- Food Packaging Forum Foundation, Zürich, Switzerland
Many nations have food contact material (FCM) legislation purporting to protect citizens from hazardous chemicals, often specifically by regulating genotoxic carcinogens. Despite such regulations, cancers that are associated with harmful chemical exposures are highly prevalent, especially breast cancer. Using the novel Key Characteristics of Toxicants framework, Kay et al. found 921 substances that are potential mammary carcinogens. By comparing Kay et al.‘s chemicals list with our own Database on migrating and extractable food contact chemicals (FCCmigex), we found that 189 (21%) of the potential mammary carcinogens have been measured in FCMs. When limiting these results to migration studies published in 2020–2022, 76 potential mammary carcinogens have been detected to migrate from FCMs sold in markets across the globe, under realistic conditions of use. This implies that chronic exposure of the entire population to potential mammary carcinogens from FCMs is the norm and highlights an important, but currently underappreciated opportunity for prevention. Reducing population-wide exposure to potential mammary carcinogens can be achieved by science-based policy amendments addressing the assessment and management of food contact chemicals.
Introduction
When travelling from source to table, foods contact a diverse array of food contact materials and articles (FCMs and FCAs), including processing equipment, packaging, and cookware. At each step along the way, substances from FCMs and FCAs, known as food contact chemicals (FCCs), can migrate into foodstuffs (Geueke et al., 2022). FCCs are regulated to ensure the safety of FCMs. United States regulations state that no (indirect) food additive, including FCCs, is considered safe if it causes cancer in humans or animals (Merrill, 1997). In the EU, materials must be manufactured so they do not transfer harmful constituents to food under normal use conditions at levels that are harmful to health (Regulation (EC) No 1935/2004) (European Commission, 2022; European Parliament and Council, 2004). Similar safety stipulations are applied by other governments and trade blocs globally (MERCOSUR, 1992; National People’s Congress, 2015). Regulatory agencies often require migration testing as part of FCM regulation and authorization, usually focused on restricting the use of cancer-causing genotoxic chemicals (Arvanitoyannis and Bosnea, 2004; Center for Food Safety and Applied Nutrition, 2021; Center for Food Safety and Applied Nutrition, 2007; Aguilar et al., 2008).
While global regulations and risk assessments of FCCs aim to prevent adverse health effects associated with FCC exposures, evidence suggests that these measures are not entirely effective. Several recent studies have identified links between FCCs and adverse health outcomes (Stevens et al., 2024; Trasande et al., 2024a; 2024b) or instances where the regulated allowable thresholds (reference dose, tolerable daily intake, etc.) are greater than the minimum observed adverse effect level in human populations (Maffini et al., 2021). Such examples indicate gaps in regulatory frameworks and assessment methodologies. Indeed, at least 127 known chemicals of concern (i.e., chemicals with hazard properties such as carcinogenic, mutagenic and toxic to reproduction (CMR), or endocrine disrupting chemicals (EDCs), etc.) have been shown to be present in FCMs, and 97 of these have evidence for migration (Zimmermann et al., 2022). Several other challenges exist for the risk assessment of food contact materials (Muncke et al., 2017), for example the lack of analytical methods and standards even for authorized food contact chemicals (Joint Research Centre, 2015) or robust exposure estimates (Alger et al., 2013). The European Parliament concluded that the implementation of EU FCM regulation is ineffective because it does not sufficiently protect human health (European Parliament, 2016). Similarly, the United States Government Accountability Office concluded in its investigation into the Food and Drug Administration’s (FDA) rules for FCMs that some FCCs may pose risks to human health (US Government Accountability Office, 2022).
To overcome these shortcomings, a generic approach to risk management (GRA) of chemicals, including FCCs, has been stipulated by the EU in its Chemicals Strategy for Sustainability (European Commission, 2020). GRA centers on identifying the intrinsic hazard properties of a substance, such as CMR, EDC, or environmental persistence. If a substance is identified as having such hazard properties of concern, restrictions or bans could be triggered without the need for extensively demonstrating human exposure.
The Key Characteristics of Toxicants (KC) framework can support GRA. KCs are inherent properties of chemical substances, derived from empirical evidence on how biological targets, such as biological molecules (DNA, proteins), cells, or tissues, are affected by types of chemicals with similar toxicity. For example, Smith et al. described the 10 KCs of carcinogens by identifying common molecular-level properties or interactions of known human carcinogens with biomolecules, like the induction of epigenetic alterations, causation of oxidative stress or chronic inflammation (Smith et al., 2016). This framework has also been expanded to other specific types of hazardous chemicals, such as endocrine disruptors (La Merrill et al., 2020), developmental toxicants (Arzuaga et al., 2019; Luderer et al., 2019), and immunotoxicants (Germolec et al., 2022). Recently, it has been suggested that the KC framework could also be used for predicting likely hazardous chemicals based on their molecular properties and interactions with biological molecular targets (Muncke et al., 2023).
Based on the KC framework, Kay et al. (2024) recently identified 921 substances with a high likelihood of contributing to breast cancer development, based on direct evidence of inducing mammary tumors in rodent models, genotoxicity testing, exhibiting endocrine disruption, or activation of other hormonal signaling pathways associated with the pathogenesis of breast cancer. In women, breast cancer is the most frequently diagnosed cancer and the globally leading cause of cancer deaths (Sung et al., 2021). As cancer is one of the few health effects specifically targeted in FCM regulation and testing, carcinogenic FCCs should be uncommon. Even so, chemical exposures, including to a few well-studied FCCs, have been linked to cancer development (Wan et al., 2022), covering the entire breast carcinogenesis process from tumor initiation (Wang et al., 2017) and growth (Koual et al., 2020), to metastasis (Koual et al., 2019) and resistance to chemotherapy (Lagunas-Rangel et al., 2022).
