and middle-income countries (LMIC) compared to high-income countries (HIC) (6, 7).
During TB infection, Mycobacterium tuberculosis (Mtb) is engulfed by innate immune cells including macrophages, which recruit other immune cells such as T cells and B cells to form a granuloma that contains Mtb (8). Cytokines and chemokines play a role in this recruitment of immune cells to the site of mycobacteria infection (9) and so they have been proposed as blood-derived biomarkers with potential for the diagnosis of TB (10–12). However, the clinical value of blood biomarkers remains to be evaluated in a setting in sub-Saharan Africa with a high prevalence of both tuberculosis and HIV.
Diagnosis of TB in HIV-positive individuals can be complex: (I) HIV infection can modify the clinical presentation of TB, leading to atypical or less specific symptoms, (II) HIV-positive individuals often have coinfections or comorbidities that can complicate the clinical picture and may mimic TB symptoms, (III) HIV-induced immunosuppression can lead to atypical immune responses to TB, affecting the performance of conventional TB diagnostic tests such as tuberculin skin tests and interferon-gamma assays (13–16). Two meta-analyses reported the potential of IP-10 and CRP for diagnosis of TB from predominantly HIV negative studies (17, 18). By comparison, this systematic review and meta-analysis focuses on the evaluation of chemokines, cytokines, and acute phase protein as diagnostic biomarkers of TB in HIV co-infected individuals in a sub-Saharan African setting with a high prevalence of both TB and HIV.
Methods
This review was developed in accordance with the Preferred Reporting for Systematic Review and Meta-Analysis protocols (PRISMA-P) diagnostic test accuracy criteria and was registered by the international database of prospectively registered systematic reviews in health and social care (PROSPERO) (CRD42021277685). Ethical approval was not required for this study.
Literature search
The search strategy was designed by combining the medical (MeSH) terms: “‘tuberculosis,' ‘latent tuberculosis,' ‘LTBI,' ‘TB,' ‘human Immunodeficiency syndrome,' ‘HIV,' ‘Sub-Saharan Africa,' ‘Biomarkers,' ‘assay,' ‘assays,' ‘bio-signature,' ‘bio-signatures,' ‘expression,' ‘marker,' ‘markers,' ‘profile,' ‘profiling,' ‘profiles,' ‘signature,' ‘signatures,' ‘surrogate endpoint,' ‘test,' ‘tests,' ‘tool,' ‘tools.”' in the following electronic databases: Embase, PubMed, and Web of Science up to and including 7 November 2023. The MeSH terms were used together using “OR,” and the results were further combined using “AND” to obtain the result. The full search strategy is described in the Supplementary data 2.
Literature selection
We included studies that assessed the diagnostic accuracy of blood-based biomarkers for TB in HIV co-infected individuals, TB negative in HIV positive individuals, in sub-Saharan Africa. Only manuscripts written in English were included. Studies focused only on human pulmonary TB. For inclusion, there must have been a confirmed TB disease by either culture (liquid or solid), Acid fast bacilli (AFB) or GeneXpert MIT/RIF, as well as confirmed HIV infection. The studies that met our inclusion criteria were published between 2009 and 2023. We included studies that reported sensitivity, specificity, or sufficient information on any biomarker/s of pulmonary TB diagnosis assessed to construct tables of outcome. We included studies focused on both hospital and community-based participants of all ages. We also included case control, cohort, cross sectional studies and only studies that have clear reference standards for laboratory diagnosis of pulmonary TB. Reviews, letters, case reports, clinical trials, conference abstracts, and animal experiments were excluded.
Data extraction
Two reviewers (AR and NM) independently performed quality assessment on the extracted data and any discrepancies were resolved by discussion and consensus. The following data were extracted: author, year, country where study was conducted, participant information, reference standards, tests index, cut-offs, true positive (TP), false positive (FP), false negative (FN) and true negative (TN).
Quality assessment
The quality assessment of diagnostic accuracy studies tool-2 (QUADAS-2) was used to evaluate the risk of bias and applicability in each study (19). Each reviewer performed an independent quality assessment of included studies by evaluating four domains (patient selection, index tests, reference standard, and flow and timing) for risk bias and three domains (patient selection, index tests and reference standard) for applicability. The Review Manager software (version 5.4, Cochrane Collaboration) was used to process the quality assessment of the included studies.
The first domain is patient selection i.e., selection of the participants based on a consecutive or at random basis, case-control design was avoided, and verifying whether the study avoided unnecessary exclusions. The participants of articles included in this review were also required to have the test condition. Thus, the risk of bias is high since only participants suspected of TB were selected. The second domain is the index test i.e., index test results interpreted without knowledge of the results of the reference standard and accurate explanation of detection threshold. The third domain is the reference standard 99% accuracy, but interpretation without considering the results of the index test and ensuring that all patients were assessed using the same reference standard. The last or fourth domain is the flow and timing (for risk bias only) describing the patients receiving the index test, the time interval between index tests, and reference standard. After independent evaluations, the reviewers discussed the article. Each domain was discussed to achieve a single view.
Statistical analysis
We used sensitivity and specificity of the biomarker reported in the studies to recalculate the sample numbers in each group. R software (version 4.12) was used to perform the statistical analyses. The pooled sensitivity, specificity, diagnostic odd ratios (DOR) were calculated, and summary receiver characteristics curves (SROC) were plotted for diagnostic efficiency of each biomarker of TB in HIV infected individuals from relevant articles relating to each biomarker. Index Q* was calculated from the corresponding value of DOR using Walter's formula (20). The DOR reflects the effectiveness of the index tests: DOR > 1 indicates that positive tests suggest active TB and DOR < 1 indicates that negative tests suggest that disease is absent. I2 statistic was used to quantify the amount of variation across studies. A p-value of < 0.05 indicated the presence of heterogeneity among included studies.
