
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Toxicol. , 06 March 2025
Sec. Immunotoxicology
Volume 7 - 2025 | https://doi.org/10.3389/ftox.2025.1558639
 Victor J. Johnson1*
Victor J. Johnson1* Cynthia V. Rider2
Cynthia V. Rider2 Michael I. Luster1
Michael I. Luster1 Cynthia J. Willson3
Cynthia J. Willson3 Shawn Harris4
Shawn Harris4 Billie Stiffler5
Billie Stiffler5 James Blake6
James Blake6 Esra Mutlu2
Esra Mutlu2 Veronica Godfrey2
Veronica Godfrey2 Brian Burback5
Brian Burback5 Reshan Fernando6
Reshan Fernando6 Suramya Waidyanatha2
Suramya Waidyanatha2 Gary R. Burleson1
Gary R. Burleson1 Dori R. Germolec2*
Dori R. Germolec2*Introduction: The ability of polycyclic aromatic compounds (PACs), most notably benzo(a) pyrene [B(a)P], to suppress antibody responses in experimental animals is well documented. Very little information, however, is available on the immunotoxicity of related PACs despite their widespread presence in the environment. Additionally, there are several weaknesses in existing immunotoxicity databases for PACs in experimental animals, limiting their applicability in quantitative risk assessment. Careful characterization of strong positive and clear negative PACs is needed in order to lay the foundation for generating robust immunotoxicity data for structurally diverse PACs that have not yet been evaluated.
Methods: In the current study, adult B6C3F1/N female mice were treated daily for 28 consecutive days by oral administration of B(a)P to provide dose levels ranging between 2 and 150 mg/kg bodyweight/day. In addition, phenanthrene and pyrene, non-carcinogenic PACs, were tested at dose ranges between 12.5 and 800 mg/kg bodyweight/day and 3.1 and 200 mg/kg bodyweight/day, respectively. Immune assessments following PAC exposure included organ weights and immunopathology, hematology, quantification of immune cell types in the spleen, and T-dependent antibody response (TDAR) to sheep red blood cells (SRBC).
Results: Benzo(a)pyrene exposure resulted in significant decreases in lymphoid organ weights, immune cell populations in the spleen and TDAR. The most sensitive indicator for immunotoxicity from B(a)P treatment was suppression of antibody responses, where an ∼75% decrease occurred at a dose level of 9 mg/kg bodyweight/day and ∼32% decrease at the lowest tested dose of 2 mg/kg bodyweight/day. Antibody suppression was associated with significant immune cell loss in the spleen; however, it was clear that the suppression of the TDAR was more sensitive than cell loss indicating that cell function impairments were involved. Phenanthrene treatment also resulted in suppression of the antibody response but only at dose levels ≥50 mg/kg bodyweight/day without significant effects on other parameters, while pyrene showed no significant immune effects.
Conclusion: Suppression of the TDAR to SRBC immunization was the most sensitive immune endpoint being 33 times more sensitive than changes in liver weight, a commonly used outcome for risk assessment for PACs. Benzo(a)pyrene was the most potent PAC regarding suppression of humoral immunity whereas pyrene did not affect the immune responses tested. These studies lay the foundation for evaluating diverse PACs with a range of immunotoxicological potencies.
Polycyclic aromatic compounds (PACs) are a structurally diverse class of chemicals containing at least two fused benzene rings and encompassing polycyclic aromatic hydrocarbons (PAHs) as well as heterocyclics that contain N, O, or S within their ring structures and N-, O-, or S- substituted PAHs (Hsieh et al., 2021). They are widespread environmental contaminants with both petrogenic (e.g., petroleum products) and pyrogenic (e.g., cigarette smoke, wildfires) sources (Al Hello et al., 2023). Exposure to PACs can occur via multiple routes including inhalation of contaminated air, ingestion, or dermal contact. People are generally exposed to complex and dynamic mixtures of PACs, not individual compounds. Exposure to PAC-containing mixtures has been associated with a broad range of toxicities. In particular, many epidemiological studies suggest that exposure to PAC mixtures leads to immunotoxicity (Biró et al., 2002; Karakaya et al., 2004; Oh et al., 2006; Szczeklik et al., 1994; Winker et al., 1997; Wu et al., 2003). Therefore, methods to evaluate the risk posed by mixtures of PACs are important for protecting public health.
A relative potency factor approach is the default method used to evaluate PAC-containing mixtures (Haber et al., 2022; US EPA, 2010; US EPA, 1993). Based on the concept of dose addition, the relative potency factor approach adds potency-adjusted doses of individual PACs to estimate the risk associated with exposure to mixtures. Potency values are calculated by dividing the reference chemical dose that elicits a certain level of effect (e.g., ED50) by the individual chemical dose that causes the same level of effect. Risk evaluations of PAC-containing mixtures have typically been limited to parent PAHs and have focused almost exclusively on carcinogenicity (Haber et al., 2022). Questions remain as to whether carcinogenicity is the most sensitive endpoint for PAC mixtures, and therefore, the most health protective. Several factors contribute to increased attention on immune suppression for PAC mixtures: the role of immune suppression in carcinogenesis, involvement of the aryl hydrocarbon receptor in both cancer and immune processes, and the ability to measure functional immune suppression in shorter-term assays, as well as other key characteristics of immunotoxicants that could help define mechanisms of immunotoxicity and mode of action (Germolec et al., 2022). Considering these factors, it was posited that immunotoxicity data could be used to enhance cancer risk assessment of PAC mixtures (Zaccaria and McClure, 2013).
Benzo(a)pyrene [B(a)P] is the most well-studied PAC and has been identified as the reference to which other chemicals in the class are compared for generating potency factors. In addition to being classified as a Group 1 carcinogen by IARC (IARC, 2010), experimental evidence indicates that B(a)P displays immunotoxicity (De Jong et al., 1999; Silkworth et al., 1995; White et al., 1985), reproductive toxicity (Archibong et al., 2002; Inyang et al., 2003), developmental toxicity (Detmar et al., 2008), and neurotoxicity (Chen et al., 2012). B(a)P is known to decrease antigen-specific humoral immunity (De Jong et al., 1999; Dean et al., 1983; Silkworth et al., 1995; Temple et al., 1993; US EPA, 2017). Other effects reported in studies of B(a)P immunotoxicity included changes in total serum immunoglobulin concentrations, immune cell numbers, cytokine levels, and T and B cell proliferation.
Phenanthrene and pyrene are at the other end of the toxicity spectrum from B(a)P. Both are in IARC Group 3 (not classifiable as to carcinogenicity in humans) (IARC, 2010) and were given a potency factor of 0 in the unreleased draft EPA document on “Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures” (US EPA, 2010). Phenanthrene contains a bay region area, like many carcinogenic PACs, and demonstrates similarities in metabolism and detoxification to B(a)P, including cytochrome P450 induction (Hecht et al., 2003; Nota et al., 2009). In addition, phenanthrene induces similar mRNA transcription profiles as B(a)P, activating an abundance of genes that not only affect biotransformation pathways, but typical stress response pathways, such as oxidative stress and immune response genes as well (Nota et al., 2009). Previous studies showed that phenanthrene had no effect on immunosuppression following a single oral dose up to 100 mg/kg, while pyrene displayed a 30% suppression of antigen-specific T-dependent antibody production following a single oral exposure of 100 mg/kg (Silkworth et al., 1995). Phenanthrene and pyrene have not been fully characterized for other toxicities (US EPA, 2009; US EPA, 2007).
The current studies are part of a larger PAC mixtures assessment program (https://ntp.niehs.nih.gov/whatwestudy/topics/pacs) aimed at addressing several uncertainties inherent in the current default relative potency factor approach. In the studies described here, we characterized the dose-response relationship for the reference PAC, B(a)P, and two additional PACs with low immunotoxicity potential, phenanthrene and pyrene, that could potentially serve as negative comparators. This work lays the foundation for additional studies exploring the immunotoxicity of structurally diverse individual PACs and mixtures of PACs.
These studies were conducted in compliance with the U.S. Food and Drug Administration Good Laboratory Practices for Nonclinical Laboratory Studies (Title 21 of the Code of Federal Regulations, Part 58) and approved by the IACUC at Burleson Research Technologies, Inc. (Morrisville, NC). The immune assessments were performed according to previous studies (Johnson et al., 2023; Watson et al., 2021) and are described below.
Female B6C3F1/N mice were obtained from Taconic Biosciences Inc. (Hudson, NY). Following acclimation, mice were randomized (±20% of mean bodyweight) and placed in individually ventilated cages (4 mice/cage). Mice were 8–12 weeks old at the start of dosing. Mice were provided NTP-2000 diet (Ziegler Bros., Inc., Gardners, PA), ad libitum, in which no contaminants were found that would interfere with the conduct of the study. Bodyweights were recorded before dose administration on Day 0 and then weekly on Days 6, 13, and 20. Terminal bodyweights were recorded following euthanasia on Day 28. All animals were euthanized by CO2 inhalation using 100% CO2 introduced at 3.65 LPM into a 7.3 L chamber to displace 50% of the atmosphere per minute; confirmation of death by severing the diaphragm.
B(a)P (Lot# CR66-25–1; >99% purity) was obtained from MRI Global (Kansas City, MO), phenanthrene (Lot# 7MP2K; >98% purity) was obtained from TCI America (Portland, OR), and pyrene (Lot# 20151201; >98% purity) was obtained from Ivy Fine Chemicals (Cherry Hill, NJ). Dose formulations were prepared in corn oil by Battelle Laboratories (Columbus, OH) and analyzed for concentration using a validated gas chromatography with flame ionization detection method [B(a)P: r2 > 0.99; precision determined as relative standard deviation (RSD) ≤ 5%; accuracy determined as relative error (RE), ≤± 3%; phenanthrene: r2 > 0.99; RSD ≤1%; RE, ≤± 5.2%; pyrene: r2 > 0.99; RSD </= 1.0%; RE, </= ± 4.0%] and were within 10% of target concentrations. Corn oil formulations of PACs were prepared at concentrations to provide dose volumes of 10 mL/kg. Cyclophosphamide monohydrate (CPS; Lot# MKBS0021V; >99% purity) was obtained from Sigma Aldrich (St. Louis, MO) and was used as a positive control for studies with B(a)P and phenanthrene, while B(a)P (10 mg/kg bodyweight/day) was the positive control for the pyrene studies. The dose formulations of CPS were prepared in 0.9% saline vehicle by RTI International (RTP, NC) and analyzed for concentration using a validated high performance liquid chromatography with evaporative light scattering detection (HPLC/ELSD) method (r > 0.99; RSD ≤6.7%; RE, ≤± 10%) and were within 10% of target concentration. Samples of the dose formulations for PACs and CPS were obtained from containers used for dosing the animals and analyzed for concentration; all animal room samples were within 10% of target. Prior to study start, stability of test article in formulations were confirmed up to a minimum of 42 days.