Here, we describe which potential mammary carcinogens identified by Kay et al. have been detected in FCMs and could contribute to human exposure because they have been shown to migrate into foodstuffs. Our findings imply that chronic exposure of the entire population to potential mammary carcinogens from FCMs is the norm and highlights an important, but currently underappreciated opportunity for prevention.
Information sources for chemical comparisons
To compile their list of potential mammary carcinogens, Kay et al. (2024) used authoritative, publicly available datasets and identified 921 potential mammary carcinogens, of which 909 have CAS registry numbers.
The chemical substances intentionally used to manufacture FCMs, or that are present but have not been intentionally added (NIAS) in FCMs (e.g., contaminants, impurities of starting substances, reaction by-products), are known collectively as food contact chemicals (FCCs). There are at least 14,000 known FCCs (Geueke et al., 2022; Groh et al., 2021) but there may be as many as 100,000 potentially migrating FCCs (Grob et al., 2006; McCombie, 2018) when including all possible NIAS. However, identifying all NIAS is challenging and testing for individual compounds is impossible (Bradley and Coulier, 2007; Muncke et al., 2017; Oldring et al., 2023). The presence of chemicals in FCMs is investigated using two types of testing approaches: extraction or migration experiments. Extraction experiments employ conditions designed to maximize the release of all potentially migrating chemicals from the material and are considered a worst-case scenario. Migration experiments are designed to mimic real-use conditions as closely as possible, to provide an estimate of the likely human exposure to chemicals diffusing from these materials into foodstuffs.
The Database of migrating and extractable food contact chemicals (FCCmigex) (Food Packaging Forum Foundation, 2022; Geueke et al., 2022) is a systematic evidence map of 4,248 FCCs with CASRNs gathered from 1,312 publicly available studies and reports describing FCM migration and extraction experiments through October 2022.
Using the CASRNs, we identified FCCs known to be present in food contact materials and articles that are also included within the list of potential mammary carcinogens by Kay et al. (2024). List comparisons were made with python v3.11.5, and pandas v2.0.3.
Potential breast carcinogens in FCMs
Of the 909 potential mammary carcinogens with CASRNs, 189 (21%) have been detected in FCMs (Excel Supplementary Table S1). Thirty of the chemicals have direct evidence of carcinogenesis in rodent models, another 67 are suspected to induce carcinogenesis based on their genotoxicity, and the remainder are highly likely or likely to be endocrine disruptors. Overall, 143 potential mammary carcinogens have been detected in plastic FCAs (76%), followed by non-specified materials (91; 48%), and paper and board (89; 47%), but all material groups except glass contained potential mammary carcinogens (Figure 1). When limiting results to FCCs migrating from FCMs into foods or food simulants, 121 potential mammary carcinogens have been detected.
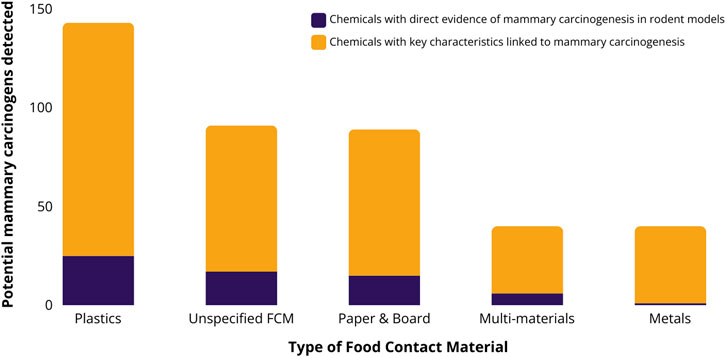
Figure 1. The number of potential mammary carcinogens as identified by Kay et al. (2024) that have been detected in migration or extraction studies of five food contact material groups. Food contact studies in FCCmigex were available online as of October 2022. Note: Potential mammary carcinogens detected, e.g., in coated cans or gaskets of metal closures, are assigned to the metal category. Columns are subdivided into food contact chemicals (FCCs) with direct evidence of carcinogenesis in rodent models (dark purple), and all other FCCs with key characteristics linked to breast carcinogenesis according to Kay et al. (yellow).
By filtering for migration studies published within the last 3 years (2020, 2021, and 2022) for which there is information in FCCmigex (Food Packaging Forum Foundation, 2022) we captured a recent picture of likely human exposure to potential mammary carcinogens from FCMs via food ingestion.
Based on 181 migration studies from these 3 years, we identified 76 potential mammary carcinogens (Table 1). Of these, 61 (80%) have been measured to transfer from plastics, 23 (30%) from unspecified materials, 21 (28%) migrated from paper and board, 8 (11%) from metals, and 6 (8%) from multi-materials. Ten of the 76 chemicals detected in FCM migration studies in recent years have direct evidence of inducing mammary tumors in rodent models, e.g., benzene, styrene and several primary aromatic amines, and 35 are genotoxic (Kay et al., 2024) (Table 1, column “Key characteristic(s)”, values: mammary carcinogen “MC”, “Genotoxic”).