Results
Literature search
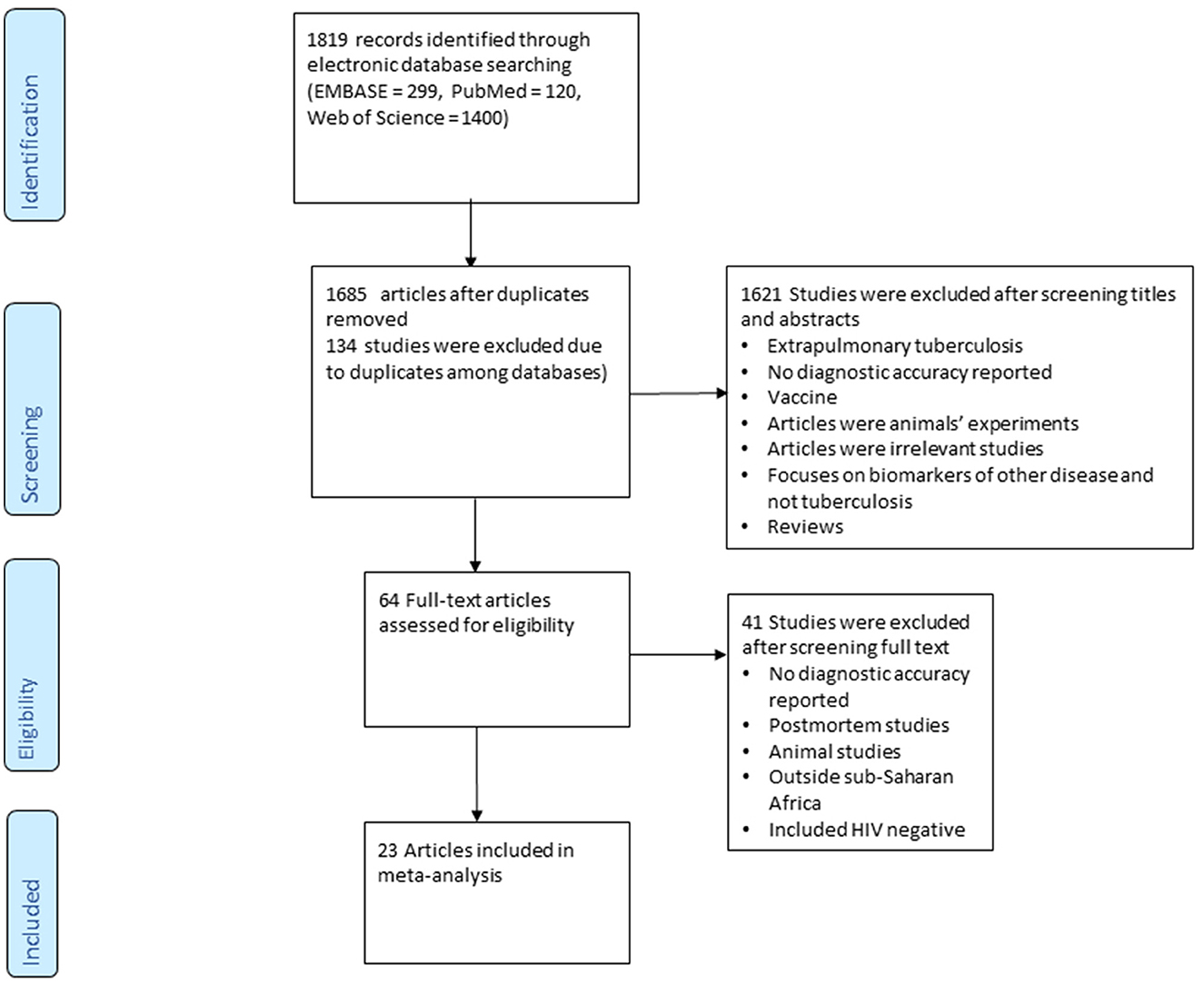
A total of 1,819 articles were identified from database searches (Embase = 299, PubMed = 120 and Web of Science = 1,400). Sixty-four papers were included and of the 1,755 excluded studies, 134 were duplicates; 1,621 studies were excluded after screening titles and abstracts relating to any of the following: (extrapulmonary tuberculosis, no diagnostic accuracy reported, vaccine studies, animal experiments, focus on biomarkers of other diseases, and reviews), 2 were reviews. Forty-one studies were excluded after full paper screening if they (Included HIV negative only, animal studies/experiments, conducted outside Sub-Saharan Africa, or Postmortem studies). Ultimately 23 studies were eligible for inclusion in this study (Figure 1).
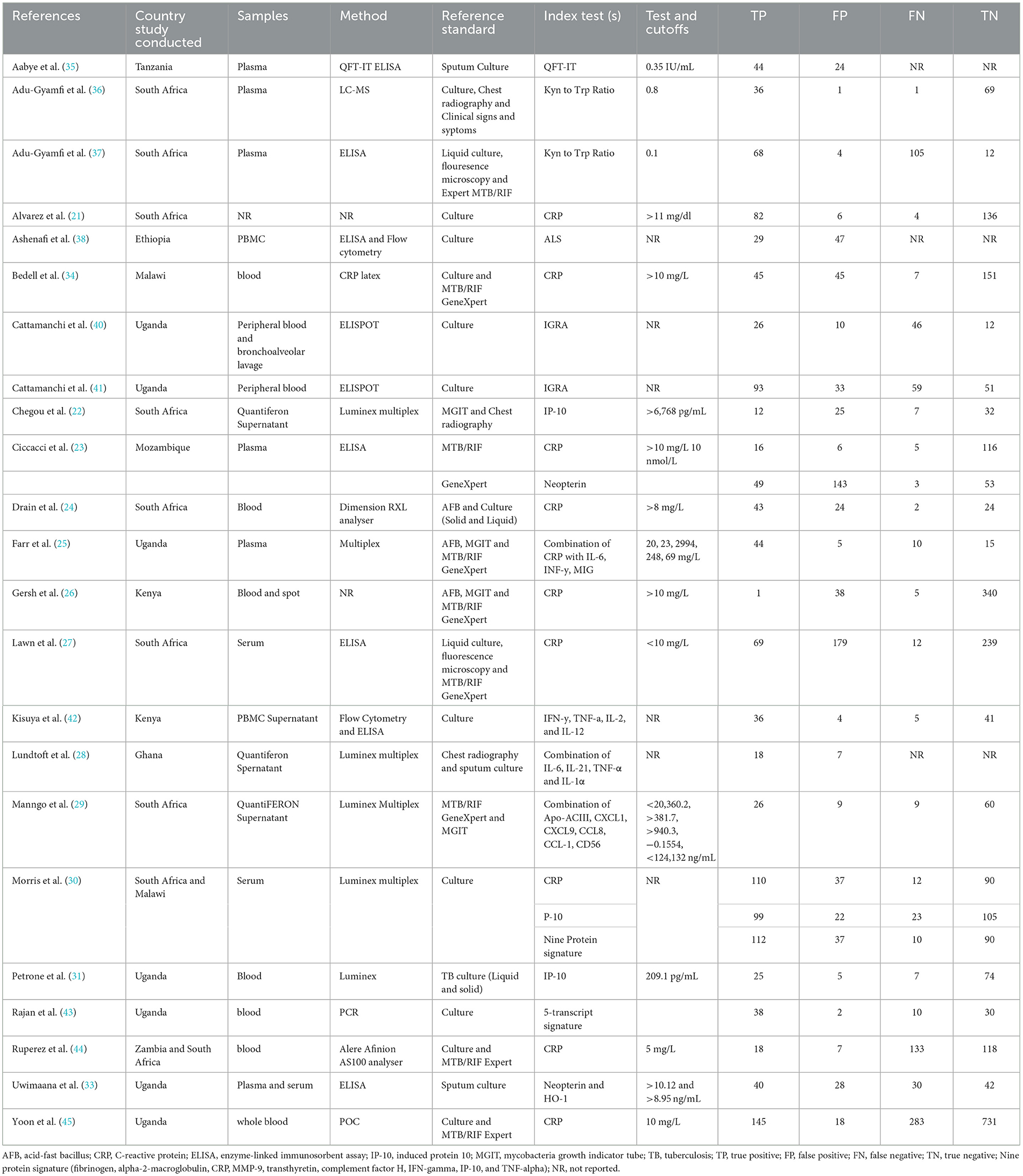
The characteristics of the 23 eligible studies are shown Table 1 (21–45). All eligible studies were published in English between 2009 and 2023. Three (13%) studies included only children (21, 28, 31), seventeen (74%) studies included only adults (23, 26–30, 33, 46), three (13%) studies did not report the ages (24, 25).