B(a)P, phenanthrene and pyrene studies were conducted at different time periods, each with their own vehicle and positive control groups, using identical experimental designs and conditions. Each study consisted of two distinct cohorts of animals. The first cohort (Cohort 1; SRBC), consisting of 8 mice per treatment group, was used to evaluate the impact of chemical treatment on the T-dependent antibody response (TDAR) to sheep red blood cells (SRBC) and splenic cell immunophenotyping. A second cohort (Cohort 2; Immunopathology), also consisting of 8 mice per treatment group, was used for collection of tissues for assessment of bone marrow cellularity, histopathology of the immune system, and hematology. Vehicle (corn oil) and test articles were administered in volumes of 10 mL/kg orally, once daily for 28 days (Day 0 through Day 27). B(a)P treatment doses were 2, 5, 9, 19, 38, 75 and 150 mg/kg bodyweight/day, phenanthrene treatment doses were 12.5, 25, 50, 100, 200, 400, and 800 mg/kg bodyweight/day, and pyrene treatment doses were 3.1, 6.3, 12.5, 25, 50, 100, and 200 mg/kg bodyweight/day. The goal in dose selection was to cover the dose-response curve for the immune function endpoints being tested. The high dose for each chemical was expected to be below the maximum tolerated dose based on available data, while the low dose was meant to approximate the no observed effect level for general toxicity. In the B(a)P and phenanthrene studies, a separate group received 50 mg/kg bodyweight/day CPS via intraperitoneal (IP) injection on Days 24–27. On Day 24, CPS was administered after immunization with SRBC. In the pyrene study, a separate group received 10 mg/kg bodyweight/day B(a)P for 28 days (Day 0 through Day 27) to serve as a calibration point across studies. No test or control article was administered on the day of euthanasia (Day 28).
Detailed clinical observations were performed for all study animals up to 2 days prior to the start of dosing and then once per week thereafter for the duration of treatment. Signs of toxicity, including onset, degree, and duration, were documented. Observations included evaluation of skin and fur, eyes and mucous membranes, respiratory and circulatory effects; autonomic effects (e.g., salivation), central nervous system effects (e.g., tremors and convulsions, changes in the level of activity, gait and posture, reactivity to handling), and behavioral changes (e.g., self-mutilation, walking backwards).
On Day 28, all surviving mice in Cohort two were anesthetized by CO2 inhalation and blood was collected by the retro-orbital route. The first drop or two of blood was discarded prior to collection into a K2EDTA blood collection tube to minimize the risk of micro-clots in the sample. The blood was shipped on ice packs on the day of blood collection for analysis (complete blood counts with white blood cell differential and reticulocyte counts) to Antech-GLP (Morrisville, NC). Blood samples were analyzed using an Advia 120 hematology analyzer with associated V.6.3.2-M software (Siemens Medical Solutions United States, Inc., Malvern, PA). The following hematologic parameters were assessed: erythrocyte count, hematocrit, hemoglobin concentration, mean cell volume, mean cell hemoglobin, mean cell hemoglobin concentration, platelet count, reticulocyte count, leukocyte count, and white blood cell differential.
Following completion of blood collection, mice were immediately euthanized with CO2 and selected tissues for histopathology were collected. Gross observations were documented and the spleen, thymus, liver, right kidney with adrenal gland, ovaries (single weight for the pair), and lungs were weighed. The spleen, thymus, mesenteric and popliteal lymph nodes, left femur with bone marrow, lung (including bronchial-associated lymphoid tissue [BALT]), right kidney with adrenal gland, left ovary, and liver (median, caudate, and right lobes) were preserved in 10% neutral buffered formalin (NBF). Other organs and tissues showing gross lesions were preserved in 10% NBF. The spleen, thymus, lymph nodes, BALT, and bone marrow were examined using the enhanced histopathology method (Elmore, 2012). Enhanced histopathology is a systematic approach used to characterize, both qualitatively and semi-quantitatively, immuno-modulatory effects within lymphoid organs after exposure to potentially immunotoxic or immunomodulatory compounds. Bone marrow cells from the right femur were removed, collected and total cell numbers determined.
SRBC in Alsever’s (Colorado Serum Company, Denver, CO) were washed 3 times in phosphate buffered saline (PBS) and resuspended to a final concentration of 3.75 × 108 SRBC/mL. Mice were intravenously immunized via the tail vein with 0.2 mL of the 3.75 × 108 SRBC/mL (7.5 × 107 SRBC/mouse) of the preparation on Day 24. Four days after immunization, animals were euthanized with CO2 and weighed. A maximum amount of blood was collected by cardiac puncture or from the inferior vena cava of each animal into a serum separator collection tube. The spleen and thymus were removed, and weight recorded. Blood was collected into tubes and allowed to clot at room temperature for 30–60 min. The tubes were centrifuged at approximately 1300xg at room temperature for approximately 10 min. Serum for each animal was then collected and stored at ≤ -70°C until evaluated for anti-SRBC IgM serum antibodies.
The antibody forming cell (AFC) response to SRBC, a T-dependent response, was used to assess the impact of PACs on humoral immunity. Spleens were processed to single cell suspensions in HBSS + HEPES and cell concentration and viability were determined. Spleen cells (1:30 and 1:120 dilution in 100 μL) and SRBC (25 μL of ∼50% suspension in HBSS) were added to 500 μL of molten agar media (at 44°C ± 1°C) and mixed with 25 μL of guinea pig complement (1/3 dilution of stock in 1 mL of HBSS with 0.1 mL of 50% SRBC suspension; Cedarlane Laboratories, Burlington, NC) in duplicate tubes. Resulting suspensions were poured onto the center of a Petri dish (in duplicate) and covered with glass. The agar was allowed to solidify prior to being placed in an incubator set to maintain 37°C for at least 3 hours and then AFC plaques were enumerated. The number of plaques were expressed per million spleen cells and per spleen.
Serum samples from individual animals were evaluated for IgM antibody titers to SRBC using a commercial ELISA kit (Life Diagnostics, St. Petersburg, FL). Briefly, diluted test samples and standards were added to microwells and incubated for 45 min. The wells were washed, and horse radish peroxidase-conjugated anti-mouse IgM added to the wells. The microplate was incubated at room temperature for 45 min, the wells washed, and the substrate solution added. Color development was stopped after 20 min by addition of the stop solution. The optical density was determined spectrophotometrically at 450 nm using a Spectramax 340 (Molecular Devices, Sunnyvale, CA). All samples and standards were run in duplicate and data analysis was performed using Softmax Pro® version 2.2.1 software (Molecular Devices).
Spleens from the SRBC cohort were subjected to ammonium chloride RBC lysis. The resulting mononuclear cells were re-suspended in RPMI with 5% FBS to 2.5 × 106 cells/mL; and 100 μL aliquots containing 2.5 × 105 cells were added to cluster tubes and the cells pelleted. The cells were then re-suspended in 50 µL stain buffer (PBS/2% BSA/0.1% NaN3) and incubated for 5–30 min on ice after addition of Fc Block solution (BioLegend, San Diego, CA). Following the blocking step, 50 μL of antibody (all BioLegend) cocktails containing antibodies to: (1) Anti-Mouse CD3, Anti-Mouse CD161a, Anti-Mouse CD45, and Anti-Mouse CD45RA; (2) Anti-Mouse CD8a, Anti-Mouse CD3, Anti-Mouse CD45, and Anti-Mouse CD4; or (3) Anti-Mouse CD11b, Anti-Mouse CD11c, Anti-Mouse Ly6G, and Anti-Mouse NKp46 were added to the appropriate tubes. Control tubes contained cells only, cells with a single antibody from the list above, or cells with a single isotype control antibody. The tubes were incubated on ice, protected from light, for 20–50 min. Following incubation, the samples were fixed using 2% paraformaldehyde for at least 30 min, followed by centrifugation and re-suspension in fresh stain buffer. The samples were then stored at 2-8°C, protected from light, until analyzed on an Accuri C6 flow cytometer using CFlow Plus v 1.0.264.15 (BD Biosciences). In all cases, a minimum of 20,000 events/sample was acquired.
Lymphocyte gating was performed on CD45+ populations. The following lymphocyte subsets were identified; T cells (CD3+CD45RA-), B cells (CD3−CD45RA+), NK cells (CD3−CD161a+), T-helper cells (CD3+CD4+), and T-cytotoxic cells (CD3+CD8+). Myeloid cells were gated based on being positive for CD11b with low-to-mid intensity staining for CD11c. Myeloid populations were differentiated from NK cells based on lack of NKp46 expression. Further differentiation was based on expression of Ly6G with positive cells being neutrophils and negative cells differentiated using SSC into monocytes/macrophages with low granularity and eosinophils with high granularity.
Data were collected into Provantis v9.2.3 (Instem, Philadelphia, PA) and calculation of endpoints was performed within this validated system. Results are presented as mean ± SEM. Bodyweight and organ weight data, which typically exhibit a normal distribution, were analyzed using a parametric multiple comparison procedure. If a significant trend was detected at p ≤ 0.01, Williams’ test was used (Williams, 1986); if the trend was not significant, Dunnett’s test was used (Dunnett, 1955). Positive control bodyweight and organ weight data were compared to the vehicle control group using a standard t-test. Data for other endpoints were analyzed using a non-parametric multiple comparison procedure. If a significant trend was observed, Shirley’s test was used (Shirley, 1977); if the trend was not significant, Dunn’s test was used (Dunn, 1964). Positive control group data were compared to the vehicle control group using the Wilcoxon rank-sum test (Mann and Whitney, 1947). Data that were different from control at p ≤ 0.05 were considered statistically significant. Extreme values were identified by an outlier test (Dixon and Massey, 1957). All flagged outliers were examined by NIEHS personnel, and statistical outliers that were biologically implausible were eliminated from the final analyses.
Histopathology data were analyzed using a Cochran-Armitage trend test and Fisher Exact pairwise tests. These tests were one-sided.
Dose response modeling was performed using BMDS Online (US EPA, 2024) to derive a benchmark dose (BMD) for organ weights and immune endpoints for each PAC. The benchmark reference used for the modeling was one standard deviation (1SD) of the control group data, as it is a commonly used reference for safety and risk assessment. All endpoints were modeled as continuous data using the models provided in BMDS Online including the following.
- Exponential M3 and M5
- Hill
- Polynomial two and 3
- Power
- Linear
Distributions for the endpoints were considered normal and the variance was set as constant or non-constant based on the data set and recommendations of BMDS Online. When no viable model (questionable and unusable output) fit the raw data, dose levels were transformed by the natural logarithm of dose +1 (constant to facilitate transformation of the vehicle group) and modeling was repeated. If no viable model was identified for the original or transformed data, no BMD or BMDL (lower limit of BMD) were presented. When more than one model was viable for a data set, BMDS Online recommended the best fit model based on the lowest BMDL or Akaike information criterion (AIC) for each data set the best fit model was used to report the BMD and BMDL. A relative change of 1xSD from control was used to provide context for the BMD calculation.