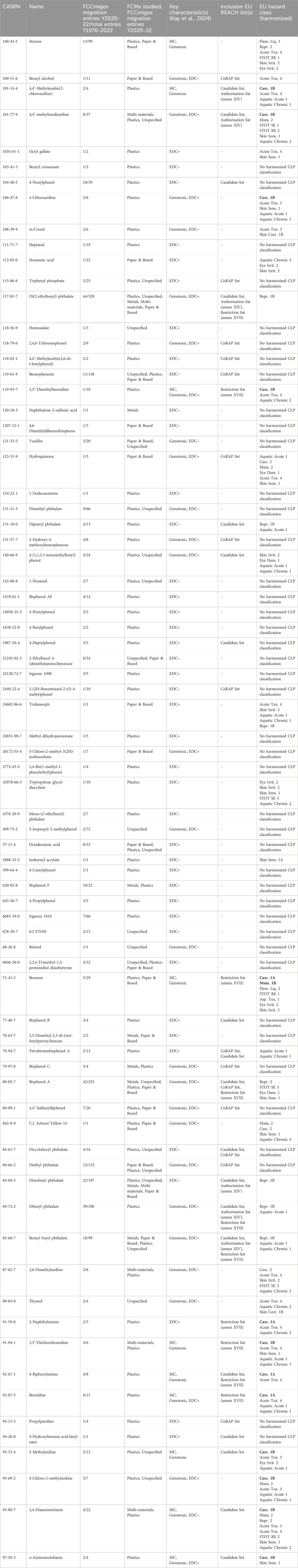
Table 1. Potential mammary carcinogens in food contact materials (FCMs) detected in migration experiments published in 2020, 2021, or 2022. Columns - FCCmigex: The number of database entries from FCCmigex related to migration studies from the years 2020–2022 compared to the total number of FCCmigex database entries where the chemical was detected in an FCM in studies published 1976–2022. FCMs studied: the FCMs tested in migration studies published in 2020–22 where the chemical was detected. Key characteristic(s): Evidence of selected KCs according to the criteria defined by Kay et al. (2024) that align with regulatory concerns in some jurisdictions, including directly inducing mammary carcinogenesis (MC) in rodent models, tested positive for genotoxicity (Genotoxic), high confidence or lower confidence endocrine disrupting (EDC+, EDC∼). EU REACH list(s): on the Community Rolling Action Plan (CoRAP) list, candidate substance of very high concern (Candidate list), or otherwise regulated. EU hazard class: Harmonized CLP classifications, known/presumed carcinogens and mutagens (1A/1B) marked in bold. Cells for which no relevant information was available are marked with (−).
At least 40 of the 76 potential mammary carcinogens detected in the recent migration studies have already been classified with some sort of hazard warning by at least one regulatory agency. For example, 19 and six potential mammary carcinogens are already included in the EU REACH Candidate and Authorisation lists, respectively (European Parliament and Council, 2006). Thirteen chemicals are classified as class-1 carcinogen or mutagen according to Annex VI of the CLP Regulation (European Parliament and Council, 2008). Proposition 65 in California lists 23 of the suspected mammary carcinogens while South Korea’s hazardous chemicals list contains 22, and the Rotterdam Convention lists nine (National Institute of Environmental Research, 2024; OEHHA, 2024; Wagner et al., 2024). At least 26 potential mammary carcinogens detected in recent migration studies belong to categories currently under regulatory scrutiny at various agencies, including six bisphenols, seven ortho-phthalates, twelve aromatic amines, and one PFAS (Lambré et al., 2023; Washington State Department of Ecology, 2023; European Parliament and Council, 2004; US FDA, 2024b).
The ten FCCs with direct evidence of mammary carcinogenesis in rodent models were recently detected in migration studies from FCMs purchased in multiple countries in the EU (Germany, Hungary, Poland, Spain), as well as the United Kingdom, United States, China, and Malaysia.
In all, the 76 recently detected potential mammary carcinogens were in FCMs purchased from markets all over the world including the United States of America (e.g., Sapozhnikova and Nuñez, 2022; Taylor and Sapozhnikova, 2022), India (e.g., Chapke et al., 2022; Mukhopadhyay et al., 2022), China (e.g., Han et al., 2021; Luo et al., 2022), Nigeria (e.g., Ibeto et al., 2022; Ucheana et al., 2022), Ghana (Angnunavuri et al., 2022; Ayamba et al., 2020), Spain (e.g., Blanco-Zubiaguirre et al., 2021; Song et al., 2022), Mexico (de Anda-Flores et al., 2021), Austria (Banaderakhshan et al., 2022), Canada (Siddique et al., 2021; Xu et al., 2023), Syria (Wissam, 2021), Poland (Marć, 2020), Iran (Cheshmazar et al., 2021), Malaysia (Naziruddin et al., 2021; Naziruddin et al., 2020), Denmark (Tisler and Christensen, 2022; Tsochatzis et al., 2021), Egypt (Gamil et al., 2022), Turkey (Alp and Yerlikaya, 2020), Greece (Kalogiouri et al., 2021), and Brazil (Oliveira et al., 2020).
Discussion
The presence of these confirmed and potential mammary carcinogens, despite regulation since 1958 in the United States (US Congress, 1958) and 1976 in the EU (European Council, 1976) specifically targeting carcinogens in FCMs, highlights the shortcomings and gaps of the current regulatory system.