The reference standard for pulmonary TB diagnosis includes the use of MTB/RIF GeneXpert, Acid Fast Bacillus (AFB) staining, culture (solid and liquid culture) and fluorescence microscopy. Study design, samples used, method, reference standard, index test, cut-off, true positive, false positive, false negative, and true negative for each study are summarized in Table 1. Five individual biomarkers CRP, IGRA, IP-10, Kynurenines to tryptophan ratio and neopterin were reported frequently. Five studies reported mixed biomarker sets for diagnosis of tuberculosis.
Ten (43%) studies used CRP as the index test (21, 23–27, 30, 34, 44, 45, 47) and a total of 1,882 participants were included. The diagnostic odds ratio (DOR) was 28.349 (8.589–93.571) (Figure 2). The pooled sensitivity was 77% (59.5–88.4) and pooled specificity 90.2% (79.5–95.6) (Figures 3, 4). The heterogeneity for CRP was significant with I2 of 96% for sensitivity and 96% for specificity with both p-value of less than < 0.01, respectively.
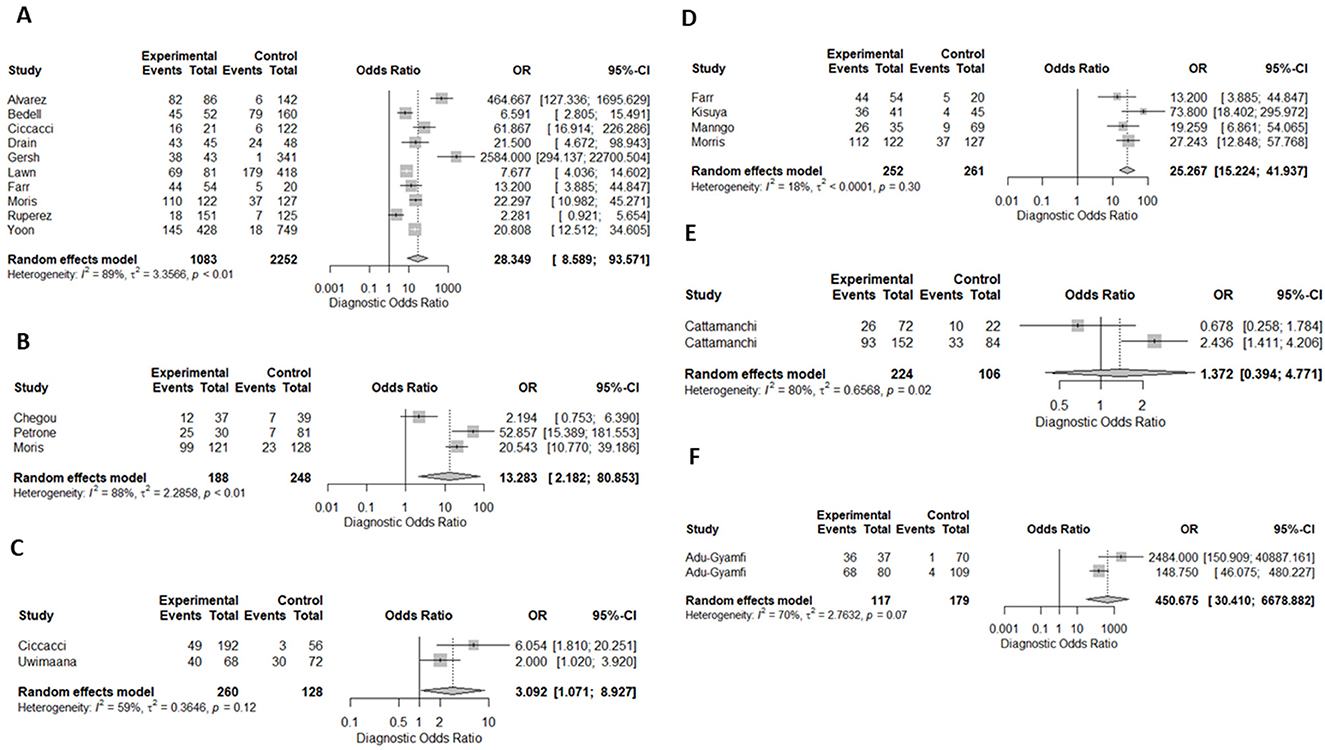
Figure 2. Forest plots of diagnostic odds ratio for (A) CRP, (B) IP-10, (C) Neopterin, (D) combination of biomarkers, (E) IGRA, and Kynurenines to tryptophan ratio in the diagnosis of TB, (F) K/T ratio in the dioagnosis of TB. OR, odds ratio; CI, confidence interval.