Summary findings relevant for evaluating immune toxicity are presented below. All study findings (including individual animal data) are available at the NTP Chemical Effects in Biological Systems (CEBS) database https://doi.org/10.22427/NTP-DATA-500-005-004-000-4.
Several mice in the 800 mg/kg bodyweight/day phenanthrene group exhibited overt toxicity within the first 2 days of dosing and the group was subsequently removed from the study due to excessive toxicity. All remaining mice dosed with 800 mg/kg bodyweight/day were humanely euthanized using CO2. Several mice in the remaining phenanthrene treatment groups showed labored breathing, hunched back and/or piloerection within the first 2 weeks of commencing dosing and subsequently were euthanized by CO2. These effects were not dose related and necropsy results indicated that they were the result of gavage trauma. There was no other clinical evidence of significant systemic toxicity in any of the other phenanthrene treated mice nor in any of the B(a)P or pyrene treated groups (CEBS Summary Tables I05). While there were no bodyweight changes related to phenanthrene or pyrene treatment, there were statistically significant treatment-related changes in bodyweight gains and bodyweights following B(a)P treatment (CEBS Summary Tables I04 and I04G). Bodyweight showed a significant negative trend with increasing dose of B(a)P on Days 20 and 28 and was significantly reduced in the 150 mg/kg bodyweight/day treatment group relative to the vehicle control group. However, the decrease in bodyweight was ∼10% compared to the vehicle control group and was unlikely to be responsible for the observed effects of B(a)P on the immune system.
At necropsy on Day 28 the spleen, thymus, liver, right kidney with adrenal gland, ovaries (single weight for the pair) were removed and weighed. In addition, popliteal and mesenteric lymph nodes were collected and examined histologically. There were significant decreases in absolute spleen weights in mice treated with 75 and 150 mg/kg bodyweight/day B(a)P, thymus weights [≥19 mg/kg bodyweight/day B(a)P] and thymus to bodyweight ratios [≥38 mg/kg bodyweight/day B(a)P] relative to the vehicle control group (Table 1; CEBS Summary Tables PA06). Absolute liver weights were increased only in mice treated with 150 mg/kg bodyweight/day B(a)P while liver to bodyweight ratios were significantly increased in groups administered doses of ≥19 mg/kg bodyweight/day (Table 1). There were also significant decreases in the absolute combined kidney and adrenal gland weight as well as relative and absolute ovary weights in mice treated with 150 mg/kg bodyweight/day B(a)P (CEBS Summary Table PA06). Although there were no significant effects on spleen or thymus weights in phenanthrene treated groups, liver weight (≥200 mg/kg bodyweight/day phenanthrene) and liver to bodyweight ratios (≥50 mg/kg bodyweight/day phenanthrene) were increased relative to the vehicle control group (Table 1). Relative spleen weight was significantly increased in mice exposed to 25 mg/kg bodyweight/day pyrene, although this isolated change is likely not biologically meaningful (Table 1). There was a negative trend for relative kidney weight with a significant reduction in mice treated with 200 mg/kg bodyweight/day pyrene (CEBS Summary Table PA06). Statistically significant changes in organ weights were observed in mice treated with CPS including decreases in the thymus and spleen weights and a slight but statistically significant increase in the liver weights in the studies conducted for B(a)P and phenanthrene. The positive control for the pyrene study, 10 mg/kg bodyweight/day B(a)P, did not result in any changes in organ weights, as expected for this dose level based on the B(a)P studies.
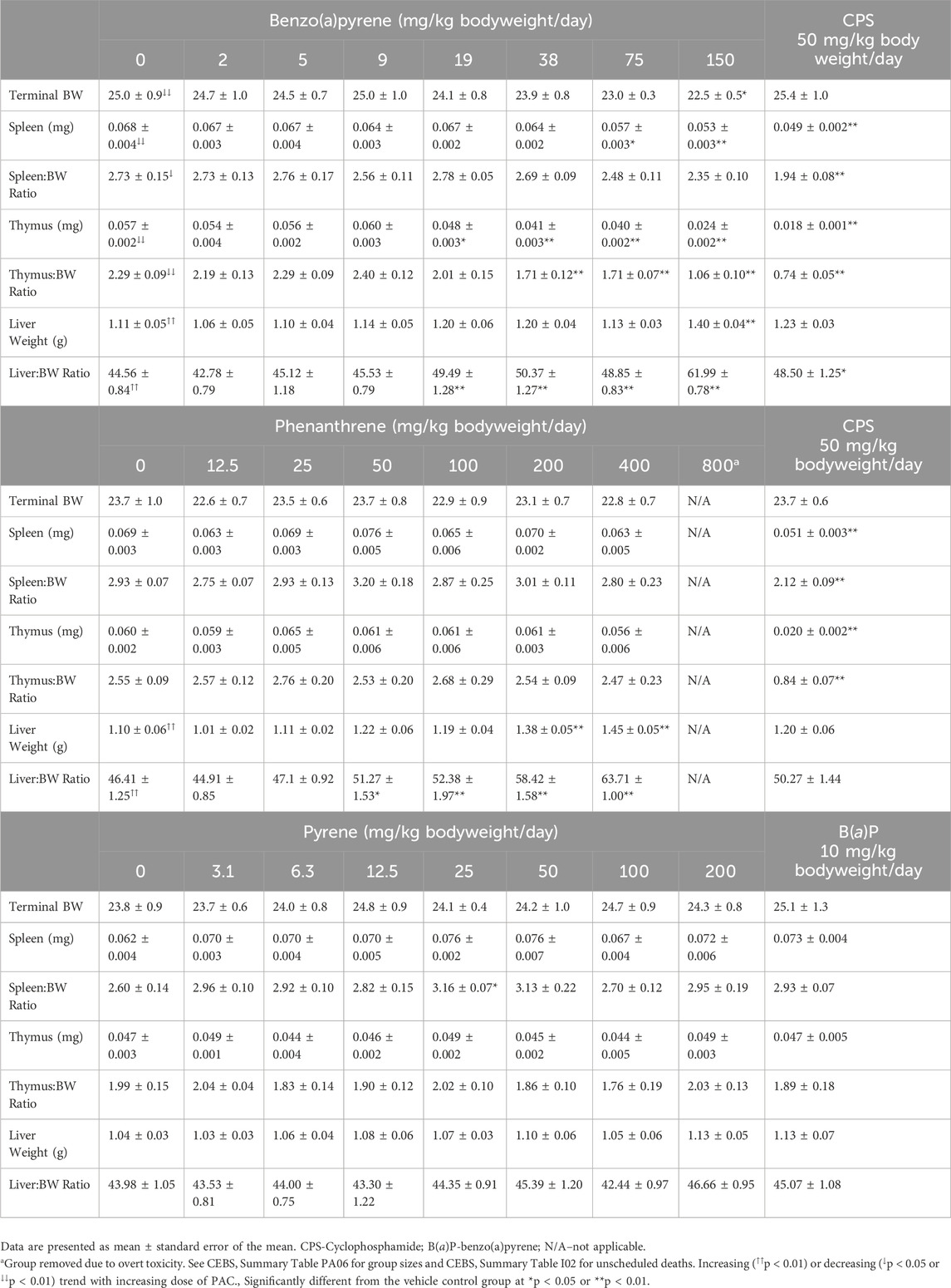
Table 1. Spleen, thymus and liver weights in female B6C3F1/N mice treated orally with benzo(a)pyrene, phenanthrene, or pyrene (Cohort 2).
Histopathological findings are provided in CEBS Summary Tables PA02, PA03, PA05, PA10, and PA18. B(a)P treatment resulted in several statistically significant histopathological changes in the thymus including minimal to moderately decreased cortical area [≥38 mg/kg bodyweight/day B(a)P], minimally to moderately decreased cortical lymphocyte numbers [≥38 mg/kg bodyweight/day B(a)P], minimally to mildly decreased medullary area [150 mg/kg bodyweight/day B(a)P], and minimally decreased medullary lymphocyte numbers [150 mg/kg bodyweight/day B(a)P] (Table 2). In addition, animals exposed to B(a)P showed minimal to moderate decreases in the thymic cortex:medulla ratio [≥38 mg/kg bodyweight/day B(a)P] and minimally increased apoptotic cells in the thymic cortex and medulla [150 mg/kg bodyweight/day B(a)P] (Table 2). Histological changes in the spleen of B(a)P treated groups included minimally to mildly decreased periarteriolar lymphoid sheath lymphocyte numbers [150 mg/kg bodyweight/day B(a)P] and a mild to moderate decrease in the number and size of follicular germinal centers [≥38 mg/kg bodyweight/day B(a)P] (Table 2). Histopathologic changes were also identified in the paracortex of the mesenteric lymph nodes associated with B(a)P treatment. These included minimally to moderately increased area and increased lymphocyte number in all groups treated with ≥38 mg/kg bodyweight/day B(a)P (Table 2). There were no attributable effects on popliteal lymph nodes, bone marrow, or BALT in B(a)P treated mice. There was a significant mild to moderate decrease in the corpus luteum numbers in the ovaries of mice treated with ≥75 mg/kg bodyweight/day B(a)P. Histopathological changes observed in mice treated with phenanthrene or pyrene were sporadic and not considered to be related to treatment. Histopathological examination was not conducted in CPS treated mice.