Of the ten recently-detected, migrating FCCs with direct evidence of mammary carcinogenesis, styrene is particularly illustrative. Styrene is a high production volume chemical with nearly 4 decades of evidence of migration into foods that continues to be allowed in FCMs (European Commission, 2024; US FDA, 2024a) despite its classification in the EU as a suspected reproductive toxicant in 2012 (ECHA, 2013), listing as a carcinogen according to California Proposition 65 in 2016 (OEHHA, 2014), and addition to the Republic of Korea’s list of toxic substances in 2021 (National Institute of Environmental Research, 2024). Additionally, styrene was highlighted as an FCC of concern that does not fit the goals outlined in the Chemicals Strategy for Sustainability in 2021 and 2022 (Groh et al., 2021; Zimmermann et al., 2022), as well the recent inclusion in the PlastChem red list and in Kay et al.‘s potential mammary carcinogens list (Kay et al., 2024; Wagner et al., 2024).
In the EU specifically, REACH (Regulation (EC) No 1907/2006) mandates the substitution of SVHCs in industrial products and consumer articles based on their intrinsic hazard properties (European Parliament and Council, 2006). However, verifying that a substance has an intrinsic hazard property, or even multiple of such properties, does not automatically translate to restrictions in FCMs (Geueke and Muncke, 2018; Zimmermann et al., 2022). This is clearly illustrated by the 19 SVHCs included in the list of potential mammary carcinogens that have been recently detected in migration studies of FCAs on the EU market. Such instances beg the question whether the current FCM regulatory system based on risk assessment creates a false sense of safety.
Mammary carcinogenicity is only one endpoint. As consensus around KCs of other health effects develops over the coming years (see www.keycharacteristics.org), the extent to which the population is being exposed to hazardous chemicals via everyday products will become clearer. Regulatory mechanisms should be in place to quickly respond to health concerns that the growing evidence brings to light.
It is important to note that a GRA-based system without consideration of alternatives could lead to regrettable substitutions (Barlow et al., 2015). A balance between protecting public health and the environment, and maintaining technological and economic viability, will need to be found. The KC framework can help with this by identifying intrinsic chemical properties in the context of biological systems that are predictive of hazard. It also simplifies identification of hazardous chemicals by eliminating the need to define each chemical’s adverse outcome pathway or critical mode of action (Guyton et al., 2018), while enabling the identification and prioritization of chemicals based on a wide range of hazardous traits (Luderer et al., 2019). With clear indicators of harmful characteristics, and how any chemical with those characteristics will be regulated, industries can be encouraged to innovate and develop safer alternatives using the KC framework.
A hazard-based regulation using GRA helps to protect against unforeseen exposures and risks that may not be adequately captured by current risk assessment methodologies and risk management approaches (e.g., Maffini et al., 2021). It acknowledges that exposure scenarios can change over time with new uses, technologies, and environmental pathways, offering a safety net against unexpected threats. Simplified chemical assessments relying on a robust, evidence-based component, such as laid out in the KC framework, streamline the assessment process, so that it can be standardized and undertaken by any regulatory agency worldwide, with minimal input data, short time requirements, and at reasonably low cost.
Moving forward, it is advisable for regulatory bodies to reconsider the integration of GRA and hazard-based criteria into the regulation of FCMs, as mandated in the EU by its Chemicals Strategy for Sustainability (European Commission, 2020). This entails not only identifying substances of concern but also addressing the broader categories of chemicals with the potential for diverse adverse health outcomes. Incorporating the KCs of chemicals that induce breast carcinogenesis via different mechanisms of action, of which genotoxicity is only one, into regulation may allow regulators to live up to the ideals espoused in FCM regulations.
Additionally, fostering international collaboration and harmonization of regulatory standards could further strengthen global efforts to manage the risks associated with chemical migration from FCMs. Such proactive measures are crucial in navigating the complexities of the modern food supply chain and ensuring the safety and wellbeing of consumers worldwide–today and for future generations.
Conclusion
While the transition to the generic approach to risk management (GRA), effectively a hazard-based regulatory framework, represents a significant shift in the approach to FCM regulation worldwide, we argue that it is an important and underappreciated opportunity for prevention. Even when considering only a single health endpoint, mammary carcinogenicity, and recent FCM migration data, there are at least 76 known or potential mammary carcinogens migrating from FCMs across the global market. This finding implies that public health protection can be significantly improved by modernized FCM regulations with a focus on hazard identification, for example by employing the KC framework.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Kay et al. 2024 (https://ehp.niehs.nih.gov/doi/10.1289/EHP13233#supplementary-materials) and FCCmigex (https://www.foodpackagingforum.org/fccmigex).
Author contributions
LP: Conceptualization, Formal Analysis, Investigation, Visualization, Writing–original draft, Writing–review and editing. BG: Visualization, Writing–review and editing, Supervision. JM: Conceptualization, Funding acquisition, Writing–review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was enabled by funding from the Minerva Foundation. FPF is a charitable organization funded by unconditional donations and project-related grants. All funding sources are listed on the FPF’s website (www.foodpackagingforum.org).