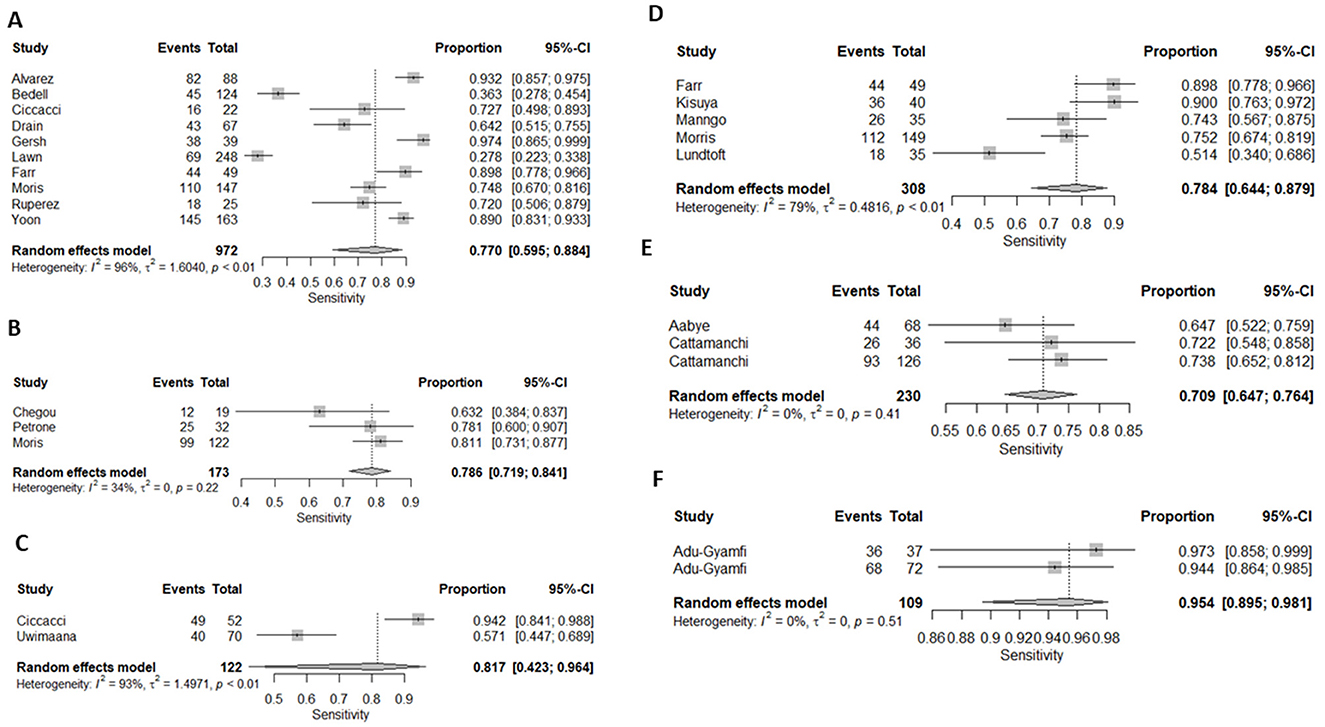
Figure 3. Forest plots of (A) CRP's, (B) IP-10, (C) Neopterin, (D) combination of biomarkers, (E) IGRA, and Kynurenines to tryptophan ratio sensitivity for diagnosis of active tuberculosis, (F) K/T ratio sensitivity for diagnosis of active tuberculosis.
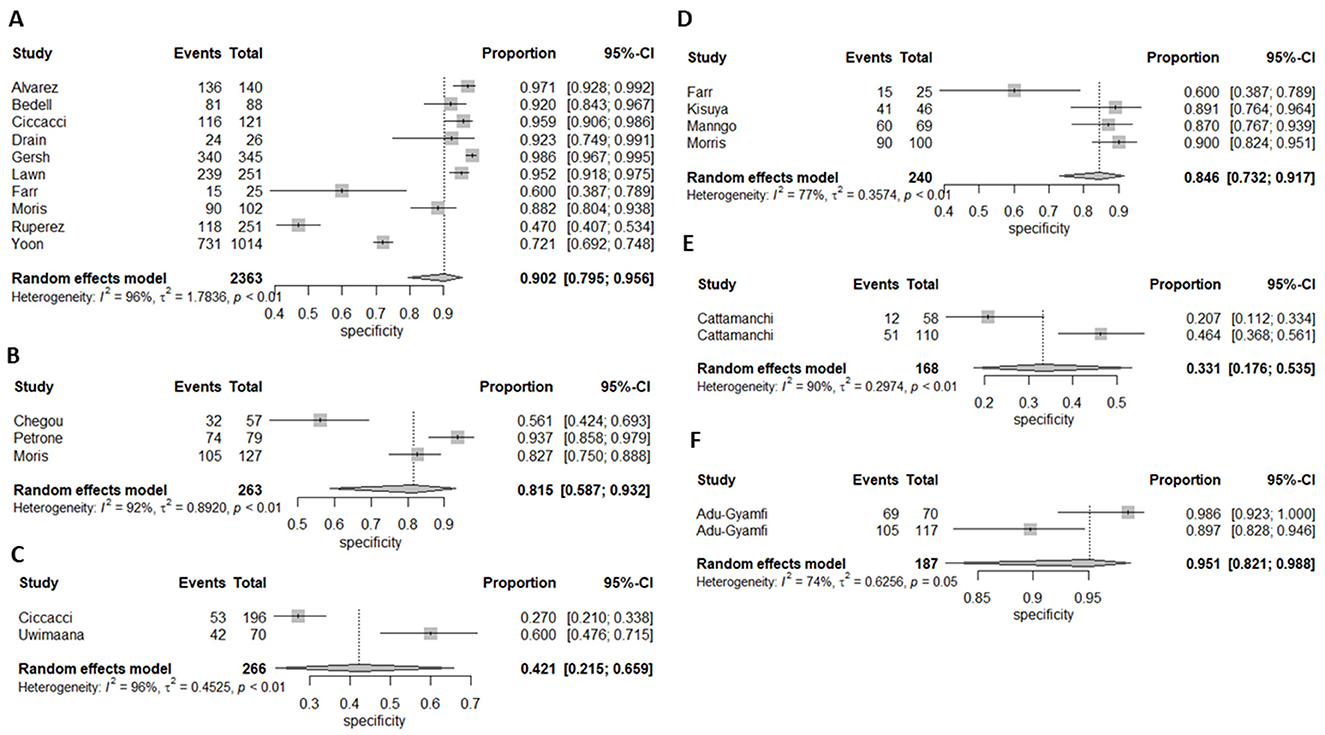
Figure 4. Forest plot of (A) CRP's, (B) IP-10, (C) Neopterin, (D) combination of biomarkers, (E) IGRA, and Kynurenines to tryptophan ratio for specificity for diagnosis of active tuberculosis, (F) K/T ratio sensitivity for diagnosis of active tuberculosis.