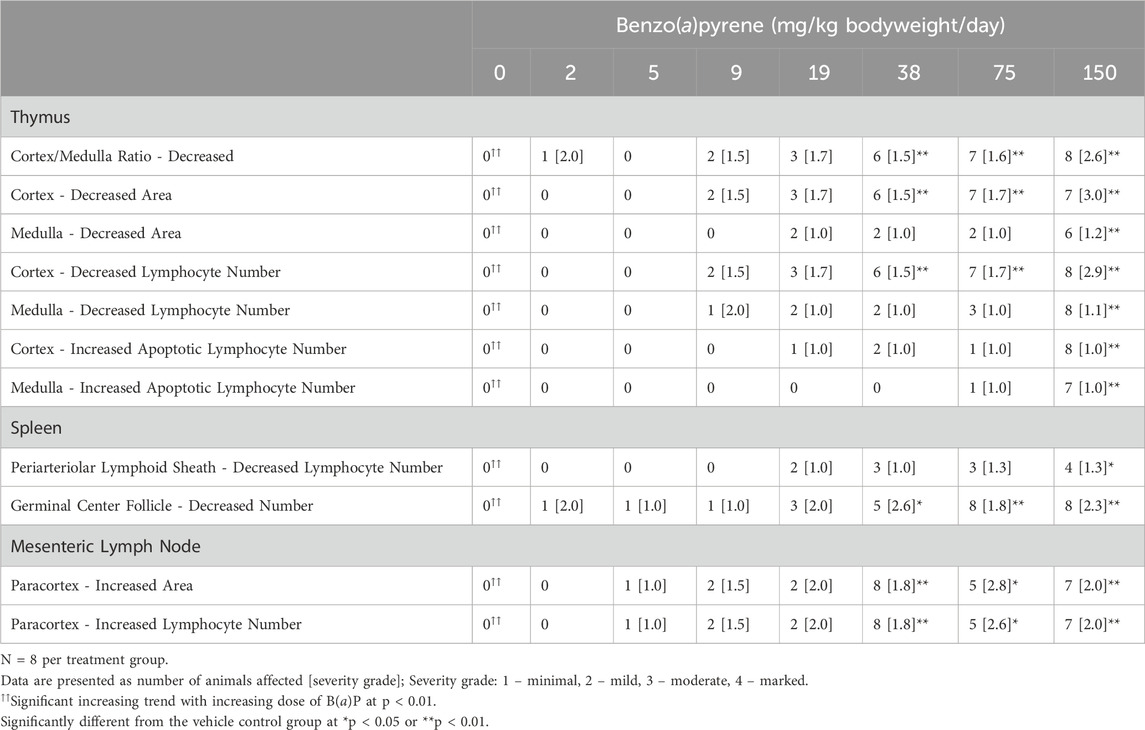
Table 2. Incidence and severity of histopathological changes in female B6C3F1/N mice treated orally with benzo(a)pyrene.
Hematological profiles and white blood cell differentials were determined on Day 28 following oral exposure to B(a)P (Table 3), phenanthrene (Table 4), or pyrene (Table 5). Significant decreases were observed in most erythrocytic parameters including erythrocyte counts (≥19 mg/kg bodyweight/day), hemoglobin and hematocrit values (≥38 mg/kg bodyweight/day), and mean cell hemoglobin concentration in mice treated with 150 mg/kg bodyweight/day B(a)P (Table 3). Mean cell hemoglobin and mean cell volumes (≥19 mg/kg bodyweight/day) and platelet counts (150 mg/kg bodyweight/day) were increased by B(a)P treatment. Increased platelet counts were observed in mice treated with 400 mg/kg bodyweight/day phenanthrene (Table 4). Mice exposed to ≥100 mg/kg bodyweight/day pyrene showed significantly decreased mean cell hemoglobin relative to the vehicle control group (Table 5).
Leukocyte counts were decreased in mice exposed to 150 mg/kg bodyweight/day B(a)P as were the number of lymphocytes (Table 6). Eosinophil counts and relative percentage were decreased in mice exposed to ≥75 mg/kg bodyweight/day B(a)P. There were no significant changes in white blood cell differentials following phenanthrene (CEBS Summary Table M03) or pyrene (CEBS Summary Table M03) exposure. CPS treatment reduced most hematological endpoints as well as produced leukocytopenia (Tables 6, 7).
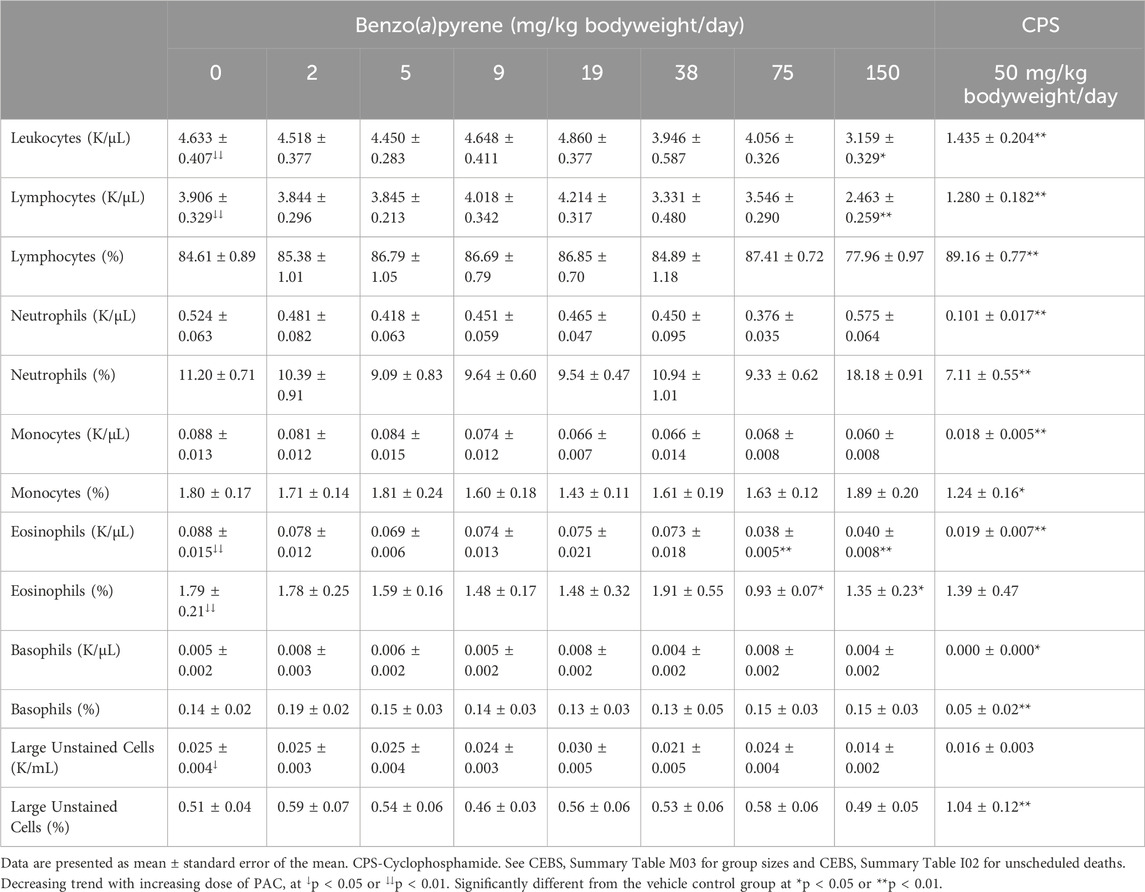
Table 6. White blood cell counts and differentials in blood of female B6C3F1/N mice treated orally with benzo(a)pyrene.
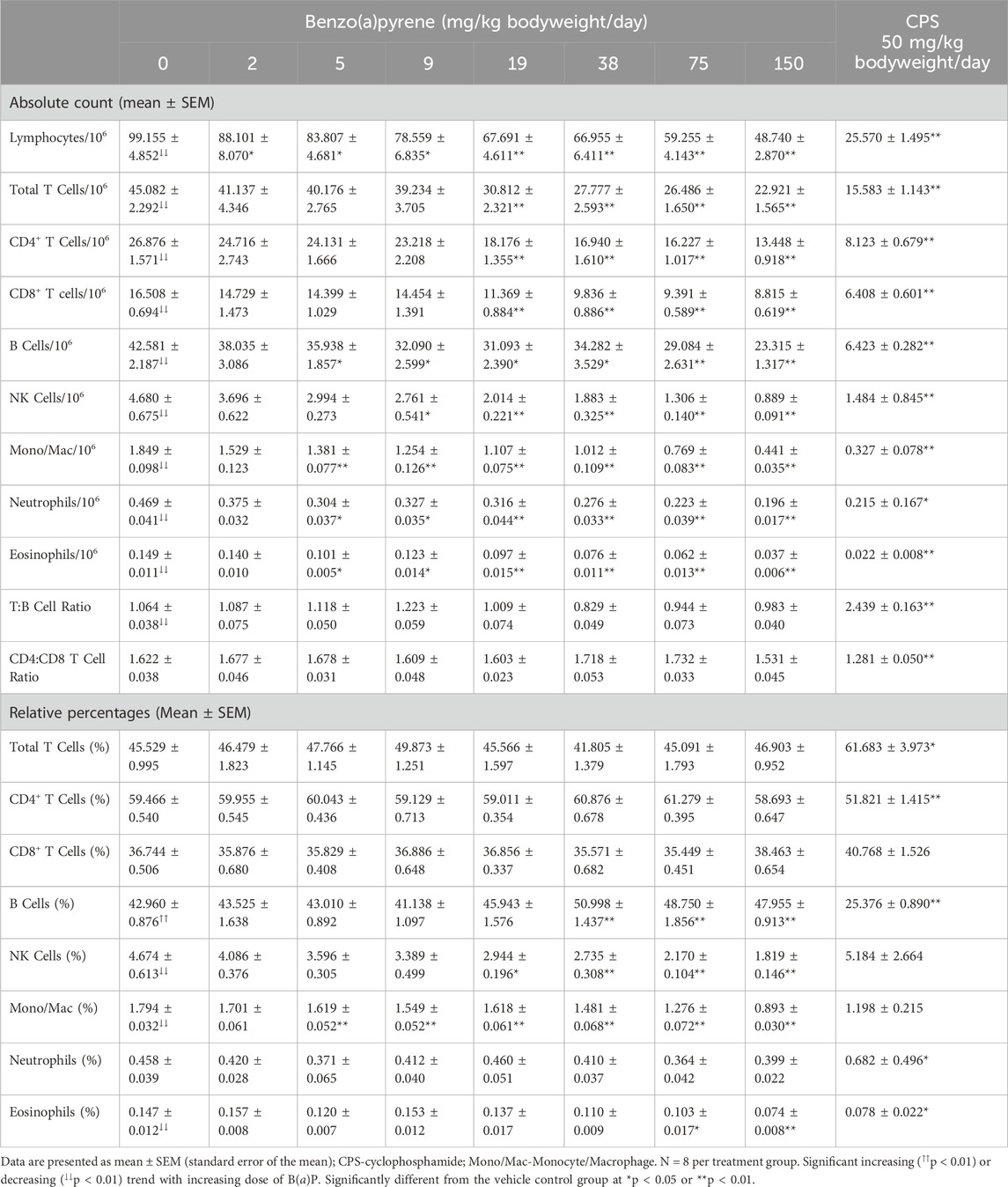
Table 7. Immunophenotypes in spleen cells of female B6C3F1/N mice treated orally for 28 days with benzo(a)pyrene.