Acknowledgments
ChatGPT 4o, a language model developed by OpenAI, was used for editorial assistance in the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor LV declared a past co-authorship with the author JM.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2024.1440331/full#supplementary-material
References
Aguilar, F., Autrup, H. N., Barlow, S., Castle, L., Crebelli, R., Engel, K., et al. (2008). Note for guidance for the preparation of an application for the safety assessment of a substance to be used in plastic food contact materials. EFSA J. 6, 21r. doi:10.2903/j.efsa.2008.21r
Alger, H. M., Maffini, M. V., Kulkarni, N. R., Bongard, E. D., and Neltner, T. (2013). Perspectives on how FDA assesses exposure to food additives when evaluating their safety: workshop proceedings. Compr. Rev. Food Sci. Food Saf. 12, 90–119. doi:10.1111/j.1541-4337.2012.00216.x
Alp, A. C., and Yerlikaya, P. (2020). Phthalate ester migration into food: effect of packaging material and time. Eur. Food Res. Technol. 246, 425–435. doi:10.1007/s00217-019-03412-y
Angnunavuri, P. N., Attiogbe, F., and Mensah, B. (2022). Effect of storage on the levels of phthalates in high-density polyethylene (HDPE) film-packaged drinking water. Sci. Total Environ. 845, 157347. doi:10.1016/j.scitotenv.2022.157347
Arvanitoyannis, I. S., and Bosnea, L. (2004). Migration of substances from food packaging materials to foods. Crit. Rev. Food Sci. Nutr. 44, 63–76. doi:10.1080/10408690490424621
Arzuaga, X., Smith, M. T., Gibbons, C. F., Skakkebæk, N. E., Yost, E. E., Beverly, B. E. J., et al. (2019). Proposed key characteristics of male reproductive toxicants as an approach for organizing and evaluating mechanistic evidence in human health hazard assessments. Environ. Health Perspect. 127, 065001. doi:10.1289/EHP5045
Ayamba, A.A.-M., Agyekum, A. A., Derick, C., and Dontoh, D. (2020). Assessment of phthalate migration in polyethylene food contact materials sold on the Ghanaian market. Cogent Environ. Sci. 6, 1794242. doi:10.1080/23311843.2020.1794242
Banaderakhshan, R., Kemp, P., Breul, L., Steinbichl, P., Hartmann, C., and Fürhacker, M. (2022). Bisphenol A and its alternatives in Austrian thermal paper receipts, and the migration from reusable plastic drinking bottles into water and artificial saliva using UHPLC-MS/MS. Chemosphere 286, 131842. doi:10.1016/j.chemosphere.2021.131842
Barlow, S. M., Boobis, A. R., Bridges, J., Cockburn, A., Dekant, W., Hepburn, P., et al. (2015). The role of hazard- and risk-based approaches in ensuring food safety. Trends Food Sci. and Technol. 46, 176–188. doi:10.1016/j.tifs.2015.10.007
Blanco-Zubiaguirre, L., Zabaleta, I., Prieto, A., Olivares, M., Zuloaga, O., and Elizalde, M. P. (2021). Migration of photoinitiators, phthalates and plasticizers from paper and cardboard materials into different simulants and foodstuffs. Food Chem. 344, 128597. doi:10.1016/j.foodchem.2020.128597
Bradley, E., and Coulier, L. (2007). An investigation into the reaction and breakdown products from starting substances used to produce food contact plastics. Food Stand. Agency. Available at: https://webarchive.nationalarchives.gov.uk/ukgwa/20100816210544/http://www.foodbase.org.uk/results.php?f_report_id=518 (Accessed May 28, 2024).
Center for Food Safety and Applied Nutrition (2007). Guidance for industry: preparation of premarket submissions for food contact substances (chemistry recommendations). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-premarket-submissions-food-contact-substances-chemistry (Accessed May 28, 2024).
Center for Food Safety and Applied Nutrition (2021). Guidance for industry: preparation of food contact substance notifications (toxicology recommendations). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-food-contact-substance-notifications-toxicology-recommendations (Accessed May 28, 2024).
Chapke, K., Gandhi, K., Lata, K., Sharma, R., Mann, B., and Singh, N. (2022). Migration study of chemical additives from low density polyethylene (LDPE) into dahi. J. Food Sci. Technol. 59, 3283–3295. doi:10.1007/s13197-022-05453-w
Cheshmazar, E., Arfaeinia, L., Vasseghian, Y., Ramavandi, B., Moradi, M., Hashemi, S. E., et al. (2021). Phthalate acid esters in pickled vegetables packaged in polyethylene terephthalate container: occurrence, migration, and estrogenic activity-associated risk assessment. J. Food Compos. Analysis 99, 103880. doi:10.1016/j.jfca.2021.103880
de Anda-Flores, Y. B., Cordón-Cardona, B. A., González-León, A., Valenzuela-Quintanar, A. I., Peralta, E., and Soto-Valdez, H. (2021). Effect of assay conditions on the migration of phthalates from polyvinyl chloride cling films used for food packaging in México. Food Packag. Shelf Life 29, 100684. doi:10.1016/j.fpsl.2021.100684
ECHA (2023). Substance infocard: styrene. Available at: https://echa.europa.eu/substance-information/-/substanceinfo/100.002.592 (Accessed May 6, 2024).
European Commission (2020). Chemicals strategy for sustainability. Available at: https://circabc.europa.eu/ui/group/8ee3c69a-bccb-4f22-89ca-277e35de7c63/library/dd074f3d-0cc9-4df2-b056-dabcacfc99b6/details?download=true (Accessed May 6, 2024).
European Commission (2022). Commission staff working document evaluation of the legislation on food contact materials - regulation (EC) No 1935/2004. Available at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=SWD:2022:0163:FIN:EN:PDF (Accessed May 6, 2024).
European Commission (2024). Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02011R0010-20230831 (Accessed May 6, 2024).
European Council (1976). Council Directive 76/893/EEC of 23 November 1976 on the approximation of the laws of the Member States relating to materials and articles intended to come into contact with foodstuffs. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31976L0893 (Accessed May 28, 2024).