Three (13%) studies used IGRA (35, 40, 41) as index test, with a total of 398 participants. The pooled sensitivity was 70.9% (64.7–76.4), pooled specificity was 33.1% (17.6–53.5), and DOR was 1.372 (0.394–4.771) (Figures 3, 4). The heterogeneity analysis of IGRA yielded an I2 of 0% for sensitivity and significant heterogeneity of pooled specificity with I2 of 90% with p- value of less than < 0.01.
The three (13%) studies which determined the diagnostic accuracy of IP-10 (22, 30, 31), included a total of 436 participants. The pooled sensitivity was 79% (72–84), pooled specificity was 82% (59–93), and DOR was 13.28 (2.18–80.85) (Figures 3, 4). The heterogeneity analysis of IP-10 yielded an I2 of 34% for sensitivity and significant heterogeneity of pooled specificity with I2 of 88% with p- value of < 0.01.
Two (8.7%) studies used K/T ratio (36, 37) as an index test, with a total of 296 participants. The pooled sensitivity was 95.4% (89.5–98.1), pooled specificity was 95.1% (82.1–98.8), and DOR was 450.675 (30.410–6678.882) (Figures 3, 4). The heterogeneity analysis of K/T ratio yielded an I2 of 0% for sensitivity and significant heterogeneity of pooled specificity with I2 of 74% with p- value of < 0.05.
Two (8.7%) studies used neopterin (23, 33) as an index test, with a total of 388 participants. The pooled sensitivity 82% (42–96), pooled specificity 42% (22–66), and DOR 3.09 (1.07–8.93) (Figures 3, 4). The heterogeneity analyses of neopterin yielded an I2 of 93% for sensitivity and 96% for specificity and p-value of < 0.01 respectively.
One (4.3%) study reported antibodies in lymphocyte supernatant (38) and another study (4.3%) reported at 5-transcript signature (43) for diagnosis of pulmonary TB in HIV co-infected participants. The pooled sensitivity and specificity were not done for ALS and 5-transcript signatures.
Among the included studies, five (21.7%) looked at a combination of biomarkers as signatures to evaluate the diagnostic accuracy of active tuberculosis. The combination of biomarker signatures in the five studies included five biomarkers (IL-6, INF-y, MIG, CRP, and IL-18) (25), four biomarkers (IL-6, IL-21, INF-y, IL-1a) (28), six biomarkers (APO-ACIII, CXCL1, CXCL9, CCL8, CCL-1, and CD56) (29), nine biomarkers (Alpha-2-macroglobulin, fibrinogen, CRP, MMP-a, transthyretin, complement factor H, INF-y, IP-10, and TNF-α) (30), and four biomarkers (INF-y, TNF-a, IL-2, and IL-12) (42). The DOR was 25.267 (15.224–41.937) (Figure 2). The pooled sensitivity and specificity were 78.4% (64.4–87.9) and 84.6% (73.2–91.7) (Figures 3, 4). There was significant heterogeneity in both pooled sensitivity and specificity with I2 of 79 and 77%, and the p-value of < 0.01 and < 0.01, respectively. We performed SROC analysis to assess the power of CRP, IP-10, Neopterin, selected combined biomarkers, IGRA, and K/T ratio and all had good diagnostic accuracy (Supplementary Figure 1).
Quality assessment
QUADAS-2 was used to assess risk bias and applicability of the study. Two studies (26, 29) showed high risk of bias and 8 (24, 25, 28, 30, 31, 38, 40, 41) had unclear risk for patient selection. Three studies (21, 28, 30) had high risk for index test and two (24, 43) showed an unclear risk of bias for index test. For applicability concerns one study (28) showed high risk of bias and one (29) showed unclear risk of bias (Supplementary Figure 2).
Discussion
The findings from this systematic review suggest that analyses of CRP (77.0 and 90.0%), IP-10 (78.6 and 81.5%), K/T ratio (95.4 and 95.1%), and a panel of multiple combined cytokines (Table 2), chemokines and acute phase proteins (78.4 and 84.6%) in TB/HIV coinfected individuals are promising potential biomarkers, with pooled sensitivity and specificity of more than 75% in diagnosing TB. With future validation, these blood-based markers may provide alternative ancillary methods for diagnosis of pulmonary TB in HIV co-infected individuals.
Blood-based biomarkers could play a crucial role in improving TB diagnosis in HIV positive individuals by providing a less invasive, more accessible, and faster diagnostic method. It has been shown that individuals with HIV-TB co-infection may have a lower bacterial load in the sputum, therefore making traditional diagnostic tool like sputum microscopy less effective (48, 49). It has been shown that in TB infection, myeloid cells, including macrophages and neutrophils, are essential in the immune response mounted to Mycobacterium tuberculosis acting as the first line of defense by phagocytosing and attempting to kill the bacteria (8, 50). Myeloid-derived suppressor cells (MDSCs) can also amass during TB infection, potentially suppressing immune responses and contributing to disease advancement (51). In HIV infection, myeloid cells such as macrophages and dendritic cells are important targets for and may serve as reservoirs of HIV, enabling the virus to endure even during antiretroviral therapy, as well as facilitate the spread to CD4+T cells (52). In the case of HIV and TB coinfection, HIV infection may impair the function of myeloid cells, reducing their ability to control M.tb which may lead to a higher risk of latent TB reactivation and more advanced disease (53). In addition, the dysfunction of macrophages and dendritic cells due to HIV can exacerbate TB outcome. Most of the studied biomarkers with potential to diagnose TB in HIV-co-infected individuals are produced by myeloid cells including macrophages and neutrophils, as well as dendritic cells (54–57). Thus, mechanistically, these myeloid specific responses would be expected to be higher in TB-HIV co-infection compared to HIV infection alone, and could aid in the diagnoses of TB in people living with HIV. However, such proposed mechanism need validation.