The T-dependent antibody response (TDAR) to SRBC following PAC treatment in mice was assessed 4 days following immunization by enumerating individual antibody forming cells (AFC) in the spleen (CEBS Summary Table M07) as well as determining the titer of IgM antibodies against SRBC in serum (CEBS Summary Table M08). The AFC responses are presented following normalization as per 106 spleen cells and per total spleen cells which allows for discrimination of direct functional effects from the potential impact of changes in cellularity of the spleen on the TDAR. There were significant negative exposure related trends for the AFC response to SRBC following B(a)P treatment (Figure 1). In addition, the number of AFC/106 spleen cells and AFC/total spleen cells were significantly decreased in mice treated with ≥5 mg/kg bodyweight/day and ≥2 mg/kg bodyweight/day, respectively (Figures 1A,B). There was also a significant decrease in nucleated spleen cells numbers at doses ≥9 mg/kg bodyweight/day B(a)P (Figure 1C). Treatment with phenanthrene also resulted in a significant negative exposure related trend in the SRBC AFC response (Figure 1D) with significant decreases in AFC/106 spleen cells (Figure 1D) and AFC/spleen (Figure 1E) in mice treated with ≥50 mg/kg bodyweight/day phenanthrene relative to the vehicle control group. In contrast to B(a)P, treatment with phenanthrene did not affect the total number of nucleated spleen cells (Figure 1F). Pyrene treatment did not affect the SRBC AFC response (Figures 1G–I). The positive control groups performed as expected, with both the number of AFCs and spleen cells markedly reduced following treatment with CPS and 10 mg/kg bodyweight/day B(a)P.
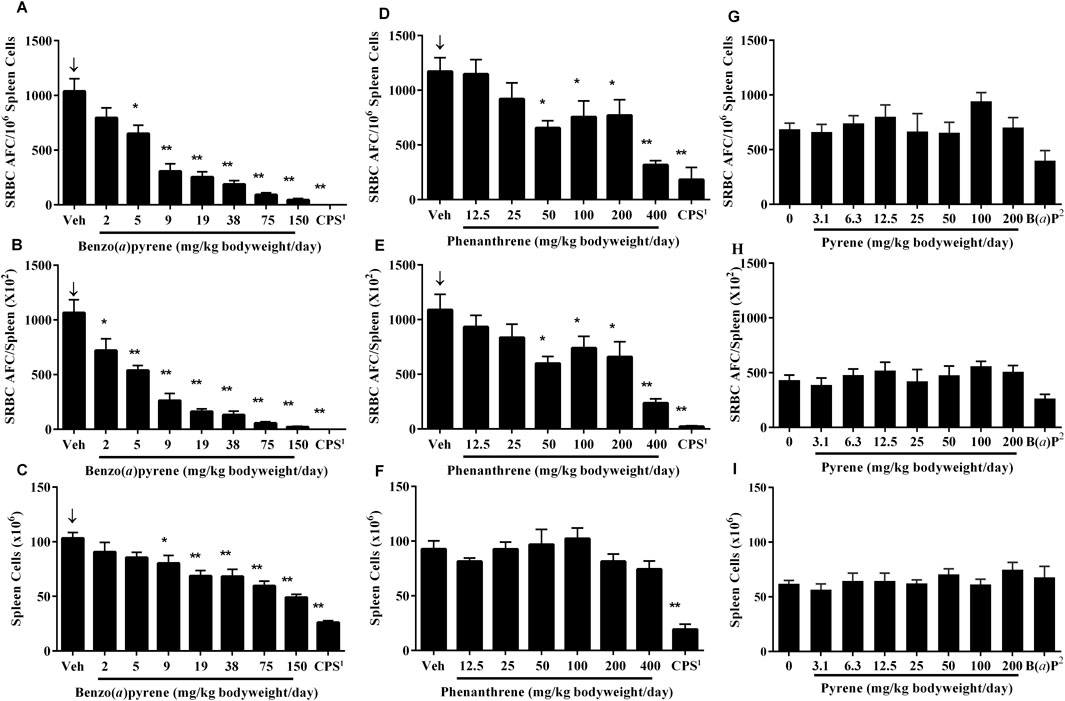
Figure 1. AFC response to SRBC in mice treated with B(a)P, phenanthrene or pyrene. Data are shown as AFC/106 spleen cells (A, D, G), AFCs per spleen (B, E, H) and total spleen cells (C, F, I). Each value represents the mean ± SEM of 5-8 mice (see CEBS Summary Tables M07 for group size and I02 for unscheduled deaths). 1Cyclophosphamide (CPS; positive control) was administered at a dose of 50 mg/kg bodyweight/day in saline, once per day via intraperitoneal (IP) injection on Days 24–27 for the B(a)P and phenanthrene studies. 2Benzo(a)pyrene [B(a)P; positive control] was administered on the same schedule as pyrene at a dose of 10 mg/kg bodyweight/day. ↓Indicates a significant decreasing trend with increasing dose of PAC (p < 0.01). Significantly different from the vehicle control group at *p < 0.05 or **p < 0.01.
A significant decrease, with increasing test concentrations of B(a)P, was observed in titers of serum anti-SRBC IgM antibodies, with statistically significant decreases observed in mice treated with ≥5 mg/kg bodyweight/day (Figure 2A). Treatment with phenanthrene (Figure 2B) or pyrene (Figure 2C) did not affect anti-SRBC IgM antibody titers. A marked decrease was observed in the positive control groups following treatment with CPS and 10 mg/kg bodyweight/day B(a)P.
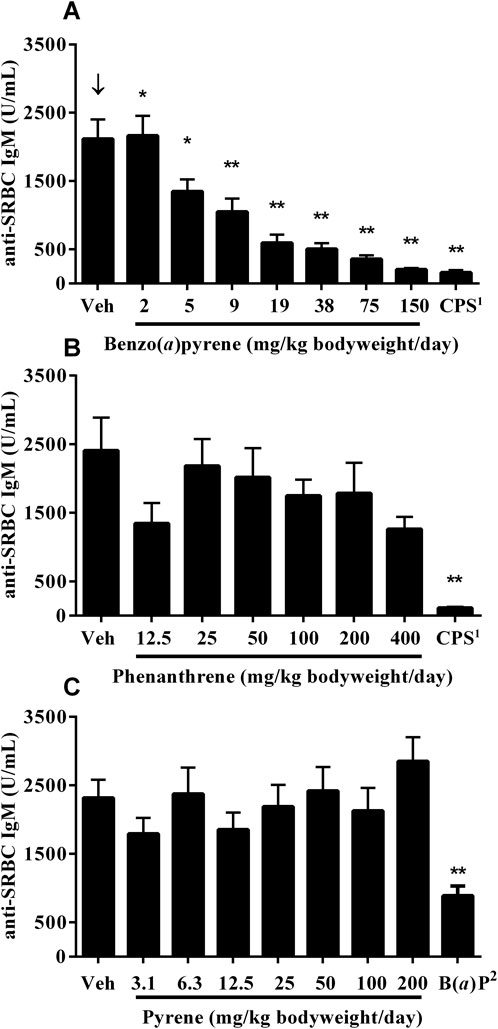
Figure 2. Anti-SRBC IgM antibody titers in serum 4 days following immunization of mice treated orally for 28 days with (A) B(a)P (B) phenanthrene, or (C) pyrene. Each value represents the Mean ± SEM of 5-8 mice (see CEBS Summary Tables M08 for group sizes and I02 for unscheduled deaths). 1Cyclophosphamide (CPS) was administered at a dose of 50 mg/kg bodyweight/day in saline, once per day via intraperitoneal (IP) injection on Days 24 (day of immunization with SRBC) to 27 for the B(a)P and phenanthrene studies. 2Benzo(a)pyrene [B(a)P; positive control] was administered on the same schedule as pyrene at a dose of 10 mg/kg body. ↓Indicates a significant decreasing trend with increasing dose of PAC (p < 0.01). Significantly different from the vehicle control group at *p < 0.05 or **p < 0.01.
Spleen cells were collected and stained with fluorescently labeled antibodies to cell surface markers for immunophenotypic analyses. Cell types enumerated included total lymphocytes, B cells, T cells, CD4+ lymphocytes, CD8+ lymphocytes, NK cells, monocytes/macrophages, eosinophils and neutrophils. In addition, CD4+:CD8+ and T:B cell ratios were determined. The results are presented as both absolute numbers and the relative percentage of nucleated spleen cells. Decreases were observed in the absolute number of all immunophenotypes examined following B(a)P treatment (Table 7). Total lymphocytes in the spleen were significantly decreased in all B(a)P treatment groups relative to the vehicle control group. Statistically significant decreases in the number of T cells, CD4+ T cells, and CD8+ T cells occurred at doses ≥19 mg/kg bodyweight/day, NK cells at doses ≥9 mg/kg bodyweight/day, and B cells, monocyte/macrophage, neutrophils, and eosinophils at doses ≥5 mg/kg bodyweight/day B(a)P. In contrast, there was little, if any, effect of B(a)P treatment on CD4+:CD8+ or T:B cell ratios compared to the vehicle control group suggesting a lack of selectivity for these immune cell populations. There was little change when analyzed as relative percentages with only small decreases in the percentage of NK cells (≥19 mg/kg bodyweight/day), monocyte/macrophage (≥5 mg/kg bodyweight/day), and eosinophils (≥75 mg/kg bodyweight/day) while the percentage of B cells showed a small but significant increase at dose levels ≥38 mg/kg bodyweight/day B(a)P.
There were minor changes in immune cell populations in the spleen following oral treatment of mice with phenanthrene (Table 8) and pyrene (Table 9) This included a significant decrease in eosinophils in the 400 mg/kg bodyweight/day phenanthrene treatment group and a slight, but significant, decrease in the T:B cell ratio in splenocytes of mice treated with ≥25 mg/kg bodyweight/day pyrene. The shift in T:B cell ratio in mice treated with pyrene corresponded with an increasing trend observed for the B cell population (absolute and relative) and a decreasing trend for T cells (relative) with the relative counts being significant for both populations in mice treated with ≥25 mg/kg bodyweight/day pyrene. Treatment of mice with ≥50 mg/kg bodyweight/day pyrene also decreased the relative percentage of eosinophils. These changes caused by phenanthrene and pyrene were small and unlikely to have a biological impact.