European Parliament (2016). European parliament resolution of 6 october 2016 on the implementation of the food contact materials regulation (EC) No 1935/2004. Available at: https://www.europarl.europa.eu/doceo/document/TA-8-2016-0384_EN.html (Accessed May 28, 2024).
European Parliament and Council (2004). Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02004R1935-20210327.
European Parliament and Council, European Commission (2006). Regulation (EC) No 1907/2006 of the European parliament and of the Council of 18 december 2006 concerning the registration. Eval. Auth. Restrict. Chem. (REACH). Available at: http://data.europa.eu/eli/reg/2006/1907/2023-12-01/eng (Accessed May 21, 2024).
European Parliament and Council (2008). Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008R1272-20231201 (Accessed May 6, 2024).
Food Packaging Forum Foundation (2022). FCCmigex database. Available at: https://www.foodpackagingforum.org/fccmigex (Accessed April 9, 2024).
Gamil, M., El Zahar, N. M., Magdy, N., and El-Kosasy, A. M. (2022). Green, bioanalytically validated chromatographic method for the determination and quantification of photoinitiators in saliva in contact with baby bibs, teethers and pacifiers. Microchem. J. 181, 107841. doi:10.1016/j.microc.2022.107841
Germolec, D. R., Lebrec, H., Anderson, S. E., Burleson, G. R., Cardenas, A., Corsini, E., et al. (2022). Consensus on the key characteristics of immunotoxic agents as a basis for hazard identification. Environ. Health Perspect. 130, 105001. doi:10.1289/EHP10800
Geueke, B., Groh, K. J., Maffini, M. V., Martin, O. V., Boucher, J. M., Chiang, Y.-T., et al. (2022). Systematic evidence on migrating and extractable food contact chemicals: most chemicals detected in food contact materials are not listed for use. Crit. Rev. Food Sci. Nutr. 63, 9425–9435. doi:10.1080/10408398.2022.2067828
Geueke, B., and Muncke, J. (2018). Substances of very high concern in food contact materials: migration and regulatory background. Packag. Technol. Sci. 31, 757–769. doi:10.1002/pts.2288
Grob, K., Biedermann, M., Scherbaum, E., Roth, M., and Rieger, K. (2006). Food contamination with organic materials in perspective: packaging materials as the largest and least controlled source? A view focusing on the European situation. Crit. Rev. Food Sci. Nutr. 46, 529–535. doi:10.1080/10408390500295490
Groh, K. J., Geueke, B., Martin, O., Maffini, M., and Muncke, J. (2021). Overview of intentionally used food contact chemicals and their hazards. Environ. Int. 150, 106225. doi:10.1016/j.envint.2020.106225
Guyton, K. Z., Rusyn, I., Chiu, W. A., Corpet, D. E., van den Berg, M., Ross, M. K., et al. (2018). Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 39, 614–622. doi:10.1093/carcin/bgy031
Han, Y., Cheng, J., Tang, Z., He, Y., and Lyu, Y. (2021). Widespread occurrence of phthalates in popular take-out food containers from China and the implications for human exposure. J. Clean. Prod. 290, 125851. doi:10.1016/j.jclepro.2021.125851
Ibeto, C., Ezeh, H., Ugwu, I., and Aju, E. (2022). Impact of storage on levels of phthalates in sachet and bottled water brands in enugu state, Nigeria. Int. J. Environ. Anal. Chem. 0, 1404–1416. doi:10.1080/03067319.2022.2038588
Kalogiouri, N. P., Pritsa, A., Kabir, A., Furton, K. G., and Samanidou, V. F. (2021). A green molecular imprinted solid-phase extraction protocol for bisphenol A monitoring with HPLC-UV to guarantee the quality and safety of walnuts under different storage conditions. J. Sep. Sci. 44, 1633–1640. doi:10.1002/jssc.202001199
Kay, J. E., Brody, J. G., Schwarzman, M., and Rudel, R. A. (2024). Application of the key characteristics framework to identify potential breast carcinogens using publicly available in vivo, in vitro, and in silico data. Environ. Health Perspect. 132, 017002. doi:10.1289/EHP13233
Koual, M., Cano-Sancho, G., Bats, A.-S., Tomkiewicz, C., Kaddouch-Amar, Y., Douay-Hauser, N., et al. (2019). Associations between persistent organic pollutants and risk of breast cancer metastasis. Environ. Int. 132, 105028. doi:10.1016/j.envint.2019.105028
Koual, M., Tomkiewicz, C., Cano-Sancho, G., Antignac, J.-P., Bats, A.-S., and Coumoul, X. (2020). Environmental chemicals, breast cancer progression and drug resistance. Environ. Health 19, 117. doi:10.1186/s12940-020-00670-2
Lagunas-Rangel, F. A., Liu, W., and Schiöth, H. B. (2022). Can exposure to environmental pollutants Be associated with less effective chemotherapy in cancer patients? Int. J. Environ. Res. Public Health 19, 2064. doi:10.3390/ijerph19042064
Lambré, C., Barat Baviera, J. M., Bolognesi, C., Chesson, A., Cocconcelli, P. S., Crebelli, R., et al. (2023). Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 21, e06857. doi:10.2903/j.efsa.2023.6857
La Merrill, M. A., Vandenberg, L. N., Smith, M. T., Goodson, W., Browne, P., Patisaul, H. B., et al. (2020). Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 16, 45–57. doi:10.1038/s41574-019-0273-8
Luderer, U., Eskenazi, B., Hauser, R., Korach, K. S., McHale, C. M., Moran, F., et al. (2019). Proposed key characteristics of female reproductive toxicants as an approach for organizing and evaluating mechanistic data in hazard assessment. Environ. Health Perspect. 127, 075001. doi:10.1289/EHP4971
Luo, R., Lin, Q., Zhu, L., Yan, J., and Li, Z. (2022). Detection of primary aromatic amines content in food packaging ink and migration from printed plastic bags. Food Packag. Shelf Life 32, 100820. doi:10.1016/j.fpsl.2022.100820
Maffini, M. V., Geueke, B., Groh, K., Carney Almroth, B., and Muncke, J. (2021). Role of epidemiology in risk assessment: a case study of five ortho-phthalates. Environ. Health 20, 114. doi:10.1186/s12940-021-00799-8
Marć, M. (2020). Emissions of selected monoaromatic hydrocarbons as a factor affecting the removal of single-use polymer barbecue and kitchen utensils from everyday use. Sci. Total Environ. 720, 137485. doi:10.1016/j.scitotenv.2020.137485
McCombie, G. (2018). Enforcement’s perspective. Available at: https://food.ec.europa.eu/system/files/2018-09/cs_fcm_eval-workshop_20180924_pres07.pdf (Accessed May 28, 2024).