The results of this study were consistent with those reported in the systematic review by Santos et al. where both high- and low-income countries were included (18), however our study considered HIV infected individuals only. The meta-analysis by Santos et al. reported higher sensitivity of IP-10, the same sensitivity of CRP and low specificity of both IP-10 and CRP in HIV uninfected participants compared to our study and showed that IP-10 and CRP can also be used to diagnose pulmonary TB in patients with other lung diseases (18). It should be noted that some studies tested different sets of biomarkers, the SROC plots include diagnostic accuracy of combined cytokines, chemokines, and acute phase proteins from those studies.
It has been estimated that the current limitation in TB diagnosis has led to ~3.6 million TB cases never being detected or properly treated (58). There is an urgent need for more sensitive and specific TB diagnostic tests with a short turnaround time. Even with the extensive rollout by WHO of MTB/RIF GeneXpert, the low sensitivity in sputum samples and challenges of diagnosis in infants and children, who may not be able to provide sputum samples (32), remains a critical limitation on improved diagnostics. The limitation of this study was the lack of data to analyze levels of the biomarkers at different time points of TB disease, from asymptomatic subclinical disease to infection and to severe advanced cavitary diseases. Secondly, we could not analyze the source of significant heterogeneity for CRP, IP-10, and Neopterin studies due to fewer number of studies, However, for CRP, the use of different sample types (blood, plasma, dried blood spots, and serum) may have contributed to the observed heterogeneity. Third limitation of the study is inability to investigate impact of CD4 count on the biomarkers.
In conclusion, the CRP, IP-10, neopterin, K/T ratio and panels of multiple combined biomarkers are promising tests for diagnosis of tuberculosis in HIV co-infected individuals. However, more studies need to be conducted to examine the combination of biomarkers in HIV infected individuals vs. HIV uninfected individuals to support the finding of this systematic review. We recommend future studies are also conducted to determine and evaluate the use of biomarker algorithms to (i) accurately diagnose active TB in HIV coinfected individuals; (ii) assess the impact of HIV treatment on TB diagnosis; (iii) determine the transition from latent to active TB; and (iv) assess if there is any strain variation in diagnosing TB using host biomarkers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. TM: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. NM: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – review & editing. TS: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. TM: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing, Formal analysis. MM: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work reported herein was made possible through funding by DST/NRF (Grant Number: MND200429517780), UKZN College of Health Science, and the South African Medical Research Council through its Division of Research Capacity Development under the Mid-Career Scientist Program (MM) from funding received from the South African National Treasury. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the funders. MM was funded by Wellcome Trust (grant# 206751/A/17/Z) and Bill & Melinda Gates Foundation (OPP1210776).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftubr.2024.1377540/full#supplementary-material
References
1. Caulfield AJ, Wengenack NL. Diagnosis of active tuberculosis disease: from microscopy to molecular techniques. J Clin Tuberc Other Mycobact Dis. (2016) 4:33–43. doi: 10.1016/j.jctube.2016.05.005
2. WHO. Guidelines Approved by the Guidelines Review Committee. Policy Statement: Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. Geneva: World Health Organization Copyright © World Health Organization (2011).
3. Ford N, Matteelli A, Shubber Z, Hermans S, Meintjes G, Grinsztejn B, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc. (2016) 19:20714. doi: 10.7448/IAS.19.1.20714
4. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113 Suppl 1:S7–s12. doi: 10.1016/j.ijid.2021.02.107
6. Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. (2006) 367:817–24. doi: 10.1016/S0140-6736(06)68337-2
7. Farahani M, Vable A, Lebelonyane R, Seipone K, Anderson M, Avalos A, et al. Outcomes of the Botswana national HIV/AIDS treatment programme from 2002 to 2010: a longitudinal analysis. Lancet Glob Health. (2014) 2:e44–50. doi: 10.1016/S2214-109X(13)70149-9
8. Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. (2012) 12:352–66. doi: 10.1038/nri3211
9. Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. (2014) 505:218–22. doi: 10.1038/nature12799
10. Chiappini E, Della Bella C, Bonsignori F, Sollai S, Amedei A, Galli L, et al. Potential role of M. tuberculosis specific IFN-γ and IL-2 ELISPOT assays in discriminating children with active or latent tuberculosis. PLoS ONE. (2012) 7:e46041. doi: 10.1371/journal.pone.0046041
11. Goletti D, Raja A, Kabeer BSA, Rodrigues C, Sodha A, Carrara S, et al. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS ONE. (2010) 5:e0012577. doi: 10.1371/journal.pone.0012577
12. Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. (2018) 18:e199–210. doi: 10.1016/S1473-3099(18)30111-7
13. Sterling TR, Pham PA, Chaisson RE. HIV infection-related tuberculosis: clinical manifestations and treatment. Clin Infect Dis. (2010) 50 Suppl 3:S223–30. doi: 10.1086/651495