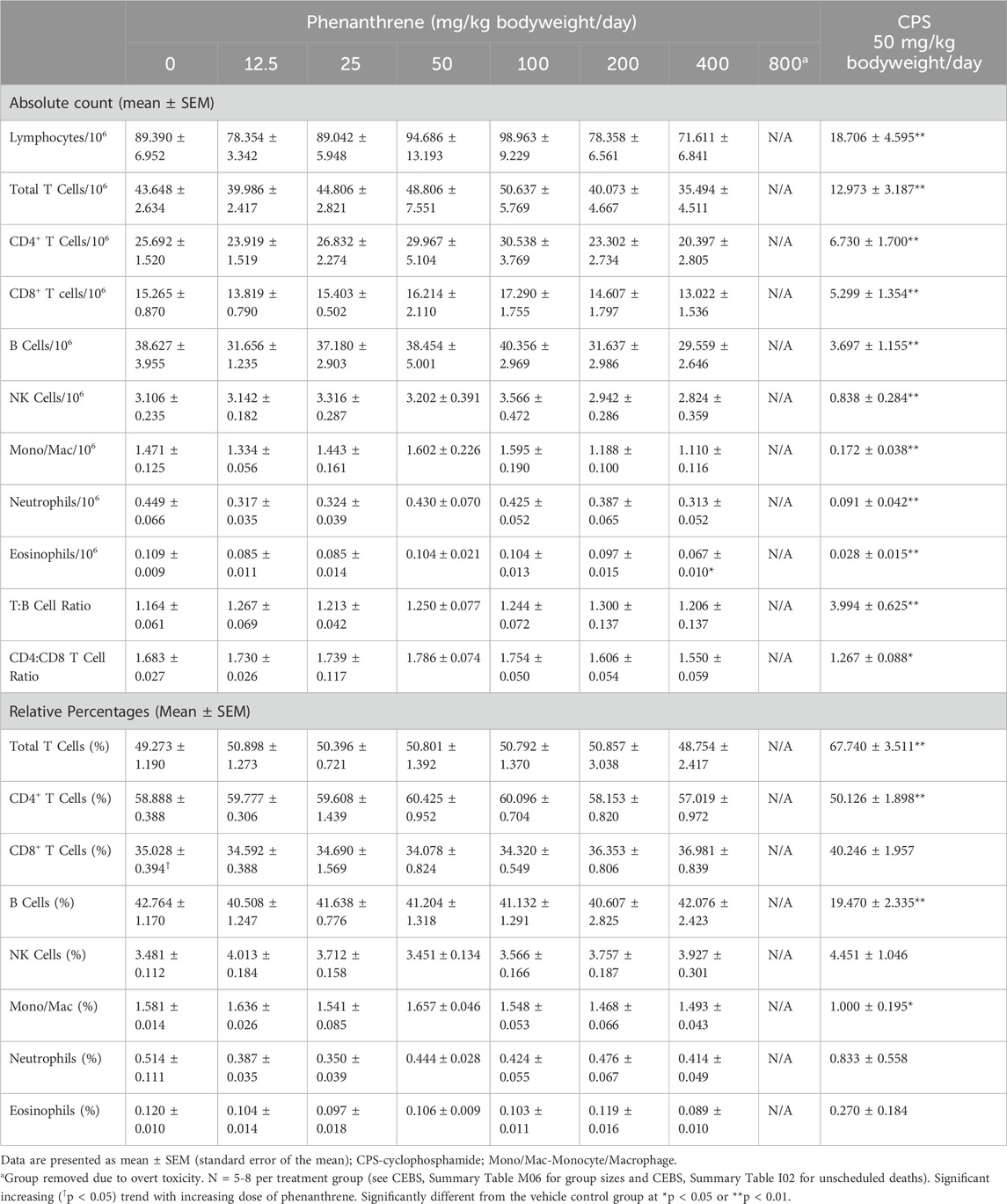
Table 8. Immunophenotypes in spleen cells of female B6C3F1/N mice treated orally for 28 days with phenanthrene.
All immune cell populations in the spleen were significantly reduced in mice treated with the positive control CPS, which is consistent with the splenic atrophy and reduced splenocyte numbers.
There were no effects following B(a)P, phenanthrene, or pyrene treatment on total cellularity (CEBS Summary Table M17) or histopathology of the bone marrow (CEBS Summary Tables PA10, PA14, and PA18), relative to vehicle controls.
The quantitative relationship between the TDAR, selected immunophenotypes, and selected organ weights to PAC treatment were determined by benchmark dose (BMD) modeling using 1SD of the control mean as the reference value (Table 10). All endpoints provided BMD estimates for B(a)P whereas fewer endpoints showed enough change to successfully model using 1SD of the control for phenanthrene and pyrene treatments. B(a)P was the most potent PAC examined (BMDs ranged from 2.00 to 144.48 mg/kg bodyweight/day) as all BMDs were lower (all but liver weight were >1 order of magnitude lower) than the corresponding BMDs for phenanthrene (BMDs ranged from 29.30 to 1044.41 mg/kg bodyweight/day) and pyrene (207.27–1251.45 mg/kg bodyweight/day). The TDAR to SRBC was the most sensitive parameter to B(a)P treatment with the BMD being 3.22 mg/kg bodyweight/day and 2.00 mg/kg bodyweight/day for the AFC/106 spleen cells and AFC/spleen, respectively. Serum levels of anti-SRBC IgM was approximately 2 times less sensitive to B(a)P with a BMD of 7.33 mg/kg bodyweight/day. The impact of B(a)P on T-cell, B-cell, macrophage/monocyte, and NK cell populations in the spleen were also less than the AFC response with BMDs ranging from 6.79–22.07 mg/kg bodyweight/day. The least sensitive parameters examined were organ weights. Spleen weight had a BMD of 74.47 mg/kg bodyweight/day which was less sensitive than the impact on immune cell populations from the spleen. Likewise, the organ weight BMDs of 28.19 mg/kg bodyweight/day for the thymus and 144.48 mg/kg bodyweight/day for the liver were substantially higher than for the TDAR in animals treated with B(a)P.
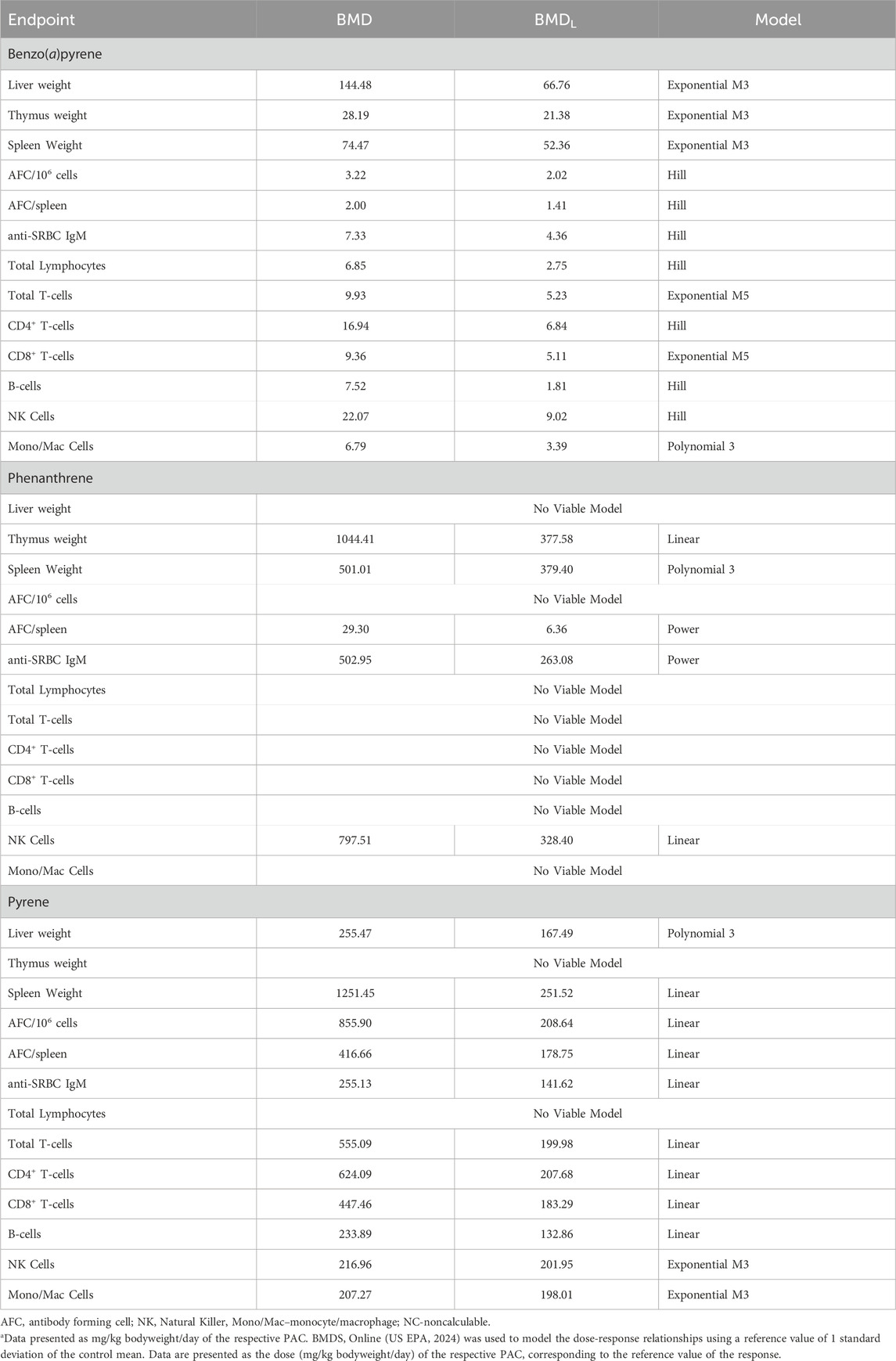
Table 10. Benchmark dose modeling for organ weights, cell population numbers, and antibody responses in mice treated orally with benzo(a)pyrene, phenanthrene, or pyrene.
The immune response profile was characterized for one potent PAC, B(a)P, and two PACs at the other end of the potency spectrum, phenanthrene and pyrene. These studies further support that B(a)P is a potent immunotoxic chemical with apparent selectivity towards disruption of humoral immunity. Several new insights into the mode of action of B(a)P on the immune system can be drawn from these data. Despite atrophy and cell loss of central and peripheral lymphoid organs (i.e., spleen, thymus, and mesenteric lymph node) following B(a)P treatment, there were no major effects identified on peripheral blood cells, as assessed by WBC counts and differentials. This would imply that B(a)P may interfere with late-stage lymphoid cell maturation that occurs predominantly in peripheral lymphoid organs. While the bone marrow is responsible for producing pro-lymphocytes, subsequently these cells migrate to peripheral lymphoid organs where they differentiate into immunocompetent B cells or T cells in an antigen-independent manner or the lymph node where they encounter antigen (Goldsby et al., 2003; Kats, 1977). Thus, while immune cells are generated, it is possible that B(a)P interferes with either cell migration to or colonization of secondary lymphoid organs. Holladay and Smith (1995), conducting studies in which differentiation markers for pro-B and pro-T cells were examined following B(a)P treatment, also concluded that late-stage maturation is compromised, although they noted that the effect may also be at the level of the bone marrow stem cell as well as central lymphoid organs as there was some depletion of bone marrow prolymphocytic cells. The present study showed that B(a)P treatment did not affect total cellularity or histopathology of the bone marrow, but these are relatively crude endpoints.
Immunophenotyping data showed that a significant loss in all immune cell types occurred in the spleen, including lymphocytes, NK cells, monocytes/macrophages and neutrophils following B(a)P treatment. This non-selective leukopenia was most evident when comparing absolute values to relative percentages where, in contrast to absolute values, there was no major effect on relative percentages in any cell population. It is important to note, however, that spleen cell depletion is likely not solely responsible for suppression of the TDAR as low dose levels of B(a)P, where spleen cell depletion was not present, still showed significant antibody suppression. This also occurred in phenanthrene studies where antibody responses were decreased without evidence of any cell depletion in central lymphoid organs.