MERCOSUR (1992). Criterios Generales de envases y equipamientos alimentarios en contacto con alimentos. Available at: https://normas.mercosur.int/public/normativas/2265 (Accessed April 9, 2024).
Merrill, R. A. (1997). Food safety regulation: reforming the delaney clause. Annu. Rev. Public Health 18, 313–340. doi:10.1146/annurev.publhealth.18.1.313
Mukhopadhyay, M., Jalal, M., Vignesh, G., Ziauddin, M., Sampath, S., Bharat, G. K., et al. (2022). Migration of plasticizers from polyethylene terephthalate and low-density polyethylene casing into bottled water: a case study from India. Bull. Environ. Contam. Toxicol. 109, 949–955. doi:10.1007/s00128-022-03474-x
Muncke, J., Andersson, A.-M., Backhaus, T., Belcher, S. M., Boucher, J. M., Carney Almroth, B., et al. (2023). A vision for safer food contact materials: public health concerns as drivers for improved testing. Environ. Int. 180, 108161. doi:10.1016/j.envint.2023.108161
Muncke, J., Backhaus, T., Geueke, B., Maffini, M. V., Martin, O. V., Myers, J. P., et al. (2017). Scientific challenges in the risk assessment of food contact materials. Environ. Health Perspect. 125, 095001. doi:10.1289/EHP644
National Institute of Environmental Research (2024). National chemicals information system. Available at: https://ncis.nier.go.kr/en/main.do (Accessed May 6, 2024).
National People’s Congress (2015). Food safety law of the People’s Republic of China. Available at: https://www.gov.cn/zhengce/2015-04/25/content_2853643.htm (Accessed April 9, 2024).
Naziruddin, M. A., Jawaid, M., Yusof, N. L., Abdul-Mutalib, N. A., Ahmad, M. F., Sanny, M., et al. (2021). Assessment and detection of the potential contaminants from oil palm empty fruit bunch fiber-based biodegradable tray. Food Packag. Shelf Life 29, 100685. doi:10.1016/j.fpsl.2021.100685
Naziruddin, M. A., Sulaiman, R., Abdul Halim Lim, S., Jinap, S., Nurulhuda, K., and Sanny, M. (2020). The effect of fat contents and conditions of contact in actual use on styrene monomer migrated from general-purpose polystyrene into selected fatty dishes and beverage. Food Packag. Shelf Life 23, 100461. doi:10.1016/j.fpsl.2019.100461
OEHHA (2024). Chemicals considered or listed under proposition 65. Available at: https://oehha.ca.gov/proposition-65/chemicals (Accessed May 6, 2024).
Oldring, P., Faust, B., Gude, T., Lesueur, C., Simat, T., Stoermer, A., et al. (2023). An overview of approaches for analysing NIAS from different FCMs. Zenodo. doi:10.5281/zenodo.7828612
Oliveira, W. da S., Monsalve, J. O., Nerin, C., Padula, M., and Godoy, H. T. (2020). Characterization of odorants from baby bottles by headspace solid phase microextraction coupled to gas chromatography-olfactometry-mass spectrometry. Talanta 207, 120301. doi:10.1016/j.talanta.2019.120301
Sapozhnikova, Y., and Nuñez, A. (2022). Non-targeted analysis with liquid chromatography - high resolution mass spectrometry for the identification of food packaging migrants. J. Chromatogr. A 1676, 463215. doi:10.1016/j.chroma.2022.463215
Siddique, S., Zhang, G., Coleman, K., and Kubwabo, C. (2021). Investigation of the migration of bisphenols from baby bottles and sippy cups. Curr. Res. Food Sci. 4, 619–626. doi:10.1016/j.crfs.2021.08.006
Joint Research Centre: Institute for Health and Consumer Protection (2015). Practical guidelines on the application of migration modelling for the estimation of specific migration. Publ. Office Eur. Union. doi:10.2788/04517
Smith, M. T., Guyton, K. Z., Gibbons, C. F., Fritz, J. M., Portier, C. J., Rusyn, I., et al. (2016). Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ. Health Perspect. 124, 713–721. doi:10.1289/ehp.1509912
Song, X.-C., Canellas, E., Dreolin, N., Goshawk, J., and Nerin, C. (2022). Identification of nonvolatile migrates from food contact materials using ion mobility–high-resolution mass spectrometry and in silico prediction tools. J. Agric. Food Chem. 70, 9499–9508. doi:10.1021/acs.jafc.2c03615
Stevens, S., McPartland, M., Bartosova, Z., Skåland, H. S., Völker, J., and Wagner, M. (2024). Plastic food packaging from five countries contains endocrine- and metabolism-disrupting chemicals. Environ. Sci. Technol. 58, 4859–4871. doi:10.1021/acs.est.3c08250
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Taylor, R. B., and Sapozhnikova, Y. (2022). Assessing chemical migration from plastic food packaging into food simulant by gas and liquid chromatography with high-resolution mass spectrometry. J. Agric. Food Chem. 70, 4805–4816. doi:10.1021/acs.jafc.2c00736