14. Mayer KH, Dukes Hamilton C. Synergistic pandemics: confronting the global HIV and tuberculosis epidemics. Clin Infect Dis. (2010) 50 Suppl 3:S67–70. doi: 10.1086/651475
15. Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis. (2011) 204 Suppl 4:S1159–67. doi: 10.1093/infdis/jir411
16. Syed Ahamed Kabeer B, Sikhamani R, Swaminathan S, Perumal V, Paramasivam P, Raja A. Role of interferon gamma release assay in active TB diagnosis among HIV infected individuals. PLoS ONE. (2009) 4:e5718. doi: 10.1371/journal.pone.0005718
17. Yoon C, Chaisson LH, Patel SM, Allen IE, Drain PK, Wilson D, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. (2017) 21:1013–9. doi: 10.5588/ijtld.17.0078
18. Santos VS, Goletti D, Kontogianni K, Adams ER, Molina-Moya B, Dominguez J, et al. Acute phase proteins and IP-10 as triage tests for the diagnosis of tuberculosis: systematic review and meta-analysis. Clin Microbiol Infect. (2019) 25:169–77. doi: 10.1016/j.cmi.2018.07.017
19. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
20. Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. (2002) 21:1237–56. doi: 10.1002/sim.1099
21. Alvarez GG, Sabri E, Ling D, Cameron DW, Maartens G, Wilson D, et al. model to rule out smear-negative tuberculosis among symptomatic HIV patients using C-reactive protein. Int J Tuberc Lung Dis. (2012) 16:1247–51. doi: 10.5588/ijtld.11.0743
22. Chegou NN, Detjen AK, Thiart L, Walters E, Mandalakas AM, Hesseling AC, et al. Utility of host markers detected in Quantiferon supernatants for the diagnosis of tuberculosis in children in a high-burden setting. PLoS ONE. (2013) 8:e64226. doi: 10.1371/journal.pone.0064226
23. Ciccacci F, Floridia M, Bernardini R, Sidumo Z, Mugunhe RJ, Andreotti M, et al. Plasma levels of CRP, neopterin and IP-10 in HIV-infected individuals with and without pulmonary tuberculosis. J Clin Tuberc Other Mycobacter Dis. (2019) 16:100107. doi: 10.1016/j.jctube.2019.100107
24. Drain PK, Mayeza L, Bartman P, Hurtado R, Moodley P, Varghese S, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis. (2014) 18:20–6. doi: 10.5588/ijtld.13.0519
25. Farr K, Ravindran R, Strnad L, Chang E, Chaisson LH, Yoon C, et al. Diagnostic performance of blood inflammatory markers for tuberculosis screening in people living with HIV. PLoS ONE. (2018) 13:e0206119. doi: 10.1371/journal.pone.0206119
26. Gersh JK, Barnabas RV, Matemo D, Kinuthia J, Feldman Z, Lacourse SM, et al. Pulmonary tuberculosis screening in anti-retroviral treated adults living with HIV in Kenya. BMC Infect Dis. (2021) 21:218. doi: 10.1186/s12879-021-05916-z
27. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. (2013) 17:636–43. doi: 10.5588/ijtld.12.0811
28. Lundtoft C, Awuah AAA, Nausch N, Enimil A, Mayatepek E, Owusu-Dabo E, et al. Alternative quantiferon cytokines for diagnosis of children with active tuberculosis and HIV co-infection in Ghana. Med Microbiol Immunol. (2017) 206:259–65. doi: 10.1007/s00430-017-0501-6
29. Manngo PM, Gutschmidt A, Snyders CI, Mutavhatsindi H, Manyelo CM, Makhoba NS, et al. Prospective evaluation of host biomarkers other than interferon gamma in QuantiFERON Plus supernatants as candidates for the diagnosis of tuberculosis in symptomatic individuals. J Infect. (2019) 79:228–35. doi: 10.1016/j.jinf.2019.07.007
30. Morris TC, Hoggart CJ, Chegou NN, Kidd M, Oni T, Goliath R, et al. Evaluation of host serum protein biomarkers of tuberculosis in sub-Saharan Africa. Front Immunol. (2021) 12:639174. doi: 10.3389/fimmu.2021.639174
31. Petrone L, Cannas A, Aloi F, Nsubuga M, Sserumkuma J, Nazziwa RA, et al. Blood or urine IP-10 cannot discriminate between active tuberculosis and respiratory diseases different from tuberculosis in children. Biomed Res Int. (2015) 2015:589471. doi: 10.1155/2015/589471
32. Petrone L, Cannas A, Aloi F, Nsubuga M, Sserumkuma J, Nazziwa RA, et al. Blood and urine IP-10 associate with active TB in HIV-infected patients. Eur Respir J. (2016) 48:OA3039. doi: 10.1183/13993003.congress-2016.OA3039
33. Uwimaana E, Bagaya BS, Castelnuovo B, Kateete DP, Godwin A, Kiwanuka N, et al. Heme oxygenase-1 and neopterin plasma/serum levels and their role in diagnosing active and latent TB among HIV/TB co-infected patients: a cross sectional study. BMC Infect Dis. (2021) 21:711. doi: 10.1186/s12879-021-06370-7
34. Bedell RA, van Lettow M, Meaney C, Corbett EL, Chan AK, Heyderman RS, et al. Predictive value of C-reactive protein for tuberculosis, bloodstream infection or death among HIV-infected individuals with chronic, non-specific symptoms and negative sputum smear microscopy. Trop Med Int Health. (2018) 23:254–62. doi: 10.1111/tmi.13025
35. Aabye MG, Ravn P, PrayGod G, Jeremiah K, Mugomela A, Jepsen M, et al. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS ONE. (2009) 4:e0004220. doi: 10.1371/journal.pone.0004220
36. Adu-Gyamfi C, Mikhathani L, Snyman T, Hoffmann C, Martinson NA, Chaisson RE, et al. Plasma indoleamine 2,3-dioxygenase, a highly sensitive blood-based screening tool for active tuberculosis disease in HIV-infected pregnant women. Int J Infect Dis. (2020) 101:456. doi: 10.1016/j.ijid.2020.09.1194
37. Adu-Gyamfi C, Savulescu D, Mikhathani L, Otwombe K, Salazar-Austin N, Chaisson R, et al. Plasma kynurenine-to-tryptophan ratio, a highly sensitive blood-based diagnostic tool for tuberculosis in pregnant women living with human immunodeficiency virus (HIV). Clin Infect Dis. (2021) 73:1027–36. doi: 10.1093/cid/ciab232