The primary TDAR is a complex immune response that involves antigen processing and presentation by antigen presenting cells including dendritic cells and macrophages leading to training of CD4+ T cells and B cells to recognize the antigen, in this case SRBC. A benchmark dose modeling approach was used to generate BMDs for the immune endpoints investigated following PAC treatment. The BMD model is a statistical approach used to determine the concentration that causes a predefined effect size in the parameters being studied and can be used to rank sensitivities. The BMD approach is preferred by many regulatory agencies, including the US EPA (US EPA, 2024) for generating points of departure for human health risk assessment and establishment of regulatory action limits. Analyses of sensitivities of the immune parameters examined in this study using BMS showed that the TDAR was the most sensitive endpoint found for both B(a)P and phenanthrene. Regarding cell numbers, macrophages and B-cells showed the same order of magnitude sensitivity to B(a)P treatment. Interestingly, CD4+ T-helper cell numbers were about 2 times less sensitive to B(a)P treatment than macrophages and B cells. These data suggest that macrophages and B cells may be more sensitive to the effects of B(a)P than CD4+ T-helper cells and the suppression of the TDAR may be driven predominantly through impacts on macrophages and B cells. Acute lymphopenia is known to result in marked lymphoproliferation and is considered a homeostatic response to the depletion of lymphocytes (Takada and Jameson, 2009). This compensatory proliferation drives activation of naïve T cells and favors expansion of regulatory T cells, an outcome that may limit downstream immune responses (Eldershaw et al., 2021). Therefore, it is possible that immune cell depletion observed following B(a)P treatment may trigger lymphoproliferation in favor of suppressive regulatory T cells, thereby contributing to the deficit in humoral immunity.
Several studies have suggested an association exists between immunotoxicity and carcinogenicity stemming from the concept that immune surveillance mechanisms may be altered by immunotoxic substances (Hanahan and Weinberg, 2011; Luster et al., 1992b; Luster et al., 1992a; Smith et al., 2016; Stjernswärd, 1966). This was highlighted in a detailed examination showing a strong correlation (R2 = 0.8976) between the relative cancer potency and relative immunotoxicity potency for 9 PACs (Zaccaria and McClure, 2013). Based on data from the later study, the authors suggested that evidence of immunotoxicity could be used to strengthen cancer risk assessments for PACs. While we found that the potent carcinogen, B(a)P, was also a potent immunotoxic agent, phenanthrene also displayed immunotoxicity despite having mostly negative carcinogenic data (IARC, 2010). Pyrene did not suppress immune activity and had mostly negative cancer data. However, it should be noted that deficiencies in the cancer database for both phenanthrene and pyrene led to an IARC conclusion of not classifiable as a human carcinogen (category 3) (IARC, 2010). Previous evaluation of the immunotoxic potential of PACs found that phenanthrene elicited no immunosuppression and pyrene suppressed the immune response by 30% following a single oral dose of 100 mg/kg in C57BL/6 mice (Silkworth et al., 1995). Ongoing immunotoxicology studies from our laboratories, using 13 individual PACs, should provide additional data to help clarify the correlation between carcinogenic and immunotoxic potencies (manuscript in preparation).
In 2017, the U.S. EPA’s Integrated Risk Information System (IRIS) program released a document that reviewed potential adverse health effects of B(a)P (US EPA, 2017). In selecting a proposed overall reference dose, multiple organ/system-specific reference doses were derived for effects identified as potential hazards from B(a)P including developmental toxicity, reproductive toxicity, and immunotoxicity. Developmental and reproductive effects were identified as more sensitive to B(a)P than the available immunotoxicity outcomes with developmental neurobehavioral effects occurring at doses as low as 0.02 mg/kg (US EPA, 2017). Although all publicly available B(a)P immunotoxicity data were evaluated, only thymus weights (Kroese et al., 2001) and baseline serum IgM levels in rats (De Jong et al., 1999), which are not sensitive targets for PAC immunotoxicity, were considered adequate for conducting quantitative risk assessment under currently used benchmark modeling as other endpoints had limitations in their experimental design. As a result, the confidence for the reference dose derived from the immunotoxicity data was low (US EPA, 2017). We believe the dose response curves obtained in the current studies for both B(a)P and phenanthrene should readily lend themselves to currently used models (Filipsson et al., 2003) to assess risk for non-cancer endpoints. The present study demonstrated significant suppression of a critical immune function, the TDAR, at the lowest dose tested of 2 mg/kg bodyweight/day B(a)P. At this dose level, the TDAR was suppressed by 32% and it is possible that further reduction in the dose would still result in significant immunosuppression bringing the sensitivity of the immune system in line with developmental and reproductive outcomes.
In summary, B(a)P produced significant immunotoxicity, in the absence of overt toxicity, as evidenced by dose related decreases in central and peripheral lymphoid organ weights, humoral antibody responses and spleen cell immunophenotypes. These effects occurred in the absence of any apparent effects on bone marrow or peripheral blood cells numbers. Suggestive evidence is also provided that antibody suppression was due partly to a loss in spleen cell numbers, particularly T cells and macrophages, although functional deficits in surviving cells are also likely involved. The most profound effect was noted in the TDAR which was decreased by >75% compared to vehicle controls at a dose of 9 mg/kg bodyweight/day B(a)P. Phenanthrene, a putative non-carcinogenic PAH, also inhibited humoral immunity, but was considerably less potent, while pyrene showed no evidence of immunotoxicity. Based on the most sensitive endpoint of AFC/spleen, the BMD of 2.00 mg/kg bodyweight/day B(a)P for the AFC/spleen was at the lowest level tested (2 mg/kg bodyweight/day), while the BMD for AFC/spleen for phenanthrene and pyrene were 29.30 and 416.66 mg/kg bodyweight/day, respectively. These studies establish the range of PAC immunotoxicity from severe immunosuppression induced by B(a)P to the lack of immunotoxicity resulting from pyrene. These PACs will be important for ranking the immunotoxic potency of additional PACs which will contribute to opportunities for modeling the impact of mixtures on the immune system and correlating immunotoxic with carcinogenic potency.
The datasets presented in this study can be found in an online repository owned by NIEHS at the following website; https://doi.org/10.22427/NTP-DATA-500-005-004-000-4.
The animal study was approved by Burleson Research Technologies, Inc. IACUC. The study was conducted in accordance with the local, state, and federal legislation and institutional requirements.
VJ: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing–original draft, Writing–review and editing. CR: Conceptualization, Data curation, Writing–review and editing. ML: Methodology, Writing–original draft, Writing–review and editing. CW: Data curation, Formal Analysis, Investigation, Supervision, Writing–review and editing. SH: Data curation, Formal Analysis, Writing–review and editing. BS: Data curation, Formal Analysis, Investigation, Writing–review and editing. JB: Data curation, Methodology, Supervision, Formal Analysis, Validation, Writing–review and editing. EM: Methodology, Supervision, Writing–review and editing. VG: Methodology, Supervision, Writing–review and editing. BB: Data curation, Formal Analysis, Investigation, Supervision, Writing–review and editing. RF: Data curation, Methodology, Supervision, Formal Analysis, Validation, Funding acquisition, Writing–review and editing. SW: Methodology, Supervision, Writing–review and editing. GB: Writing–review and editing. DG: Conceptualization, Funding acquisition, Methodology, Project administration, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) Intramural Research Programs, Research Triangle Park, NC (ZIA ES103373 and ZIC ES103391-02) and contracts HHSN273201400017C (Burleson Research Technologies, Morrisville, NC), HHSN273201400027C (Battelle, Columbus, OH), HHSN273201400022C (RTI International, RTP, NC), and GS-00F-173CA/75N96022F00055 (Social and Scientific Systems, Inc., A DLH Holdings Corp Company).
The authors would like to thank Rachel Frawley for her critical review of the manuscript. We also would like to thank Helen Cunny and Kristen Ryan for curating and organizing the public data page for this work.
Authors VJ, ML, and GB were employed by Burleson Research Technologies, Inc. Author CW was employed by Integrated Laboratory Systems, LLC. Author SH was employed by DLH, LLC. Authors BS, and BB was employed by Battelle.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al Hello, M., Burris, D. R., Chitsaz, M., and Rodenburg, L. A. (2023). Source apportionment of polycyclic aromatic hydrocarbons in New York/New Jersey Harbour sediment. Water Environ. J. 37, 527–537. doi:10.1111/WEJ.12856
Archibong, A. E., Inyang, F., Ramesh, A., Greenwood, M., Nayyar, T., Kopsombut, P., et al. (2002). Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod. Toxicol. 16, 801–808. doi:10.1016/S0890-6238(02)00058-8
Biró, A., Pállinger, É., Major, J., Jakab, M. G., Klupp, T., Falus, A., et al. (2002). Lymphocyte phenotype analysis and chromosome aberration frequency of workers occupationally exposed to styrene, benzene, polycyclic aromatic hydrocarbons or mixed solvents. Immunol. Lett. 81, 133–140. doi:10.1016/S0165-2478(01)00342-X
Chen, C., Tang, Y., Jiang, X., Qi, Y., Cheng, S., Qiu, C., et al. (2012). Early postnatal benzo(a)pyrene exposure in Sprague-Dawley rats causes persistent neurobehavioral impairments that emerge postnatally and continue into adolescence and adulthood. Toxicol. Sci. 125, 248–261. doi:10.1093/TOXSCI/KFR265
Dean, J. H., Luster, M. I., Boorman, G. A., Lauer, L. D., Leubket, R. W., and Lawson, L. (1983). Selective immunosuppression resulting from exposure to the carcinogenic congener of benzopyrene in B6C3F1 mice. Clin. Exp. Immunol. 52, 199–206.
De Jong, W. H., Dinant Kroese, E., Vos, J. G., and Loveren, H. V. (1999). Detection of immunotoxicity of benzo[a]pyrene in a subacute toxicity study after oral exposure in rats. Toxicol. Sci. 50, 214–220. doi:10.1093/toxsci/50.2.214
Detmar, J., Rennie, M. Y., Whiteley, K. J., Qu, D., Taniuchi, Y., Shang, X., et al. (2008). Fetal growth restriction triggered by polycyclic aromatic hydrocarbons is associated with altered placental vasculature and AhR-dependent changes in cell death. Am. J. Physiol. Endocrinol. Metab. 295, E519–E530. doi:10.1152/AJPENDO.90436.2008
Dixon, W. J., and Massey, F. J. (1957). Introduction to statistical analysis. International student edition. New York, NK: McGraw-Hill Book Company, Inc.