Tisler, S., and Christensen, J. H. (2022). Non-target screening for the identification of migrating compounds from reusable plastic bottles into drinking water. J. Hazard. Mater. 429, 128331. doi:10.1016/j.jhazmat.2022.128331
Trasande, L., Krithivasan, R., Park, K., Obsekov, V., and Belliveau, M. (2024a). Chemicals used in plastic materials: an estimate of the attributable disease burden and costs in the United States. J. Endocr. Soc. 8, bvad163. doi:10.1210/jendso/bvad163
Trasande, L., Nelson, M. E., Alshawabkeh, A., Barrett, E. S., Buckley, J. P., Dabelea, D., et al. (2024b). Prenatal phthalate exposure and adverse birth outcomes in the USA: a prospective analysis of births and estimates of attributable burden and costs. Lancet Planet. Health 8, e74–e85. doi:10.1016/S2542-5196(23)00270-X
Tsochatzis, E. D., Lopes, J. A., Gika, H., Dalsgaard, T. K., and Theodoridis, G. (2021). A fast SALLE GC–MS/MS multi-analyte method for the determination of 75 food packaging substances in food simulants. Food Chem. 361, 129998. doi:10.1016/j.foodchem.2021.129998
Ucheana, I. A., Ihedioha, J. N., Njoku, J. B. C., Abugu, H. O., and Ekere, N. R. (2022). Migration of bisphenol A from epoxy-can malt drink under various storage conditions and evaluation of its health risk. Int. J. Environ. Anal. Chem. 0, 1–20. doi:10.1080/03067319.2022.2098473
US Congress. (1958). Food additives amendment of 1958. Available at: https://uscode.house.gov/statutes/pl/85/929.pdf [Accessed May 28, 2024].
US FDA (2024a). Cumulative estimated daily intake (CEDI). Available at: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=CEDI&id=5003&sort=Substance&order=ASC&startrow=1&type=basic&search=100%2D42%2D5 (Accessed May 21, 2024).
US FDA (2024b). FDA, industry actions end sales of PFAS used in US food packaging. Available at: https://www.fda.gov/news-events/press-announcements/fda-industry-actions-end-sales-pfas-used-us-food-packaging (Accessed April 11, 2024).
US Government Accountability Office (2022). Food safety: FDA oversight of substances used in manufacturing, packaging, and transporting food could Be strengthened | U.S. GAO. Available at: https://www.gao.gov/products/gao-23-104434 (Accessed May 28, 2024).
Wagner, M., Monclus, L., Arp, H. P., Groh, K. J., Løseth, M. E., Muncke, J., et al. (2024). State of the science on plastic chemicals - identifying and addressing chemicals and polymers of concern. Available at: https://plastchem-project.org/.
Wan, M. L. Y., Co, V. A., and El-Nezami, H. (2022). Endocrine disrupting chemicals and breast cancer: a systematic review of epidemiological studies. Crit. Rev. Food Sci. Nutr. 62, 6549–6576. doi:10.1080/10408398.2021.1903382
Wang, Z., Liu, H., and Liu, S. (2017). Low-dose bisphenol A exposure: a seemingly instigating carcinogenic effect on breast cancer. Adv. Sci. 4, 1600248. doi:10.1002/advs.201600248
Washington State Department of Ecology (2023). Phthalates action plan. Available at: https://apps.ecology.wa.gov/publications/SummaryPages/2304067.html (Accessed July 1, 2024).
Wissam, Z. (2021). Levels of BPA in makdous, a traditional Syrian food, using solid-phase extraction followed by HPLC. Braz. J. Pharm. Sci. 57, e19094. doi:10.1590/s2175-97902020000419094
Xu, Z., Chughtai, H., Tian, L., Liu, L., Roy, J.-F., and Bayen, S. (2023). Development of quantitative structure-retention relationship models to improve the identification of leachables in food packaging using non-targeted analysis. Talanta 253, 123861. doi:10.1016/j.talanta.2022.123861
Keywords: food packaging, food contact chemicals, breast cancer, hazard assessment, chemical safety, regulation
Citation: Parkinson LV, Geueke B and Muncke J (2024) Potential mammary carcinogens used in food contact articles: implications for policy, enforcement, and prevention. Front. Toxicol. 6:1440331. doi: 10.3389/ftox.2024.1440331
Received: 29 May 2024; Accepted: 27 August 2024;
Published: 24 September 2024.
Edited by:
Laura N. Vandenberg, University of Massachusetts Amherst, United StatesReviewed by:
Jennifer Kay, Silent Spring Institute, United StatesCopyright © 2024 Parkinson, Geueke and Muncke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jane Muncke, amFuZS5tdW5ja2VAZnAtZm9ydW0ub3Jn
 Lindsey V. Parkinson
Lindsey V. Parkinson Birgit Geueke
Birgit Geueke Jane Muncke
Jane Muncke