38. Ashenafi S, Aderaye G, Zewdie M, Raqib R, Bekele A, Magalhaes I, et al. BCG-specific IgG-secreting peripheral plasmablasts as a potential biomarker of active tuberculosis in HIV negative and HIV positive patients. Thorax. (2013) 68:269–76. doi: 10.1136/thoraxjnl-2012-201817
39. Bwanga F, Disque C, Lorenz MG, Allerheiligen V, Worodria W, Luyombya A, et al. Higher blood volumes improve the sensitivity of direct PCR diagnosis of blood stream tuberculosis among HIV-positive patients: an observation study. BMC Infect Dis. (2015) 15:48. doi: 10.1186/s12879-015-0785-3
40. Cattamanchi A, Ssewanyana I, Magala R, Miller CR, Den Boon S, Davis JL, et al. Performance of bronchoalveolar lavage enzyme-linked immunospot for diagnosis of smear-negative tuberculosis in HIV-infected patients. Am J Respir Crit Care Med. (2011) 183:A1202. doi: 10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A1202
41. Cattamanchi A, Ssewenyana I, Davis JL, Huang L, Worodria W, den Boon S, et al. Role of interferon-gamma release assays in the diagnosis of pulmonary tuberculosis in patients with advanced HIV infection. BMC Infect Dis. (2010) 10:75. doi: 10.1186/1471-2334-10-75
42. Kisuya J, Chemtai A, Raballah E, Okumu W, Keter A, Ouma C. The role of Mycobacterium tuberculosis antigen specific cytokines in determination of acid fast bacilli culture status in pulmonary tuberculosis patients co-infected with human immunodeficiency virus. Pan Afr Med J. (2018) 31:17294. doi: 10.11604/pamj.2018.31.166.17294
43. Rajan JV, Semitala FC, Mehta T, Seielstad M, Montalvo L, Andama A, et al. A novel, 5-transcript, whole-blood gene-expression signature for tuberculosis screening among people living with human immunodeficiency virus. Clin Infect Dis. (2019) 69:77–83. doi: 10.1093/cid/ciy835
44. Ruperez M, Shanaube K, Mureithi L, Wapamesa C, Burnett MJ, Kosloff B, et al. Use of point-of-care C-reactive protein testing for screening of tuberculosis in the community in high-burden settings: a prospective, cross-sectional study in Zambia and South Africa. Lancet Global Health. (2023) 11:E704–E14. doi: 10.1016/S2214-109X(23)00113-4
45. Yoon C, Semitala FC, Atuhumuza E, Katende J, Mwebe S, Asege L, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis. (2017) 17:1285–92. doi: 10.1016/S1473-3099(17)30488-7
46. Petrone L, Cannas A, Vanini V, Cuzzi G, Aloi F, Nsubuga M, et al. Blood and urine inducible protein 10 as potential markers of disease activity. Int J Tuberc Lung Dis. (2016) 20:1554–61. doi: 10.5588/ijtld.16.0342
47. Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med. (2013) 11:231. doi: 10.1186/1741-7015-11-231
48. Yang Q, Han J, Shen J, Peng X, Zhou L, Yin X. Diagnosis and treatment of tuberculosis in adults with HIV. Medicine. (2022) 101:e30405. doi: 10.1097/MD.0000000000030405
49. Hanrahan CF, Theron G, Bassett J, Dheda K, Scott L, Stevens W, et al. Xpert MTB/RIF as a measure of sputum bacillary burden. Variation by HIV status and immunosuppression. Am J Respir Crit Care Med. (2014) 189:1426–34. doi: 10.1164/rccm.201312-2140OC
50. Scordo JM, Olmo-Fontánez AM, Kelley HV, Sidiki S, Arcos J, Akhter A, et al. The human lung mucosa drives differential Mycobacterium tuberculosis infection outcome in the alveolar epithelium. Mucosal Immunol. (2019) 12:795–804. doi: 10.1038/s41385-019-0156-2
51. Leukes V, Walzl G, du Plessis N. Myeloid-derived suppressor cells as target of phosphodiesterase-5 inhibitors in host-directed therapeutics for tuberculosis. Front Immunol. (2020) 11:451. doi: 10.3389/fimmu.2020.00451
52. Herskovitz J, Gendelman HE. HIV and the macrophage: from cell reservoirs to drug delivery to viral eradication. J Neuroimmune Pharmacol. (2019) 14:52–67. doi: 10.1007/s11481-018-9785-6
53. Hoerter A, Arnett E, Schlesinger LS, Pienaar E. Systems biology approaches to investigate the role of granulomas in TB-HIV coinfection. Front Immunol. (2022) 13:1014515. doi: 10.3389/fimmu.2022.1014515
54. Ganor Y, Real F, Sennepin A, Dutertre CA, Prevedel L, Xu L, et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol. (2019) 4:633–44. doi: 10.1038/s41564-018-0335-z
55. Watters SA, Mlcochova P, Gupta RK. Macrophages: the neglected barrier to eradication. Curr Opin Infect Dis. (2013) 26:561–6. doi: 10.1097/QCO.0000000000000014
56. Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. (2013) 254:54–64. doi: 10.1111/imr.12066
57. Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. (2019) 568:244–8. doi: 10.1038/s41586-019-1027-4
Keywords: cytokines, chemokine, systematic review, TB diagnosis, biomarkers, HIV-TB co-infection
Citation: Rapulana AM, Mpotje T, Mthiyane N, Smit TK, McHugh TD and Marakalala MJ (2025) Analyses of blood-derived host biomarkers for pulmonary tuberculosis diagnosis in human immunodeficiency virus co-infected individuals in sub-Saharan Africa: a systematic review and meta-analysis. Front. Tuberc. 2:1377540. doi: 10.3389/ftubr.2024.1377540
Received: 27 January 2024; Accepted: 06 December 2024;
Published: 08 January 2025.
Edited by:
Robert Jansen, Radboud University, NetherlandsReviewed by:
Luis Anibarro, Complexo Hospitalario Universitario Pontevedra, SpainSridhar Rathinam, Government of Tamil Nadu, India
Copyright © 2025 Rapulana, Mpotje, Mthiyane, Smit, McHugh and Marakalala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohlopheni J. Marakalala, bW9obG9waGVuaS5tYXJha2FsYWxhQGFocmkub3Jn
 Antony M. Rapulana
Antony M. Rapulana Thabo Mpotje
Thabo Mpotje Nondumiso Mthiyane2,4
Nondumiso Mthiyane2,4 Theresa K. Smit
Theresa K. Smit Timothy D. McHugh
Timothy D. McHugh Mohlopheni J. Marakalala
Mohlopheni J. Marakalala