Dunn, O. J. (1964). Multiple comparisons using rank sums. Technometrics 6, 241–252. doi:10.1080/00401706.1964.10490181
Dunnett, C. W. (1955). A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 50, 1096–1121. doi:10.1080/01621459.1955.10501294
Eldershaw, S., Verma, K., Croft, W., Rai, T., Kinsella, F. A. M., Stephens, C., et al. (2021). Lymphopenia-induced lymphoproliferation drives activation of naive T cells and expansion of regulatory populations. iScience 24, 102164. doi:10.1016/J.ISCI.2021.102164
Filipsson, A. F., Sand, S., Nilsson, J., and Victorin, K. (2003). The benchmark dose method—review of available models, and recommendations for application in health risk assessment. Crit. Rev. Toxicol. 33, 505–542. doi:10.1080/10408440390242360
Germolec, D. R., Lebrec, H., Anderson, S. E., Burleson, G. R., Cardenas, A., Corsini, E., et al. (2022). Consensus on the key characteristics of immunotoxic agents as a basis for hazard identification. Environ. Health Perspect. 130, 105001–105016. doi:10.1289/EHP10800
Goldsby, R., Kindt, T. J., Osborne, B. A., and Kuby, J. (2003). “Chapter 2: cells and organs of the immune system,” in Immunology (New York: W. H. Freeman and Company), 24–56.
Haber, L. T., Pecquet, A. M., Vincent, M. J., and White, L. M. (2022). The long goodbye: finally moving on from the relative potency approach to a mixtures approach for polycyclic aromatic hydrocarbons (PAHs). Int. J. Environ. Res. Public Health 19, 9490. doi:10.3390/IJERPH19159490
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi:10.1016/J.CELL.2011.02.013
Hecht, S. S., Chen, M., Yagi, H., Jerina, D. M., and Carmella, S. G. (2003). r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: a potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol Biomarkers Prev 12, 1501–1508.
Holladay, S., and Smith, B. J. (1995). Benzo[a]pyrene-induced alterations in total immune cell number and cell-surface antigen expression in the thymus, spleen and bone marrow of B6C3F1 mice. Vet. Hum. Toxicol. 37, 99–104.
Hsieh, J., Sedykh, A., Mutlu, E., Germolec, D. R., Auerbach, S. S., and Rider, C. V. (2021). Harnessing in silico, in vitro, and in vivo data to understand the toxicity landscape of polycyclic aromatic compounds (PACs). Chem. Res. Toxicol. 34, 268–285. doi:10.1021/acs.chemrestox.0c00213
IARC (2010). “Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures,” in (IARC monographs on the evaluation of carcinogenic risks to humans; v.92). IARCV Monographs on the Evaluation of Carcinogenic Risks to Humans (Lyon, France).
Inyang, F., Ramesh, A., Kopsombut, P., Niaz, M. S., Hood, D. B., Nyanda, A. M., et al. (2003). Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod. Toxicol. 17, 527–537. doi:10.1016/S0890-6238(03)00071-6
Johnson, V. J., Rider, C. V., Luster, M. I., Brix, A., Burleson, G. R., Cora, M., et al. (2023). Immunotoxicity of N-butylbenzenesulfonamide: impacts on immune function in adult mice and developmentally exposed rats. Toxicol. Sci. 196, 71–84. doi:10.1093/TOXSCI/KFAD083
Karakaya, A., Ates, I., and Yucesoy, B. (2004). Effects of occupational polycyclic aromatic hydrocarbon exposure on T-lymphocyte functions and natural killer cell activity in asphalt and coke oven workers. Hum. Exp. Toxicol. 23, 317–322. doi:10.1191/0960327104HT455OA
Kats, D. H. (1977). Lymphocyte differentiation, recognition, and regulation. New York, NY: Academic Press.
Kroese, E. D., Muller, J. J. A., Mohn, G. R., Dortant, P. M., and Wester, P. W. (2001). Tumorigenic effects in Wistar rats orally administered benzo[a]pyrene for two years (gavage studies): implications for human cancer risks associated with oral exposure to polycyclic aromatic hydrocarbons. Bilthoven, Netherlands: National Institute for Public Health and the Environment.
Luster, M. I., Pait, D. G., Portier, C., Rosenthal, G. J., Germolec, D. R., Comment, C. E., et al. (1992a). Qualitative and quantitative experimental models to aid in risk assessment for immunotoxicology. Toxicol. Lett. 64-65, 71–78. doi:10.1016/0378-4274(92)90174-i
Luster, M. I., Portier, C., Pait, D. G., White, K. L., Gennings, C., Munson, A. E., et al. (1992b). Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 18, 200–210. doi:10.1016/0272-0590(92)90047-l
Mann, H. B., and Whitney, D. R. (1947). On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Statistics 18, 50–60. doi:10.1214/aoms/1177730491
Nota, B., Bosse, M., Ylstra, B., van Straalen, N. M., and Roelofs, D. (2009). Transcriptomics reveals extensive inducible biotransformation in the soil-dwelling invertebrate Folsomia candida exposed to phenanthrene. BMC Genomics 10, 236. doi:10.1186/1471-2164-10-236
Oh, E., Im, H., Kang, H. S., Jung, W., Won, N. H., Lee, E., et al. (2006). Comparison of immunnological and genotoxicological parameters in automobile emission inspectors exposed to polycyclic aromatic hydrocarbons. Environ. Toxicol. Pharmacol. 21, 108–117. doi:10.1016/J.ETAP.2005.08.004
Shirley, E. (1977). A non-parametric equivalent of Williams’ test for contrasting increasing dose levels of a treatment. Biometrics 33, 386–389. doi:10.2307/2529789
Silkworth, J. B., Lipinskas, T., and Stoner, C. R. (1995). Immunosuppressive potential of several polycyclic aromatic hydrocarbons (PAHs) found at a Superfund site: new model used to evaluate additive interactions between benzo[a]pyrene and TCDD. Toxicology 105, 375–386. doi:10.1016/0300-483X(95)03235-8
Smith, M. T., Guyton, K. Z., Gibbons, C. F., Fritz, J. M., Portier, C. J., Rusyn, I., et al. (2016). Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ. Health Perspect. 124, 713–721. doi:10.1289/EHP.1509912
Stjernswärd, J. (1966). Effect of noncarcinogenic and carcinogenic hydrocarbons on antibody-forming cells measured at the cellular level in vitro. JNCI J. Natl. Cancer Inst. 36, 1189–1195. doi:10.1093/JNCI/36.6.1189
Szczeklik, A., Szczeklik, J., Galuszka, Z., Musial, J., Kolarzyk, E., and Targosz, D. (1994). Humoral immunosuppression in men exposed to polycyclic aromatic hydrocarbons and related carcinogens in polluted environments. Environ. Health Perspect. 102, 302–304. doi:10.1289/EHP.94102302
Takada, K., and Jameson, S. C. (2009). Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 9, 823–832. doi:10.1038/NRI2657
Temple, L., Kawabata, T., Munson, A., and White, K. (1993). Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 21, 412–419. doi:10.1006/FAAT.1993.1116
US EPA (1993). “Provisional guidance for quantitative risk assessment of polycyclic aromatic hydrocarbons (PAH),” in U.S. Environmental protection agency, office of research and development (Washington DC: Office of Health and Environmental Assessment).
US EPA (2007). “Provisional peer reviewed toxicity values for pyrene (CASRN 129-00-0),” in Superfund health risk technical support center, national center for environmental assessment, office of research and development (Cincinnati, OH: U.S. Environmental Protection Agency).
US EPA (2009). “Provisional peer-reviewed toxicity values for phenanthrene (CASRN 85-01-8),” in Superfund health risk technical support center, national center for environmental assessment, office of research and development (Cincinnati, OH: U.S. Environmental Protection Agency).
US EPA (2010). Development of a relative potency factor (rpf) approach for polycyclic aromatic hydrocarbon (PAH) mixtures (external review draft, suspended). Washington, DC: U.S. Environmental Protection Agency. EPA/635/R-08/012A.
US EPA (2017). IRIS toxicological review of benzo[A]pyrene (final report). Washington, DC: U.S. Environmental Protection Agency. EPA/635/R-17/003F.
US EPA (2024). BMDS-online (build d5f505c8; model library version 2023.03.1). Available at: https://bmdsonline.epa.gov.
Watson, A. L. T. D., Johnson, V. J., Luster, M. I., Burleson, G. R., Fallacara, D. M., Sparrow, B. R., et al. (2021). Immunotoxicity studies of sulfolane following developmental exposure in Hsd:Sprague Dawley SD rats and adult exposure in B6C3F1/N mice. J. Immunotoxicol. 18, 1–12. doi:10.1080/1547691X.2020.1869355
White, K. L., Lysy, H. H., and Holsapple, M. P. (1985). Immunosuppression by polycyclic aromatic hydrocarbons: a structure-activity relationship in B6C3F1 and DBA/2 mice. Immunopharmacology 9, 155–164. doi:10.1016/0162-3109(85)90011-6
Williams, D. A. (1986). A note on Shirley’s nonparametric test for comparing several dose levels with a zero-dose control. Biometrics 42, 183–186. doi:10.2307/2531254
Winker, N., Tuschl, H., Kovac, R., and Weber, E. (1997). Immunological investigations in a group of workers exposed to various levels of polycyclic aromatic hydrocarbons. J. Appl. Toxicol. 17, 23–29. doi:10.1002/(sici)1099-1263(199701)17:1<23::aid-jat387>3.0.co;2-o
Wu, M. T., Pan, C. H., Huang, Y. L., Tsai, P. J., Chen, C. J., and Wu, T. N. (2003). Urinary excretion of 8-hydroxy-2-deoxyguanosine and 1-hydroxypyrene in coke-oven workers. Environ. Mol. Mutagen 42, 98–105. doi:10.1002/EM.10176
Keywords: immunotoxicity, immunosuppression, polycyclic aromatic hydrocarbons (PAH), benzo(a)pyrene, phenanthrene, pyrene, polycyclic aromatic compounds (pac)
Citation: Johnson VJ, Rider CV, Luster MI, Willson CJ, Harris S, Stiffler B, Blake J, Mutlu E, Godfrey V, Burback B, Fernando R, Waidyanatha S, Burleson GR and Germolec DR (2025) Suppression of the T-dependent antibody response following oral exposure to selected polycyclic aromatic compounds in B6C3F1/N mice. Front. Toxicol. 7:1558639. doi: 10.3389/ftox.2025.1558639
Received: 10 January 2025; Accepted: 30 January 2025;
Published: 06 March 2025.
Edited by:
Emanuela Corsini, University of Milan, ItalyCopyright © 2025 Johnson, Rider, Luster, Willson, Harris, Stiffler, Blake, Mutlu, Godfrey, Burback, Fernando, Waidyanatha, Burleson and Germolec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dori R. Germolec, Z2VybW9sZWNAbmloLmdvdg==; Victor J. Johnson, dmpvaG5zb25AYnJ0LWxhYnMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.